Abstract
Heterozygous loss-of-function mutations in the GRN gene lead to progranulin (PGRN) haploinsufficiency and cause frontotemporal lobar degeneration with TDP-43 pathology type A (FTLD-TDP type A). PGRN is a highly conserved, secreted glycoprotein and functions in the central nervous system as a key modulator of microglial function. Hence, altered microglial function caused by PGRN deficiency may be tied to the pathogenesis of FTLD-TDP. Our previous studies showed that haploinsufficiency of GRN mutations extends to microglial PGRN expression in the hippocampal CA1 region. In this study, we found that the CA1 sector was associated with less neuronal loss and more frequent TDP-43 inclusions in FTLD-TDP type A cases with GRN mutations than in sporadic cases. In addition, the CA1 region in GRN mutation cases contained more rod-like microglia, which also had reduced PGRN expression. These findings suggest that the profile of TDP-43 inclusions, neuronal number, and microgliosis in the CA1 sector of FTLD-TDP type A cases may be influenced by GRN gene expression status.
Keywords: Frontotemporal lobar degeneration, Hippocampal sclerosis, Microglia, Neuroinflammation, Progranulin, TAR DNA-binding protein 43
INTRODUCTION
Frontotemporal lobar degeneration (FTLD) is a neuropathologic process characterized by atrophy in the frontal and temporal lobes of the brain, with the parietal and occipital lobes usually being spared. Common proteinopathies that are found in FTLD include either tau-positive (FTLD-tau) or TAR DNA-binding protein 43-positive (FTLD-TDP) inclusion bodies. FTLD-TDP can be classified into 4 distinct histopathologic types, A–D (1).
Among the 4 types, type A cases are clinically associated with behavioral variant frontotemporal degeneration or nonfluent/agrammatic primary progressive aphasia (PPA-G) (2–9). FTLD-TDP type A is characterized by TDP-43-immunopositive inclusions in the neocortex, which are mainly localized in the upper layers, and are composed of short dystrophic neurites (DNs), neuronal cytoplasmic inclusions, and lentiform to round neuronal intranuclear inclusions. In the hippocampus, the CA1 region commonly has frequent TDP-43-positive DNs; however, the number of neuronal cytoplasmic inclusions varies in dentate granule cells (10–12). Genetically, FTLD-TDP type A is associated with heterozygous loss-of-function mutations in GRN (13–17).
Progranulin (PGRN), encoded by GRN, is a 63.5 kDa cysteine-rich, secreted protein with a predicted molecular mass of 63.5 kDa (18–20). PGRN is expressed in many peripheral tissues serving functions in cancer, inflammation, and metabolic diseases (21, 22). In the central nervous system, it is a growth factor (23, 24) or modulator of microglial cells (25, 26). Altered microglial function caused by PGRN deficiency is associated with the pathogenesis of FTLD-TDP. GRN knockout mice showed a robust increase in microglial activation and neuronal loss, and PGRN-deficient macrophages and microglia were shown to be cytotoxic to hippocampal cells in vitro (25, 26). In addition, human carriers of the GRN mutation have increased microglial infiltration in disease-affected regions, for example, in the frontal and temporal cortices (27). Our previous studies showed that haploinsufficiency of GRN mutations extends to microglial PGRN expression in the CA1 region of the hippocampus in patients with FTLD-TDP with GRN mutations (28). We hypothesize that GRN haploinsufficiency in CA1 leads to unique neuropathology in this region.
In this study, we aimed to evaluate the relationship between low CA1 microglial PGRN expression in the brains of patients with FTLD-TDP with GRN mutations and other CA1 pathologies, such as neuronal loss and gliosis, TDP-43 pathology, and density of IBA-1-positive microglia.
MATERIALS AND METHODS
Human Samples
Paraformaldehyde-fixed, paraffin-embedded human brain samples from 48 cases were acquired from the Neuropathology Core of the NIA-funded Alzheimer’s Disease Center within the Mesulam Center for Cognitive Neurology and Alzheimer’s Disease at Northwestern University’s Feinberg School of Medicine. Demographic and neuropathologic data for these cases are presented in Table 1. Five groups of cases were included in this study: normal controls (n = 6), Alzheimer disease (AD, n = 5), AD with hippocampal sclerosis (ADHS, n = 6), and FTLD-TDP type A with or without GRN mutations (n = 14 and 17, respectively). Pathologic characterization was made by 2 board-certified neuropathologists following consensus criteria (1, 2, 29, 30). Cases with GRN gene mutations are listed in Table 2. Among these cases, the clinical diagnosis of PPA was based on the criteria of Mesulam (31, 32), the clinical FTD diagnosis was based on the Rascovsky et al 2011 criteria (33), and the clinical PRAD diagnosis was based on the McKhann et al 2011 criteria (34). Subjects who had a history of epilepsy or cerebrovascular accidents, and who had a neuropathologic diagnosis of hippocampal ischemic injury, including microinfarcts, were excluded. Table 1 also includes the ABC scores of AD neuropathologic change (35, 36) for each case. Informed consent was obtained for all studies.
TABLE 1.
Sample Demographics
| Neuropathologic Diagnosis | Number of Cases | Gender, M/F | Age at Death (Mean ± SD) |
|---|---|---|---|
| CON | 6 | 3/3 | 77.8 ± 7.8 |
| AD | 5 | 2/3 | 77.3 ± 8.4 |
| ADHS | 6 | 2/4 | 78.3 ± 5.2 |
| FTLD-TDP type A | 17 | 9/8 | 74.0 ± 11.8 |
| FTLD-TDP type A with GRN mutation | 14 | 8/6 | 63.1 ± 5.2 |
AD, Alzheimer disease; ADHS, AD with hippocampal sclerosis; CON, normal control; FTLD-TDP type A, FTLD-TDP type A without GRN mutation.
TABLE 2.
Study Subjects With Identified GRN Mutations
| Case | Sex | Clinical Diagnosis | ADNC Score (A, B, C) | Age at Death (Years) | Symptom Duration (Years) | GRN Mutation |
|---|---|---|---|---|---|---|
| 1 | M | FTD | 0, 1, 0 | 53 | 3.0 | IVS6 + 2 del TGAG |
| 2 | M | FTD | 0, 1 , 0 | 61 | 2.0 | c.1477C>T |
| 3 | M | FTD | 0, 1, 0 | 64 | 6.5 | IVS6 + 2 del TGAG |
| 4 | F | PPA | 1, 1, 0 | 61 | 4.0 | c.675_676delCA |
| 5 | F | PPA | 1, 1, 2 | 67 | 6.0 | c.1477 C > T |
| 6 | F | PPA | 0, 0, 0 | 56 | 6.0 | c.910_911insTG |
| 7 | M | PPA | 0, 0, 0 | 61 | 8.0 | c.5913 A > G |
| 8 | M | PPA | 0, 0, 0 | 64 | 4.0 | c.3240C>T |
| 9 | F | PRAD | 0, 1, 0 | 62 | 7.0 | c.102delC |
| 10 | M | PPA | 0, 0, 0 | 70 | 4.0 | c.−8 + 3A>G |
| 11 | M | PPA | 0, 0, 0 | 65 | 8.0 | c.385_388del |
| 12 | M | FTD | 0, 1, 0 | 63 | 6.0 | c.1317delC |
| 13 | F | PPA | 1, 1, 1 | 74 | 7.0 | c.1477C>T |
| 14 | F | FTD | 0, 0, 0 | 65 | 8.0 | c.675_676delCA |
ADNC, Alzheimer disease neuropathologic change; FTD, frontotemporal dementia; PPA, primary progressive aphasia; PRAD, probable Alzheimer disease.
Tissue Staining
Paraffin-embedded tissue sections from the hippocampus were cut to a thickness of 5 μm for immunohistochemical analysis. The staining procedure has been described in our previous study (28). The following primary antibodies were used: anti-IBA-1 antibody (goat polyclonal, 1:1000, Abcam, Boston, MA), anti-PGRN antibody (mAbs, 1:100, homemade [37, 38]), and anti-phosphorylated TDP-43 antibody (pS409/410-2, rabbit polyclonal, 1:2500, Cosmo Bio, Carlsbad, CA). Biotinylated secondary antibodies (DAKO, Carpinteria, CA) were amplified using avidin-biotin substrate (ABC solution, DAKO), followed by color development in DAB chromogen (K4007, DAKO). Control sections were incubated either with antiserum pre-adsorbed with 1 mg/mL antigenic peptide (28) or in the absence of the primary antibody.
Semiquantitative Analysis and Statistics
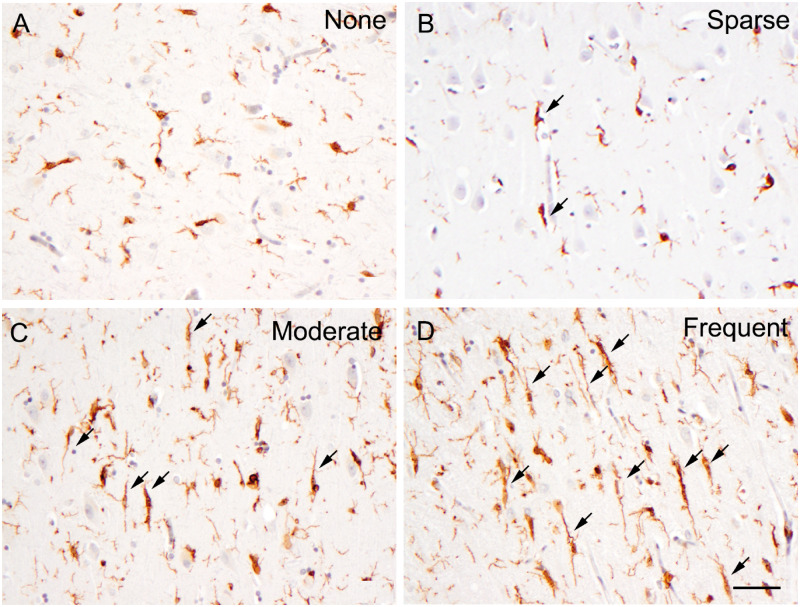
Cases were examined by neuropathologists (Q.M. and E.B.) blinded to clinical and pathologic diagnoses, as well as GRN status. The hippocampal CA1 region of each case was analyzed for neuronal loss and gliosis, TDP-43 pathology, and the density of PGRN- and IBA-1-positive microglia cells. The delineation of the hippocampus was performed according to the previously described method (39). The density of PGRN-positive and rod-shaped microglia was graded as none, sparse, moderate, or frequent. Figure 1 shows examples of none, sparse, moderate, and frequent rod-like microglia. A similar scale was used to grade neuronal loss and gliosis and the severity of TDP-43 pathology. Differences in the frequency of neuronal loss and gliosis, rod-like microglia, PGRN-positive microglia, and TDP-43 DNs were analyzed according to presence or absence of a GRN mutation using Fisher exact tests for data from the CA1 region and then for subiculum (39). Differences in the frequency data of hippocampal CA1 pathology of FTLD-TDP type A were tested by presence of a GRN mutation using a Fisher exact test. Two-sample t-tests were used to test for differences in the age of onset, age of death, and duration of disease for FTLD-TDP type A patients with Type 1 and Type 2 CA1 pathology. SAS 9.4 statistical software was used with a p = 0.05 level of statistical significance.
FIGURE 1.
Illustrations of none (A), sparse (B), moderate (C), and frequent (D) IBA-1-positive rod-like microglia. In this paper, we specifically defined a rod-shaped microglial cell as 1 having a cell length equal to or more than 40 µm. Arrow, rod-shaped microglial cell. Bar: 50 µm.
RESULTS
Our previous studies showed that haploinsufficiency of GRN mutations extends to microglial PGRN expression in the CA1 region of the hippocampus (28). We then postulated that the hippocampal CA1 region of the brain with GRN mutations may demonstrate other region-specific neuropathologies. We first compared the neuronal loss and gliosis in the CA1 region and subiculum between FTLD-TDP cases with and without GRN mutations. As previously reported, hippocampal sclerosis (HS), which is characterized by severe neuronal loss and gliosis in both the hippocampal CA1 region and subiculum, is seen in 79% of FTLD-TDP type A patients (40, 41). To our surprise, in FTLD-TDP type A with GRN mutations, the neuronal loss and gliosis in CA1 versus subiculum was not synchronized; CA1 typically appeared intact or had only mild neuronal loss, while its adjacent subiculum showed severe neuronal loss and gliosis. In addition, neuronal loss and gliosis in the CA1 region followed a dichotomous severity distribution, being either mild or severe. Specifically, only 4 out of 14 FTLD-TDP type A with GRN mutation cases showed severe neuronal loss and gliosis in CA1, with the remaining 10 showing only mild neuronal loss and gliosis in that region. However, the majority of these cases (i.e. 12 out of 14) showed severe neuronal loss and gliosis in the subiculum, with the remaining 2 showing mild neuronal loss and gliosis in both the subiculum and CA1. However, in the 17 FTLD-TDP type A without GRN mutations, 13 cases showed severe neuronal loss and gliosis in both the subiculum and CA1, while the remaining 4 cases showed mild neuronal loss in both regions (Table 3; Fig. 2). Mild or no neuronal loss and gliosis in the CA1 region was significantly associated with GRN mutations (Fisher exact test p = 0.01). The hippocampal CA3 region showed no obvious neuronal loss and gliosis in FTLD-TDP type A cases, both those with and without GRN mutations.
TABLE 3.
Evaluating Neuropathological Associations of GRN Mutations in Hippocampal CA1 and Subiculum Regions Using Fisher Exact Tests
| CA1 |
Subiculum |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No GRN Mutation |
GRN Mutation |
No GRN Mutation |
GRN Mutation |
|||||||
| N | n (%) | N | n (%) | p | N | n (%) | N | n (%) | p | |
| Neuronal loss | 17 | 14 | 0.01 | 17 | 14 | 0.66 | ||||
| Mild | 4 (23.53) | 10 (71.43) | 4 (23.53) | 2 (14.29) | ||||||
| Severe | 13 (76.47) | 4 (28.57) | 13 (76.47) | 12 (85.71) | ||||||
| TDP-43 DN | 17 | 14 | 0.01 | 17 | 14 | NA | ||||
| Sparse | 13 (76.47) | 4 (28.57) | 17 (100.00) | 14 (100.00) | ||||||
| Frequent | 4 (23.53) | 10 (71.43) | 0 (0.00) | 0 (0.00) | ||||||
| Rod-like microglia | 17 | 14 | 0.01 | 17 | 14 | NA | ||||
| Sparse | 13 (76.47) | 4 (28.57) | 17 (100.00) | 14 (100.00) | ||||||
| Frequent | 4 (23.53) | 10 (71.43) | 0 (0.00) | 0 (0.00) | ||||||
| PGRN+ microglia | 17 | 14 | <0.01 | 17 | 14 | 0.81 | ||||
| Sparse | 1 (5.88) | 10 (71.43) | 4 (23.53) | 2 (14.29) | ||||||
| Moderate | 3 (17.65) | 4 (28.57) | 1 (5.88) | 0 (0.00) | ||||||
| Frequent | 13 (76.47) | 0 (0.00) | 12 (70.59) | 12 (85.71) | ||||||
DN, dystrophic neurites.
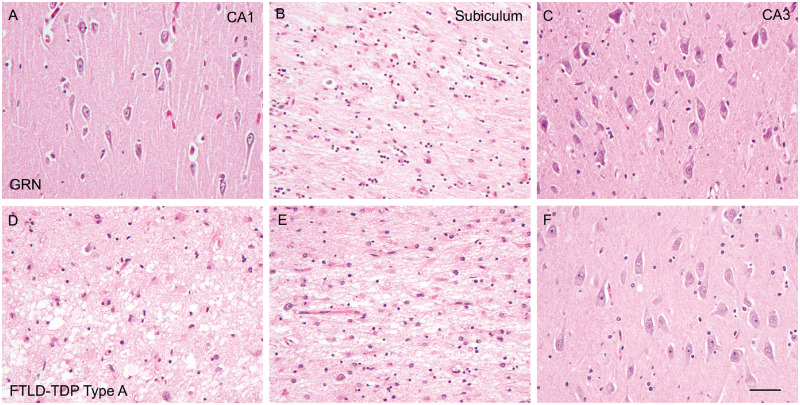
FIGURE 2.
Hematoxylin and eosin stains reveal unsynchronized neuronal loss in the CA1 region and subiculum of brains with GRN mutations. Neuronal loss is mostly mild in the CA1 region (A), severe in the subiculum (B), and minimal in the CA3 region (C) of FTLD-TDP type A with GRN mutation (GRN+/−) brains. However, neuronal loss is severe in both the CA1 region (D) and subiculum (E), and minimal in the CA3 region (F) of FTLD-TDP type A without GRN mutation brains (FTLD-TDP type A). Bar: 50 µm.
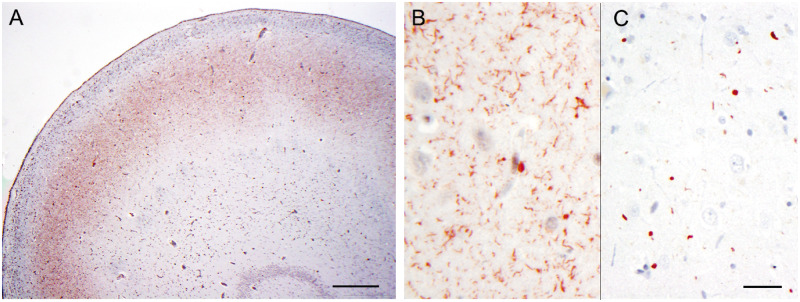
We then examined the whole microglial population in the CA1 region of different diseases. The whole microglial population can be revealed by IBA-1 immunostaining (42–44). IBA-1-positive microglia showed morphologic heterogeneity in the hippocampus (Fig. 3), consistent with findings previously described by Bachstetter et al (45). Specifically, ramified microglial cells were mainly seen in the hippocampi of brains from normal control and AD patients; hypertrophic microglial cells were more commonly seen in the hippocampi of ADHS and FTLD-TDP type A without GRN mutation patient brains (Fig. 3). Rod-shaped microglia, which are characterized by a narrow cell body and few planar processes, were sparse in all disease groups, though that they were more frequent in the FTLD-TDP type A with GRN mutations group (Fig. 3). In addition, rod-shaped microglia in the CA1 region of FTLD-TDP type A with GRN mutations brains were longer than in FTLD-TDP type A without GRN mutation brains, and formed trains of cells (Fig. 3F). To make microglial semiquantification easier, in this study, we only counted rod-shaped microglial cells in which cell length was equal to or more than 40 µm. The density of rod-shaped microglia was graded as none, sparse, moderate, or frequent, as illustrated in Figure 1. The frequency of rod-shaped microglia followed a dichotomous distribution in the hippocampal CA1 region as well, that is cases falling into the moderate category were rare. Frequent rod-shaped microglia were present in 10 out of 14 cases of FTLD-TDP type A with GRN mutations and sparse rod-shaped microglia were seen in the remaining 4 of these cases (Table 3). Conversely, rod-shaped microglia were frequent in only 4 out of 17 and sparse in 13 out of 17 FTLD-TDP type A without GRN mutations cases (Table 3). Immunostains with anti-PGRN antibody were performed in parallel to reveal PGRN-immunopositive microglia. The morphology of PGRN-positive microglia has been described in our previous work (28). The quantification of PGRN-positive microglia showed that hippocampal CA1 in FTLD-TDP type A with GRN mutations had sparse PGRN-positive microglial cells in 10 out of 14 cases and moderate PGRN-positive microglial cells in 4 out of 14 cases. The CA1 region in FTLD-TDP type A without GRN mutations brains had frequent PGRN-positive microglia in 13 out of 17 cases and sparse to moderate PGRN-positive microglial cells in 4 out of 14 cases (Fig. 4; Table 3). The CA1 region in ADHS brains had frequent PGRN-positive microglia (Fig. 4). Frequent rod-like microglia and sparse PGRN-positive microglia were significantly associated with GRN mutations (Fisher exact test p ≤ 0.01).
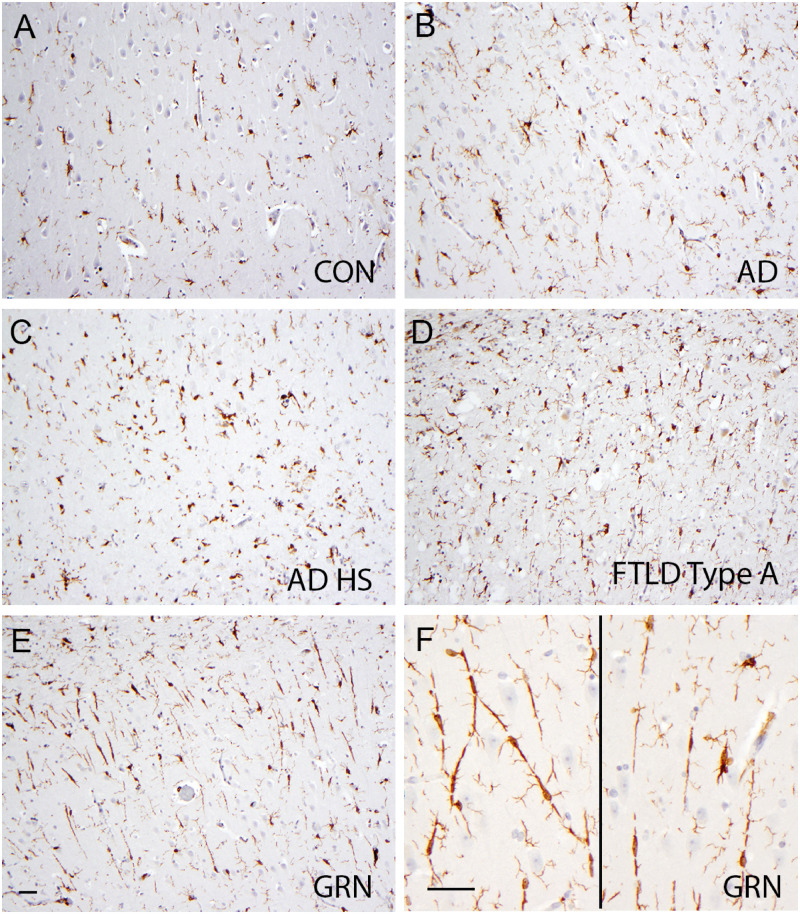
FIGURE 3.
IBA-1-positive microglia in the hippocampi of brains with different diseases. Sparse IBA-1-positive, rod-shaped microglia are present in the CA1 region in normal controls (CON, A), in Alzheimer disease (AD, B), in AD with hippocampal sclerosis (ADHS, C), and in FTLD-TDP type A without GRN mutations (FTLD-TDP type A, D), and are frequent in FTLD-TDP type A with GRN mutations (GRN, E and F). The rod-like microglia in FTLD-TDP type A with GRN mutations form end-to-end alignments (F). Bar: 50 µm.
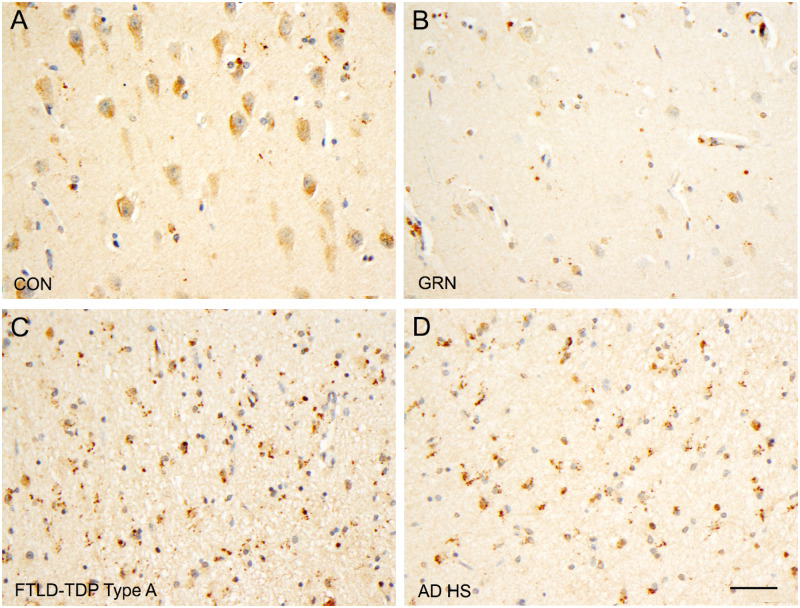
FIGURE 4.
PGRN-positive microglia in the hippocampi of brains with different diseases. Sparse PGRN-positive microglia are present in the CA1 region in normal controls (CON, A) and in FTLD-TDP type A with GRN mutations (GRN+/−, B). Frequent PGRN-positive microglia are found in the CA1 region in Alzheimer disease with hippocampal sclerosis (ADHS, C) and FTLD-TDP type A without GRN mutations (FTLD-TDP type A, D). Bar: 50 µm.
We next compared the density of the TDP-43-positive DNs in the CA1 region between FTLD-TDP cases with and without GRN mutations. DNs are abnormal neuronal processes with aberrant sprouting, dystrophic expansion, and accumulation of abnormal protein aggregates (46). Consistent with the previous study (11), frequent TDP-43-positive DNs were more commonly found in the brains of patients with FTLD-TDP type A with GRN mutations than in those of FTLD-TDP type A without GRN mutations; the area of frequent DNs was typically well demarcated and limited to the CA1 region (Fig. 5A, B). The severity of TDP-43-positive DNs in the CA1 region showed a dichotomous distribution. Most cases showed either sparse neurites or frequent neurites. Ten out of 14 cases of GRN mutations showed frequent DNs, while only 4 out of 17 of the cases without GRN mutations showed frequent DNs (Table 3). Frequent TDP-43-positive DNs were significantly associated with GRN mutations (Fisher exact test p = 0.01). The CA1 region of FTLD-TDP type A without GRN mutations showed only sparse TDP-43-positive DNs (Fig. 5C). TDP-43-positive neuronal cytoplasmic inclusions were rare in the hippocampal CA1 region in FTLD-TDP type A both with and without GRN mutations. Inter-rater reliability was tested, which showed high inter-rater reliability with neuronal loss and gliosis, TDP-43 DNs, and IBA-1-positive rod-like microglia matching perfectly, and PGRN-positive microglia having a weighted Cohen’s Kappa of 0.86 (CA1) and 0.78 (subiculum), which is very good and good agreement, respectively.
FIGURE 5.
Frequent TDP-43 dystrophic neurites (DNs) in the CA1 region of the brain with FTLD-TDP type A with GRN mutations (A, B). Sparse TDP-43 DNs in the CA1 region of the brain with FTLD-TDP type A without GRN mutations (C). Bar in A, 500 µm; bar in C, 20 µm for B and C.
We then grouped the CA1 pathology of FTLD-TDP-43 cases into Type 1 and Type 2 (Table 4). FTLD-TDP-43 Type 1 pathology is defined as having mild neuronal loss and gliosis, frequent TDP-43 DNs, frequent rod-shaped microglia, and sparse PGRN-positive microglia. On the contrary, FTLD-TDP-43 Type 2 pathology has severe neuronal loss and gliosis, sparse TDP-43 DNs, sparse rod-shaped microglia, and frequent PGRN-positive microglia. Interestingly, 10 out of 14 of FTLD-TDP type A with GRN mutation cases showed FTLD-TDP-43 Type 1 CA1 pathology, while the majority (13 out of 17) of FTLD-TDP type A without GRN mutation cases showed FTLD-TDP-43 Type 2 CA1 pathology (Table 5). FTLD-TDP-43 Type 1 CA1 pathology was significantly associated with GRN mutations (Fisher exact test p = 0.01).
TABLE 4.
Classification of Hippocampal CA1 Pathology
| Type 1 | Type 2 | |
|---|---|---|
| Neuronal loss | Mild | Severe |
| TDP-43 neurites | Frequent | Sparse |
| PGRN-positive microglia | Sparse | Frequent |
| IBA-1-positive rod-like microglia | Frequent | Sparse |
TABLE 5.
Hippocampal CA1 Pathology of FTLD-TDP Type A With or Without GRN Mutations Using a Fisher Exact Test
| GRN Mutation | No GRN Mutation | p | |
|---|---|---|---|
| Type 1 | 10 (0.71) | 4 (0.24) | 0.01 |
| Type 2 | 4 (0.29) | 13 (0.76) |
Lastly, clinicopathologic correlation revealed that FTLD-TDP type A cases with Type 1 CA1 pathology had a younger age of onset (p < 0.05), and age of death (p < 0.01) than FTLD-TDP type A patients with Type 2 pathology (Table 6). However, the disease duration, which is a variable that is subject to bias as it is based on historical report, showed no significant differences between the 2 groups (p > 0.05). The correlation between CA1 pathology and Clinical Dementia Rating (CDR) score (47) 1 year before death was evaluated in 12 cases for which CDR scores were available (Table 7). There were no differences in CDRs 1 year before death between cases with Type 1 and Type 2 pathology, and all cases showed severe dementia.
TABLE 6.
Mean ± Standard Deviation Value of Age at Onset, Age at Death and Duration of FTLD-TDP Type A Cases With Type 1 or Type 2 CA1 Pathology
| Type 1 (n = 14) | Type 2 (n = 17) | |
|---|---|---|
| Age at onset (years) | 56.4 ±5.3* | 65.2 ± 10.8 |
| Age at death (years) | 62.9 ± 5.6** | 73.7 ± 12.1 |
| Duration (years) | 6.5 ± 2.8 | 8.0 ± 4.3 |
p < 0.05.
p < 0.01, Type 1 versus Type 2 pathology.
TABLE 7.
Clinical and Pathologic Features of a Subset of FTLD-TDP Type A Cases With or Without GRN Mutations
| GRN Mutation | Sex | Clinical Dx | Death (Years) | Disease Duration (Years) | CDR Global | CDR Memory | CA1 Pathology |
|---|---|---|---|---|---|---|---|
| c.910_911insTG | F | PPA | 56 | 6.0 | 3 | 3 | Type 1 |
| c.102delC | F | PRAD | 62 | 7.0 | 3 | 3 | Type 1 |
| c.−8 + 3A>G | M | PPA | 70 | 4.0 | 3 | 3 | Type 1 |
| c.1317delC | M | FTD | 63 | 6.0 | 3 | 3 | Type 1 |
| c.1477C>T | F | PPA | 74 | 7.0 | 3 | 3 | Type 2 |
| – | F | FTD | 80 | 11 | 3 | 3 | Type 2 |
| – | M | LBD | 73 | 6 | 3 | 3 | Type 2 |
| - | F | PPA | 69 | 10 | 3 | 3 | Type 2 |
| – | F | PPA | 58 | 4 | 3 | 3 | Type 2 |
| – | F | PRAD | 84 | 9 | 3 | 3 | Type 2 |
| – | M | PRAD | 96 | 14 | 2 | 3 | Type 2 |
| – | M | CBS | 82 | 11 | 3 | 3 | Type 2 |
-, no GRN mutations.
CBD, corticobasal syndrome; CDR, Clinical Dementia Rating; FTD, frontotemporal dementia; LBD, Lewy body disease; PPA, primary progressive aphasia; PRAD, probable Alzheimer disease.
DISCUSSION
In this study, we found a unique CA1 pathology in FTLD-TDP type A with GRN mutations. In contrast to HS, which is seen in 79% of cases with sporadic FTLD-TDP type A (40, 41), the CA1 of those with FTLD-TDP type A with GRN mutations showed more viable neurons, more TDP-43 DNs, and more rod-like microglia, whereas the CA1 of cases with sporadic FTLD-TDP type A showed less viable neurons, less TDP-43 DNs, less rod-like microglia, and higher CA1 microglial PGRN expression.
Similar to the previous study (11), we found (i) frequent TDP-43-positive DNs in hippocampal CA1 were significantly associated with GRN mutations; and (ii) dichotomous severity of distribution of the DNs and neuronal loss in the CA1 region. In this study, we expand upon prior findings and demonstrated that in FTLD-TDP type A with GRN mutation brains, frequent TDP-43-positive DNs in the hippocampus corresponded closely to the area affected by mild neuronal loss, instead of severe neuronal loss and gliosis. We also found (i) non-synchronized neuronal loss and gliosis in the CA1 region and subiculum of patients with FTLD-TDP type A with GRN mutations; (ii) dichotomous severity distribution of the rod-shaped microglia and PGRN-positive microglia; and (iii) frequent rod-shaped microglia and sparse PGRN-positive microglia in the hippocampus corresponding closely to the area affected by mild neuronal loss.
PGRN has been found to be associated with neurite outgrowth and neuroinflammation in the central nervous system (24, 48–52). PGRN is constitutively expressed and secreted in microglia (26, 53) and carries out an anti-inflammatory role: the absence of PGRN in microglia causes increased production and release of proinflammatory cytokines in response to an inflammatory stimulus (26, 53). Brains of GRN knockout mice displayed greater activation of microglia and astrocytes than aged wild-type mice (25). In addition, PGRN-deficient macrophages and microglia were cytotoxic to neurons in the hippocampus and substantia nigra (25). Furthermore, Lui et al recently reported that GRN knockout mice showed substantial dysregulation of microglial complement gene expression and of lysosome maturation (54). Their findings were associated with evidence of loss of inhibitory synapses from parvalbumin-positive neurons in the ventral thalamus. Similarly, human GRN mutation carriers showed increased microglia infiltration in diseased brain regions, most prominently in the frontal and temporal cortices, which paradoxically led to increased levels of GRN mRNA transcription (27). These results indicated that PGRN deficiency is associated with overactivation of microglia and astrocytes, as well as with neuronal cytotoxicity.
Our previous studies demonstrated that GRN mutants had lower microglial PGRN expression in hippocampal CA1 than patients with FTLD-TDP type A without GRN mutations and with ADHS (28). Hence, we predicted that the CA1 of GRN mutants would show worse neuronal loss due to the neurotoxicity of microglial PGRN deficiency. However, surprisingly, the CA1 of FTLD-TDP type A with GRN mutations showed more viable neurons with more TDP-43 neurites, whereas sporadic TDP-A was associated with fewer neurons and fewer TDP-43 neurites. In other words, being intraneuronal, TDP-43 did not stay around when the neuron died. Does this mean that GRN mutations are protective of TDP-43 neurotoxicity when compared to sporadic TDP-43 disease? Even more puzzling is that homozygous GRN mutations may have an even slower neurotoxic effect, causing neuronal lipofuscinosis, which in the case of Kufs disease may be quite indolent (55). But, if that is the case, why does GRN mutation cause FTLD-TDP at all? Maybe the real disease is in the rod-shaped microglia. A recent publication demonstrated that rod-shaped microglia are uniquely present in the brains of patients with neurodegenerative diseases, including AD, HS-aging, and Lewy body disease (45). Studies on rod-shaped microglia are rare, and little is known about their functions (56). In this study, we present the novel association between PGRN deficiency and frequent rod-shaped microglia and other neuropathological features in the CA1 region of GRN mutant brains. Future clinicopathologic studies can help further elucidate the mechanistic functions of rod-shaped microglia.
Clinicopathologic correlation revealed that FTLD-TDP type A patients with Type 1 CA1 pathology died at a younger age than FTLD-TDP type A patients with Type 2 pathology. This finding, together with the milder neuronal loss and gliosis in Type 1 pathology, suggested that Type 1 CA1 pathology might represent an early stage of Type 2 pathology. However, those cases for which CDRs are available, with either Type 1 or Type 2 CA1 pathology, all showed severe dementia and equivalent CDRs 1 year before death. In addition, the disease duration showed no significant differences between these 2 groups. These results may actually suggest that Type 1 CA1 pathology constitutes a different type rather than reflecting a function of extent.
In conclusion, unique CA1 pathology in the brains of patients with FTLD-TDP type A with GRN mutations will provide important insights into the pathogenesis of hippocampal pathology of FTLD-TDP type A with or without GRN mutations and may facilitate the future development of PGRN-based treatments for dementia.
ACKNOWLEDGMENTS
We would like to thank the patients and their families who donated brains to advance our understanding of neurodegeneration. We gratefully acknowledge the assistance of the Mesulam Center for Cognitive Neurology and Alzheimer’s disease and its participants.
This study was supported by the Alzheimer’s Disease Research Fund Grant (83282003F and 93282003G) from the Illinois Department of Public Health (to Q.M.), an Alzheimer’s Disease Core Center grant (P30 AG013854) from the National Institute on Aging to Northwestern University, Chicago, Illinois (R01 DC008552 to M.M.), (R35 NS097261 to R.R.), as well as research grants from the National Natural Science Foundation of China (81471772 and 81773265 to H.X.) and the Fundamental Research Funds for the Central Universities (GK201706002 to H.X.).
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: Consensus recommendations. Acta Neuropathol 2009;117:15–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 2007;114:5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: Clinicopathological correlations. Ann Neurol 2006;59:952–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geser F, Martinez-Lage M, Robinson J, et al. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol 2009;66:180–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Josephs KA, Stroh A, Dugger B, et al. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol 2009;118:349–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mackenzie IR, Baborie A, Pickering-Brown S, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: Classification and relation to clinical phenotype. Acta Neuropathol 2006;112:539–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mackenzie IR, Neumann M, Baborie A, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 2011;122:111–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neumann M, Mackenzie IR, Cairns NJ, et al. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J Neuropathol Exp Neurol 2007;66:152–7 [DOI] [PubMed] [Google Scholar]
- 9. Sampathu DM, Neumann M, Kwong LK, et al. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol 2006;169:1343–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mackenzie IR, Baker M, Pickering-Brown S, et al. The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 2006;129:3081–90 [DOI] [PubMed] [Google Scholar]
- 11. Hatanpaa KJ, Bigio EH, Cairns NJ, et al. TAR DNA-binding protein 43 immunohistochemistry reveals extensive neuritic pathology in FTLD-U: A midwest-southwest consortium for FTLD study. J Neuropathol Exp Neurol 2008;67:271–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Josephs KA, Ahmed Z, Katsuse O, et al. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol 2007;66:142–51 [DOI] [PubMed] [Google Scholar]
- 13. Gass J, Cannon A, Mackenzie IR, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 2006;15:2988–3001 [DOI] [PubMed] [Google Scholar]
- 14. Huey ED, Grafman J, Wassermann EM, et al. Characteristics of frontotemporal dementia patients with a progranulin mutation. Ann Neurol 2006;60:374–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Ber I, van der Zee J, Hannequin D, et al. Progranulin null mutations in both sporadic and familial frontotemporal dementia. Hum Mutat 2007;28:846–55 [DOI] [PubMed] [Google Scholar]
- 16. Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006;442:916–9 [DOI] [PubMed] [Google Scholar]
- 17. Cruts M, Gijselinck I, van der Zee J, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 2006;442:920–4 [DOI] [PubMed] [Google Scholar]
- 18. Bateman A, Belcourt D, Bennett H, et al. Granulins, a novel class of peptide from leukocytes. Biochem Biophys Res Commun 1990;173:1161–8 [DOI] [PubMed] [Google Scholar]
- 19. Bhandari V, Palfree RG, Bateman A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci USA 1992;89:1715–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shoyab M, McDonald VL, Byles C, et al. Epithelins 1 and 2: Isolation and characterization of two cysteine-rich growth-modulating proteins. Proc Natl Acad Sci USA 1990;87:7912–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med 2003;81:600–12 [DOI] [PubMed] [Google Scholar]
- 22. Petkau TL, Leavitt BR. Progranulin in neurodegenerative disease. Trends Neurosci 2014;37:388–98 [DOI] [PubMed] [Google Scholar]
- 23. Tapia L, Milnerwood A, Guo A, et al. Progranulin deficiency decreases gross neural connectivity but enhances transmission at individual synapses (Research Support, Non-U.S. Gov't). J Neurosci 2011;31:11126–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Damme P, Van Hoecke A, Lambrechts D, et al. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol 2008;181:37–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yin F, Banerjee R, Thomas B, et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't). J Exp Med 2010;207:117–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martens LH, Zhang J, Barmada SJ, et al. Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury (Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.). J Clin Invest 2012;122:3955–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen-Plotkin AS, Xiao J, Geser F, et al. Brain progranulin expression in GRN-associated frontotemporal lobar degeneration. Acta Neuropathol 2010;119:111–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mao Q, Wang D, Li Y, et al. Disease and region specificity of granulin immunopositivities in Alzheimer disease and frontotemporal lobar degeneration. J Neuropathol Exp Neurol 2017;76:957–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McKhann GM, Albert MS, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia: Report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol 2001;58:1803–9 [DOI] [PubMed] [Google Scholar]
- 30. Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathol 2010;119:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mesulam MM. Primary progressive aphasia. Ann Neurol 2001;49:425–32 [PubMed] [Google Scholar]
- 32. Mesulam MM. Primary progressive aphasia—A language-based dementia. N Engl J Med 2003;349:1535–42 [DOI] [PubMed] [Google Scholar]
- 33. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Demen 2011;7:263–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Demen 2012;8:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: A practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chai L, Mao Q, Liu S, et al. Domain-specific monoclonal antibodies produced against human PGRN. Hybridoma (Larchmt) 2011;30:271–8 [DOI] [PubMed] [Google Scholar]
- 38. Li Y, Li Y, Ye M, et al. Biological function analysis of monoclonal antibodies against human granulins in vitro using U251 cells as a model. Protein Expr Purif 2017;130:55–62 [DOI] [PubMed] [Google Scholar]
- 39. Hatanpaa KJ, Raisanen JM, Herndon E, et al. Hippocampal sclerosis in dementia, epilepsy, and ischemic injury: Differential vulnerability of hippocampal subfields. J Neuropathol Exp Neurol 2014;73:136–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Josephs KA, Dickson DW. Hippocampal sclerosis in tau-negative frontotemporal lobar degeneration. Neurobiol Aging 2007;28:1718–22 [DOI] [PubMed] [Google Scholar]
- 41. Knopman DS, Mastri AR, Frey WH 2nd, et al. Dementia lacking distinctive histologic features: A common non-Alzheimer degenerative dementia. Neurology 1990;40:251–6. [DOI] [PubMed] [Google Scholar]
- 42. Ito D, Imai Y, Ohsawa K, et al. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res 1998;57:1–9 [DOI] [PubMed] [Google Scholar]
- 43. Okere CO, Kaba H. Heterogeneous immunohistochemical expression of microglia-specific ionized calcium binding adaptor protein (Iba1) in the mouse olfactory bulb. Brain Res 2000;877:85–90 [DOI] [PubMed] [Google Scholar]
- 44. Hirayama A, Okoshi Y, Hachiya Y, et al. Early immunohistochemical detection of axonal damage and glial activation in extremely immature brains with periventricular leukomalacia. Clin Neuropathol 2001;20:87–91 [PubMed] [Google Scholar]
- 45. Bachstetter AD, Van Eldik LJ, Schmitt FA, et al. Disease-related microglia heterogeneity in the hippocampus of Alzheimer's disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol Commun 2015;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dickson TC, King CE, McCormack GH, et al. Neurochemical diversity of dystrophic neurites in the early and late stages of Alzheimer's disease. Exp Neurol 1999;156:100–10 [DOI] [PubMed] [Google Scholar]
- 47. McCulla MM, Coats M, Van Fleet N, et al. Reliability of clinical nurse specialists in the staging of dementia. Arch Neurol 1989;46:1210–1 [DOI] [PubMed] [Google Scholar]
- 48. Ryan CL, Baranowski DC, Chitramuthu BP, et al. Progranulin is expressed within motor neurons and promotes neuronal cell survival. BMC Neurosci 2009;10:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gao X, Joselin AP, Wang L, et al. Progranulin promotes neurite outgrowth and neuronal differentiation by regulating GSK-3beta. Protein Cell 2010;1:552–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tapia L, Milnerwood A, Guo A, et al. Progranulin deficiency decreases gross neural connectivity but enhances transmission at individual synapses. J Neurosci 2011;31:11126–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gass J, Lee WC, Cook C, et al. Progranulin regulates neuronal outgrowth independent of sortilin. Mol Neurodegen 2012;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Petkau TL, Neal SJ, Milnerwood A, et al. Synaptic dysfunction in progranulin-deficient mice. Neurobiol Dis 2012;45:711–22 [DOI] [PubMed] [Google Scholar]
- 53. Suh HS, Choi N, Tarassishin L, et al. Regulation of progranulin expression in human microglia and proteolysis of progranulin by matrix metalloproteinase-12 (MMP-12). PLoS One 2012;7:e35115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lui H, Zhang J, Makinson SR, et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell 2016;165:921–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith KR, Damiano J, Franceschetti S, et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet 2012;90:1102–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Graeber MB. Changing face of microglia. Science 2010;330:783–8 [DOI] [PubMed] [Google Scholar]