Abstract
STUDY QUESTION
Are urinary levels of oxidative stress biomarkers associated with reproductive outcome success following fertility treatments?
SUMMARY ANSWER
Levels of oxidative stress in the middle tertile for women are associated with the highest levels of reproductive success while no associations were noted for men.
WHAT IS KNOWN ALREADY
Oxidative stress may contribute to adverse fertility outcomes in the general population, but findings from couples undergoing fertility treatments are sparse.
STUDY DESIGN, SIZE, DURATION
This prospective cohort study included 481 women and 249 of their male partners undergoing fertility treatments from 2007 to 2015, from the Environment and Reproductive Health (EARTH) study in Boston, MA.
PARTICIPANTS/MATERIALS, SETTING, METHODS
One urine sample per participant was collected at each cycle and analysed for two oxidative stress markers: 8-isoprostane-PGF2α (8-iso-PGF2α) and 8-isoprostane-PGF2α metabolite (F2-isoP-M). Reproductive outcomes were abstracted from medical records and included the fertilization rate, for IVF (oocytes fertilized/mature oocytes retrieved), and rates of implantation, clinical pregnancy and live birth, for both IVF and IUI. Cluster-weighted generalized estimating equations were used to analyse adjusted associations between exposure tertiles and outcomes.
MAIN RESULTS AND THE ROLE OF CHANCE
Levels of F2-isoP-M in the middle tertile were associated with the most success among women. Women in the upper tertile of F2-isoP-M had an adjusted mean live birth rate after IVF and IUI of 23% (95% CI: 17, 29) compared to 38% (95% CI: 31, 45) for women in the middle tertile and 27% (95% CI: 21, 34) in the lower tertile. The fertilization rate during IVF was higher for women with 8-iso-PGF2α in the middle tertile (0.77 [95% CI: 0.73, 0.80]) compared to women in the lower (0.69 [95% CI: 0.64, 0.73]) or upper tertiles (0.66 [95% CI: 0.61, 0.71]). No significant associations were found for other measured outcomes with 8-iso-PGF2α, or between any oxidative stress biomarker in men and reproductive outcomes in their partners.
LIMITATIONS, REASONS FOR CAUTION
Isoprostanes are short-lived biomarkers and this study may not have captured the most relevant window of susceptibility for oxidative stress on the outcomes of interest. Findings from this study may not be generalizable to couples attempting conception without fertility assistance.
WIDER IMPLICATIONS OF THE FINDINGS
This study suggests that a non-linear association may exist between oxidative stress and reproductive outcomes in a population undergoing fertility treatment, a finding not previously identified in the literature. Oxidative stress may represent the mechanism through which environmental chemicals are associated with adverse reproductive outcomes.
STUDY FUNDING/COMPETING INTEREST(S)
This research was supported by the Intramural Research Program of the National Institutes of Environmental Health Sciences (NIEHS) (ZIA ES103314) and by NIEHS grants R01ES022955, R01ES009718 and R01ES00002. There are no competing interests to report.
TRIAL REGISTRATION NUMBER
N/A
Keywords: oxidative stress, IVF, fertility determinant, assisted reproductive techniques, isoprostane
Introduction
Impaired fecundity, characterized by difficulty either getting pregnant or carrying a pregnancy to a live birth, affects an estimated 12% of United States (US) women aged 15–44 (CDC, 2017). Over 7 million women in the US have used fertility services in their lifetimes and utilization of services has been increasing in recent years (CDC, 2018b; 2017). IVF is one of the most common fertility treatments and involves retrieval of a woman’s mature oocytes, fertilization by sperm in a lab and subsequent transfer into the woman. In 2016, over 260 000 cycles of IVF were performed in the US, with roughly 25% of those cycles resulting in live birth (CDC, 2018a). IUI is a fertility treatment in which sperm is inserted directly into a woman’s uterus and has lower success rates compared to IVF. Given the increasing use of IVF and IUI and the stable live birth rates among sub-fertile populations, investigation of potential determinants of outcome is warranted.
Oxidative stress is a biological imbalance between reactive oxygen species (ROS) and antioxidants that leads to cellular damage. Levels are influenced by factors such as diet (Vetrani et al., 2013), exercise (Carraro et al., 2018), age (Cui et al., 2012) and smoking status (Nagai et al., 2006). Environmental exposures have also been implicated as a cause of oxidative stress and represent a modifiable risk factor (Al-Gubory, 2014).
Furthermore, oxidative stress may play a role in infertility as a balance between ROS and antioxidants is needed for proper reproductive function in both women and men. In women, processes including follicular development (Sabatini et al., 1999), oocyte maturation (Shiotani et al., 1991, Martin-Romero et al., 2008), implantation (Jauniaux et al., 2000) and embryo development (Bedaiwy et al., 2004) can be affected by oxidative stress. In men, ROS are necessary for sperm capacitation (Ford, 2004), but spermatozoa can also be damaged by an overproduction of ROS (Aitken and Clarkson, 1987, Bykova et al., 2007).
Results from previous epidemiologic studies suggest that among women seeking fertility treatments, intrafollicular concentrations of oxidative stress biomarkers are higher among those with high rates of degenerate oocytes and are associated with lower fertilization potential of oocytes (Tamura et al., 2008, Das et al., 2006). Additionally, women with unexplained infertility had lower levels of total antioxidant status compared to fertile women (Polak et al., 2001). Reproductive conditions associated with impaired fertility (e.g. endometriosis, polycystic ovary syndrome) have also been associated with higher levels of oxidative stress (Fenkci et al., 2003, Bedaiwy et al., 2002, Dong et al., 2001). However, research has primarily examined biomarkers of oxidative stress within follicular or peritoneal fluid, rather than circulating measures of oxidative stress.
In men, the link between oxidative stress and fertility difficulties is well-established and thought to be mediated through DNA damage in spermatozoa (Simon et al., 2011, Dorostghoal et al., 2017, Oleszczuk et al., 2016). However, previous studies have measured oxidative stress in sperm or ejaculates, rather than circulating measures. Circulating measures are more easily collected and may serve as a more accessible diagnostic tool if an association with outcomes can be established.
The Environment and Reproductive Health (EARTH) cohort was designed to investigate dietary and environmental influences on fertility and results from this paper may help elucidate findings from previous studies; oxidative stress has previously been associated with various environmental and dietary exposures and may be the mechanism through which these exposures are associated with fertility outcomes. Using a prospective cohort of couples undergoing fertility treatments, we examined the association between systemic oxidative stress levels in urine and reproductive success across multiple IVF and IUI cycles among couples attending a fertility centre in Boston, MA.
Materials and Methods
Study population
This prospective analysis comprises women and men enrolled in the EARTH study, a prospective cohort designed to examine determinants of fertility (Messerlian et al., 2018). Women undergoing fertility treatment and their partners were recruited at the Massachusetts General Hospital (MGH) Fertility Center from November 2007 through April 2015. Women were eligible for inclusion in the study if they were between the ages of 18 and 45 and contributed their own oocytes for the fertility treatment. Men between the ages of 18 and 51 were eligible. For the present analysis, we included women who contributed at least one urine sample during an IUI and/or IVF cycle and, where available, their male partners who also provided a urine sample for at least one cycle. Oxidative stress measurements were also required for inclusion in this analysis.
Ethical approval
EARTH protocols were approved by the Human Studies Institutional Review Boards at MGH and Harvard T.H. Chan School of Public Health. All participants provided written informed consent before any study involvement.
Clinical data
Prior to any treatments, all couples met with a physician at the MGH Fertility Center who assigned a primary infertility diagnosis based on definitions from the Society for Assisted Reproductive Technology (Mok-Lin et al., 2010). Oocytes underwent either conventional IVF or intracytoplasmic sperm injection as clinically indicated. Women participating in conventional IVF underwent one of three controlled ovarian stimulation treatment protocols: (i) luteal phase GnRH-agonist protocol; (ii) follicular-phase GnRH-agonist/flare protocol; or (iii) GnRH-antagonist protocol. Oocytes were subsequently retrieved when the dimensions of at least three follicles on a transvaginal ultrasound reached 16–18 mm and estradiol levels reached ≥500 pg/ml. The fertilization rate was defined as the number of fertilized oocytes with two pronuclei divided by the total number of metaphase II oocytes inseminated, as determined by embryologists 17–20 hours after insemination. Clinical outcomes of implantation, clinical pregnancy and live birth rates were measured per IVF cycle initiated. Implantation was defined as a serum β-hCG level >6m IU/ml, usually measured at approximately 17 days after oocyte retrieval. Clinical pregnancy was ascertained through the elevated β-hCG measures and confirmation of an intrauterine pregnancy via ultrasound at 6 weeks. Live birth was defined as the birth of a neonate on or after 24 weeks completed gestation.
For couples undergoing IUI, women were treated with clomiphene citrate or gonadotropins on cycle day 3 or 5. Ovulation was induced using recombinant human chorionic gonadotropin (hCG) and artificial insemination followed approximately 36 hours later. Implantation, clinical pregnancy and live birth following IUI were defined with the same criteria used for IVF. Success rates presented reflect success among the total cycles, not among those with a previous successful step (i.e. clinical pregnancy success rate is among all cycles, not among women with a successful implantation).
Oxidative stress measures
Men and women provided one spot urine sample per cycle at the time of oocyte retrieval during the IVF procedure or at the time of insemination for IUI. Urine was collected at the MGH Fertility Center in a sterile, clean polypropylene specimen cup using midstream clean catch urine protocol. Specific gravity (sg), a measure of urine dilution, was measured within an hour of the sample collection using a handheld refractometer. Urine was aliquoted and frozen at −20°C and later stored at −80°C prior to shipment to the Eicosanoid Core Laboratory at Vanderbilt University Medical Center (Nashville, TN).
Two compounds were measured and used as biomarkers of oxidative stress: free 8-iso-prostaglandin F2α (8-iso-PGF2α) and 2,3-dinor-5,6-dihydro-15-F2t-isoprostane (F2-IsoP-M), a main metabolite of 8-iso-PGF2α. F2-IsoP-M may be a more sensitive biomarker of oxidative stress than the traditionally used 8-iso-PGF2α (Dorjgochoo et al., 2012). 8-iso-PGF2α is produced in the kidney and directly excreted. The contribution of F2-IsoP-M from the kidney is minimal and therefore urinary levels of F2-IsoP-M may better reflect contributions from the whole body.
Biomarker concentrations were measured using gas chromatography–negative ion chemical ionization–mass spectrometry employing stable isotope dilution. [2H4]- 8-iso-PGF2α was added as an internal standard to 0.2 ml of urine in 5 ml of water. The concentration of 8-iso-PGF2α was calculated as the ratio of intensities of the m/z 569: m/z 573. Further detail on the analytic process has been previously provided (Milne et al., 2007).
Statistical analyses
Demographic characteristics of participants at their first cycle were reported using median (interquartile range [IQR]) or n (%). Oxidative stress measures were corrected for urinary dilution using the following formula: Os = O([1.015 − 1]/(SG − 1)) where Os is the sg-corrected oxidative stress measure, O is the raw measure (ng/ml) and 1.015 represents the average sg level from all samples in the study. Median (IQR) levels of sg-corrected oxidative stress biomarkers at the first cycle were examined, stratified by sex. Oxidative stress levels were further summarized within levels of type of procedure (IUI vs. IVF) and by demographic characteristics.
Cluster-weighted generalized estimating equation models (CWGEE) were applied to evaluate the relationship between oxidative stress biomarkers and fertilization rate, and probabilities of implantation, clinical pregnancy and live birth. Men and women were evaluated separately and both IUI and IVF cycles were included in the models, except for the fertilization rate analysis that only applies to IVF cycles. CWGEE allows for the use of repeated measures among individuals and provides unbiased estimates in the presence of an unbalanced design (i.e. participants contributing different numbers of cycles). Weights were assigned to participants using the inverse number of cycles contributed to account for the lower likelihood of success among participants with more cycles, and all analyses were weighted. We have recently shown that CWGEE may provide narrower CIs as compared with multivariable generalized linear mixed models and may more accurately account for the within-subject correlation pattern (Yland et al., 2019).
Weights were not used in the crude examination of cycle outcomes and thus success rates in crude summaries are lower than success rates generated from the models. A binomial distribution and logit link function were used for all outcomes. For all analyses, the individual was treated as the unit of observation. Oxidative stress levels were categorized into tertiles based on preliminary analyses, which suggested a non-linear association between oxidative stress measures and clinical outcomes. The association with quartiles and outcome was also assessed. Tertiles were established using oxidative stress measures at all time points. The middle tertile of oxidative stress biomarkers was treated as the referent group and P-values were generated comparing the lower and upper tertiles to the referent group. We also examined associations between oxidative stress measures and clinical outcomes stratified by treatment type (IUI vs. IVF).
Confounding was evaluated using a priori knowledge of factors associated with both oxidative stress measures and fertility outcomes. All covariates presented in Table I were evaluated as potential confounders. Final covariates for statistical models were then identified within the data as those that had a >10% change in effect estimates, and included age (continuous), BMI (continuous), smoking (never vs. ever), primary infertility diagnosis (male, female, unexplained), race (white vs. other) and type of procedure (IVF vs. IUI).
Table I.
Demographic characteristics at first cycle for women and men enrolled in the Environment and Reproductive Health Study (EARTH)—median (IQR) or n (%).
| Women (n = 481) | Men (n = 249) | |
|---|---|---|
| Age (years) | 35 (32, 38) | 36 (33, 40) |
| missing | 0 | 1 |
| BMI (kg/m2) | 23.4 (21.2, 26.4) | 26.9 (24.3, 29.5) |
| missing | 2 | 1 |
| Race | ||
| White | 400 (83.2) | 218 (87.9) |
| Black/African American | 13 (2.70) | 6 (2.42) |
| Asian | 45 (9.36) | 15 (6.05) |
| Other | 23 (4.78) | 9 (3.63) |
| missing | 0 | 1 |
| Education | ||
| <College graduate | 39 (9.03) | 34 (16.8) |
| ≥College graduate | 393 (91.0) | 168 (83.2) |
| missing | 49 | 47 |
| Smoking status | ||
| Never | 350 (72.8) | 167 (67.3) |
| Current | 13 (2.70) | 17 (6.85) |
| Former | 118 (24.5) | 64 (25.8) |
| missing | 0 | 1 |
| Primary infertility diagnosis at study entry | ||
| Female factor | ||
| Diminished ovarian reserve | 42 (8.73) | 18 (7.23) |
| Ovulation disorders | 55 (11.4) | 30 (12.1) |
| Endometriosis | 29 (6.03) | 13 (5.22) |
| Uterine disorders | 5 (1.04) | 2 (0.80) |
| Tubal factor | 28 (5.82) | 19 (7.63) |
| Male factor | 137 (28.5) | 77 (30.9) |
| Unexplained | 185 (38.5) | 90 (36.1) |
Abbreviations: body mass index (BMI); interquartile range (IQR).
Results
Our sample included 481 women contributing 1001 total cycles (575 IUI and 426 IVF) and 249 men contributing 472 cycles (232 IUI and 240 IVF). At the first cycle, women were on average 35 years old, of normal BMI (mean = 23.4 kg/m2), primarily white (83%), college-educated (91%) and never smokers (73%) (Table I). Among participating women, female factor accounted for 33% of primary infertility diagnoses, with male factor providing 29% of primary diagnoses and the remaining infertility cases were of unexplained origin. The demographics of women in EARTH who were excluded from this analysis (either due to a pregnancy before treatment or missing oxidative stress measures) were similar to women included in this analysis (Supplementary Table SI). Women who were excluded were more likely to be black, to have a college education and had a slightly different distribution of primary infertility diagnoses. Men were on average 36 years old at the first cycle and overweight (26.9 kg/m2). They were also predominantly white (88%), most had a college degree (84%) and 67% never smoked. The distribution of infertility diagnoses among the participating male partners was similar to what we observed for all participating women.
Oxidative stress levels at the first cycle were higher in men than in women for both biomarkers measured. The median (IQR) level of 8-iso-PGF2α in women was 1.30 (0.91, 1.79) ng/ml and in men, it was 1.43 (1.08, 1.92) ng/ml. The median (IQR) level of F2-IsoP-M in women was 0.50 (0.39, 0.69) ng/ml and among men, 0.76 (0.54, 1.22) ng/ml. The correlation coefficient between 8-iso-PGF2α and F2-IsoP-M was 0.55 for women and 0.52 for men.
Levels of the two oxidative stress biomarkers did not meaningfully differ by cycle (data not shown). Oxidative stress levels differed across demographic categories (Table II). Among women, levels of F2-IsoP-M were significantly higher among those younger than 30 compared to those older. Levels of both 8-iso-PGF2α and F2-IsoP-M were positively associated with BMI. F2-IsoP-M levels were significantly higher among women with an infertility diagnosis of female factor compared to a diagnosis of male factor or unexplained infertility. There were no statistically significant differences in oxidative stress levels by race, education or IVF treatment protocol. Higher levels of 8-iso-PGF2α were noted among men younger than 30 years old compared to men ages 40 or older.
Table II.
Specific gravity-corrected biomarker levels (ng/ml) at first cycle by demographic characteristics [geometric mean (geometric standard deviation)].
| Women (n = 481) | Men (n = 249) | |||
|---|---|---|---|---|
| 8-iso-PGF2α | F2-IsoP-M | 8-iso-PGF2α | F2-IsoP-M | |
| Age (years) | ||||
| <30 (ref) | 1.45 (1.9) | 0.62 (1.9) | 1.68 (1.6) | 0.90 (2.1) |
| 30–40 | 1.28 (1.6) | 0.52 (1.7)* | 1.44 (1.6) | 0.89 (2.1) |
| >40 | 1.27 (1.5) | 0.50 (1.5)* | 1.27 (1.6)* | 0.78 (1.8) |
| BMI (kg/m2) | ||||
| <25 (ref) | 1.24 (1.6) | 0.48 (1.6) | 1.40 (1.6) | 0.84 (2.1) |
| 25–30 | 1.38 (1.6)* | 0.58 (1.8)* | 1.41 (1.5) | 0.87 (2.0) |
| >30 | 1.47 (1.7)* | 0.66 (1.7)* | 1.43 (1.6) | 0.89 (2.0) |
| Race | ||||
| White (ref) | 1.28 (1.6) | 0.52 (1.7) | 1.43 (1.6) | 0.88 (2.0) |
| Other | 1.36 (1.7) | 0.54 (1.7) | 1.33 (1.6) | 0.77 (2.0) |
| Education | ||||
| <College graduate (ref) | 1.39 (1.6) | 0.59 (1.7) | 1.55 (1.6) | 0.90 (2.0) |
| ≥College graduate | 1.27 (1.6) | 0.52 (1.7) | 1.42 (1.6) | 0.85 (2.0) |
| Smoking status | ||||
| Never (ref) | 1.31 (1.6) | 0.53 (1.7) | 1.41 (1.6) | 0.85 (2.0) |
| Ever | 1.26 (1.6) | 0.49 (1.7) | 1.43 (1.6) | 0.89 (2.1) |
| Primary infertility diagnosis at study entry | ||||
| Female factor | 1.34 (1.7) | 0.56 (1.8)* | 1.45 (1.5) | 0.99 (2.4) |
| Male infertility | 1.31 (1.7) | 0.53 (1.7) | 1.40 (1.6) | 0.81 (1.9) |
| Unexplained (ref) | 1.24 (1.6) | 0.48 (1.6) | 1.40 (1.6) | 0.81 (1.8) |
| Treatment (IVF only) | ||||
| Antagonist (ref) | 1.30 (1.4) | 0.53 (1.5) | 1.44 (1.8) | 0.73 (2.0) |
| Flare | 1.37 (1.6) | 0.47 (1.6) | 1.34 (1.5) | 0.67 (1.6) |
| Luteal phase agonist | 1.39 (1.7) | 0.54 (1.8) | 1.37 (1.5) | 0.85 (2.0) |
Abbreviations: 8-isoprostane-PGF2α (8-iso-PGF2α); 8-isoprostane-PGF2α metabolite (F2-isoP-M); body mass index (BMI); in-vitro fertilization (IVF).
*Indicates significant difference from the referent group at P < 0.05.
A total of 426 of the initiated IVF cycles in women had at least one embryo transferred with 246 (58%) resulting in implantation, 218 (51%) in clinical pregnancy and 185 (43%) in live birth (Fig. 1a). Of the 575 IUI cycles performed among women, approximately 17% led to successful implantation (97 cycles), 15% resulted in a clinical pregnancy (84 cycles) and 11% led to a live birth (64 cycles) (Fig. 1b). Success rates were similar among the subset of men whose female partners underwent IVF and IUI. Of the 240 IVF cycles in female partners of participating men with stress biomarkers, 60% led to implantation, 52% to a clinical pregnancy and 43% to a live birth (Fig. 2a). Similarly, IUI was much less successful and only 19% of the 232 cycles in female partners of participating men led to implantation, 18% resulted in a clinical pregnancy and 13% resulted in a live birth (Fig. 2b). Among men with partners undergoing IUI, there were 2 cycles with unknown outcomes that were excluded from analyses.
Figure 1.
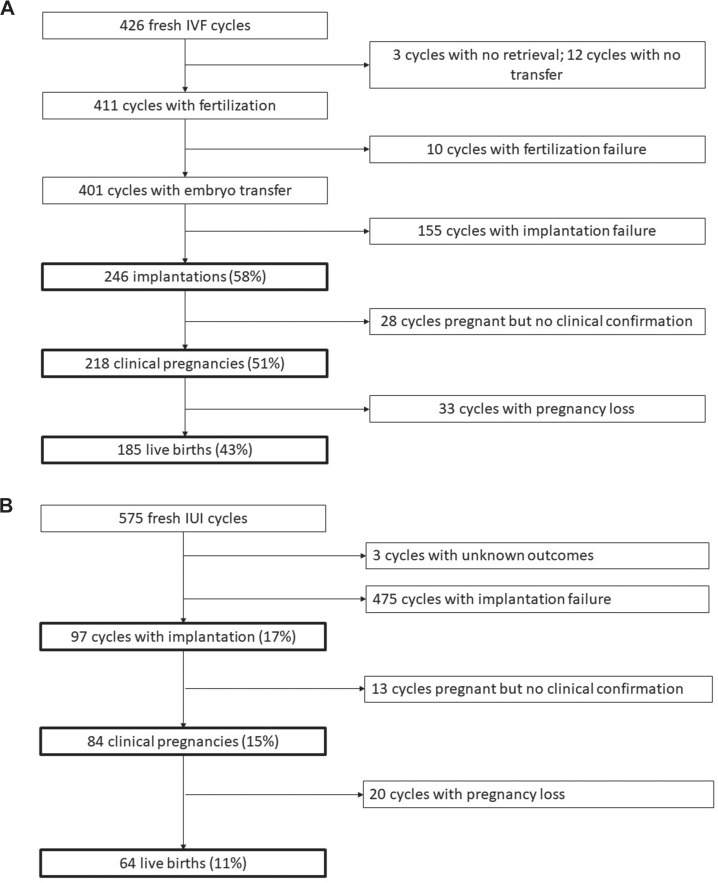
Cycle outcomes among women. (a) Women undergoing in-vitro fertilisation (IVF); (b) Women undergoing intrauterine insemination (IUI).
Figure 2.
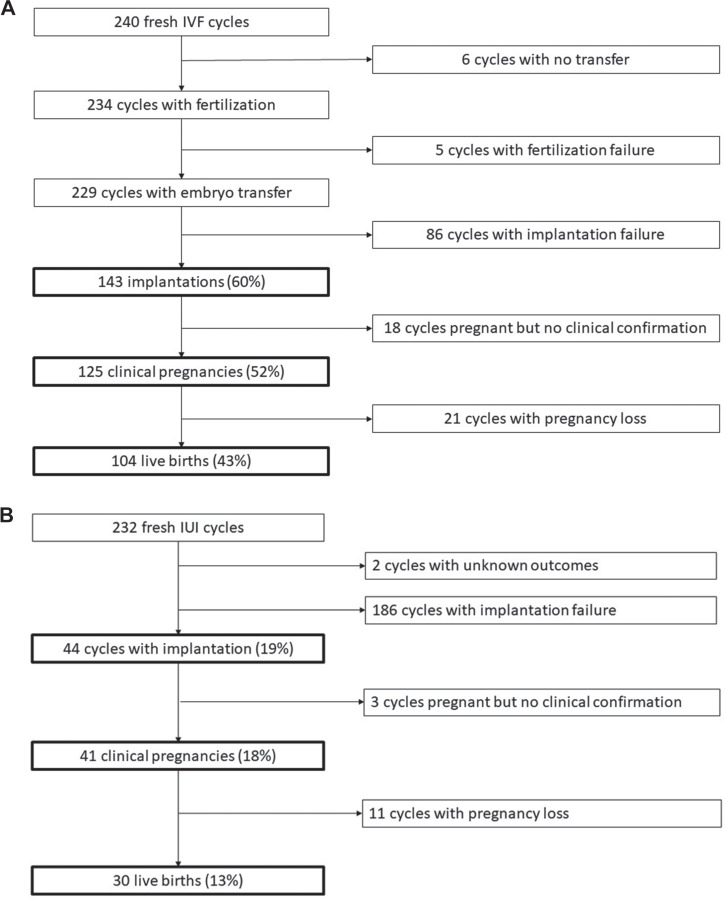
Cycle outcomes among men. (a) Men with partners undergoing IVF; (b) Men with partners undergoing IUI.
IVF and IUI cycles were first examined independently but subsequently combined to increase statistical power after noting similar trends. Models examining associations with maternal oxidative stress levels were run separately from models examining paternal oxidative stress levels. In adjusted models of maternal oxidative stress biomarkers and clinical outcomes, success rates tended to be higher among women with levels of 8-iso-PGF2α and F2-IsoP-M in the middle tertile (Table III). However, these differences only reached statistical significance when measuring the association of F2-IsoP-M and clinical pregnancy and live birth.
Table III.
Adjusteda and weightedb mean proportion of cycles (95% CI) resulting in implantation, clinical pregnancy and live birth in association with urinary oxidative stress biomarkers among 478 women undergoing 992 IVF and IUI cycles.
| Implantation | p-valuec | Clinical pregnancy | p-valuec | Live birth | p-valuec | |
|---|---|---|---|---|---|---|
| 8-iso-PGF 2α | ||||||
| T1 lower | 0.40 (0.33, 0.47) | 0.49 | 0.36 (0.29, 0.43) | 0.45 | 0.30 (0.24, 0.37) | 0.57 |
| T2 middle | 0.44 (0.37, 0.51) | 0.39 (0.33, 0.47) | 0.33 (0.26, 0.40) | |||
| T3 upper | 0.39 (0.32, 0.46) | 0.32 | 0.33 (0.27, 0.40) | 0.20 | 0.26 (0.21, 0.33) | 0.17 |
| F 2 -IsoP-M | ||||||
| T1 lower | 0.39 (0.33, 0.46) | 0.21 | 0.34 (0.28, 0.41) | 0.04 | 0.27 (0.21, 0.34) | 0.03 |
| T2 middle | 0.46 (0.38, 0.54) | 0.44 (0.37, 0.52) | 0.38 (0.31, 0.45) | |||
| T3 upper | 0.36 (0.30, 0.44) | 0.07 | 0.29 (0.23, 0.36) | P < 0.01 | 0.23 (0.17, 0.29) | P < 0.01 |
aAdjusted for maternal age (continuous), maternal BMI (continuous), smoking (never vs. ever), primary infertility diagnosis (male, female, unexplained), race (white vs. other) and type of procedure (IVF vs. IUI).
bModels were weighted to account for number of cycles with weights assigned using inverse number of cycles.
c P-value represents differences compared to the 2nd tertile.
Note: There were 5 cycles missing information on BMI (2 women), 1 cycle missing 8-iso-PGF2α (1 woman) and 3 cycles missing F2-IsoP-M (3 women).
Abbreviations: 8-isoprostane-PGF2α (8-iso-PGF2α); 8-isoprostane-PGF2α metabolite (F2-isoP-M); IVF and IUI.
Women with levels of F2-IsoP-M in the middle tertile had a significantly higher proportion of cycles leading to clinical pregnancy (44%, 95% CI: 37, 52) than women in either the upper (29%, 95% CI: 23, 36) or lower tertile (34%, 95% CI: 28, 41). A similar pattern was observed when live birth was the outcome of interest; the middle tertile had significantly higher proportion of successful cycles (38%, 95% CI: 31, 45) compared to the upper or lower tertile (23% successful cycles and 27% successful cycles), respectively. There were no statistically significant associations for 8-iso-PGF2α in association with clinical outcomes.
These results were similar to analyses stratified by treatment type (i.e. IVF and IUI; Supplementary Tables SII and SIII). In a similar analysis among men, there were no significant associations between paternal oxidative stress levels and success rate of implantation, clinical pregnancy or live birth (Table IV). There was a slight inverse linear trend between both biomarkers and live birth. These results also showed no association in models stratified by treatment type (Supplementary Tables SIV and SV).
Table IV.
Adjusteda and weightedb mean proportion of cycles (95% CI) resulting in implantation, clinical pregnancy and live birth in association with urinary oxidative stress biomarkers among 246 men with partners undergoing 468 IVF and IUI cycles.
| Implantation | p-valuec | Clinical pregnancy | p-valuec | Live birth | p-valuec | |
|---|---|---|---|---|---|---|
| 8-iso-PGF 2α | ||||||
| T1 lower | 0.51 (0.40, 0.61) | 0.90 | 0.46 (0.37, 0.57) | 0.77 | 0.42 (0.33, 0.53) | 0.18 |
| T2 middle | 0.50 (0.39, 0.60) | 0.44 (0.34, 0.55) | 0.33 (0.24, 0.44) | |||
| T3 upper | 0.46 (0.36, 0.57) | 0.64 | 0.40 (0.31, 0.51) | 0.60 | 0.31 (0.22, 0.41) | 0.81 |
| F 2 -IsoP-M | ||||||
| T1 lower | 0.49 (0.39, 0.58) | 0.68 | 0.43 (0.34, 0.52) | 0.51 | 0.40 (0.31, 0.49) | 0.60 |
| T2 middle | 0.52 (0.42, 0.62) | 0.47 (0.38, 0.57) | 0.36 (0.27, 0.46) | |||
| T3 upper | 0.46 (0.35, 0.57) | 0.45 | 0.41 (0.31, 0.52) | 0.39 | 0.31 (0.21, 0.42) | 0.45 |
aAdjusted for paternal age (continuous), paternal BMI (continuous), smoking (never vs. ever), primary infertility diagnosis (male, female, unexplained), race (white vs. other) and type of procedure (IVF vs. IUI).
bModels were weighted to account for number of cycles with weights assigned using inverse number of cycles.
c P-value represents differences compared to the 2nd tertile.
Note: There were 2 cycles missing information on age, BMI, smoking and race (1 man). There were 2 cycles missing information on F2-IsoP-M (2 men).
Abbreviations: 8-isoprostane-PGF2α (8-iso-PGF2α); 8-isoprostane-PGF2α metabolite (F2-isoP-M); in-vitro fertilisation (IVF); intrauterine insemination (IUI).
Among IVF cycles, we additionally examined associations with oocyte fertilization rate, or the proportion of fertilized oocytes among mature oocytes retrieved. There were 302 women who contributed 421 IVF cycles and 167 men who contributed 240 IVF cycles for this analysis (Table V). Women with middle tertile levels of 8-iso-PGF2α had significantly higher rates of fertilization than women in either the lower or upper tertile. In the middle tertile, the fertilization rate was 0.77 (95% CI: 0.73, 0.80), while the rate in the upper tertile was 0.66 (95% CI: 0.61, 0.71), and lower tertile rate was 0.69 (95% CI: 0.64, 0.73). No statistically significant differences were noted among F2-IsoP-M and fertilization rate, or either paternal oxidative stress markers or fertilization rate in the partners of the participating men.
Table V.
Adjusted and weighteda estimated fertilization rateb (95% CI) among women undergoing IVF cycles.
| Womenc (n = 302 women, 421 cycles) |
p-valued | Mene (n = 167 men, 240 cycles) |
p-valued | |
|---|---|---|---|---|
| 8-iso-PGF 2α | ||||
| T1 lower | 0.69 (0.64, 0.73) | P < 0.01 | 0.73 (0.68, 0.77) | 0.89 |
| T2 middle | 0.77 (0.73, 0.80) | 0.73 (0.67, 0.79) | ||
| T3 upper | 0.66 (0.61, 0.71) | P < 0.01 | 0.65 (0.57, 0.72) | 0.11 |
| F 2 -IsoP-M | ||||
| T1 lower | 0.68 (0.63, 0.73) | 0.19 | 0.73 (0.67, 0.78) | 0.43 |
| T2 middle | 0.72 (0.68, 0.76) | 0.70 (0.63, 0.76) | ||
| T3 upper | 0.71 (0.66, 0.76) | 0.67 | 0.69 (0.62, 0.75) | 0.81 |
aModels were weighted to account for number of cycles with weights assigned using inverse number of cycles.
bFertilization rate is defined as the ratio of fertilized oocytes to mature oocytes retrieved.
cAdjusted for maternal age (continuous), maternal BMI (continuous), smoking (never vs. ever), primary infertility dx (male, female, unexplained) and race (white vs. other).
d P-value represents differences in adjusted mean proportion compared to the 2nd tertile.
eAdjusted for paternal age (continuous), BMI (continuous), smoking (never vs. ever), primary infertility dx (male, female, unexplained) and race (white vs. other).
Note: There was 1 cycle missing information on BMI (1 woman). There were 3 cycles missing information on fertilization rate (3 women).
Abbreviations: 8-isoprostane-PGF2α (8-iso-PGF2α); 8-isoprostane-PGF2α metabolite (F2-isoP-M); in-vitro fertilisation (IVF).
Discussion
In this large, prospective cohort of women and men undergoing fertility treatments, we found evidence for non-linear relationships between oxidative stress biomarkers and reproductive outcomes in women. Women with levels of oxidative stress biomarkers in the middle tertile had higher rates of success for both clinical pregnancy and live birth than women with levels in the upper or lower tertiles. Similarly, women with levels of oxidative stress biomarkers in the middle tertile had higher fertilization rates than women with levels in the lower or upper tertile. Among men, there were no differences detected in their partners for any outcome according to urinary levels of oxidative stress biomarkers in the men. In contrast with many previous studies, our analysis measured oxidative stress using systemic measures rather than localized measures (i.e. ejaculate, follicular fluid, tubal fluid, etc.)
We noted inconsistencies in findings between F2-IsoP-M and 8-iso-PGF2α in this study, where F2-IsoP-M levels were associated with significant differences in clinical outcomes but no association was noted for fertilization rate. Inversely, we identified associations between 8-iso-PGF2α levels and fertilization rate, but no effects for clinical outcomes. This inconsistency may reflect differences in accuracy between the two biomarkers; F2-IsoP-M is thought to be more reliable and thus we believe the effect estimates produced from this analysis to be more accurate (Dorjgochoo et al., 2012).
There is extensive literature on oxidative stress and fertility in women (reviewed in Ruder et al., 2008, Lu et al., 2018), but few studies have specifically examined oxidative stress biomarkers and outcomes following IVF and IUI procedures. Generally, successful reproduction is dependent on many factors and oxidative stress may interfere at multiple points. It is believed to have an adverse effect on oocyte quality and maturation, subsequently influencing later development (Yang et al., 1998, Bosco et al., 2005). Higher levels of ROS in culture media have been associated with slow early embryo development, high fragmentation and decreased formation of morphologically normal blastocysts (Bedaiwy et al., 2004). The endometrium, responsible for embryo implantation and development, can become defective in the presence of oxidative stress (Iborra et al., 2005). Additionally, oxidative stress may cause luteal regression and insufficient luteal hormone levels, hindering the necessary support to sustain a pregnancy (Agarwal and Allamaneni, 2004).
Among the studies examining oxidative stress and outcomes in women undergoing fertility treatments, there is consistent evidence that higher levels of oxidative stress are associated with poorer outcomes. However, these studies had small sample sizes, exposure and outcome data at only one time point, and no control for potential confounders.
Lin et al., (2005) measured 8-iso-PGF2α in follicular fluid of 29 women undergoing IVF and found lower levels in women who went on to become pregnant compared to those who did not achieve pregnancy. Ahelik et al., (2015) measured urinary 8-iso-PGF2α in 79 infertile couples and detected the highest levels in women who had a biochemical pregnancy that did not develop into a clinical pregnancy, although this group contained only seven women. These levels were higher than those of women who did not achieve pregnancy at all and women who had a clinical pregnancy.
8-hydroxy-2′-deoxyguanosine (8-OHdG) is a marker of oxidative DNA damage and was measured in two studies. Both found associations between elevated 8-OHdG levels and decreased reproductive success. A study of 96 Japanese women measured 8-OHdG in granulosa cells and found an association with oxidative stress levels and statistically significant decreased rates of fertilization (Seino et al., 2002). In an additional study measuring 8-OHdG in follicular fluid, higher oxidative stress levels were associated with lower fertilization rates and lower proportion of quality blastocysts (Nishihara et al., 2018).
Although these studies are not directly comparable to ours due to different matrices, biomarkers, endpoints and study design, the consistency of findings support adverse effects of elevated oxidative stress levels on assisted reproductive outcomes.
Among men, oxidative stress may cause decreased reproductive success through DNA damage. Oxidative stress is considered to be one of the primary causes of sperm DNA damage and current research suggests that sperm lack the necessary DNA repair mechanisms, making this damage irreversible (Simon et al., 2017). One study measured malondialdehyde (MDA), a marker of lipid peroxidation, in seminal plasma and found no difference in average concentration between those with successful fertilization and those without (Jedrzejczak et al., 2005). Another study examined male oxidative stress levels in association with IVF outcomes and noted no associations between urinary 8-iso-PGF2α and successful pregnancies (Ahelik et al., 2015). Lastly, a study evaluating IUI outcomes found that 8-OHdG levels were significantly lower in men whose partners achieved clinical pregnancy compared to those who did not (Thomson et al., 2011).
A number of studies have found associations between DNA damage in sperm and decreased reproductive success following IVF (Simon et al., 2010, Meseguer et al., 2008, Simon et al., 2011, De Sutter et al., 2012). MDA and 8-iso-PGF2α induce oxidative stress through lipid peroxidation, whereas 8-OHdG generates oxidative stress through its oxidative effects on DNA. Because the male contribution of infertility is hypothesized to be mediated through DNA damage, 8-OHdG may be a more useful biomarker and oxidative stress levels measured this way may be more biologically meaningful. Systemic measures of 8-iso-PGF2α may not be sufficient to capture the localized DNA-damage leading to impaired reproduction.
No identified studies, male or female, have allowed for potential non-linear effects of oxidative stress, despite research that suggests that some level of oxidative stress may be necessary for proper reproductive processes. In a recent review, Lu et al., (2018) identify a number of reproductive processes for which oxidative stress has a beneficial role including promotion of the development of follicles, ovulation, protection of cells from apoptosis and synthesis of progesterone. In epidemiologic research, some studies have identified that a certain threshold of ROS in follicular fluid is necessary, indicative of healthy and developing oocytes (Attaran et al., 2000, Pasqualotto et al., 2004, Wiener-Megnazi et al., 2004). The role of elevated ROS in other matrices (i.e. urine) remains unknown and it is important to note that while a certain level of ROS may be necessary, it must be carefully balanced with antioxidant levels to protect against cellular damage.
If findings from this analysis can be reproduced, oxidative stress represents a potentially modifiable determinant of IVF/IUI success. Oxidative stress levels have previously been linked to exposures such as smoking, diet and environmental chemicals (Vetrani et al., 2013, Carraro et al., 2018, Nagai et al., 2006, Al-Gubory, 2014). Altering these exposures and subsequently lowering oxidative stress levels may result in improved fertility outcomes following IVF and IUI. Our findings of a non-linear association somewhat complicate potential implications and this association should be examined more carefully in additional studies before suggesting exposures that may increase a woman’s oxidative stress levels.
This study is not without limitations. Our results may not be generalizable to the general population as couples who seek infertility treatment tend to be older, more educated and of a higher socioeconomic status than those who do not. However, given the prevalence of infertility, findings from our study are relevant to a non-negligible percentage of the population. Additionally, isoprostanes are stable but short-lived markers of oxidative stress. We do not know the windows of susceptibility for oxidative stress on the outcomes of interest and misclassification of oxidative stress biomarkers is possible. However, we would expect this misclassification to be non-differential with respect to outcome. Isoprostanes specifically measure oxidative stress through lipid peroxidation and other markers of oxidative stress (such as those capturing DNA damage) should also be assessed before drawing firm conclusions. It is also possible that vulvar contamination occurred during collection of urine samples. However, we believe that any contamination would be non-differential with respect to outcome.
Furthermore, previous studies have found that 8-OHdG levels increase following repeated ovarian stimulations in mice (Chao et al., 2005, Miyamoto et al., 2010). A study performed in humans identified a correlation between the number of ampules of gonadotropin stimulation and the amount of DNA damage measured by 8-OHdG, even after adjusting for maternal age, baseline FSH levels and infertility etiology (Polgar et al., 2001). In the last study, it is unclear if oxidative stress levels were higher due to increased stimulation or rather the aggressive stimulation represented an increased requirement. In this analysis, oxidative stress biomarker levels were measured following ovarian stimulation, at the time of oocyte retrieval. The stimulation itself may have elevated oxidative stress levels and our exposure may be subsequently misclassified. Lastly, we examined many associations without any control for multiple comparisons, but this is solely an exploratory analysis.
This study provides a large sample of men and women and is sufficiently powered to examine the association between oxidative stress and fertility treatment success. Additionally, we had exposure information available at multiple time points, potentially reducing issues of misclassification. This study builds on previous studies of oxidative stress and reproductive outcomes through its inclusion of outcomes other than live birth (i.e. implantation and clinical pregnancy) and its examination of IUI. To our knowledge, the relationship between oxidative stress and IUI outcomes in women has previously been unstudied. Circulating oxidative stress measures are more easily collected than localized measures. Utilizing urinary oxidative stress markers may be more feasible in a clinical setting to assess a patient’s oxidative stress burden and predict IVF/IUI outcomes. Results from this study suggest that among men, circulating levels may not be sufficient for prediction and that more localized measures are necessary.
In conclusion, we found preliminary evidence that a moderate degree of oxidative stress in women, but not in men, is associated with improved outcomes following IVF/IUI treatment. Additional cellular and animal studies are needed to further unravel the effects of oxidative stress on female reproductive processes while future epidemiologic studies should be longitudinal in nature and validate the utility of measuring oxidative stress in different biologic compartments.
Supplementary Material
Funding
We gratefully acknowledge the EARTH participants for contributing to this study.
Authors’ roles
E.M.R. performed the statistical analyses and drafted the manuscript. L.M.-A. assisted with the statistical analyses and served as an expert on study design and cohort. J.D.M. contributed to the study question and facilitated data acquisition. P.W. provided statistical guidance and served as an expert on the cohort. G.L.M. analysed and prepared the exposure data for analysis. R.H. conceived of the cohort and was responsible for data collection and cohort maintenance. K.K.F. helped develop the study question and collaborated on the statistical strategy. All authors contributed to interpretation of the data and to drafting and revising the manuscript, and approved the final version.
Funding
Intramural Research Program of the National Institutes of Environmental Health Sciences (ZIA ES103314); National Institutes of Environmental Health Sciences grants (R01ES022955, R01ES009718 and R01ES00002).
Conflict of interest
There are no competing interests to report.
References
- Agarwal A, Allamaneni SS. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod Biomed Online 2004;9:338–347. [DOI] [PubMed] [Google Scholar]
- Ahelik A, Mandar R, Korrovits P, Karits P, Talving E, Rosenstein K, Jaagura M, Salumets A, Kullisaar T. Systemic oxidative stress could predict assisted reproductive technique outcome. J Assist Reprod Genet 2015;32:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 1987;81:459–469. [DOI] [PubMed] [Google Scholar]
- Al-Gubory KH. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reprod Biomed Online 2014;29:17–31. [DOI] [PubMed] [Google Scholar]
- Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, Sharma RK. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med 2000;45:314–320. [PubMed] [Google Scholar]
- Bedaiwy MA, Falcone T, Mohamed MS, Aleem AA, Sharma RK, Worley SE, Thornton J, Agarwal A. Differential growth of human embryos in vitro: role of reactive oxygen species. Fertil Steril 2004;82:593–600. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, Agarwal A. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod 2002;17:426–431. [DOI] [PubMed] [Google Scholar]
- Bosco L, Ruvolo G, Morici G, Manno M, Cittadini E, Roccheri MC. Apoptosis in human unfertilized oocytes after intracytoplasmic sperm injection. Fertil Steril 2005;84:1417–1423. [DOI] [PubMed] [Google Scholar]
- Bykova M, Athayde K, Sharma R, Jha R, Sabanegh E, Agarwal A. Defining the reference value of seminal reactive oxygen species in a population of infertile men and normal healthy volunteers. Fertil Steril 2007;88:S305. [Google Scholar]
- Carraro E, Schilirò T, Biorci F, Romanazzi V, Degan R, Buonocore D, Verri M, Dossena M, Bonetta S. Physical activity, lifestyle factors and oxidative stress in middle age healthy subjects. Int J Environ Res Public Health 2018;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . 2017. Key Statistics from the National Survey of Family Growth. CDC/National Center for Health Statistics 2017. https://www.cdc.gov/nchs/nsfg/key_statistics/i.htm#impaired (27 February 2019, date last accessed).
- CDC . 2016 Assisted Reproductive Technology Fertility Clinic Success Rate Report. Atlanta, GA: Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology, 2018a [Google Scholar]
- CDC . 2016 Assisted Reproductive Technology National Summary Report. Atlanta, GA: Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology, 2018b [Google Scholar]
- Chao HT, Lee SY, Lee HM, Liao TL, Wei YH, Kao SH. Repeated ovarian stimulations induce oxidative damage and mitochondrial DNA mutations in mouse ovaries. Ann N Y Acad Sci 2005;1042:148–156. [DOI] [PubMed] [Google Scholar]
- Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct 2012;2012:646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Chattopadhyay R, Ghosh S, Ghosh S, Goswami SK, Chakravarty BN, Chaudhury K. Reactive oxygen species level in follicular fluid—embryo quality marker in IVF? Hum Reprod 2006;21:2403–2407. [DOI] [PubMed] [Google Scholar]
- De Sutter P, Stadhouders R, Dutre M, Gerris J, Dhont M. Prevalence of chromosomal abnormalities and timing of karyotype analysis in patients with recurrent implantation failure (RIF) following assisted reproduction. Facts Views Vis Obgyn 2012;4:59–65. [PMC free article] [PubMed] [Google Scholar]
- Dong M, Shi Y, Cheng Q, Hao M. Increased nitric oxide in peritoneal fluid from women with idiopathic infertility and endometriosis. J Reprod Med 2001;46:887–891. [PubMed] [Google Scholar]
- Dorjgochoo T, Gao YT, Chow WH, Shu X, Yang G, Cai Q, Rothman N, Cai H, Li H, Deng Xet al. Major metabolite of F(2)-isoprostane in urine may be a more sensitive biomarker of oxidative stress than isoprostane itself. Am J Clin Nutr 2012;96:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorostghoal M, Kazeminejad SR, Shahbazian N, Pourmehdi M, Jabbari A. Oxidative stress status and sperm DNA fragmentation in fertile and infertile men. Andrologia 2017;49. [DOI] [PubMed] [Google Scholar]
- Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril 2003;80:123–127. [DOI] [PubMed] [Google Scholar]
- Ford WC. Regulation of sperm function by reactive oxygen species. Hum Reprod Update 2004;10:387–399. [DOI] [PubMed] [Google Scholar]
- Iborra A, Palacio JR, Martinez P. Oxidative stress and autoimmune response in the infertile woman. Chem Immunol Allergy 2005;88:150–162. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol 2000;157:2111–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrzejczak P, Fraczek M, Szumala-Kakol A, Taszarek-Hauke G, Pawelczyk L, Kurpisz M. Consequences of semen inflammation and lipid peroxidation on fertilization capacity of spermatozoa in in vitro conditions. Int J Androl 2005;28:275–283. [DOI] [PubMed] [Google Scholar]
- Lin K, Barnhart K, Shaunik A, Butts S, Fitzgerald GA, Coutifaris C. Follicular fluid F2-isoprostanes: a novel assessment of oxidative stress in IVF patients. Fertil Steril 2005;84:S47. [Google Scholar]
- Lu J, Wang Z, Cao J, Chen Y, Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol 2018;16:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Romero FJ, Ortiz-de-Galisteo JR, Lara-Laranjeira J, Dominguez-Arroyo JA, Gonzalez-Carrera E, Alvarez IS. Store-operated calcium entry in human oocytes and sensitivity to oxidative stress. Biol Reprod 2008;78:307–315. [DOI] [PubMed] [Google Scholar]
- Meseguer M, Martinez-Conejero JA, O'Connor JE, Pellicer A, Remohi J, Garrido N. The significance of sperm DNA oxidation in embryo development and reproductive outcome in an oocyte donation program: a new model to study a male infertility prognostic factor. Fertil Steril 2008;89:1191–1199. [DOI] [PubMed] [Google Scholar]
- Messerlian C, Williams PL, Ford JB, Chavarro JE, Minguez-Alarcon L, Dadd R, Braun JM, Gaskins AJ, Meeker JD, James-Todd Tet al. The environment and reproductive health (EARTH) study: a prospective preconception cohort. Hum Reprod Open 2018;2018. doi: 10.1093/hropen/hoy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc 2007;2:221–226. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Sato EF, Kasahara E, Jikumaru M, Hiramoto K, Tabata H, Katsuragi M, Odo S, Utsumi K, Inoue M. Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free Radic Biol Med 2010;49:674–681. [DOI] [PubMed] [Google Scholar]
- Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl 2010;33:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K, Betsuyaku T, Kondo T, Nasuhara Y, Nishimura M. Long term smoking with age builds up excessive oxidative stress in bronchoalveolar lavage fluid. Thorax 2006;61:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara T, Matsumoto K, Hosoi Y, Morimoto Y. Evaluation of antioxidant status and oxidative stress markers in follicular fluid for human in vitro fertilization outcome. Reprod Med Biol 2018;17:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszczuk K, Giwercman A, Bungum M. Sperm chromatin structure assay in prediction of in vitro fertilization outcome. Andrology 2016;4:290–296. [DOI] [PubMed] [Google Scholar]
- Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA, Joshi NJ, Rose BI. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril 2004;81:973–976. [DOI] [PubMed] [Google Scholar]
- Polak G, Koziol-Montewka M, Gogacz M, Blaszkowska I, Kotarski J. Total antioxidant status of peritoneal fluid in infertile women. Eur J Obstet Gynecol Reprod Biol 2001;94:261–263. [DOI] [PubMed] [Google Scholar]
- Polgar K, Mukherjee T, McCaffrey C, Levin I, Gordon J, Copperman AB. Understanding the relationship between controlled ovarian hyperstimulation and intra-follicular oxidative damage. Fertil Steril 2001;76:S132. [Google Scholar]
- Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update 2008;14:345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini L, Wilson C, Lower A, Al-Shawaf T, Grudzinskas JG. Superoxide dismutase activity in human follicular fluid after controlled ovarian hyperstimulation in women undergoing in vitro fertilization. Fertil Steril 1999;72:1027–1034. [DOI] [PubMed] [Google Scholar]
- Seino T, Saito H, Kaneko T, Takahashi T, Kawachiya S, Kurachi H. Eight-hydroxy-2′-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization-embryo transfer program. Fertil Steril 2002;77:1184–1190. [DOI] [PubMed] [Google Scholar]
- Shiotani M, Noda Y, Narimoto K, Imai K, Mori T, Fujimoto K, Ogawa K. Immunohistochemical localization of superoxide dismutase in the human ovary. Hum Reprod 1991;6:1349–1353. [DOI] [PubMed] [Google Scholar]
- Simon L, Aston KI, Emery BR, Hotaling J, Carrell DT. Sperm DNA damage output parameters measured by the alkaline comet assay and their importance. Andrologia 2017;49. [DOI] [PubMed] [Google Scholar]
- Simon L, Brunborg G, Stevenson M, Lutton D, McManus J, Lewis SE. Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum Reprod 2010;25:1594–1608. [DOI] [PubMed] [Google Scholar]
- Simon L, Lutton D, McManus J, Lewis SE. Sperm DNA damage measured by the alkaline comet assay as an independent predictor of male infertility and in vitro fertilization success. Fertil Steril 2011;95:652–657. [DOI] [PubMed] [Google Scholar]
- Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura Ket al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res 2008;44:280–287. [DOI] [PubMed] [Google Scholar]
- Thomson LK, Zieschang JA, Clark AM. Oxidative deoxyribonucleic acid damage in sperm has a negative impact on clinical pregnancy rate in intrauterine insemination but not intracytoplasmic sperm injection cycles. Fertil Steril 2011;96:843–847. [DOI] [PubMed] [Google Scholar]
- Vetrani C, Costabile G, Di Marino L, Rivellese AA. Nutrition and oxidative stress: a systematic review of human studies. Int J Food Sci Nutr 2013;64:312–326. [DOI] [PubMed] [Google Scholar]
- Wiener-Megnazi Z, Vardi L, Lissak A, Shnizer S, Reznick AZ, Ishai D, Lahav-Baratz S, Shiloh H, Koifman M, Dirnfeld M. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil Steril 2004;82:1171–1176. [DOI] [PubMed] [Google Scholar]
- Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod 1998;13:998–1002. [DOI] [PubMed] [Google Scholar]
- Yland J, Messerlian C, Minguez-Alarcon L, Ford JB, Hauser R, Williams PL. Methodological approaches to analyzing IVF data with multiple cycles. Hum Reprod 2019;34:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


