Abstract
Background
Despite wide clinical acceptance, the use of weight-banded dosing regimens for the treatment of TB in adults has been defined on an empirical basis. The potential impact of known covariate factors on exposure to different drugs has not been taken into account.
Objectives
To evaluate the effect of demographic factors on the exposure to standard of care drugs after weight-banded dosing, as currently recommended by TB treatment guidelines. In addition, we aim to identify alternative dosing regimens that ensure comparable systemic exposure across the overall patient population.
Methods
Clinical trial simulations were performed to assess the differences in systemic exposure in a cohort of virtual patients. Secondary pharmacokinetic parameters were used to evaluate the adequacy of each regimen along with the percentage of patients achieving predefined thresholds.
Results
Our results show that patients weighing less than 40 kg are underexposed relative to patients with higher body weight. The opposite trend was observed following a crude weight band-based dosing regimen with 50 kg as the cut-off point. Simulations indicate that a fixed-dose regimen based on three (<40 kg), four (40–70 kg) or five (>70 kg) tablets of 150 mg rifampicin, 75 mg isoniazid, 400 mg pyrazinamide and 275 mg ethambutol reduces variability in exposure, increasing the overall probability of favourable long-term outcome across the population.
Conclusions
These findings suggest the need to revisit current guidelines for the dose of standard of care drugs for TB treatment in adults. The proposed fixed-dose regimen should be considered in future clinical trials.
Introduction
The WHO and International Union Against Tuberculosis and Lung Disease (IUATLD) have published guidelines for treatment of TB that include recommendations for standardized first-line dosing regimens.1,2 Although considerably effective in clinical trial settings, the rationale underpinning dosing regimens of modern short-course therapy has been empirical.3 As a result, the dosing regimens that are currently used for TB treatment have never been optimized taking into account the known sources of variability in the pharmacokinetics (PK) of each drug. Consequently, the implications of variability in exposure for the evaluation of efficacy after treatment over periods shorter than 6 months have not been considered in recent clinical trials.
The lack of consensus regarding the optimal regimen and limited evidence on the impact of different regimens on the efficacy and safety profile of the standard of care drugs may partly explain the discrepancies in the choice of dosing regimens used in current clinical practice. Moreover, there are no data showing that each of these regimens warrants comparable exposure across the trial population. A few studies in the published literature have focused on the evaluation of PK variability of first-line drugs across the WHO weight bands.4–8 A correlation between weight and drug concentrations was found in all analyses, implying that patients with lower body weight will be exposed to lower drug levels despite the use of weight band-based dosing regimens. These findings support the need to revisit the current dosing recommendations.9
Growing evidence suggests that the currently recommended dosing regimens are suboptimal.10–13 Assessing the impact of different regimens on drug exposure variability across weight bands used in TB treatment would, however, require a complicated, expensive and ethically complex clinical study. In fact, considering the reality of poverty-related diseases and the limited funding available, such clinical trials are unlikely to be conducted in the near future.
Nevertheless, such limitations should not prevent us from evaluating and optimizing the dose rationale for the first-line treatment of TB. In fact, this has been one focus of the clinical debate regarding the effective use of antibiotics for more than a decade, as it represents the most direct method for improving treatment outcome, potentially allowing for shorter intervention and tackling resistance.14
The aim of the present study was therefore to evaluate the implications of different dosing regimens for all four drugs used as standard of care in adults, taking into account the effect of body size on systemic drug exposure. Whereas pharmacodynamic (PD), immunological and microbiological aspects also contribute to variability in response, the ultimate goal of this analysis was to minimize the impact of differences in drug disposition by identifying the optimal ratio for standard care fixed-dose combination (FDC) regimens that ensure comparable systemic exposure across the patient population.
Methods
Patient population
Individual data sets from five clinical studies were obtained from the Innovative Medicines Initiative- (IMI) funded PreDiCT-TB consortium and three clinical studies from the Critical Path to TB Drug Regimens (CPTR) database. The CPTR initiative is a public–private partnership launched in March 2010 by the Critical Path Institute, the Bill & Melinda Gates Foundation and the Global Alliance for TB Drug Development (TB Alliance). The baseline demographic characteristics of the patient population in each study are summarized in Table S1 (available as Supplementary data at JAC Online). Age, weight, height and sex were the covariates of interest. Only patients who were between 18 and 65 years of age were included in the analysis. Patients who were HIV positive or had unknown HIV status were excluded. The final patient population for the PK simulations will be referred to as the ‘trial population’ from here onwards.
Population PK analysis
Published PK models of rifampicin,15 isoniazid,16 pyrazinamide17 and ethambutol18 were used to simulate concentration versus time profiles during the intensive phase of TB treatment (Figure S1). The chosen PK sampling times were: 0, 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12 and 24 h post-dose. No changes with respect to the model structure (i.e. random and fixed effect, or covariate effect) were made. An exception was made with regard to interoccasion variability (IOV). These parameters were excluded in our analysis to minimize study-related bias in the simulated PK variability. Sources of parameter variability in the simulations were hence derived from interindividual variability and covariate effect. Body size was included as covariate for clearance (CL/F) in all PK models, using either weight (isoniazid, pyrazinamide and ethambutol) or normal fat mass (NFM; rifampicin). NFM can be predicted from sex, weight and height as described by Holford and Anderson.19 NAT2 genotype was an additional covariate for CL/F in the isoniazid PK model. PK simulations were performed in the trial population (200 trial simulations) to assess the magnitude of the differences in drug exposure following currently recommended dosing regimens, as well alternative regimens identified in the present analysis.
Assessment of PK variability following the current dosing regimens
Considering that systemic exposure is probably the most relevant index for efficacy,20,21 area under the plasma concentration–time curve (AUC0–24) at steady-state was derived following dosing regimens based on WHO guidelines and crude weight band where 50 kg was chosen as the cut-off value. According to WHO guidelines,2 patients in the <40, 40–54, 55–70 or >70 kg weight band, respectively, received a daily fixed dose of two, three, four or five tablets of 150 mg rifampicin, 75 mg isoniazid, 400 mg pyrazinamide and 275 mg ethambutol for 2 months. Patients treated with the crude weight band-based regimen were given a daily fixed dose of either three (<50 kg) or four tablets (≥50 kg). The trapezoidal rule was used to calculate AUC0–24. Taking into account accepted variation due to differences in formulation,22 variation in exposure across weight bands was considered acceptable if the average AUC0–24 did not vary by more than 20% (80%–120%) relative to the highest WHO weight band (>70 kg), which was treated as the reference. Lastly, the relationship between weight, CL/F and drug exposure was evaluated to demonstrate the contribution of body size to PK variability.
Software
The statistical software R (version 3.2.5)23 was used for data preparation, data analysis and statistical and graphical summaries. NONMEM 7.324 was used to simulate concentration versus time profiles.
Results
Patient population
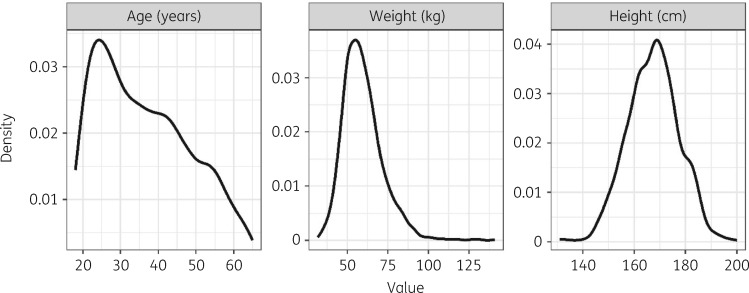
A total of 2231 patients (676 female and 1555 male) were included in this analysis. Baseline characteristics of this main population are summarized in Table 1. The median height stratified by sex and WHO weight band population (Table S2) was imputed for, respectively, 123 female (5.5%) and 275 male patients (12.3%) for whom NFM (a covariate in the rifampicin PK model) otherwise could not be calculated due to missing height. The distributions of the continuous covariates of interest in the trial population are presented in Figure 1.
Table 1.
Summary of demographic characteristics of the trial population
| Characteristic | |
|---|---|
| Age (years) | 35 (18–65) |
| Height (cm)a | 168 (131–200) |
| BMI (kg/m2)a | 20.3 (12–57) |
| Male patients, n (%) | 1555 (69.7) |
| Weight (kg) | 58 (32–141) |
| Patients per WHO weight band, n (%) | |
| <40 kg | 46 (2.1) |
| 40–54 kg | 759 (34) |
| 55–70 kg | 1054 (47.2) |
| >70 kg | 372 (16.7) |
| Patients per crude weight band, n (%) | |
| <50 kg | 460 (21) |
| ≥50 kg | 1771 (79) |
Data are presented as median (range) unless stated otherwise.
Data available from 1833 patients.
Figure 1.
Density plots of the baseline demographic characteristics of the trial population (N = 2231). Height distribution was available from 1833 patients only.
PK variability associated with currently recommended dosing regimens
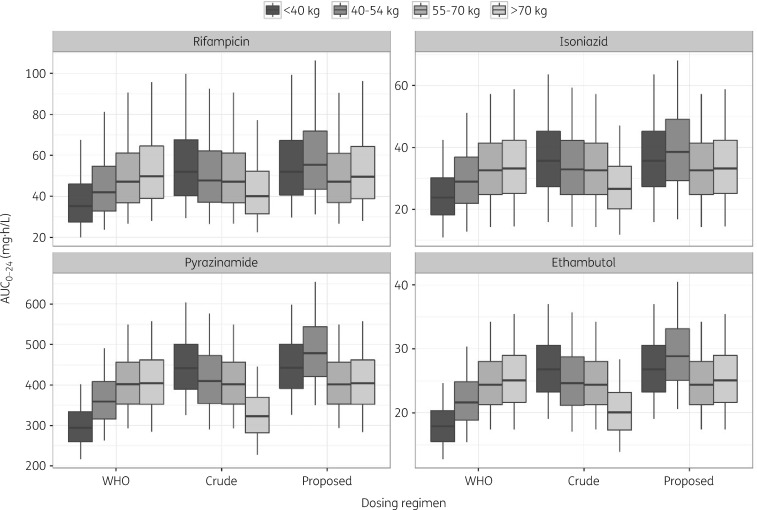
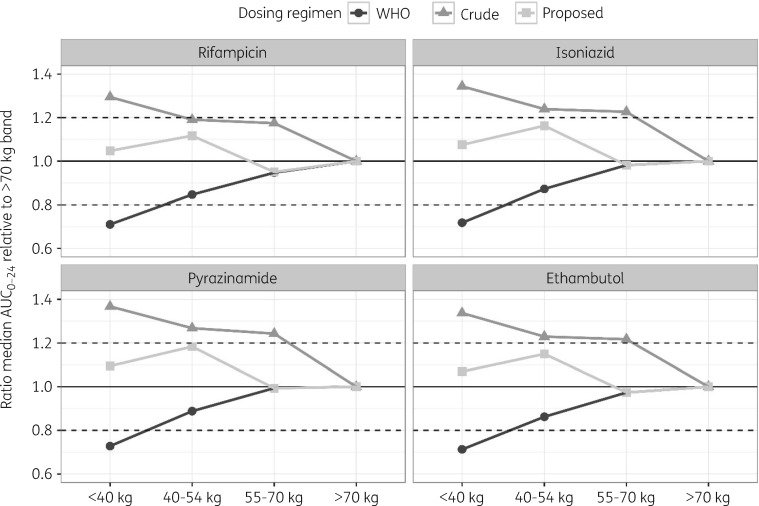
AUC0–24 at steady-state was derived from the simulated concentration–time profiles at the end of the intensive phase. Dosing regimens based on WHO-recommended weight bands (<40, 40–54, 55–70 and >70 kg) yielded highly variable exposure across the population (Figure 2). Subjects who weighed less than 40 kg appeared to be underdosed when compared with the rest of the population and displayed on average a >20% lower AUC0–24 as compared with patients in the highest WHO weight band (Figure 3). Conversely, comparable exposure between patients weighing up to 70 kg was achieved when using a crude weight band in which 50 kg was used as cut-off value (Figure 2). However, in this case, underdosing occurred in patients in the highest weight band (>70 kg), wherein the simulated AUC0–24 was on average >20% lower than in patients weighing <40 kg (Figure 3). This finding was somewhat expected as heavier patients received less than the WHO-recommended dosage (four instead of five tablets).
Figure 2.
Comparison of simulated AUC0–24 at steady-state following proposed adjusted dosing regimens versus regimens based on the WHO-recommended weight band and a cut-off value of 50 kg (N = 2231; 200 clinical trial simulations). Box-plots depict the 5th, 25th, median, 75th and 95th percentiles of the simulated data.
Figure 3.
Comparison of predicted relative variability in median steady-state exposure across the trial population following the proposed adjusted dosing regimens (filled squares) versus regimens based on the WHO-recommended weight band (filled circles) and a cut-off value of 50 kg (filled triangles). Patients in the highest WHO weight band (>70 kg) were selected as the reference population. Dashed horizontal lines represent the variability acceptance interval (80%–120%).
Proposal for an adjusted weight-banded dosing regimen
The weight band-based dosing regimen that yields comparable exposure range across the population is presented in Table 2. Based on this regimen, patients weighing <40 kg were given three instead of two FDC tablets, whereas patients between 40 and 54 kg received four (instead of three) FDC tablets. Simulations revealed that the use of the proposed regimen resulted in considerably lower variation in drug exposure (<20%) across the population (Figures 2 and 3).
Table 2.
Proposed adjusted weight band-based dosing regimen for first-line antitubercular drugs
| Drug (tablet strength) | Daily dose for each weight band (no. of tablets) |
|||
|---|---|---|---|---|
| <40 kg | 40–54 kg | 55–70 kg | >70 kg | |
| Rifampicin (150 mg) | 3 | 4 | 4 | 5 |
| Isoniazid (75 mg) | ||||
| Pyrazinamide (400 mg) | ||||
| Ethambutol (275 mg) | ||||
Under the proposed dosing regimen, one additional fixed-dose combination tablet was given to patients in the <40 kg (from two to three) and 40–54 kg (from three to four) weight bands, in comparison with WHO recommendations.
Assessment of body size-specific effect on exposure variability
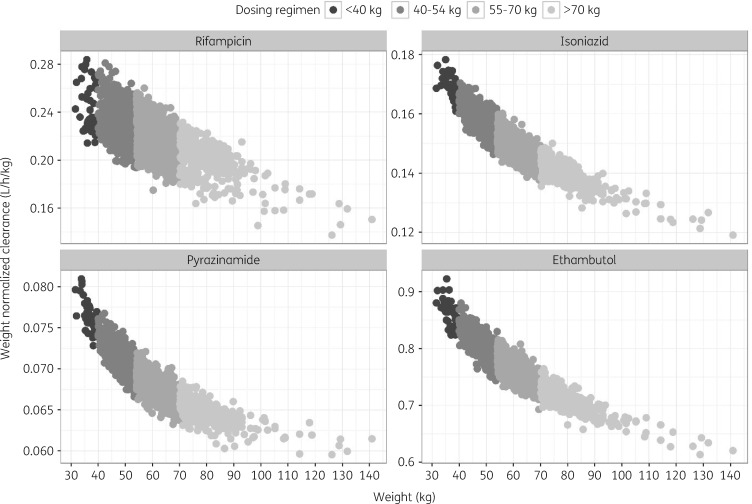
Our analysis showed that currently recommended dosing regimens fail to take into account the non-linear relationship between body size and drug clearance (Figure 4). Using rifampicin as an example, Figure 4 showed that rifampicin CL/F (per kilogram of body weight) in patients weighing <40 kg was in fact on average 1.2-fold faster than in patients weighing >70 kg. Similar observations were also found for isoniazid, pyrazinamide and ethambutol. Consequently, these results demonstrate that a more than proportional dose change is needed for standard of care drugs, especially for patients in the lowest weight band, to account for the higher clearance per kilogram of body weight.
Figure 4.
Predicted non-linear relationship between weight and clearance (CL/F) of the standard of care drugs in the trial population (N = 2231). Filled circles represent the simulated median individual CL/F at steady-state, normalized to body weight.
Discussion
Our results show that despite the use of weight bands, the recommended dosing regimens do not correct for the influence of body size on drug disposition. None of the currently used regimens yields satisfactory exposure variability across all the population of TB patients. The use of clinical trial simulations showed that currently recommended dosing regimens result in wide variation in drug exposure across patients with different body weight. The usage of a crude weight band based on a 50 kg cut-off value seemed to improve the variability in exposure in patients up to 70 kg but simultaneously yielded lower exposure levels in patients at the highest weight band. Such differences in exposure can contribute to treatment failure and should not be overlooked. Of interest are the implications for patients in the lowest weight band (<40 kg), who according to our analysis appear to be underexposed when treated according to the WHO weight-banded regimens. Indeed, studies have found an association between low body weight and unsuccessful treatment outcome or delayed culture conversion.25–27
The current dosing recommendations assume a linear correlation between weight and drug elimination, whereas a non-linear relationship between body weight and systemic exposure can be observed for many drugs.28 Such non-linearity was clearly demonstrated in our analysis. A non-linear correlation between body weight and drug clearance has been found for numerous compounds, supporting the importance of acknowledging this relationship when defining doses and dosing regimens.28
Reducing variability in drug exposure is critical for the optimization of treatment response
A fixed dosing regimen of three (<40 kg), four (40–70 kg) and five tablets (>70 kg) of 150 mg rifampicin, 75 mg isoniazid, 400 mg pyrazinamide and 275 mg ethambutol was found to yield the desired target drug exposure as compared with the standard WHO regimen whilst reducing overall variability of all first-line drugs. The proposed increase in the doses of all four drugs for patients below 54 kg is expected not to lead to a higher increase in adverse events as was shown recently with higher doses of rifampicin.11
We acknowledge that better target exposure achievement, efficacy and potentially shorter treatment duration may require much higher doses than are currently prescribed.11,29 Nonetheless, reducing variability is in itself an important step in improving therapeutics. We have identified an optimal ratio for standard care FDC regimens that has the potential to immediately benefit TB patients. In the long term, we envisage that addition of higher doses of key sterilizing drugs such as rifampicin or pyrazinamide to our proposed dosing regimen will lead to a truly optimized TB treatment as a result of maximizing efficacious exposure and minimizing PK variability across the patient population.
We also acknowledge that currently used weight band cut-offs may not be optimal for reducing variability in exposure. On the other hand, given that these cut-offs have been used in clinical practice for a long time, we believe that adhering to currently used weight groups would facilitate the implementation of the proposed dose recommendation.
Limitations
Our analysis has several limitations. First, only a small fraction of the patient population that were included weighed less than 40 kg (2.1%) which might be lower than in a real setting. A survey performed in 2001 as part of the National TB programme in Kenya, Nepal and Senegal (n = 8640) showed that the fraction of patients weighing below 40 kg could be as much as ∼30%.30
Second, the PK models we used were developed based on a relatively small number of patients. As such, we may have imposed factors on our predicted drug that may have been specific to those studies only (such as formulation effects or genetic variants). The PK models did not include additional covariates that may also be relevant for PK variability such as genotype (except for isoniazid), race or other co-morbidities (e.g. HIV). Consequently, we were not able to take into account the potential effect of covariates other than size and/or sex on PK variability in our proposed dosing regimen. The effect of genotype on isoniazid PK has been evaluated earlier16 and was therefore not explored in detail in this analysis. Most importantly, we have not included HIV patients in the analysis, who are affected by drug–drug interactions with antiretrovirals.31,32 Further assessment of the role of antiretrovirals for dose optimization in TB-HIV patients is the scope of a future investigation by our group.
Finally, we recognize that the limited microbiological and clinical cure data may weaken the inferences regarding the impact of underexposure to standard of care drugs, i.e. that those patients are effectively at a higher risk of treatment failure or relapse. Given that current first-line treatment can already achieve as much as 83% success rate,33 further testing of this hypothesis would be desirable to demonstrate that PK factors may partly explain the observed efficacy rates, especially if one considers that the frequency of low body weight patients in the real population is far larger than that of those enrolled in clinical trials.30
Conclusions
The impact of body size on PK variability highlights the relevance of discriminating patient- from drug-related factors during the development of novel treatments for TB. Clinical trial simulations showed that regimens based either on the currently recommended WHO weight bands or crude weight bands lead to inadequate drug exposure variability across the population. In contrast, an adjusted fixed-dose regimen based on three (<40 kg), four (40–70 kg) or five (>70 kg) tablets of 150 mg rifampicin, 75 mg isoniazid, 400 mg pyrazinamide and 275 mg ethambutol was shown to reduce the variability in systemic exposure. This may have direct implications for efficacy rates and long-term outcome across the population. Our findings suggest the need to revisit current guidelines on the use of standard of care drugs for TB.
Supplementary Material
Acknowledgements
The authors thank Oxford University Clinical Research Unit (funded by the Wellcome Trust), Janssen, Novosibirsk Tuberculosis Research Institute and the TB Research Unit at Case Western Reserve University (established with funds from the United States National Institutes of Allergy and Infectious Diseases, National Institutes of Health and Human Services, under Contract No. NO1-AI95383 and HHSN266200700022C/NO1-AI-70022) for their contribution in providing individual patient data to the PreDiCT-TB database. The authors are also grateful to Critical Path to TB Drug Regimens (CPTR) for providing data used in the preparation of this article. The investigators within CPTR contributed to the design and implementation of the CPTR database and/or provided data but did not participate in the analysis of the data or the writing of this report.
Funding
The research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking (grant agreement no. 115337), resources of which are made up of a financial contribution from the European Union's Seventh Framework Programme (FP7/2007–2013) and European Federation of Pharmaceutical Industries and Associations companies’ in-kind contribution.
Transparency declarations
None to declare.
References
- 1. Aït-Khaled N, Alarcón E, Armengol R et al. Management of Tuberculosis: A Guide to the Essentials of Good Practice. Paris, France: International Union Against Tuberculosis and Lung Disease, 2010. https://www.theunion.org/what-we-do/publications/technical/english/pub_orange-guide_eng.pdf.
- 2.World Health Organization. Treatment of Tuberculosis: Guidelines for National Programmes 2003. WHO/CDS/TB/2003.313. https://apps.who.int/iris/bitstream/handle/10665/67890/WHO_CDS_TB_2003.313_eng.pdf.
- 3. Muliaditan M, Davies GR, Simonsson US et al. The implications of model-informed drug discovery and development for tuberculosis. Drug Discov Today 2017; 22: 481–6. [DOI] [PubMed] [Google Scholar]
- 4. Chirehwa MT, Rustomjee R, Mthiyane T et al. Model-based evaluation of higher doses of rifampin using a semimechanistic model incorporating autoinduction and saturation of hepatic extraction. Antimicrob Agents Chemother 2015; 60: 487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McIlleron H, Rustomjee R, Vahedi M et al. Reduced antituberculosis drug concentrations in HIV-infected patients who are men or have low weight: implications for international dosing guidelines. Antimicrob Agents Chemother 2012; 56: 3232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rockwood N, Meintjes G, Chirehwa M et al. HIV-1 coinfection does not reduce exposure to rifampin, isoniazid, and pyrazinamide in South African tuberculosis outpatients. Antimicrob Agents Chemother 2016; 60: 6050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sahota T, Della Pasqua O. Feasibility of a fixed-dose regimen of pyrazinamide and its impact on systemic drug exposure and liver safety in patients with tuberculosis. Antimicrob Agents Chemother 2012; 56: 5442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chirehwa MT, McIlleron H, Rustomjee R et al. Pharmacokinetics of pyrazinamide and optimal dosing regimens for drug-sensitive and -resistant tuberculosis. Antimicrob Agents Chemother 2017; 61: e00490–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnston JC, Khan FA, Dowdy DW. Reducing relapse in tuberculosis treatment: is it time to reassess WHO treatment guidelines? Int J Tuberc Lung Dis 2015; 19: 624.. [DOI] [PubMed] [Google Scholar]
- 10. Ahmad Z, Fraig MM, Bisson GP et al. Dose-dependent activity of pyrazinamide in animal models of intracellular and extracellular tuberculosis infections. Antimicrob Agents Chemother 2011; 55: 1527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boeree MJ, Heinrich N, Aarnoutse R et al. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 2017; 17: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donald PR, Parkin DP, Seifart HI et al. The influence of dose and N-acetyltransferase-2 (NAT2) genotype and phenotype on the pharmacokinetics and pharmacodynamics of isoniazid. Eur J Clin Pharmacol 2007; 63: 633–9. [DOI] [PubMed] [Google Scholar]
- 13. Steingart KR, Jotblad S, Robsky K et al. Higher-dose rifampin for the treatment of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis 2011; 15: 305–16. [PubMed] [Google Scholar]
- 14. Goutelle S, Bourguignon L, Maire P et al. The case for using higher doses of first line anti-tuberculosis drugs to optimize efficacy. CPD 2014; 20: 6191–206. [DOI] [PubMed] [Google Scholar]
- 15. Smythe W, Khandelwal A, Merle C et al. A semimechanistic pharmacokinetic–enzyme turnover model for rifampin autoinduction in adult tuberculosis patients. Antimicrob Agents Chemother 2012; 56: 2091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilkins JJ, Langdon G, McIlleron H et al. Variability in the population pharmacokinetics of isoniazid in South African tuberculosis patients. Br J Clin Pharmacol 2011; 72: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilkins JJ, Langdon G, McIlleron H et al. Variability in the population pharmacokinetics of pyrazinamide in South African tuberculosis patients. Eur J Clin Pharmacol 2006; 62: 727–35. [DOI] [PubMed] [Google Scholar]
- 18. Jonsson S, Davidse A, Wilkins J et al. Population pharmacokinetics of ethambutol in South African tuberculosis patients. Antimicrob Agents Chemother 2011; 55: 4230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holford NHG, Anderson BJ. Allometric size: the scientific theory and extension to normal fat mass. Eur J Pharm Sci 2017; 109S: S59–64. [DOI] [PubMed] [Google Scholar]
- 20. Gumbo T, Louie A, Liu W et al. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob Agents Chemother 2007; 51: 2329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jayaram R, Gaonkar S, Kaur P et al. Pharmacokinetics–pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 2003; 47: 2118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Peer A. Variability and impact on design of bioequivalence studies. Basic Clin Pharmacol Toxicol 2010; 106: 146–53. [DOI] [PubMed] [Google Scholar]
- 23.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2016. https://www.R-project.org/.
- 24. Beal S, Sheiner LB, Boekmann A et al. NONMEM’s User’s Guides. Ellicott City, MD, USA: ICON Development Solutions, 2009. [Google Scholar]
- 25. Sinshaw Y, Alemu S, Fekadu A et al. Successful TB treatment outcome and its associated factors among TB/HIV co-infected patients attending Gondar University Referral Hospital, Northwest Ethiopia: an institution based cross-sectional study. BMC Infect Dis 2017; 17: 132.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Biruk M, Yimam B, Abrha H et al. Treatment outcomes of tuberculosis and associated factors in an Ethiopian University Hospital. Adv Public Health 2016; 2016: 8504629. [Google Scholar]
- 27. Sekaggya-Wiltshire C, von Braun A, Lamorde M et al. Delayed sputum culture conversion in tuberculosis–human immunodeficiency virus-coinfected patients with low isoniazid and rifampicin concentrations. Clin Infect Dis 2018; 67: 708–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 2008; 48: 303–32. [DOI] [PubMed] [Google Scholar]
- 29. Alsultan A, Savic R, Dooley KE et al. Population pharmacokinetics of pyrazinamide in patients with tuberculosis. Antimicrob Agents Chemother 2017; 6: e02625-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diop AH, Gakiria G, Pande SB et al. Dosages of anti-tuberculosis medications in the national tuberculosis programs of Kenya, Nepal, and Senegal. Int J Tuberc Lung Dis 2002; 6: 215–21. [PubMed] [Google Scholar]
- 31. Semvua HH, Kibiki GS, Kisanga ER et al. Pharmacological interactions between rifampicin and antiretroviral drugs: challenges and research priorities for resource-limited settings. Ther Drug Monit 2015; 37: 22–32. [DOI] [PubMed] [Google Scholar]
- 32. Daskapan A, Idrus LR, Postma MJ et al. A systematic review on the effect of HIV infection on the pharmacokinetics of first-line tuberculosis drugs. Clin Pharmacokinet 2019; 58: 747–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Global Tuberculosis Report 2017. https://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.