Abstract
Determining the spatial distribution of human papillomavirus (HPV) and performing accurate public health analyses helps to distinguish areas of healthcare that require further research, and enables therapeutic techniques and approaches in healthcare to be focused more accurately. A total of 4,560 women were enrolled in the present study. Flow-through hybridization and gene chip assays were used to detect the genotypes of HPV infection. Heat maps were then generated to present the spatial distribution of HPV infections in Zhejiang Province according to genotype. Of the exfoliated cervical cell samples from the 4,560 women, HPV was detected in 1,886 samples. HPV-16, −58, −52 and −18 were the most prevalently identified genotypes in the population included in the present study. HPV-16 and −58 infections were mainly distributed in the northern and central regions of Zhejiang Province, such as in Hangzhou and Shaoxing, where the prevalence was higher than that in the southern regions (P<0.05). HPV-18 infection was widespread throughout Zhejiang Province, but had a much lower infection rate in Ningbo and Huzhou (P<0.05). High infection rates of HPV-52 were mainly detected in Hangzhou and the eastern coastal areas of Wenzhou, with a relatively low rate of infection in the center of the province (P<0.05). In conclusion, HPV-16, −58, −52 and −18 were the four most prevalent HPV genotypes observed in Zhejiang Province. Heat maps were created to display the spatial distribution of HPV infection according to genotype, which varied by geographical regions. The results indicate that for individuals in Ningbo or Wenzhou, bivalent or quadrivalent vaccines may be suitable, but for those in Hangzhou and Shaoxing, nonavalent vaccines are strongly recommended.
Keywords: human papillomavirus, distribution, heat maps, genotype
Introduction
Cervical cancer ranks fourth in terms of both incidence and mortality, according to data reported by GLOBOCAN 2018, with an estimated 311,000 deaths annually in 2018 (1).
Human papillomavirus (HPV), which is spread primarily through sexual contact, has been established as the essential causative agent for cervical intraepithelial lesions and cervical cancer (2,3). There are >200 genotypes of HPV that have been identified, of which 15 are oncogenic and classified as high-risk HPVs (hr-HPVs) (4,5), and result in the development of ≥99.7% of cervical cancer cases (6).
The prevalence of HPV is 11–12% worldwide (7), and the two most widespread types of hr-HPV are HPV-16 and −18. A survey of healthy women conducted by the International Agency for Research on Cancer confirmed that there is geographic variation in HPV infection rates and types; for example, HPV-45 and −33 are most prevalent in Africa, HPV-33 and −31 are most prevalent across Europe, HPV-31, −33 and −45 are most prevalent in the USA, and HPV-58 and −52 are the most prevalent in Asia (5). Furthermore, the Retrospective International Survey and HPV Time Trends Study Group analyzed the prevalence of HPV subtypes in cervical cancer and observed that the geographical variations in HPV distribution in healthy women was associated with the variation in cancer biology (4). Therefore, it is important to survey the distribution of regional HPV genotypes, in order to guide the effectiveness of vaccination programs, calculate health economics and develop improved vaccines (8).
China, with its vast territory, has a population that comprises 19% of the total global population and is currently unvaccinated (8). Although several reports have demonstrated that HPV-16, −18, −52 and −58 are the most common genotypes (9–11) in Zhejiang Province, no specific type of HPV has been demonstrated to be of the greatest concern. The present study utilized a model based on a Geographic Information System (GIS) to represent the spatial distribution of HPV and perform accurate public health analyses (12–14). This should aid in determining future healthcare, and enable new therapeutic techniques and approaches to be more accurately focused.
The aim of the present study was to establish the urban baseline distribution of hr-HPV genotypes in Zhejiang Province, China, in order to recommend appropriate specific vaccines for this population.
Patients and methods
Patients
Participants were enrolled during their health examination between January 2016 and December 2017 in Zhejiang Cancer Hospital (Hangzhou, China). The inclusion criteria were as follows: i) Mentally and physically competent; ii) never received a HPV vaccine; iii) stably located (census registration) in Zhejiang; iv) a permanent resident of Zhejiang (>60% of the year resides in a permanent residence), and v) had previously had sexual intercourse. Of these 8,897 participants, 2,669 did not meet the inclusion criteria and 1,668 missed the follow-up. Thus, a total of 4,560 women were enrolled in the present study. Each participant provided written informed consent prior to the start of the study. The protocol was approved by the Ethics Committee of Zhejiang Cancer Hospital (approval no. IRB-2019-75).
HPV detection and typing
A senior gynecologist performed the pelvic examination, and collected samples of exfoliated cervical cells for HPV DNA detection. The HPV GenoArray Test kit (HybriBio Ltd.) was used to differentiate between 13 hr-HPV genotypes (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59 and −68).
All the exfoliated cervical cell samples were obtained by cytobrush and collected in Surepath™ solution (TriPath Imaging, Inc.). Next, 0.05 g of the samples was centrifuged at 20,784 × g for 1 min at 4°C to remove supernatant and the pellet was resuspended in 200 ml PBS buffer. DNA extracts were prepared with the QIAamp DNA Blood Mini kit 3 (Qiagen, Inc.) following the Blood and Body Fluid Spin protocol. HPV genotyping by HybriMax used an HPV GenoArray Test kit (HybriBio Ltd.) according to the manufacturer's instructions (15). A negative control (pure water) and a positive control (HPV DNA from low density gene chip hybrid membrane in HPV GenoArray test kit) were used to avoid false-positive reactions. HPV genotyping was performed following a HPV GenoArray test kit (HybriBio, Ltd.).
Heat maps
All the detailed geographical information of the participants in the present study was obtained using EsgynDB (Esgyn Corporation). EsgynDB is a large database with platforms such as Hadoop, Mobile Measurement Partner, stream processing and in-memory databases, and fusion of internal data (operation, business and management support systems), that can use the internet to extract multidimensional data. The GIS is one of these functions, which possesses omnibearing position data, and has improved data acquisition and analysis capability when compared with GRPS positioning. In order to determine the spatial distribution of the 4,560 women, their locations were pin-pointed according to longitude and latitude coordinates using the GIS. The accuracy of detection is 0.01 degrees of longitude and latitude (the longitude distance is ~1,000 m, and the latitude distance is ~1,113 m; thus, each unit is 1,000×1,113 m). To decrease the risk of bias, data for the unit were discarded if the total population in each unit was <3 study participants. The heat maps were generated directly in the database, based on the HPV positive infection rate (0–100%). EsgynDB is a platform for managing big data and performing analyses, but is not available populated with the information on subject location that was used in the present study.
Statistical analysis
Data analyses were performed using SPSS 20 software (IBM Corp.). The χ2 test was performed to compare hr-HPV genotype distributions across regions. P<0.05 was considered to indicate a statistically significant difference.
Results
HPV positive rate and infection status
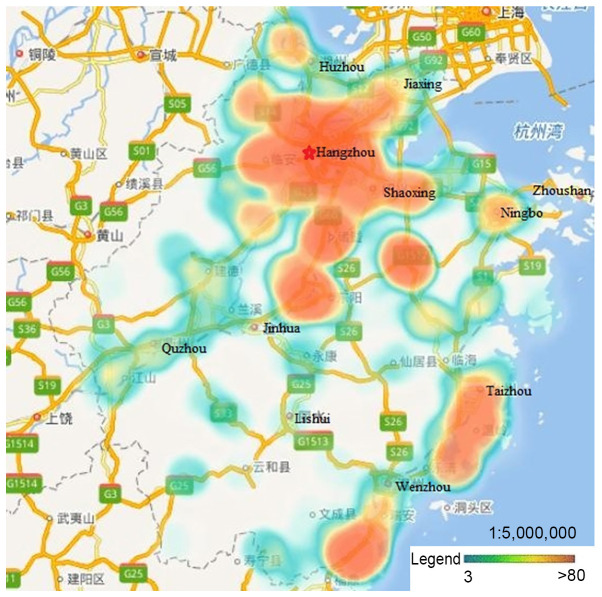
The mean age of the enrolled women was 49.05 years (range, 12–90 years). Of the 4,560 cervical samples, 1,886 (41.4%) had detectable HPV. The 13 hr-HPV genotypes had infection rates that increased with age; the most prevalent type was HPV-16 (19.85%), followed by HPV-58, −52 and −18 with lower rates (7.74, 5.15 and 3.55%, respectively), and other HPV types with rates ranging from 2.96 down to 0.35% (Table I). The participants were mainly from Hangzhou, Ningbo, Wenzhou, Huzhou, Jiaxing, Shaoxing, Jinhua and Taizhou. There were few data available from Zhoushan, Quzhou and Lishui, as well as other rural or remote areas (Fig. 1). The proportions of single-, co-, tri-, tetra- and more genotype infections are presented in Table II. A total of 1,604 (35.18%) participants were infected with a single HPV genotype, and 282 participants were infected with more than one HPV genotype, with 242 (5.31%), 34 (0.75%), and 5 (0.11%) having co-, tri- and tetra-genotype infections, respectively.
Table I.
Epidemiological distribution of HPV genotype infection rates (%) according to age.
| Age (years) | |||||||
|---|---|---|---|---|---|---|---|
| Genotype | <30 | 30–39 | 40–49 | 50–59 | 60–69 | ≥70 | Total |
| HPV16 | 15.53 | 14.23 | 17.72 | 21.59 | 26.71 | 26.78 | 19.85 |
| HPV18 | 2.48 | 2.53 | 2.72 | 4.60 | 5.04 | 2.73 | 3.55 |
| HPV31 | 0.00 | 1.33 | 1.46 | 2.88 | 2.52 | 3.28 | 2.01 |
| HPV33 | 3.73 | 3.06 | 2.79 | 2.42 | 4.45 | 1.64 | 2.96 |
| HPV35 | 0.00 | 0.13 | 0.33 | 0.39 | 0.74 | 0.00 | 0.35 |
| HPV39 | 4.35 | 1.86 | 2.06 | 2.34 | 2.67 | 0.01 | 2.24 |
| HPV45 | 0.62 | 0.53 | 0.13 | 0.70 | 0.30 | 0.00 | 0.39 |
| HPV51 | 2.48 | 1.46 | 1.53 | 2.49 | 2.82 | 3.28 | 2.08 |
| HPV52 | 3.73 | 3.06 | 5.04 | 4.52 | 7.57 | 11.48 | 5.15 |
| HPV56 | 0.00 | 0.53 | 0.46 | 0.86 | 0.89 | 2.73 | 0.72 |
| HPV58 | 5.59 | 5.19 | 6.64 | 8.03 | 11.42 | 13.66 | 7.74 |
| HPV59 | 0.62 | 0.13 | 0.40 | 1.17 | 0.45 | 0.00 | 0.57 |
| HPV68 | 1.86 | 1.20 | 0.86 | 0.94 | 0.74 | 0.55 | 0.94 |
| Total | 32.92 | 31.38 | 42.14 | 44.58 | 54.45 | 55.19 | 41.36 |
HPV, human papillomavirus.
Figure 1.
Distribution of study participants. Participants were mainly from Hangzhou, Ningbo, Wenzhou, Huzhou, Jiaxing, Shaoxing, Jinhua and Taizhou, with few data from Zhoushan, Quzhou, Lishui, and other rural or remote areas.
Table II.
Numbers of positive HPV tests according to genotype and infection status.
| Multiple-genotype infection | |||||
|---|---|---|---|---|---|
| Genotype | Single-genotype infection | Co- | Tri- | Tetra- | Penta- |
| HPV16 | 735 | 149 | 17 | 3 | 1 |
| HPV18 | 120 | 37 | 3 | 2 | 0 |
| HPV31 | 62 | 21 | 7 | 1 | 1 |
| HPV33 | 81 | 38 | 14 | 2 | 0 |
| HPV35 | 6 | 7 | 2 | 1 | 0 |
| HPV39 | 61 | 33 | 7 | 1 | 0 |
| HPV45 | 8 | 9 | 0 | 1 | 0 |
| HPV51 | 51 | 31 | 12 | 0 | 1 |
| HPV52 | 161 | 58 | 12 | 3 | 1 |
| HPV56 | 18 | 10 | 3 | 1 | 1 |
| HPV58 | 267 | 67 | 17 | 2 | 0 |
| HPV59 | 15 | 7 | 3 | 1 | 0 |
| HPV68 | 19 | 17 | 5 | 2 | 0 |
| Total | 1,604 | 484 | 102 | 20 | 5 |
| No. of patients | 1,604 | 242 | 34 | 5 | 1 |
HPV, human papillomavirus.
HPV geographical distribution
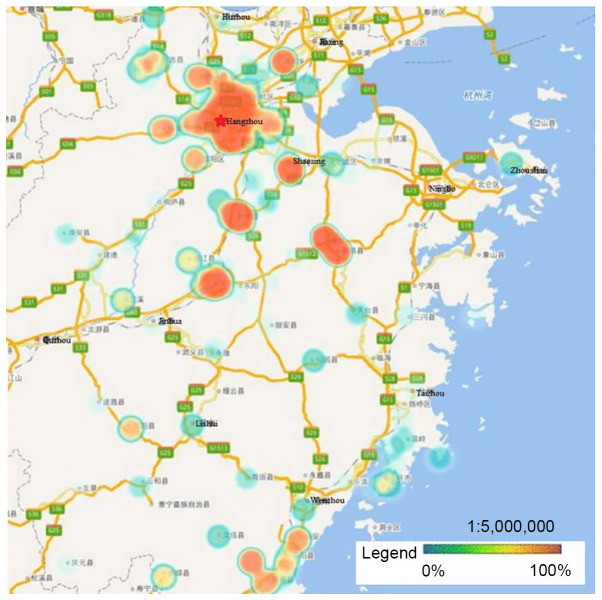
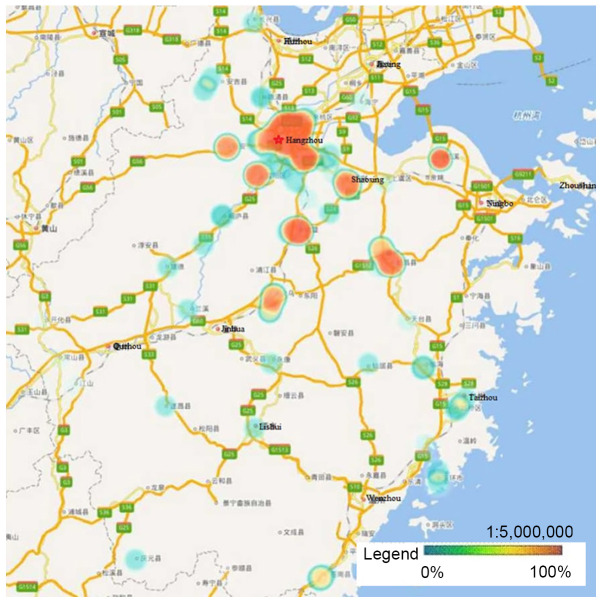
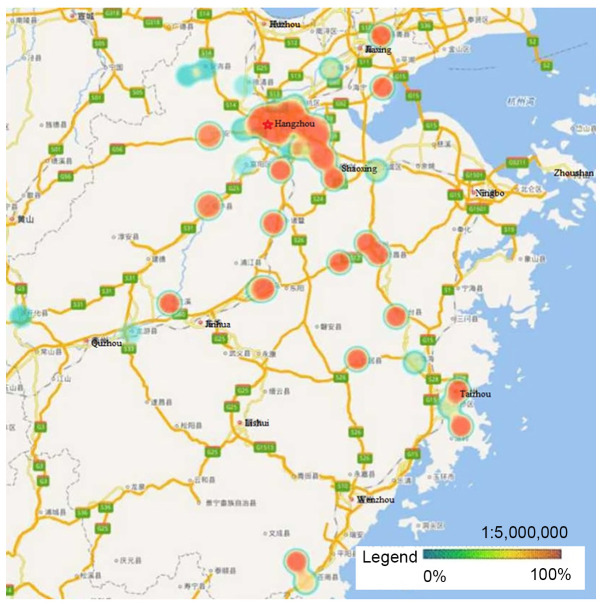
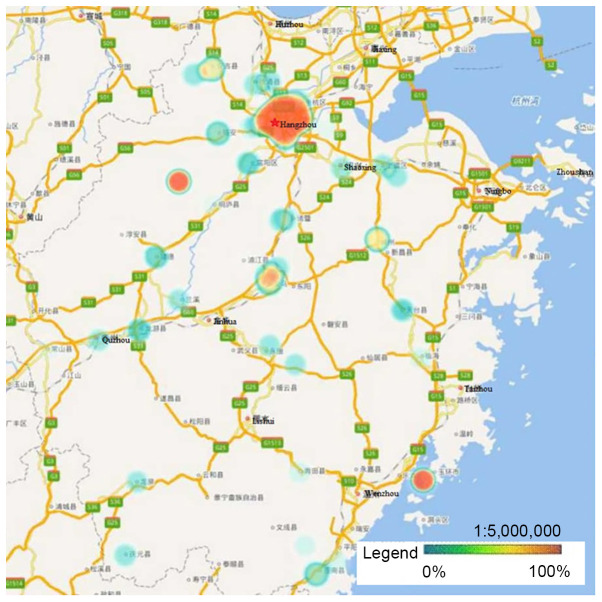
HPV-16, −58, −52 and −18 were the four most prevalent genotypes observed in Zhejiang Province, and the ranking of each varied by geographical region. Hangzhou, the capital city of Zhejiang Province, had a significantly higher rate of HPV infection than other regions. Heat maps revealed that areas with high HPV infection rates tended to be have dense traffic networks, particularly transport nodes. Specifically, HPV-16 and −58 infections were mainly distributed in the northern and central regions of Zhejiang Province, including Hangzhou and Shaoxing, where they were more prevalent than in the southern regions (P<0.05; Figs. 2 and 3). Unlike HPV-16 and −58, HPV-18 infection was widespread throughout Zhejiang Province, but had much lower infection rates in Ningbo and Huzhou compared with the rest of the province (P<0.05; Fig. 4). Notably, the high infection rate of HPV-52 was mainly distributed in Hangzhou and the eastern coastal areas of Wenzhou, with a lower infection rate in the center of Zhejiang Province (P<0.05; Fig. 5).
Figure 2.
Heat maps of HPV-16 infection in Zhejiang Province. The locations with high overall HPV-16 infection rates were mainly distributed in the northern and central regions of Zhejiang Province, such as Hangzhou and Shaoxing, where the prevalence was higher compared with that in the southern regions (P<0.05). HPV, human papillomavirus.
Figure 3.
Heat maps of HPV-58 infection in Zhejiang Province. HPV-58 infection was mainly distributed in the northern and central regions of Zhejiang Province, where the prevalence was higher compared with that in the southern regions (P<0.05). HPV, human papillomavirus.
Figure 4.
Heat maps of HPV-18 infection in Zhejiang Province. HPV-18 infection was widespread throughout Zhejiang Province, with a much lower infection rate in Ningbo and Huzhou (P<0.05). HPV, human papillomavirus.
Figure 5.
Heat maps of HPV-52 infection in Zhejiang Province. High infection rates of HPV-52 were mainly detected in Hangzhou and the eastern coastal areas of Wenzhou, with a lower infection rate in the center of Zhejiang Province (P<0.05). HPV, human papillomavirus.
Discussion
To the best of our knowledge, no urban-specific baseline prevalence data of HPV genotypes according to location in Zhejiang Province have been described. Such data would help in healthcare planning, and enable researchers to identify new therapeutic techniques and approaches more accurately. The present study revealed this information using heat maps, which showed that high HPV infection rates were associated with areas with dense traffic networks, particularly transport nodes, and were much higher in such areas than in remote areas. This finding indicates that high population density and mobility may be risk factors for HPV infection. Other possible reasons are that people from urban areas usually are more sexually active, and have larger incomes, higher education levels, improved hygiene standards and greater medical check-up awareness compared with those from rural areas, which results in a high infection and detection rates in the urban population (16). However, this finding contrasts with previous findings from a study in French Guiana, in which the prevalence of HPV infection was high in remote villages (17). The reasons for this may be that the HPV vaccine is more accessible in urban populations, which reduces HPV infection (18,19).
HPV-16, −58, −52 and −18 were the most prevalent types of HPV in the population analyzed in the present study, which was consistent with previous studies conducted in Asia (20,21). Therefore, it is likely that bivalent or quadrivalent vaccines alone will not meet regional requirements due to the prevalence of other HR-HPV genotypes, such as HPV-52 and HPV-58, that are not targeted by these vaccines. Further analysis revealed that the distribution of HPV genotypes varied by geographical region; Hangzhou had a high incidence of the four most prevalent HPV genotypes, while Ningbo had a low incidence of these HPVs. Another phenomenon was that HPV-16 and −58 infections were mainly distributed in the northern and central regions, where their infection rates were significantly higher than in the southern regions. HPV-18 infection was found to be widespread throughout Zhejiang Province, but had a much lower infection rate in Ningbo and Huzhou. Wenzhou had relatively low rates of HPV-52 and −58 infections, but higher rates of HPV-16 and −18 infections, which is consistent with the epidemiological characteristics of European countries (3). A potential reason for this is emigration from Europe and the USA. Furthermore, infection with a single genotype of HPV was more frequently observed (35.18%) than multiple infections (6.18%). Given the high prevalence rate of hr-HPV infection in Zhejiang, an emphasis on vaccination and expanded and innovative screening is important, particularly in large cities. Specific vaccination programs have been implemented in clinical practice since 2017 in Australia (22), and Nygård et al (23) demonstrated that an effective HPV vaccination program could decrease the incidence of cervical pre-invasive neoplasia by 51.5-66.6%. In the present study, one of the positive aspects of using heat maps to display the spatial distribution of HPV infection by geographical region in Zhejiang Province is that it enables potentially appropriate vaccines to be identified. The data suggest that for individuals in Ningbo or Wenzhou, bivalent or quadrivalent vaccines may be suitable, but for those in Hangzhou and Shaoxing, nonavalent vaccines are strongly recommended.
The present study has several limitations. First, only participants who received health examinations in Zhejiang Cancer Hospital were enrolled, and this could produce a certain level of bias. Another potential bias is that urban populations are more likely than rural populations to have health examinations, mainly due to greater income followed by check-up awareness. More importantly, the genotypes of HPV that were detected and the presence of multi-infections cause bias. If the patterns of multi-infection elsewhere in China are consistent with the pattern of geographical distribution of the virus detected in the present study, the cities of China with the most migration, the communities that mostly migrate to these cities, and whether the migration is temporary or permanent should be determined; this would help to distinguish whether the HPV geographical distribution is specific or imported. In addition to analyzing the large-scale geographic variations in HPV genotype distribution, a future larger scale, population-based study should be conducted to obtain comprehensive information on the prevalence and genotype distribution of HPV in Chinese populations.
In conclusion, the data in the present study indicate that HPV-16, −58, −52 and −18 are the four most prevalent genotypes of HPV in Zhejiang Province. Heat maps displaying the spatial distribution of HPV infection demonstrate that the genotypes vary by geographical region.
Acknowledgements
Not applicable.
Funding Statement
The present study was funded by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (grant nos. 2018KY276 and 2019PY022).
Funding
The present study was funded by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (grant nos. 2018KY276 and 2019PY022).
Availability of data and materials
The data that support the findings of this study are available from EsgynDB but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Authors' contributions
ZN and JX are responsible for the original concept of the study and, with all co-authors, designed the study. TT and YLG were responsible for data processing. JQZ, AWZ and AJY were responsible for data cleaning and analyses. JX drafted the manuscript, which was revised by ZN. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Each participant provided written informed consent prior to the start of the study. The protocol was approved by the Ethics Committee of Zhejiang Cancer Hospital (approval no. IRB-2019-75).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Burchell AN, Winer RL, de Sanjosé S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24(Suppl 3):S3/52–61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 3.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ, International Agency for Research on Cancer Multicenter Cervical Cancer Study Group Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 4.De Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 5.Clifford GM, Gallus S, Herrero R, Muñoz N, Snijders PJ, Vaccarella S, Anh PT, Ferreccio C, Hieu NT, Matos E, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: A pooled analysis. Lancet. 2005;366:991–998. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 6.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–1799. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 8.Xu XX, Zhou JS, Yuan SH, Yu H, Lou HM. Distribution of HPV genotype in invasive cervical carcinoma and cervical intraepithelial neoplasiain zhejiang province, southeast China: Establishing the baseline for surveillance. Int J Environ Res Public Health. 2015;12:10794–10805. doi: 10.3390/ijerph120910794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang S, Afonina I, Miller BA, Beckmann AM. Human papillomavirus types 52 and 58 are prevalent in cervical cancers from Chinese women. Int J Cancer. 1997;70:408–411. doi: 10.1002/(SICI)1097-0215(19970207)70:4<408::AID-IJC6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Lo KW, Wong YF, Chan MK, Li JC, Poon JS, Wang VW, Zhu SN, Zhang TM, He ZG, Wu QL, et al. Prevalence of human papillomavirus in cervical cancer: A multicenter study in China. Int J Cancer. 2002;100:327–331. doi: 10.1002/ijc.10506. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Rose B, Huang X, Liao G, Carter J, Wu X, Thompson C. Comparative analysis of characteristics of women with cervical cancer in high-versus low-incidence regions. Gynecol Oncol. 2004;94:803–810. doi: 10.1016/j.ygyno.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Requia WJ, Roig HL, Adams MD, Zanobetti A, Koutrakis P. Mapping distance-decay of cardiorespiratory disease risk related to neighborhood environments. Environ Res. 2016;151:203–215. doi: 10.1016/j.envres.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 13.Svendsen ER, Gonzales M, Mukerjee S, Smith L, Ross M, Walsh D, Rhoney S, Andrews G, Ozkaynak H, Neas LM. GIS-modeled indicators of traffic-related air pollutants and adverse pulmonary health among children in El Paso, Texas. Am J Epidemiol. 2012;176(Suppl 7):S131–S141. doi: 10.1093/aje/kws274. [DOI] [PubMed] [Google Scholar]
- 14.Khoury MJ, Iademarco MF, Riley WT. Precision Public Health for the Era of Precision Medicine. Am J Prev Med. 2016;50:398–401. doi: 10.1016/j.amepre.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao P, Zheng W, Wang Y, Bian ML. Sensitive HPV genotyping based on the flow-through hybridization and gene chip. J Biomed Biotechnol. 2012;2012:938780. doi: 10.1155/2012/938780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou H, Meng X, Jia T, Zhu C, Chen X, Li X, Xu J, Ma W, Zhang X. Awareness and acceptance of human papillomavirus (HPV) vaccination among males attending a major sexual health clinic in Wuxi, China: A cross-sectional study. Hum Vaccin Immunother. 2016;12:1551–1559. doi: 10.1080/21645515.2015.1099771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adenis A, Dufit V, Douine M, Corlin F, Ayhan G, Najioullah F, Molinie V, Brousse P, Carles G, Lacoste V, et al. High prevalence of HPV infection in the remote villages of French Guiana: An epidemiological study. Epidemiol Infect. 2017;145:1276–1284. doi: 10.1017/S0950268816003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynge E, Clausen LB, Guignard R, Poll P. What happens when organization of cervical cancer screening is delayed or stopped. J Med Screen. 2006;13:41–46. doi: 10.1258/096914106776179773. [DOI] [PubMed] [Google Scholar]
- 19.Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, Hingmire S, Malvi SG, Thorat R, Kothari A, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 20.Sukvirach S, Smith JS, Tunsakul S, Muñoz N, Kesararat V, Opasatian O, Chichareon S, Kaenploy V, Ashley R, Meijer CJ, et al. Population-based human papillomavirus prevalence in Lampang and Songkla, Thailand. J Infect Dis. 2003;187:1246–1256. doi: 10.1086/373901. [DOI] [PubMed] [Google Scholar]
- 21.Li N, Hang D, Yang L, Feng X, Lyu Z, Xie S, Zhou J, Wu L, Li X, Li N, et al. Persistence of type-specific human papillomavirus infection among Daqing City women in China with normal cytology: A pilot prospective study. Oncotarget. 2017;8:81455–81461. doi: 10.18632/oncotarget.20188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mammas IN, Spandidos DA, Sourvinos G. Genomic diversity of human papillomaviruses (HPV) and clinical implications: An overview in adulthood and childhood. Infect. Genet Evol. 2014;21:220–226. doi: 10.1016/j.meegid.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Nygård M, Hansen BT, Dillner J, Munk C, Oddsson K, Tryggvadottir L, Hortlund M, Liaw KL, Dasbach EJ, Kjær SK. Targeting human papillomavirus to reduce the burden of cervical, vulvar and vaginal cancer and pre-invasive neoplasia: Establishing the baseline for surveillance. PLoS One. 2014;9:e88323. doi: 10.1371/journal.pone.0088323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from EsgynDB but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.