Abstract
Background
The mechanisms behind ART-induced bone changes in HIV-infected patients are poorly known. We aimed to analyse changes in inflammatory and bone markers in HIV after tenofovir disoproxil fumarate initiation, and the associations with changes in the bone strength parameters.
Methods
HIV-positive participants starting tenofovir disoproxil fumarate-based ART underwent dual-energy X-ray absorptiometry (QDR 4500 SL®, Hologic, Waltham, MA, USA) for bone mineral density (BMD), a microindentation test (OsteoProbe®, Active Life Scientific, Santa Barbara, CA, USA) for bone quality [bone material strength index (BMSi)] and phlebotomy at baseline and 48 weeks after ART. A panel of inflammatory biomarkers and bone turnover markers were measured by ELISA. HIV-negative controls underwent identical procedures once. Values are expressed as medians and IQRs, and non-parametric tests were used to perform the analysis.
Results
Twenty HIV-infected individuals and 20 HIV-negative control individuals were matched in terms of age and gender. HIV individuals showed higher levels of inflammatory markers. We found no differences in bone turnover markers. HIV-positive individuals presented lower BMSi values at baseline compared with controls [86 (83–90) versus 89 (88–93), respectively; P = 0.034]. We found no difference in BMD (at either of the sites evaluated). BMSi tended to increase with treatment. IL-1β at baseline was positively correlated with changes in BMSi after ART (rho = 0.564, P = 0.014). Baseline levels of sclerostin tended to be negatively correlated with changes in BMSi (rho = −0.402, P = 0.097). We found a negative correlation between time since HIV diagnosis and changes in BMSi (rho = −0.466, P = 0.04).
Conclusions
We observed a correlation between changes in bone quality and the inflammatory environment in HIV-positive individuals. Moreover, among the underlying mechanisms we highlight the Wnt pathway as having a potentially significant role in ART bone quality recovery.
Introduction
HIV infection is known to induce bone loss, but the underlying factors and mechanisms remain elusive. One of the most relevant hypotheses is that HIV-induced immune activation is responsible for bone loss.1 This immune response includes the production of TNF-α and IL-6, inflammatory cytokines that also appear to contribute to bone loss in other inflammatory conditions.2–4 Bone tissue and the immune system share many common signalling pathways that form what is known as the ‘immunoskeletal interface’. As bone remodelling is a tightly regulated balance between bone-forming osteoblasts and bone-resorbing osteoclasts, inflammatory cytokines produced during chronic inflammation could induce an uncoupling of bone formation and resorption, leading to significant bone loss. Indeed, both IL-6 and TNF-α have been reported to exert a negative effect on osteoblast differentiation.5,6
Moreover, bone loss in HIV patients can become aggravated after beginning ART, probably due to the adverse effects of treatments on various body tissues. Therefore, the bone of patients initiating ART treatment is impacted by an effect of HIV itself, plus the effect of ART toxicity.
Compared with the general population, the accelerated level of bone loss experienced by HIV patients leads to increased risk of osteoporosis and associated bone fractures.7–10 In this respect, it has become essential to improve the diagnostic tools for bone disease, such as the ability to make an earlier diagnosis. Traditionally, bone tissue has been assessed using dual-energy X-ray absorptiometry (DXA) to determine bone mineral density (BMD). Previous studies have demonstrated that BMD decreases after patients begin tenofovir disoproxil fumarate and PI treatment regimens.11 However, newer techniques, such as trabecular bone score (TBS) and microindentation, which assess bone microarchitecture and bone tissue quality, respectively, can provide additional information regarding bone health for both the general population12–15 and for HIV-infected individuals in particular.7 For example, in contrast to the aforementioned bone loss (i.e. decreases in BMD), our group has recently found that bone tissue quality improves once immune reconstitution has occurred, suggesting that more complex processes occur in bone both before and after initiating tenofovir disoproxil fumarate-based ART.16
The aim of this study was to explore the mechanisms behind ART-induced bone changes in HIV-infected patients. Using the healthy population as a reference, we analysed changes in the plasma concentrations of inflammatory and bone markers in HIV-infected patients after tenofovir disoproxil fumarate initiation and investigated associations with changes in the bone parameters (i.e. bone density, microarchitecture and bone tissue quality).
Methods
Study population
HIV participants were selected from a clinical HIV cohort that was established in Hospital del Mar, Barcelona, Spain. Eligible participants included individuals who were ≥18 years of age and were treatment naive. We excluded any patients who had previously received treatments that affect bone remodelling (e.g. systemic glucocorticoids or anti-osteoporotic medications) and any patients who had previously been diagnosed with chronic kidney disease, chronic endocrine conditions, malabsorption syndrome, advanced liver disease, neoplasia or bone diseases. An HIV-negative control group was recruited from a primary care centre associated with Hospital del Mar, matched by age and sex with the same exclusion criteria.
HIV-negative participants were visited only once. HIV participants were visited at entry, at 24 weeks and at 48 weeks. Measurements were taken at entry and at 48 weeks.
All patients received tenofovir disoproxil fumarate/emtricitabine (as a backbone drug) with elvitegravir/cobicistat, in a single pill, once a day and were monitored for 48 weeks. Before each visit, patients fasted for 8 h and then had their plasma samples collected by phlebotomy. Demographic and HIV-related variables, such as year of diagnosis, nadir CD4+ count, peak viral load and opportunistic infections, were also recorded at entry.
Ethics
The study protocol was approved by the Ethics Committee of Parc de Salut Mar (2016/6803/I) and was carried out in accordance with the Declaration of Helsinki. All participants gave written informed consent to take part in this study after having read the study information sheet and having any questions answered. The privacy rights of human subjects were observed at all times.
Evaluation of bone parameters
We obtained bone measurements for HIV patients both at entry (baseline) and 48 weeks after starting ART, as previously described.17 In contrast, control individuals underwent the same procedures, but only once. Briefly, BMD was measured at the lumbar spine (L1–L4) and femoral neck using a DXA densitometer (QDR 4500 SL®; Hologic, Waltham, MA, USA). The in vivo coefficient of variation ranged from 1% at the lumbar spine to 1.65% at the femoral neck. We performed spine TBS measurements using the TBS software supplied with the densitometer (TBS iNsight® v2.1, Med-Imaps, Pessac, France). Bone material strength index (BMSi) was measured by bone microindentation at the tibia using the OsteoProbe® (Active Life Scientific, Santa Barbara, CA, USA) as previously described.11
Biomarker assays
The plasma samples obtained at baseline and at 48 weeks were stored at −80°C until analysis. We used an ELISA kit (Roche Diagnostics, Indianapolis, IN, USA) to measure the following bone turnover markers: amino propeptide of type 1 collagen (P1NP; mg/L), C-telopeptide of type 1 collagen (CTX; ng/mL), 25-OH vitamin D, intact parathyroid hormone (PTH) and bone-specific alkaline phosphatase. Furthermore, as part of the routine clinical care, we also analysed serum concentrations of calcium, phosphorus, creatinine, thyroid stimulating hormone (TSH) and aspartate transferase using an electro-chemiluminescent immunoassay (ECLIA) (Roche Diagnostics). In addition, we used a multiassay ELISA panel (Merck Millipore, Munich, Germany) to measure the following inflammatory markers: IFN-γ, IL-10, IL-17A, IL-1β, IL-2, IL-4, IL-6, IL-8, soluble CD40, TNF-α and the soluble receptors of IL-1, IL-6 and TNF-α (soluble receptors 1 and 2). In this ELISA multiassay panel, bone remodelling factors such as DKK1, osteoprotegerin and sclerostin were also measured. Other parameters measured were: high-sensitivity C-reactive protein (hs-CRP) [CLIA, Immulite 2000 (Siemens)], erythrocyte sedimentation rate (ESR), β2-microglobulin [CLIA, Immulite 2000 (Siemens)], D-dimer [immunoturbidimetry (ACL TOP 300)] and fibrinogen [Clauss method (ACL TOP 300)].
Statistical analysis
Sample size was calculated considering changes in hs-CRP. Assuming an alpha risk of 0.05 and a beta risk of 0.20 in a two-sided test, we required at least 20 subjects to detect a statistically significant difference of ≥0.2 mg/dL. We assumed the standard deviation to be 0.3 units. We anticipated a dropout rate of 10%.
In our analysis, we describe continuous measures, using medians and IQRs, and categorical variables, using frequencies and percentages.
For each parameter, we calculated the percentage change at 48 weeks with respect to the baseline value.
The Wilcoxon rank-sum test was used to compare the quantitative variables between HIV participants and controls, as well as the biomarker levels in HIV patients before and after ART initiation. Comparisons were done between baseline and 48 weeks in the HIV group, as well as between the HIV group at baseline and controls and between the HIV group at 48 weeks and controls.
In addition, we used Spearman correlation coefficients to study the relationships between the changes (i.e. prior to ART versus 48 weeks after ART) in bone parameters and the bone turnover and inflammatory markers.
We fitted multivariate linear regression models for each dependent variable (i.e. percentage change for each bone parameter) using the baseline inflammatory or bone turnover markers as independent variables. Models were adjusted for potential confounding variables including age, sex, BMI, pre-ART CD4+ T cell count and pre-ART HIV RNA. We established a significance level of <0.05 for all analyses. Analyses were performed using STATA software 14.2 (College Station, TX, USA).
Results
Subject characteristics
We recruited a total of 20 HIV-infected individuals who were about to start ART and compared these with 20 HIV-negative control individuals matched in terms of age and gender. Table 1 shows the baseline characteristics of the two groups along with relevant HIV infection parameters. We found no marked differences between groups with respect to anthropometric features, smoking and alcohol consumption, or the use of intravenous drugs (neither prior nor current use).
Table 1.
Baseline characteristics of the study population
| Controls | HIV-positive individuals | P | |
|---|---|---|---|
| N | 20 | 20 | |
| Age (years), median (IQR) | 38 (35–42) | 37 (31–43) | 0.423 |
| Male, n (%) | 15 (75) | 16 (80) | 0.336 |
| BMI (kg/m2), median (IQR) | 23 (20–24) | 24 (22–26) | 0.281 |
| Smoking, n (%) | 8 (40) | 8 (40) | |
| Alcohol >10 g/day, n (%) | 0 (0) | 1 (5) | 0.314 |
| Ex-IVDU, n (%) | 0 (0) | 1 (5) | 0.314 |
| Recreational drugs, n (%) | 3 (15) | 7 (35) | 0.097 |
| Previous fracture, n (%) | 0 (0) | 0 (0) | |
| Family history of fracture, n (%) | 2 (10) | 1 (5) | 0.605 |
| Prevalent spine fractures, n (%) | 0 (0) | 0 (0) | |
| eGFR <60 mL/min, n (%) | 0 (0) | 0 (0) | |
| eGFR (CKD-EPI) (mL/min), median (IQR) | 92 (75–97) | 88 (72–91) | 0.345 |
| Years since HIV diagnosis, median (IQR) | 1 (0–2) | ||
| Nadir CD4+ count (per mL), median (IQR) | 364 (327–443) | ||
| Current CD4+ count (per mL), median (IQR) | 748 (678–1437) | 374 (341–460) | 0.093 |
| Ever met AIDS criteria, n (%) | 1 (5) |
eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Analysis of inflammatory markers, bone turnover markers and bone strength parameters in treatment-naive HIV patients compared with control individuals
As expected, we detected a significantly higher inflammation status among treatment-naive HIV patients compared with the control group (Table 2). Of all inflammatory markers, we found the greatest differences for hs-CRP [0.04 (0.002–0.19) versus 0.31 (0.10–0.60) mg/dL in the control group versus treatment-naive HIV patients, respectively; P = 0.003], ESR [3 (2–9) versus 16 (10–22) mm/h in the control group versus treatment-naive HIV patients, respectively; P = 0.003], IL-17A [13.60 (4.90–23.80) versus 3.21 (1.46–5.28) pg/mL in the control group versus treatment-naive HIV patients, respectively; P = 0.002] and TNF-α soluble receptor 2 [7.5 (6.7–9.1) versus 13.7 (11.4–15.6) ng/mL in the control group versus treatment-naive HIV patients, respectively; P = 0.001]. In contrast, we found no differences in bone turnover markers.
Table 2.
Bone strength parameters, inflammation markers and bone turnover markers for the various groups
| Controls | HIV group at baseline | P a | HIV group at 48 weeks | P b | P c | |
|---|---|---|---|---|---|---|
| Bone parameters | ||||||
| BMSi | 89 (88–93) | 86 (83–90) | 0.034 | 90 (88–93) | 0.02 | 0.765 |
| TBS | 1.374 (1.29–1.39) | 1.339 (1.28–1.38) | 0.336 | 1.291 (1.225–1.397) | 0.007 | 0.002 |
| lumbar spine BMD (g/cm2) | 0.988 (0.92–1.06) | 1.019 (0.91–1.11) | 0.929 | 0.97 (0.88–1.091) | 0.001 | 0.951 |
| femoral neck BMD (g/cm2) | 0.831 (0.75–0.98) | 0.841 (0.79–1.02) | 0.734 | 0.818 (0.733–0.957) | 0.001 | 0.092 |
| Inflammation markers | ||||||
| hs-CRP (mg/dL) | 0.04 (0.002–0.19) | 0.31 (0.1–0.6) | 0.003 | 0.08 (0.06–0.24) | 0.026 | 0.142 |
| ESR (mm/h) | 3 (2–9) | 16 (10–22) | 0.003 | 6 (3–14) | 0.003 | 0.255 |
| fibrinogen (mg/dL) | 350 (289–395) | 320 (290–410) | 0.734 | 366 (306–391) | 0.753 | 0.688 |
| D-dimer (IU/mL) | 97 (63–144) | 131 (87–257) | 0.242 | 100 (76–180) | 0.601 | 0.982 |
| IFN-γ (pg/mL) | 16.9 (10.4–38.4) | 11.5 (7.4–20.5) | 0.183 | 7.58 (4.5–15.9) | 0.394 | 0.023 |
| IL-10 (pg/mL) | 3.24 (3.24–3.57) | 3.57 (3.24–4.15) | 0.163 | 3.24 (3.24–3.57) | 0.182 | 0.881 |
| IL-17A (pg/mL) | 13.6 (4.9–23.8) | 3.21 (1.46–5.28) | 0.002 | 5.1 (3.2–13.8) | 0.169 | 0.003 |
| IL-1β (pg/mL) | 1.87 (1.28–2.79) | 1.87 (1.28–2.79) | 0.707 | 2.03 (1.65–2.41) | 0.332 | 0.705 |
| IL-2 (pg/mL) | 2.41 (0.58–2.91) | 2.41 (0.58–2.91) | 0.535 | 2.41 (0.58–2.89) | 0.317 | 0.683 |
| IL-4 (pg/mL) | 5.78 (2.52–10.72) | 2.52 (1.27–5.78) | 0.023 | 4.01 (2.66–5.37) | 0.186 | 0.024 |
| IL-6 (pg/mL) | 2.86 (2.86–2.99) | 2.92 (2.86–2.99) | 0.895 | 2.86 (1.24–2.99) | 0.516 | 0.438 |
| IL-8 (pg/mL) | 21.1 (6.7–37.3) | 9.31 (4.51–15.46) | 0.144 | 5.14 (2.99–9.85) | 0.033 | 0.005 |
| soluble CD40 (ng/mL) | 6.47 (4.4–7.5) | 5.35 (2.6–8.3) | 0.342 | 5.5 (4–8.1) | 0.609 | 0.446 |
| TNF-α (pg/mL) | 12.6 (9.8–19.3) | 18.9 (16.7–26.6) | 0.026 | 13.9 (12–17.3) | 0.003 | 0.668 |
| IL-6 soluble receptor (ng/mL) | 34.7 (32.1–41.1) | 41.3 (31.5–49.7) | 0.343 | 34.1 (26–47.4) | 0.05 | 0.766 |
| TNF-α soluble receptor 1 (ng/mL) | 1.9 (1.6–2.3) | 1.9 (1.7–2.3) | 0.825 | 1.4 (1.1–1.8) | 0.03 | 0.015 |
| TNF-α soluble receptor 2 (ng/mL) | 7.5 (6.7–9.1) | 13.7 (11.4–15.6) | 0.001 | 9.2 (8.5–10.4) | 0.003 | 0.006 |
| Bone metabolism markers | ||||||
| DKK1 (ng/mL) | 3.8 (2.7–4.6) | 3.1 (2.6–4.1) | 0.179 | 2.7 (2.4–3.4) | 0.177 | 0.022 |
| osteoprotegerin (ng/mL) | 0.5 (0.4–0.6) | 0.4 (0.3–0.5) | 0.428 | 0.4 (0.3–0.5) | 0.461 | 0.272 |
| sclerostin (ng/mL) | 1.5 (0.9–1.8) | 1.5 (1–2) | 0.819 | 1.2 (0.8–1.6) | 0.05 | 0.545 |
| P1NP (ng/mL) | 45.6 (37.1–71.3) | 50.9 (34–59.5) | 0.571 | 71.3 (54.7–95.3) | 0.003 | 0.035 |
| CTX (ng/mL) | 0.31 (0.14–0.46) | 0.26 (0.21–0.41) | 0.968 | 0.38 (0.31–0.46) | 0.007 | 0.105 |
| bone alkaline phosphatase (μg/mL) | 11.7 (6.6–16.3) | 13.4 (10.1–16.8) | 0.319 | 16.1 (12.5–23.1) | 0.005 | 0.021 |
| 25-OH vitamin D (ng/mL) | 28.3 (14.1–43) | 21.4 (10.3–28.6) | 0.145 | 23.4 (14.2–31.6) | 0.356 | 0.704 |
| PTH (pg/mL) | 23 (14–31) | 27 (22–32) | 0.317 | 42 (32–67) | 0.434 | 0.009 |
Results are shown as median (IQR).
Bold font represents significant differences between groups.
Corresponds to the P value when comparing controls with the HIV group at baseline.
Corresponds to the P value when comparing the HIV group at baseline with the HIV group at 48 weeks.
Corresponds to the P value when comparing controls with the HIV group at 48 weeks.
In terms of bone parameters, we only detected significant differences in BMSi. More specifically, HIV patients presented significantly lower BMSi values and thus poorer bone tissue quality compared with control individuals [86 (83–90) versus 89 (88–93), respectively; P = 0.034]. We found no significant difference in the BMD levels (at either of the sites evaluated) or in the TBS between HIV-positive and control individuals (Table 2).
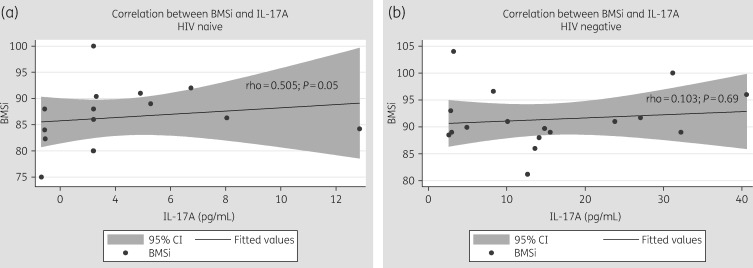
We found a slight correlation between baseline BMSi and IL-17A levels in HIV patients (rho = 0.505, P = 0.050), but not in control individuals (rho = 0.103, P = 0.69) (Figure 1). In contrast, we found no correlation with any plasma marker for TBS or BMD.
Figure 1.
Correlation between bone tissue quality (BMSi) and IL-17A at baseline in (a) HIV-positive individuals and (b) HIV-negative individuals.
Changes in inflammatory markers, bone turnover markers and bone strength parameters in HIV patients after ART initiation
Forty-eight weeks after starting tenofovir disoproxil fumarate-based ART, most of the inflammatory markers that were significantly increased by HIV tended to return toward levels similar to the HIV-uninfected controls (Table 2).
Moreover, ART appeared to significantly increase bone remodelling in HIV patients. This is shown by the increase in bone turnover markers including CTX, bone alkaline phosphatase and P1NP (Table 2). In contrast, levels of sclerostin decreased after 48 weeks of ART [1.5 (1–2) versus 1.2 (0.8–1.6) ng/mL in the HIV group at baseline and the HIV group at 48 weeks, respectively; difference −20%; P = 0.05]. In accordance with that, DKK1 levels were also reduced by ART compared with controls [2.7 (2.4–3.4) versus 3.8 (2.7–4.6) ng/mL in the HIV group at 48 weeks and controls, respectively; P = 0.022] (Table 2).
While BMSi increased after 48 weeks of ART treatment, reaching similar levels as in the control group [90 (88–93) in HIV patients versus 89 (88–93) in control individuals, P = 0.765], the other bone parameters, namely BMD and TBS, significantly decreased after ART initiation. See Table 2.
Factors associated with ART-related changes of bone strength components
Changes in bone tissue quality—BMSi
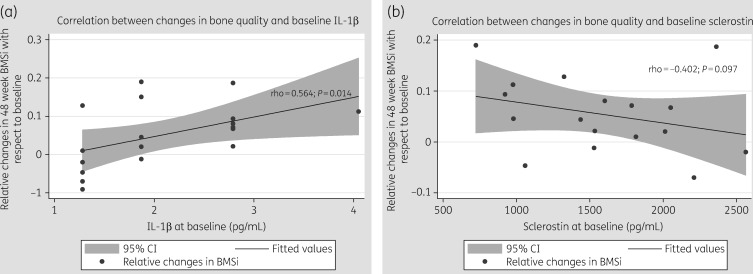
We found that levels of IL-1β at baseline were positively correlated with changes in BMSi after 48 weeks of ART (rho = 0.564, P = 0.014). In other words, patients who had higher IL-1β levels before ART initiation presented greater increases in BMSi after ART. This association persisted after adjusting by CD4+ T cell count and viral load. See Figure 2(a). Moreover, baseline levels of sclerostin tended to be negatively correlated with the relative changes in BMSi (rho = −0.402, P = 0.097) (Figure 2b).
Figure 2.
Correlation between relative changes at week 48 with respect to baseline in bone tissue quality (BMSi) and (a) IL-1β at baseline and (b) sclerostin at baseline.
We also found a negative correlation between time since HIV diagnosis and changes in BMSi (rho = −0.466, P = 0.04) with a beta coefficient of −0.01 (P = 0.05) in the linear regression after adjusting for age, sex and CD4+ T cell count.
Changes in BMD
In terms of the percentage change in lumbar spine BMD, we found that it was negatively correlated with baseline levels of IL-6 (rho = −0.704, P = 0.001), but positively correlated with baseline levels of IL-17A (rho = 0.537, P = 0.04).
Changes in microarchitecture—TBS
No associations were found between changes in TBS and any of the aforementioned markers.
Correlations between inflammatory and bone markers at baseline with percentage changes in bone strength parameters are found in Table 3.
Table 3.
Spearman correlation rhos between baseline inflammation and bone markers and percentage change in bone parameters
| Percentage change in BMSi | Percentage change in thBMD | Percentage change in lsBMD | Percentage change in TBS | |
|---|---|---|---|---|
| IFN-γ | −0.134 | 0.254 | 0.176 | 0.374 |
| IL-10 | 0.127 | 0.253 | −0.054 | 0.258 |
| IL-17A | −0.147 | 0.295 | 0.537 a | 0.419 |
| IL-1β | 0.564 a | −0.095 | 0.141 | 0.392 |
| IL-2 | 0.578 a | −0.157 | 0.104 | 0.356 |
| IL-4 | −0.406 | −0.154 | 0.325 | 0.149 |
| IL-6 | 0.173 | −0.001 | −0.704 b | −0.224 |
| IL-8 | 0.019 | −0.282 | −0.193 | −0.107 |
| Soluble CD40 | −0.471 a | 0.046 | −0.216 | 0.091 |
| TNF-α | −0.218 | −0.017 | 0.194 | 0.161 |
| IL-1 soluble receptor | −0.004 | −0.165 | 0.489 a | 0.386 |
| IL-6 soluble receptor | −0.249 | −0.214 | 0.332 | 0.101 |
| TNF-α soluble receptor 1 | −0.191 | −0.117 | −0.014 | −0.168 |
| TNF-α soluble receptor 2 | −0.143 | 0.183 | 0.181 | 0.237 |
| Sclerostin | −0.402 c | −0.397 | 0.105 | −0.155 |
| DKK1 | 0.248 | −0.358 | 0.101 | 0.067 |
| Osteoprotegerin | 0.068 | −0.332 | 0.052 | −0.853 |
thBMD, total hip BMD; lsBMD, lumbar spine BMD.
Bold font represents significant correlations.
P < 0.05.
P < 0.01.
P = 0.09.
Discussion
We report an association between inflammation levels (as measured by different markers) in HIV individuals and an improvement in bone tissue quality, measured by microindentation, after starting ART. We observed that changes in different inflammation and immunological pathways induced by ART might be contributing to the observed increase in bone tissue quality. In contrast, BMD and TBS seemed to be modulated by other pathways mainly affected by ART. Since bone quality is measuring bone propensity to fracture, the inflammatory status induced by HIV is probably contributing to the increase in fracture risk.9,10 In this study, we also present the implication of the Wnt/β-catenin pathway as one of the players in bone strength in HIV-infected individuals.
Bone strength components such as BMD, bone microarchitecture and bone tissue quality can be easily measured in the clinical setting.11 These components describe different properties of bone and are regulated by different pathways. BMD is the amount of the mineral component of bone, whereas TBS reflects the structural condition of bone microarchitecture, and BMSi measures the quality of the material itself. Although the risk of fracture can increase significantly with decreasing BMD, several studies showed that osteoporotic fractures can occur across a wide spectrum of BMDs.17 Most likely, these events are related to bone quality, a component not captured by DXA measurements. Hence, bone quality measured by microindentation has been reported to predict fracture better than BMD.18 We have the hypothesis that microindentation is more dynamic than BMD. Mellibovsky et al.14 reported how corticosteroids diminished bone tissue quality before any change was detected in BMD and Sundh et al.19 reported how high-impact mechanical loading improved bone tissue quality before any change was detected with DXA. Notably, the present paper confirms previous findings where BMSi values, but not TBS or BMD values, were found to be lower in HIV patients than in healthy subjects.11 Moreover, in studies conducted in rodent sepsis inflammatory models a reduction in bone strength independently of BMD within 24 h after the inflammatory injury was described. This was not because of increased bone turnover, but due to the loss of collagen fibre crosslinks that lead to a reduction in collagen and mineral elastic modulus.20 Similarly, in HIV infection, the inflammatory environment could be affecting collagen fibre crosslinks that are partially restored once ART reduces inflammation. Meanwhile, tenofovir disoproxil fumarate induced bone loss through a reduction in the mineral component, not only due to tubular toxicity but also through the induction of osteoclastogenesis.21
Our study provides insight into how inflammation decreases after the initiation of ART. We have found significant differences in the levels of inflammatory markers between HIV and non-HIV subjects and some of these changes persisted after 48 weeks of treatment. It has previously been demonstrated that persistent inflammation is the ultimate mechanism behind many of the comorbidities observed in HIV patients.22,23 Interestingly, we detected significantly lower levels of IL-17A among HIV individuals. This could be the consequence of the early loss of Th17 cells, one of the hallmarks of HIV infection.24 Moreover, the correlation found at baseline among IL-17A and bone tissue quality probably reflects the interaction of bone and an intense inflammatory milieu, that is partially reversed with treatment. However, after treatment, IL-17A does not recover completely while BMSi improves, showing that there is probably not a direct causality association between these two events. IL-17A is produced by CD4+ Th17 cells, a target of HIV from the early stages of infection.25,26 These cells play a key role in the pathogenesis of HIV since a significant decrease in their levels during infection leads to immune dysregulation.27 As expected, we detected a significantly lower level of IL-17A in treatment-naive HIV individuals compared with healthy individuals.
We identified a positive correlation between IL-17A levels and BMSi at baseline; patients with lower IL-17A levels had poorer bone material properties. However, this correlation disappeared after the immune reconstitution, suggesting that bone tissue quality is affected by a myriad of factors.
Moreover, IL-17A levels were also correlated with ART-induced changes in lumbar spine BMD; higher IL-17A levels at baseline were associated with lower ART-related bone loss, suggesting that less HIV-induced immune impairment could prevent ART-induced bone damage.
IL-17A illustrates the close relationship between the immune system and the skeleton. IL-17A could suppress osteoclast differentiation,28 whereas it could accelerate bone formation by stimulating proliferation of mesenchymal progenitor cell and osteoblast differentiation.29 Therefore, IL-17A levels seem to be important for the optimal maintenance of bone strength components and, in particular, bone tissue quality.
We also report that two key inhibitors of the Wnt/β-catenin pathway, sclerostin and DKK1, were significantly modified by treatment and also had a significant impact on bone tissue quality. This pathway regulates bone development and its later adult homeostasis.30 We found that sclerostin was significantly reduced in antiretroviral-treated participants, whereas DKK1 was reduced after ART and was significantly lower among treated individuals with respect to controls. Moreover, higher baseline levels of sclerostin were associated with a poorer evolution of bone tissue quality during treatment. Sclerostin and DKK1 bind to LRP5/6 receptors and inhibit the Wnt signalling pathway, thereby leading to decreased bone formation.30,31 The fact that Wnt signalling is closely associated with the immune system32 could be one of the mechanisms by which ART improves bone quality.
The widely reported bone loss induced by tenofovir disoproxil fumarate has been attributed to tubular toxicity inducing excessive renal phosphate wasting and osteomalacia33 resulting in a decrease in BMD and increase in bone turnover makers. However, no prior data on how tenofovir disoproxil fumarate impacts on bone tissue quality are available. The restoration of the immune dysregulation once ART is started and its indirect influence on other pathways such as Wnt could improve bone tissue quality. The role that ART-induced bone remodelling markers play in different bone strength components is uncertain but seems to be opposite between BMSi and BMD and TBS. Indeed, the increased bone turnover could explain the bone loss and deteriorated microarchitecture after ART,34 but bone protein transcription could explain bone quality. Moreover, further studies are needed to explore which mechanisms are involved in ART-triggered BMD and TBS impairment.
This work has some noteworthy limitations; the main limitation is the small sample recruited, based on a single centre, so some correlations have probably been missed and others overestimated. Moreover, as we have not evaluated the tubular toxicity of tenofovir disoproxil fumarate, we do not know its exact role in BMD loss. Moreover, in order to reduce ART bias we included only one regimen.
Nonetheless, as reflected by the capacity of ART to recover BMSi levels, we have uncovered a clear connection between a patient’s inflammatory status and bone quality. Moreover, these results were confirmed by the fact that ART-related BMSi changes are correlated with time since HIV diagnosis.
Furthermore, since ART-induced stabilization of the immune system is associated with improved bone quality, but impaired BMD and microarchitecture, we can assume that different mechanisms are probably involved in regulating each of these bone strength parameters.
In summary, we have observed a correlation between changes in bone tissue quality and the inflammatory environment in HIV-positive individuals. Moreover, among the underlying mechanisms, we highlight the Wnt pathway as having a potentially significant role in ART-related bone quality recovery.
Funding
This work was supported by the Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES) (grant number CB16/10/00245), the Fondos de Investigación en Salud (FIS) Project from Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación (grant numbers PI13/00589 and PI16/01860) and FEDER funds. T. T. B. is supported in part by K24 AI120834.
Transparency declarations
None to declare.
References
- 1. Barkhordarian A, Ajaj R, Ramchandani MH et al. Osteoimmunopathology in HIV/AIDS: a translational evidence-based perspective. Patholog Res Int 2011; 2011: 359242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nguyen L, Dewhirst FE, Hauschka PV et al. Interleukin-1β stimulates bone resorption and inhibits bone formation in vivo. Lymphokine Cytokine Res 1991; 10: 15–21. [PubMed] [Google Scholar]
- 3. Guo H, Gao J, Taxman DJ et al. HIV-1 infection induces interleukin-1β production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J Biol Chem 2014; 289: 21716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown TT, Ross AC, Storer N et al. Bone turnover, osteoprotegerin/RANKL and inflammation with antiretroviral initiation: tenofovir versus non-tenofovir regimens. Antivir Ther 2011; 16: 1063–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaneshiro S, Ebina K, Shi K et al. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J Bone Miner Metab 2014; 32: 378–92. [DOI] [PubMed] [Google Scholar]
- 6. Zhu S, He H, Gao C et al. Ovariectomy-induced bone loss in TNFα and IL6 gene knockout mice is regulated by different mechanisms. J Mol Endocrinol 2018; 60: 185–98. [DOI] [PubMed] [Google Scholar]
- 7. Brown TT, McComsey GA, King MS et al. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr 2009; 51: 554–61. [DOI] [PubMed] [Google Scholar]
- 8. Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 2006; 20: 2165–74. [DOI] [PubMed] [Google Scholar]
- 9. Prieto-Alhambra D, Güerri-Fernández R, De Vries F et al. HIV infection and its association with an excess risk of clinical fractures: a nationwide case-control study. J Acquir Immune Defic Syndr 2014; 66: 90–5. [DOI] [PubMed] [Google Scholar]
- 10. Güerri‐Fernandez R, Vestergaard P, Carbonell C et al. HIV infection is strongly associated with hip fracture risk, independently of age, gender, and comorbidities: a population‐based cohort study. J Bone Miner Res 2013; 28: 1256–8. [DOI] [PubMed] [Google Scholar]
- 11. Güerri-Fernández R, Molina D, Villar-García J et al. HIV infection is associated with worse bone material properties, independently of bone mineral density. J Acquir Immune Defic Syndr 2016; 72: 314–8. [DOI] [PubMed] [Google Scholar]
- 12. Duarte Sosa D, Fink Eriksen E. Women with previous stress fractures show reduced bone material strength. Acta Orthop 2016; 87: 626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malgo F, Hamdy NAT, Papapoulos SE et al. Bone material strength as measured by microindentation in vivo is decreased in patients with fragility fractures independently of bone mineral density. J Clin Endocrinol Metab 2015; 100: 2039–45. [DOI] [PubMed] [Google Scholar]
- 14. Mellibovsky L, Prieto-Alhambra D, Mellibovsky F et al. Bone tissue properties measurement by reference point indentation in glucocorticoid-induced osteoporosis. J Bone Miner Res 2015; 30: 1651–6. [DOI] [PubMed] [Google Scholar]
- 15. Farr JN, Drake MT, Amin S et al. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res 2014; 29: 787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Güerri-Fernández R, Lerma-Chippirraz E, Fernandez Marron A et al. Bone density, microarchitecture and tissue quality after 1 year of treatment with tenofovir disoproxil fumarate. AIDS 2018; 32: 913–20. [DOI] [PubMed] [Google Scholar]
- 17. LaFleur J, Rillamas-Sun E, Colón-Emeric CS et al. Fracture rates and bone density among postmenopausal Veteran and non-Veteran women from the Women’s Health Initiative. Gerontologist 2016; 56 Suppl 1: S78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diez-Perez A, Güerri R, Nogues X et al. Microindentation for in vivo measurement of bone tissue mechanical properties in humans. J Bone Miner Res 2010; 25: 1877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sundh D, Nilsson M, Zoulakis M et al. High-impact mechanical loading increases bone material strength in postmenopausal women—a 3-month intervention study. J Bone Miner Res 2018; 33: 1242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puthucheary ZA, Sun Y, Zeng K et al. Sepsis reduces bone strength before morphologic changes are identifiable. Crit Care Med 2017; 45: e1254–61. [DOI] [PubMed] [Google Scholar]
- 21. Ofotokun I, Titanji K, Vunnava A et al. Antiretroviral therapy induces a rapid increase in bone resorption that is positively associated with the magnitude of immune reconstitution in HIV infection. AIDS 2016; 30: 405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hunt PW, Brenchley J, Sinclair E et al. Relationship between T cell activation and CD4+ T cell count in HIV‐seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 2008; 197: 126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis 2016; 214 Suppl 2: S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bixler SL, Sandler NG, Douek DC et al. Suppressed Th17 levels correlate with elevated PIAS3, SHP2, and SOCS3 expression in CD4 T cells during acute simian immunodeficiency virus infection. J Virol 2013; 87: 7093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008; 28: 454–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bixler SL, Mattapallil JJ. Loss and dysregulation of Th17 cells during HIV infection. Clin Dev Immunol 2013; 2013: 852418.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol 2013; 21: 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kitami S, Tanaka H, Kawato T et al. IL-17A suppresses the expression of bone resorption-related proteinases and osteoclast differentiation via IL-17RA or IL-17RC receptors in RAW264.7 cells. Biochimie 2010; 92: 398–404. [DOI] [PubMed] [Google Scholar]
- 29. Ono T, Okamoto K, Nakashima T et al. IL-17-producing γδT cells enhance bone regeneration. Nat Commun 2016; 7: 10928.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zimmerman ZF, Moon RT, Chien AJ. Targeting Wnt pathways in disease. Cold Spring Harb Perspect Biol 2012; 4: a008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Negredo E, Diez-Pérez A, Bonjoch A et al. Switching from tenofovir to abacavir in HIV-1-infected patients with low bone mineral density: changes in bone turnover markers and circulating sclerostin levels. J Antimicrob Chemother 2015; 70: 2104–7. [DOI] [PubMed] [Google Scholar]
- 32. Blumenthal A, Ehlers S, Lauber J et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 2009; 108: 965–73. [DOI] [PubMed] [Google Scholar]
- 33. Fux CA, Christen A, Zgraggen S et al. Effect of tenofovir on renal glomerular and tubular function. AIDS 2007; 21: 1483–5. [DOI] [PubMed] [Google Scholar]
- 34. Gerdhem P, Ivaska KK, Alatalo SL et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res 2003; 19: 386–93. [DOI] [PubMed] [Google Scholar]