Abstract
This study investigated the influence of the rate of nicotine metabolism, as indicated by the nicotine metabolite ratio (NMR), on tobacco dependence. We stratified 136 smokers on the basis of saliva NMR as fast (n = 65) and slow (n = 71) metabolizers. Two “loading cigarettes” were smoked after overnight, and a “reward cigarette” was smoked after 6 hours of daytime, abstinence. Blood nicotine concentrations, expired carbon monoxide, withdrawal/craving, and reward questionnaires were collected before/after smoking and during daytime abstinence. Compared with slow metabolizers, fast metabolizers had a shorter nicotine elimination half-life (P < 0.001), lower plasma nicotine concentrations (P < 0.001), and higher withdrawal/craving scores (P < 0.05) for most times during daytime abstinence, indicating that fast metabolizers are likely smoking more to relieve withdrawal symptoms (negative reinforcement). Reward/satisfaction scores were similar in fast and slow metabolizers, suggesting that faster nicotine metabolism, assessed by NMR, is not associated with greater positive reinforcement. CYP2A6 normal (n = 82) and reduced (n = 42) genotype predicted plasma nicotine concentrations but not withdrawal symptoms.
Nicotine dependence underlies tobacco dependence and sustains cigarette smoking, which remains a major cause of premature death.1 Nicotine dependence is motivated by seeking rewarding effects (e.g., stimulation and pleasure), also termed positive reinforcement, and reversing aversive effects of nicotine withdrawal (irritability, anxiety, and difficulty concentrating), also called negative reinforcement.
Nicotine is metabolized primarily by the hepatic cytochrome P450 enzyme CYP2A6, with ≈80% of nicotine converted to cotinine (COT), which is further metabolized by the same enzyme to 3′-hydroxycotinine (3HC).1 There is wide individual variability in the clearance of nicotine, because of both genetic variation and environmental and hormonal factors. The ratio of 3HC/COT, also called the nicotine metabolite ratio (NMR), is a phenotypic biomarker that can be measured in plasma, urine, and saliva and is correlated with the rate of nicotine clearance.2 The NMR accounts for both genetic and nongenetic influences of CYP2A6 activity, is reproducible within subjects, and is independent of the time since last cigarette smoked.3–5
The rate of nicotine metabolism is an important determinant of tobacco and nicotine dependence. Faster nicotine metabolism is associated with greater dependence/higher tobacco consumption and lower rates of quitting without pharmacotherapy and with transdermal nicotine patch compared with slower metabolizers.6–11 One potential mechanism for this association is that fast metabolizers experience more severe craving/withdrawal and, thus, are more likely to smoke to relieve such symptoms (i.e., for negative reinforcement).7,12 This hypothesis is supported by findings showing that smokers with higher NMR experience more anxiety, insomnia, difficulty concentrating, anger, and impatience during abstinence.6,13 Another possible mechanism is that because of the faster elimination of nicotine, tolerance to psychoactive effects dissipates more rapidly and, therefore, subsequent nicotine exposures (i.e., cigarette smoking) are more rewarding (i.e., positive reinforcement may be greater among faster metabolizers). In support of this idea, brain imaging studies show that fast metabolizers exhibit greater reactivity in dopamine-dependent reward circuitry when given visual smoking cues than slow metabolizers (defined herein as the highest and lowest NMR quartiles, respectively).14
Smoking behavior, severity of dependence, and the rate of nicotine metabolism vary by race.15,16 On average, blacks metabolize nicotine more slowly, because of a higher frequency of several slow metabolism variants of CYP2A6 and UGT2B10 genes, the latter of which codes the uridine diphosphate glucuronlyltransferase isoform mainly responsible for nicotine glucuronidation,10,17–21 and smoke fewer cigarettes per day (CPDs).22 Although one may expect that their slower nicotine metabolism and reduced CPDs may be associated with less severe nicotine dependence, blacks report greater difficulty quitting than whites,15 suggesting higher nicotine dependence. The paradox between slower metabolism and higher dependence may be related to greater smoking intensity observed previously in blacks compared with whites,22 but also other factors unrelated to nicotine metabolism.
Sex differences also present some paradoxes; women metabolize nicotine faster than men because of estrogen-mediated induction of CYP2A6,23,24 but they smoke, on average, fewer CPDs,22 whereas the nicotine intake per cigarette is, on average, the same in both sexes.25 Most clinical trials studying NMR do not find a sex difference in quit rates, indicating that the nicotine metabolism rate predicts cessation success in women as well as in men.7–10
The aim of the present study was to investigate the association of NMR with withdrawal/craving symptoms after abstinence from smoking and the response to smoking a cigarette, as assessed by questionnaire scores, nicotine plasma concentration, and expired carbon monoxide (CO) and heart rate (HR) changes after two different abstinence periods (i.e., overnight and 6 hours during the day). We hypothesized that fast, relative to slow, metabolizers will demonstrate more severe withdrawal symptoms and greater craving to smoke during abstinence and more reward after subsequent smoking. Furthermore, we aimed to investigate possible racial differences of these mechanistic relationships and to compare NMR with CYP2A6 genotype as a biomarker of withdrawal effects.
RESULTS
A total of 552 potential participants were scheduled for an inperson screening visit. Among them, 275 did not meet eligibility (e.g., COT <50 ng/mL or vital signs not within normal range) and 106 did not meet the study’s NMR cut points. From the remaining 171, 34 declined/did not complete the study. A total of 137 participants completed the study, but one participant with nicotine concentrations below the limit of quantification (LOQ) and low CO was excluded from analysis because it is assumed that this participant did not inhale smoke from the cigarettes during the study. Finally, 136 participants were included in the final analysis, 71 slow metabolizers and 65 fast metabolizers by NMR.
Baseline characteristics
The NMR frequency histogram can be found as supporting information (Figure S1). Table 1 shows the baseline characteristics, as well as comparisons based on NMR, race, and sex. Significant correlations were found between saliva and plasma NMR (r = 0.708, P < 0.001), saliva NMR and nicotine half-life (r = −0.432, P < 0.001), and saliva NMR and saliva COT (r = −0.349, P < 0.001). Comparisons within NMR groups by race and by sex can be found as supporting information (Tables S1 and S2). Complete CYP2A6 genotyping was available for 124 participants (91.2%; not available in 12 cases because of incomplete genotype results (n = 11) or DNA not available (n = 1)). Among them, 82 (66.1%) were normal metabolizers (NMs) (median NMR, 0.47; range, 0.08–1.1) and 42 (33.9%) were reduced metabolizers (RMs) (median NMR, 0.18; range, 0.06–0.72). Of 42 RMs, 33 (78.6%) were NMR slow metabolizers, whereas for 50 of the 82 NMs (61%), the NMR was indicative for fast metabolism (Figure 1). Analysis of the baseline characteristics using the genotype (i.e., NMs vs. RMs) showed significantly higher NMR in the NM group (P < 0.001), but no significant differences regarding other parameters.
Table 1.
Baseline characteristics (mean (SD), median (range), or number (percentage))
| Characteristics | All (n = 136) | Slow NMR (n = 71) | Fast NMR (n = 65) | P value | Whites (n = 98) | Blacks (n = 38) | P value | Male (n = 83) | Female (n = 53) | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 36.3 (12.3) | 34.4 (12.3) | 38.3 (12) | 0.065 | 35 (12.1) | 39.6 (12.3) | 0.05 | 34.7 (11.5) | 38.8 (13.1) | 0.054 |
| Sex (female) | 53 (39) | 21 (30) | 32 (49) | 0.019 | 35 (36) | 18 (47) | 0.211 | NA | NA | NA |
| Race (blacks) | 38 (28) | 22 (31) | 16 (25) | 0.408 | NA | NA | NA | 20 (24) | 18 (34) | 0.211 |
| BMI (kg/m2) | 25.7 (4.6) | 25.6 (4.6) | 25.8 (4.7) | 0.793 | 25.1 (4.6) | 27.2 (4.1) | 0.016 | 25.5 (4.3) | 25.9 (5.1) | 0.649 |
| FTCD | 4.2 (2.1) | 4.3 (2.1) | 4.1 (2.2) | 0.69 | 4.3 (2.1) | 3.8 (2.2) | 0.15 | 4 (2.1) | 4.3 (2.2) | 0.495 |
| CPD | 12 (5–40) | 12 (5–40) | 13 (5–35) | 0.42 | 13 (5–40) | 10 (5–20) | 0.001 | 13 (5–40) | 10 (5–30) | 0.054 |
| Smoking time (years) | 14.5 (2–43) | 13 (2–43) | 15 (2–41) | 0.231 | 13.5 (2–43) | 16 (2–43) | 0.515 | 15 (2–43) | 14 (2–41) | 0.561 |
| TFC (minutes) | 30 (0.5–480) | 20 (0.5–180) | 30 (2–480) | 0.033 | 28 (1–480) | 30 (0.5–240) | 0.967 | 30 (0.5–480) | 30 (2–240) | 0.682 |
| Alcohol, self-reported (g/week) | 37 (0–486) | 41 (0–486) | 13 (0–235) | 0.156 | 42.5 (0–486) | 13 (0–333) | 0.13 | 52 (0–390) | 10 (0–486) | <0.001 |
| Menthol cigarettes | 38 (28) | 20 (28) | 18 (28) | 0.95 | 14 (14) | 24 (63) | <0.001 | 21 (25) | 17 (32) | 0.39 |
| Saliva COT (ng/mL) | 145.2 (48.2–653.8) | 189.8 (48.2–653.8) | 119.3 (49.5–481.7) | <0.001 | 138.3 (48.2–653.8) | 148.2 (74–610.3) | 0.308 | 147.8 (48.2–653.8) | 137.5 (52.4–481.7) | 0.384 |
| Saliva NMR | 0.25 (0.06–1.32) | 0.16 (0.06–0.27) | 0.55 (0.37–1.32) | <0.001 | 0.36 (0.07–1.32) | 0.19 (0.06–0.57) | 0.005 | 0.21 (0.08–1.32) | 0.46 (0.06–1.1) | 0.034 |
| Plasma NMR | 0.39 (0–2) | 0.27 (0–1) | 0.66 (0–2) | <0.001 | 0.45 (0–2) | 0.33 (0–2) | 0.007 | 0.36 (0–2) | 0.46 (0–2) | 0.044 |
| Nicotine elimination half-life (minutes) | 111.3 (41.1–272.4) | 130.4 (50.3–255.5) | 93.0 (41.1–272.4) | <0.001 | 105.9 (41.1–272.4) | 151.2 (50.3–255.5) | 0.001 | 112.0 (50–272.4) | 109.1 (41.1–255.5) | 0.854 |
| Urine TNE (nmol/mg creatinine) | 53.3 (0.9–195.5) | 51.6 (1–195.5) | 55.0 (0.9–173.8) | 0.812 | 62.1 (1–195.5) | 36.8 (0.9–116.2) | 0.009 | 45.0 (3.9–195.5) | 67.4 (0.9–150.2) | 0.098 |
Bold numbers indicate significant differences (P < 0.05) between groups (between slow and fast metabolizers and between whites and blacks).
BMI, body mass index; CPD, cigarettes per day; COT, cotinine; FTCD, Fagerstrom Test for Cigarette Dependence; NA, not applicable. NMR, nicotine metabolite ratio; TFC, time to first cigarette after awakening in the morning; TIME, total nicotine equivalent.
Figure 1.
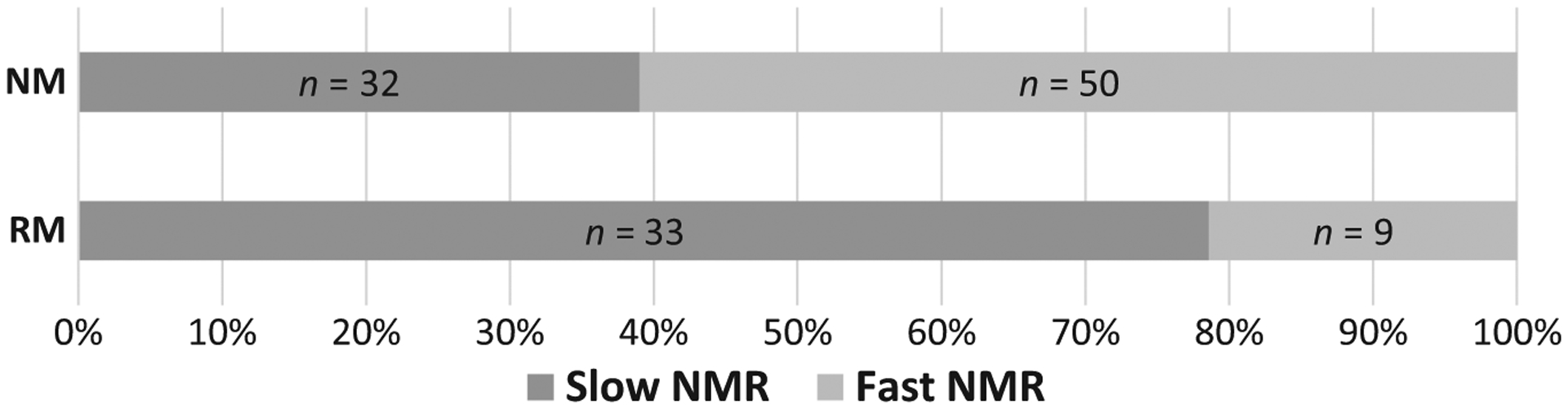
Frequency of slow and fast NMR in the CYP2A6 genotype groups (n = 124). NMs and RMs assessed by CYP2A6 genotype. NM, normal metabolizer; NMR, nicotine metabolite ratio; RM, reduced metabolizer.
Association of nicotine metabolism, as assessed by NMR, with withdrawal/craving
Table 2 shows the association of NMR with withdrawal/craving outcomes after the two abstinence periods. In a within-subject comparison, the Positive and Negative Affect Schedule (PANAS) negative score was significantly higher after overnight compared with daytime abstinence (P < 0.001), whereas the Tiffany Questionnaire on Smoking Urges-Brief (QSU) Global Craving Score neared significance (P = 0.05). Compared with slow metabolizers, fast metabolizers did not demonstrate more severe craving/withdrawal symptoms after overnight abstinence; however, significantly higher craving and withdrawal and PANAS negative scores nearing significance were seen after 6 hours of daytime abstinence. When comparing only whites (n = 98), significant differences were seen regarding nicotine concentrations (lower in fast compared with slow metabolizers both after overnight and daytime abstinence (P < 0.001)), but not for HR, CO, or craving scores. When analyzing only blacks (n = 38), fast metabolizers had significantly lower nicotine concentrations (P = 0.008) and expired CO (P = 0.022) after overnight abstinence compared with slow metabolizers, as well as after daytime abstinence (P = 0.01 and P = 0.003, respectively). Using the genotypes, significant differences were seen only regarding nicotine concentrations (lower in NMs compared with RMs both after overnight (P = 0.011) and after 6 hours of daytime (P = 0.003) abstinence).
Table 2.
Associations of NMR with withdrawal and physiological measurements (mean (SD) or median (range))
| After overnight abstinence (i.e., before Cig 1) | After 6 hours of abstinence (i.e., before Cig 3) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | All (n = 136) | Slow NMR (n = 71) | Fast NMR (n = 65) | P value | All (n = 136) | Slow NMR (n = 71) | Fast NMR (n = 65) | P value |
| Plasma nicotine (ng/mL) | 1.0 (0.7–6.7) | 1.5 (0.7–6.7) | 0.7 (0.7–6.6) | <0.001 | 1.4 (0.7–8.9) | 2.25 (0.7–8.9) | 1.2 (0.7–3.4) | <0.001 |
| Expired CO (ppm) | 9 (2–22) | 9 (3–22) | 8 (2–21) | 0.31 | 8 (2–16) | 9 (3–16) | 8 (2–14) | 0.022 |
| HR (bpm) | 69.8 (9.1) | 69.6 (9.4) | 70 (8.9) | 0.816 | 72.2 (10.2) | 72.4 (10.2) | 72 (10.2) | 0.804 |
| QSU Global Craving Score | 4 (1–7) | 3.9 (1–6.4) | 4.3 (1–7) | 0.203 | 3.85 (1–7) | 3.7 (1–7) | 3.9 (1.5–7) | 0.016 |
| MNWS | 7 (0–32) | 6 (0–22) | 8 (1–32) | 0.087 | 8 (0–48) | 8 (0–21) | 9 (1–48) | 0.057 |
| PANAS negative | 12 (10–35) | 12 (10–24) | 13 (10–35) | 0.178 | 11 (10–30) | 11 (10–20) | 12 (10–30) | 0.059 |
| PANAS positive | 26 (3–46) | 26.5 (3–45) | 26 (11–46) | 0.790 | 24 (10–50) | 24 (10–50) | 22 (10–50) | 0.493 |
Bold numbers indicate significant differences (P < 0.05) between slow and fast metabolizers.
Cig 1 and 3, first and third cigarettes of the day, respectively; CO, carbon monoxide; HR, heart rate; MNWS, Minnesota Nicotine Withdrawal Scale; NMR, nicotine metabolite ratio; PANAS, Positive and Negative Affect Schedule; QSU, Questionnaire on Smoking Urges-Brief.
Association of nicotine metabolism, as assessed by NMR, with rewarding effects
Table 3 shows the association of NMR with the response to smoking after abstinence. Significant score differences (higher craving and withdrawal and lower satisfaction in fast metabolizers compared with slow metabolizers) were seen after the first cigarette of the day (Cig 1) but not after Cig 3. When analyzing only whites or using the genotypes, no significant differences were found. Analysis of blacks only revealed significantly lower nicotine concentrations (P = 0.04), expired CO (P = 0.003), and higher craving (P = 0.019) after Cig 1, as well as lower expired CO (P = 0.024) after Cig 3, in fast compared with slow metabolizers.
Table 3.
Associations of NMR with the response to smoking after abstinence (mean (SD) or median (range))
| After first cigarette of the day (i.e., after Cig 1, except indicated otherwise) | After Cig 3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | All (n = 136) | Slow NMR (n = 71) | Fast NMR (n = 65) | P value | All (n = 136) | Slow NMR (n = 71) | Fast NMR (n = 65) | P value |
| Plasma nicotine (ng/mL) | 12.9 (0.7–40.8) | 13.6 (2.2–40.8) | 10.9 (0.7–32.3) | 0.038 | 17.3 (3.6–51.8) | 18.2 (6.1–51) | 16.2 (3.6–51.8) | 0.077 |
| Expired CO (ppm) | 15 (6–32) | 16 (8–32) | 15 (6–29) | 0.107 | 16 (3–42) | 16 (7–42) | 16 (3–31) | 0.097 |
| HR | 85.5 (12.7) | 84 (12.4) | 87.1 (12.9) | 0.149 | 85.1 (11.2) | 83.9 (11.4) | 86.3 (10.9) | 0.224 |
| QSU Global Craving Score | 1.3 (1–7) | 1.2 (1–4.8) | 1.6 (1–7) | 0.022 | 1.2 (1–5.5) | 1.1 (1–4.9) | 1.4 (1–5.5) | 0.067 |
| MNWS | 4 (0–22) | 3 (0–22) | 5 (0–21) | 0.02 | 3 (0–17) | 3 (0–15) | 4 (0–17) | 0.512 |
| PANAS negative | 11 (10–35)a | 11 (10–35)a | 12 (10–28)a | 0.508 | 11 (10–28) | 11 (10–26) | 11 (10–28) | 0.393 |
| PANAS positive | 26 (10–47)a | 25 (10–47)a | 26 (10–43)a | 0.613 | 25 (10–50) | 26 (10–50) | 24 (10–48) | 0.732 |
| mCES aversion | 6 (2–14) | 6 (2–14) | 6 (2–13) | 0.128 | 4 (2–13) | 4 (2–12) | 4 (2–13) | 0.552 |
| mCES craving | 6 (1–7) | 6 (1–7) | 6 (1–7) | 0.207 | 6 (1–7) | 6 (1–7) | 6 (1–7) | 0.235 |
| mCES reward | 18 (5–35) | 18 (5–35) | 17 (5–35) | 0.624 | 17 (5–35) | 16 (5–35) | 18 (5–35) | 0.247 |
| mCES satisfaction | 14.5 (3–21) | 16 (3–21) | 12 (3–21) | 0.048 | 15 (3–21) | 15 (3–21) | 15 (4–21) | 0.482 |
| mCES respiratory tract sensations | 4 (1–7) | 4 (1–7) | 3 (1–7) | 0.421 | 4 (1–7) | 4 (1–7) | 4 (1–7) | 0.205 |
Bold numbers indicate significant difference (P < 0.05) comparing slow vs fast metabolizers.
Cig 1 and 3, first and third cigarettes of the day, respectively; CO, carbon monoxide; HR, heart rate; mCES, modified Cigarette Evaluation Scale; MNWS, Minnesota Nicotine Withdrawal Scale; NMR, nicotine metabolite ratio; PANAS, Positive and Negative Affect Schedule; QSU, Questionnaire on Smoking Urges-Brief.
After Cig 2.
Nicotine concentrations and scores over time
Figure 2 shows the nicotine plasma concentrations over time, by NMR and genotype. Fast metabolizers had significantly lower area under the nicotine concentration-time curve compared with slow metabolizers by NMR (34.1 (8.4–68.6) vs. 48.8 (15.0–125.4); P < 0.001) and by genotype (37.0 (8.4–79.4) vs. 46.2 (18.0–125.4); P = 0.001). When analyzing the two races separately, these differences remained among whites by NMR (34.9 (10.9–68.6) vs. 49.0 (15.0–91.2); P < 0.001) and by genotype (36.6 (10.9–79.4) vs. 45.4 (18.2–91.2); P = 0.012), and among blacks by NMR (30.2 (8.4–50.5) vs. 46.6 (20.2–125.4); P = 0.003) but not by genotype (P = 0.058). Whites had significant differences (fast lower concentrations compared with slow NMR) for every timepoint except post-Cig 1, post-Cig 2, and post-Cig 3, whereas blacks had significant differences for every timepoint except post-Cig 3.
Figure 2.
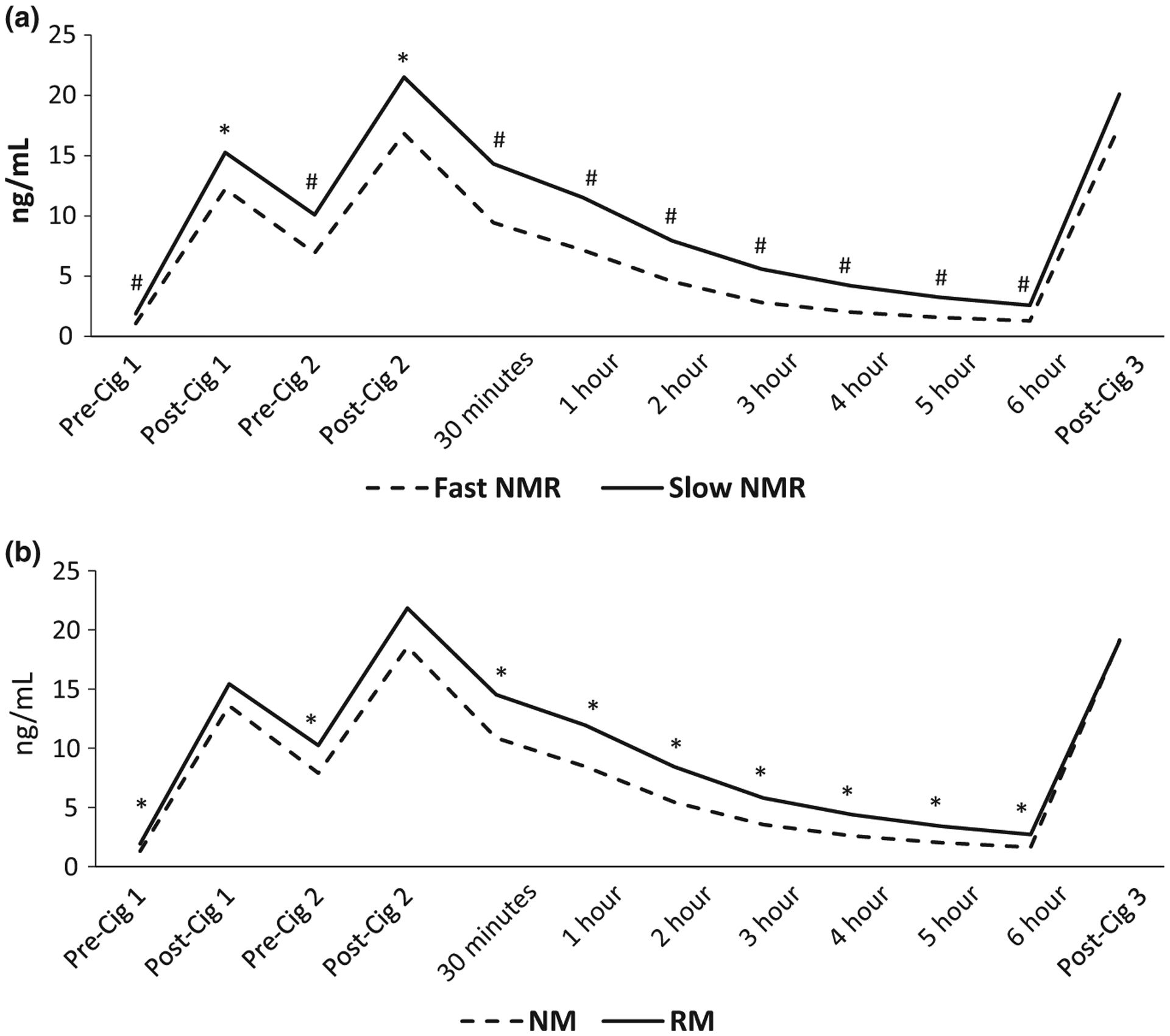
Average plasma nicotine concentrations over time based on NMR (a) and genotype (b). *P < 0.05, #P < 0.001 for differences between groups. NMs and RMs assessed by CYP2A6 genotype. Cig 1, 2, and 3, first, second, and third cigarettes of the day, respectively; NM, normal metabolizer; NMR, nicotine metabolite ratio; RM, reduced metabolizer.
Figures 3 and 4 show the Minnesota Nicotine Withdrawal Scale (MNWS) and QSU, respectively, over time, by NMR and genotype. Significant differences between fast and slow metabolizers were seen for the MNWS and QSU craving scores at various timepoints using the NMR but not with the genotype. Using the area under the effect-time curve (AUEC), fast metabolizers had significantly higher MNWS AUEC (39.3 (10.9–185.3) vs. 35.9 (0.32–112.3); P = 0.02) and QSU AUEC (20.8 (8.4–42.0) vs. 16.6 (6.7–36.5); P = 0.005) compared with slow metabolizers by NMR, but not by genotype. No significant MNWS differences were seen between fast and slow metabolizers when analyzing only whites or blacks. Analysis of the QSU craving over time in whites showed significantly higher scores among fast compared with slow metabolizers at 1, 2, and 3 hours, and in blacks post-Cig 1. In whites, the QSU AUEC was higher among fast compared with slow metabolizers by NMR (20.8 (8.4–42.0) vs. 17.6 (8.4–36.5); P = 0.029) but not by genotype, whereas no significant differences were found in blacks. Regarding other scores (i.e., PANAS and modified Cigarette Evaluation Scale (mCES)), no significant differences were seen by NMR, except higher mCES satisfaction in slow compared with fast metabolizers post-Cig 1 (Table 3). Analysis of only blacks or whites showed no significant differences by NMR regarding those scores. Using the genotypes, a significant difference was seen for the 4-hour PANAS negative score (higher among NMs compared with RMs; P = 0.036).
Figure 3.
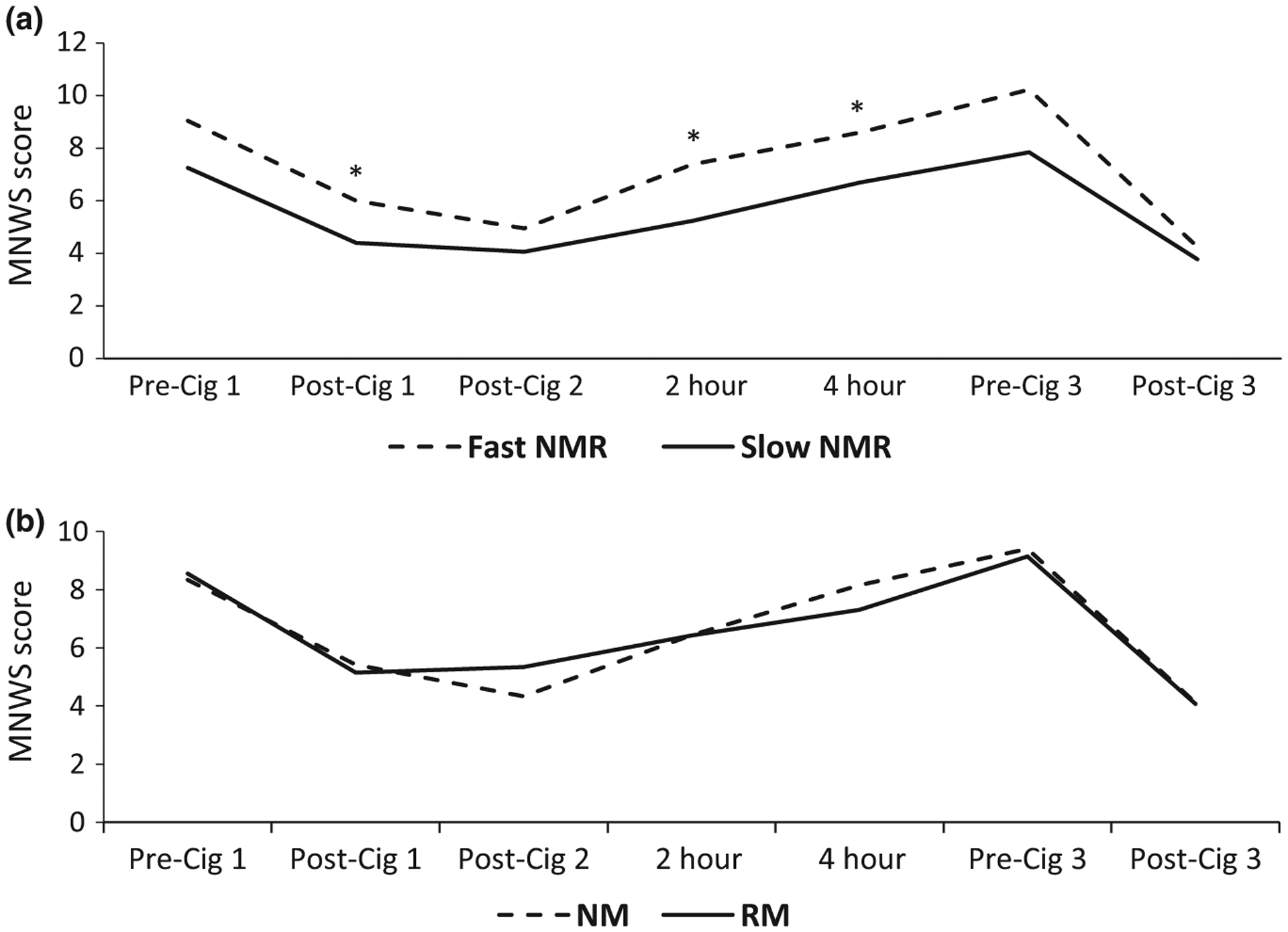
Average MNWS score over time based on NMR (a) and genotype (b). *P < 0.05 for differences between groups. NMs and RMs assessed by CYP2A6 genotype. Cig 1, 2, and 3, first, second, and third cigarettes of the day, respectively; MNWS, Minnesota Nicotine Withdrawal Scale; NM, normal metabolizer; NMR, nicotine metabolite ratio; RM, reduced metabolizer.
Figure 4.
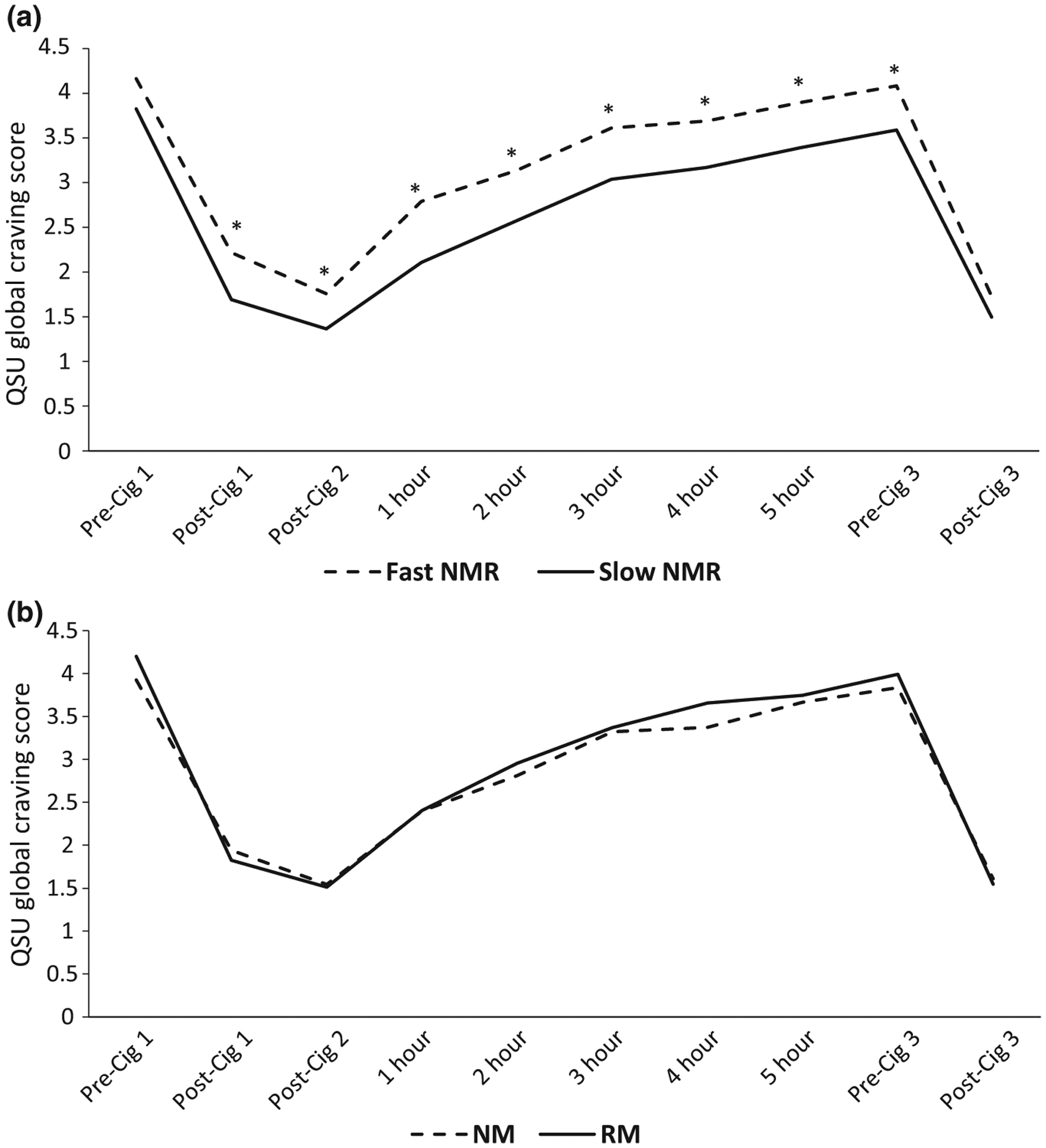
Average QSU Global Craving Score over time based on NMR (a) and genotype (b). *P < 0.05 for differences between groups. NMs and RMs assessed by CYP2A6 genotype. Cig 1, 2, and 3, first, second, and third cigarettes of the day, respectively; NM, normal metabolizer; NMR, nicotine metabolite ratio; QSU, Questionnaire on Smoking Urges; RM, reduced metabolizer.
Boosts and changes
Investigation of presmoking to postsmoking boosts and changes of nicotine, CO, HR, MNWS, QSU craving, and PANAS showed no significant differences between NMR groups. Similar results were found when analyzing only whites or blacks, whereas analysis based on genotypes showed greater CO boost after Cig 3 in RMs compared with NMs (P = 0.009). Cig 3 CO and nicotine boosts were significantly higher than Cig 1 (P < 0.001), whereas Cig 3 HR changes were significantly lower than Cig 1 (P = 0.003).
Explorative general linear model analysis
After individual exploration of age, body mass index (BMI), and urine total nicotine equivalents (TNEs), age emerged as a potentially significant covariate and was included in the general linear model (GLM) analysis together with sex and race. NMR emerged as a significant covariate for mCES respiratory tract sensations (P = 0.047) but not for other responses. Sex emerged as a significant covariate for MNWS pre-Cig 1 (P = 0.022), MNWS and craving during daytime abstinence (P = 0.011 and P = 0.007, respectively; higher scores in women), and PANAS positive post-Cig 3 (P = 0.027; higher scores in men). Age was significant for PANAS negative during daytime abstinence (P = 0.047; higher scores among older participants), PANAS positive post-Cig 3 (P = 0.022), mCES reward (Cig 1, P = 0.007; Cig 2, P = 0.045; Cig 3, P = 0.045), and respiratory tract sensations (Cig 1, P = 0.001; Cig 2, P = 0.002; Cig 3, P = 0.004) (higher scores among younger participants). Race emerged as a statistically significant covariate for mCES satisfaction (post-Cig1, P = 0.017; post-Cig 2, P = 0.021) (higher scores in whites).
DISCUSSION
We found that fast metabolizers by the phenotypic biomarker NMR had shorter nicotine elimination half-lives, lower plasma nicotine concentrations, and greater craving/withdrawal (as assessed by significantly higher AUEC and withdrawal/craving scores for most times during abstinence and PANAS negative scores nearing significance at 6 hours) compared with slow metabolizers over 6 hours of daytime cigarette abstinence. This supports the hypothesis that the NMR is associated with physical dependence and the idea that fast metabolizers are likely smoking more for negative reinforcement. The differences in withdrawal symptoms appear relative quickly after smoking the last cigarette. Many of the effects persisted when analyzing blacks and whites separately, thus indicating that associations with NMR were not attributable to confounding by race.
We did not confirm the hypothesis of positive reinforcement, as indicated by the absence of significant NMR group differences in satisfaction/reward and PANAS positive questionnaires and physiological measurements (e.g., HR changes). Furthermore, despite significant differences in nicotine concentration and the longer abstinence duration, withdrawal/craving scores were higher, but not significantly different, in fast compared with slow metabolizers after the overnight abstinence, possibly because the latter usually represents a normality in smokers’ daily routine, thus triggering less withdrawal/craving effects than during 6 hours of daytime abstinence. On the basis of our study, the noninvasive NMR appears to be a better biomarker of withdrawal effects than the CYP2A6 genotype. This is consistent with the idea that nicotine metabolism is also mediated by other genes and environmental factors.
Previous studies have shown an association of fast NMR with lower nicotine levels and stronger craving 1 week into a quit attempt with transdermal nicotine7 and higher withdrawal after 24-hour abstinence in adolescent smokers.6 Smokers in the top NMR quartile had greater craving compared with those in the lowest quartile after overnight abstinence,12 whereas in a study with community-based samples, fast metabolizers experienced significantly higher anxiety compared with slow metabolizers with transdermal nicotine treatment.13 Despite differences and limitations of those studies (e.g., small sample size in the adolescent study), these findings all support that fast metabolizers experience more severe withdrawal/craving during abstinence associated with differences in the rate of nicotine metabolism.
The observation that faster nicotine metabolism is associated with more severe withdrawal symptoms has important clinical implications. Withdrawal symptoms can present a major obstacle to smoking cessation and increase relapse risk.26,27 Therefore, specific supporting measures (e.g., higher than standard nicotine replacement doses,7 behavioral intervention,13 and use of varenicline to relieve craving/withdrawal symptoms11,28) should be considered for smokers with high NMR attempting to quit. However, other studies8,9 failed to find an association between high NMR and craving/withdrawal, possibly because of methodological and sample differences (e.g., use of bupropion and not nicotine replacement therapy8).
Positive reinforcement is another suggested mechanism for the greater dependence and lower quitting rates in fast metabolizers. This is supported by a brain imaging study in which fast metabolizers exhibited greater reactivity in dopamine-dependent reward circuitry after visual smoking cues14 and a study with administration of nicotine intravenously after overnight abstinence.12 Possible reasons for the different findings in our study might be stronger reward effects after intravenous administration of nicotine compared with smoking and differences between neural responses in functional magnetic resonance imaging and subjective feelings of reward.
Our data are consistent with previous findings that blacks have, on average, slower nicotine metabolism compared with whites.10,17–21 Blacks also smoke fewer CPDs,29 which could be related to slower metabolism,30 but they also smoke more intensively31 and have higher nicotine intake per cigarette.22 Blacks and slow NMR participants had higher COT levels despite lower TNEs, consistent with other pharmacokinetic observations on effects of reduced CYP2A6 activity on COT levels.32,33 Separate analysis of blacks showed significant differences in nicotine concentration and expired CO between fast and slow NMR at more timepoints compared with whites, but less significant differences in craving scores, possibly because of the smaller sample size. Our findings further indicate possible higher reward effects in whites, which might be associated with racial or NMR differences.
Regarding sex differences, similar to previous studies,23 female participants were significantly faster metabolizers than male participants. Our findings suggest that women might experience more negative effects during abstinence. The higher NMR and withdrawal do not offer an explanation regarding the fewer CPDs reported in women, which may be associated with non-nicotine-related behavior factors.
Concordance between genotype and NMR was not complete. Although several CYP2A6 gene variants have been shown to have an effect on smoking behavior,21,34 the currently identified variants explain only a small percentage of the variation in nicotine metabolism.7,35 In a recent genome-wide association study of NMR, >700 significantly associated variants were identified on chromosome 19q13 (the loci of CYP2A6)36; further characterization will improve utility of the CYP2A6 genotype. In addition, there are likely unaccounted for effects of uridine diphosphate glucuronlyltransferase and other genetic variations and hormonal and environmental effects. Next to estrogen, other substances (e.g., phenobarbital, rifampin, and broccoli) can induce CYP2A6, whereas others (e.g., grapefruit and menthol) can inhibit its activity.21 It is, therefore, not surprising that the CYP2A6 genotype did not predict outcomes as well as the NMR.
Limitations of our study include that, despite our intention, achieved plasma nicotine baseline after the loading cigarettes was not equal in the two NMR groups. The numbers of blacks and women were relatively small. Furthermore, we studied only 6-hour abstinence, whereas longer abstinence periods would be associated with more intense withdrawal/craving, and factors other than nicotine dependence might also affect the desire to smoke/craving for tobacco. Despite these limitations, the present study clearly demonstrates in a prospectively stratified design that high NMR is associated with lower blood nicotine levels and higher craving/withdrawal during brief smoking abstinence.
In conclusion, our study provides evidence that fast metabolizers by NMR are likely smoking more for negative reinforcement (i.e., to relieve craving/withdrawal symptoms) and, to a lesser extent, if at all, for positive reinforcement (i.e., greater reward effects after smoking). The noninvasive saliva NMR appears to be a better biomarker of withdrawal/craving effects than the CYP2A6 genotype.
METHODS
Participants and recruitment
Participants were healthy, or with stable medical or psychiatric conditions, volunteers, between the ages of 18 and 70 years, of self-reported black or white descent (both parents and grandparents of same race), who smoked at least five CPDs regularly for the past year. Participants were recruited through Craigslist, flyers, and newspaper advertisements. At an initial screening visit, medical history was provided by a questionnaire. A physical examination was performed on entry on the research ward. Participants provided a saliva sample for COT to verify smoking status (i.e., COT ≥50 ng/mL) and to assess NMR, and they completed a demographics form, the Fagerstrom Test for Cigarette Dependence (including time to first cigarette after awakening in the morning and CPDs),37 and questions regarding amount and frequency of alcohol use. On the basis of the saliva NMR, participants were stratified as fast or slow metabolizers. On the basis of prior studies,38 cut points were ≤0.20 and ≥0.37, respectively, in blacks, and ≤0.26 and ≥0.45, respectively, in whites (all within 0.01). The study was approved by the University of California, San Francisco, Institutional Review Board.
Study procedures
Eligible participants were admitted to the research ward at Zuckerberg San Francisco General Hospital the evening before the study to enforce 12 hours of overnight abstinence (last cigarette at 9 PM). In the evening, a urine sample was collected for TNEs. In the morning of the study day, participants had an intravenous catheter inserted for blood drawing, and a light breakfast was served. One hour later (≈9 AM), participants smoked two cigarettes (Marlboro Regular for nonmenthol smokers and Marlboro Menthol for menthol smokers) with a standardized puffing protocol (i.e., one puff of 2-second duration every 38 seconds for a total of 10 puffs from each cigarette). Cig 2 was smoked approximately 26 minutes after Cig 1. These “loading cigarettes” were intended to relieve overnight withdrawal symptoms and place participants in a similar nicotine-satiated baseline state across the study groups. Participants then abstained from smoking for 6 hours, which represents on average three half-lives of nicotine, allowing an estimate of elimination half-life and adequate time for the development of significant withdrawal/craving symptoms.39–41 After 6 hours of daytime abstinence, Cig 3 of the smoker’s own brand was smoked in the smoker’s usual way. This “reward cigarette” was followed by a 90-minute period of monitored “free” ad libitum smoking (these results will be described in another publication). A modified version of the MNWS42 was administered before Cig 1 and Cig 3; immediately after Cig 1, Cig 2, and Cig 3; and 2 and 4 hours after Cig 2. The QSU43 was administered at the same timepoints with the MNWS, as well as 1, 3, and 5 hours after Cig 2. The mCES44 was administered after each cigarette. The PANAS45 was assessed before Cig 1 and Cig 3; as well as immediately and 2 and 4 hours after Cig 2 and immediately after Cig 3. Nicotine blood concentrations were measured before and 2 minutes after each loading cigarette; then at 30 minutes and 1, 2, 3, 4, and 5 hours after Cig 2; and before and 2 minutes after Cig 3. One sample (baseline) was also used to calculate plasma NMR to validate saliva NMR. CO and HR were measured before each blood sample.
Study measures
The modified MNWS (excluding items relating to sleep disturbance and constipation) included eight items (angry/irritable/frustrated, anxious/nervous, depressed mood/sad, desire or craving to smoke, difficulty concentrating, increased appetite/hungry, restless, and impatient), rated on a scale from 0 (none) to 4 (severe). The QSU total (global) craving score is the average of all responses (mean of 10 items, rated on a scale from 1 (strongly disagree) to 7 (strongly agree)). The mCES (total of 12 items) was used to assess satisfaction (three items), psychological reward (five items), aversion (two items), craving reduction (one item), and respiratory tract sensations as responses to smoking (one item). The mCES is rated on a scale from 1 (not at all) to 7 (extremely), and scoring is done by adding the item scores for each scale. The PANAS included items assigned as positive or negative affect (each score is the sum of 10 items, rated on a scale from 1 (very slightly/not at all) to 5 (extremely)). Plasma nicotine and CO boosts represent changes in levels between presmoking and postsmoking.
Laboratory methods
Saliva 3HC and COT were measured by liquid chromatography-tandem mass spectrometry,46 and plasma nicotine was measured by gas chromatography-tandem mass spectrometry47 (LOQ, 1 ng/mL). TNE in urine was calculated by taking the total (molar sum of free and glucuronide conjugate) of six metabolites of nicotine (COT, 3HC, nicotine-n-oxide, cotinine-n-oxide, nornicotine, and norcotinine) assayed by liquid chromatography-tandem mass spectrometry and normalized by urine creatinine.2
Genotyping of CYP2A6*1X2, CYP2A6*2, CYP2A6*4, CYP2A6*9, CYP2A6*12, CYP2A6*20, CYP2A6*23, CYP2A6*24, CYP2A6*25, CYP2A6*26, CYP2A6*27, CYP2A6*28, and CYP2A6*35 was performed at the University of Toronto, according to previously described protocols.18,48 Those without variants (or with the duplication CYP2A6*1X2 variant) were characterized as NMs, those with a single copy of CYP2A6*9 or CYP2A6*12 were characterized as intermediate metabolizers, and those with two copies or any other reduced or loss-of-function variant were characterized as slow metabolizers. For analyses using the genotype, slow and intermediate metabolizers were grouped together as RMs.
Data cleaning and analysis
Numerical data are presented as arithmetic mean and SD if normally distributed or median and range if not normally distributed, and nominal data are presented as proportion (percentage). Measurements below the LOQ were replaced by .49 Because creatinine levels can vary by sex, age, BMI, and race, we also used a covariate-adjusted standardization method50 to control measurement error bias. Skewed score values were log transformed before analysis. Differences were tested using the χ2 test for categorical variables, the t test for normally distributed continuous variables, and the Mann-Whitney test for non-parametric variables. Missing data were not imputed. P < 0.05 was considered statistically significant. Subjective withdrawal (MNWS) and craving (QSU) effects were also determined as the AUEC using the trapezoidal method. Next to our main analysis investigating differences between slow and fast NMR, we also performed an additional analysis of whites and blacks only to investigate possible racial differences. Furthermore, in an additional explorative analysis, we included sex and race, which have been shown to influence NMR as covariates in a GLM, with fast and slow NMR entered as a categorical between-subject factor predicting withdrawal and reward outcomes. Other covariates (i.e., age, BMI, creatinine-corrected TNEs, and covariate-adjusted TNEs) were also individually explored for their potential contributions on the NMR effects. For withdrawal after overnight abstinence and reward outcomes after each cigarette, a univariate GLM was used, whereas for the 6-hour abstinence period, a repeated-measures GLM over time was used with the first timepoint (i.e., post-Cig 2) as a covariate. Analyses were conducted using SPSS statistical software (IBM SPSS Statistics 23.0). Covariate adjustment of TNEs was performed using SAS version 9.4 (SAS Institute, Cary, NC). Nicotine elimination half-lives were estimated from plasma nicotine concentrations using Phoenix WinNonlin 6.3 (Pharsight, Mountain View, CA).
Supplementary Material
Table S1. Comparisons of slow and fast metabolizers in the two racial subgroups (mean (SD), median (range), or number (percentage)).
Table S2. Comparisons of slow and fast metabolizers in the two sex subgroups (mean (SD), median (range), or number (percentage)).
Figure S1. NMR frequency histogram.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The nicotine metabolite ratio (NMR) is a phenotypic biomarker that is highly correlated with the rate of nicotine clearance, which is an important determinant of smoking behavior and nicotine dependence.
WHAT QUESTION DID THIS STUDY ADDRESS?
The present study aimed to examine the effect of NMR on nicotine withdrawal symptoms and the response to smoking a cigarette after overnight abstinence and 6 hours of daytime abstinence.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Fast metabolizers by NMR had lower blood nicotine concentrations and greater craving/withdrawal scores compared with slow metabolizers, but not greater reward after smoking, thus supporting the idea that fast metabolizers are likely smoking more to relieve craving/withdrawal symptoms.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Selection of medications and/or doses of medications on the basis of the importance of relieving craving and nicotine withdrawal symptoms, guided by NMR, may be useful in optimizing smoking cessation therapy.
ACKNOWLEDGMENTS
We thank Drs. Caryn Lerman and Andrew Strasser (University of Pennsylvania) for advice in experimental design; Trisha Mao, Lisa Yu, and Lawrence Chan for performing analytical chemistry; Faith Allen for data management; and Newton Addo-Otto for statistical support.
FUNDING
This research was supported by grants from the National Institute on Drug Abuse (R01 DA031193, P30 DA012393, and U01 020830) and from the National Center for Research Resources (S10 RR026437). E.L.’s research fellowship was supported by the Bangerter-Rhyner Foundation. We acknowledge support from the Canada Research Chairs program (R.F.T., the Canada Research Chair in Pharmacogenomics), Canadian Institutes of Health Research grant (FDN-154294), and the Campbell Family Mental Health Research Institute of Centre for Addiction and Mental Health. Clinical Trials Registry: NCT01627392.
Footnotes
SUPPORTING INFORMATION
Supplementary information accompanies this paper on the Clinical Pharmacology & Therapeutics website (www.cpt-journal.com).
CONFLICT OF INTEREST
As an Associate Editor for Clinical Pharmacology & Therapeutics, R.F.T. was not involved in the review or decision process for this article. N.L.B. is a consultant to Pfizer and Achieve Life Sciences, companies that market or are developing smoking cessation medications, and has been a paid expert witness in litigation against tobacco companies. R.F.T. has served as a paid consultant to Apotex and Quinn Emmanuel and received unrestricted research funding from Pfizer as part of the Global Research Awards for Nicotine Dependence, an independently reviewed competitive grants program.
References
- 1.Benowitz NL Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu. Rev. Pharmacol. Toxicol 49, 57–71 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dempsey D et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin. Pharmacol. Ther 76, 64–72 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Lea RA, Dickson S & Benowitz NL Within-subject variation of the salivary 3HC/COT ratio in regular daily smokers: prospects for estimating CYP2A6 enzyme activity in large-scale surveys of nicotine metabolic rate. J. Anal. Toxicol 30, 386–389 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Mooney ME, Li ZZ, Murphy SE, Pentel PR, Le C & Hatsukami DK Stability of the nicotine metabolite ratio in ad libitum and reducing smokers. Cancer Epidemiol. Biomarkers Prev 17, 1396–1400 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.St Helen G et al. Reproducibility of the nicotine metabolite ratio in cigarette smokers. Cancer Epidemiol. Biomarkers Prev 7, 1105–1114 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubinstein ML, Benowitz NL, Auerback GM & Moscicki AB Rate of nicotine metabolism and withdrawal symptoms in adolescent light smokers. Pediatrics 122, e643–e647 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lerman C et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin. Pharmacol. Ther 79, 600–608 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Patterson F et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin. Pharmacol. Ther 84, 320–325 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N & Lerman C Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol. Biochem. Behav 92, 6–11 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho MK et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin. Pharmacol. Ther 85, 635–643 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerman C et al. Use of nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomized, double-blind placebo-controlled trial. Lancet Respir. Med 3, 131–138 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sofuoglu M, Herman AI, Nadim H & Jatlow P Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology 37, 1509–1516 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufmann A et al. Rate of nicotine metabolism and smoking cessation outcomes in a community-based sample of treatment-seeking smokers. Addict. Behav 51, 93–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang DW, Hello B, Mroziewicz M, Fellows LK, Tyndale RF & Dagher A Genetic variation in CYP2A6 predicts neural reactivity to smoking cues as measured using fMRI. NeuroImage 60, 2136–2143 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Kulak JA, Cornelius ME, Fong GT & Giovino GA Differences in quit attempts and cigarette smoking abstinence between whites and African Americans in the United States: literature review and results from the International Tobacco Control US Survey. Nicotine Tob. Res 18 (suppl. 1), 79–87 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benowitz NL, Perez-Stable EJ, Fong I, Modin G, Herrera B & Jacob P Ethnic differences in N-glucuronidation of nicotine and cotinine. J. Pharmacol. Exp. Ther 291, 1196–1203 (1999). [PubMed] [Google Scholar]
- 17.Perez-Stable EJ, Herrera B, Jacob P & Benowitz NL Nicotine metabolism and intake in black and white smokers. JAMA 280, 152–156 (1998). [DOI] [PubMed] [Google Scholar]
- 18.Mwenifumbo JC et al. Novel and established CYP2A6 alleles impair in vivo nicotine metabolism in a population of black African descent. Hum. Mutat 29, 679–688 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Mwenifumbo JC, Sellers EM & Tyndale RF Nicotine metabolism and CYP2A6 activity in a population of black African descent: impact of gender and light smoking. Drug Alcohol Depend. 89, 24–33 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Kaivosaari S, Toivonen P, Hesse LM, Koskinen M, Court MH & Finel M Nicotine glucuronidation and the human UDP-glucuronosyltransferase UGT2B10. Mol. Pharmacol 72, 761–768 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Tanner JA & Tyndale RF. Variation in CYP2A6 activity and personalized medicine. J. Pers. Med 7, pii: E18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB & Wang J Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am. J. Epidemiol 169, 236–248 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Benowitz NL, Lessov-Schlaggar CN, Swan GE & Jacob P Female sex and oral contraceptive use accelerate nicotine metabolism. Clin. Pharmacol. Ther 79, 480–488 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Higashi E et al. Human CYP2A6 is induced by estrogen via estrogen receptor. Drug Metab. Dispos 35, 1935–1941 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Benowitz NL & Hatsukami D Gender differences in the pharmacology of nicotine addiction. Addict. Biol 3, 383–404 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Killen JD & Fortmann SP Craving is associated with smoking relapse: findings from three prospective studies. Exp. Clin. Psychopharmacol 5, 137–142 (1997). [DOI] [PubMed] [Google Scholar]
- 27.Shiffman S, West R, Gilbert D, & SRNT Work Group on the Assessment of Craving and Withdrawal in Clinical Trials. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine Tob. Res 6, 599–614 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Prochaska JJ & Benowitz NL The past, present, and future of nicotine addiction therapy. Annu. Rev. Med 67, 467–486 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muscat JE, Richie JP Jr & Stellman SD Mentholated cigarettes and smoking habits in whites and blacks. Tob. Control 11, 368–371 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benowitz NL, Pomerleau OF, Pomerleau CS & Jacob P Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob. Res 5, 621–624 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Cropsey KL, Weaver MF, Eldridge GD, Villalobos GC, Best AM & Stitzer ML Differential success rates in racial groups: results of a clinical trial of smoking cessation among female prisoners. Nicotine Tob. Res 11, 690–697 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu AZ et al. The ability of plasma cotinine to predict nicotine and carcinogen exposure is altered by differences in CYP2A6: the influence of genetics, race, and sex. Cancer Epidemiol. Biomarkers Prev 22, 708–718 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benowitz NL, St Helen G, Dempsey DA, Jacob P & Tyndale RF Disposition kinetics and metabolism of nicotine and cotinine in African American smokers: impact of CYP2A6 genetic variation and enzymatic activity. Pharmacogenet. Genomics 26, 340–350 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyndale RF & Sellers EM Genetic variation in CYP2A6-mediated nicotine metabolism alters smoking behavior. Ther. Drug Monit 24, 163–171 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Swan GE, Benowitz NL, Lessov CN, Jacob P, Tyndale RF & Wilhelmsen K Nicotine metabolism: the impact of CYP2A6 on estimates of additive genetic influence. Pharmacogenet. Genomics 15, 115–125 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Loukola A et al. A genome-wide association study of a biomarker of nicotine metabolism. PLoS Genet. 11, e1005498 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heatherton TF, Kozlowski LT, Frecker RC & Fagerstrom KO The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br. J. Addict 86, 1119–1127 (1991). [DOI] [PubMed] [Google Scholar]
- 38.Benowitz NL, Dains KM, Dempsey D, Wilson M & Jacob P Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob. Res 13, 772–783 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN., Elins, J.L. & Benowitz, N.L. Nicotine blood levels and subjective craving for cigarettes. Pharmacol. Biochem. Behav 66, 553–558 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Teneggi V, Tiffany ST, Squassante L, Milleri S, Ziviani L & Bye A Smokers deprived of cigarettes for 72 h: effect of nicotine patches on craving and withdrawal. Psychopharmacology 164, 177–187 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Schuh KJ & Stitzer ML Desire to smoke during spaced smoking intervals. Psychopharmacology 120, 289–295 (1995). [DOI] [PubMed] [Google Scholar]
- 42.Hughes JR & Hatsukami D Signs and symptoms of tobacco withdrawal. Arch. Gen. Psychiatry 43, 289–294 (1986). [DOI] [PubMed] [Google Scholar]
- 43.Cox LS, Tiffany ST & Christen AG Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob. Res 3, 7–16 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Cappelleri J, Bushmakin A, Baker C, Merikle E, Olufase A & Gilbert D Confirmatory analysis and reliability of the modified cigarette evaluation questionnaire. Addict. Behav 32, 912–923 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Watson D, Clark LA & Tellegen A Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol 54, 1063–1070 (1988). [DOI] [PubMed] [Google Scholar]
- 46.Jacob P, Yu L, Duan M, Ramos L, Yturralde O & Benowitz NL Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 879, 267–276 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacob P, Yu L, Wilson M & Benowitz NL Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3′,3′-d2 in humans. Biol. Mass Spectrom 20, 247–252 (1991). [DOI] [PubMed] [Google Scholar]
- 48.Wassenaar CA, Zhou Q & Tyndale RF CYP2A6 genotyping methods and strategies using real-time and end point PCR platforms. Pharmacogenomics 17, 147–162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacob P et al. Nicotine, carbon monoxide, and carcinogen exposure after a single use of a waterpipe. Cancer Epidemiol. Biomarkers Prev 20, 2345–2353 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Brien KM, Upson K, Cook NR & Weinberg CR Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ. Health Perspect 124, 220–227 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparisons of slow and fast metabolizers in the two racial subgroups (mean (SD), median (range), or number (percentage)).
Table S2. Comparisons of slow and fast metabolizers in the two sex subgroups (mean (SD), median (range), or number (percentage)).
Figure S1. NMR frequency histogram.


