Abstract
Background
We previously observed a rapid increase in the incidence of renal cell carcinoma (RCC) in men and women between 1935 and 1989 in the USA, using data from the Connecticut Tumor Registry. This increase appeared to be largely explained by a positive cohort effect, but no population-based study has been conducted to comprehensively examine age-period-cohort effects by histologic types for the past decade.
Methods
We calculated age-adjusted and age-specific incidence rates of the two major kidney-cancer subtypes RCC and renal urothelial carcinoma, and conducted an age-period-cohort analysis of 114 138 incident cases of kidney cancer reported between 1992 and 2014 to the Surveillance, Epidemiology, and End Results programme.
Results
The age-adjusted incidence rates of RCC have been increasing consistently in the USA among both men and women (from 12.18/100 000 in 1992–1994 to 18.35/100 000 in 2010–2014 among men; from 5.77/100 000 in 1992–1994 to 8.63/100 000 in 2010–2014 among women). Incidence rates generally increased in successive birth cohorts, with a continuing increase in rates among the younger age groups (ages 0–54 years) in both men and women and among both Whites and Blacks. These observations were confirmed by age-period-cohort modelling, which suggested an increasing birth-cohort trend for RCC beginning with 1955 birth cohorts, regardless of the assumed value for the period effect for both men and women and for Whites and Blacks.
Conclusions
Known risk factors for kidney cancer may not fully account for the observed increasing rates or the birth-cohort pattern for RCC, prompting the need for additional etiologic hypotheses (such as environmental exposures) to investigate these descriptive patterns.
Keywords: Renal cell carcinoma, descriptive epidemiology, SEER
Key Messages
We previously observed a rapid increase in the incidence of renal cell carcinoma (RCC) in both men and women from 1935 to 1989 using data from the Connecticut (USA) Tumor Registry.
We conducted age-period-cohort analyses of kidney cancer rates in the USA using data from the population-based Surveillance, Epidemiology, and End Results programme from 1992 to 2014.
Our findings suggest a rapid increase in RCC rates among younger age groups between 0 and 54 years of age in both men and women in the USA, and an increasing birth-cohort trend for RCC beginning with 1955 birth cohorts.
Known risk factors for RCC may not fully account for these patterns, prompting the need for additional etiologic hypotheses.
Introduction
The American Cancer Society estimates that at least 65 340 new cases of kidney cancer will be diagnosed in 2018 in the USA.1 Kidney cancer is one of the most common malignancies in adults1 and includes two major histologic types: renal cell carcinoma (RCC, also known as renal cell adenocarcinoma) and renal urothelial carcinoma (RUC). RCC originates in the renal parenchyma and is the most common type of kidney cancer in adults, responsible for about 90–95% of cases. It is also the most lethal of the malignant urological tumours.2 RUC originates in the lining of the renal pelvis (also known as urothelial carcinomas) and contributes to about 5–10% of kidney cancers. Kidney cancer also includes several other rare histologic types, such as renal sarcoma (<1%) and Wilms tumour (occurring in children).2
Using data from the Connecticut Tumor Registry, the oldest population-based cancer registry in the USA, we previously observed a rapid increase in the incidence of RCC in both men and women from 1935 to 1989, and this observed increase appeared to be largely explained by a positive cohort effect (i.e. from the changes in the risk factors of the disease of interest).3 Based on this cohort analysis, we predicted that rates of RCC would rise continuously in the immediate future. An increase in the incidence of kidney cancer was also reported by others,4,5 but no population-based study has been conducted to comprehensively examine age-period-cohort effects by histologic types for the past decade.
To better understand contemporary time trends and the potential birth-cohort pattern of histologic types of kidney cancer in the USA, we conducted a birth-cohort analysis utilizing data from the US Surveillance, Epidemiology, and End Results (SEER) programme for the time period 1992–2014, the most recent matured data available to us. Birth-cohort analyses and age-period-cohort (APC) modelling allow us to determine the relative importance of age, period and cohort effects on the observed time trends, and thus may aid in understanding the risk factors that might be responsible for the observed time trends.
Methods
The current study was based on 114 138 incident cases of kidney cancer reported between 1992 and 2014 to the SEER programme from 13 SEER registries: Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound and Utah, Los Angeles, San Jose-Monterey, Rural Georgia and the Alaska Native Tumor Registry.6 Based on the coding for the site (topography) of cancer as found in the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3), cases with site code C64.9 (kidney parenchyma or kidney) and site code C65.9 (renal pelvis) were included in this study. We did not attempt to analyse the data by anatomic sub-site, due to the high percentage of cases without specification of anatomic site (ICD-O-3 C69).
Analysis by histology (as defined by ICD-O-3) was limited to the two major subtypes: adenocarcinomas (ICD-O-3 codes 8140–8389, RCC) and RUC (ICD-O-3 codes 8120, 8123 and 8130). The proportion of cases with histological confirmation was 90% in 1992 and had increased to 94% by 2014.
Age-specific and age-adjusted incidence rates were calculated for RCC and RUC by sex (men vs women) for all the cases reported to the SEER registries during the study period. Analysis by race was limited to Whites and Blacks only because of the small numbers for other racial groups. To calculate age-specific incidence rates, cases were grouped into 5-year age intervals. Data were also presented for those aged 0–54 and 54 years and older, and for all age groups. Age-adjusted incidence rates were calculated using the direct method, standardized to the 2000 Standard US population.
The data are presented by calendar year and by cohort year of birth to explore the secular trends and potential birth-cohort patterns. An APC model was used to analyse age-specific incidence rates for both RCC and RUC. Methodological details regarding this analysis have been described elsewhere.7–9 Briefly, this analytical method allows a simultaneous evaluation of the effects of age, years of diagnosis (period) and generation or year of birth (cohort). APC models were based on 12 5-year age intervals beginning at age 30 years (there are very few cases reported for persons under age 30) and five period intervals (1992–1994, 1995–1999, 2000–2004, 2005–2009 and 2010–2014).
Because of the non-identifiability problem (cohort = year – age), the independent effects of age, period and cohort cannot be evaluated.10,11 The cohort effect can be evaluated by constraining the period effect to different assumptions (here, the parameter values βp = 0, –0.01 or 0.01), where βp = 0 represents a slope of zero, βp = –0.01 indicates that the period slope was decreasing and βp = 0.01 denotes that the period slope was increasing during the study period. All models were fit using SAS (version 9.3).12 The significance level was set at 0.05 for a two-sided test.
Results
A total of 114 138 incident cases of kidney cancer were reported to the SEER programme between 1992 and 2014. Of these, 99 062 (86.79%) were RCC, 8717 (7.64%) were RUC and 6359 (5.57%) were of another histologic type. In men, the overall age-adjusted incidence rates for RCC increased from 12.18/100 000 in 1992–1994 to 18.35/100 000 in 2010–2014 (Table 1). The overall age-adjusted incidence rates for RUC, on the other hand, were low and showed a slight decrease from 1.54/100 000 in 1992–1994 to 1.32/100 000 in 2010–2014. In women, the incidence rates showed similar trends during the study period: a continuing increase in RCC from 5.77/100 000 in 1992–1994 to 8.63/100 000 in 2010–2014 (Table 2), and a slight decrease in RUC from 0.80/100 000 in 1992–1994 to 0.74/100 000 in 2010–2014.
Table 1.
Kidney cancer by two main histological types for men only
| Period | Renal cell carcinoma |
Renal urothelial carcinoma |
||||
|---|---|---|---|---|---|---|
| Age-adjusted rate | Crude rate | Number of cases | Age-adjusted rate | Crude rate | Number of cases | |
| 1992–1994 | 12.18 | 9.51 | 5092 | 1.54 | 1.13 | 606 |
| 1995–1999 | 12.95 | 10.43 | 9703 | 1.45 | 1.08 | 1005 |
| 2000–2004 | 15.02 | 12.83 | 12 492 | 1.42 | 1.09 | 1065 |
| 2005–2009 | 17.87 | 16.52 | 16 565 | 1.32 | 1.09 | 1093 |
| 2010–2014 | 18.35 | 18.37 | 19 198 | 1.32 | 1.19 | 1241 |
Table 2.
Kidney cancer by two main histological types for women only
| Period | Renal cell carcinoma |
Renal urothelial carcinoma |
||||
|---|---|---|---|---|---|---|
| Age-adjusted rate | Crude rate | Number of cases | Age-adjusted rate | Crude rate | Number of cases | |
| 1992–1994 | 5.77 | 5.51 | 3030 | 0.80 | 0.79 | 435 |
| 1995–1999 | 6.15 | 5.99 | 5715 | 0.81 | 0.82 | 778 |
| 2000–2004 | 7.35 | 7.39 | 7377 | 0.75 | 0.78 | 775 |
| 2005–2009 | 8.73 | 9.23 | 9508 | 0.74 | 0.80 | 826 |
| 2010–2014 | 8.63 | 9.68 | 10 382 | 0.74 | 0.83 | 893 |
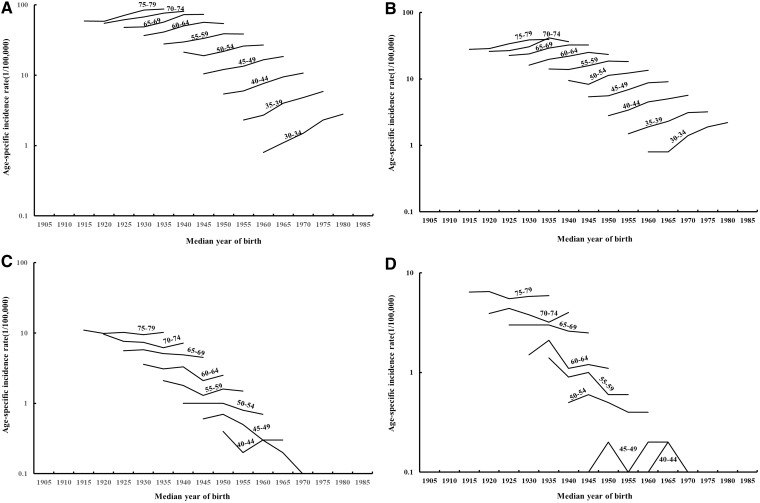
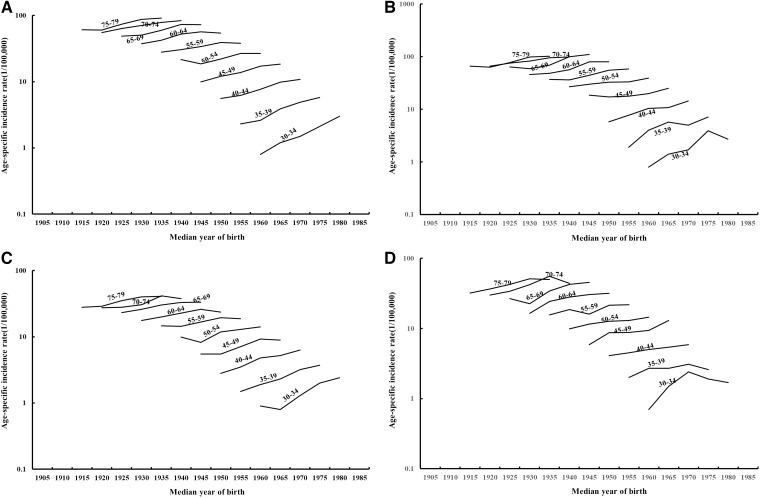
The age-specific incidence rates of RCC by median year of birth are shown in Figure 1. Examination of rates by birth cohort showed that the incidence rates for RCC generally increased in successive birth cohorts, with a more rapid increase in rates among the younger age groups under age 55 years in men (Figure 1A) and women (Figure 1B). On the other hand, incidence rates for RUC showed a decrease in successive birth cohorts in both men (Figure 1C) and women (Figure 1D), whereas the very low rates of RUC made the cohort trend unstable. Further examination of the rates for Whites and Blacks (Figure 2A–D) showed similar patterns, whereas the rates are less stable among Blacks due to the smaller numbers of cases.
Figure 1.
Incidence rates of kidney cancer presented by age and median year of birth for renal cell carcinomas for men (A) and women (B) and for renal urothelial carcinomas for men (C) and women (D).
Figure 2.
Incidence rates for renal cell carcinoma presented by age and median year of birth for (A) White men; (B) Black men; (C) White women; and (D) Black women.
Because the birth-cohort analyses showed a continuing rapid increase in RCC among the younger age group (<55 years), we analysed the time trends of RCC by age (0–54 and 55+ years) for Whites and Blacks and presented for men in Table 3 and women in Table 4. Black men and women had higher incidence rates in both younger and older age groups during the study period. The incidence rates of RCC increased continuously for the younger age group during the study period, whereas the rates for those aged 55 years and over levelled off in the last time period for both men and women and in both Whites and Blacks.
Table 3.
Crude and age-adjusted incidence rates for renal cell carcinoma (1/100 000) by age (0–54 and 55+ years) for Whites and Blacks among men only
| Year of diagnosis | Whites |
Blacks |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–54 |
55+ |
0–54 |
55+ |
|||||||||
| Crude rate | Age-adjusted ratea | Count | Crude rate | Age-adjusted ratea | Count | Crude rate | Age-adjusted ratea | Count | Crude rate | Age-adjusted ratea | Count | |
| 1992–1994 | 3.0 | 3.6 | 1036 | 44.2 | 44.6 | 3224 | 3.1 | 4.8 | 157 | 51.6 | 51.7 | 349 |
| 1995–1999 | 3.4 | 3.7 | 1996 | 47.8 | 48.4 | 6049 | 4.0 | 5.4 | 348 | 52.9 | 54.4 | 638 |
| 2000–2004 | 4.4 | 4.4 | 2664 | 54.5 | 56.1 | 7573 | 5.2 | 6.2 | 478 | 61.7 | 64.7 | 847 |
| 2005–2009 | 5.8 | 5.5 | 3400 | 61.8 | 64.9 | 9789 | 5.9 | 6.6 | 560 | 80.3 | 85.7 | 1326 |
| 2010–2014 | 6.2 | 6.0 | 3612 | 61.6 | 65.3 | 11 297 | 7.3 | 8.1 | 715 | 80.5 | 83.3 | 1629 |
Rates are age-adjusted to the 2000 US Standard Population.
Table 4.
Crude and age-adjusted incidence rates for renal cell carcinoma (1/100 000) by age (0–54 and 55+ years) for Whites and Blacks among women only
| Year of diagnosis | Whites |
Blacks |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–54 |
55+ |
0–54 |
55+ |
|||||||||
| Crude rate | Age-adjusted ratea | Count | Crude rate | Age-adjusted ratea | Count | Crude rate | Age-adjusted ratea | Count | Crude rate | Age-adjusted ratea | Count | |
| 1992–1994 | 1.7 | 1.9 | 555 | 21.3 | 20.9 | 2005 | 1.7 | 2.2 | 88 | 22.9 | 23.1 | 217 |
| 1995–1999 | 1.8 | 1.8 | 1010 | 23.0 | 22.6 | 3688 | 2.2 | 2.7 | 210 | 25.9 | 26.3 | 436 |
| 2000–2004 | 2.6 | 2.5 | 1511 | 26.2 | 26.2 | 4477 | 2.7 | 3.0 | 270 | 29.8 | 30.8 | 564 |
| 2005–2009 | 3.2 | 3.1 | 1832 | 30.0 | 30.9 | 5659 | 3.0 | 3.1 | 301 | 36.3 | 38.8 | 815 |
| 2010–2014 | 3.5 | 3.3 | 1958 | 28.6 | 29.8 | 6070 | 3.5 | 3.5 | 354 | 34.2 | 35.9 | 922 |
Rates are age-adjusted to the 2000 US Standard Population.
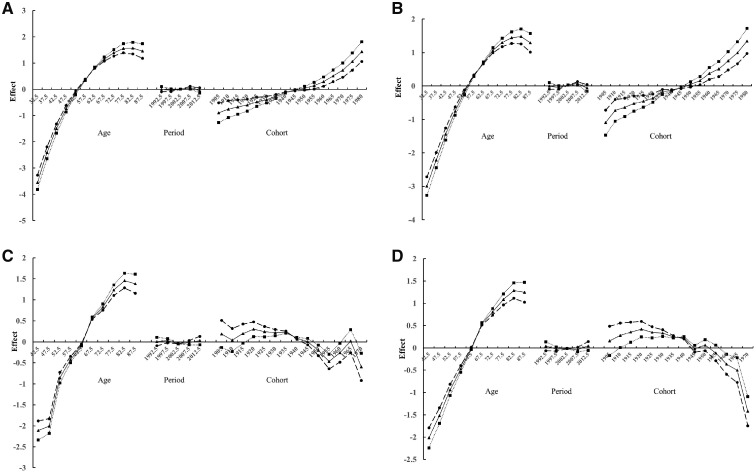
Figure 3 presents the results of APC modelling for RCC for men (Figure 3A) and women (Figure 3B). Each figure shows plots of the estimated effects for age, period and cohort, using three plausible assumptions for the period effect (βp = 0, –0.01 or 0.01). As described previously, several sets of parameters are used, as a unique set of parameters is impossible to ascertain. The resulting plots suggested an increasing birth-cohort trend for RCC, beginning with 1955 birth cohorts, regardless of the assumed value for the period effect, for both men and women. In contrast, a decreasing birth-cohort trend was observed for RUC in both men (Figure 3C) and women (Figure 3D). Stratified analyses for Whites and Blacks showed similar birth-cohort trends as observed for the all racial groups (data not shown).
Figure 3.
Age-period-cohort effects on the incidence of renal cell carcinomas for men (A) and for women (B) and on renal urothelial carcinomas for men (C) and for women (D).
Discussion
The results of our time-series analysis of SEER incidence data from 1992 to 2014 indicate that RCC rates have indeed been increasing consistently in the USA, in contrast to RUC, for which the incidence rates have stabilized. Our cohort analysis and APC modelling confirmed that the observed increase in RCC is a birth-cohort phenomenon, which was previously observed in our study of Connecticut Tumor Registry data alone over the period 1935–1989.
What factors may be responsible for this observed long-term increase and the birth-cohort pattern for RCC in the USA? Since image acquisition and interpretation of RCC may benefit from more advanced techniques,13 increasing access to improved imaging technology may have led to increased earlier detection of localized kidney cancer. Several observations, however, do not support that a period effect is likely the sole reason for the observed long-term increase in RCC rates. Whereas RCC rates have been increasing, the RUC has been decreasing during the same study period; the RCC rates increase continuously in the young age groups that clearly show a birth-cohort pattern whereas the RCC rates in the older age groups started to level off in the recent period. Taking together our previous study of Connecticut Tumor Registry data,3 the rate of RCC has been increasing in Connecticut for decades, dating back to the period 1935–1939. In fact, if the current increasing trend in the young cohorts does not stabilize, we would anticipate a much more rapid increase in RCC in the coming years in the USA. Others have also argued against the idea that the observed increase in RCC among Blacks could be a result of long-term greater access to and utilization of imaging technologies by Blacks.14,15 Whereas some have suggested that kidney-cancer incidence has shown signs of stabilizing,16 this phenomenon can mostly be attributed to the flat rate among older age groups during the most recent period of observation. Our results clearly show a continuing increase in birth-cohort effects, without any apparent deceleration, among younger age groups.
Several factors have been linked to kidney-cancer risk, including tobacco smoking, chronic kidney diseases and dialysis treatment, obesity, hypertension, diabetes, family history of kidney cancer and rare genetic conditions.14–18 But, as pointed out by Lipworth et al.,14 ‘the factors most consistently associated with increased renal cell cancer risk in epidemiologic studies—obesity, hypertension, cigarette smoking—likely account for less than half of these cancers among Whites, and there is scant published evidence pertaining to their association among Blacks with renal cell cancer’. These factors together also do not appear to be able to completely explain the observed time trends and the birth-cohort pattern for RCC in the USA during the past decades, as we further consider below.
Tobacco smoking
Tobacco smoking has been linked to kidney cancer and has been identified as the most significant risk factor for RUC.14,19,20 Based on the report from CDC,21 the smoking rates have steadily declined over the past decade, from 20.9% in 2005 to 15.5% in 2016. Those who continue using cigarettes are smoking less, with the average number of cigarettes per day decreasing from 17 to 14 during the same time period. The number of people who have quit smoking has increased from 50.8% in 2005 to 59.0% in 2016, with the age range of 25–44 years making the most progress. Thus, smoking could explain the observed decrease in RUC. Smoking may even explain part of the flat rates of RCC in older age groups, especially in the last time period. However, smoking cannot explain the long-term continuing increase in rates of RCC, especially the increase in RCC incidence for those under age 54 years that have the most people quitting smoking. Indeed, almost all smoking-related cancer-incidence rates have either stabilized or started decreasing in recent birth cohorts, including adenocarcinoma of the lung in women,22 reflecting the effects of anti-smoking campaigns during the past several decades.
Chronic kidney diseases and dialysis treatment
RCC of the kidney has long been associated with chronic kidney conditions and long-term dialysis.14,23–27 Thus, increases in the prevalence of well-managed end-stage renal disease and of long-term dialysis may explain, in part, the high rate and increasing rate of RCC, especially among older adults.
Obesity and hypertension
The global epidemic of overweight and obesity has become a major public-health problem in the world, with recent WHO statistics revealing that 39% of adults aged 18 years and over (39% of men and 40% of women) were overweight in 2016.28 In the USA, the data from the National Health and Nutrition Examination Survey (NHANES) showed that the prevalence of obesity was 39.8% in adults and 18.5% in the youth in 2015–2016.29
Epidemiologic studies have linked overweight and obesity to increased risk of kidney cancer,14,30,31 particularly the risk of clear-cell RCC.32 Based on the literature, this relationship between obesity and risk of RCC is established. However, additional studies are needed to evaluate the risk of RCC in relation to obesity across the lifecourse.33 In our study, the cohort analysis showed a continuing and more rapid increase in rates among younger age groups under age 55 years of both sexes in cohorts born in late 1950s and later. It is also unknown to what extent observed associations between excessive body weight and kidney-cancer risk may actually be related to initiation or the promotion and progression of existing kidney cancers. For example, obesity-related agents such as adipokines have been shown to be both initiators of new cancerous tumours and stimulators of existing cancerous tumours.34
Hypertension is an established risk factor for RCC with a dose-dependent relationship per increase in blood pressure measurement.14,35 Several studies have estimated the proportion of RCC cases attributable to this risk factor. In a population-based case–control study conducted in Minnesota, an estimated 21% of the RCC cases were attributable to hypertension.36 Subsequently, a population-based case–control study of kidney cancer in Detroit and Chicago estimated that incidence rates of RCC would be 44% lower for Black men and 35% lower for White men in the absence of hypertension. The corresponding percentages for Black and White women were 51 and 30%, respectively.37 An estimated 1 in 17 young adults under the age of 40 meets diagnostic thresholds for hypertension,38 but the prevalence of this condition generally increases with age. The rising prevalence of hypertension likely contributes to the overall increasing rates of RCC in the USA, although it is currently unclear the extent to which this risk factor is driving the rate increase among younger individuals specifically.
Diabetes
Diabetes mellitus has also been associated with the risk of kidney cancer, as demonstrated, e.g., in the findings of the Nurses’ Health Study (for women). Insulin, blood glucose and inflammation phenomena may have contributed to the observed association.39–42 A meta-analysis of cohort studies reported an overall relative risk of 1.42 (95% confidence interval = 1.06–1.91) for diabetes and kidney-cancer risk, with a stronger association in women than men. However, these results were attenuated after restricting to studies that adjusted for body mass index and cigarette smoking.43
Genetics and family history
About 2–3% of kidney cancers are related to familial syndromes and several autosomal-dominant syndromes,44,45 but these factors cannot explain the observed birth-cohort patterns.
Occupational and environmental factors
Occupational and environmental exposure to solvents, particularly trichloroethylene (TCE), and cadmium have been associated with RCC risk.14,16,46,47 IARC has concluded that TCE is a human carcinogen (Group 1) that causes kidney cancer.48 Occupational exposure to perchloroethylene (PERC) has also been inconsistently associated with kidney cancer risk, including in a larger population-based case–control study in the USA that observed an increased risk of kidney cancer in relation to higher occupational exposure to PERC, independently of TCE.49 Cadmium is mainly stored in the kidney50 and positive associations with kidney cancer have been reported for cadmium and cadmium compounds.51
Concern is growing about the potential relationship between exposure to perfluorinated compounds (PFCs) and risk of kidney cancer. Humans are exposed to PFCs on a daily basis through intake of contaminated food, water, air and dermal exposure as a result of the widespread use of PFCs in consumer and industrial products since the 1950s.52–57 The kidney is the main organ for PFCs bioaccumulation58–62 and also the ‘target organ’ of PFCs. PFCs accumulated in the kidneys were shown to cause serious kidney damage and increased uric-acid levels even at the levels commonly experienced by US children/adolescents and adults.63–68 Higher uric-acid levels are associated with increased risk of hypertension, metabolic syndrome and chronic kidney disease in adults and children.69–73 PFCs at concentrations similar to exposures observed in the US population increase the production of reactive oxidative species74 that cause renal microvascular disease75 and result in alterations in renal microvascular endothelial cell permeability,74,76–78 which plays a critical role in ischemic renal injury.79–81 Higher body PFC levels have been linked to higher cholesterol levels in humans,82–86 which, in turn, is associated with an increased risk of chronic kidney disease.87,88 PFCs also cause a reduction in renal tubular secretion of uric acid, and thus cause an increase in body levels of uric acid,89 which in turn is associated with increased systemic inflammation, insulin resistance, metabolic syndrome, diabetes mellitus, hypertension and chronic kidney disease.69,90–95 Hypertension, chronic kidney diseases and diabetes are risk factors for kidney cancer. PFCs can cross the placental barrier, reaching fetal circulation, and can also pass to infants through breast-feeding.96–100 PFC levels in children are generally higher than PFC levels in adults58,101 and, thus, children are at greater risk of developing disrupted renal physiologic functions,64 which might make children more susceptible to the PFC exposure. Increased production of PFC products since the late 1950s and the subsequent increased population exposure also align with our APC modelling results.52,102 To support the hypothesis, PFC-exposed workers and residents living in PFC-contaminated communities have an increased risk of kidney cancer,103–107 whereas not others.108,109 It is interesting to note that the liver is also an organ (other than the kidney) for PFC bioaccumulation110 and liver cancer has been reported to be increasing during the past decades.111 Epidemiological studies have also implicated obesity, diabetes and cigarette smoking as risk factors for liver cancer in addition to hepatitis B and C infection.111
Conclusion
Our analysis of the incidence of kidney cancer using SEER data suggests that RCC has increased during the past several decades and that the observed increase is a birth-cohort phenomenon. The known risk factors for kidney cancer may not fully account either for the observed increase or for the birth-cohort pattern. Population-based epidemiologic studies are urgently needed to identify the risk factors for kidney cancer that can be used to explain the observed increase and the birth-cohort pattern of kidney cancer.
Conflict of interest: None declared.
References
- 1.American Cancer Society. Cancer Facts & Figures 2018. Atlanta: American Cancer Society, 2018. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html. [Google Scholar]
- 2. Muglia VF, Prando A. Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras 2015;48:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katz DL, Zheng T, Holford TR, Flannery J. Time trends in the incidence of renal carcinoma: analysis of Connecticut Tumor Registry data, 1935–89. Int J Cancer 1994;58:57–63. [DOI] [PubMed] [Google Scholar]
- 4. Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA 1999;281:1628–31. [DOI] [PubMed] [Google Scholar]
- 5. Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. JNCI 2006;98:1331–34. [DOI] [PubMed] [Google Scholar]
- 6. United States Surveillance, Epidemiology, and End Results (SEER) Program. https://seer.cancer.gov/data/ (26 March 2018, date last accessed).
- 7. Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics 1983;39:311–24. [PubMed] [Google Scholar]
- 8. Holford TR. Understanding the effects of age, period and cohort on incidence and mortality rates. Annu Rev Public Health 1991;12:425–57. [DOI] [PubMed] [Google Scholar]
- 9. Holford TR. Analyzing the temporal effects of age, period and cohort. Stat Methods Med Res 1992;1:317–37. [DOI] [PubMed] [Google Scholar]
- 10. Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: age-period and age-cohort models. Stat Med 1987;6:449–67. [DOI] [PubMed] [Google Scholar]
- 11. Clayton D, Schifflers E. Models for temporal variation in cancer rates. II Age-period and age-cohort models. Stat Med 1987;6:469–81. [DOI] [PubMed] [Google Scholar]
- 12.Statistical Analysis System SAS 9.3. http://support.sas.com/software/93/ (1 June 2019, date last accessed).
- 13. Bagheri MH, Ahlman MA, Lindenberg L et al. Advances in medical imaging for the diagnosis and management of common genitourinary cancers. Urol Oncol 2017;35:473–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lipworth L, Tarone RE, McLaughlin JK. Renal cell cancer among African Americans: an epidemiologic review. BMC Cancer 2011;11:133.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lipworth L, McLaughlin JK, Tarone RE, Blot WJ. Renal cancer paradox: higher incidence but not higher mortality among African Americans. Eur J Cancer Prev 2011;20:331–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010;7:245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chow WH, Devesa SS. Contemporary renal cell cancer epidemiology. Cancer J 2008;14:288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ronchi E, Pizzocaro G, Miodini P, Piva L, Salvioni R, Di Fronzo GJ. Steroid hormone receptors in normal and malignant human renal tissue: relationship with progestin therapy. Steroid Biochem 1984;21:329–35. [DOI] [PubMed] [Google Scholar]
- 19. Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer 2005;114:101–08. [DOI] [PubMed] [Google Scholar]
- 20. Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE. Risk factors for renal cell cancer: the multiethnic cohort. Am J Epidemiol 2007;166:932–40. [DOI] [PubMed] [Google Scholar]
- 21. Jamal A, Phillips E, Gentzke AS et al. Current cigarette smoking among adults—United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng T, Holford TR, Boyle P et al. Time trend and the age-period-cohort effect on the incidence of histologic types of lung cancer in Connecticut, 1960–1989. Cancer 1994;74:1556–67. [DOI] [PubMed] [Google Scholar]
- 23. Engels EA, Pfeiffer RM, Fraumeni JF Jr et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011;306:1891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stewart JH, Vajdic CM, van Leeuwen MT et al. The pattern of excess cancer in dialysis and transplantation. Nephrol Dial Transplant 2009;24:3225–31. [DOI] [PubMed] [Google Scholar]
- 25. Vajdic CM, McDonald SP, McCredie MR. et al. Cancer incidence before and after kidney transplantation. JAMA 2006;296:2823–31. [DOI] [PubMed] [Google Scholar]
- 26. Stewart JH, Buccianti G, Agodoa L et al. Cancers of the kidney and urinary tract in patients on dialysis for end-stage renal disease: analysis of data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol 2003;14:197–207. [DOI] [PubMed] [Google Scholar]
- 27. Maisonneuve P, Agodoa L, Gellert R et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet 1999;354:93–99. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Obesity and Overweight.http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (16 February 2018, date last accessed)
- 29. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity among Adults and Youth: Unites States, 2015–2016. NCHS Data Brief, No. 288. Hyattsville, MD: National Center for Health Statistics, 2017. [PubMed]
- 30. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78. [DOI] [PubMed] [Google Scholar]
- 31. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, for the International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer—viewpoint of the IARC working group. N Engl J Med 2016;375:794–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lowrance WT, Thompson RH, Yee DS, Kaag M, Donat SM, Russo P. Obesity is associated with a higher risk of clear-cell renal cell carcinoma than with other histologies. BJU Int 2010;105:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song MY, Willett WC, Hu FB et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer 2016;138:2383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gati A, Kouidhi S, Marrakchi R et al. Obesity and renal cancer: role of adipokines in the tumor-immune system conflict. Oncoimmunology 2014;3:e-27810-1–e-27810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kabaria R, Klaassen Z, Terris MK. Renal cell carcinoma: links and risks. Int J Nephrol Renovasc Dis 2016;9:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benichou J, Chow WH, McLaughlin JK, Mandel JS, Fraumeni JF Jr. Population attributable risk of renal cell cancer in Minnesota. Am J Epidemiol 1998;148:424–30. [DOI] [PubMed] [Google Scholar]
- 37. Colt JS, Schwartz K, Graubard BI, Davis F, Ruterbusch J, DiGaetano R. Hypertension and risk of renal cell carcinoma among white and black Americans. Epidemiology 2011;22:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leeson P. Hypertension and cardiovascular risk in young adult life: insights from CAVI. Eur Heart J Suppl 2017;19(Suppl_B):B24–9. doi:10.1093/eurheartj/suw061. [Google Scholar]
- 39. Habib SL, Prihoda TJ, Luna M, Werner SA. Diabetes and risk of renal cell carcinoma. J Cancer 2012;3:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin CC, Chiang JH, Li C et al. Cancer risks among patients with type 2 diabetes: a 10-year follow-up study of a nationwide population-based cohort in Taiwan. BMC Cancer 2014;14:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sanfilippo KM, McTigue KM, Fidler CJ et al. Hypertension and obesity and the risk of kidney cancer in two large cohorts of us men and women. Hypertension 2014;63:934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Graff RE, Sanchez A, Tobias DK. Type 2 diabetes in relation to the risk of renal cell carcinoma among men and women in two large prospective cohort studies. Diabetes Care 2018;41:1432–7. doi:10.2337/dc17-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Larsson SC, Wolk A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia 2011;54:1013–18. [DOI] [PubMed] [Google Scholar]
- 44. Haas NB, Nathanson KL. Hereditary renal cancer syndromes. Adv Chronic Kidney Dis 2014;21:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Costa WH, Jabboure Netto G, Cunha IW. Urological cancer related to familial syndromes. Int Braz J Urol 2017;43:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pesch B, Haerting J, Ranft U, Klimpel A, Oelschlägel B, Schill W. Occupational risk factors for renal cell carcinoma: agent-specific results from a case-control study in Germany. Int J Epidemiol 2000;29:1014–24. [DOI] [PubMed] [Google Scholar]
- 47. Song J, Luo H, Yin X et al. Association between cadmium exposure and renal cancer risk: a meta-analysis of observational studies. Sci Rep 2015;5:17976.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 106: Trichloroethylene, Tetrachloroethylene, and Some Other Chlorinated Agents. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono106.pdf (28 April 2019, date last accessed). [PMC free article] [PubMed]
- 49. Purdue MP, Stewart PA, Friesen MC et al. Occupational exposure to chlorinated solvents and kidney cancer: a case-control study. Occup Environ Med 2017;74:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kollmeier H, Seeman J, Wittig P, Witting C, Rothe G. Metallanreicherungen in Humangeweben. Schriftenreihe Der Bundesanstalt Für Arbeitsschutz Fb 347. Bremerhaven: Wirtschaftsverlag NW, 1985.
- 51.International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 100C. Arsenic, Metals, Fibres and Dusts. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono100C.pdf (15 September 2018, date last accessed). [PMC free article] [PubMed]
- 52. Wang ZY, DeWitt JC, Higgins CP, Cousins IT. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ Sci Technol 2017;51:2508–18. [DOI] [PubMed] [Google Scholar]
- 53. White SS, Fenton SE, Hines EP. Endocrine disrupting properties of perfluorooctanoic acid. J Steroid Biochem Mol Biol 2011;127:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Environmental Health Agency(EPA). Emerging Contaminants—Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA). 2014. http://www2.epa.gov/fedfac/emerging-contaminants-perfluorooctane-sulfonate-pfos-and-perfluorooctanoic-acid-pfoa.
- 55. Déon S, Escoda A, Fievet P. A transport model considering charge adsorption inside pores to describe salts rejection by nanofiltration membranes. Chem Eng Sci 2011;66:2823–32. [Google Scholar]
- 56. Shoeib M, Harner T, Webster GM, Sverko E, Cheng Y. Legacy and current-use flame retardants in house dust from Vancouver, Canada. Environ Pollut 2012;169:175–82. [DOI] [PubMed] [Google Scholar]
- 57.National Health and Nutrition Examination Survey (NHANES). 2015. http://wwwn.cdc.gov/nchs/nhanes/search/nhanes15_16.aspx (15 September 2018, date last accessed).
- 58. Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 2007;99:366–94. [DOI] [PubMed] [Google Scholar]
- 59. Butenhoff JL, Kennedy GL Jr, Hinderliter PM et al. Pharmacokinetics of perfluorooctanoate in cynomolgus mondeys. Toxicol Sci 2004;8:394–406. [DOI] [PubMed] [Google Scholar]
- 60. Olsen GW, Burris JM, Ehresman DJ et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate and perfluorooctanoate in retired fluorochemical production worrkers. Environ Health Perspect 2007;115:1298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61., Yoo H, Guruge KS, Yamanaka N. et al. Depuration kinetics and tissue disposition of PFOA and PFOS in white leghorn chickens (Gallus gallus) administered by subcutaneous implantation. Ecotoxicol Environ Saf 2009;72:26–36. [DOI] [PubMed] [Google Scholar]
- 62. Han X, Kemper RA, Jepson GW. Subcellular distribution and protein binding of perfluorooctanoic acid in rat liver and kidney. Drug Chem Toxicol 2005;28:197–209. [DOI] [PubMed] [Google Scholar]
- 63. Shankar A, Xiao J, Ducatman A. Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am J Epidemiol 2011;174:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kataria A, Trachtman H, Malaga-Dieguez L, Trasande L. Association between perfluoroalkyl acids and kidney function in a cross-sectional study of adolescents. Environ Health 2015;14:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Geiger SD, Xiao J, Shankar A. Positive association between perfluoroalkyl chemicals and hyperuricemia in children. Am J Epidemiol 2013;177:1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gleason JA, Post GB, Fagliano JA. Association of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007–2010. Environ Res 2015;136:8–14. [DOI] [PubMed] [Google Scholar]
- 67. Steeland K, Tinker S, Shankar A, Jucatman A. Association of perfluorooctanoic acid and perfluorooctane sulfonate with uric acid among adults with elevated community exposure to PFOA. Environ Health Perspect 2010;118:229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Watkins DJ, Josson J, Elston B et al. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ Health Perspect 2013;121:625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cain L, Shankar A, Ducatman AM, Steenland K. The relationship between serum uric acid and chronic kidney disease among Appalachian adults. Nephrol Dial Transplant 2010;25:3593–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alper AB Jr, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension 2005;45:34–38. [DOI] [PubMed] [Google Scholar]
- 71. Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension 2003;42:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee MS, Wahlqvist ML, Yu HL, Pan WH. Hyperuricemia and metabolic syndrome in Taiwanese children. Asia Pac J Clin Nutr 2007;16:594–600. [PubMed] [Google Scholar]
- 73. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Qian Y, Ducatman A, Ward R et al. Perfluorooctane sulfonate iunduces reactive oxygen species (ROS) production in human microvascular endothelial cells: role in endothelial permeability. J Toxicol Environ Health 2010;73:819–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cui L, Zhou QF, Liao CY, Fu JJ, Jiang GB. Studies on the toxicological effects of PFOA and OFOS on rats using histological observation and chemical analysis. Arch Environ Contam Toxicol 2009;56:338–49. [DOI] [PubMed] [Google Scholar]
- 76. Hu W, Jones PD, DeCoen W et al. Alterations in cell membrane properties caused by perfluorinated compounds. Comp Biochem Physiol C Toxicol Pharmacol 2003;135:77–88. [DOI] [PubMed] [Google Scholar]
- 77. Yao X, Zhong L. Genotoxic risk and oxidative DNA damage in HepG2 cells exposed to perfluorooctanoic acid. Mutat Res 2005;587:38–44. [DOI] [PubMed] [Google Scholar]
- 78. Panaretakis T, Shabalina IG, Grander D, Shoshan MC, DePierre JW. Reactive oxygen species and mitochondria mediate the induction of apoptosis in human hepatoma HepGe cells by the rodent peroxisome proliferator and hepatocarcinogen, perfluorooctanoic acid. Toxicol Appl Pharmacol 2001;173:56–64. [DOI] [PubMed] [Google Scholar]
- 79. Sutton TA. Alteration of microvascular permeability in acute kidney injury. Microvasc Res 2009;77:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol 2003;285:F191–98. [DOI] [PubMed] [Google Scholar]
- 81. Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 2002;62:1539–49. [DOI] [PubMed] [Google Scholar]
- 82. Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general US population. Environ Health Perspect 2010;118:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Steeland K, Tinker S, Frishee S, Ducatman A, Vaccarino V. Associations of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am. J Epidemiol 2009;170:1268–78. [DOI] [PubMed] [Google Scholar]
- 84. Sakr CJ, Kreckmann KH, Green JW, Gillies PJ, Reynolds JL, Leonard RC. Cross-sectional study of lipids and liver enzymes related to a serum biomarker of exposure (ammonium perfluorooctanoate or APFO) as part of a general health survey in a cohort of occupationally exposed workers. J Occup Environ Med 2007;49:1086–96. [DOI] [PubMed] [Google Scholar]
- 85. Sakr CJ, Leonard RC, Kreckmann KH, Slade MD, Cullen MR. Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate. J Occup Environ Med 2007;49:872–79. [DOI] [PubMed] [Google Scholar]
- 86. Olsen GW, Zobel LR. Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int Arch Occup Environ Health 2007;81:231–46. [DOI] [PubMed] [Google Scholar]
- 87. Shankar A, Klein R, Moss SE, Klein BE, Wong TY. The relationship between albuminuria and hypercholesterolemia. J Nephrol 2004;17:658–65. [PubMed] [Google Scholar]
- 88. Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int 2000;58:293–301. [DOI] [PubMed] [Google Scholar]
- 89. Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des 2005;11:4145–51. [DOI] [PubMed] [Google Scholar]
- 90. Bandaru P, Shankar A. Association between serum uric acid levels and diabetes mellitus. Int J Endocrinol 2011;2011:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shankar A, Klein R, Klein BE, Nieto FJ. The association between serum uric acid level and long-term incidence of hypertension: population-based cohort study. J Hum Hypertens 2006;20:937–45. [DOI] [PubMed] [Google Scholar]
- 92. Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. JASN 2005;16:3553–62. [DOI] [PubMed] [Google Scholar]
- 93. Chen J, Muntner P, Hamm LL et al. The metabolic syndrome and chronic kidney disease in US adults. Ann Intern Med 2004;140:167–74. [DOI] [PubMed] [Google Scholar]
- 94. Chen J, Muntner P, Hamm LL et al. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol 2003;14:469–77. [DOI] [PubMed] [Google Scholar]
- 95. Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 1991;266:3008–11. [PubMed] [Google Scholar]
- 96. Grasty RC, Wolf DC, Grey BE, Lau CS, Rogers JM. Prenatal window of susceptibility to perfluorooctane sulfonate-induced neonatal mortality in the Sprague-Dawley rat. Birth Defects Res B Dev Reprod Toxicol 2003;68:465–71. [DOI] [PubMed] [Google Scholar]
- 97. Luebker DJ, York RG, Hansen KJ, Moore JA, Butenhoff JL. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague–Dawley rats: dose–response, and biochemical and pharamacokinetic parameters. Toxicology 2005;215:149–69. [DOI] [PubMed] [Google Scholar]
- 98. Thibodeaux JR, Hanson RG, Rogers JM et al. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol Sci 2003;74:369–81. [DOI] [PubMed] [Google Scholar]
- 99. Lee YJ, Kim MK, Bae J, Yang JH. Concentrations of perfluoroalkyl compounds in maternal and umbilical cord sera and birth outcomes in Korea. Chemosphere 2013;90:1603–09. [DOI] [PubMed] [Google Scholar]
- 100. Apelberg BJ, Witter FR, Herbstman JB et al. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect 2007;115:1670–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Post GB, Cohn PD, Cooper KR. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environ Res 2012;116:93–117. [DOI] [PubMed] [Google Scholar]
- 102.U.S. EPA. Office of Land and Emergency Management. Technical Fact Sheet-Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA). EPA 505-F-17–001, November 2017. https://www.epa.gov/sites/production/files/2017-12/documents/ffrrofactsheet_contaminants_pfos_pfoa_11-20-17_508_0.pdf (1 March 2019, date last accessed).
- 103. Steenland K, Woskie S. Cohort mortality study of workers exposed to perfluorooctanoic acid. Am J Epidemiol 2012;176:909–17. [DOI] [PubMed] [Google Scholar]
- 104. Leonard RC, Kreckmann KH, Sakr CJ, Dymons JM. Retrospective cohort mortality study of workers in a polymer production plant including a reference population of regional workers. Ann Epidemiol 2008;18:15–22. [DOI] [PubMed] [Google Scholar]
- 105. Barry V, Winquist A, Steenland K. Perfluorooctanoic acid exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect 2013;121:1313–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect 2013;121:318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.C8 Science Panel. Probable Link Evaluation of Cancer. 2012. http://www.c8sciencepanel.org/pdfs/Probable_Link_C8_Cancer_16April2012_v2.pdf (2 October 2018, date last accessed).
- 108. Lundin JI, Alexander BH, Olsen GW, Church TR. Ammonium perfluorooctanoate production and occupational mortality. Epidemiology 2009;20:921–28. [DOI] [PubMed] [Google Scholar]
- 109. Raleigh KK, Alexander BH, Olsen GW et al. Mortality and cancer incidence in ammonium perfluorooctanoate production workers. Occup Environ Med 2014;71:500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hundley SG, Sarrif AM, Kennedy GL. Absorption, distribution, and excretion of ammonium perfluorooctanoate (APFO) after oral administration to various species. Drug Chem Toxicol 2006;29:137–45. [DOI] [PubMed] [Google Scholar]
- 111. Islami F, Miller KD, Siegel RL, Fedewa SA, Ward EM, Jemal A. Disparities in liver cancer occurrence in the United States by race/ethnicity and state. CA Cancer J Clin 2017;67:273–89. [DOI] [PubMed] [Google Scholar]