Abstract
Excitotoxic neuronal injury is associated with numerous acute and chronic neurological disorders, such as Alzheimer's disease and glaucoma. Neuroprotection is a direct and effective therapeutic approach, with small-molecule bioactive peptides displaying certain advantages, including high membrane permeability, low immunogenicity and convenient synthesis and modification. FK18 is a novel peptide derived from basic fibroblast growth factor, which is a protein with neuroprotective effects. The present study aims to evaluate the neuroprotective effect of FK18 against excitotoxic injury. For this purpose, cell viability was determined by the MTS assay, cell apoptosis was assessed by flow cytometry and the TUNEL assay; expression of antiapoptotic proteins Bcl-2, proapoptotic protein Bax and caspase-3 as well as the phosphorylation of Akt and Erk was estimated by western blotting. The results of the present study demonstrated that FK18 effectively increased the viability of, and attenuated glutamate-induced apoptosis of SH-SY5Y cells. In addition, FK18 significantly increased Akt phosphorylation and decreased Erk phosphorylation in SH-SY5Y cells. FK18 also increased the Bcl-2/Bax ratio and decreased the level of cleaved-caspase-3 in SY5Y cells, which was reversed by the Akt pathway inhibitor LY294002, but not by the Erk pathway inhibitor U0126. The findings of the present study suggested that FK18 may be a promising therapeutic agent for the inhibition of neuronal cell death in multiple neurological diseases involving excitotoxicity.
Keywords: excitotoxicity, peptide, neuroprotection, Akt, Erk
Introduction
Glutamate-induced excitotoxicity is one of the major pathological mechanisms associated with a number of chronic neurodegenerative disorders, such as Alzheimer's disease and Parkinson's disease in the brain (1-3) and glaucoma, retinitis pigmentosa and age-associated macular degeneration in the eyes (4-6). Excessive accumulation of extracellular glutamate may lead to the hyperexcitability of neurons, calcium overload and increased intracellular oxygen free radicals, resulting in DNA injury and apoptosis (7,8). Massive death of neurons inevitably leads to the dysfunction of nervous system, irreversible vision loss and is life threatening; therefore, minimizing neuronal apoptosis is the most direct and effective therapeutic approach for patients with the aforementioned diseases (9,10).
Small-molecular chemicals, including glutamate antagonists, calcium channel blockers and antioxidant/radical scavenging agents, are primarily used to provide pharmacological neuroprotection (11-13); however, the unexpected side effects associated with their low target specificity have impeded their wider application (14). Supplementation of downregulated neurotrophic factors is another approach to neuroprotection, such as growth factors and erythropoietin (15-17). Nevertheless, it requires repeated invasive administration, which is not suitable for long-term therapy in chronic diseases, such as glaucoma and diabetic retinopathy (18,19). In contrast, small-molecule bioactive peptides are becoming a hotspot for research, due to their relatively high membrane permeability, low immunogenicity and convenient synthesis and modification (20-22).
FK18 is a small peptide composed of 18 amino acids that is derived from basic fibroblast growth factor (bFGF) using bioinformatics screening methods (23). The sequence is located in the conserved receptor-binding domain of human bFGF, which is known for its neuroprotective activity against a series of cerebral and ocular diseases, such as cerebral ischemia and light damage in eyes (24-26). SH-SY5Y cells were chosen in the current study as they share similar morphological, neurochemical and electrophysiological properties to neurons. The aim of the present study was to examine the protective effect of FK18 on neurodegenerative diseases and further investigated its mechanisms.
Materials and methods
Preparation of peptides
FK18 peptide (sequence, FFFERLESNNYNTYSRK) and a scrambled negative control peptide, SCpep (sequence, SFLNKTNFREFRNYSEYF), were prepared using a high-efficiency solid-phase method by China Peptides Co., Ltd. All the peptides had a purity >98% as tested by high-performance liquid chromatography (Shimadzu Corporation) and were freeze-dried and stored at -20˚C until further use.
Cell culture and treatment
SH-SY5Y cells were purchased from the Shanghai Cell Bank at the Chinese Academy of Sciences (cat. no. SCSP-5014) and were authenticated using STR profiling. The cells were maintained in a DMEM/F-12 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in a humidified atmosphere containing 5% CO2. Cells between the fourth and the seventh passages were used in the present study, to ensure their stable properties, including morphology, function and viability. Cells were seeded in 96-well plates to test cell viability, in 24-well plates to conduct TUNEL assay, in 6-well plates to perform flow cytometry and 10-cm dishes to perform western blotting. A cell density of 1x106/ml was used for each type of plate in following experiments. Excitotoxicity was induced by exposing the cultures to different concentrations of glutamate (4, 6, 8 and 10 mM; Sigma-Aldrich; Merck KGaA) for 24 h at 37˚C.
To determine the effect of FK18, its dose-response effect was tested, and the result demonstrated a plateau of protection reached under the dose of 10 µg/ml (data not shown), which was similar to that against oxygen and glucose deprivation-induced injury as previously reported (23). Hence, 10 µg/ml was selected as the dosage in subsequent experiments. The cells were pretreated with normal saline, 10 µg/ml FK18, or recombinant human bFGF (100 ng/ml; R&D Systems, Inc.) 2 h before, at the same time or 30 min after the induction of excitotoxicity at 37˚C. The timepoint was chosen according to preliminary experiment results (data not shown). For the detection of Akt, phospho-Akt, Erk1/2, and phospho-Erk1/2, the cells were treated with either LY294002 (20 µM; Beyotime Institute of Biotechnology), a PI3K inhibitor, or U0126, a highly selective inhibitor of MEK1/2 (20 µM; Beyotime Institute of Biotechnology ), 2 h prior to the addition of FK18.
Cell viability assay
Viability of SH-SY5Y cells was quantitatively evaluated using an MTS kit (Promega Corporation). Briefly, 20 µl MTS was added to each well of a 96-well plate for 2 h at 37˚C. Absorbance was measured photometrically at 490 nm and cell viability was expressed as a percentage of the optical density measured in the control group treated with normal saline.
Annexin V staining and flow cytometry for measuring apoptosis
The percentage of SH-SY5Y apoptotic cells following glutamate injury was determined by flow cytometry using an Annexin V-FITC/propidium iodide (PI) Detection kit (BD Pharmingen; BD Biosciences), according to the manufacturer's instructions. Briefly, the cells were digested with 0.05% trypsin and collected by centrifugation (1,000 x g for 5 min) at room temperature. The pellets were washed in PBS and resuspended in binding buffer. A 100 µl volume of the cell suspension (~1x105 cells) was treated in the dark with 5 µl FITC Annexin V and 5 µl PI at room temperature for 15 min. The stained cells were analyzed using a FACSCalibur (Becton-Dickinson and Company) and FlowJo version 10.4 (Becton-Dickinson and Company). Both early and late apoptotic cells were included in the final analysis.
TUNEL assay
TUNEL staining was performed according to the manufacturer's instructions for 1 h at 37 ˚C (DeadEnd™ Fluorometric TUNEL System; Promega Corporation) to detect apoptotic cells. For the in vitro experiments, following glutamate treatment, SH-SY5Y cells were fixed with 4% PFA for 25 min at 4˚C. After fixation, the cells were permeabilized with 0.2% Triton X-100 for 5 min. The nuclei were imaged after counterstaining with 0.5 µg/ml DAPI at room temperature for 10 min. TUNEL-positive cells were viewed using a laser scanning confocal microscope (magnification, x20; Zeiss LSM 510; Carl Zeiss AG) in six random fields (at least 100 DAPI-positive cells per field).
Western blotting
SH-SY5Y cells were lysed in RIPA lysis buffer containing PMSF and phosphatase inhibitor (v/v=98:1:1) for 5, 10, 20, 30 and 60 min after 8 mM glutamate addition for detecting phosphorylated related proteins. For detection of apoptosis-associated markers, cell lysates were prepared 24 h after 8 mM glutamate addition. The cell lysates were then prepared and the amount of protein in each lysate was determined using a bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of Biotechnology). The mass of protein loaded per lane was 10 µl. The extracted proteins were subjected to either 10 or 12% SDS-PAGE and transferred onto PVDF membranes electrophoretically. After blocking non-specific sites with 5% non-fat milk for 1 h at 37 ˚C, the membrane was incubated with primary antibodies (all from Cell Signaling Technology, Inc.) against phosphorylated-(p) Akt (1:1,000; cat. no. 4060S), Akt (1:1,000; cat. no. 4691S), p-Erk (1:1,000; cat. no. 4370S), Erk (1:1,000; cat. no. 4695S), Bcl-2 (1:1,000; cat. no. 4223S), Bax (1:1,000; cat. no. 5023S), cleaved caspase-3 (1:1,000; cat. no. 9664S), full-length caspase-3 (1:1,000; cat. no. 14220S) or GAPDH (1:1,000; cat. no. 2118L) overnight at 4˚C and detected with HRP-conjugated anti-rabbit IgG secondary antibody (1:10;000; cat. no. 111-035-003; Jackson ImmunoResearch Laboratories, Inc.) for 2 h at room temperature. Bands were visualized using an enhanced chemiluminescence detection system (Merck KGaA), and band densities were quantified using Adobe Photoshop version 19.1.8 (Adobe Systems, Inc.).
Statistical analysis
All experiments were performed at least three times. Data are expressed as the mean ± SD and analyzed using IBM SPSS statistics version 24.0 (IBM Corp). The distribution of data was examined using the Kolmogorov-Smirnov test. Differences were evaluated by one-way ANOVA followed by a post hoc Tukey's HSD test. Non-parametric data were analyzed using the Kruskal-Wallis test and Bonferroni's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
FK18 improved cell viability in SH-SY5Y cells after glutamate injury
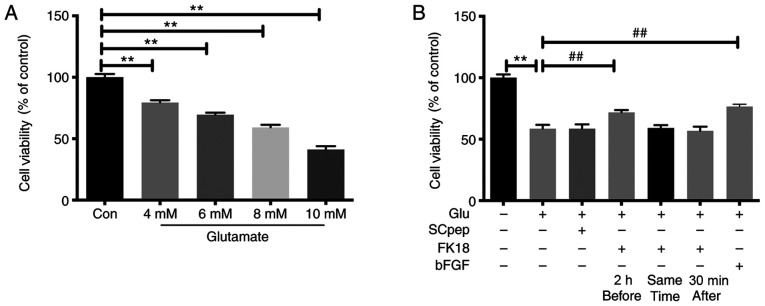
The cell viability of SH-SY5Y cells decreased gradually as the concentration of glutamate increased, with the survival rates being 79.48±1.84, 69.58±1.59, 59.16±2.21 and 41.18±2.77% after exposure to 4, 6, 8 and 10 mM of glutamate, respectively (Fig. 1A). The insult of 8 mM glutamate was chosen for the present study. Pretreatment with FK18 dramatically increased cell viability, with an increase in cell viability from 58.50±3.22 to 71.78 ± 1.87% (P<0.01; Fig. 1B). However, when cells were exposed to FK18, either simultaneously or 30 min after adding glutamate, the neuroprotective effect was lost (Fig. 1B).
Figure 1.
Neuroprotective effect of FK18 on cell viability tested by the MTS assay. (A) Viability of SH-SY5Y cells following exposure to different concentrations of glutamate for 24 h. (B) Viability of SH-SY5Y cells incubated with SCpep, FK18 and bFGF, at three different time points: 2 h before, simultaneously and 30 min after glutamate addition. The data are expressed as the mean ± SD. **P<0.01 vs. control group; ##P<0.01 vs. glutamate group. Con, control group treated with normal saline; Glu, glutamate; bFGF, basic fibroblast factor.
FK18 attenuated glutamate-induced cell apoptosis in SH-SY5Y cells
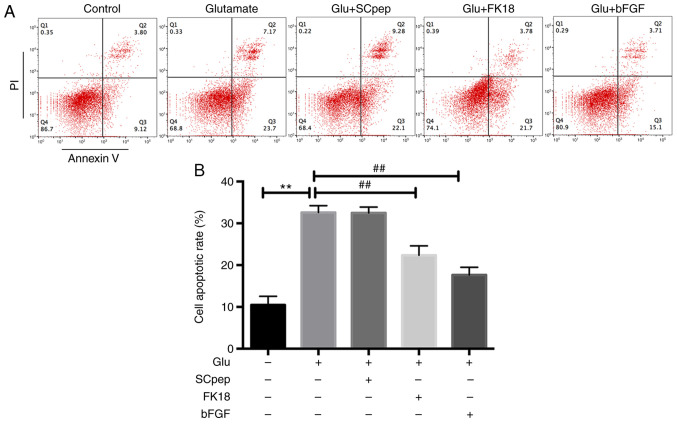
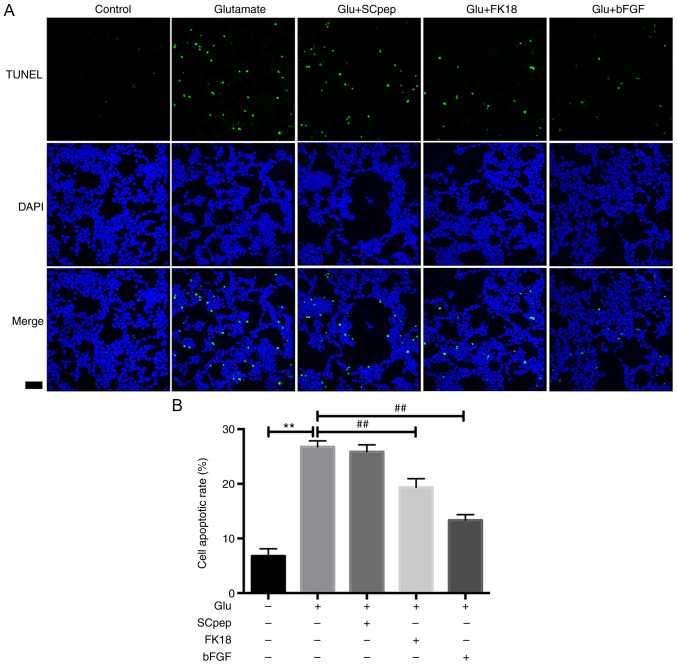
FK18 protective ability against glutamate-induced apoptosis was assessed using flow cytometry and TUNEL assay. The flow cytometry results demonstrated that pretreatment with FK18 reduced cell apoptosis from 32.58±1.63 to 22.35±2.26% in the glutamate group (Fig. 2A and B). The TUNEL assay demonstrated that the percentage of TUNEL-positive cells decreased from 26.77±1.10 to 19.34±1.60% in the glutamate group following the application of FK18 (Fig. 3A and B). The bFGF group demonstrated a decrease in apoptosis to 17.62±1.83 and 13.33±1.03% compared with the glutamate group according to the flow cytometry and the TUNEL assay, respectively. The SCpep group demonstrated no significant difference compared with the glutamate group (Figs. 2 and 3).
Figure 2.
Neuroprotective effects of FK18 against glutamate-induced apoptosis of SH-SY5Y cells tested by flow cytometry. (A) Apoptotic cells assessed by flow cytometry. (B) Histogram indicating the percentages of apoptotic cells following various treatments. The data are expressed as the mean ± SD. **P<0.01 vs. control group; ##P<0.01 vs. glutamate group. Control group, cells treated with normal saline; Glu, glutamate; bFGF, basic fibroblast factor; PI, propidium iodide.
Figure 3.
Neuroprotective effects of FK18 against glutamate-induced apoptosis of SH-SY5Y cells tested by TUNEL assay. (A) Representative immunofluorescence images of TUNEL-positive (green) SH-SY5Y cells. Scale bar, 50 µm (magnification, x20). (B) Histogram indicating the percentages of TUNEL-positive cells relative to the total number of neurons following various treatments. The data are expressed as the mean ± SD. **P<0.01 vs. control group; ##P<0.01 vs. glutamate group. Control group, cells treated with normal saline; Glu, glutamate; bFGF, basic fibroblast factor.
Effect of FK18 on phosphorylation of Akt and Erk in SH-SY5Y cells
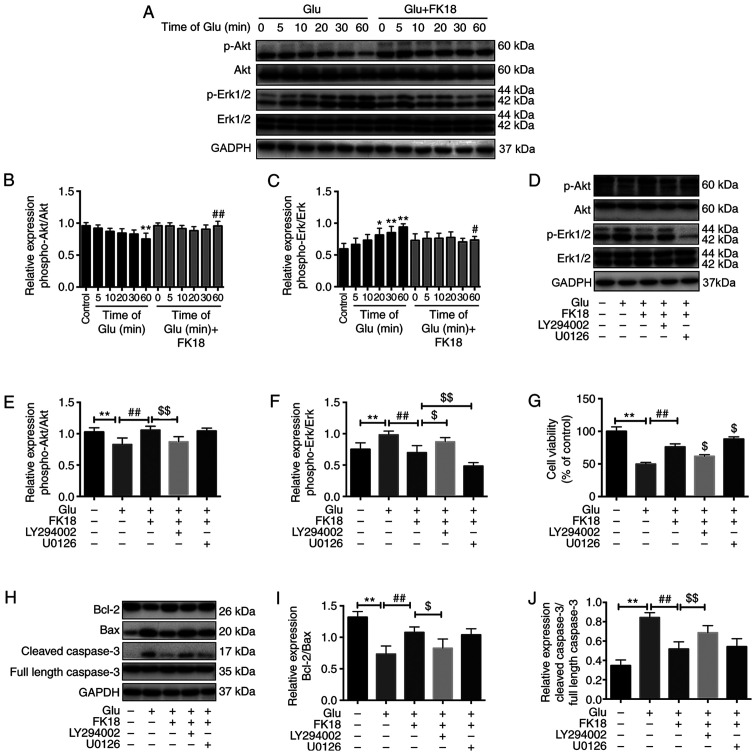
To investigate the potential mechanisms underlying the protective effects of FK18, the present study investigated p-Akt and p-Erk expression with the treatment of glutamate or FK18 at different time points using western blotting. Treatment with 8 mM glutamate decreased the basal p-Akt level in a time-dependent manner by 4.17, 9.38, 12.50, 13.50 and 21.88% compared with the control group after 5, 10, 20, 30 and 60 min of treatment, respectively, while it significantly increased the basal p-Erk level in a time-dependent manner 1.12-, 1.23-, 1.37-, 1.43- and 1.58-fold, respectively (Fig. 4A-C). The decrease of Akt phosphorylation reached significance 60 min after the glutamate injury when compared with the control group, while the increase of Erk phosphorylation reached significance 20 min and peaked at 60 min, following glutamate injury (Fig. 4A-C). FK18 pretreatment maintained a relatively high level of p-Akt and a low level of p-Erk compared with the glutamate group (Fig. 4A-C). The addition of LY294002 significantly blocked the Akt phosphorylation caused by FK18 and promoted the Erk phosphorylation that had been decreased by FK18 (Fig. 4D-F). The addition of U0126 had no effect on the p-Akt level, but significantly downregulated p-Erk expression (Fig. 4D-F) compared with the FK18 group. LY294002 abolished the neuroprotective effect of FK18, with a decrease in cell viability from 76.23±4.58 to 61.83±2.40%, while U0126 facilitated the effect of FK18, increasing cell viability from 76.23±4.58 to 88.23±3.33% (Fig. 4G).
Figure 4.
Effect of FK18 on Akt, Erk phosphorylation and apoptosis-associated proteins in SH-SY5Y cells. (A) Representative western blot image demonstrating the effect of FK18 on p-Akt and p-Erk expression following the induction of glutamate in a time-dependent manner. (B and C) Semi-quantification of the relative expression of p-Akt/total Akt and p-Erk/total Erk following glutamate injury in a time-dependent manner. (D) Representative western blot image demonstrating the effect of FK18 on p-Akt and p-Erk expression in the presence or absence of LY294002 or U0126. (E and F) Semi-quantification of the relative expression of p-Akt/total Akt and p-Erk/total Erk in the presence or absence of LY294002 and U0126. (G) Cell viability of SH-SY5Y cells following glutamate injury in the presence or absence of LY294002 or U0126, as determined by MTS assay. (H) Representative western blots demonstrating the expression of Bcl-2, Bax and caspase-3 following glutamate injury, with or without FK18 pretreatment, in the presence or absence of LY294002 or U0126. (I) Bcl-2/Bax protein expression ratio following various treatments. (J) Semi-quantification of cleaved caspase-3 protein expression expressed as a ratio full length caspase-3 expression. The data is expressed as the mean ± SD. *P<0.05 and **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. glutamate group; $P<0.05 and $$P<0.01 vs. glutamate-plus-FK18 group. Control group, treated with normal saline; Glu, glutamate; bFGF, basic fibroblast factor; PI, propidium iodide; p, phosphorylated; LY294002, Akt pathway inhibitor; U0126, Erk pathway inhibitor.
Effect of FK18 on apoptosis-associated proteins in SH-SY5Y cells
Bcl-2/Bax ratio was a sensitive determinant of the regulatory effects of glutamate, with or without FK18 and pathway inhibitor pretreatment (27). The ratio significantly decreased following glutamate injury, whereas it significantly increased following the addition of FK18 and was further inhibited by the effect of LY294002, but not affected by U0126 compared with the FK18 group (Fig. 3H and I). The relative protein expression level of cleaved caspase-3 significantly increased following glutamate injury, whereas FK18 pretreatment suppressed this change. The addition of LY294002 reversed this effect, but U0126 was not able to do so (Fig. 3H and J).
Discussion
The present study demonstrated, for the first time that FK18 protects against neuronal cell death induced by glutamate in SH-SY5Y cells. The findings of the present study suggested that the FK18 neuroprotective role is mediated via the Akt and Erk pathways, which may be affected by Akt and Erk phosphorylation, but independently of the caspase apoptotic pathway.
The human neuroblastoma cell line SH-SY5Y possesses similar morphological, neurochemical and electrophysiological properties to neurons and has been widely used for researching the disease pathogenesis and mechanism underlying drugs in the nervous system (28-30). Glutamate-induced injury of SH-SY5Y cells provides a rapid and sensitive in vitro model of neuronal excitotoxicity, with concentrations of glutamate ranging from 2-100 mM (28,31-33). In the present study, it was demonstrated that the injurious effect of glutamate was concentration-dependent using MTS assay; 8 mM of glutamate decreased cell viability by ~50% and was the most suitable concentration for the subsequent observation of drug effects.
The results of the present study demonstrated that FK18 protected against neuronal excitatory injury in SH-SY5Y cells. However, the neuroprotective effect of FK18 only occurred when the peptide was applied before the excitotoxic stimulus and did not occur when it was added simultaneously or after glutamate. This is a common difficulty in other neuroprotective agents, such as neuropeptide Y (34). As for peptides, it may be improved by chemical modification and optimization of its sequence and structure, or by the development of a targeted drug delivery system (35). In addition, the neuroprotective effects of FK18 against excitotoxicity in vitro were relatively weak in the present study, which could be associated with the non-specific mechanism of FK18 against excitotoxic injury. The aforementioned anti-excitotoxic effect of FK18 may be a part of its neuroprotection and other mechanisms that may be involved need further investigation.
The Akt pathway is a central signal transduction pathway involved in cell proliferation, survival and metabolism (36,37). A previous study demonstrated that Akt activity is responsible for as much as 80% of neurotrophin-regulated cell survival, indicating that Akt is the major survival-promoting protein for neurons (38). Akt signaling inhibits apoptosis by regulating the expression of Bcl-2 and Bax and finally, the expression of caspase-3 (39,40). The findings of the present study were consistent with those of the aforementioned studies. As demonstrated by western blotting in the present study, FK18 activated the Akt pathway, significantly promoted Akt phosphorylation and increased the cell viability of SH-SY5Y cells. With the addition of the Akt inhibitor LY294002, protection of SH-SY5Y cells by FK18 was significantly reversed. In addition, the present study revealed that the expression of the Bcl-2/Bax ratio decreased significantly after exposure to glutamate and that this process could be reversed in the presence of FK18 (which was accompanied by lower cleaved-caspase-3 activity). When adding the Akt inhibitor LY294002 to FK18, this effect was eliminated in the present study.
The Erk signaling pathway is not only involved in synaptic plasticity and neuronal development under physiological conditions (41,42), but is associated with cell apoptosis and neurodegeneration under pathological conditions (43,44). In the present study, a role that the Erk pathway serves was revealed, albeit a smaller one, in the protective effect of FK18. When glutamate-injured SH-SY5Y cells were treated with FK18, Erk1/2 phosphorylation was significantly suppressed, this decrease was further promoted by the addition of the Erk1/2 inhibitor U0126. In the present study, cell viability increased by FK18 was further improved by U0126; however, there were no significant changes of the Bcl-2/Bax ratio and cleaved-caspase-3 with the addition of U0126 compared with the FK18 group, suggesting that the Erk-mediated neuroprotective effects of FK18 may be independent of caspase-3. Previous studies have also demonstrated that persistent active Erk translocates to the nucleus, causing cell apoptosis by regulating gene expression and cell differentiation in a caspase-3-independent manner (45,46). In addition, cross-talk between the Akt and Erk pathways was observed in the present study, with the Akt inhibitor LY294002 promoting the phosphorylation of Erk. Other studies have also suggested a negative regulatory effect of Akt on the Erk pathway (47,48), although the exact mechanisms need to be further investigated.
Several limitations should be acknowledged. First, the effects of FK18 in animal disease models have not been evaluated in the current study, which needs further investigation. Second, the specific target of FK18 has not been fully elucidated; its effects on the Akt and Erk pathways as suggested in the current study might provide some clues for future investigation Third, the protection of FK18 against excitotoxic injury is relatively weak, future modifications of the peptide would be helpful to improve its effect.
In conclusion, the present study extended present knowledge of the application of FK18 to neuronal excitatory diseases by demonstrating the protective effects of FK18 against excitotoxic injury in SH-SY5Y cells. In addition, the present study elucidated the mechanism of FK18 by demonstrating that apart from activating the Akt pathway, FK18 could exert its neuroprotective effect through the suppression of the Erk pathway, which cross-talks with the Akt pathway, but involves a caspase-3-independent mechanism. The findings of the present study indicated that FK18, a novel peptide derived from human bFGF may be a promising therapeutic agent for the inhibition of neuronal death in multiple neurological diseases involving excitotoxicity, including Alzheimer's disease and Parkinson's disease in the brain and glaucoma, retinitis pigmentosa and age-associated macular degeneration in the eyes.
Acknowledgements
Not applicable.
Funding Statement
Funding: This work was financially supported by the Shanghai Sailing Program (grant no. 18YF1420200) and the Program of the National Natural Science Foundation of China (grant no. 81970812).
Availability of data and methods
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SX and MM designed the study. SX drafted the manuscript. SX, YX, FW and QG were responsible for the collection and analysis of the experimental data. XH and XX made substantial contributions to conception and design of the research, were involved in revising the manuscript critically for important intellectual content and approved the final version to be published. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 2.Wang R, Reddy PH. Role of Glutamate and NMDA Receptors in Alzheimer's Disease. J Alzheimers Dis. 2017;57:1041–1048. doi: 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pisanò CA, Brugnoli A, Novello S, Caccia C, Keywood C, Melloni E, Vailati S, Padoani G, Morari M. Safinamide inhibits in vivo glutamate release in a rat model of Parkinson's disease. Neuropharmacology. 2020;167(108006) doi: 10.1016/j.neuropharm.2020.108006. [DOI] [PubMed] [Google Scholar]
- 4.Connaughton V. Glutamate and Glutamate Receptors in the Vertebrate Retina. In: Webvision: The Organization of the Retina and Visual System. Kolb H, Fernandez E and Nelson R (eds) University of Utah Health Sciences Center Copyright, Webvision, Salt Lake City, UT, 1995. [PubMed] [Google Scholar]
- 5.Charles-Messance H, Blot G, Couturier A, Vignaud L, Touhami S, Beguier F, Siqueiros L, Forster V, Barmo N, Augustin S, et al. IL-1β induces rod degeneration through the disruption of retinal glutamate homeostasis. J Neuroinflammation. 2020;17(1) doi: 10.1186/s12974-019-1655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delyfer MN, Forster V, Neveux N, Picaud S, Léveillard T, Sahel JA. Evidence for glutamate-mediated excitotoxic mechanisms during photoreceptor degeneration in the rd1 mouse retina. Mol Vis. 2005;11:688–696. [PubMed] [Google Scholar]
- 7.Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruman II, Mattson MP. Pivotal role of mitochondrial calcium uptake in neural cell apoptosis and necrosis. J Neurochem. 1999;72:529–540. doi: 10.1046/j.1471-4159.1999.0720529.x. [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg MD. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osborne NN, Casson RF, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Binda NS, Carayon CP, Agostini RM, Pinheiro AC, Cordeiro MN, Silva MA, Silva JF, Pereira EM, da Silva CA Jr, Castro CJ Jr, et al. PhTx3-4, a spider toxin calcium channel blocker, reduces NMDA-induced injury of the retina. Toxins (Basel) 2016;8(70) doi: 10.3390/toxins8030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yiğit U, Erdenöz S, Uslu U, Oba E, Cumbul A, Cağatay H, Aktaş S, Eskicoğlu E. An immunohistochemical analysis of the neuroprotective effects of memantine, hyperbaric oxygen therapy, and brimonidine after acute ischemia reperfusion injury. Mol Vis. 2011;17:1024–1033. [PMC free article] [PubMed] [Google Scholar]
- 13.Stankowska DL, Dibas A, Li L, Zhang W, Krishnamoorthy VR, Chavala SH, Nguyen TP, Yorio T, Ellis DZ, Acharya S. Hybrid compound SA-2 is neuroprotective in animal models of retinal ganglion cell death. Invest Ophthalmol Vis Sci. 2019;60:3064–3073. doi: 10.1167/iovs.18-25999. [DOI] [PubMed] [Google Scholar]
- 14.Craik DJ, Fairlie DF, Liras S, Liras S, Price D. The future of peptide-based drugs. Chem Biol Drug Des. 2013;81:136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, Wei N, Lu T, Zhu J, Xu G, Liu X. Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience. 2011;172:398–405. doi: 10.1016/j.neuroscience.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 16.Larpthaveesarp A, Georgevits M, Ferriero DM, Gonzalez FF. Delayed erythropoietin therapy improves histological and behavioral outcomes after transient neonatal stroke. Neurobiol Dis. 2016;93:57–63. doi: 10.1016/j.nbd.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao YZ, Lin M, Lin Q, Yang W, Yu XC, Tian FR, Mao KL, Yang JJ, Lu CT, Wong HL. Intranasal delivery of bFGF with nanoliposomes enhances in vivo neuroprotection and neural injury recovery in a rodent stroke model. J Control Release. 2016;224:165–175. doi: 10.1016/j.jconrel.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Harrell CR, Fellabaum C, Arsenijevic A, Markovic BS, Djonov V, Volarevic V. Therapeutic potential of mesenchymal stem cells and their secretome in the treatment of glaucoma. Stem Cells Int. 2019;2019(7869130) doi: 10.1155/2019/7869130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minhas G, Prabhakar S, Morishita R, Shimamura M, Bansal R, Anand A. Transplantation of lineage-negative stem cells in pterygopalatine artery ligation induced retinal ischemia-reperfusion injury in mice. Mol Cell Biochem. 2017;429:123–136. doi: 10.1007/s11010-017-2941-0. [DOI] [PubMed] [Google Scholar]
- 20.Cervia D, Catalani E, Casini G. Neuroprotective peptides in retinal disease. J Clin Med. 2019;8(1146) doi: 10.3390/jcm8081146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Q, Shen Y, Su L, Xu X. Inhibition of pathological retinal neovascularization by a small peptide derived from human tissue-type plasminogen kringle 2. Front Pharmacol. 2020;10(1639) doi: 10.3389/fphar.2019.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu T, Cheng L, Wang H, Zhu S, Yang X, Liu K, Jin H, Xu X. KS23, a novel peptide derived from adiponectin, inhibits retinal inflammation and downregulates the proportions of Th1 and Th17 cells during experimental autoimmune uveitis. J Neuroinflammation. 2019;16(278) doi: 10.1186/s12974-019-1686-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong S, Xu Y, Ma M, Wang H, Wei F, Gu Q, Xu X. Neuroprotective effects of a novel peptide, FK18, under oxygen-glucose deprivation in SH-SY5Y cells and retinal ischemia in rats via the Akt pathway. Neurochem Int. 2017;108:78–90. doi: 10.1016/j.neuint.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Fernández-Sánchez MT, Novelli A. Basic fibroblast growth factor protects cerebellar neurons in primary culture from NMDA and non-NMDA receptor mediated neurotoxicity. FEBS Lett. 1993;335:124–131. doi: 10.1016/0014-5793(93)80453-2. [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Qiu J, Hirt L, Dalkara T, Moskowitz MA. Synergistic protective effect of caspase inhibitors and bFGF against brain injury induced by transient focal ischaemia. Br J Pharmacol. 2001;133:345–350. doi: 10.1038/sj.bjp.0704075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Driscoll C, O'Connor J, O'Brien CJ, Cotter TG. Basic fibroblast growth factor-induced protection from light damage in the mouse retina in vivo. J Neurochem. 2008;105:524–536. doi: 10.1111/j.1471-4159.2007.05189.x. [DOI] [PubMed] [Google Scholar]
- 27.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 28.Taveira M, Sousa C, Valentão P, Ferreres F, Teixeira JP, Andrade PB. Neuroprotective effect of steroidal alkaloids on glutamate-induced toxicity by preserving mitochondrial membrane potential and reducing oxidative stress. J Steroid Biochem Mol Biol. 2014;140:106–115. doi: 10.1016/j.jsbmb.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Ito S, Ménard M, Atkinson T, Brown L, Whitfield J, Chakravarthy B. Relative expression of the p75 neurotrophin receptor, tyrosine receptor kinase A, and insulin receptor in SH-SY5Y neuroblastoma cells and hippocampi from Alzheimer's disease patients. Neurochem Int. 2016;101:22–29. doi: 10.1016/j.neuint.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Ma H, DaSilva NA, Rose KN, Johnson SL, Zhang L, Wan C, Dain JA, Seeram NP. Development of a neuroprotective potential algorithm for medicinal plants. Neurochem Int. 2016;100:164–177. doi: 10.1016/j.neuint.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao M, Zhang WC, Liu QS, Hu JJ, Liu GT, Du GH. Pinocembrin prevents glutamate-induced apoptosis in SH-SY5Y neuronal cells via decrease of bax/bcl-2 ratio. Eur J Pharmacol. 2008;591:73–79. doi: 10.1016/j.ejphar.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y, Li J, Liu P, Chen X, Guo DH, Li QS, Rahman K. Protection of SH-SY5Y neuronal cells from glutamate-induced apoptosis by 3,6'-disinapoyl sucrose, a bioactive compound isolated from Radix Polygala. J Biomed Biotechnol. 2012;2012:1–5. doi: 10.1155/2012/728342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu MF, Xiong YY, Liu JK, Qian JJ, Zhu L, Gao J. Asiatic acid, a pentacyclic triterpene in Centella asiatica, attenuates glutamate-induced cognitive deficits in mice and apoptosis in SH-SY5Y cells. Acta Pharmacol Sin. 2012;33:578–587. doi: 10.1038/aps.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santos-Carvalho A, Elvas F, Alvaro AR, Ambrósio AF, Cavadas C. Neuropeptide Y receptors activation protects rat retinal neural cells against necrotic and apoptotic cell death induced by glutamate. Cell Death Dis. 2013;4(e636) doi: 10.1038/cddis.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahrens VM, Bellmann-Sickert K, Beck-Sickinger AG. Peptides and peptide conjugates: Therapeutics on the upward path. Future Med Chem. 2012;4:1567–1586. doi: 10.4155/fmc.12.76. [DOI] [PubMed] [Google Scholar]
- 36.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT - a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Lim JY, Park SI, Oh JH, Kim SM, Jeong CH, Jun JA, Lee KS, Oh W, Lee JK, Jeun SS. Brain-derived neurotrophic factor stimulates the neural differentiation of human umbilical cord blood-derived mesenchymal stem cells and survival of differentiated cells through MAPK/ERK and PI3K/Akt-dependent signaling pathways. J Neurosci Res. 2008;86:2168–2178. doi: 10.1002/jnr.21669. [DOI] [PubMed] [Google Scholar]
- 40.Luo C, Huang Q, Yuan X, Yang Y, Wang B, Huang Z, Tang L, Sun H. Abdominal paracentesis drainage attenuates severe acute pancreatitis by enhancing cell apoptosis via PI3K/AKT signaling pathway. Apoptosis. 2020;25:290–303. doi: 10.1007/s10495-020-01597-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Fukunaga K, Miyamoto E. Role of MAP kinase in neurons. Mol Neurobiol. 1998;16:79–95. doi: 10.1007/BF02740604. [DOI] [PubMed] [Google Scholar]
- 43.Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, Klann E, Angiolieri MR, Johnson JW, et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000;275:12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- 44.Chu CT, Levinthal DJ, Kulich SM, Chalovich EM, DeFranco DB. Oxidative neuronal injury. The dark side of ERK1/2. Eur J Biochem. 2004;271:2060–2066. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramaniam S, Zirrgiebel U, von Bohlen Und Halbach O, Strelau J, Laliberté C, Kaplan DR, Unsicker K. ERK activation promotes neuronal degeneration predominantly through plasma membrane damage and independently of caspase-3. J Cell Biol. 2004;165:357–369. doi: 10.1083/jcb.200403028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolch W. Meaningful relationships: The regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351:289–305. [PMC free article] [PubMed] [Google Scholar]
- 47.Wennström S, Downward J. Role of phosphoinositide 3-kinase in activation of ras and mitogen-activated protein kinase by epidermal growth factor. Mol Cell Biol. 1999;19:4279–4288. doi: 10.1128/mcb.19.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rommel C, Clarke BA, Zimmermann S, Nuñez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.