Gardner highlights work from Ayukawa et al. that reveals that microtubule oligomer curvature, rather than length, is the limiting factor that slows the microtubule nucleation rate.
Abstract
In this issue, Ayukawa, Iwata, Imai, and colleagues (2021. J. Cell Biol. https://doi.org/10.1083/jcb.202007033) use rapid temporal and high-spatial-resolution electron microscopy imaging to examine the earliest stages of new microtubule nucleation. They discover that straightening of curved tubulin oligomers increases the efficiency of microtubule nucleation.
Microtubules are long cytoskeletal filaments that provide structure to cells, allow for transport within the cells, and participate in cell division by acting together with molecular motors to build a mitotic spindle. While microtubules act as the stiff “bones” of the cell, they also have a unique and important ability to rapidly restructure their length and organization in response to cellular cues. Therefore, large arrays of microtubules, such as in a mitotic spindle, can rapidly depolymerize and disappear as needed. However, in order to rebuild these microtubule networks, the nucleation of new microtubules is required. This nucleation of new microtubules, whether from existing templates such as centrosomes or spindle poles, or via the de novo organization of the tubulin subunits that make up microtubules, remains a poorly understood process.
In this issue, Ayukawa, Iwata, Imai, and colleagues used rapid temporal and high-spatial-resolution imaging to study the earliest stages of microtubule nucleation (1). First, the authors purified αβ-tubulin heterodimers with a Y222F mutation in the β-tubulin subunit. This Y222F β-tubulin mutation increased the rate of microtubule assembly and, importantly, greatly accelerated the nucleation rate of new microtubules. Thus, a comparison between wild-type and mutant tubulin allowed the authors to dissect differences in the early nucleation process that could explain the increased nucleation rate for the mutant tubulin. Importantly, a “rapid flush method” was used to capture high-resolution transmission electron microscopy images of tubulin subunits very early in the nucleation process. The rapid flush method revealed “oligomers” of tubulin subunits: chains of tubulin subunits linked together along their long axis.
The authors reasoned that these oligomers are likely on-pathway intermediates that are crucial for new microtubule nucleation. Examination of the oligomers revealed differences in curvature between wild-type and mutant tubulin: the mutant tubulin that nucleated more readily had oligomers that were straighter and less curled than the wild-type tubulin. Further, a comparison of GTP tubulin and GDP tubulin early nucleation intermediates revealed that, for both wild-type and mutant tubulin, the GDP tubulin oligomers, which did not nucleate efficiently, were more curved than the GTP tubulin oligomers that nucleated more efficiently (Fig. 1 A, left, red versus blue). Importantly, the fraction of nearly straight oligomers in each sample directly corresponded to the respective nucleation rate for each tubulin type. Thus, the degree of curvature of the oligomers predicted the overall nucleation rate, such that an increase in the fraction of straight oligomers was directly correlated to an increase in the microtubule nucleation rate (Fig. 1 A).
Figure 1.
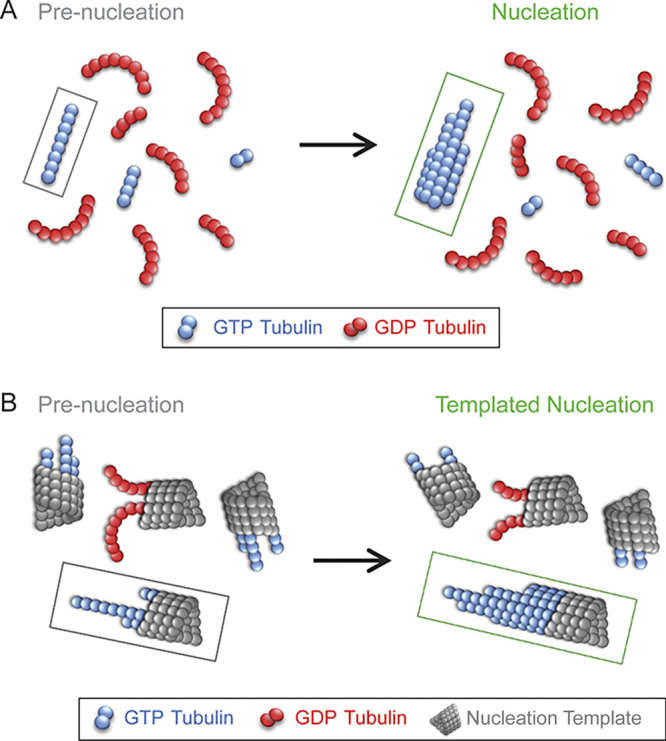
Long, straight oligomers promote microtubule nucleation. (A) The nucleation of new microtubules is limited by the availability of critical-length, straight GTP tubulin (blue) oligomers. GDP tubulin oligomers (red) are more curved, with reduced nucleation efficiency. (B) Straight GTP tubulin protofilaments (blue) that are attached to templates (gray) could also be required to facilitate the nucleation of new microtubules from templates such as in centrosomes. Curved GDP tubulin protofilaments (red) attached to templates (gray) would not efficiently facilitate nucleation of new microtubules.
The authors then examined the role of oligomer length in the nucleation rate, to determine whether there was a critical minimum oligomer length that could predict efficient microtubule nucleation. They fit their bulk microtubule growth curves (turbidity) to a standard nucleation-and-growth model (2) to estimate the minimum size of the oligomers that were likely to grow into microtubules, i.e., the critical length. For wild-type tubulin, ∼4 tubulin dimers were required in order for oligomers to grow into microtubules. However, this critical length was common, and indeed prevalent, within the early nucleation mixtures. Therefore, it seems likely that oligomer curvature, rather than length, is the limiting factor that slows the microtubule nucleation rate (Fig. 1 A).
Why would oligomer curvature limit microtubule nucleation rate? While oligomers of sufficient length were readily observed in early nucleation mixtures, lateral association of new oligomers with existing oligomers was rare. Further, when multiple oligomers had indeed associated laterally to form a new, multi-protofilament assembly, the length of the longest strand greatly exceeded the maximum size of the single-stranded oligomers. Thus, it is likely that straight oligomers facilitate the lateral association of new tubulin subunits or oligomers along their length, stabilizing the nascent microtubules and allowing for their stable growth as a multistranded filament.
While this work sheds light on the nucleation of new microtubules using purified tubulin, the described results provide interesting insights into potential nucleation mechanisms inside of cells. In cells, microtubules predominately grow from templates, such as centrosomes, and various microtubule-associated proteins may also influence the nucleation process. A recent study of microtubule nucleation from templates found that there was a significant time lag between the arrival of new tubulin subunits to a template and the growth of a new microtubule from the template (3). That study concluded that GTP hydrolysis inhibits microtubule nucleation by destabilizing the nascent microtubule. These results are consistent with the conclusion from Ayukawa, Iwata, Imai, and colleagues that GDP tubulin oligomers are curved and therefore unable to efficiently make lateral associations with other oligomers to stabilize the new microtubule and allow for growth (Fig. 1 B; 1). However, recent electron microscopy studies have also revealed that the GTP tubulin–containing ends of growing microtubules show extended protofilaments with a gentle curvature (4, 5, 6, 7, 8). Thus, one additional barrier to the nucleation of new microtubules on templates may be the straightening of GTP tubulin oligomers. Here, the straightening of template-attached, gently curved GTP tubulin oligomers would then allow for lateral binding of new tubulin dimers and oligomers (Fig. 1 B). The transient straightening of gently curved GTP tubulin oligomers could potentially be accomplished via thermal forces and the resulting protofilament curvature fluctuations. However, this straightening process could also be facilitated by microtubule-associated proteins that promote nucleation, such as TPX2 and XMAP215 (9, 10). In support of this idea, Ayukawa, Iwata, Imai, and colleagues found that the distribution of oligomer curvatures appeared similar to what has been reported for the protofilaments at the growing ends of microtubules (5).
In light of these results, interesting future work could explore whether microtubule-associated proteins act to regulate the curvature of the oligomers involved in microtubule nucleation. While many aspects of the mechanisms of microtubule nucleation remain unknown, this new work sheds light on the very earliest stages of microtubule nucleation, which may have wide-ranging implications in future studies of microtubule nucleation and growth in cells.
Acknowledgments
M.K. Gardner is supported by National Institutes of Health grant NIGMS R35-GM126974.
The author declares no competing financial interests.
References
- 1.Ayukawa, R., et al. 2021. J. Cell Biol. 10.1083/jcb.202007033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oosawa, F., and Asakura S.. 1975. Thermodynamics of the polymerization of protein. Academic Press [Google Scholar]
- 3.Wieczorek, M., et al. 2015. Nat. Cell Biol. 10.1038/ncb3188 [DOI] [PubMed] [Google Scholar]
- 4.Chrétien, D., et al. 1995. J. Cell Biol. 10.1083/jcb.129.5.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guesdon, A., et al. 2016. Nat. Cell Biol. 10.1038/ncb3412 [DOI] [Google Scholar]
- 6.McIntosh, J.R., et al. 2018. J. Cell Biol. 10.1083/jcb.201802138 [DOI] [Google Scholar]
- 7.Muller-Reichert, T., et al. 1998. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.95.7.3661 [DOI] [Google Scholar]
- 8.Vitre, B., et al. 2008. Nat. Cell Biol. 10.1038/ncb1703 [DOI] [PubMed] [Google Scholar]
- 9.Roostalu, J., et al. 2015. Nat. Cell Biol. 10.1038/ncb3241 [DOI] [Google Scholar]
- 10.King, B.R., et al. 2020. Mol. Biol. Cell. 10.1091/mbc.E20-02-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]


