Abstract
Impaired function of regulatory T cells (Tregs) contributes to the pathogenesis of systemic lupus erythematosus (SLE). Our previous study demonstrated aberrant responses of T lymphocytes to endoplasmic reticulum (ER) stress in patients with SLE. The present study investigated whether ER stress inhibition by 4-phenylbutyric acid (4-PBA) ameliorated lupus manifestations in an experimental lupus model and the effect of ER stress inhibition on the frequency and function of Tregs. A murine lupus model was induced through a 4-week treatment with Resiquimod, a toll-like receptor (TLR) 7 agonist. From the 8th week, the mice were treated with 4-PBA for 4 weeks. 4-PBA significantly decreased the levels of anti-dsDNA antibodies and serum TNF-α. A significant decrease in glomerulonephritis score was also observed in the 4-PBA-treated group. ER stress inhibition decreased the activated T and B lymphocytes population of splenocytes; however, the population of Tregs was not significantly different between the vehicle and 4-PBA group. However, a markedly enhanced suppressive capacity of Treg was detected in the 4-PBA-treated group. The present results suggest that ER stress inhibition attenuated disease activity in an experimental model by improving the suppressive capacity of Tregs. Therefore, reduction of ER stress could be used as a beneficial therapeutic strategy in SLE.
Keywords: endoplasmic reticulum stress, systemic lupus erythematosus, regulatory T cell, lupus nephritis
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease with a wide spectrum of clinical manifestations and a prevalence of 20-50 persons per 100,000 population. The pathogenesis of SLE is related to a disruption of immune homeostasis, resulting from loss of self-tolerance, presence of autoantibodies and formation of immune complexes, and dysregulation of autoreactive lymphocytes, which are responsible for damage to various systemic organs (1,2). Especially, the failure of regulatory T cells (Treg)-mediated suppression is considered as a factor implicated in the loss of immune homeostasis (3,4). The elimination of Treg can lead to the development of lupus-like manifestations, including glomerulonephritis and production of autoantibodies, which indicates that failure of Treg-mediated suppression is implicated in the pathogenesis of SLE, even though the reports on the counts and function of Treg in patients with SLE have shown conflicting results (5,6).
In recent years, several studies have revealed the pathological role of endoplasmic reticulum (ER) stress in the autoimmune and inflammatory diseases (7-10). ER is the largest cellular organelle that is responsible for protein synthesis and folding, transportation and storage of calcium, and lipid synthesis (11-13). In the process of ER proteostasis, the proteins are supposed to be modified and folded correctly by the folding enzymes and chaperone. However, these elaborate processes are prone to be disrupted during diseased status, resulting in overwhelming protein folding demand over protein folding capacity (14). Consequently, the misfolded proteins are accumulated in the ER, which is known as ER stress, and it activates unfolded protein response (UPR) to acquire opportunities to correct misfolded proteins. However, if the UPR signaling pathway is affected, the cell apoptosis is initiated (15). We also have shown that aberrant UPRs were found in T lymphocytes of patients with SLE and that higher levels of apoptosis response to ER stress may contribute to the pathogenesis of SLE (16). Based on previous findings, we investigated whether ER stress inhibition can alleviate clinical manifestations in lupus and the effect of ER stress inhibition on the Treg. To address this issue, we used 4-phenylbutyric acid (4-PBA) to suppress the ER stress signaling. 4-PBA, a low molecular weight chemical chaperone, increases protein folding capacity of ER and consequently prevents accumulation of misfolded protein. Because of the action to mitigate ER stress, several researches have used 4-PBA for the purpose of amelioration of ER stress-related inflammatory diseases (17,18). In our experiments, we administrated 4-PBA intraperitoneally in an induced SLE murine model generated by application of toll-like receptor (TLR) 7 agonist. Herein, we report that ER stress inhibition ameliorates systemic autoimmunity and consequently improves symptoms in the murine lupus model by Treg modulation.
Materials and methods
Mice and in vivo treatment
BALB/C mice were purchased from Central Lab animal Inc.. All mice were 7-8-week-old females. They were maintained in the conventional cage with 12 h/12 h light/dark cycle and fed with standard diet. The Resiquimod (R848)-induced model has been used for our experiments. Several studies have demonstrated that systemic autoimmune features developed following topical treatment with Resiquimod. The induced mouse models of lupus have presented phenotypic changes including marked splenomegaly, edematous and a swollen appearance, elevated level of autoantibodies and multiple organ involvement (19,20). The mice were divided into four groups, namely controls, lupus model treated with vehicle, lupus model treated with 4-PBA, and lupus model treated with steroid. First, to induce the experimental lupus model, the skin on the back of mice was treated with 100 µg of Resiquimod (R848), which is a TLR7 agonist (Enzo) in 100 µg of acetone, 3 times weekly from week 0 to week 4. Second, the mice were treated with phosphate buffered saline (PBS), 4-PBA dissolved in 100 µl of PBS intraperitoneally three times weekly (500 mg/kg) (Calbiocam), and dexamethasone dissolved in 100 µl of PBS intraperitoneally once a day (1 mg/kg) (Daewon Pharm) for 4 weeks, from week 8 to week 12. At the age of 12 weeks, mice were sacrificed. All mice were anaesthetized by an intraperitoneal injection of sodium pentobarbital (50 mg/kg). The blood was collected from retro-orbital sinus after anaesthesia procedure. Following collection of blood sample, the all mice were immediately euthanized by cervical dislocation. Following the completion of the euthanasia procedure, death was confirmed the combination of signs including ascertaining cardiac arrest, lack of breathing and loss of a corneal reflex.
All mouse experiments were performed according to the protocols approved by the Institutional Animal Care and Use Committee of the Chonbuk National University, Jeonju, Korea (CBNU 2017-0027).
Histopathologic assessment and immunofluorescence
Kidneys were harvested after perfusion with buffered saline, fixed with 4% formaldehyde (Biosesang) for 24 h, and embedded in paraffin. For the determination of renal histopathology, 10-µm sections of the kidney were stained with hematoxylin and eosin and periodic acid-Schiff. Renal pathology was evaluated based on a previously described scoring scale (21). Glomerular pathology and interstitial pathology were assessed semiquantitatively on a scale of 0-3 in 10 randomly selected high-power fields for each mouse.
The 10-µm-thick acetone-fixed sections were stained with rabbit anti-mouse IgG-heavy and light chain antibody FITC-conjugated, goat anti-mouse IgM cross-absorbed antibody FITC-conjugated (Bethyl Laboratories Inc.), and were then incubated at room temperature for 1 h. For C3 staining, the kidney sections were stained with rat anti-mouse C3 (Abcam) and then incubated at room temperature overnight, followed by staining with goat anti-rat IgG-heavy and light chain antibody FITC-conjugated and then incubated for 1 h at room temperature. Sections were mounted with glycerin and the images were acquired by a Nikon Eclipse E600 fluorescence microscopy (Nikon). In addition, fluorescence intensity scores were assessed based on the methods described in a previous study (21). The intensity scores were evaluated for each section with at least 10 glomeruli.
For detection of antinuclear antibodies (ANAs), serum was diluted 1:40 and placed on HEp-2 slides (Antibodies Incorporated) with rabbit anti-mouse IgG-heavy and light chain antibody FITC-conjugated. After mounting with glycerin, images were analyzed by Nikon Eclipse E600 fluorescence microscopy.
Serologic analysis and urinalysis
Urine albumin and creatinine (Exocell Inc.), Serum anti-double stranded DNA (anti-dsDNA) antibodies (Shibayagi Co.) and serum cytokines, including TNF-α (Enzo), were quantified by ELISA according to the manufacturers' instructions.
Cell isolation and flow cytometry
Spleens were harvested and weighed, and splenocytes were isolated by passing tissues through a 70-µm cell strainer (BD Biosciences). For fluorescence-activated cell sorting analysis of splenocytes, the following antibodies from commercial sources were used: anti-CD45 (B220), anti-CD69, anti-CD3, anti-CD4, anti-CD25, and anti-Foxp3 (BD Biosciences). All antibodies were fluorochrome-conjugated. The stained cells were analyzed using FACSCalibur flow cytometer (BD Biosciences) and data analysis was performed using FlowJo software (TreeStar Inc.).
Western blot analysis
Proteins were extracted from the spleen tissue samples using a lysis buffer. Protein levels were determined using Bio-Rad DC Protein assay (Bio-Rad Laboratories, Inc.). Proteins were separated on 10% SDS-PAGE gels and were then transferred to nitrocellulose membranes. Membranes were blocked in 5% fat-free milk in Tris-buffered saline for 60 min at room temperature with shaking and then probed with primary antibodies (1:1,000) against GRP78 (Bioworld Technology), PERK (Santa Cruz Biotechnology, Inc.), IRE1α (Cell Signaling Technology, Inc.), eIF2α (Cell Signaling Technology, Inc.), ATF-6α (Bioworld Technology), CHOP (Bioworld Technology), cleaved ATF4 (Cell Signaling Technology, Inc.), p-PERK (Santa Cruz Biotechnology, Inc.), p-IRE1α (Thermo Fisher Scientific, Inc.), p-eIF2α (Cell Signaling Technology, Inc.), and Actin (Bethyl Laboratories) overnight at 4˚C. After three washes, the membranes were incubated with secondary horseradish peroxidase-conjugated antibodies (1:3,000) for 2 h at room temperature. The reactive proteins were detected using ECL (Amersham Life Sciences/GE Healthcare) and the intensity of bands was quantified densitometrically using the Vilber Lourmat Fusion fx7 system (Vilber Lourmat).
Inhibition assays
CD4+CD25- target cells that were labelled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) were co-cultured for 5 days with regulatory CD4+CD25+ T cells at target cell: Treg ratio of 1:1. The cells were incubated in the presence of anti-CD3 and anti-CD28 antibodies coated beads (cells to beads ratio 10:1) (Life Technologies; Thermo Fisher Scientific, Inc.) as previously described (22). The stained cells were analyzed using FACSCalibur flow cytometer (BD Biosciences) and data analysis was performed using FlowJo software (TreeStar Inc.). We calculated inhibitory index (%) of cell proliferation in a same manner as described previously (22).
Statistical analysis
SPSS 22.0 software (SPSS Inc.) was used for statistical analysis. Kruskal-Wallis tests for group comparisons and Mann-Whitney test with Bonferroni's correction for comparisons between pairs of groups were applied to the analysis of pathology and IF scoring. All other data, which were parametically distributed, were analyzed by one-way ANOVA with a Tukey post hoc test for significance when comparing groups including proteinuria, autoantibody, cytokine, flow cytometry assay. P values <0.05 were considered statistically significant. The results were expressed as mean ± EM.
Results
4-PBA decreased lupus manifestations and disease activity in murine lupus model
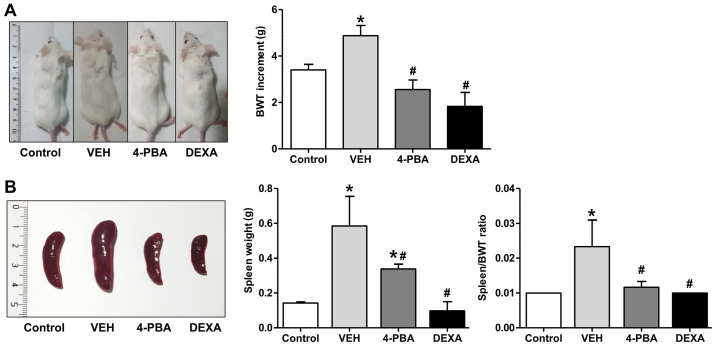
We investigated the effect of ER stress inhibition on the development of clinical manifestations in murine lupus. The murine lupus model which is induced with TLR7 agonist exhibits severe SLE-phenotypes, such as splenomegaly, edema, presence of autoantibodies to nuclear antigen, and glomerulonephritis. To determine whether ER stress suppression prevents lupus immunopathology, 4-PBA was administrated in induced lupus group at 8 weeks of age, when mice did not present overt lupus manifestations. Additionally, steroid treatment was initiated in another group with induced lupus to compare the inhibitory effects of 4-PBA with those of steroids. At 12 weeks, vehicle-treated mice appeared edematous and swollen compared to 4-PBA or steroid-treated mice (Fig. 1A). Regarding body weight change, The TLR7 agonist-stimulated group showed an increase in weight compared to the control mice. The TLR7 agonist-stimulated group also presented marked splenomegaly from the expansion of lymphocytes due to plasma dendritic cell activation following TLR7 stimulation and displayed an edematous and swollen appearance because of systemic inflammation as treatment continued. Body weight likely increased in these mice due to splenomegaly, which was increased over three times compared to the control mice by the 12th week. The increment of body weight from baseline to 12th week was significantly higher in vehicle-treated lupus mice (4.875±1.246 g) compared to 4-PBA-treated (2.556±1.236 g) and steroid-treated group (1.833±1.472 g) (Fig. 1A). Marked splenomegaly also was detected in vehicle-treated mice (weight, 0.585±0.416 g; ratio 0.023±0.018), while 4-PBA (weight 0.338±0.066 g; ratio 0.011±0.004) and steroid-treated mice (weight 0.097±0.105 g; ratio 0.010±0) showed a decrease in spleen size and spleen to body weight ratio (Fig. 1B).
Figure 1.
Clinical manifestations in lupus mice following 4-PBA and steroid treatment. (A) BWT increment from BALB/c (n=5) and murine lupus models that were treated with VEH (n=5), 4-PBA (n=5) and DEXA (n=5) for 4 weeks. (B) Comparisons of weight of the spleen and spleen to weight ratio among BALB/c murine lupus models treated with VEH, 4-PBA and DEXA. *P<0.05 vs. BALB/c mice; #P<0.05 vs. VEH-treated mice. Values are presented as the mean ± SEM of three independent experiments. 4-PBA, 4-phenylbutyric acid; BWT, body weight; DEXA, dexamethasone; VEH, vehicle.
4-PBA reduced tumor necrosis factor-α level and production of auto-antibodies
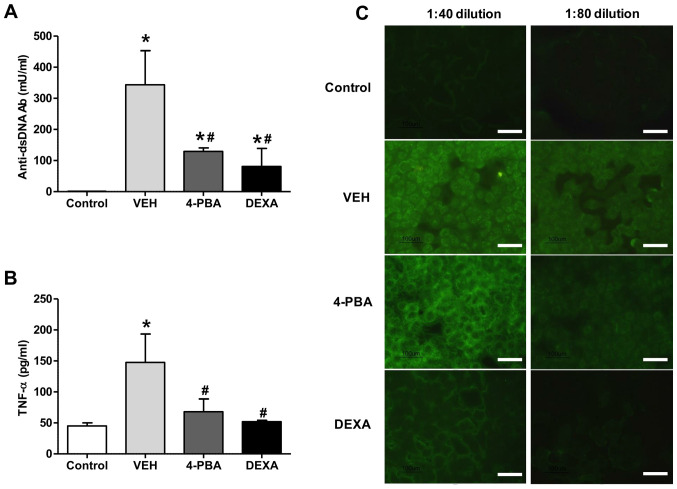
To assess the effects of ER stress suppression on autoantibody production, the serum level of anti-dsDNA antibody was measured. The sera from vehicle-treated mice showed higher level of anti-dsDNA (343.5±219.6 mU/ml), whereas the mice treated with 4-PBA showed decreased level of anti-dsDNA (129.5±21.9 mU/ml) at 12th week and the level was similar to that of the steroid-treated group (81.0±82.0 mU/ml) (Fig. 2A). The presence of ANAs in sera of murine lupus model was assayed by indirect immunofluorescence using HEp-2 slides. The serum was diluted at the concentration of 1:40 and 1:80 to determine the difference of fluorescence intensity between the groups. Enhanced expression of ANA with speckled pattern was detected in the serum of vehicle group with 1:40 dilution and the fluorescent activity was also detected at 1:80 dilution, even though the activity was decreased. In the serum of 4-PBA-treated mice, the fluorescent activity against ANA was detected at 1:40 concentration; however, it was rarely detected in the serum diluted at the concentration of 1:80 (Fig. 2C). These results indicate that 4-PBA treatment blocked the production of autoantibodies.
Figure 2.
Effect of 4-PBA on inflammatory cytokines and antibody production. (A) Serum anti-dsDNA and (B) TNF-α levels in BALB/c mice and murine lupus mice treated with VEH, 4-PBA and DEXA were determined by ELISA. (C) Indirect immunofluorescence analysis of sera from BALB/c mice and murine lupus groups treated with VEH, 4-PBA and DEXA (1:40 and 1:80 sera dilution). Scale bar, 100 µm. *P<0.05 vs. BALB/c mice; #P<0.05 vs. VEH-treated mice. Data are presented as the mean ± SEM (n=5). Representative images are shown on the right. Data were obtained from three paired experiments. 4-PBA, 4-phenylbutyric acid; anti-dsDNA, anti-double-stranded DNA; DEXA, dexamethasone; TNF-α, tumor necrosis factor-α; VEH, vehicle.
Our experiments revealed an increased concentration of TNF-α (147.7±79.4 pg/ml) in vehicle-treated group, in agreement with the results of previous reports. However, treatment with 4-PBA reduced the levels of TNF-α (68.0±35.6 pg/ml) in murine lupus model (Fig. 2B). The steroid-treated group showed similar level of TNF-α (52.0±2.8 pg/ml). Taken together, these data suggest that 4-PBA administration decreases autoantibodies production and pro-inflammatory cytokine production, consequently ameliorating the disease activity of murine lupus model.
4-PBA ameliorated lupus nephritis
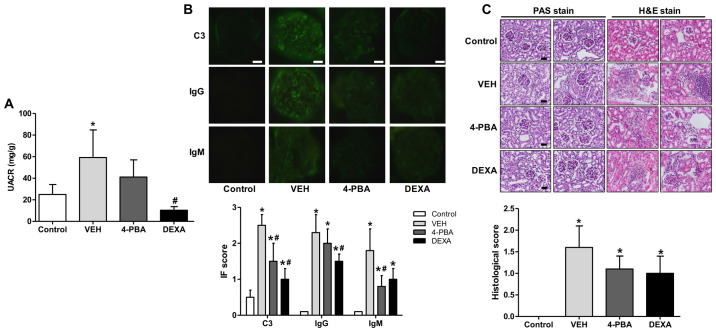
SLE exhibits a broad range of clinical manifestations, of which lupus nephritis is the most serious form of major organ involvement and the leading cause of high morbidity and mortality (23). Thus, it is essential to assess the effects of 4-PBA on renal pathology in murine lupus model. The BALB/c mice treated with TLR7 agonist developed spontaneously a disease with characteristic features of human SLE, including nephritis (19). In the present experiment, progressive albuminuria was observed over time in vehicle-treated group with a urine albumin-to-creatinine ratio (UACR) of up to 59±44 mg/g. On the other hand, 4-PBA group exhibited decreased albuminuria compared to the vehicle group at 12th week, with an UACR of 41±27 mg/g, but the difference was not statistically significance. The steroid-treated group showed the lower range of UACR, 10±5 mg/g, that was significantly lower compared to that of vehicle group (Fig. 3A).
Figure 3.
Decreased albuminuria and renal deposition of immune complex and C3 and ameliorated pathologic severity of kidney injury induced by 4-PBA treatment. (A) UACR in BALB/c and lupus mice that were treated with VEH, 4-PBA and DEXA. The mouse urine was collected for 24 h at the age of 12 week using a metabolic cage, and urinary albumin and creatinine levels were quantified using ELISA. (B) Representative renal sections stained for C3, IgG and IgM (original magnification, x400) and IF staining scores are shown. Scale bar, 10 µm. (C) Representative kidney sections stained with PAS stain and hematoxylin and eosin stain (original magnification, x400) and histologic scores are shown. Scale bar, 25 µm. *P<0.05 vs. BALB/c mice; #P<0.05 vs. vehicle-treated mice. Results are presented as the mean ± EM of three independent experiments per group. 4-PBA, 4-phenylbutyric acid; DEXA, dexamethasone; IF, immunofluorescence; PAS, periodic acid-Schiff; UACR, urine albumin-to-creatinine ratio; VEH, vehicle.
To determine whether 4-PBA attenuates immunopathology, we performed indirect fluorescent assay of kidney to detect IgG, IgM, and C3 deposition. In the vehicle group, IgG, IgM, and C3 were heavily deposited within glomeruli; however, 4-PBA treatment significantly reduced deposition of C3 and IgM (Fig. 3B). We also compared the histopathology of kidneys between the groups. The assessment revealed obvious mesangial hypercellularity and thickening of glomerular basement membrane in the vehicle-treated lupus mice compared to BALB/c mice. Moreover, the interstitium also showed advanced fibrosis and mononuclear inflammatory cell infiltration. However, compared to vehicle group, the glomeruli of 4-PBA-treated or dexamethasone-treated groups showed relatively mild mesangial hypercellularity and decreased interstitial fibrosis and inflammatory cell infiltration (Fig. 3C), even though there was not a statistical significance.
Elevated expression of ER stress markers in murine lupus and attenuation of ER stress by 4-PBA treatment
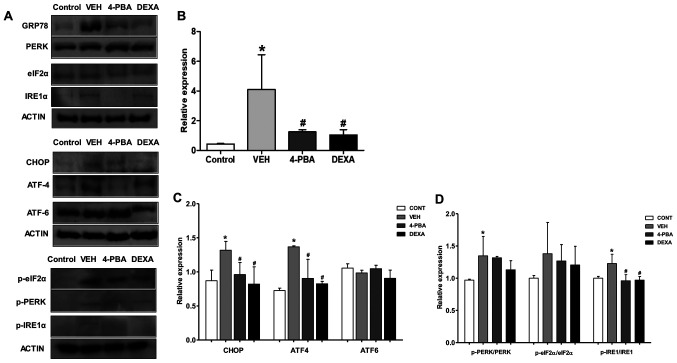
Our next goal was to demonstrate whether ER stress inhibition is involved in the amelioration of lupus phenotypes, including nephritis, in murine model. The immunoblot analysis of the spleen, a largest lymphatic organ and the site of innate and adaptive immune processes, was performed for measuring ER stress markers. Immunoblot analysis revealed that ER stress markers, including GRP78, CHOP, p-PERK, and p-IRE1α, were elevated in the vehicle-treated murine lupus group compared to wild-type mice. However, GRP78, p-IRE1α, and CHOP were greatly suppressed by treatment of the mice with 4-PBA (Fig. 4A-C). Accordingly, these results indicate that: i) 4-PBA suppressed ER stress signaling that was upregulated in the murine lupus model; and ii) 4-PBA treatment induced a marked improvement in lupus manifestation, including nephritis; thus, these results suggest the beneficial effects of ER stress inhibition on murine lupus.
Figure 4.
Upregulated ER stress markers in murine lupus models and their attenuation by 4-PBA treatment. (A) Representative western blotting images of the ER stress markers from spleen tissue lysates. (B) The pan form or phosphorylated form of GRP78 and (C) CHOP, ATF4, ATF6, (D) PERK, eIF2α and IRE1 were semiquantified by densitometric analysis and normalized to ACTIN expression or their unphosphorylated proteins. *P<0.05 vs. BALB/c mice; #P<0.05 vs. vehicle-treated mice. Values are presented as the mean ± EM of three independent experiments per group. 4-PBA, 4-phenylbutyric acid; CONT, control; DEXA, dexamethasone; ER, endoplasmic reticulum; p-, phosphorylated-; TNF-α, tumor necrosis factor-α; VEH, vehicle.
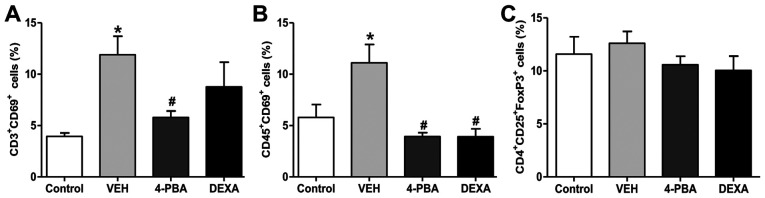
The proportion of activated T and B lymphocytes was decreased by 4-PBA treatment
Next, we analyzed the immune cell populations, including activated T and B lymphocytes, and Treg from the spleen to determine the roles of these immune cells on the improvement of lupus phenotypes by 4-PBA treatment. The frequency of activated T and B lymphocyte by analysis of CD69+ expression was higher in the vehicle group compared to that in the wild-type mice. The murine lupus with 4-PBA treatment showed a significantly reduced proportion of activated T and B lymphocyte and the same results were observed in steroid-treated group (Figs. 5 and S1). Thus, these findings led us to speculate that the reduction of frequencies of activated T and B lymphocytes by 4-PBA may contribute to ameliorate clinical manifestation, organ damage, and decrease autoantibodies production. However, Treg, which play an important role in the negative regulation of dysregulated lymphocytes, did not show significant differences in the population of the four groups (Fig. 5). The decreased proportion of activated T and B lymphocytes by 4-PBA and steroid treatment was in accordance with previous results; however, a paradoxical finding was that no changes were observed in the frequency of Treg among the groups. Thus, qualitative evaluation in the suppressive function of Treg in vehicle-, 4-PBA-, and steroid-treated group was performed to define the exact effect of ER stress inhibitor on Treg.
Figure 5.
Altered population of activated T and B lymphocytes and regulatory T cells. Bar graphs showing the proportions of activated (A) T and (B) B lymphocytes. and (C) regulatory T cells from BALB/c mice, murine lupus models treated with VEH, 4-PBA and DEXA. *P<0.05 vs. BALB/c mice; #P<0.05 vs. vehicle-treated mice. Results are presented as the mean ± EM of three independent experiments per group. 4-PBA, 4-phenylbutyric acid; DEXA, dexamethasone; VEH, vehicle.
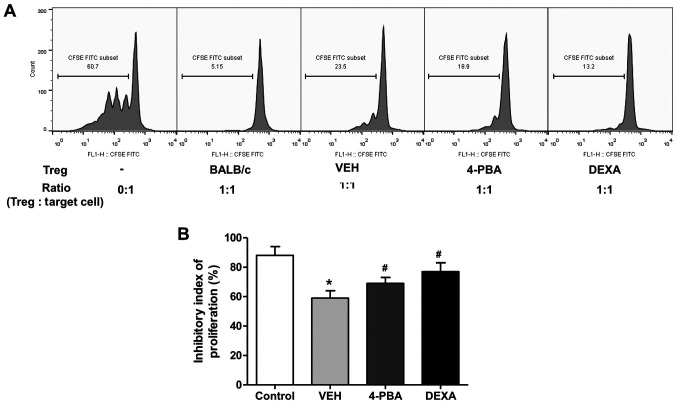
4-PBA improved the suppressive capacity of Treg in murine lupus
We evaluated the suppressive function of CD4+CD25+ Treg in vehicle-, 4-PBA-, and dexamethasone-treated groups by measuring the proliferation of CD4+CD25- T cells, as target cells, in vitro. Freshly isolated CD4+CD25+ T cells were co-cultured with CFSE-stained target cells at a ratio of 1:1 for 5 days and the inhibitory index of proliferation was acquired using the same equation as described above. The ability of Treg to suppress target cell proliferation was significantly lower in vehicle-treated group than in wild-type mice. The Treg of 4-PBA group and steroid group exhibited markedly improved suppressive activity compared to that of vehicle-treated group (vehicle, 59.2±6.8%; 4-PBA 68.8±6.2%, steroid, 77.2±7.1%; P=0.03 and P=0.01) (Fig. 6A and B). Thus, these results suggested that ER stress inhibition improves the function of Treg in murine lupus, even if it did not affect the frequency of Treg.
Figure 6.
Increased suppressive activity of Tregs by 4-PBA treatment in murine lupus. (A) Flow cytometric analysis of proliferation of target cells (CD4+CD25- T cells) in the presence or absence of Tregs of VEH-, 4-PBA- and DEXA-treated mice groups. CD4+CD25+ regulatory T cells (3x104 cells/well) from each group were co-cultured with CFSE-labeled CD4+CD25- target cells of BALB/c at a ratio of 1:1 for 5 days. (B) Inhibitory index of target cell proliferation indicating the suppressive capacity of Tregs. *P<0.05 vs. BALB/c mice; #P<0.05 vs. vehicle-treated mice. Results are presented as the mean ± EM of three independent experiments per group. 4-PBA, 4-phenylbutyric acid; Tregs, regulatory T cells; DEXA, dexamethasone; VEH, vehicle.
Discussion
By administration of 4-PBA, clinical manifestations of SLE, such as splenomegaly or generalized edema, were improved and serum levels of anti-dsDNA antibodies and inflammatory cytokines were also decreased. In particular, with respect to the renal involvement, 4-PBA treatment showed a trend of attenuating the albuminuria and histological damage, including inflammatory cell infiltration and IgM and C3 deposition. The deposition of IgG was not affected by 4-PBA treatment in our experiment, but there are two possible explanations for this result. First, the characteristics of immunoglobulin can influence this finding. Contrary to IgG, IgM is the first antibody to appear in the response to the initial exposure to an antigen. Therefore, it is possible that the antigen presentation of dendritic cells was suppressed by the suppression of regulatory T cells by 4-PBA. Second, this result could arise due to variation between the lupus mice. The level of IgG in the 4-PBA group was lower than that in the vehicle group, although the difference was not statistically significant. It is possible that variations between mice have suppressed the derivation of statistically significant results, even though a reduction trend is seen. Further studies will be needed to identify the precise mechanism behind this reduction.
To evaluate the effects of ER stress on the immune cells, we investigated the population of activated T and B cells and function of Treg. The cell population showed different percentages among the treatment groups and a higher proportion of activated T and B cells was observed in the vehicle group compared to the 4-PBA group; however, no significant statistical difference in Treg population was detected between the two groups. However, enhanced suppressive capacity of Treg in 4-PBA treated group was observed. These results suggest that the ER stress suppression through 4-PBA may improve the function of Treg, resulting in more efficacious regulation of over-activated T and B cells. Consequently, 4-PBA may limit the exaggerated immune response, leading to improvement of overall SLE manifestations, including lupus nephritis. Numerous attempts have been made to prove the role of Treg by its modulation, such as adoptive transfer or depletion of Treg, and many animal studies have shown different effects of Treg on prevention or development of autoimmune diseases (24,25). Especially, adoptive transfer of Treg from ‘young’ F1 mice or from the mice that transduced to express Ccr2 to recipients showed ameliorated immune-mediated manifestations, including pneumonitis or autoimmune sialoadenitis, in (NZB x NZW)F1 mice or MRL-Faslpr mice (26,27). Taken together these reports, even though we did not modulate Treg directly, our experiments indicate that enhanced suppressive function of Treg in 4-PBA-treated mice substantially exert therapeutic effects on the disease.
Recently, a number of researches have reported that ER stress suppression blocks disease progression and ameliorates clinical manifestations (7-9). Our experiments are the first to show the ameliorative effect of 4-PBA in murine lupus nephritis. Based on our current results, we can speculate that increasing ER folding capacity and facilitating misfolded proteins translocation by chemical ER stress inhibitor may result in improvement of suppressive capacity of Treg, although no statistical difference was detected in studied population.
Treg occupies only 5-10% of total CD4+ T lymphocytes in the healthy controls; consequently, the transfer of Tregs to the patients with lupus is limited. Thus, the efforts in expansion of Treg in vivo or in vitro have been made and their efficacy and safety profile has been evaluated in several studies that studied the immune disorders (28-30). Together with these approaches, improving the function of Treg by ER stress inhibition might be an option to optimize immunological homeostasis. Furthermore, we investigated the effects of steroids in order to estimate and compare the efficacy of 4-PBA with clinical improvements obtained by steroid treatment, and the results showed that the ameliorating effects of 4-PBA in murine lupus were comparable with steroid treatment. However, more researches are needed to elucidate how ER stress inhibition is involved in restoration of the Treg function. The dynamic organelle, ER, is responsible for the calcium storage, gluconeogenesis, cholesterol and lipid synthesis, as well as proteostasis (31); therefore, the impaired function of Treg under the condition of elevated ER stress could be based on these disrupted metabolic processes; however, the accurate mechanism remains unknown. The clarification of this link between improved function of Treg and ER stress inhibition is necessary.
Some limitations of this study should be acknowledged. First, absence of several serum cytokines or serial urine proteins of lupus murine, and unidentified identification of various cell markers in flow cytometry analysis. However, we quantified important and basic cytokines and autoantibodies that should be identified in the lupus murine model and analyzed the flow cytometry using a representative cell activation marker, and the data obtained through these analyses are judged to support our results meaningfully. Second, we focused only on the Treg and did not evaluate the effects of 4-PBA on other innate immune cells, including macrophage, dendritic cells or neutrophils, and the subsets of B and T lymphocytes. Because immune responses occur in synergy of diverse types of cells, clinical improvement induced by 4-PBA in murine lupus may be affected by the other altered function of different subset of cells. However, it is noteworthy that 4-PBA ameliorates disease severity and that the improved suppressive function of Treg is associated with the phenomenon, at least in part. Another limitation is that the expression of ER stress markers was evaluated in the entire population of splenocytes, not the Treg. Because the limited cell counts of Treg, assessment of markers in specific subsets of cells was technically difficult. Third, the murine lupus model we used was not a SLE-prone mouse model, but a TLR7-agonist induced model. Differences in types of manifestations, autoantibodies levels, and disease severity could exist between the mice. However, because the enhanced sensing of RNA-containing antigen, overproduction of autoantibodies, aberrant activation of T and B lymphocytes are the mechanisms in the TLR7 agonist-induced mice model (19), it was more apparent to evaluate the function of Treg in this murine lupus model. Fourth, we did not show changes in regional lymph nodes, which could clarify the effect of 4-PBA in the lupus model. The spleen showed marked changes in the expansion of myeloid and lymphoid cells between vehicle- and 4-PBA-treated mice and may be representative of regional lymph nodes as the spleen is the largest lymphatic organ in the body. However, further studies analyzing immune cell expansion and activation using regional lymph nodes are necessary to prove the suppressive capacity of 4-PBA.
In conclusion, the present study demonstrated for the first time that ER stress inhibition can improve Treg function, thereby inducing proper regulation of aberrant immune hyper-activation, and consequently leading to phenotypical improvements, especially in lupus nephritis, in lupus model. These findings provide strong support for a potential therapeutic effect of 4-PBA in patients with SLE.
Supplementary Material
Acknowledgements
This abstract was presented at the Annual European Congress of Rheumatology June 12-15, 2019, Madrid, Spain and was published as Abstract no. THU0209, at the ACR/ARHP Annual Meeting November 8-13, 2019, Atlanta, USA, and was published as Abstract no. 995, and at the Annual Meeting of the Korean College of Rheumatology May 18-19, 2018, Seoul, South Korea, and was published as Abstract no. 17S-0063.
Funding Statement
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (grant no. 2015006120) and Fund of Biomedical Research Institute, Chonbuk National University Hospital (grant no. CUH2018-0006).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YC and WHY designed the experiment. JHJ and EGL performed the main experiment. KMK performed the histological examination of the kidney. YC and WHY statistically analyzed the data. YC, JHJ, EGL and KMK interpreted the results. YC prepared a draft of the manuscript. WHY and KMK revised the manuscript. WHY acquired funding and contributed to resources. YC and WHY authenticate the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Chonbuk National University, Jeonju, Korea (CBNU 2017-0027).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests
References
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Kiriakidou M, Cotton D, Taichman D, Williams S. Systemic lupus erythematosus. Ann Intern Med. 2013;159:ITC4–ITC1. doi: 10.7326/0003-4819-159-7-201310010-01004. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Regulatory T cells: Key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 4.Shevach EM. CD4+ CD25+ suppressor T cells: More questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 6.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukai S, Ogawa Y, Urano F, Kudo-Saito C, Kawakami Y, Tsubota K. Novel Treatment of Chronic Graft-Versus-Host Disease in Mice Using the ER Stress Reducer 4-Phenylbutyric Acid. Sci Rep. 2017;7(41939) doi: 10.1038/srep41939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng M, Sang W, Chen S, Chen R, Zhang H, Xue F, Li Z, Liu Y, Gong Y, Zhang H, et al. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol Lett. 2017;271:26–37. doi: 10.1016/j.toxlet.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Mohammed-Ali Z, Lu C, Marway MK, Carlisle RE, Ask K, Lukic D, Krepinsky JC, Dickhout JG. Endoplasmic reticulum stress inhibition attenuates hypertensive chronic kidney disease through reduction in proteinuria. Sci Rep. 2017;7(41572) doi: 10.1038/srep41572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng P, Lin Y, Wang F, Luo R, Zhang T, Hu S, Feng P, Liang X, Li C, Wang W. 4-PBA improves lithium-induced nephrogenic diabetes insipidus by attenuating ER stress. Am J Physiol Renal Physiol. 2016;311:F763–F776. doi: 10.1152/ajprenal.00225.2016. [DOI] [PubMed] [Google Scholar]
- 11.Braakman I, Hebert DN. Protein folding in the endoplasmic reticulum. Cold Spring Harb Perspect Biol. 2013;5(a013201) doi: 10.1101/cshperspect.a013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagone P, Jackowski S. Membrane phospholipid synthesis and endoplasmic reticulum function. J Lipid Res. 2009;50 (Suppl):S311–S316. doi: 10.1194/jlr.R800049-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 14.Bhandary B, Marahatta A, Kim HR, Chae HJ. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci. 2012;14:434–456. doi: 10.3390/ijms14010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Lee WS, Sung MS, Lee EG, Yoo HG, Cheon YH, Chae HJ, Yoo WH. A pathogenic role for ER stress-induced autophagy and ER chaperone GRP78/BiP in T lymphocyte systemic lupus erythematosus. J Leukoc Biol. 2015;97:425–433. doi: 10.1189/jlb.6A0214-097R. [DOI] [PubMed] [Google Scholar]
- 17.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2(799) doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokogawa M, Takaishi M, Nakajima K, Kamijima R, Fujimoto C, Kataoka S, Terada Y, Sano S. Epicutaneous application of toll-like receptor 7 agonists leads to systemic autoimmunity in wild-type mice: A new model of systemic lupus erythematosus. Arthritis Rheumatol. 2014;66:694–706. doi: 10.1002/art.38298. [DOI] [PubMed] [Google Scholar]
- 20.Hasham MG, Baxan N, Stuckey DJ, Branca J, Perkins B, Dent O, Duffy T, Hameed TS, Stella SE, Bellahcene M, et al. Systemic autoimmunity induced by the TLR7/8 agonist Resiquimod causes myocarditis and dilated cardiomyopathy in a new mouse model of autoimmune heart disease. Dis Model Mech. 2017;10:259–270. doi: 10.1242/dmm.027409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan JJ, Lee JG, Jang JY, Koo TY, Ahn C, Yang J. IL-2/anti-IL-2 complexes ameliorate lupus nephritis by expansion of CD4+CD25+Foxp3+ regulatory T cells. Kidney Int. 2017;91:603–615. doi: 10.1016/j.kint.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Lee YA, Kim HR, Lee JS, Jung HW, Kim HY, Lee GM, Lee J, Sim JH, Oh SJ, Chung DH, et al. CD4+ FOXP3+ Regulatory T Cells Exhibit Impaired Ability to Suppress Effector T Cell Proliferation in Patients with Turner Syndrome. PLoS One. 2015;10(e0144549) doi: 10.1371/journal.pone.0144549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu F, Haas M, Glassock R, Zhao MH. Redefining lupus nephritis: Clinical implications of pathophysiologic subtypes. Nat Rev Nephrol. 2017;13:483–495. doi: 10.1038/nrneph.2017.85. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 25.Masteller EL, Tang Q, Bluestone JA. Antigen-specific regulatory T cells - ex vivo expansion and therapeutic potential. Semin Immunol. 2006;18:103–110. doi: 10.1016/j.smim.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa H, Inoue A, Muraoka M, Yamanouchi J, Miyazaki T, Yasukawa M. Therapy for pneumonitis and sialadenitis by accumulation of CCR2-expressing CD4+CD25+ regulatory T cells in MRL/lpr mice. Arthritis Res Ther. 2007;9(R15) doi: 10.1186/ar2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weigert O, von Spee C, Undeutsch R, Kloke L, Humrich JY, Riemekasten G. CD4+Foxp3+ regulatory T cells prolong drug-induced disease remission in (NZBxNZW) F1 lupus mice. Arthritis Res Ther. 2013;15(R35) doi: 10.1186/ar4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamarche C, Levings MK. Guiding regulatory T cells to the allograft. Curr Opin Organ Transplant. 2018;23:106–113. doi: 10.1097/MOT.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 29.Ukena SN, Höpting M, Velaga S, Ivanyi P, Grosse J, Baron U, Ganser A, Franzke A. Isolation strategies of regulatory T cells for clinical trials: Phenotype, function, stability, and expansion capacity. Exp Hematol. 2011;39:1152–1160. doi: 10.1016/j.exphem.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Donnelly C, Dykstra B, Mondal N, Huang J, Kaskow BJ, Griffin R, Sackstein R, Baecher-Allan C. Optimizing human Treg immunotherapy by Treg subset selection and E-selectin ligand expression. Sci Rep. 2018;8(420) doi: 10.1038/s41598-017-17981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.