Abstract
Background
Previous studies reported that compared with percutaneous coronary interventions (PCIs), coronary artery bypass grafting (CABG) is associated with a reduced risk of mortality and repeat revascularization in patients with mild to moderate chronic kidney disease (CKD) and end-stage renal disease (ESRD). Information about outcomes associated with CABG versus PCI in patients with advanced stages of CKD is limited. We evaluated the incidence and relative risk of acute kidney injury (AKI) associated with CABG versus PCI in patients with advanced CKD.
Methods
We examined 730 US veterans with incident ESRD who underwent a first CABG or PCI up to 5 years prior to dialysis initiation. The association of CABG versus PCI with AKI was examined in multivariable adjusted logistic regression analyses.
Results
A total of 466 patients underwent CABG and 264 patients underwent PCI. The mean age was 64 ± 8 years, 99% were male, 20% were African American and 84% were diabetic. The incidence of AKI in the CABG versus PCI group was 67% versus 31%, respectively (P < 0.001). The incidence of all stages of AKI were higher after CABG compared with PCI. CABG was associated with a 4.5-fold higher crude risk of AKI {odds ratio [OR] 4.53 [95% confidence interval (CI) 3.28–6.27]; P < 0.001}, which remained significant after multivariable adjustments [OR 3.50 (95% CI 2.03–6.02); P < 0.001].
Conclusion
CABG was associated with a 4.5-fold higher risk of AKI compared with PCI in patients with advanced CKD. Despite other benefits of CABG over PCI, the extremely high risk of AKI associated with CABG should be considered in this vulnerable population when deciding on the optimal revascularization strategy.
Keywords: acute kidney injury, chronic kidney disease, coronary artery bypass grafting, percutaneous coronary interventions
ADDITIONAL CONTENT
An author video to accompany this article is available at: https://academic.oup.com/ndt/pages/author_videos.
INTRODUCTION
Cardiovascular diseases (CVDs) are a dominant cause of mortality globally, representing 31.5% of all deaths [1, 2]. The relative risk of all-cause and cardiovascular mortality is 5–30 times higher in patients with advanced chronic kidney disease (CKD) compared with the general population [3]. Patients with CVD and advanced CKD are 5–10 times more likely to die prior to transition to end-stage renal disease (ESRD) [4]. Surgical or nonsurgical methods of coronary artery revascularization have significantly decreased cardiovascular mortality over the last decades, but there is a lack of consensus on the optimal coronary revascularization strategy for patients with advanced CKD and acute coronary syndrome (ACS) [5].
Coronary artery bypass grafting (CABG) and percutaneous coronary interventions (PCIs) are the most often used and compared methods of coronary artery revascularization [6–8]. Recent American College of Cardiology and American Association for Thoracic Surgery [6] guidelines recommended CABG, as opposed to PCI, in left main or equivalent and multivessel coronary artery diseases as an optimal revascularization strategy, although debates continue regarding the optimal revascularization strategy in vulnerable patients such as those with CKD [6]. Previous studies reported that compared with PCI, CABG is associated with a reduced risk of mortality and repeat revascularization in patients with mild to moderate CKD, ESRD and diabetics [7–9]. However, most of the studies have not addressed short-term complications such as acute kidney injury (AKI) in CKD patients.
AKI is a common complication after both CABG and PCI [10, 11]. AKI is associated with high in-hospital and long-term mortality and with progression to ESRD [12–15]. The incidence of AKI has been found to be higher after CABG compared with PCI in patients with normal kidney function and with mild and moderate CKD [16, 17]. However, the relative risk of AKI associated with CABG versus PCI in patients with advanced CKD (who may be more susceptible to radiocontrast-associated AKI) is unclear. Considering this knowledge gap, we examined the incidence and relative risk of AKI associated with CABG versus PCI in advanced CKD patients using a large nationally representative cohort of US veterans with incident ESRD who underwent a first CABG or PCI up to 5 years prior to dialysis initiation. We hypothesized that CABG is associated with a higher incidence and increased relative risk of AKI compared with PCI in patients with advanced CKD.
MATERIALS AND METHODS
Study population
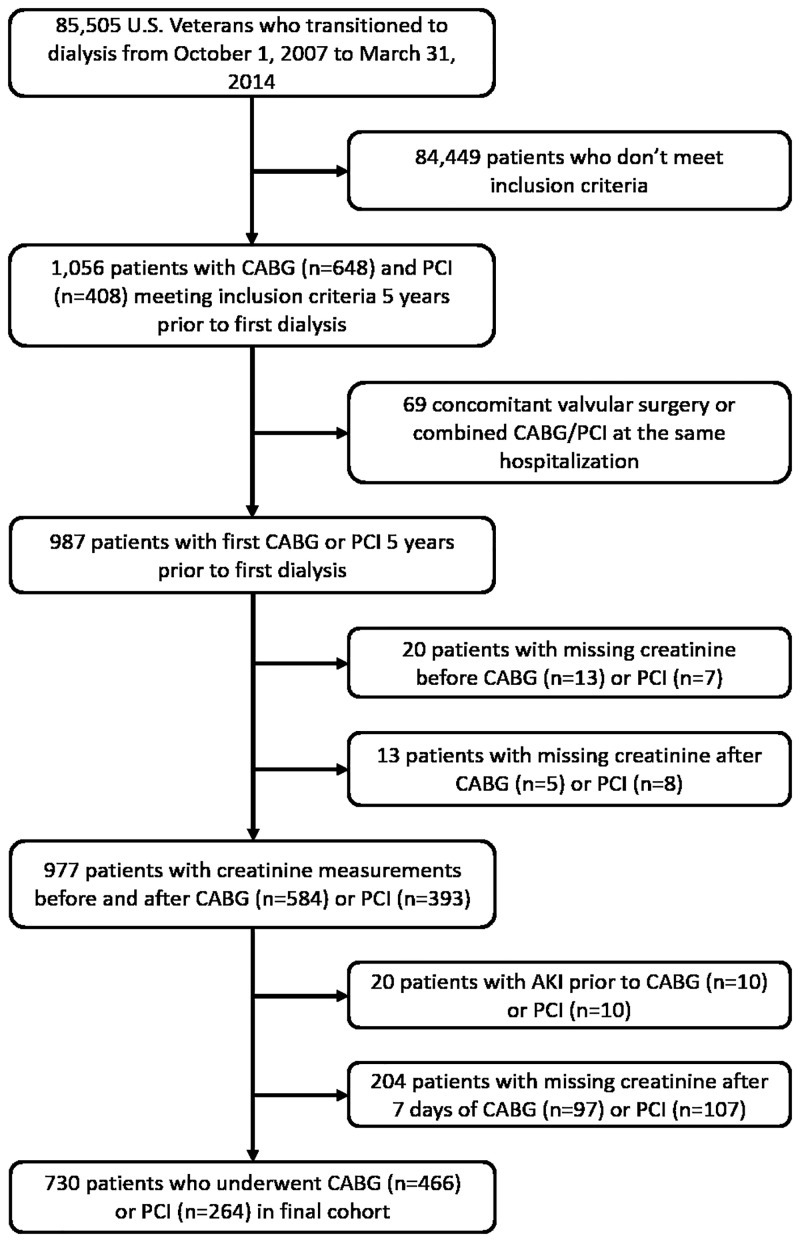
We studied longitudinal data from the Transition of Care in CKD (TC-CKD) study, a historical cohort study examining US veterans with incident ESRD transitioning to dialysis from 1 October 2007 through 31 March 2014 [18]. A total of 85 505 US veterans were identified from the US Renal Data System (USRDS) [19] as a source population. The algorithm for the cohort definition is shown in Figure 1. For the present study, 1056 patients who underwent PCI or CABG up to 5 years prior to ESRD (defined as the date of first maintenance dialysis service) were included. Patients receiving both CABG and PCI during the same hospitalization and patients undergoing concomitant ventricular reconstruction or pericardial or valve surgery were excluded. Patients with pre-procedural AKI (defined as a >25% increase in serum creatinine at the last measurement before the procedure compared with the one before the last measurement) and with missing creatinine measurements prior to and after coronary artery revascularization (CABG or PCI) were excluded as well. The final study population consisted of 730 patients, of whom 466 underwent CABG and 264 underwent PCI.
FIGURE 1.
Flow chart of the study population.
Definition of post-coronary artery revascularization AKI
Post-coronary artery revascularization AKI was classified according to Kidney Disease: Improving Global Outcomes (KDIGO) creatinine-based criteria from the date of CABG or PCI, identifying AKI as an increase in serum creatinine of ≥0.3 mg/dL from baseline within 48 h or ≥50% within 7 days [20]. Stage 1 was classified as a creatinine increase of 0.3 mg/dL over 48 h or a 50–99% increase within 7 days; Stage 2, a 100–200% increase within 7 days; and Stage 3, a ≥200% increase. Baseline serum creatinine was defined as the most recent outpatient or inpatient serum creatinine measurement prior to CABG or PCI during the last 365 days.
Exposures and covariates
CABG surgery and PCI procedure types were determined from International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) procedure codes and Current Procedural Terminology (CPT) procedure codes in the Veterans Affairs (VA) Inpatient or Outpatient Medical SAS datasets and categorized according to the Clinical Classifications Software procedural classification system (Supplementary data, Table S1). Based on CPT and ICD-9 secondary codes, revascularizations were stratified as single- or multivessel procedures. Information about baseline age, race, sex, marital status, per capita income and body mass index (BMI) were obtained from national VA research data files, as previously described [21]. Information about comorbidities (diabetes, hypertension, ischemic heart disease, myocardial infraction, cerebrovascular disease, congestive heart failure, peripheral vascular disease, atrial fibrillation, chronic pulmonary disease, connective tissue disease, paraplegia and hemiplegia, hyperlipidemia, liver disease, peptic ulcer disease, depression, dementia, malignancy and anemia) within 6 months prior to the studied coronary artery revascularization procedure was extracted from the VA Inpatient and Outpatient Medical SAS datasets and from the Centers for Medicare & Medicaid Services (CMS) datasets using diagnostic and procedure codes [22]. The Charlson Comorbidity Index score was calculated using the Deyo modification for administrative datasets, without including kidney disease [23]. Medication use [angiotensin-converting enzyme inhibitors (ACEIs) angiotensin receptor blockers (ARBs), beta-blockers, alpha-blockers, statins, calcium channel blockers (CCBs), thiazide diuretics, loop diuretics, potassium-sparing diuretics, anticoagulants, aspirin, digitalis, antianginals, vasodilators and antidiabetic agents] was determined from VA pharmacy dispensation records in the 6 months prior to coronary artery revascularization [24].
Blood hemoglobin and serum albumin levels were obtained from VA research databases as previously described [21] and their baseline values were defined as the average of each covariate during the 6-month period preceding CABG or PCI. Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration equation [25].
Statistical analysis
Data are summarized as percentages for categorical variables and as mean ± standard deviation (SD) or median (interquartile range), as appropriate. Categorical variables were compared using χ2 tests. Continuous variables were compared using t tests, Mann–Whitney U tests or analysis of variance, as appropriate. The odds of AKI overall and staged by severity in patients undergoing CABG versus PCI were determined using unadjusted and adjusted logistic and ordinal logistic regression models, respectively. Models were incrementally adjusted for the following potential confounders based on the theoretical considerations and their availability in this study: Model 1: adjusted for demographics (age, sex, race/ethnicity, marital status and income); Model 2: additionally adjusted for comorbidities (diabetes, malignancy, liver diseases, hypertension, ischemic heart disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, anemia, atrial fibrillation, depression, hyperlipidemia) and BMI; Model 3: additionally adjusted for medications (anticoagulants, aspirin, digitalis, beta-blockers, alpha-blockers, CCBs, antianginals, statins, vasodilators, thiazide diuretics, loop diuretics, potassium-sparing diuretics, ACEIs/ARBs and antidiabetic agents), procedure type (single versus multivessel) and Model 4: additionally adjusted for baseline blood hemoglobin, serum albumin, eGFR and systolic and diastolic blood pressure.
In all, 689 (94%) patients had complete data for analysis in Model 3. In Model 4 an additional 287 cases (39% of cohort) had missing variables. Therefore we regarded Model 3 as our main multivariable adjusted model and Model 4 was performed as a sensitivity analysis using multiple imputations to account for missingness [18]. All covariates were tested for multicollinearity; the highest variance inflation factor (VIF) was 2.79 (mean VIF = 1.39).
The associations of AKI (both overall and staged by severity) with revascularization type were also examined in subgroups of patients stratified by age (<65 or ≥65 years), race (African American or others), baseline eGFR (<30 or ≥30 mL/min/1.73 m2), BMI (<25 or ≥25 kg/m2) and type of intervention (single or multivessel). Potential interactions were formally tested by including relevant interaction terms.
P-values are two-sided and reported as significant at <0.05 for all analyses. All analyses were conducted using STATA MP version 15 (StataCorp, College Station, TX, USA). The study was approved by the institutional review boards of the Memphis and Long Beach VA Medical Centers, with exemption from informed consent.
RESULTS
Patients’ baseline characteristics in the overall cohort and stratified by type of coronary artery revascularization are presented in Table 1. The overall mean ± SD age at baseline was 64 ± 8 years, 99% were male, 20% were African American and 84% were diabetic. Compared with patients who underwent CABG, those who underwent PCI had a higher prevalence of hypertension, chronic pulmonary disease, atrial fibrillation, liver disease and dementia and had a higher Charlson Comorbidity Index. The baseline eGFR was similar in the PCI and CABG groups, but patients who underwent CABG had significantly lower levels of blood hemoglobin and serum albumin. BMI and systolic and diastolic blood pressures did not differ between the PCI and CABG groups. In the PCI and CABG groups, 85% of patients received single-vessel treatments and 89% of patients received multivessel treatments, respectively (P < 0.001).
Table 1.
Baseline characteristics of patients
| All (N = 730) | PCI (n = 264) | CABG (n = 466) | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years), mean ± SD | 64 ± 8 | 64 ± 9 | 64 ± 8 | 0.156 |
| Gender (male), n (%) | 724 (99) | 262 (99) | 462 (99) | 0.885 |
| Race, n (%) | 0.860 | |||
| White | 537 (73) | 196 (74) | 341 (73) | |
| African American | 145 (20) | 55 (21) | 90 (19) | |
| Others | 13 (2) | 4 (2) | 9 (2) | |
| Unknown | 35 (5) | 9 (3) | 26 (6) | |
| Ethnicity (Hispanic), n (%) | 55 (8) | 11 (4) | 44 (9) | 0.009 |
| Marital status, n (%) | 0.001 | |||
| Married | 350 (48) | 149 (56) | 201 (43) | |
| Single | 68 (9) | 13 (5) | 55 (12) | |
| Divorced | 244 (34) | 76 (29) | 168 (36) | |
| Widowed | 68 (9) | 26 (10) | 42 (9) | |
| Income (US$), median (IQR) | 20 044 (10 812–34 992) | 27 109 (11 910–36 876) | 17 702 (10 024–33 228) | 0.093 |
| Comorbidities, n (%) | ||||
| Diabetes | 616 (84) | 219 (83) | 397 (85) | 0.423 |
| Hypertension | 693 (95) | 258 (98) | 435 (93) | 0.010 |
| Ischemic heart disease | 659 (90) | 231 (88) | 428 (92) | 0.057 |
| Myocardial infraction | 327 (45) | 124 (47) | 203 (44) | 0.374 |
| Cerebrovascular disease | 269 (37) | 94 (36) | 175 (38) | 0.600 |
| Congestive heart failure | 370 (51) | 145 (55) | 225 (48) | 0.085 |
| Peripheral vascular disease | 331 (45) | 120 (45) | 211 (45) | 0.963 |
| Atrial fibrillation | 53 (7) | 26 (10) | 27 (6) | 0.043 |
| Chronic pulmonary disease | 321 (44) | 133 (50) | 188 (40) | 0.009 |
| Connective tissue disease | 30 (4) | 15 (6) | 15 (3) | 0.107 |
| Paraplegia and hemiplegia | 32 (4) | 14 (5) | 18 (4) | 0.361 |
| Hyperlipidemia | 619 (85) | 228 (86) | 391 (84) | 0.374 |
| Liver disease | 84 (12) | 42 (16) | 42 (9) | 0.005 |
| Peptic ulcer disease | 46 (6) | 21 (8) | 25 (5) | 0.166 |
| Depression | 226 (31) | 88 (33) | 138 (30) | 0.296 |
| Dementia | 6 (1) | 6 (2) | 0 | 0.001 |
| Malignancy | 120 (16) | 51 (19) | 69 (15) | 0.114 |
| Anemia | 252 (35) | 96 (36) | 156 (33) | 0.430 |
| Charlson Comorbidity Index, median (IQR) | 4 (3-6) | 5 (3-6) | 4 (3-6) | 0.022 |
| Vital parameters, mean ± SD | ||||
| Body mass index (kg/m2) | 30 ± 6 | 30 ± 6 | 30 ± 5 | 0.099 |
| Systolic BP (mmHg) | 143 ± 17 | 143 ± 18 | 143 ± 16 | 0.850 |
| Diastolic BP (mmHg) | 75 ± 10 | 75 ± 11 | 75 ± 10 | 0.891 |
| Laboratory parameters, mean ± SD | ||||
| Blood hemoglobin (g/dL) | 11.5 ± 1.6 | 11.9 ± 1.8 | 11.3 ± 1.4 | <0.001 |
| Serum albumin (g/dL) | 3.4 ± 0.6 | 3.5 ± 0.6 | 3.3 ± 0.5 | <0.001 |
| eGFR (mL/min/1.73 m2), median (IQR) | 34 (22–53) | 33 (22–51) | 35 (23–54) | 0.541 |
| Medications, n (%) | ||||
| ACEIs/ARBs | 575 (79) | 201 (76) | 374 (80) | 0.191 |
| Beta-blockers | 675 (92) | 238 (90) | 437 (94) | 0.075 |
| Alpha-blockers | 210 (29) | 90 (34) | 120 (26) | 0.017 |
| CCBs | 485 (66) | 166 (63) | 319 (68) | 0.125 |
| Statins | 655 (90) | 229 (87) | 426 (91) | 0.046 |
| Thiazide diuretics | 221 (30) | 77 (29) | 144 (31) | 0.624 |
| Loop diuretics | 481 (66) | 176 (67) | 305 (65) | 0.739 |
| Potassium-sparing diuretics | 65 (9) | 28 (11) | 37 (8) | 0.224 |
| Anticoagulants | 329 (45) | 115 (44) | 214 (46) | 0.538 |
| Aspirin | 606 (83) | 205 (78) | 401 (86) | 0.004 |
| Digitalis | 37 (5) | 18 (7) | 19 (4) | 0.052 |
| Antianginals | 523 (72) | 163 (62) | 360 (77) | <0.001 |
| Vasodilatators | 269 (37) | 99 (37) | 170 (36) | 0.784 |
| Antidiabetics | 576 (79) | 162 (61) | 414 (89) | <0.001 |
| Treated vessels, n (%) | <0.001 | |||
| Single vessel | 278 (38) | 225 (85) | 53 (11) | |
| Multivessel | 452 (62) | 39 (15) | 413 (89) | |
| Outcome | ||||
| AKI (any stage), n (%) | 392 (54) | 81 (31) | 311 (67) | <0.001 |
BP, blood pressure; IQR, interquartile range.
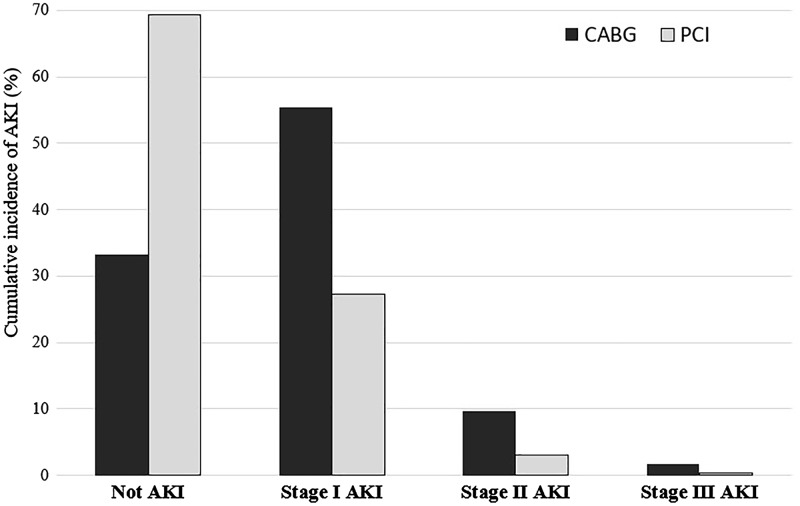
The overall incidence of AKI was higher in the CABG group compared with the PCI group (67% versus 31%; P < 0.001) (Table 1). The incidences of Stage 1, 2 and 3 AKI were also higher in the CABG group compared with the PCI group (55% versus 27%, 10% versus 3% and 2% versus 0.4%, respectively; P < 0.001) (Figure 2).
FIGURE 2.
Cumulative incidence of AKI stratified by type of revascularization (CABG versus PCI).
Unadjusted and adjusted odds ratios (ORs) and 95% confidence interval (CIs) of overall AKI associated with CABG versus PCI are presented in Table 2. CABG was associated with a 4.5-fold higher crude risk of AKI [OR 4.53 (95% CI 3.28–6.27)]. Upon further multivariable adjustments, CABG was associated with 3.5- to 4.3-fold higher risk of AKI in various models. Results were similar when modeling AKI by stages in ordinal logistic regression models (Table 2) and after multiple imputations for Model 4 [OR 3.87 (95% CI 2.19–6.84)].
Table 2.
Association between the presence of overall AKI and the severity of AKI with the type of revascularization [CABG versus PCI (reference)] using logistic and ordinal logistic regression models
| Models | Overall AKI, OR (95% CI) | P-value | Severity of AKI, OR (95% CI) | P-value |
|---|---|---|---|---|
| Unadjusted (n = 730) | 4.53 (3.28–6.27) | <0.001 | 4.46 (3.24–6.14) | <0.001 |
| Model 1 (n = 695) | 4.27 (3.04–5.99) | <0.001 | 4.08 (2.93–5.69) | <0.001 |
| Model 2 (n = 689) | 4.08 (2.86–5.83) | <0.001 | 3.94 (2.79–5.58) | <0.001 |
| Model 3 (n = 689) | 3.50 (2.03–6.02) | <0.001 | 3.10 (1.85–5.17) | <0.001 |
Models are as follows: Unadjusted model: only exposure variable included. Model 1 adjusted for age, sex, race/ethnicity, marital status and income. Model 2 additionally adjusted for comorbidities (diabetes, malignancy, liver diseases, hypertension, ischemic heart disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, anemia, atrial fibrillation, depression, hyperlipidemia) and BMI. Model 3 additionally adjusted for medications (anticoagulants, aspirin, digitalis, beta-blockers, alpha-blockers, CCBs, antianginals, statins, vasodilatators, thiazide diuretics, loop diuretics, potassium-sparing diuretics, ACEIs/ARBs and antidiabetics) and vessels.
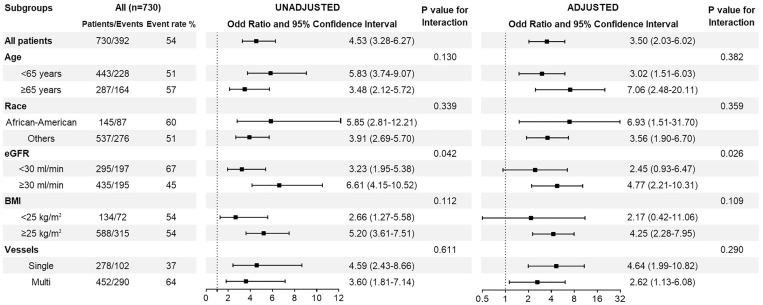
In subgroup analyses, compared with PCI, patients who underwent CABG had a higher relative risk of AKI across all subgroups (Figure 3 and Supplementary data, Figure S1). A statistically significant interaction was present for eGFR, with stronger associations between CABG and AKI risk among patients with eGFR ≥30 mL/min/1.73 m2 versus eGFR <30 mL/min/1.73 m2.
FIGURE 3.
Association between the presence of AKI and type of revascularization [CABG versus PCI (reference)] using unadjusted and adjusted logistic regression models in selected subgroups.
DISCUSSION
In this large, nationally representative cohort of US veterans with advanced CKD, we found that CABG was associated with a higher incidence and 4.5-fold increased relative risk of AKI compared with PCI. This association remained present after adjustment for potential confounders such as demographics, comorbidities and medications and in selected subgroups stratified by age, race, baseline eGFR, BMI and number of treated vessels.
Several studies report an AKI incidence ranging from 1% to 17% following PCI [26–28] and up to 36% after CABG [29–31]. There are few clinical trials [9, 32] and prospective studies [33] examining the incidence of AKI after PCI compared with CABG populations with multivessel coronary artery disease. In the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREDOM) trial, where PCI and CABG (n = 953 and 947) were compared to define the better revascularization strategy in diabetic patients with multivessel coronary artery disease, the incidence of AKI was 0.1% versus 0.8% (P = 0.02) [9], whereas in the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial the incidence of AKI was 14.2% after PCI (n = 4412) compared with 31.7% after CABG (n = 1215) in patients with multivessel coronary artery disease at high risk for ACS [32]. However, these studies did not examine CKD patients. Patients with multiple comorbidities and those with advanced CKD are often excluded from clinical trials or cardiovascular interventional studies because of a higher risk of complications and adverse events, making retrospective studies the sole source of information. Large retrospective studies comparing AKI following CABG versus PCI reported 1.6-, 2.6- and 2-fold higher risks of AKI after CABG compared with PCI [16, 17], and a higher incidence of AKI in patients with CKD compared with patients with normal kidney function, with an incrementally higher frequency of AKI in patients with more advanced stages of CKD [16, 17]. Contrasting these findings, another VA-based study [14] described a relatively higher incidence of AKI after cardiac surgery in patients with baseline eGFR >45 mL/min/1.73 m2 compared with baseline eGFR <45 mL/min/1.73 m2. One potential explanation for this observation is that patients with a lower eGFR may receive heightened scrutiny and may receive targeted interventions and optimized medical management to mitigate the risk of AKI. Similar mechanisms may explain the finding in our study that the risk of AKI associated with CABG versus PCI was relatively higher in patients with higher baseline eGFR and single-vessel disease.
The main results of our study are comparable with the results of other large cohort studies [16, 17] in terms of the higher risk of AKI after CABG compared with PCI. However, we examined patients with moderate and advanced CKD, whereas the previous cohort studies included variable proportions of patients with mild to moderate CKD. Furthermore, we used changes in serum creatinine to define AKI as opposed to ICD-9-based definitions in previous large cohorts.
Most of the studies comparing outcomes of CABG versus PCI in CKD and ESRD have examined patients with multivessel coronary artery diseases, demonstrating a reduced risk of long-term mortality and repeat revascularizations with CABG [7–9, 34]. There is a lack of knowledge regarding the ideal intervention strategy in patients with advanced CKD who have single-vessel coronary artery disease. In the general population with single-vessel coronary artery disease, PCI is considered the optimal revascularization strategy [6], but the risk:benefit ratio may be altered in patients with advanced CKD, who have increased risk of bleeding, fluid overload, AKI, infections and other complications [35]. Various risk scores can aid in decision making about the optimal revascularization strategy in an individual patient [36–38], but these scores have not been validated for patients with advanced CKD. In our study, CABG was associated with a higher risk of AKI compared with PCI in all subgroups.
Recent guidelines recommend using CABG as opposed to PCI in patients with severe coronary disease or other poor prognostic indicators (e.g. diabetes mellitus, reduced ejection fraction or mechanical complications) [6, 39]. However, certain comorbid conditions may represent relative contraindications for CABG and may lead to choosing PCI as the preferred intervention. In our cohort, patients who underwent PCI (compared with CABG) had a significantly higher prevalence of hypertension, chronic pulmonary disease, atrial fibrillation, liver disease and dementia and had a higher Charlson Comorbidity Index, emphasizing that these patients with advanced CKD were sicker than patients who underwent CABG. Despite this, patients who underwent CABG had a higher incidence and relative risk of AKI compared with patients who underwent PCI.
Several potential explanations have been suggested for the underlying mechanisms of AKI after coronary artery revascularization. Cardiac surgery is one of the most important risk factors for postoperative AKI (up to 30% incidence) compared with other types of surgery [40, 41]. There are several preoperative, intraoperative and postoperative factors increasing the risk of AKI after cardiac surgery, including hemodynamic instability, fluid overload, the length of cardiopulmonary bypass, rewarming after mild hypothermia, hemoglobinemia and lipid peroxidation [42–45]. In patients with PCI, radiocontrast (including the type of contrast and the administered volume) and cholesterol embolism are the most important risk factors [46]. All of these risk factors may be exacerbated in patients with more advanced CKD; the fact that in our study the risk of AKI associated with CABG versus PCI was mitigated (albeit still significantly higher) in the subgroup with CKD Stage 4 and above suggests that the risks associated with PCI are magnified to a larger extent by advancing CKD, which could be considered when making individualized decisions.
Potential strategies to mitigate the risk of AKI following coronary revascularization include operative interventions that minimize the duration of surgery, hypotensive episodes and aortic cross-clamp time for CABG, lower radiocontrast volume and a transradial cannulation approach for PCI [47]. Off-pump CABG has been suggested as a potential strategy to improve postoperative outcomes in CABG, but the effects of it on outcomes in patients with advanced CKD are controversial [48–51]. The decision about the optimal revascularization strategy in patients with advanced CKD should consider both long-term and short-term complications. While there is compelling evidence that CABG is associated with a higher risk of AKI compared with PCI (including the results of our present study), previous studies have suggested that CABG results in better long-term outcomes, such as lower risk of mortality and repeat revascularizations [7–9, 34], although information about this in patients with advanced CKD is also scarce. Decisions about the optimal revascularization strategy need to balance short-term and long-term risks and benefits of CABG versus PCI; short of randomized controlled trials informing about the best strategy in patients with advanced CKD, such decisions need to be individualized using data from available observational studies and extrapolations from randomized controlled trials performed in patients with normal kidney function.
The strengths of this study include its large size, its nationally representative nature, the examination of patients with advanced CKD and the rigorous assessment of AKI using detailed laboratory data. Our study also has limitations that deserve to be mentioned. First, because this was a retrospective observational study, we were unable to collect information on the severity and complexity of coronary artery lesions and acute or elective indications, which were used to decide the type of revascularization. Second, most of our patients were male US veterans, hence the results may not be generalizable to women or other patient populations, in particular those outside the USA. Third, due to the observational nature of our study, adjusted analyses were limited to preprocedural (preoperative) confounders measured and available in our cohort, and therefore our study may be limited by potential residual confounding from unmeasured confounders such as contrast type and volume, baseline left ventricle ejection fraction and proteinuria, as well as intraoperative and postoperative risk factors such as on-pump or off-pump open heart surgery, type and dosage of inotropic support, management of fluid-volume balance and complications. Differences in outcomes may be affected by indication bias, as the decision to perform CABG versus PCI may have been affected by risk factors of AKI in individual patients; while we adjusted for many such risk factors (e.g. baseline eGFR and comorbid conditions), there may have been unmeasured ones that could have affected the outcome of our study. Finally, our data were derived from a cohort of incident ESRD patients, hence we could not include information from patients with advanced CKD who died before dialysis start.
In conclusion, CABG was associated with a 4.5-fold higher risk of AKI compared with PCI in patients with advanced CKD, which remained present after adjustment for potential confounders. Despite other benefits of CABG over PCI, the extremely high risk of AKI associated with CABG should be considered in this vulnerable population when deciding on the optimal revascularization strategy.
Supplementary Material
ACKNOWLEDGEMENTS
C.P.K. and K.K.Z. are employees of the Department of Veterans affairs. Opinions expressed in this article are those of the authors and do not necessarily represent the opinion of the Department of Veterans Affairs. The results of this article have not been published previously in whole or part.
FUNDING
A.G. was supported by the International Society of Nephrology (ISN) research fellowship program and this work has been made possible through an ISN-funded fellowship. This study is supported by grant 5U01DK102163 from the National Institute of Health to K.K.Z. and C.P.K. and by resources from the US Department of Veterans Affairs. The data reported here have been supplied in part by the USRDS. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (project numbers SDR 02-237 and 98-004).
AUTHORS’ CONTRIBUTIONS
A.G., M.Z.M., C.P.K. provided contributions to study concept and design. M.Z.M., P.K.P., E.S., K.K.Z., C.P.K. were responsible for the acquisition of data. A.G., M.Z.M., C.P.K. provided analysis and interpretation of the data. A.G., M.Z.M., C.P.K. drafted the article. A.G., M.Z.M., P.K.P., K.S., Z.S., O.A., E.S., C.M.R., S.K.G.K., R.B.C., K.K.Z. and C.P.K. were responsible for critical revision of the article for important intellectual content and approval of the final version. Each author contributed important intellectual content during article drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. C.P.K. and M.Z.M. take responsibility that this study has been reported honestly, accurately and transparently; that no important aspects of the study have been omitted and that any discrepancies from the study as planned (and registered, if relevant) have been explained. Funders of this study had no role in study design; collection, analysis and interpretation of data; writing the report or the decision to submit the report for publication.
CONFLICT OF INTEREST STATEMENT
None of the authors have relevant conflicts of interest.
REFERENCES
- 1. Roth GA, Huffman MD, Moran AE et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015; 132: 1667–1678 [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Blaha MJ, Chiuve SE et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017; 135: e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weiner DE, Tabatabai S, Tighiouart H et al. Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis 2006; 48: 392–401 [DOI] [PubMed] [Google Scholar]
- 4. Collins AJ, Li S, Gilbertson DT et al. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int 2003; 64(Suppl 87): S24–S31 [DOI] [PubMed] [Google Scholar]
- 5. Fox C, Muntner P, Chen A et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network Registry. Circulation 2010; 121: 357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel MR, Calhoon JH, Dehmer GJ et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology appropriate use criteria task force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol 2017; 69: 2212–2241 [DOI] [PubMed] [Google Scholar]
- 7. Weintraub WS, Grau-Sepulveda MV, Weiss JM et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012; 366: 1467–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Charytan DM, Desai M, Mathur M et al. Reduced risk of myocardial infarct and revascularization following coronary artery bypass grafting compared with percutaneous coronary intervention in patients with chronic kidney disease. Kidney Int 2016; 90: 411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farkouh ME, Domanski M, Sleeper LA et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367: 2375–2384 [DOI] [PubMed] [Google Scholar]
- 10. James MT, Ghali WA, Tonelli M et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int 2010; 78: 803–809 [DOI] [PubMed] [Google Scholar]
- 11. Mangano CM, Diamondstone LS, Ramsay JG et al. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. Ann Intern Med 1998; 128: 194–203 [DOI] [PubMed] [Google Scholar]
- 12. Hobson CE, Yavas S, Segal MS et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009; 119: 2444–2453 [DOI] [PubMed] [Google Scholar]
- 13. Dasta JF, Kane-Gill SL, Durtschi AJ et al. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008; 23: 1970–1974 [DOI] [PubMed] [Google Scholar]
- 14. Ishani A, Nelson D, Clothier B et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med 2011; 171: 226–233 [DOI] [PubMed] [Google Scholar]
- 15. Chawla LS, Amdur RL, Amodeo S et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 2011; 79: 1361–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang TI, Leong TK, Boothroyd DB et al. Acute kidney injury after CABG versus PCI: an observational study using 2 cohorts. J Am Coll Cardiol 2014; 64: 985–994 [DOI] [PubMed] [Google Scholar]
- 17. Shen W, Aguilar R, Montero AR et al. Acute kidney injury and in-hospital mortality after coronary artery bypass graft versus percutaneous coronary intervention: a nationwide study. Am J Nephrol 2017; 45: 217–225 [DOI] [PubMed] [Google Scholar]
- 18. Molnar MZ, Streja E, Sumida K et al. Pre-ESRD depression and post-ESRD mortality in patients with advanced CKD transitioning to dialysis. Clin J Am Soc Nephrol 2017; 12: 1428–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saran R, Robinson B, Abbott KC et al. US Renal Data System 2016 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2017; 69: A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care 2013; 17: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kovesdy CP, Norris KC, Boulware LE et al. Association of race with mortality and cardiovascular events in a large cohort of US veterans. Circulation 2015; 132: 1538–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Department of Veterans Affairs. VIReC Research User Guide; VHA Medical SAS Inpatient Datasets FY2006-2007. Hines, IL: VA Information Resource Center, 2007
- 23. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45: 613–619 [DOI] [PubMed] [Google Scholar]
- 24.VA Information Resource Center. VIReC Research User Guide: VHA Pharmacy Prescription Data, 2nd edn. Hines, IL: VA Information Resource Center, 2008 [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown JR, Solomon RJ, Robey RB et al. Chronic kidney disease progression and cardiovascular outcomes following cardiac catheterization—a population-controlled study. J Am Heart Assoc 2016; 5: e003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kooiman J, Seth M, Dixon S et al. Risk of acute kidney injury after percutaneous coronary interventions using radial versus femoral vascular access: insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. Circ Cardiovasc Interv 2014; 7: 190–198 [DOI] [PubMed] [Google Scholar]
- 28. Cortese B, Sciahbasi A, Sebik R et al. Comparison of risk of acute kidney injury after primary percutaneous coronary interventions with the transradial approach versus the transfemoral approach (from the PRIPITENA urban registry). Am J Cardiol 2014; 114: 820–825 [DOI] [PubMed] [Google Scholar]
- 29. Ryden L, Sartipy U, Evans M et al. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130: 2005–2011 [DOI] [PubMed] [Google Scholar]
- 30. Helgadottir S, Sigurdsson MI, Palsson R et al. Renal recovery and long-term survival following acute kidney injury after coronary artery surgery: a nationwide study. Acta Anaesthesiol Scand 2016; 60: 1230–1240 [DOI] [PubMed] [Google Scholar]
- 31. Nina VJ, Matias MM, Brito DJ et al. Acute kidney injury after coronary artery bypass grafting: assessment using RIFLE and AKIN criteria. Rev Bras Cir Cardiovasc 2013; 28: 231–237 [DOI] [PubMed] [Google Scholar]
- 32. Ben-Gal Y, Moses JW, Mehran R et al. Surgical versus percutaneous revascularization for multivessel disease in patients with acute coronary syndromes: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. JACC Cardiovasc Interv 2010; 3: 1059–1067 [DOI] [PubMed] [Google Scholar]
- 33. Mack MJ, Prince SL, Herbert M et al. Current clinical outcomes of percutaneous coronary intervention and coronary artery bypass grafting. Ann Thorac Surg 2008; 86: 496–503 [DOI] [PubMed] [Google Scholar]
- 34. Chang TI, Shilane D, Kazi DS et al. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol 2012; 23: 2042–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krishnan M. Preoperative care of patients with kidney disease. Am Fam Physician 2002; 66: 1471–1379 [PubMed] [Google Scholar]
- 36. Farooq V, van Klaveren D, Steyerberg EW et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet 2013; 381: 639–650 [DOI] [PubMed] [Google Scholar]
- 37. Tsai TT, Patel UD, Chang TI et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath-PCI Registry. J Am Heart Assoc 2014; 3: e001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nashef SA, Roques F, Sharples LD et al. EuroSCORE II. Eur J Cardiothorac Surg 2012; 41: 734–744; 744–735 [DOI] [PubMed] [Google Scholar]
- 39. Cutlip D, Levin T, Aroesty JM. Revascularization in patients with stable coronary artery disease: coronary artery bypass graft surgery versus percutaneous coronary intervention. In: Aldea G, Windecker S (eds). UpToDate. Waltham, MA: Wolters Kluwer [Google Scholar]
- 40. Grams ME, Sang Y, Coresh J et al. Acute kidney injury after major surgery: a retrospective analysis of veterans health administration data. Am J Kidney Dis 2016; 67: 872–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1: 19–32 [DOI] [PubMed] [Google Scholar]
- 42. O'Neal JB, Shaw AD, Billings FTt. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care 2016; 20: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lannemyr L, Lundin E, Reinsfelt B et al. Renal tubular injury during cardiopulmonary bypass as assessed by urinary release of N-acetyl-β-D-glucosaminidase. Acta Anaesthesiol Scand 2017; 61: 1075–1083 [DOI] [PubMed] [Google Scholar]
- 44. Boodhwani M, Rubens FD, Wozny D et al. Effects of mild hypothermia and rewarming on renal function after coronary artery bypass grafting. Ann Thorac Surg 2009; 87: 489–495 [DOI] [PubMed] [Google Scholar]
- 45. Newland RF, Baker RA, Mazzone AL et al. Rewarming temperature during cardiopulmonary bypass and acute kidney injury: a multicenter analysis. Ann Thorac Surg 2016; 101: 1655–1662 [DOI] [PubMed] [Google Scholar]
- 46. Alonso A, Lau J, Jaber BL et al. Prevention of radiocontrast nephropathy with N-acetylcysteine in patients with chronic kidney disease: a meta-analysis of randomized, controlled trials. Am J Kidney Dis 2004; 43: 1–9 [DOI] [PubMed] [Google Scholar]
- 47. Steinvil A, Garcia-Garcia HM, Rogers T et al. Comparison of propensity score-matched analysis of acute kidney injury after percutaneous coronary intervention with transradial versus transfemoral approaches. Am J Cardiol 2017; 119: 1507–1511 [DOI] [PubMed] [Google Scholar]
- 48. Seabra VF, Alobaidi S, Balk EM et al. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010; 5: 1734–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Di Mauro M, Gagliardi M, Iaco AL et al. Does off-pump coronary surgery reduce postoperative acute renal failure? The importance of preoperative renal function. Ann Thorac Surg 2007; 84: 1496–1502 [DOI] [PubMed] [Google Scholar]
- 50. Charytan DM, Yang SS, McGurk S et al. Long and short-term outcomes following coronary artery bypass grafting in patients with and without chronic kidney disease. Nephrol Dial Transplant 2010; 25: 3654–3663 [DOI] [PubMed] [Google Scholar]
- 51. Garg AX, Devereaux PJ, Yusuf S et al. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA 2014; 311: 2191–2198 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.