Abstract
STUDY QUESTION
Are non-esterified fatty acid (NEFA) kinetics altered in women with polycystic ovary syndrome (PCOS)?
SUMMARY ANSWER
Women with PCOS, particularly obese subjects, have dysregulated plasma NEFA kinetics in response to changes in plasma insulin and glucose levels, which are associated with insulin resistance (IR) independently of the fasting plasma NEFA levels.
WHAT IS KNOWN ALREADY
Elevated plasma NEFA levels are associated with IR in many disorders, although the homeostasis of NEFA kinetics and its relationship to IR in women with PCOS is unknown.
STUDY DESIGN, SIZE, DURATION
We prospectively compared insulin sensitivity and NEFA kinetics in 29 PCOS and 29 healthy controls women matched for BMI.
PARTICIPANTS/MATERIALS, SETTING, METHODS
This study was conducted in a tertiary institution. Plasma NEFA, glucose and insulin levels were assessed during a modified frequently sampled intravenous glucose tolerance test (mFSIVGTT). Minimal models were used to assess insulin sensitivity (Si) and NEFA kinetics (i.e. model-derived initial plasma NEFA level [NEFA0], phi constant [Φ], reflecting glucose-mediated inhibition of lipolysis and measures of maximum rate of lipolysis [SFFA] and NEFA uptake from plasma [KFFA]).
MAIN RESULTS AND THE ROLE OF CHANCE
The study provides new evidence that women with PCOS have defective NEFA kinetics characterized by: (i) lower basal plasma NEFA levels, measured directly and modeled (NEFA0), and (ii) a greater glucose-mediated inhibition of lipolysis in the remote or interstitial space (reflected by a lower affinity constant [Φ]). There were no differences, however, in the maximal rates of adipose tissue lipolysis (SFFA) and the rate at which NEFA leaves the plasma pool (KFFA). The differences observed in NEFA kinetics were exacerbated, and almost exclusively observed, in the obese PCOS subjects.
LIMITATIONS, REASONS FOR CAUTION
Our study did not study NEFA subtypes. It was also cross-sectional and based on women affected by PCOS as defined by the 1990 National Institutes of Health (NIH) criteria (i.e. Phenotypes A and B) and identified in the clinical setting. Consequently, extrapolation of the present data to other phenotypes of PCOS should be made with caution. Furthermore, our data is exploratory and therefore requires validation with a larger sample size.
WIDER IMPLICATIONS OF THE FINDINGS
Dysfunction in NEFA kinetics may be a marker of metabolic dysfunction in nondiabetic obese women with PCOS and may be more important than simply assessing circulating NEFA levels at a single point in time for understanding the mechanism(s) underlying the IR of PCOS.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by NIH grants R01-DK073632 and R01-HD29364 to R.A.; a Career Development Award from MD Medical Group, Moscow, RF, to D.L. and Augusta University funds to Y.-H.C. RA serves as consultant to Ansh Labs, Medtronics, Spruce Biosciences and Latitude Capital. U.E., Z.A., D.L., R.M., Y.-H.C., R.C.B. and Y.D.I.C. have no competing interests to declare.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: PCOS, free fatty acids, non-esterified fatty acids, NEFA, kinetics, insulin sensitivity, insulin resistance
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine-metabolic disorder, affecting 7–10% of women even when the most conservative definition of PCOS is used. PCOS is frequently associated with insulin resistance (IR) and compensatory hyperinsulinemia (DeUgarte et al., 2005), which increases their risk of developing type 2 diabetes (T2DM). However, little is known about the underlying cause of IR in PCOS.
Most of the PCOS-related investigations of IR have focused on insulin-mediated glucose uptake in insulin-sensitive tissues and suppression of hepatic endogenous glucose production (Bergman, 1989; Ezeh et al., 2013a), even though impaired insulin-mediated suppression of lipolysis and plasma non-esterified fatty acids (NEFA) levels are also associated with IR (Chen et al., 1987; Santomauro et al., 1999). Furthermore, plasma NEFA is an important substrate for triglyceride synthesis and provides fuel for β-oxidation in non-adipose tissues. Data concerning the contribution of abnormalities in plasma NEFA to the IR of PCOS is limited (Phelan et al., 2011; Bellanger et al., 2012).
In the present study, we aimed to test the hypothesis that abnormalities in plasma NEFA levels and NEFA kinetics exist in PCOS and that these abnormalities are associated with the IR of these women. We applied the Boston NEFA minimal model using an insulin-modified frequently sampled intravenous glucose tolerance test (mFSIVGTT) (Boston and Moate, 2008a,b; Boston et al., 2008). This model or its modifications has been successfully used in numerous studies to predict the dynamic changes that occur in plasma NEFA concentrations in response to FIVGTT (Boston and Moate, 2008a,b; Boston et al., 2008; Vethakkan et al., 2012; Anholm et al., 2014).
Materials and Methods
Study population
A cohort of 29 women aged 18–45 years with PCOS and 29 healthy controls were prospectively recruited at the Center for Androgen-Related Disorders (CARD) at Cedars Sinai Medical Center (CSMC) in Los Angeles, CA, USA. As we were interested in studying metabolic dysfunction in classic PCOS, we chose to use the 1990 National Institutes of Health (NIH) criteria for PCOS, defined by the presence of (a) oligo-ovulation, (b) biochemical or clinical hyperandrogenism and (c) the exclusion of other known endocrinopathies, as previously described (Azziz et al., 2004). Control women comprised healthy premenopausal women with long-term predictable eumenorrhea, and no evidence of hyperandrogenism or endocrine disorders. Study exclusion criteria included other endocrine disorders, inability to assess menstruation or ovulation status (e.g. prior hysterectomy, bilateral oophorectomy, vaginal agenesis or postmenopausal or premenarcheal state) and use of hormonal medication within the previous three months.
Research subjects were recruited through advertisements or the clinical practice of CARD. To ensure the diagnostic groups matched as closely as possible, we generally first recruited a PCOS subject, then selected a control to match (either from our pool of controls or by specifically seeking a matching control). Our overall recruitment protocol matched controls and PCOS subjects to within a BMI of ±3 kg/m2, an age of ±5 years and race and has been previously described (Ezeh et al., 2013a).
All qualifying subjects underwent a history and physical exam with blood sampling, as previously described (Knochenhauer et al., 1998). All subjects were normoglycemic by a prior 2-hr. oral glucose tolerance test (if PCOS) or a fasting glucose level (if controls). Fasting blood samples were obtained on days 3–8 of a spontaneous or progesterone-induced withdrawal bleed and were assessed for total testosterone (T), free T, dehydroepiandrosterone sulfate (DHEAS) and sex hormone-binding globulin. Height, weight, waist circumference (WC), modified Ferriman-Gallwey (mF-G) hirsutism score and calculated waist-to-hip ratio were determined, as previously described (Azziz et al., 2004). The study was approved by the institutional review board at CSMC, and all subjects provided written informed consent before study entry.
Modified frequently sampled intravenous glucose tolerance test (mFSIVGTT)
All subjects underwent an mFSIVGTT on days 3–8 of a spontaneous or induced withdrawal bleed. In brief, after an overnight fast, two intravenous catheters were placed in each forearm between 8:00 and 9:00 am. Thereafter, intravenous (iv) glucose (0.3 g/kg) was injected at time 0 min. followed by an iv bolus of regular insulin (0.03 U/kg) at time 20 min. Blood samples (2.0 ml) were collected 34 times from −20 min. (relative to the time of glucose administration) to +180 min. Plasma samples were drawn into pre-chilled tubes containing EDTA (for insulin), sodium fluoride potassium oxalate (for glucose) or paraoxon (for NEFA), and samples were frozen at −80°C until assayed. After assaying the plasma glucose, insulin and NEFA, the levels at −20, −15 and 0 min. were averaged to yield respective fasting values. Data were additionally analyzed using minimal models to determine glucose/insulin (Bergman, 1989; Boston et al., 2003) and NEFA kinetics (Boston and Moate, 2008a; Boston et al., 2008), as described below.
Determination of dynamic state insulin sensitivity and NEFA kinetics using minimal models
Glucose and NEFA kinetics were determined using minimal models as previously described (Bergman, 1989; Boston and Moate, 2008a). Of note, until recently, complex intravenous infusion of isotopic tracers to measure glycerol appearance rates and colorimetry were required to estimate whole-body lipolysis and NEFA oxidation rates, respectively (Magkos et al., 2012). More recently, the use of minimal models (i.e. mathematical models using a minimum of inputs) have been demonstrated to be reliable for the assessment of NEFA kinetics (and insulin/glucose kinetics), with many advantages over traditional tracer methods, including avoiding the use of isotope tracer and calorimetry, and their relative practicality, simplicity and lower cost (Thomaseth and Pavan, 2003; Periwal et al., 2008; Roy and Parker, 2006; Boston and Moate, 2008a,b; Boston et al., 2008).
In the minimal model described by Boston and Moate to assess NEFA kinetics during an FSIVGTT (Boston and Moate, 2008a,b; Boston et al., 2008), glucose in a remote compartment, considered a proxy for insulin action in adipose tissue or acting on its own, is the principal driver of adipose lipolysis and NEFA kinetics. Briefly, plasma glucose levels provide a time-delayed input function to a remote glucose compartment via an intracellular signaling pathway and the increase in remote glucose concentration results in a Michaelis–Menten type inhibition of lipolysis, which causes a unique decline from a basal NEFA level, nadir and rebound in the rate of NEFA output into the plasma compartment (Boston and Moate, 2008a,b; Boston et al., 2008). The Boston NEFA model has been demonstrated to predict all the dynamic changes that occur in plasma NEFA concentrations in response to FIVGTT (Boston and Moate, 2008a,b; Boston et al., 2008; Vethakkan et al., 2012; Anholm et al., 2014). The following indices were obtained.
Estimates of glucose/insulin kinetics (Bergman, 1989):
acute insulin response to glucose (AIRg): the first phase endogenous insulin secretion in response to a bolus glucose injection;
insulin sensitivity index (Si): the extent of insulin-mediated glucose uptake;
glucose effectiveness (Sg): the capacity of glucose, at basal insulin level, to mediate its own glucose uptake;
disposition index (Di): the ability of the β-cell to compensate for the prevailing peripheral IR (i.e. Di = Si × AIRg).
Estimates of NEFA kinetics (Boston and Moate, 2008a; Boston et al., 2008):
initial NEFA level (NEFA0): the model-derived NEFA level at time 0;
adjustable Michaelis-Menten type affinity constant (Φ): the extent to which remote compartment (interstitial) glucose inhibits lipolysis (i.e. the lower the constant, the higher the reaction affinity and the faster the glucose-mediated inhibition of lipolysis);
lipolysis (SFFA): the maximum rate at which NEFA enters the plasma pool;
NEFA uptake (KFFA): the maximum rate at which NEFA leaves the plasma pool.
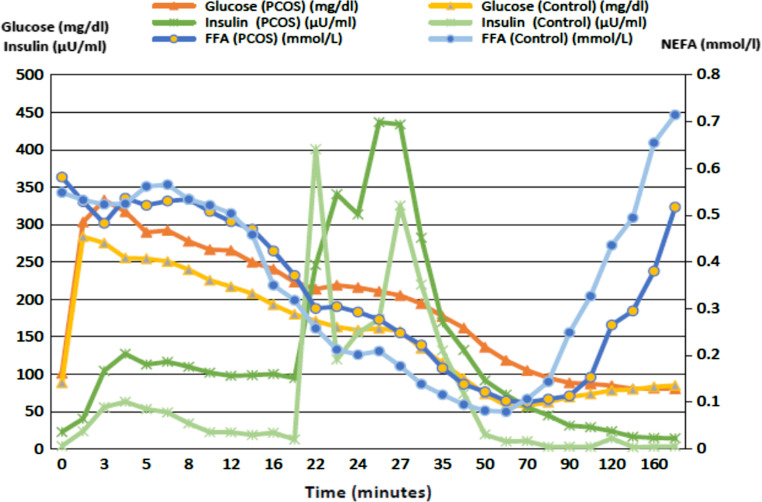
Of note, the Boston minimal model also calculates and uses additional parameters of glucose utilization to estimate NEFA kinetics (i.e. initial glucose level [Gb]: the model-derived glucose level at time 0; initial remote glucose level [R0]: the initial glucose level in the remote (interstitial) compartment; threshold glucose level [gs]: the threshold plasma glucose level above which plasma glucose enters the remote compartment, after a delay of time τ; latency [τ]: the time in minutes that it takes plasma glucose to enter the remote compartment; and dissipation constant [KC]: a rate constant reflecting the ease of movement of plasma glucose in and out of the remote (interstitial) compartment (the lower the KC the less the movement in and out of the remote compartment)), although these were not primary endpoints of the study. See Fig. 1 for example depicting glucose, insulin and NEFA changes during the FSIVGTT.
Figure 1.
Plasma glucose, insulin and NEFA levels in PCOS versus control subjects during an FSIVGTT. Data were generated from 13 insulin resistant PCOS patients (Si < 2.0 min−1 μU−1 mL−1) and 12 insulin sensitive controls (Si > 5.2 min−1 μU−1 mL−1). Data are means.
Determination of basal state insulin sensitivity
Basal state IR and insulin secretion were assessed by the homeostasis model assessment of IR (HOMA-IR) and insulin secretion (HOMA-β%), calculated from fasting glucose and insulin levels, as previously described (Matthews et al., 1985).
Assessment of total, visceral and subcutaneous fat content
There were 22 PCOS (76%) and 14 (48%) controls who also underwent a single-slice computerized axial tomography scan to determine their subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT) and total adipose tissue (TAT) contents, as previously described (Dey et al., 2008; Ezeh et al., 2013a).
Biochemical analysis
Total testosterone (T) was measured using high-turbulence liquid chromatography tandem mass spectrometry (LC–MS/MS). Free T was determined by equilibrium dialysis (Quest Diagnostics, San Juan Capistrano, CA, USA), as previously described (Salameh et al., 2014). In eight patients, total T levels were determined by an extraction chromatography and radioimmunoassay (RIA) method, as previously described (Salameh et al., 2014) and results were converted to LC–MS/MS values (Supplementary Figure S1). DHEAS was measured by a competitive immunoassay (Modular E170; Roche Diagnostics, Indianapolis, IN, USA).
Insulin was assayed by chemiluminescence (ADVIA Centaur chemiluminescent immunoassay system; Siemens Healthcare, Deerfield, IN, USA). Serum glucose levels were measured using the hexokinase/glucose-6-phosphate dehydrogenase method (Roche Applied Sciences, Indianapolis, IN, USA).
Plasma NEFA levels were determined using a colourimetric method (Noma et al., 1973) from Wako Diagnostics (Richmond, VA; catalog no. 991-34691) on the automated instrument, Elan ATAC8000 (Elan Diagnostics, Athlone, Ireland; interassay variation: 2.3–4.8%; intra-assay variation: 5.1–8.6%) (Miller et al., 2012). The method relies on the acylation of coenzyme A (CoA) by NEFA in the presence of added acyl-CoA synthatase. The resulting acyl-CoA is oxidized by added acyl-CoA oxidase with generation of hydrogen peroxide. In the presence of peroxidase, hydrogen peroxide converts the substrate into a colored product, which can be read at 550 nm by the instrument.
Statistical analysis
Data were expressed as mean ± SEM or geometrical mean (range) if log-transformed. The Shapiro–Wilks W test was used to determine if continuous variables were normally distributed. All continuous variables, except for the mF-G score, reasonably followed a parametric normal distribution on the original or log scales, with six variables requiring log transformation (fasting glucose, fasting insulin, HOMA-IR, Si, KC and Φ). Initial differences in PCOS versus control women were evaluated using the Student’s unpaired t-test for normally distributed continuous variables or the Wilcoxon rank-sum test for the mF-G score.
Analysis of covariance models were used with the entire cohort to model the relationship between NEFA kinetics and measures of metabolic dysfunction determined by insulin sensitivity index (Si) while adjusting for differences in other parameters between the groups (PCOS vs. controls). We further assessed the association of each covariate (i.e. mF-G score, free T, total T and DHEAS) with the study groups by modeling group, the potential covariate and group by covariate interactions. If the interaction with group was not significant, we interpreted the model without the interaction term. A similar analysis was conducted to assess the differences between the obese groups (i.e. obese vs. not obese) and the interaction of groups and each covariate (i.e. NEFA0, gs, KC, τ, Φ, SFFA and KFFA).
In determining whether or not to adjust α (P) values for multiple testing, we considered the following. First, only four variables denoting NEFA kinetics (NEFA0, Φ, SFFA and KFFA) were considered primary under the hypothesis explored in this study. All other comparisons were secondary, ancillary or merely descriptive. Second, while failure to adjust for multiple comparisons may result in false positive rates being larger than the set α level, alternatively, the use of these adjustments will adversely impact false negative rates. Since our study is exploratory, affecting false negative rates was of greater concern than impacting false positives. Considering these issues, we chose not to adjust our results for multiple comparisons. All statistical analyses were conducted using the Stats Direct statistics software package, version 2.7.8 2010 (Cheshire, UK).
Results
Baseline features of PCOS and control subjects
The basal characteristics of the study subjects are depicted in Table I. We studied 58 nondiabetic subjects (29 PCOS and 29 healthy controls). As expected, women with PCOS had higher mean mF-G scores and higher mean baseline total T, free T and DHEAS levels than controls. Despite a proactive group matching strategy, controls were slightly older than PCOS subjects; the mean age of PCOS women was 29.2 years (range: 22.0–42.8 years) and 34.5 years for controls (range: 21–45.8 years). There were 17 (58.6%) PCOS and 14 (48.3%) control women who obese (BMI ≥ 30 kg/m2), a non-significant difference. Overall, there were no differences in mean VAT, SAT and TAT between PCOS and matched controls. The results were similar when the study subjects were subcategorized into obese and nonobese subgroups (Table I).
Table I.
Basic characteristics of PCOS and control subjects.
| Variable | All | Obese | Lean | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PCOS (n = 29) | Controls (n = 29) | P value | PCOS (n = 17) | Controls (n = 14) | P value | PCOS (n = 12) | Controls (n = 15) | P value | |
| Age (years) | 29.2 ± 0.9 | 34.5 ± 1.2 | 0.001 | 30. 0 ± 1.3 | 35.9 ± 1.7 | 0.008 | 28.1 ± 1.1 | 33.1 ± 1.8 | 0.035 |
| Body mass index (kg/m2) | 32.1 ± 1.4 | 30.4 ± 1.4 | 0.388 | 36.9 ± 1.4 | 36.5 ± 1.5 | 0.881 | 25.4 ± 0.7 | 24.7 ± 0.7 | 0.504 |
| Waist-Hip Ratio | 0.87 ± 0.02 | 0.85 ± 0.02 | 0.348 | 0.9 0 ± 0.02 | 0.89 ± 0.03 | 0.664 | 0.83 ± 0.02 | 0.81 ± 0.02 | 0.472 |
| Visceral Adipose Tissue (cm2)* | 120.3 ± 15.6 | 108.8 ± 14.3 | 0.617 | 145.8 ± 17.3 | 141.1 ± 15.0 | 0.871 | 52.4 ± 8.2 | 76.5 ± 17.8 | 0.275 |
| Subcutaneous Adipose Tissue (cm2)* | 436.9 ± 15.6 | 370.2 ± 14.3 | 0.282 | 511.4 ± 39.0 | 487.8 ± 47.4 | 0.728 | 238.1 ± 26.9 | 252.743.5 | 0.789 |
| Total Adipose Tissue (cm2)* | 557.2 ± 49.5 | 479.1 ± 58.5 | 0.321 | 657.2 ± 46.0 | 628.9 ± 60.3 | 0.729 | 290.4 ± 33.6 | 329.2 ± 60.8 | 0.605 |
| mF-G score | 7.6 ± 5.1 | 0.89 ± 1.3 | 0.001 | 8.5 ± 1.3 | 0.6 ± 0.4 | 0.001 | 6.25 ± 1.3 | 1.2 ± 0.3 | 0.001 |
| Free Testosterone (pg/mL) | 5.2 ± 0.6 | 2.8 ± 0.3 | 0.001 | 5.8 ± 0.8 | 3.1 ± 0.4 | 0.007 | 4.2 ± 0.7 | 2.3 ± 0.5 | 0.032 |
| Total Testosterone (ng/dL) | 43.8 ± 5.2 | 29.9 ± 2.8 | 0.005 | 42.1 ± 7.6 | 31.4 ± 3.2 | 0.238 | 46.3 ± 6.5 | 27.9 ± 5.0 | 0.036 |
| DHEAS (ug/dL) | 313.7 ± 26.8 | 168.6 ± 21.7 | 0.001 | 331.6 ± 40.6 | 138.1 ± 24.7 | 0.001 | 286 ± 27.0 | 202 ± 35 | 0.073 |
| Fasting plasma NEFA (mg/dL) | 0.493 ± 0.334 | 0.604 ± 0.049 | 0.069 | 0.567 ± 0.0.045 | 0.694 ± 0.052 | 0.074 | 0.388 ± 0.343 | 0.579 ± 0.078 | 0.167 |
| Fasting plasma glucose (mg/dL)** | 91.7 (68.7–110.4) | 89.8 (76.1–106) | 0.414 | 96.3 ± 2.2 | 88.1 ± 2.3 | 0.016 | 86.2 (68.7–100.9) | 91.7 (76.1–106.0) | 0.146 |
| Fasting plasma insulin (μIU/mL)** | 7.1 (1.5–50.6) | 5.0 (1.6–27.3) | 0.11 0 | 15.4 (4.5–50.6) | 8.0 (2.9–27.3) | 0.023 | 3.5 (1.5–12.2) | 3.9 (1.6–17.7) | 0.706 |
| HOMA-IR** | 1.61 (0.27–13.51) | 1.11 (0.35–5.95) | 0.112 | 3.83 (1.03–13.51) | 1.77 (0.15–5.95) | 0.016 | 0.74 (0.27–2.90) | 0.8 (0.35) | 0.572 |
| HOMA-β% | 36.23 ± 7.29 | 22.38 ± 3.98 | 0.101 | 52.1 ± 10.8 | 28.7 ± 6.7 | 0.076 | 13.8 ± 3.2 | 16.5 ± 4.2 | 0.630 |
| AIRg (μU−1/mL) | 559.6 ± 76.9 | 476.2 ± 98.1 | 0.506 | 681.9 ± 101.4 | 679.5 ± 185.8 | 0.991 | 386.3 ± 102.8 | 286.4 ± 43.7 | 0.345 |
| Di | 1593.6 ± 224.7 | 1846.8 ± 242.1 | 0.447 | 1174.2 ± 162.2 | 1863.3 ± 446.6 | 0.13 0 | 2187.7 ± 449.2 | 1831.4 ± 231.7 | 0.462 |
| Si (min−1 . μU−1 . mL−1)** | 2.68 (0.51–19.22) | 4.65 (0.58–14. 00) | 0.012 | 2.02 (0.51–4.54) | 3.71(0.58–7.32) | 0.006 | 5.04 (0.82–19.22) | 6.9 0 (4.06–4.00) | 0.218 |
| Sg (min -1) | 0.0218 ± 0.0019 | 0.0224 ± 0.0022 | 0.833 | 0.0169 ± 0.0021 | 0.0200 ± 0.0030 | 0.389 | 0.0287 ± 0.0026 | 0.0245 ± 0.003 | 0.349 |
Values are expressed as mean ± SE, or mean and range if log transformed. Significant P values are denoted in bold and italics.
Analysis by unpaired t-test p values reported for all variable, except by Wilcoxon p-value for mFG score and HOMA% β-function.
*Assessment of adipose tissue content was performed in 22 PCOS and 14 controls.
**Data were log-transformed prior to analysis.
See text for key to abbreviations.
Insulin sensitivity in PCOS and control subjects
When comparing all PCOS to controls, no significant differences in glucose/insulin parameters were observed, with the exception of insulin sensitivity (as assessed by mean log Si), which was lower in women with PCOS than controls (Table I). Higher fasting plasma glucose and insulin levels and HOMA-IR values and a lower Si and a trend towards a higher HOMA-β% values, were observed when comparing obese PCOS and controls (Table I). Alternatively, there were no differences for any of these parameters, including Si, when comparing nonobese PCOS and nonobese controls (Table I).
NEFA kinetics in PCOS and control subjects
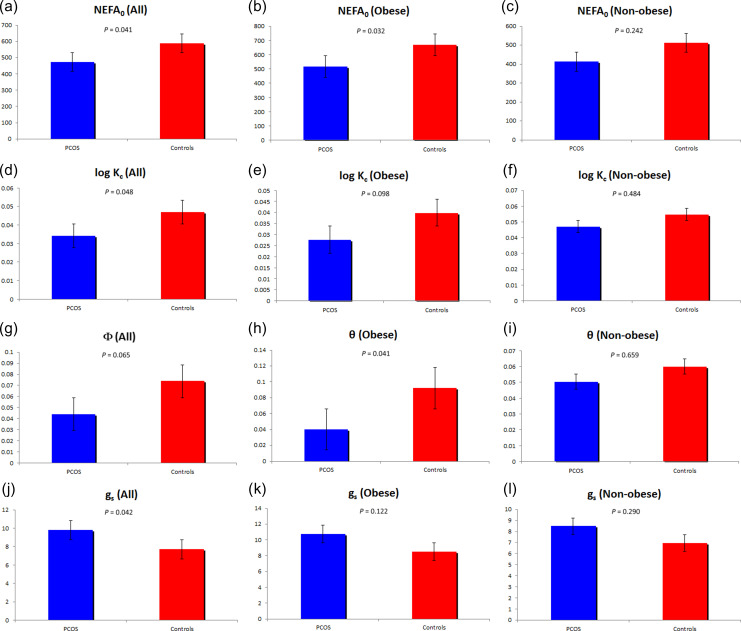
The comparison of NEFA kinetics between PCOS and controls is depicted in Table II and Figure 2. Comparing directly measured fasting plasma NEFA levels, PCOS women tended to have lower levels than controls, although the difference did not reach significance for both the entire cohort and the obese subgroup (Table I). Consistent with this trend, comparing the calculated NEFA value modeled at time 0 (NEFA0), PCOS women had significantly lower values than controls, for the entire cohort and the obese subgroup. PCOS women tended to have a lower affinity constant (Φ) for the entire cohort, reflecting the extent to which the remote compartment glucose inhibited the provision of NEFA to the plasma pool in PCOS women. Alternatively, PCOS and controls were similar in terms of the maximal rates of adipose tissue lipolysis (SFFA) and the rate at which NEFA leaves the plasma pool (KFFA).
Table II.
Non-esterified free fatty acid (NEFA) kinetics in PCOS and control women.
| Variables | All | Obese | Lean | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PCOS (n = 29) | Controls (n = 29) | P value | PCOS (n = 17) | Controls (n = 14) | P value | PCOS (n = 12) | Controls (n = 15) | P value | |
| NEFA0 (μmol/l) | 473. 0 ± 33.0 | 587.3 ± 43.4 | 0.041 | 515.9 ± 45.7 | 668.5 ± 49.9 | 0.032 | 412.1 ± 42.8 | 511.4 ± 65.5 | 0.242 |
| log KC (%/min) | 0.041 (0.007–0.107) | 0.055 (0.021–0.149) | 0.048 | 0.033 (0.007–0.079) | 0.044 (0.021–0.104) | 0.098 | 0.052 (0.024–0.149) | 0.064 (0.026–0.149) | 0.484 |
| SFFA (μmol·l−1·min−1) | 82.3 ± 7.8 | 77.6 ± 5.8 | 0.628 | 89.0 ± 9.6 | 72.9 ± 8.4 | 0.228 | 72.9 ± 13.2 | 81.9 ± 8.2 | 0.553 |
| KFFA (%/min) | 0.088 ± 0.006 | 0.093 ± 0.008 | 0.587 | 0.074 ± 0.007 | 0.071 ± 0.006 | 0.713 | 0.107 ± 0.009 | 0.114 ± 0.013 | 0.659 |
| gs (mmol/l) | 9.8 ± 0.7 | 7.7 ± 0.7 | 0.042 | 10.7 ± 0.9 | 8.5 ± 1.1 | 0.122 | 8.5 ± 0.9 | 7.0 ± 1.0 | 0.29 0 |
| log θ (mmol/l) | 0.044 (0.010–1.300) | 0.075 (0.008–0.409) | 0.065 | 0.040 (0.011–1.300) | 0.092 (0.013–0.409) | 0.041 | 0.050 (0.0208–0.461) | 0.060 (0.008–0.401) | 0.659 |
| τ (min) | 11.2 ± 1.1 | 10.9 ± 0.8 | 0.817 | 10.5 ± 1.5 | 11.5 ± 0.8 | 0.555 | 12.3 ± 1.5 | 10.3 ± 1.3 | 0.335 |
Values are expressed as mean ± SE, or mean and range if log transformed.
See text for key to abbreviations.
Figure 2.
Comparison of indices of plasma NEFA kinetics in PCOS (n = 29) and controls (n = 29). The NEFA indices NEFA0 (Figs. 2a-c), Kc (Figs. 2d-f), (Figs. 2g-i) and gs (Figs. 2j-l) are depicted. All parameters were significantly different, or tended to be, between PCOS and controls when considering all subjects (Figs. 2a, d, g, j), while only NEFA0 and Φ differed in obese subjects (Figs. 2b and h). Non-obese subjects did not differ in any of the NEFA kinetic parameters depicted.
Comparing obese and nonobese subjects separately, we observed that the difference in affinity constant (Φ) became clearly significant when comparing obese PCOS women and controls while the difference in mean dissipation constant (KC) and threshold plasma glucose parameter (gs) values observed for the entire PCOS versus control cohort were no longer observed. Alternatively, when comparing nonobese PCOS and controls, all differences in NEFA kinetics disappeared.
Interaction models of NEFA parameters with obesity status or androgens
Since differences were present in NEFA parameters between obese and nonobese women with PCOS compared to their respective controls, the interactions of NEFA parameters with obesity status (i.e. obese vs. nonobese) were examined. Supplementary Table SI depicts the models with and without the interaction of each covariate with subgroup (obese and nonobese) with respect to log Si. A significant interaction of obese status was found with each of the covariates (P < 0.0001), indicating that differences in obesity status affected the NEFA parameters.
Because women with PCOS demonstrated greater degrees of hyperandrogenism than controls (Table I), the relation of measures of androgenicity (i.e. mF-G score, free T, total T and DHEAS) to NEFA parameters were examined with or without interactions of each covariate with the diagnostic group (i.e. PCOS and control) (Supplementary Table SII). No significant interactions were found between NEFA parameters and mF-G score. However, significant interactions of diagnostic group (i.e. PCOS vs. controls) with free T (P = 0.049) or total T (P = 0.026) were found for affinity constant (Φ). Alternatively, significant interaction of diagnostic group with DHEAS (P = 0.045) was found with respect to threshold plasma glucose parameter (gs) outcome.
Association of indices of NEFA kinetics with insulin sensitivity
There was a lineal correlation between fasting total plasma NEFA levels and log SI in both PCOS (Pearson’s r = −0.38, P-value = 0.045) and controls (r = −0.38, P-value = 0.041). log Si was also used as the dependent variable in multiple linear regression models including diagnostic group (PCOS and control), age, BMI, measures of androgenicity, fasting plasma NEFA level and indices of NEFA kinetics (NEFA0, log KC, gs, log Φ, τ, SFFA and KFFA) as potential independent variables (Table III). In addition to independent association of the group, age and BMI with log Si, log KC and gs were positively associated and NEFA0 and τ were negatively associated, with log Si. About 67.1% (i.e. r2 = 0.671) of the variation in Si could be explained by seven variables included in the model (i.e. diagnostic group, age, BMI, NEFA0, gs, KC and τ).
Table III.
Multple lineal regression analysis predicting insulin sensitivity (Si) in PCOS and controls.
| Independent variables | β-Coefficient ± SE | P value |
|---|---|---|
| Diagnostic group | −0.22 ± 0.10 | 0.037 |
| Age (years) | 0.02 ± 0.01 | 0.030 |
| Body mass index (kg/m2) | −0.02 ± 0.01 | 0.001 |
| NEFA0 (μmol/l) | −0.24 ± 0.10 | 0.027 |
| gs (mmol/l) | 0.68 ± 0.33 | 0.044 |
| log KC (%/min) | 0.56 ± 0.19 | 0.007 |
| Latency (τ) (mins) | −0.47 ± 0.19 | 0.018 |
Gb, R0, log θ, SFFA and KFFA and androgen values demonstrated no independent association with log Si.
Diagnostic group was coded as PCOS = 1 and Control = 0.
R2, % = 67.1 (R2 is the regression coefficient of determination, estimating the percent of the total variation in the outcome that is accounted for by predicting variables).
See text for key to abbreviations.
Discussion
This study evaluated the kinetics of NEFA and their relationship with whole-body insulin sensitivity in nondiabetic women with PCOS. In line with other studies, we found that PCOS women overall had impaired whole-body insulin sensitivity compared to controls matched for adiposity (Ezeh et al., 2013a). The study also provides new evidence that women with PCOS have defective NEFA kinetics characterized by (i) lower basal plasma NEFA levels, measured directly and modeled (NEFA0), and (ii) a greater glucose-mediated inhibition of lipolysis in the remote or interstitial space (i.e. reflected by a lower affinity constant [Φ]). There were no differences, however, in the maximal rates of adipose tissue lipolysis (SFFA) and the rate at which NEFA leaves the plasma pool (KFFA). The differences observed in insulin sensitivity and NEFA kinetics were exacerbated and almost exclusively observed in the obese PCOS subjects.
Overall, these data suggest that in nondiabetic women with PCOS, particularly obese individuals, there is a lower capacity to facilitate the provision of NEFA into the circulation. Additionally, our data suggested that adipose tissue metabolism of NEFA itself is not altered in PCOS. Our results also suggested that the differences between women with PCOS and controls with regards to NEFA kinetics may depend, to some extent, on differences in androgenicity and obesity.
We also observed that dysfunction in NEFA kinetics was independently associated with differences in mean Si in PCOS and control women, regardless of the subjects’ age, BMI and basal plasma NEFA levels. In fact, ~70% of the variation in Si could be explained by the variables included in our models, suggesting that our study was able to capture a significant proportion of the factors determining whole-body insulin sensitivity. These data suggest that the higher degrees of metabolic dysfunction in PCOS patients, particularly in obese individuals, may be associated, at least in part, with dysfunction in NEFA kinetics and not directly due to excess circulating NEFA levels.
The mechanisms underlying the observed dysfunction in NEFA kinetics, while preserving adipose tissue metabolism of NEFA itself, remain to be determined. Postprandial spillover of NEFA from hydrolysis of circulating triglycerides (Fielding, 2011) or dysfunction in adipose NEFA re-esterification (Pereira et al., 2016), which were not assessed in this study, may affect plasma NEFA levels without manifestation of defects in lipolysis or lipid β-oxidation. Our data may also be the result of the high sensitivity of lipolysis to small increases in circulating insulin levels (Jensen et al., 1989; Magkos et al., 2012) with the result that the degrees of hyperinsulinemia present in PCOS women could be sufficient to potentially normalize the rates of lipolysis and NEFA uptake. Furthermore, IR can result from other causes including disturbance in mitochondrial oxidative function, intracellular NEFA trafficking and lipotoxicity of non-adipose tissues, without necessarily manifesting evidence of abnormalities in plasma NEFA levels, lipolysis and NEFA uptake (Mittendorfer, 2011). Because NEFA levels are associated with increased fat mass (Mittendorfer, 2011), the fact that our PCOS patients were matched for BMI and abdominal adiposity may also explain the similar rate of lipolysis and NEFA uptake between the PCOS group and the controls.
Our findings of a tendency to lower fasting plasma NEFA levels and a lower calculated NEFA0 value in PCOS compared to controls were surprising, giving reports of dyslipidemia in PCOS (Lim et al., 2013) and impaired insulin-mediated suppression of lipolysis in many IR states (Mittendorfer, 2011). Our results also differ from previous studies in adult populations, which either did not observe a difference in circulating NEFA levels between PCOS and controls (Ciampelli et al., 2005; Patel et al., 2017) or found higher levels of circulating NEFA in PCOS subjects, particularly those who were obese (Holte et al., 1994; Zhao et al., 2014).
The differing circulating NEFA levels may be due to a number of factors, including the impact of the antagonistic effects of hyperinsulinemia and hyperandrogenemia on plasma NEFA levels in PCOS (Li et al., 2017), adaptation of adipose tissue to obesity in terms of whole-body NEFA output into the circulation (McQuaid et al., 2011) and marked diurnal variability in NEFA levels (Boston and Moate, 2008a; Boston et al., 2008). Furthermore, fasting plasma NEFA levels do not appear to reflect day-long NEFA activities in insulin-sensitive tissues (McQuaid et al., 2011). The poor independent association between fasting plasma NEFA levels and insulin sensitivity in our study is in line with other large studies (Baldeweg et al., 2000; Magkos et al., 2012), indicating that other factors may be involved.
It should be noted that there are very few studies that have addressed NEFA dysfunction in PCOS, some of which agree and others disagree with our findings. A study of nine adolescent girls (8–14 years) with a family history of PCOS versus ten controls found that the PCOS-at risk group had impaired insulin sensitivity (Si), β-cell function and insulin-mediated suppression of plasma NEFA levels assessed by an mFSIVGTT, although they had greater BMI and WC (Trottier et al., 2012). Another study investigated NEFA dysfunction in 21 obese PCOS girls versus 21 matched controls, assessing Si by hyperinsulinemic–euglycemic clamp and NEFA dynamics by tracer techniques (Kim et al., 2018). The investigators reported that PCOS girls had larger VAT and diminished Si, suppression of plasma NEFA, lipolysis and lipid oxidation, although fasting plasma NEFA levels were similar between the groups. Another study conducted in adult obese PCOS women found increased fasting plasma NEFA levels and abdominal adiposity in PCOS compared to controls (Holte et al., 1994). Therefore, the results of these studies may be confounded by the impact of visceral adiposity, which is usually associated with impaired insulin-mediated suppression of plasma NEFA levels, IR and lipid oxidation (Mittendorfer, 2011).
Our study has a number of major strengths. First, it includes well phenotyped and matched subjects with PCOS and controls. Second, our sample size is greater than that in most prior studies. Third, this study includes a comprehensive and novel assessment of plasma NEFA kinetics, in addition to assessing insulin-mediated glucose uptake in both static and dynamic states.
However, our study also has potential limitations. First, a comprehensive profile of NEFA by subtype was not performed and there are data to suggest that the various subtypes of NEFA vary differently according to pathology (Zhao et al., 2014). Second, the cross-sectional nature of our study does not allow us to examine causal relationships between IR and NEFA kinetics in PCOS. Third, the fact that the minimal model calculations for NEFA kinetics uses glucose-related parameters (e.g. KC and gs) implies that the estimation may be highly susceptible to severe alterations in glucose/insulin kinetics and the model may not adequately describe NEFA kinetics in such situations. Fourthly, our study was based on women affected by PCOS as defined by the 1990 NIH criteria (i.e. phenotypes A and B as currently defined) and who were recruited from the clinical setting. These women tend to have a more severe metabolic and androgenic phenotype than other phenotypes of the syndrome (Ezeh et al., 2013b), and consequently, we should be cautious when extrapolating the results this study (and any other similar study) to the entirety of the PCOS population.
In conclusion, our data suggest that nondiabetic women with PCOS, particularly obese subjects, may have dysregulated plasma NEFA kinetics in response to changes in circulating insulin and glucose and that this dysfunction appears to be associated with the insulin sensitivity of these patients. Our data also suggest that understanding NEFA kinetics may be more important than simply assessing circulating NEFA levels at a single point in time when assessing the mechanism(s) underlying the IR of PCOS. Longitudinal and interventional studies will be required to determine which parameters of NEFA kinetics may prove to be markers of early metabolic dysfunction and which are causative. Finally, the results of our study are preliminary and further research will be required to confirm these findings, elucidate the underlying molecular etiology and determine whether current treatments that improve insulin sensitivity also correct the dysfunction in NEFA kinetics.
Supplementary Material
Acknowledgments
The authors thank Laura Sherrouse Hubbard for assistance with manuscript preparation and Dr. Marita Pall with the identification of subjects and performance of testing.
Authors’ roles
U.E., Y.D.I.C. and R.A. designed the study, identified and phenotyped subjects, researched data, contributed to discussion, wrote the manuscript and reviewed and edited the manuscript. R.M. assisted in phenotyping subjects, obtained study samples, researched data and reviewed the manuscript. D.L. and Y.D.I.C. researched data and reviewed and edited the manuscript. Y.-H.C. and Z.A. performed the mFSIVGTT sample analyses and reviewed and edited the manuscript. R.C.B. analyzed the data for NEFA kinetics and reviewed and edited the manuscript. U.E. also performed additional statistical analysis. R.A. is the guarantor of this work and, as such, takes responsibility for the data integrity and accuracy of the data analysis.
Funding
National Institutes of Health (1-K24-HD01346 and R01-DK073632 to R.A.); a Career Development Award from MD Medical Group, Moscow, RF (to D.L.); and Augusta University funds (to Y.-H.C.).
Conflict of interest
R.A. serves as consultant to Ansh Labs, Medtronics, Spruce Biosciences and Latitude Capital. U.E., Z.A., D.L., R.M., Y.-H.C., R.C.B. and Y.D.I.C. have no competing interests to declare.
References
- Anholm C, Kumarathurai P, Klit MS, Kristiansen OP, Nielsen OW, Ladelund S, Madsbad S, Sajadieh A, Haugaard SB. Adding liraglutide to the backbone therapy of biguanide in patients with coronary artery disease and newly diagnosed type-2 diabetes (the AddHope2 study): a randomized controlled study protocol. BMJ Open 2014;4:e005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745–2749. [DOI] [PubMed] [Google Scholar]
- Baldeweg SE, Golay A, Natali A, Balkau B, Del Prato S. Coppack. Insulin resistance, lipid and fatty acid concentrations in 867 healthy Europeans. European Group for the Study of Insulin Resistance (EGIR). Eur J Clin Invest 2000;30:45–52. [DOI] [PubMed] [Google Scholar]
- Bellanger S, Battista MC, Fink GD, Baillargeon JP. Saturated fatty acid exposure induces androgen overproduction in bovine adrenal cells. Steroids 2012;77:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman RN. Lilly lecture. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes 1989;38:1512–1517. [DOI] [PubMed] [Google Scholar]
- Boston RC, Moate PJ. A novel minimal model to describe NEFA kinetics following an intravenous glucose challenge. Am J Physiol Regul Integr Comp Physiol 2008. a;294:R1140–R1147. [DOI] [PubMed] [Google Scholar]
- Boston RC, Moate PJ. NEFA minimal model parameters estimated from the oral glucose tolerance test and the meal tolerance test. Am J Physiol Regul Integr Comp Physiol 2008. b;295:R395–R403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boston R, Roche JR, Ward GM, Moate PJ. A novel minimal model to describe non-esterified fatty acid kinetics in Holstein dairy cows. J Dairy Res 2008;75:13–18. [DOI] [PubMed] [Google Scholar]
- Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 2003;5:1003–1015. [DOI] [PubMed] [Google Scholar]
- Chen YD, Golay A, Swislocki AL, Reaven GM. Resistance to insulin suppression of plasma free fatty acid concentrations and insulin stimulation of glucose uptake in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1987;64:17–21. [DOI] [PubMed] [Google Scholar]
- Ciampelli M, Leoni F, Cucinelli F, Mancuso S, Panunzi S, De Gaetano A, Lanzone A. Assessment of insulin sensitivity from measurements in the fasting state and during an oral glucose tolerance test in polycystic ovary syndrome and menopausal patients. J Clin Endocrinol Metab 2005;90:1398–1406. [DOI] [PubMed] [Google Scholar]
- DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril 2005;83:1454–1460. [DOI] [PubMed] [Google Scholar]
- Dey D, Suzuki Y, Suzuki S, Berman DS. Automated quantification of pericardiac fat from non-contrast CT. Invest Radiol 2008;43:145–153. [DOI] [PubMed] [Google Scholar]
- Ezeh U, Pall M, Mathur R, Dey D, Berman D, Chen IY, Dumesic DA, Azziz R. Effects of endogenous androgens and abdominal fat distribution on the interrelationship between insulin and non-insulin-mediated glucose uptake in females. J Clin Endocrinol Metab 2013. a;98:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeh U, Yildiz BO, Azziz R. Referral bias in defining the phenotype and prevalence of obesity in polycystic ovary syndrome . J Clin Endocrinol Metab 2013. b;98:E1088–E1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding B. Tracing the fate of dietary fatty acids: metabolic studies of postprandial lipaemia in human subjects. Proc Nutr Soc 2011;70:342–350. [DOI] [PubMed] [Google Scholar]
- Holte J, Bergh T, Berne C, Lithell H. Serum lipoprotein lipid profile in women with the polycystic ovary syndrome: relation to anthropometric, endocrine and metabolic variables. Clin Endocrinol (Oxf) 1994;41:463–471. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Caruso M, Heiling V, Miles JM. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes 1989;38:1595–1601. [DOI] [PubMed] [Google Scholar]
- Kim JY, Tfayli H, Michaliszyn SF, Arslanian S. Impaired Lipolysis, Diminished Fat Oxidation, and Metabolic Inflexibility in Obese Girls With Polycystic Ovary Syndrome. J Clin Endocrinol Metab 2018;103:546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 1998;83:3078–3082. [DOI] [PubMed] [Google Scholar]
- Li S, Chu Q, Ma J, Sun Y, Tao T, Huang R, Liao Y, Yue J, Zheng J, Wang Let al. Discovery of novel lipid profiles in PCOS: do insulin and androgen oppositely regulate bioactive lipid production? J Clin Endocrinol Metab 2017;102:810–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev 2013;14:95–109. [DOI] [PubMed] [Google Scholar]
- Magkos F, Fabbrini E, Conte C, Patterson BW, Klein S. Relationship between adipose tissue lipolytic activity and skeletal muscle insulin resistance in nondiabetic women. J Clin Endocrinol Metab 2012;97:E1219–E1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- McQuaid SE, Hodson L, Neville MJ, Dennis AL, Cheeseman J, Humphreys SM, Ruge T, Gilbert M, Fielding BA, Frayn KNet al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 2011;60:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Pereira RI, Langefeld CD, Lorenzo C, Rotter JI, Chen YD, Bergman RN, Wagenknecht LE, Norris JM, Fingerlin TE. Levels of free fatty acids (FFA) are associated with insulin resistance but do not explain the relationship between adiposity and insulin resistance in Hispanic Americans: the IRAS Family Study. J Clin Endocrinol Metab 2012;97:3285–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorfer B. Origins of metabolic complications in obesity: adipose tissue and free fatty acid trafficking. Curr Opin Clin Nutr Metab Care 2011;14:535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A, Okabe H, Kita M. A new colorimetric micro-determination of free fatty acids in serum. Clin Chim Acta 1973;43:317–320. [DOI] [PubMed] [Google Scholar]
- Patel SS, Truong U, King M, Ferland A, Moreau KL, Dorosz J, Hokanson JE, Wang H, Kinney GL, Maahs DMet al. Obese adolescents with polycystic ovarian syndrome have elevated cardiovascular disease risk markers. Vasc Med 2017;22:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MJ, Skrtic S, Katsogiannos P, Abrahamsson N, Sidibeh CO, Dahgam S, Månsson M, Risérus U, Kullberg J, Eriksson JW. Impaired adipose tissue lipid storage, but not altered lipolysis, contributes to elevated levels of NEFA in type 2 diabetes. Degree of hyperglycemia and adiposity are important factors. Metabolism 2016;65:1768–1780. [DOI] [PubMed] [Google Scholar]
- Periwal V, Chow CC, Bergman RN, Ricks M, Vega GL, Sumner AE. Evaluation of quantitative models of the effect of insulin on lipolysis and glucose disposal. Am J Physiol Regul Integr Comp Physiol 2008;295:R1089–R1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan N, O’Connor A, Kyaw Tun T, Correia N, Boran G, Roche HM, Gibney J. Hormonal and metabolic effects of polyunsaturated fatty acids in young women with polycystic ovary syndrome: results from a cross-sectional analysis and a randomized, placebo-controlled, crossover trial. Am J Clin Nutr 2011;93:652–662. [DOI] [PubMed] [Google Scholar]
- Roy A, Parker RS. Dynamic modeling of free fatty acid, glucose, and insulin: an extended ‘minimal model’. Diabetes Technol Ther 2006;8:617–626. [DOI] [PubMed] [Google Scholar]
- Salameh WA, Redor-Goldman MM, Clarke NJ, Mathur R, Azziz R, Reitz RE. Specificity and predictive value of circulating testosterone assessed by tandem mass spectrometry for the diagnosis of polycystic ovary syndrome by the National Institutes of Health 1990 criteria. Fertil Steril 2014;101:1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 1999;48:1836–1841. [DOI] [PubMed] [Google Scholar]
- Thomaseth K, Pavan A. Model-based analysis of glucose and free fatty acid kinetics during glucose tolerance tests. In: Hargrove JL, Berdanier CD (eds). Mathematical Modeling in Nutrition and Toxicology. Athens, GA: Mathematical Biology Press, 2003, 21–40. [Google Scholar]
- Trottier A, Battista MC, Geller DH, Moreau B, Carpentier AC, Simoneau-Roy J, Baillargeon JP. Adipose tissue insulin resistance in peripubertal girls with first-degree family history of polycystic ovary syndrome . Fertil Steril 2012;98:1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vethakkan SR, Walters JM, Gooley JL, Boston RC, Kay TW, Goodman DJ, Jenkins AJ, Ward GM. Normalized NEFA dynamics during an OGTT after islet transplantation. Transplantation 2012;94:e49–e51. [DOI] [PubMed] [Google Scholar]
- Zhao X, Xu F, Qi B, Hao S, Li Y, Li Y, Zou L, Lu C, Xu G, Hou L. Serum metabolomics study of polycystic ovary syndrome based on liquid chromatography-mass spectrometry. J Proteome Res 2014;13:1101–1111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.