Abstract
One of the best strategies for healthy brain aging is regular aerobic exercise. Commonly studied “anti-aging” compounds may mimic some effects of exercise on the brain, but novel approaches that target energy-sensing pathways similar to exercise will probably be more effective in this context. We review evidence in support of this hypothesis by focusing on biological hallmarks of brain aging.
Keywords: Brain aging, exercise, anti-aging compounds, neurodegenerative disease, energy-sensing pathways
Summary:
This review examines the influence of exercise, pharmaceutical compounds, and select interventions on the hallmarks of brain aging.
INTRODUCTION
As the world’s population ages, the incidence of dementia and neurodegenerative diseases is expected to markedly increase. One key precursor to dementia and neurodegeneration is brain aging itself. Brain aging is characterized by declines in cognitive function (primarily memory and learning, attention/processing speed, and executive function) (1). In some people, these declines may develop into mild cognitive impairment (MCI), which increases the risk for dementia and neurodegenerative diseases like Alzheimer’s disease (AD) (2).
Age-related cognitive declines are caused in part by adverse biological events in the brain known as the “hallmarks of brain aging” (Figure 1). This collection of processes mirrors the hallmarks of aging (3) in peripheral tissues in many ways, but it also includes several brain-specific phenomena. Importantly, the hallmarks of brain aging are also involved, and often more pronounced, in most neurodegenerative diseases. Thus, brain aging and neurodegeneration may be part of a continuum, and a combination of hallmarks/risk factors may determine if dementia or disease develops (2). As a result, there is growing interest in strategies for inhibiting the hallmarks of brain aging, which could potentially delay or prevent the onset of neurodegenerative diseases (4).
Figure 1.
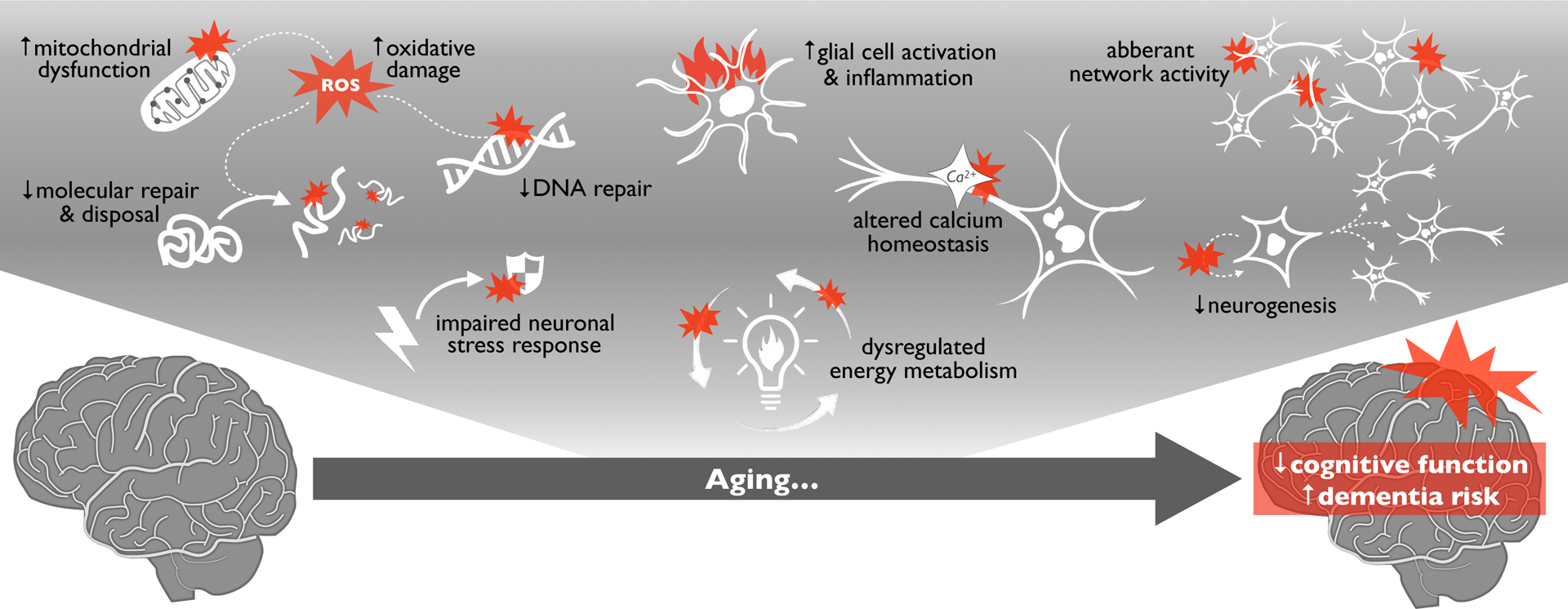
Aging is the primary risk factor for the development of cognitive dysfunction, mild cognitive impairment (MCI) and dementia. Numerous, established biological “hallmarks of brain aging” (shown at top) contribute to age-related reductions in cognitive function and increased neurodegeneration/dementia risk with aging.
Regular aerobic exercise is one of the few evidence-based ways to prevent age-related cognitive decline and neurodegenerative diseases (5). As such, it has been suggested that exercise should be a first line strategy for healthy brain aging. Still, there is significant interest in exercise alternatives, including pharmacological “anti-aging” compounds that may have similar or complementary effects (especially in Western societies where sedentary lifestyles are common and many find it difficult to maintain an exercise routine). Many compounds have been tested for their effects on health and longevity in model organisms, and several in particular have been shown to suppress hallmarks of aging in peripheral tissues and increase healthy lifespan in the National Institute on Aging’s Intervention Testing Program (ITP) (6). These compounds are some of the most-studied in the broad field of aging research, and may hold some promise for attenuating hallmarks of brain aging specifically.
The literature on how exercise affects the hallmarks of brain aging is relatively new, and the extent to which select/ITP anti-aging compounds influence the hallmarks of brain aging per se (and whether these agents can mimic the effects of exercise on the brain) is also an emerging topic. However, we and others have shown that exercise remains among the best approaches to reduce hallmarks of aging and increase health/function in peripheral tissues (4, 7). Here, we hypothesize that: 1) although most pharmacological agents known to increase lifespan in the ITP may reduce some hallmarks of brain aging, exercise will likely remain a more effective intervention for healthy brain aging because it stimulates key energy-sensing pathways that modulate multiple hallmarks; and 2) novel (non-ITP) compounds and/or interventions that target energy-sensing pathways similar to exercise will prove to be more effective for promoting healthy brain aging in this context. In the following sections, we review the hallmarks of brain aging and the effects of exercise on these hallmarks, and we compare these to the effects of key ITP compounds on the same hallmarks as a framework for understanding the importance of energy-sensing pathways in healthy brain aging. Then, we discuss newer treatments and ideas for targeting bioenergetic signaling pathways similar to exercise that may hold the most promise as exercise alternatives.
Dysregulated Energy Metabolism: A Central Mechanism
Dysregulated energy metabolism is an underlying contributor to all hallmarks of brain aging. During aging, fasting glucose levels increase as cells become less effective at importing glucose in response to insulin (insulin resistance). Peripheral insulin resistance and elevated fasting glucose are linked with accelerated brain aging, poorer cognitive function, and dementia. Brain function is particularly sensitive to the adverse effects of insulin resistance as glucose is a key energy source for neurons, and this can be compounded by metabolic and cardiovascular changes with aging (e.g., dyslipidemia, increased triglycerides, and LDL cholesterol). Consequently, sedentary lifestyles and unhealthy diets that impair peripheral function and metabolic health are also linked with accelerated brain aging (4, 8).
Importantly, exercise is an energetic stress (reduced cellular energy levels) that peripheral tissues and the brain react to with many metabolic, mitochondrial, and cellular responses (known as hormesis). These adaptive responses involve the activation of energy-sensitive pathways that restore bioenergetic homeostasis in both peripheral tissues and in the brain. Key examples include upregulation of glucose transporters (GLUT) and increased signaling through the low-energy sensors AMP protein kinase (AMPK), Sirtuin 1 (SIRT1, an NAD+ sensor), and the amino acid-sensing mammalian target of rapamycin (mTOR) pathway (Figure 2) (8, 9). The activation of these systems results in enhanced glucose utilization (GLUT), supports neuronal energy levels by regulating glycolysis and respiration (AMPK), controls the activity of transcription factors and energy-metabolizing enzymes (SIRT1), and modulates the turnover of proteins/macromolecules that can be used for energy (mTOR). These signaling proteins also may directly impact neural gene expression, synaptic plasticity, and memory formation. Moreover, prolonged exercise leads to glycogen depletion, fatty acid oxidation and the production of ketone bodies, which are another important energy source for the brain and can independently activate these same sensors (8). In addition to all of this, exercise increases the expression of brain-derived neurotrophic factor (BDNF), which powerfully regulates neuron function and development, energy intake/behavior, and can interact directly with and/or be activated by energy sensors like AMPK, mTOR and SIRT1 (10, 11). Importantly, all of these signaling pathways: 1) contribute to stress resistance by enhancing cellular function and quality control; 2) have been reported to decline with aging; and 3) modulate other hallmarks of aging/brain aging, as emphasized in Figure 2 and described in the following sections (3, 8, 9). Thus, the activation of these energy-regulating pathways is central, and perhaps the most important mechanism underlying the beneficial influence of exercise on the the aging brain.
Figure 2.
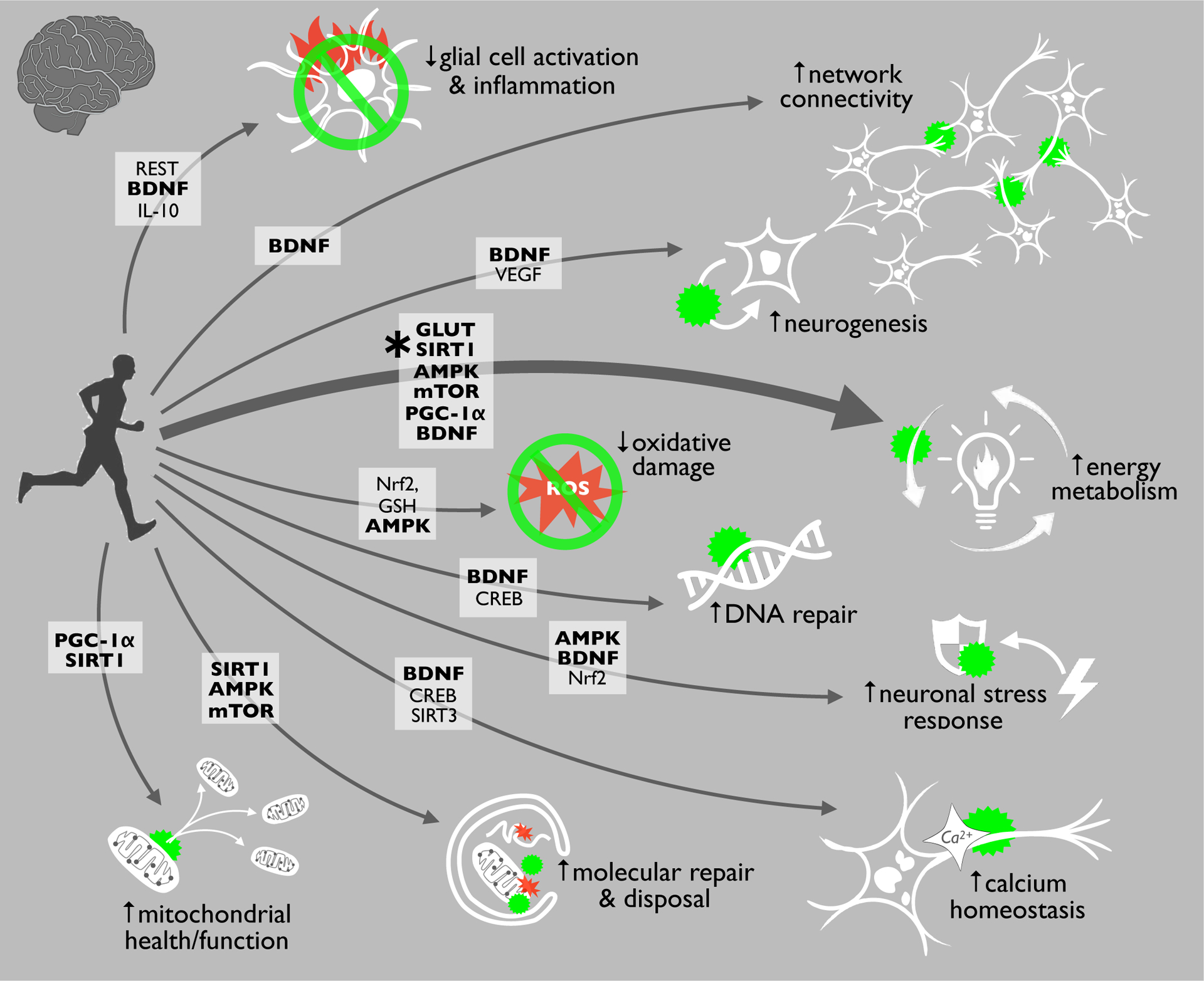
Exercise is perhaps the best strategy for inhibiting all major hallmarks of brain aging, largely because it activates key cellular energy sensing pathways. This enhanced energy metabolism/regulation with exercise is important because the proteins and cellular pathways involved (in bold) can also directly influence other hallmarks. Abbreviations: REST: RE1-silencing transcription factor; BDNF: brain-derived neurotrophic factor; IL-10: Interleukin 10; VEGF: Vascular Endothelial Growth Factor; GLUT: glucose transporters; SIRT1: Sirtuin 1; AMPK: AMP protein kinase; mTOR: mammalian target of rapamycin; PGC-1α: proliferator-activated receptor gamma coactivator-1α; Nrf2: nuclear factor erythroid 2-related factor 2; GSH: Glutathione; CREB: cyclic AMP response element-binding protein; SIRT3: Sirtuin 3.
Mitochondrial Dysfunction
Aging is associated with a decline in efficiency of the electron transport chain, resulting in reduced ATP generation and electron leakage that propagates reactive oxygen species (ROS). These ROS can damage cellular structures, including mitochondria and even mitochondrial DNA (mtDNA). This mitochondrial dysfunction is a universal hallmark of aging that impairs overall tissue function, and it is exacerbated by age-related declines in mitochondrial biogenesis and disposal of damaged mitochondria (mitophagy) (3).
Mitochondria are centrally important in the brain, where they play key roles in calcium homeostasis and neuronal metabolism. Numerous studies have shown that mitochondrial dysfunction, especially in neurons, increases with brain aging (8). Dysfunctional mitochondria are also centrally implicated in neurodegenerative diseases, including Parkinson’s disease (PD) and AD. However, evidence shows that exercise reduces mitochondrial dysfunction in peripheral tissues and the brain. For example, in old mice, chronic high-intensity running increases mtDNA copy number and enhances mitochondrial biogenesis by activating proliferator-activated receptor gamma coactivator-1α (PGC-1α) (12), which is downstream of both the key energy sensors AMPK and SIRT1. Long-term wheel running also activates autophagy (the cellular process of recycling damaged internal components, including mitochondria) (13), and similar experiments in rats show that running enhances mitochondrial network formation and mitochondrial respiration (14).
As with many of the hallmarks of brain aging, there is limited direct evidence for the effects of exercise on brain mitochondrial function with aging in humans (because access to brain tissue/cells is limited). However, at least one study has shown that hippocampal gene expression patterns in older adults who are more physically active reflect enhanced mitochondrial energy production (15). As methods for monitoring mitochondrial function in vivo become more advanced, there may be opportunities to demonstrate such cellular effects of exercise more directly.
Accumulation of Oxidatively Damaged Molecules
Oxidatively damaged biomolecules accumulate with aging as a result of oxidative stress, an imbalance between antioxidant defenses and ROS production (3). These ROS can damage biomolecules like proteins, lipids and DNA, as well as organelles—all of which can impair cellular function directly (because damaged components do not function properly) or indirectly (because damaged biomolecules may interfere with function in other cellular compartments). Neurons are especially sensitive to ROS due to their high, oxidizable lipid content and rate of oxidative metabolism (8).
Interestingly, exercise is associated with oxidative stress, as increased metabolism with exercise increases ROS generation. However, this effect is likely hormetic because exercise-associated oxidative stress leads to greater long-term antioxidant capacity (9). In fact, habitual aerobic exercise is associated with reduced levels of biomolecule damage in the brain. In old rodents, for example, both long-term running and swimming improve cognitive function and reduce ROS, oxidized proteins (carbonyls), and peroxidized lipids in the brain, in part by increasing antioxidants like glutathione and various neurotrophic factors (16)—many of which are modulated by AMPK, mTOR and SIRT1. Long term wheel running even reduces peroxidized lipids, improves memory, and protects against AD pathology in transgenic mouse models (17). Some evidence suggests that these beneficial effects require longer exercise interventions in older mice, which is consistent with the idea that exercise is a hormetic stress on the brain (18). Although there is little direct evidence for the effects of exercise on oxidative damage in the human brain, clinical studies have shown that exercise increases antioxidant defenses and reduces pro-oxidant processes systemically (9), so similar effects likely occur in the brain.
Reduced Molecular Repair and Disposal
One main reason for both mitochondrial dysfunction and oxidative damage accumulation with aging is reduced activity of cellular repair and disposal systems. Many reports have documented systemic, age-related reductions in: 1) autophagic-lysosomal degradation of proteins and organelles; and 2) activity of the proteasome (degrading old/damaged proteins). These systems are critical in neurons, which are post-mitotic and must maintain their functional capacity throughout the lifespan. Moreover, the accumulation of undegraded, damaged, and/or aggregated proteins due to reduced autophagy and proteasome activity is central to the pathology of many neurodegenerative diseases (e.g., amyloid beta plaques in AD, alpha synuclein deposits in PD, etc.) (2).
Exercise increases autophagy and proteasome activity in both pre-clinical models and clinical settings (9), and while there is limited direct evidence for these effects in the brain, rodent studies indicate that swimming enhances autophagy and mitochondrial function in the hippocampus of older animals (19) and may protect against pharmacologically-induced “premature” brain aging by activating the same systems (20). Running also increases proteasome activity in the brain (21), which may explain reductions in smaller, damaged biomolecules (e.g., protein carbonyls). These effects of exercise on molecular quality control likely involve multiple signaling networks for which autophagy and the proteasome are common downstream effector mechanisms. For example, studies in mice show that exercise activates autophagy in the brain by increasing the activity of AMPK and SIRT1 (13). Exercise also increases mitophagy and enhances mitochondrial network dynamics, in part by activating PGC-1α (14). In transgenic mice, the activation of these protective quality control systems by exercise may even reduce neurodegenerative protein aggregation (22).
Dysregulated Calcium Homeostasis
Cellular control of important ions and minerals becomes dysregulated in most aged tissues, but impaired calcium (Ca2+) homeostasis with aging is a particular problem in neurons. Ca2+ plays a role in numerous brain functions, including neurotransmission, neuronal excitability, synaptic plasticity and long-term memory consolidation. Ca2+ levels also modulate gene expression, as Ca2+ influx through NMDA receptors can activate transcription factors like cyclic AMP response element-binding protein (CREB) and PGC-1α. With aging, neuronal Ca2+ homeostasis becomes impaired due to increased Ca2+ influx and dysregulation of intracellular Ca2+ modulators like mitochondria. This leads to altered gene expression and neuronal function that are linked with cognitive decline, and can also contribute directly to neuronal death via Ca2+-driven excitotoxicity (8).
There is limited direct evidence for the effects of exercise on calcium homeostasis in the context of brain aging per se. However, many studies show that exercise increases BDNF, which has been reported to reduce neurotoxic extrasynaptic Ca2+ influx mediated by the NMDA receptor (23). Some of the beneficial effects of exercise on neuronal mitochondria likely improve Ca2+ homeostasis as well, as exercise enhances mitochondrial Ca2+ handling. In fact, studies in transgenic mice show that exercise increases the expression of the protective mitochondrial sirtuin, SIRT3, and that this is required for protection against Ca2+-related excitotoxicity (24). Studies of neuronal Ca2+ homeostasis in humans are lacking, but there is strong interest in related systemic biomarkers like S100 calcium-binding protein β (S100β), which is linked with brain aging, injury and neurodegeneration, and is reduced by exercise (25).
Impaired adaptive cellular stress response
All cells activate stress resistance networks (e.g., antioxidant and anti-inflammatory pathways) in response to stressors. In the brain, these adaptive responses are important in settings of electrochemical, ionic, and even psychological stress. With aging, however, most cells become markedly less effective at mounting adaptive stress responses (3). In neurons, this is partly due to age-related decreases in the expression/activity of stress sensors like AMPK and SIRT1, and protective neurotrophic factors, such as BDNF and nerve growth factor (NGF). These proteins stimulate gene expression that promotes antioxidant defenses, healthy mitochondrial function, and Ca2+ handling (8).
As a hormetic stress itself, exercise activates adaptive cellular stress responses, and it increases the ability of these same systems to respond to other stressors. The metabolic stress associated with exercise activates antioxidant systems in mouse neurons, such as those controlled by nuclear factor erythroid 2-related factor 2 (Nrf2), and these protect against aging-relevant neurotoxic stressors (8, 26). This exercise-induced Nrf2 activation increases endogenous antioxidants like catalase and superoxide dismutases (SODs) that directly reduce ROS, and it activates other protective processes (e.g., autophagy) that further protect against oxidative stress. Studies in aged rodents also show that exercise potentiates stress response systems that protect neurons against inflammatory insults, in part by activating BDNF and anti-inflammatory cytokines like interleukin 10 (IL-10) (27).
In humans, there is some evidence that routine exercise increases tolerance for psychological stressors with aging, but direct data on cellular stress responses are limited. Still, many clinical studies have shown that aerobic exercise increases the activity of systemic antioxidant and anti-inflammatory defense systems, and some have even demonstrated that these effects are associated with increases in BDNF and reductions in S100β (28).
Aberrant Neuronal Network Activity
The brain relies on communication among billions of neurons. Optimal function of neuronal networks requires balanced activity of glutamatergic (excitatory) neurons and GABAergic (inhibitory) interneurons. However, during aging, activity within these circuits (largely white matter communication via myelinated axons) is dysregulated, which can result in hyperexcitability and excitotoxic damage. This may lead to degeneration of fiber systems involved in decision-making and learning/memory (8, 26).
Some studies have investigated the influence of exercise on neuronal network activity. One showed that pharmacologically-induced brain aging is mitigated by swimming, which reduces neurotoxicity and improves synaptic transmission by influencing the expression of glutamate-receptor 1 and synaptophysin (a marker of synaptic integrity) in rats (29). Exercise may also confer neuroprotection by modulating GABA disinhibition (26), and by enhancing dendritic outgrowth, maintaining structural integrity, and improving long-term potentiation in aged animals via BDNF and other proteins also involved in energy sensing. These changes coincide with increased hippocampal glutamate synthesis and an increase in nerve growth factors (30).
In humans, there is evidence that exercise may positively influence neuronal transmission and connectivity. For example, greater cardiovascular fitness in older adults is associated with improved pre-frontal cortex (important for decision making) processing speed and heightened synaptic plasticity (31), and meta-analyses find that exercise is associated with greater hippocampus volume and may influence network functional connectivity in this region (32).
Inflammation
Neuroinflammation is characterized by increased numbers of immune-activated, pro-inflammatory astrocytes and microglia that secrete neurotoxic cytokines (26). This glial cell activation is linked with brain aging, MCI and most neurodegenerative diseases. In fact, markers of neuroinflammation and related neurodegeneration (e.g., glial fibrillary acidic protein [GFAP] and neurofilament light chain [NFL]) have even been shown to increase with aging in cognitively unimpaired people (2). Evidence shows that exercise decreases brain inflammation, marked by lower levels of the pro-inflammatory transcription factor nuclear factor κB (NF-κB) and tumor necrosis factor α (TNF-α), and that these changes are associated with improved spatial memory (26). Exercise even prevents obesity-induced cognitive decline in rodents by influencing inflammatory genes, reducing the number of reactive astroglia and microglia, and reducing major markers of hippocampal inflammation (33). Finally, in old mice, running reduces brain pro-inflammatory cytokine levels, in part by activating BDNF and RE1-silencing transcription factor (REST), which is linked with numerous neurological disorders (34).
In humans, peripheral inflammation has been linked with brain structural abnormalities, reduced cognitive function, and greater dementia risk. As an example, recent studies demonstrate that high levels of C-reactive protein (CRP, a common clinical marker of inflammation) are associated with reduced brain white matter integrity in older adults (35), and epidemiological data show that systemic inflammation may precede neuro-degenerative diseases by decades. However, meta-analyses show that habitual exercise is associated with reduced pro-inflammatory cytokines including CRP and TNF-α (36).
Impaired DNA Repair
The accumulation of DNA damage is an important hallmark of aging and it plays a causal role in several premature aging syndromes. DNA damage is also closely linked with brain aging, cognitive decline, and neurodegenerative diseases (8). The genomic damage that accumulates with aging can contribute to cellular senescence (in which cells cease dividing and begin to produce pro-inflammatory molecules) and apoptosis (programmed cell death), leading to functional decline of organs/systems (including the brain) and reduced longevity (3).
The mechanisms by which exercise reduces DNA damage during aging are not well understood, but evidence points towards several DNA-repair pathways, many of which are modulated by SIRT1 and other energy sensors (37). Rodent studies have shown that running stimulates DNA repair in brain tissue by upregulating the expression of CREB and apurinic/apyrimidinic endonuclease 1 (APE1), which is a key enzyme for DNA base excision repair (38). Treadmill exercise has also been shown to reduce hippocampal DNA fragmentation and neuronal apoptosis in rat models of traumatic brain injury (39). Moreover, in animal models of AD, treadmill exercise is associated with reduced DNA damage and fewer double-stranded breaks in the hippocampus (40).
Impaired Neurogenesis/Stem Cell Exhaustion
Most neurons do not proliferate, but the dentate gyrus of the hippocampus, subventricular zone of the lateral ventricle, and the olfactory bulbs all contain stem cells (41). As is the case with most stem cells, the ability of these stem cells to self-renew and generate new progenitors (neurogenesis) declines with aging, which may account for some of the structural declines observed in the aging brain (8). However, exercise enhances neurogenesis within these areas, and there is strong interest in determining underlying mechanisms in the hippocampus, which is crucial for learning and memory.
To date, most studies on exercise and hippocampal neurogenesis have been in rodents. Some studies have demonstrated that running induces neurogenesis in the hippocampal dentate gyrus, and is associated with increased neurogenesis and improved memory (42). Others have investigated the influence of long-term exercise on hippocampal neurogenesis in old mice and found that exercise increases neurogenesis and improves neuronal structure (43), and these results are likely linked with increased activity of BDNF. In humans, neurogenesis may correlate with cerebral blood flow, which has been shown to increase with exercise.
Other: Telomere Attrition and Cellular Senescence
Telomeres are repetitive DNA sequences that cap the ends of chromosomes and protect DNA from degradation. They also protect overall cellular function, as critically short telomeres can lead to cell death or senescence. Telomere shortening and cellular senescence are established hallmarks of aging in peripheral tissues. Their role in brain aging is less clear, but telomere maintenance, which contributes to the production of neuronal stem cells, may protect against neurodegenerative diseases—and there is evidence that habitual exercise is associated with longer telomeres and reduced cellular senescence in both mice and humans (3, 8, 9).
ITP ALTERNATIVES TO AEROBIC EXERCISE FOR HEALTHY BRAIN AGING
Aerobic exercise is perhaps the strongest way to protect brain health during aging, but it may not be feasible for some people. Additionally, in Western societies where sedentary lifestyles have become common, many may find it difficult to exercise regularly. It is therefore an opportune time to consider whether anti-aging compounds may recapitulate some benefits of exercise on the brain. The National Institute on Aging’s ITP has tested the effects of many compounds on healthspan and lifespan in genetically heterogeneous mice. To date, a number of compounds tested by the ITP have been shown to improve health, increase lifespan, and reduce many hallmarks of aging in vitro and in vivo. The six ITP compounds that are the most heavily studied include: aspirin, rapamycin, 17-⍺-estradiol, acarbose, nordihydroguaiaretic acid, and Protandim. All of these compounds elicit beneficial health responses in peripheral tissues. Thus, these pharmacological interventions may also improve brain health (44) and reduce some hallmarks of brain aging (Figure 3). As these are among the most-studied pharmacological anti-aging interventions, comparing the evidence for the effects of these ITP compounds to those of exercise on the brain may provide insight on key mechanisms/targets for healthy brain aging.
Figure 3.
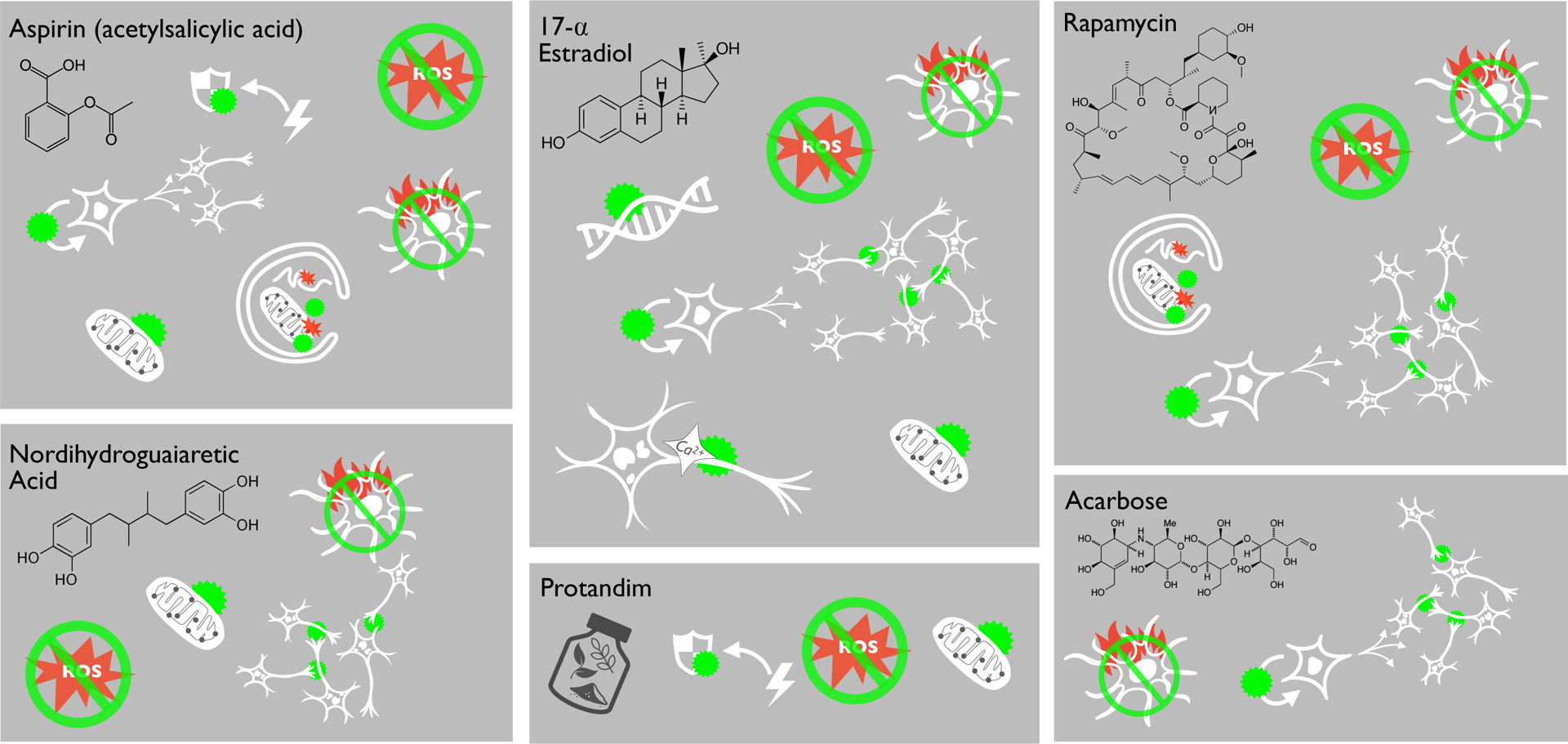
Select pharmacological “anti-aging” treatments that have been shown to increase lifespan/healthspan in the NIA Intervention Testing Program may inhibit certain hallmarks of brain aging, but some are supported by more evidence than others. To date, rapamycin and 17-α-estradiol appear to be the most effective in this context. However, more research is needed to determine how these and other compounds affect additional hallmarks of brain aging, and to fully characterize bioavailability and safety in humans.
Aspirin
Aspirin may enhance health/lifespan both by suppressing inflammation and through its antioxidant properties (44). Evidence suggests that aspirin supplementation improves brain health during aging by reducing oxidative stress and inflammation, and by protecting against neurotoxic stressors in dopaminergic neurons (which are important for learning and memory) via enhanced mitochondrial function and reduced ROS production (45). In addition, aspirin improves lysosomal biogenesis and autophagy, and reduces plaque pathology in transgenic AD mice (46). Aspirin treatment also reduces stress sensitivity in mice by lowering the activity of the hypothalamic-pituitary-adrenal stress axis and decreasing the expression of GFAP (a marker of pro-inflammatory astrocytes) (47). Finally, both low-dose and high-dose aspirin supplementation improve neurogenesis by increasing oligodendrocyte proliferation and white matter integrity in rats (48).
In humans, there is mixed evidence that aspirin supplementation in older age may protect against cognitive decline. For example, low-dose aspirin intake is associated with higher scores on cognitive tests in older adults in some studies; however, some meta-analyses have found no association between low-dose aspirin supplementation and improved cognitive health or protection against dementia in older adults (49). Other promising anti-inflammatories currently under investigation for healthy aging/brain aging in humans include salsalate and naproxen (50).
Rapamycin
Rapamycin inhibits intracellular growth cascades (largely by inhibiting mTOR), and it increases median and maximal lifespan in male and female mice (6). There is evidence that rapamycin influences most hallmarks of aging in peripheral tissues and growing support for similar effects in the brain. For example, rapamycin reduces oxidative stress in the brains of old rats by activating autophagy, decreasing neuroinflammation, and improving neuronal integrity (51). Acute injection of rapamycin also reduces pro-inflammatory cytokines and chemokines, and inhibits the activity of macrophages and microglia (4).
Research on rapamycin and the brain is evolving, and the compound may reduce some markers of brain aging via recently discovered mechanisms. For example, rapamycin supplementation is reported to influence the expression of brain telomerase reverse transcriptase (TERT), which maintains telomere integrity and reduces ROS (52). Chronic rapamycin supplementation also improves NMDA (a glutamate and ion channel receptor that plays a role in synaptic plasticity) signaling. Additionally, rapamycin suppresses senescence in rat models of accelerated aging and improves myelination and neuronal structure (53). Long-term rapamycin supplementation even enhances vasculature and brain/neuronal metabolism in mouse models of AD (54). To date, there have been no clinical trials investigating the influence of rapamycin on the hallmarks of brain aging. However, some argue that such trials should soon occur due to the large number of positive results in animal models. There is also a current Phase 1b/2a trial underway to determine whether mTOR inhibition with the novel compound RTB101 may benefit PD patients (ACTRN12619000372189).
17-⍺ Estradiol
17-⍺-estradiol (17aE) is an endogenous steroid with an affinity for estrogen receptors. Long-term treatment with 17aE increases median lifespan in male mice but not females (6). Interestingly, the brain has many estrogen receptors (which decline in number during aging), and it has therefore been suggested that 17aE might reduce hallmarks of brain aging. Estrogen also modulates neuron-to-neuron communication, helps in the production of brain growth factors, and has a supportive role for glial cells. In fact, 17aE has been shown to reduce age-associated hypothalamic inflammation, in part by diminishing the activity of reactive/pro-inflammatory microglia and suppressing TNF-α and GFAP (i.e., astrocyte activation) (55). Other reports show that 17aE protects against intraneuronal ROS production and reduces markers of oxidative stress in the brain (56).
There is also evidence that estrogen may improve neuronal calcium homeostasis by suppressing intracellular calcium accumulation, reduce DNA damage in the brain, and stimulate neurogenesis in the hippocampus. Moreover, it has been shown that estradiol can have a direct impact on neuronal network activity by stimulating synaptic excitatory activity, enhancing calcium dynamics, and improving neuronal structure (57). 17aE may even reduce amyloid beta-induced neuronal cell death (a feature of AD), in part by enhancing mitochondrial function (56).
Although pre-clinical evidence in favor of 17aE are promising, existing data in humans are less clear. Studies on post-menopausal women given hormone replacement therapy have documented a mild association with self-reported estrogen supplementation and reduced risk of dementia (58). However, others have reported that long-term 17-β-estradiol (an isomer of 17aE) does not influence verbal memory, executive function, or global cognitive ability (59). Thus, more studies are needed to determine if estrogen or related hormones may influence cognitive function or dementia in larger cohorts, and if these compounds may do so by modulating hallmarks of brain aging.
Acarbose
Acarbose is an inhibitor of small intestinal α-glucosidase that prevents the breakdown of complex carbohydrates into glucose, resulting in decreased glucose absorption and lower blood glucose levels. Acarbose increased median lifespan in the ITP, but the effect was greater in male mice (6). One recent study has shown that chronic acarbose treatment improves synaptic integrity and increases the expression of nerve growth factors, which coincides with improved memory in a mouse model of accelerated aging (60). Acarbose also decreases age-associated hypothalamic inflammation in male mice but not females (55). One population-based retrospective cohort study showed that acarbose use is associated with a reduced risk of dementia in type-2 diabetics; however, this effect was only seen in women (61).
Nordihydroguaiaretic Acid
Nordihydroguaiaretic acid (NDGA), an antioxidant compound found in the creosote bush, has reported anti-inflammatory and antioxidant capabilities. NDGA improved median lifespan in the ITP, but only in male mice (6). There is limited evidence that NDGA may protect against several hallmarks of brain aging in rodents. For example, NDGA supplementation is associated with reduced age-related hypothalamic inflammation and glial cell reactivity in old mice (55). Additionally, NDGA improves mitochondrial function, membrane potential, ATP generation and morphology, as well as synaptic structure in the striatum of mice. These positive changes are associated with reduced lipid peroxidation and attenuated oxidative stress (62). Finally, NDGA protects cerebellar neurons against H2O2-induced oxidative stress by activating Nrf2 antioxidant pathways (63). Despite these few studies suggesting that NDGA may be protective against brain aging and neurodegenerative diseases in animal models, there have been no studies investigating the influence of this compound on brain aging in humans.
Protandim
Protandim is a mixture of five botanical compounds (curcumin, bacosides, silymarin, withaferin A, and epigallocatechin-3-gallate). It is a Nrf2 activator and may benefit health by increasing endogenous antioxidant activity. In the ITP, Protandim improved median lifespan, but the effect was only seen in male mice (6). Many studies have investigated the benefits of Nrf2 activation in brain aging and neurodegenerative diseases; however, only a few have specifically looked at the effects of Protandim supplementation on the brain. A recent study showed that Protandim reduces oxidative stress and mitochondrial oxidizing species in rat brains (64), but further studies are needed to determine the effects of Protandim on other hallmarks of brain aging. There is also some interest in additional/novel Nrf2 activators, such as sulforaphane and dimethyl fumarate, and these may have promise in this context.
Others
Several other promising compounds and phytochemicals (some currently being tested in the ITP) may have the ability to influence the hallmarks of brain aging. While not described in detail in the current review, these compounds include the polyphenol resveratrol (65), senolytic compounds (which clear senescent cells) (66), curcumin (derived from the Indian spice Tumeric) (67), and spermidine (an autophagy activator) (68). Most of these compounds have been shown to influence some hallmarks of brain aging and are well-tolerated in humans. These compounds, in addition to those described above, may have great potential to positively influence brain health during aging and reduce the risk for neurodegenerative disease. However, more rigorous research is needed to determine effective doses, bioavailability, and mechanisms of action before clinical trials commence.
FUTURE DIRECTIONS: NOVEL ENERGY-TARGETING STRATEGIES FOR HEALTHY BRAIN AGING
Aerobic exercise clearly has a strong, inhibitory influence on all hallmarks of brain aging. Several popular pharmacological candidates for improving healthspan may exert similar effects on certain hallmarks, but most are not as broadly effective as exercise. Of the most commonly studied compounds in the ITP, rapamycin, 17aE, and (to a lesser extent) aspirin appear to be supported by the most encouraging evidence (Figure 3). Of these compounds, rapamycin may be the most promising candidate, as others have already yielded mixed results in clinical settings. Still, clinical data are limited, and further research is needed to determine if rapamycin and/or other ITP compounds may be truly effective for inhibiting the hallmarks of brain aging, improving cognitive function, and preventing neurodegenerative diseases in humans.
One key reason that aerobic exercise outperforms common pharmacological healthspan enhancers in the context of brain aging is that exercise provides a strong physiologic/metabolic stimulus. As described above, exercise causes metabolic stress (low cellular energy) linked with accumulation of important energetic signaling molecules (e.g., AMP, NAD+, ketones) (Figures 1 and 4). These and other exercise-induced metabolites flip a “metabolic switch” that activates numerous protective cellular pathways with pleiotropic effects. Interestingly, although anti-aging compounds are not associated with the same hormetic energy stress, a common denominator among the ITP compounds that most favorably modulate hallmarks of brain aging (rapamycin, 17aE and aspirin) is that they have all been linked with activation of exercise-relevant, energy-sensing pathways. Rapamycin specifically modulates mTOR signaling, and both 17aE and salicylate (the active moiety in aspirin) have been shown to increase signaling/activity of AMPK, SIRT1, and BDNF (69–71). This observation is consistent with the idea that activation of these energy-sensing pathways is likely required to truly mimic the effects of exercise on the brain. Thus, we hypothesize that several less-studied exercise alternatives which focus on activating energy-sensing pathways may be more effective for healthy brain aging than traditional ITP compounds (Figure 4).
Figure 4.
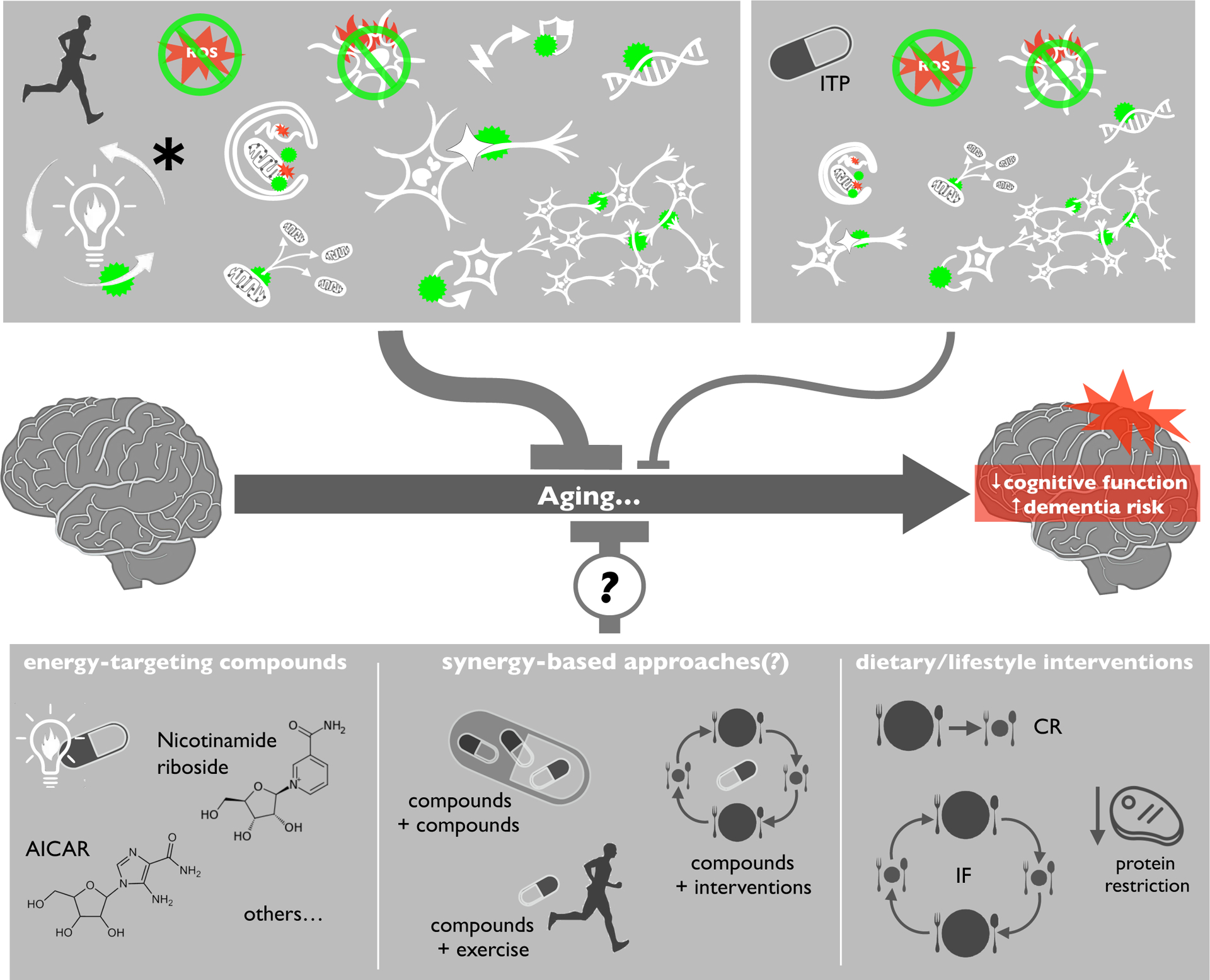
Exercise is more broadly effective than traditional anti-aging compounds for preserving cognitive function and reducing dementia risk, because it is associated with a strong bioenergetic stimulus that directly influences all hallmarks of brain aging. However, some energy-targeting pharmacological agents (e.g., AICAR, nicotinamide riboside) and lifestyle interventions (e.g., calorie restriction [CR], protein restriction, intermittent fasting) may better mimic effects of exercise on the hallmarks of brain aging. Potential synergistic effects among these interventions and compounds could hold particular promise for promoting healthy brain aging.
1. Novel (non-ITP) compounds targeting energy-sensing pathways
Many compounds have not been investigated in the ITP but could have powerful effects on brain aging, and those that directly stimulate energy-sensing systems may hold the most promise. Key examples include: NAD+-boosting compounds like nicotinamide mononucleotide (NMN) or nicotinamide riboside (NR, currently under testing in the ITP); 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) and/or various small molecules that directly activate AMPK (e.g., Compound 13, PT-1) (72), as well as metformin (no effect on lifespan in the ITP). There is limited but mostly positive evidence for the effects of these compounds on the brain (73–75). Future studies will need to determine the bioavailability, ideal dosing, and efficacy of these and other related compounds for healthy brain aging—but given that they directly target exercise-like pathways, this is likely a compelling area for future research.
2. Novel dietary/lifestyle interventions targeting energy-sensing pathways
Growing evidence suggests that select nutritional interventions (especially those that target energy-sensing pathways) may be similarly/as effective as exercise for healthy brain aging. For example, calorie restriction (CR, 10–50% reduction in daily caloric intake) and intermittient fasting (IF, periods of time without food) are considered perhaps the strongest nutritional interventions to reduce hallmarks of brain aging (4). Both CR and IF contribute to hormesis by increasing cellular stress resistance and stress responses (76), and both have been reported to positively influence the brain and reduce essentially all hallmarks of brain aging (77, 78). The mechanisms underlying CR and IF include powerful modulation of key energy-sensing proteins like AMPK, SIRT1, BDNF, and mTOR (79). IF and related approaches (e.g., time-restricted feeding (80)), as well as other strategies for mimicking CR (e.g., protein restriction (81)), may be the most practical dietary strategies, as CR is difficult to implement clinically (82)—but the influence of these interventions on human cognitive function/brain aging warrants significant study in the future, as most current evidence is pre-clinical and/or limited to peripheral tissues (83).
3. Synergistic, energy-targeted compound/intervention combinations
An interesting direction for future research on strategies for healthy brain aging may be to study similarities/interactions among compounds and/or interventions. For example, could combinations of compounds targeting energy-sensing pathways more effectively mimic the effects of exercise on the hallmarks of brain aging (i.e., vs. one compound alone)? Or, could exercise, CR or IF in combination with select compounds have synergistic, protective effects on the aged brain? These ideas are intriguing and could lead to highly translatable strategies for healthy brain aging by reducing the inherent challenges of individual approaches. For instance, a synergistic cocktail of compounds targeting multiple energy-sensing pathways might stimulate greater hormesis (like exercise), and the compounds could potentially be included in lower doses to avoid side effects. Similarly, exercise, CR or IF could be combined with compounds that complementary compounds (i.e., that stimulate similar signaling pathways).
There is conceptual precedent for novel, combinatorial approaches to promote healthy brain aging in other settings. Indeed, the “polypill” concept (combining multiple drugs) is a current and popular research topic in the effort to prevent cardiovascular diseases (84). Endpoints in any study of an energy pathway-focused polypill for healthy brain aging would be less specific, but focusing on cognitive function and hallmarks of brain aging, as described here, should be a starting point. As for lifestyle intervention/compound synergy, some studies in older adults have pointed to adverse interactions between exercise and otherwise protective compounds like metformin (85) and antioxidants (86) which may blunt the beneficial hormetic influence of exercise. These reports have focused on peripheral effects of the interventions (e.g., exercise tolerance, performance) rather than brain health, but they suggest a cautionary approach to this area of research. One possible strategy for avoiding these problems might be to combine less intense exercise, or partial CR or IF with compounds that target complementary energy-sensing pathways. Such interventions could be more manageable for the average adult who might otherwise not adhere, and they would be associated with fewer risks (e.g., injury, malnourishment, etc.), which are especially significant concerns among older adults (82).
CONCLUSIONS
Based on the available evidence, it seems likely that developing novel strategies to target central, exercise-relevant energy-sensing pathways is a particularly promising direction for future research aimed at promoting healthy brain aging. New strategies like those outlined above will need to be tested in pre-clinical models and then carefully controlled clinical studies for feasibility, safety and efficacy—but given the importance of healthy brain aging and dementia prevention, we suspect that such endeavors will be well worth the effort.
Key points:
Brain aging is the greatest risk factor for neurodegenerative disease.
Exercise reduces all major hallmarks of brain aging.
Anti-aging compounds may recapitulate some but not all of the effects of exercise on the hallmarks of brain aging.
Emerging compounds and interventions that target energy-sensing pathways similar to exercise may be more effective in this context.
ACKNOWLEDGEMENTS:
The authors were supported by National Institutes of Health grant AG060302 (TJL).
Funding: This work is supported by the National Institute on Aging, National Institutes of Health (AG060302).
References
- 1.Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29(4):737–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565–81. [DOI] [PubMed] [Google Scholar]
- 3.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The Hallmarks of Aging. Cell. 2013;153(6):1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahl D, Solon-Biet SM, Cogger VC, Fontana L, Simpson SJ, Le Couteur DG, et al. Aging, lifestyle and dementia. Neurobiol Dis. 2019;130:104481. [DOI] [PubMed] [Google Scholar]
- 5.In: Downey A, Stroud C, Landis S, Leshner AI, editors. Preventing Cognitive Decline and Dementia: A Way Forward. Washington (DC)2017. [PubMed] [Google Scholar]
- 6.Gonzalez-Freire M, Diaz-Ruiz A, Hauser D, Martinez-Romero J, Ferrucci L, Bernier M, et al. The road ahead for health and lifespan interventions. Ageing Res Rev. 2020;59:101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol. 2016;594(8):2001–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattson MP, Arumugam TV. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018;27(6):1176–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garatachea N, Pareja-Galeano H, Sanchis-Gomar F, Santos-Lozano A, Fiuza-Luces C, Moran M, et al. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015;18(1):57–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue DS, Monteiro PA, Gerosa-Neto J, Santana PR, Peres FP, Edwards KM, et al. Acute increases in brain-derived neurotrophic factor following high or moderate-intensity exercise is accompanied with better cognition performance in obese adults. Sci Rep. 2020;10(1):13493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang W, Cao J, Liu X, Meng F, Li M, Chen B, et al. AMPK Plays a Dual Role in Regulation of CREB/BDNF Pathway in Mouse Primary Hippocampal Cells. J Mol Neurosci. 2015;56(4):782–8. [DOI] [PubMed] [Google Scholar]
- 12.E L, Burns JM, Swerdlow RH. Effect of high-intensity exercise on aged mouse brain mitochondria, neurogenesis, and inflammation. Neurobiol Aging. 2014;35(11):2574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Wang X, Zhu Y, Li Z, Zhu YT, Wu JC, et al. Exercise activates lysosomal function in the brain through AMPK-SIRT1-TFEB pathway. CNS Neurosci Ther. 2019;25(6):796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marques-Aleixo I, Santos-Alves E, Balca MM, Rizo-Roca D, Moreira PI, Oliveira PJ, et al. Physical exercise improves brain cortex and cerebellum mitochondrial bioenergetics and alters apoptotic, dynamic and auto(mito)phagy markers. Neuroscience. 2015;301:480–95. [DOI] [PubMed] [Google Scholar]
- 15.Berchtold NC, Prieto GA, Phelan M, Gillen DL, Baldi P, Bennett DA, et al. Hippocampal gene expression patterns linked to late-life physical activity oppose age and AD-related transcriptional decline. Neurobiol Aging. 2019;78:142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radák Z, Kaneko T, Tahara S, Nakamoto H, Pucsok J, Sasvári M, et al. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochemistry International. 2001;38(1):17–23. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Mesa Y, Colie S, Corpas R, Cristofol R, Comellas F, Nebreda AR, et al. Oxidative Stress Is a Central Target for Physical Exercise Neuroprotection Against Pathological Brain Aging. J Gerontol A Biol Sci Med Sci. 2016;71(1):40–9. [DOI] [PubMed] [Google Scholar]
- 18.Cui L, Hofer T, Rani A, Leeuwenburgh C, Foster TC. Comparison of lifelong and late life exercise on oxidative stress in the cerebellum. Neurobiol Aging. 2009;30(6):903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo L, Dai JR, Guo SS, Lu AM, Gao XF, Gu YR, et al. Lysosomal Proteolysis Is Associated With Exercise-Induced Improvement of Mitochondrial Quality Control in Aged Hippocampus. J Gerontol A Biol Sci Med Sci. 2017;72(10):1342–51. [DOI] [PubMed] [Google Scholar]
- 20.Kou X, Li J, Liu X, Chang J, Zhao Q, Jia S, et al. Swimming attenuates d-galactose-induced brain aging via suppressing miR-34a-mediated autophagy impairment and abnormal mitochondrial dynamics. J Appl Physiol (1985). 2017;122(6):1462–9. [DOI] [PubMed] [Google Scholar]
- 21.Bayod S, Mennella I, Sanchez-Roige S, Lalanza JF, Escorihuela RM, Camins A, et al. Wnt pathway regulation by long-term moderate exercise in rat hippocampus. Brain Res. 2014;1543:38–48. [DOI] [PubMed] [Google Scholar]
- 22.Zhao N, Zhang X, Song C, Yang Y, He B, Xu B. The effects of treadmill exercise on autophagy in hippocampus of APP/PS1 transgenic mice. Neuroreport. 2018;29(10):819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau D, Bengtson CP, Buchthal B, Bading H. BDNF Reduces Toxic Extrasynaptic NMDA Receptor Signaling via Synaptic NMDA Receptors and Nuclear-Calcium-Induced Transcription of inhba/Activin A. Cell Rep. 2015;12(8):1353–66. [DOI] [PubMed] [Google Scholar]
- 24.Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, et al. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 2016;23(1):128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barha CK, Hsiung GYR, Liu-Ambrose T. The Role of S100B in Aerobic Training Efficacy in Older Adults with Mild Vascular Cognitive Impairment: Secondary Analysis of a Randomized Controlled Trial. Neuroscience. 2019;410:176–82. [DOI] [PubMed] [Google Scholar]
- 26.Fan X, Wheatley EG, Villeda SA. Mechanisms of Hippocampal Aging and the Potential for Rejuvenation. Annu Rev Neurosci. 2017;40:251–72. [DOI] [PubMed] [Google Scholar]
- 27.Gomes da Silva S, Simoes PS, Mortara RA, Scorza FA, Cavalheiro EA, da Graca Naffah-Mazzacoratti M, et al. Exercise-induced hippocampal anti-inflammatory response in aged rats. J Neuroinflammation. 2013;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roh H-T, So W-Y. The effects of aerobic exercise training on oxidant–antioxidant balance, neurotrophic factor levels, and blood–brain barrier function in obese and non-obese men. Journal of Sport and Health Science. 2017;6(4):447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaRocca TJ, Mariani A, Watkins LR, Link CD. TDP-43 knockdown causes innate immune activation via protein kinase R in astrocytes. Neurobiol Dis. 2019;132:104514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai SF, Ku NW, Wang TF, Yang YH, Shih YH, Wu SY, et al. Long-Term Moderate Exercise Rescues Age-Related Decline in Hippocampal Neuronal Complexity and Memory. Gerontology. 2018;64(6):551–61. [DOI] [PubMed] [Google Scholar]
- 31.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101(9):3316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li MY, Huang MM, Li SZ, Tao J, Zheng GH, Chen LD. The effects of aerobic exercise on the structure and function of DMN-related brain regions: a systematic review. Int J Neurosci. 2017;127(7):634–49. [DOI] [PubMed] [Google Scholar]
- 33.Jahangiri Z, Gholamnezhad Z, Hosseini M. The effects of exercise on hippocampal inflammatory cytokine levels, brain oxidative stress markers and memory impairments induced by lipopolysaccharide in rats. Metab Brain Dis. 2019;34(4):1157–69. [DOI] [PubMed] [Google Scholar]
- 34.Dallagnol KMC, Remor AP, da Silva RA, Prediger RD, Latini A, Aguiar AS, Jr. Running for REST: Physical activity attenuates neuroinflammation in the hippocampus of aged mice. Brain Behav Immun. 2017;61:31–5. [DOI] [PubMed] [Google Scholar]
- 35.Walker KA, Windham BG, Power MC, Hoogeveen RC, Folsom AR, Ballantyne CM, et al. The association of mid-to late-life systemic inflammation with white matter structure in older adults: The Atherosclerosis Risk in Communities Study. Neurobiology of Aging. 2018;68:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khosravi N, Stoner L, Farajivafa V, Hanson ED. Exercise training, circulating cytokine levels and immune function in cancer survivors: A meta-analysis. Brain Behav Immun. 2019;81:92–104. [DOI] [PubMed] [Google Scholar]
- 37.Sarga L, Hart N, Koch LG, Britton SL, Hajas G, Boldogh I, et al. Aerobic endurance capacity affects spatial memory and SIRT1 is a potent modulator of 8-oxoguanine repair. Neuroscience. 2013;252:326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang JL, Lin YT, Chuang PC, Bohr VA, Mattson MP. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromolecular Med. 2014;16(1):161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Y, Dong Y, Tucker D, Wang R, Ahmed ME, Brann D, et al. Treadmill Exercise Exerts Neuroprotection and Regulates Microglial Polarization and Oxidative Stress in a Streptozotocin-Induced Rat Model of Sporadic Alzheimer’s Disease. J Alzheimers Dis. 2017;56(4):1469–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim DH, Ko IG, Kim BK, Kim TW, Kim SE, Shin MS, et al. Treadmill exercise inhibits traumatic brain injury-induced hippocampal apoptosis. Physiol Behav. 2010;101(5):660–5. [DOI] [PubMed] [Google Scholar]
- 41.Ming G-L, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wahl D, Anderson RM, Le Couteur DG. Anti-aging therapies, cognitive impairment and dementia. J Gerontol A Biol Sci Med Sci. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maharaj H, Maharaj DS, Daya S. Acetylsalicylic acid and acetaminophen protect against MPP+-induced mitochondrial damage and superoxide anion generation. Life Sci. 2006;78(21):2438–43. [DOI] [PubMed] [Google Scholar]
- 46.Chandra S, Jana M, Pahan K. Aspirin Induces Lysosomal Biogenesis and Attenuates Amyloid Plaque Pathology in a Mouse Model of Alzheimer's Disease via PPARα. The Journal of Neuroscience. 2018;38(30):6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerber AR, Bale TL. Antiinflammatory treatment ameliorates HPA stress axis dysfunction in a mouse model of stress sensitivity. Endocrinology. 2012;153(10):4830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Zuo S, Wang J, Huang J, Zhang X, Liu Y, et al. Aspirin Promotes Oligodendrocyte Precursor Cell Proliferation and Differentiation after White Matter Lesion. Frontiers in Aging Neuroscience. 2014;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veronese N, Stubbs B, Maggi S, Thompson T, Schofield P, Muller C, et al. Low-Dose Aspirin Use and Cognitive Function in Older Age: A Systematic Review and Meta-analysis. J Am Geriatr Soc. 2017;65(8):1763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH, et al. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7(4):402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh AK, Singh S, Tripathi VK, Bissoyi A, Garg G, Rizvi SI. Rapamycin Confers Neuroprotection Against Aging-Induced Oxidative Stress, Mitochondrial Dysfunction, and Neurodegeneration in Old Rats Through Activation of Autophagy. Rejuvenation Res. 2019;22(1):60–70. [DOI] [PubMed] [Google Scholar]
- 52.Miwa S, Czapiewski R, Wan T, Bell A, Hill KN, von Zglinicki T, et al. Decreased mTOR signalling reduces mitochondrial ROS in brain via accumulation of the telomerase protein TERT within mitochondria. Aging (Albany NY). 2016;8(10):2551–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kolosova NG, Vitovtov AO, Muraleva NA, Akulov AE, Stefanova NA, Blagosklonny MV. Rapamycin suppresses brain aging in senescence-accelerated OXYS rats. Aging (Albany NY). 2013;5(6):474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lesniewski LA, Seals DR, Walker AE, Henson GD, Blimline MW, Trott DW, et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell. 2017;16(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sadagurski M, Cady G, Miller RA. Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell. 2017;16(4):652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med. 2013;19(3):197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Finney CA, Shvetcov A, Westbrook RF, Jones NM, Morris MJ. The role of hippocampal estradiol in synaptic plasticity and memory: A systematic review. Front Neuroendocrinol. 2020;56:100818. [DOI] [PubMed] [Google Scholar]
- 58.Imtiaz B, Tuppurainen M, Rikkonen T, Kivipelto M, Soininen H, Kroger H, et al. Postmenopausal hormone therapy and Alzheimer disease: A prospective cohort study. Neurology. 2017;88(11):1062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Shoupe D, et al. Cognitive effects of estradiol after menopause: A randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tong JJ, Chen GH, Wang F, Li XW, Cao L, Sui X, et al. Chronic acarbose treatment alleviates age-related behavioral and biochemical changes in SAMP8 mice. Behav Brain Res. 2015;284:138–52. [DOI] [PubMed] [Google Scholar]
- 61.Chin-Hsiao T Dementia Risk in Type 2 Diabetes Patients: Acarbose Use and Its Joint Effects with Metformin and Pioglitazone. Aging and disease. 2019;11(3):0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J, Kosaras B, Del Signore SJ, Cormier K, McKee A, Ratan RR, et al. Modulation of lipid peroxidation and mitochondrial function improves neuropathology in Huntington’s disease mice. Acta Neuropathol. 2011;121(4):487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guzman-Beltran S, Espada S, Orozco-Ibarra M, Pedraza-Chaverri J, Cuadrado A. Nordihydroguaiaretic acid activates the antioxidant pathway Nrf2/HO-1 and protects cerebellar granule neurons against oxidative stress. Neurosci Lett. 2008;447(2–3):167–71. [DOI] [PubMed] [Google Scholar]
- 64.Priestley JRC, Fink KE, McCord JM, Lombard JH. NRF2 activation with Protandim attenuates salt-induced vascular dysfunction and microvascular rarefaction. Microcirculation. 2019;26(7):e12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wahl D, Bernier M, Simpson SJ, de Cabo R, Le Couteur DG. Future directions of resveratrol research. Nutr Healthy Aging. 2018;4(4):287–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baker DJ, Petersen RC. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. The Journal of Clinical Investigation. 2018;128(4):1208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhat A, Mahalakshmi AM, Ray B, Tuladhar S, Hediyal TA, Manthiannem E, et al. Benefits of curcumin in brain disorders. Biofactors. 2019;45(5):666–89. [DOI] [PubMed] [Google Scholar]
- 68.Schwarz C, Stekovic S, Wirth M, Benson G, Royer P, Sigrist SJ, et al. Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging (Albany NY). 2018;10(1):19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hardie DG, Ross Fiona A, Hawley Simon A. AMP-Activated Protein Kinase: A Target for Drugs both Ancient and Modern. Chemistry & Biology. 2012;19(10):1222–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hardie DG. AMPK--sensing energy while talking to other signaling pathways. Cell Metab. 2014;20(6):939–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.López M, Tena-Sempere M. Estradiol effects on hypothalamic AMPK and BAT thermogenesis: A gateway for obesity treatment? Pharmacology & Therapeutics. 2017;178:109–22. [DOI] [PubMed] [Google Scholar]
- 72.Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Experimental & Molecular Medicine. 2016;48(4):e224–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garg G, Singh S, Singh AK, Rizvi SI. Antiaging Effect of Metformin on Brain in Naturally Aged and Accelerated Senescence Model of Rat. Rejuvenation Research. 2016;20(3):173–82. [DOI] [PubMed] [Google Scholar]
- 74.Kobilo T, Guerrieri D, Zhang Y, Collica SC, Becker KG, van Praag H. AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learn Mem. 2014;21(2):119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mo Y, Zhu J-L, Jiang A, Zhao J, Ye L, Han B. Compound 13 activates AMPK-Nrf2 signaling to protect neuronal cells from oxygen glucose deprivation-reoxygenation. Aging. 2019;11(24):12032–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008;7(1):43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhatti GK, Reddy AP, Reddy PH, Bhatti JS. Lifestyle Modifications and Nutritional Interventions in Aging-Associated Cognitive Decline and Alzheimer’s Disease. Front Aging Neurosci. 2019;11:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cerqueira FM, Chausse B, Kowaltowski AJ. Intermittent Fasting Effects on the Central Nervous System: How Hunger Modulates Brain Function. In: Preedy VR, Patel VB, editors. Handbook of Famine, Starvation, and Nutrient Deprivation: From Biology to Policy. Cham: Springer International Publishing; 2019. p. 1243–60. [Google Scholar]
- 79.Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59(2):293–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manoogian ENC, Panda S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing research reviews. 2017;39:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wahl D, Solon-Biet SM, Wang Q-P, Wali JA, Pulpitel T, Clark X, et al. Comparing the Effects of Low-Protein and High-Carbohydrate Diets and Caloric Restriction on Brain Aging in Mice. Cell Reports. 2018;25(8):2234–43.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martens CR, Seals DR. Practical alternatives to chronic caloric restriction for optimizing vascular function with ageing. J Physiol. 2016;594(24):7177–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Research Reviews. 2017;39:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roshandel G, Khoshnia M, Poustchi H, Hemming K, Kamangar F, Gharavi A, et al. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster-randomised trial. Lancet. 2019;394(10199):672–83. [DOI] [PubMed] [Google Scholar]
- 85.Konopka AR, Laurin JL, Schoenberg HM, Reid JJ, Castor WM, Wolff CA, et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell. 2019;18(1):e12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proceedings of the National Academy of Sciences. 2009;106(21):8665. [DOI] [PMC free article] [PubMed] [Google Scholar]


