Abstract
Microorganisms such as viruses, bacteria, and protozoa are the cause of many waterborne human infections. These microbes are either naturally present in aquatic environments or transferred within them by fecal sources. They remain in these environments for varying lengths of time before contaminating a new host. With the emergence of the COVID-19 pandemic, some studies have reported the presence of viral nucleic acids in stool samples from COVID-19 patients, suggesting the possibility of fecal-oral transmission. The SARS-CoV-2 RNA was thereby detected in the wastewater of symptomatic and asymptomatic people with a risk to human and environmental health. In this work, we try to discuss the different potential sources of this contamination, the forms of persistence in the environment, the techniques of partial elimination, and the possibility of creating new reservoirs.
Keywords: SARS-CoV-2, SARS-CoV, wastewater, environmental risk, enteric viruses, dissemination
Background: From Enteric Virus to SARS-CoV-2
Viruses are very small microorganisms, with a size ranging from 0.02 to 0.4 μm in diameter. Their replication mechanism requires a host where they inject their genome, which can be DNA or RNA, in double or single-stranded form. Each group has its own host range and tropism.1 They can be transmitted by different routes: respiratory, via aerosols, or via the fecal-oral route. Transmission of the virus through sexual contact, with contaminated blood products, contact with infected animals (zoonotic viruses), or through vectors such as mosquitoes or ticks have also been documented.2
Enteric viruses are known by their direct or indirect fecal-oral mode of transmission, with the ability to reach the intestinal mucosa and multiply in enterocytes.3 With a diameter varying between 25 and 220 nm, they have a genome consisting of RNA (except for adenoviruses). The majority of human enteric viruses, including rotaviruses, noroviruses, and astroviruses, are characterized by a non-enveloped, naked capsid of a protein nature,4 which presumably can tolerate gastro-intestinal fluids and enzymes. They present a great diversity and are classified according to the diseases they induce in humans: acute hepatitis, gastroenteritis, or other diseases (Table 1).5-7 Caliciviruses, rotaviruses, adenoviruses, astroviruses, and coronaviruses, are viruses that use the enteric tract as a route of entry to the human, animal, or avian host. These “enteric” viruses occur globally and share similar features. Most are RNA viruses that replicate in the cytoplasm of mature absorptive epithelial cells lining the villi of the small intestine, leading to inflammation and villus atrophy. Vomiting and diarrhea can result in dehydration and death if untreated.8
Table 1.
Various enteric viruses are known to cause gastroenteritis.
| Family | Genius | Species | |
|---|---|---|---|
| Gastroenteritis virus | Calciviridae | Norovirus | Norovirus |
| Saprovirus | Virus Sapporo | ||
| Astroviridae | Astrovirus | Astrovirus | |
| Reoviridae | Rotavirus | Rotavirus (groups A&C) | |
| Reovirus | Reovirus | ||
| Coronaviridae | Coronavirus | Coronavirus | |
| Torovirus | Torovirus | ||
| Adenoviridae | Mastadenovirus | Adenovirus types 40&41 | |
| Parvoviridae | Parvovirus | Parvovirus | |
| Hepatitis virus | Picornaviridae | Hepatovirus | Hepatitis A virus (HAV) |
| Hepeviridae | VHE | Hepatitis E virus (HEV) | |
| Other diseases | Piconaviridae | Enterovirus | Poliovirus, coxsackievirus A & B, ECHOvirus, enterovirus 68-71 |
| Adenoviriadae | Mastadenovirus | Adenovirus 40 & 41 | |
Coronaviruses are enteric viruses,3 which have been associated with gastroenteritis in humans,9 sclerosing enterocolitis in neonates,10 and fatal fulminant gastroenteritis in neonates,11 but it appears that in almost 90% of cases the infection is asymptomatic.12 Moreover, they are associated with respiratory, hepatic, and neurological diseases.13,14 Their first infection occurred in the 1960s in the context of benign upper respiratory infections. They have long been considered one of the main agents, along with rhinoviruses, of the common cold.15 coronaviruses are also single-stranded RNA viruses, with a genome of about 30 kb, which explains their instability in bacterial plasmids.16 Characterized by a tubular capsid with an envelope bristling with large spicules that gives the virion a crown-like appearance.17 Their diameter is about 120 nm and their size ranges from 60 to 220 nm.10,16 Enveloped viruses are often more easily inactivated than non-enveloped viruses because the envelope is less resistant to environmental conditions and disinfectants.18 This envelope consists of the glycoproteins M, E, and S (protein S is a membrane protein that organizes itself as a trimer to form spicules) and hemaglutinin-esterase HE (only for group 2 coronaviruses). The viral capsid consists of protein N (bound to viral RNA) and is helical in shape.17 Tissue tropism and host spectrum are largely determined by the S protein, which is responsible for the attachment of the virion to the cell receptor and allows membrane fusion.17,19,20
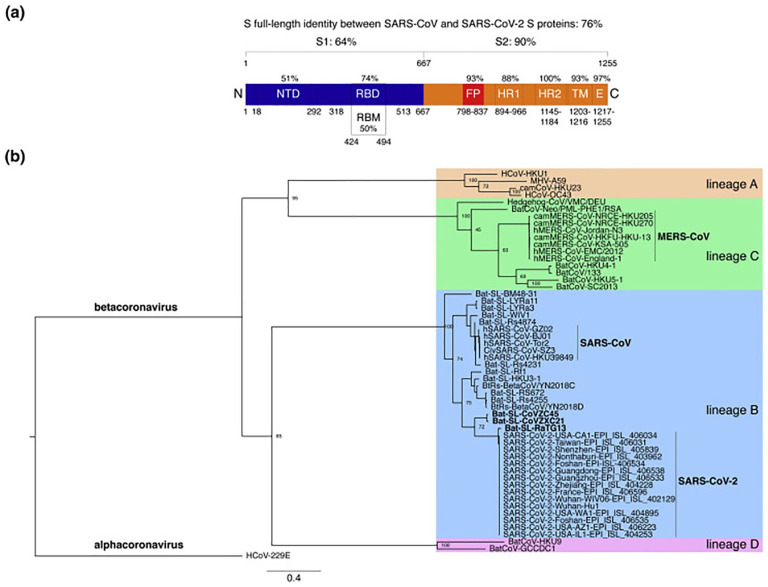
The family Coronaviridae is divided into 2 subfamilies, the Coronavirinae and the Torovirinae. Coronaviruses, belonging to the subfamily Coronavirinae, are subdivided into 3 groups (1-3) on a serological and molecular basis, groups 1 and 2 infecting mammals and group 3 infecting birds. There are 5 human coronaviruses (HCoV): 229E, OC43 (both described since 1960), SARS-CoV (Severe Acute Respiratory Syndrome, described since the 2003 epidemic), NL63 (described in 2004), and HKU1 (described in 2005) (Figure 1).17,22-24
Figure 1.
Comparative analyses of the SARS-CoV-2 S protein sequence. (a) Protein sequence identities between SARS-CoV-2 S and SARS-CoV S. (b) The S protein sequence was aligned with representatives of the 4 betacoronavirus lines.21
Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) are 2 coronaviruses of zoonotic origin from a family of 6 coronaviruses that can cause severe respiratory illness and high mortality. Both share a similar genomic organization with other coronaviruses, but also have a unique genomic structure that includes several specific accessory genes, including ORF3a, 3b, ORF6, ORF7a, 7b, ORF8a, 8b, and 9b.25 In December 2019 in China, the 7th member of the coronavirus family emerged as the cause of Coronavirus Disease 2019 (COVID-19). This disease is due to zoonotic transmission related to a large seafood market that also included trade in live wild animals. The first published studies on this subject identified bats as the possible origin of SARS-CoV-2 and the angiotensin converting enzyme 2 (ACE2) as its cell surface receptor.26 Despite the fact that these publications have advanced our non-compliance with SARS-CoV-2, our knowledge of this virus is still limited. Phylogenetic analysis of the whole viral genome showed that COVID-19 was closely related to a group of SARS-like coronaviruses.27
Noroviruses, rotaviruses, toroviruses, coronaviruses, astroviruses, enteroviruses, and adenoviruses are enteric viruses that are responsible for 50% of cases of diarrhea and vomiting (Table 1).28,29 Coronaviruses have been detected in the stools of patients suffering from digestive disorders associated with respiratory symptoms.17,30-32 These digestive disorders have thus been validated in more than 60% of patients with COVID-19.33,34 It is clear that SARS-CoV-2 is primarily a respiratory virus, but some studies have indicated possible infestation and replication in the gastrointestinal tract, because of the high levels of ACE2 the gut.35-37 This is probably true because between 2% and 80% of patients confirmed with SARS-CoV-2 have experienced gastrointestinal illness including diarrhea and vomiting.38,39 Moreover, SARS-CoV-2 RNA is often present in the stool after the resolution of the respiratory infection and respiratory samples are negative.40,41 However, to date, the source of the presence of SARS-CoV-2 RNA in feces has not been identified, which puts certain hypotheses related to swallowed sputum or active replication at the game, as well as suggesting a spreading of infectious virions via sewage.
In order to identify this source, several studies were launched, including those of Zhang et al who had indicated an infection and replication of virions in the gastrointestinal tract,35,36 and that of Danchin et al who have indicated as well that the fecal-oral route is putatively important for the transmission of the virus.42 The replication of the virus in the intestinal tract suggests an integration of fecal matter into wastewater,40,43 and the presence of the envelope may be related to the difficulty of isolating and detecting infectious virions in feces and sewage.
RNA and/or SARS-CoV Virion in Effluents: Would It Be Disseminated in the Same Way as Enteric Viruses?
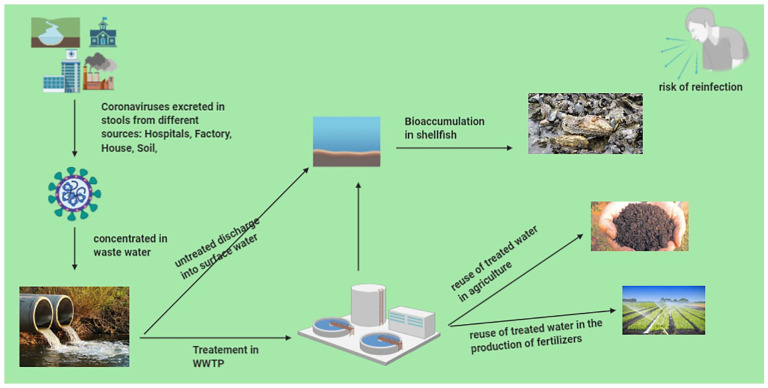
Enteric viruses are rejected, and dispersed in large amounts via the fecal-oral route through human excretions, including feces (103-1010 viral particles/fecal gram) into the environment.44 They enter the gastrointestinal tract, survive the acidity of the stomach, and initiate their infectious cycle. Viral particles are then excreted in high doses in the stool (10−7 infectious particles per gram of stool),45 and are transferred to surface waters and environments via untreated or treated wastewater discharged from several sources.46 The absence of a lipidic layer makes them highly resistant to physicochemical environmental conditions such as acids, temperature, dryness, pressure, disinfectants, and ultra-violet radiation. They are also able to retain their infectious character after several hours or days in seawater or on inert or biological surfaces (Figure 2).47,48
Figure 2.
The discharge of enteric viruses into the environment through treated or untreated wastewater involves some forms of the persistence of the virus in the environment, including the formation of aerosols from activated sludge, contamination of agricultural products, bioaccumulation in aquatic resources, and so on.
Billions of liters of untreated sewage enter the coastal ocean every year, containing enteric microbes.49 As a result of this environmental situation, water quality has deteriorated and is unfit for human consumption in some developed and less developed countries.50,51 From the environment, the fecal-oral transmission of enteric viruses occurs mainly through the consumption of contaminated food, eaten fresh or not having undergone sufficient industrial or domestic treatment, as well as through human-to-human contact. Waterborne transmission of enteric viruses can cause epidemics in countries with poor sanitary conditions.47,52 Danchin et al in their study suggested that natural water bodies contaminated by wastewater could become environmental reservoirs for SARS-CoV-2, this is probably true because the high infectivity of SARS-CoV-2 could lead to the transmission of COVID-19 in such environments.42 During the current COVID-19 pandemic, people with poor sanitation, consuming food irrigated with wastewater and having direct contact with water resources contaminated by enteric viruses will be a danger to unexposed people.53 It should be noted that to date no study has been carried out in this context for SARS-CoV-2, which encourages such research in countries with a weak sanitation network.54
SARS-CoV in wastewater: Persistence or Elimination?
In aquatic environments, the evolution and persistence of native and non-native enteric microbes are governed by biotic and abiotic factors, which may in some cases favor their appearance or inhibit their growth or persistence outside the primary host.55,56 In relation to COVID-19, temperature, organic matter content, and pH have been reported as factors influencing the infectivity of SARS-CoV.57
In general, fecal-oral transmitted viruses are persistent in the environment and are able to persist in the processes used to inactivate or control pathogens in contaminated food.58 Most foodborne viruses are non-enveloped and therefore fairly stable outside the host, and are resistant to extreme pH (acid and alkaline), drought, radiation, and so on.59
In a previous study, a fecal-oral contamination was reported in a residential complex in Hong Kong, where a large group of people with symptoms of diarrhea and oral infection were reported to be infected with SARS-CoV as a result of its spread from a poor sanitation system. It has been suggested that this SARS-CoV infection occurred through the breathing of aerosols created by flushing toilets or faulty plumbing systems.60 The detection of SARS-CoV-2 RNA in feces and sewage,43,61 makes us think of the detection of his virions as well in wastewater and contaminated water systems (freshwater source case),62,63 as indirect routes of infection. Even if, to date, no SARS-CoV2 infectious viruses have been recovered from untreated or treated wastewater.64
While to date, SARS-CoV-2 virions have not been detected in the environment, its RNA fragments have been detected in untreated sewage from several countries including Italy65; Spain66; Australia61; Netherlands43; United States of America67,68; France.69 Moreover, a positive correlation between the concentrations of RNA of SARS-CoV-2 detected in wastewater and the number of clinical cases infected by this virus has been confirmed by researchers in France,69 America,67,68 and the Netherlands.43 And, its detection is not only limited to symptomatic but also asymptomatic people,40,70-72 because wastewater contains viruses shed by both types of people,73 as previously demonstrated for enteric viruses, such as norovirus, hepatitis A virus, and poliovirus.74,75
The SARS-CoV-2 RNAs detected in different countries have often been localized in large cities, which makes us think of large sewage systems that contain gray water from different sources (hospitals, houses, soil leaching, rainwater runoff, and so on), this may amplify the potential for collection and dissemination, increasing the risk of transmission.76 Without forgetting that these different sources of wastewater, both point and diffuse, contain components such as disinfectants, detergents, soaps, etc., which can minimize the concentration of viral load,77 so there is also the dilution factor in the receiving environment, this is important because the concentration of viral load in body fluids such as saliva and sputum is potentially high.40
Discharge of wastewater containing SARS-CoV into the environment: New risks?
In the environment, septic tank sewage leaks, pipe failures, lack of treatment at wastewater treatment plants (WWTPs), or lack of infrastructure in some countries are factors that can result in the direct release of SARS-CoV into receiving water systems (e.g. streams, rivers, ponds, estuaries, lakes, and groundwaters). In addition, treated wastewater such as secondary effluents that are discharged may also transport viruses into the environment.69 This is true because SARS-CoV-2 RNA has been detected in a Japanese and Italian river,65 even though there is no real detection of its virions in aquatic environments.78
During production, irrigation of fruit and vegetable crops with water of poor sanitary quality is an important factor for viral contamination; such an observation has been documented in SARS-CoV.79 The same is true for the use of organic fertilizers (animal dejecta, sludge not having undergone heat treatment) during traditional farming practices, which are particularly important for agricultural and vegetable production in developing countries,80,81 with a risk of food contamination that can occur throughout the agro-industrial chain, from the production of raw materials to the consumption of marketed products, through the stages of processing and development of finished products.
Treated wastewater has been documented to have SARS-CoV-2 RNA,69 thus suggesting potential risks associated with wastewater reuse for agriculture. As long as the oral-fecal transmission of SARS-CoV-2 has not been studied so far, it should be noted that such a risk has been documented with viruses of the same family.82 Infectivity of human bovine 229E CoV was detected on lettuce leaves even after a storage time of 2 days at 4°C.82,83 In addition, washing the products does not eliminate virions.83
Viral inactivation is highly variable and depending on the type of virus, the type of treatment, the type of matrix, the concentration of viral load, and the inactivation parameter. It is therefore not easy to determine the most resistant virus for a particular treatment in a given matrix, and there is no single treatment regime applicable to all viruses in all matrices.84 Temperature is widely known as the main factor determining virus inactivation in the environment and is also widely applied in the food industry.57 The inactivation of infectivity of CoV-SARS has been studied during low temperatures, for example, 14 days at 4°C and 2 days at 25°C in wastewater.85 This allows us to think about cold and temperate seasons where the environmental survival of SARS-CoV-2 could increase, which is interesting because according to recent studies, total inactivation of SARS-CoV and SARS-CoV-2 was detected at 56°C for 30 and 90 minutes respectively.86,87
Composting is an aerobic process that facilitates and accelerates the transformation of fermentable organic matter by many microorganisms naturally present in the material to be composted. During this process, temperature changes within the compost induce changes in the composition and activity of microbial communities and promote the elimination of pathogenic microorganisms.88 Numerous studies have focused on the dynamics of pathogenic microbes during the composting process and have shown, the growth of some pathogenic at the beginning of the thermophilic phase, or a resumption of growth of the populations during the cooling phase.89 In some cases, compost may be re-contaminated during storage, handling, watering, or by animals.90 The use of re-contaminated compost as fertilizer may cause contamination of plant materials with pathogenic microorganisms.91
In composts, especially when manipulating windrows (turning), bioaerosols are generated. This transition can be considered as a form of persistence,92 which represents a significant public health problem for humans both directly (contact) and indirectly (airborne contamination). During the first SARS-CoV outbreak, the aerosol formation was detected as a key mechanism of fecal-oral transmission, making us think of SARS-CoV-2 as the new suspect.93,94 In particular, 1 study suggested the aerosol viability of SARS-CoV-2 up to 16 hours,95,96 and that HCoV 229E remains infectious for 6 days at 25°C and longer periods at 6°C.97 Even though no aerosol analyses of SARS-CoV-2 in sewage treatment plants have been reported, the formation of aerosols during the treatment process could be a risk for sewage treatment plant operators and facilitate dissemination, especially for sewage treatment plants in densely populated areas.98,99
Dissemination of SARS-CoV-2 in the environment
Groundwater contamination occurs from a variety of sources (natural, agricultural, industrial, residential, etc.), residential wastewater systems can be a source of various types of contaminants, including bacteria, viruses, nitrates, and organic compounds. Wells used for the disposal of domestic wastewater (septic systems, cesspools, drainage wells for exceptional rainfall-runoff, groundwater recharge wells) are particularly concerned about groundwater quality if they are located near drinking water wells.100
The morphological characteristics of viruses confer them high mobility in groundwater,101 which is possible because of their external peak glycoproteins, which give them interaction with flow paths and fractures.102 The SARS-CoV-2 known by its size ~100 nm and its long survival time in water and surfaces,77 indicate that the virus can migrate into the subsoil and contaminate aquifers. However, the presence of envelope, extracellular enzymes in bacteria such as hydrolases and proteases are able to inactivate SARS-CoV-2.103,104
While enveloped viruses in the environment are likely to be eliminated more than non-enveloped viruses,103,104 studies targeting the detection of RNA in sludge had documented the presence of CoV genes in 80% of untreated sewage sludge samples, of which HKU1 CoV was the most dominant.61 The lack of data in this context for SARS-CoV,105 calls molecular techniques such as Virome metagenomics106 and Transcriptomics,107 studies, because the low concentration of viral load will be an obstacle for its detection. In some countries with partial or non-existent sewerage systems, there are still people who practice open defecation,108 which can amplify the viral load locally, especially in the presence of rainfall.
In addition to groundwater contamination, the release of wastewater into surface water leads to the bioaccumulation of certain microbes in fisheries resources.49 Bathing and consumption of raw or undercooked seafood from coastal waters polluted by enteric viruses leads to more than 120 million cases of gastrointestinal (GI) diseases and 50 million cases of respiratory diseases.109
Bivalve molluscs (oysters, mussels, clams. . .) are one of the fishery resources that filter, through their gills, large quantities of water (100-650 L/hour/kg of mussels), thus accumulating enteric viruses in their digestive gland.110,111 Unlike bacterial contaminants, the adhesion of viruses to the cells of the digestive mucosa involves specific bonds that strongly limit the effectiveness of the depurative periods that precede marketing.112
In a study conducted by Gabrieli et al, 2007, 137 bivalves were collected for environmental and market monitoring; all samples were analyzed by RT-PCR assay. Bacteriological enumerations meeting European Union criteria for molluscs were performed in 69.5% of all samples, while overall positive values for the presence of enteric viruses were obtained: 25.5%, 18.2%, 8.0%, and 2.1% for rotaviruses, astroviruses, enteroviruses, and noroviruses, respectively. Mussels appeared to be the most contaminated bivalves, with 64.8% of samples positive, 55.7% and 22.7% respectively for clams and oysters, while in bivalves collected for human consumption 50.7% were positive for enteric viruses, compared to 56.4% of samples collected for the classification of growing areas. The overall positive sample was 54.0%.111
It is true that the COVID-19 pandemic originated from a live animal and seafood market in Wuhan, China.26 But it should be noted also that SARS-CoV-2 belongs to the family Coronaviridae and the genus Betacoronavirus – which have only been reported to infect mammals.17,22-24 At present, there is no evidence to suggest that SARS-CoV-2 can infect aquatic food animals (e.g. fish, crustaceans, molluscs, amphibians) and therefore these animals do not play an epidemiological role in the spread of COVID-19 to humans.113
Aquatic food-producing animals and their products, like any other surface, can potentially be contaminated with SARS-CoV-2, particularly when handled by persons infected with the virus. Nevertheless, with proper food handling and sanitation, the likelihood of contamination of aquatic animals or their products with SARS-CoV-2 should be negligible.
In the current context of trade liberalization and globalization, these productions represent a significant risk that must be taken into consideration.114 An infected individual, whether or not he or she develops clinical symptoms, will excrete enteric viruses in his or her feces for relatively long periods of time (from a few days to several weeks depending on the virus).115 In 94 norovirus epidemics recently studied in the United States, the involvement of a handler at 1 level of the production chain was demonstrated in 48% of cases.116
Also, article 14 of the European regulation EC n°178/2002 indicates that “no food shall be placed on the market if it is unsafe” and that “food is considered unsafe if it is: (a) injurious to health; (b) unfit for human consumption.” In its recital 2, Regulation EC No 2073/2005 specifies that “food must not contain micro-organisms or their toxins or metabolites in quantities which present an unacceptable risk to human health.” Enteric viruses are therefore perfectly within the regulatory framework of these texts which are the basis of the Hygiene Package in force since 2006.
SARS-CoV: Host changing and adaptive capacity
Coronaviruses are both highly adaptive and capable of infecting different human tissues. They transfer easily between new host species and adapt to a variety of ecological conditions through the accumulation of point mutations and homologous recombination.117 The research carried out to identify the animal reservoirs of SARS-CoV has highlighted the strong evolutionary potential of these viruses, their very broad host spectrum, and their high genetic diversity.118-120
It is certainly true that to date there have been no SARS-CoV-2 virions in the environment,78 and this may be related to the lack of environmental monitoring. But, the phenomena of its bioaccumulation in bivalves and its dispersion in water environments, makes us think about the possible interactions and genetic exchanges between the different viruses present in wastewater.121
It is true that the rate of mutation in SARS-CoV-2 is not yet clear,122,123 but, the rate of intra-host variants observed and shared between different individuals suggests adaptive evolution of the virus in patients. This high level of diversity could potentially affect the antigenicity, virulence, and infectivity of the virus, making its elimination more difficult during a reinfection.124
In addition, for the detection of SARS-CoV-2 RNA in wastewater, viruses are considered infectious when they can penetrate the cell membrane and express at least 1 viral gene or replicate their intact genome, as well as if the viral capsid is undamaged and retains its ability to bind to the cell receptor,125,126 therefore, the persistence of “naked” nucleic acid (DNA/RNA) in the environment suggests a possible horizontal gene transfer between non-indigenous and indigenous communities by the transformation. These processes of horizontal gene transfer may participate in the diffusion of genes and virulence.55,127
Conclusion
Zoonotic infections are a separate category for human health but unfortunately, to date, few studies have been published on the use of environmental surveillance to monitor the contamination of water systems by SARS-CoV.
In both developed and less developed countries, wastewater treatment is an incomplete process that leads to the accumulation of enteric microbes in the receiving environment. It is essential to control and safeguard the quality of wastewater containing enteric viruses and their products. The health risks of COVID-19 through waterborne transmission may be greater than originally thought, and wastewater should be further investigated as a potential pathway for COVID-19 transmission.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contribution: All authors contributed conception and design of the study YS wrote different sections of the manuscript. FC and FB contributed to manuscript revision. All authors contributed to manuscript revision, read and approved the submitted version.
ORCID iD: Yousra Sbaoui  https://orcid.org/0000-0002-6919-4929
https://orcid.org/0000-0002-6919-4929
References
- 1. Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Viruses: structure, function, and uses. In: Molecular Cell Biology. 4th ed. W. H. Freeman; 2000. Accessed October 06, 2020. https://www.ncbi.nlm.nih.gov/books/NBK21523/
- 2. The routes of infection and paths of transmission of viruses. Proc R Soc Med. 1936;29(6):563-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saif LJ. Comparative pathogenesis of enteric viral infections of swine. In: Paul PS, Francis DH, eds. Mechanisms in the Pathogenesis of Enteric Diseases 2. Springer; 1999:47-59. [DOI] [PubMed] [Google Scholar]
- 4. Bushman FD, McCormick K, Sherrill-Mix S. Virus structures constrain transmission modes. Nat Microbiol. 2019;4(11):1778-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mead PS, Slutsker L, Dietz V, et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5(5):607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Y, Guo H, Xu Z, et al. An outbreak of norovirus gastroenteritis associated with a secondary water supply system in a factory in south China. BMC Public Health. 2013;13(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lopman B, Vennema H, Kohli E, et al. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. The Lancet. 2004;363(9410):682-688. [DOI] [PubMed] [Google Scholar]
- 8. Bishop RF, Kirkwood CD. Enteric viruses. Encycl Virol. 2008:116-123. Published online July 30, 2008. doi: 10.1016/B978-012374410-4.00386-1 [DOI] [Google Scholar]
- 9. Mortensen ML, Ray CG, Payne CM, Friedman AD, Minnich LL, Rousseau C. Coronavirus like particles in human gastrointestinal disease: epidemiologic, clinical, and laboratory observations. Am J Dis Child. 1985;139(9):928-934. [DOI] [PubMed] [Google Scholar]
- 10. Chany C, Moscovici O, Lebon P, Rousset S. Association of coronavirus infection with neonatal necrotizing enterocolitis. Pediatrics. 1982;69(2):209-214. [PubMed] [Google Scholar]
- 11. Vaucher YE, Ray CG, Minnich LL, Payne CM, Beck D, Lowe P. Pleomorphic, enveloped, virus-like particles associated with gastrointestinal illness in neonates. J Infect Dis. 1982;145(1):27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bar-On YM, Flamholz A, Phillips R, Milo R. SARS-CoV-2 (COVID-19) by the numbers. eLife. 2020;9:e57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lai MM, Cavanagh D. The molecular biology of coronaviruses. Adv Virus Res. 1997;48:1-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woo PCY, Lau SKP, Yip CCY, et al. Comparative analysis of 22 coronavirus HKU1 genomes reveals a novel genotype and evidence of natural recombination in coronavirus HKU1. J Virol. 2006;80(14):7136-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang Y, Lau SKP, Woo PCY, Yuen KY. CoVDB: a comprehensive database for comparative analysis of coronavirus genes and genomes. Nucleic Acids Res. 2008;36(Database issue):D504-D511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ding S, Liang TJ. Is SARS-CoV-2 also an enteric pathogen with potential fecal–oral transmission? A COVID-19 virological and clinical review. Gastroenterology. 2020;159(1):53-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vabret A, Dina J, Brison E, Brouard J, Freymuth F. Coronavirus humains (HCoV). Pathol Biol. (Paris) 2009;57(2):149-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Madigan MT, Bender KS, Buckley DH, Sattley WM, Stahl DA. Brock biology of microorganisms. Accessed September 29, 2020. https://content/one-dot-com/one-dot-com/us/en/higher-education/product.html
- 19. van Boheemen S, de Graaf M, Lauber C, et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio. 2012;3(6):e00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jaimes JA, André NM, Chappie JS, Millet JK, Whittaker GR. Phylogenetic analysis and structural modeling of SARS-CoV-2 spike protein reveals an evolutionary distinct and proteolytically sensitive activation loop. J Mol Biol. 2020;432(10):3309-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967-1976. [DOI] [PubMed] [Google Scholar]
- 23. Rota PA, Oberste MS, Monroe SS, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300(5624):1394-1399. [DOI] [PubMed] [Google Scholar]
- 24. Williams WB, Liao HX, Moody MA, et al. Diversion of HIV-1 vaccine-induced immunity by gp41-microbiota cross-reactive antibodies. Science. 2015;349(6249):aab1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Al-Qahtani AA. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): emergence, history, basic and clinical aspects. Saudi J Biol Sci. 2020;27(10):2531-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alain S, Denis F. Épidémiologie des diarrhées aiguës infectieuses en France et en Europe. Arch Pédiatrie. 2007;14:S132-S144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. La Rosa G, Pourshaban M, Iaconelli M, Muscillo M. Quantitative real-time PCR of enteric viruses in influent and effluent samples from wastewater treatment plants in Italy. Ann Ist Super Sanita. 2010;46(3):266-273. [DOI] [PubMed] [Google Scholar]
- 30. Kuypers J, Martin ET, Heugel J, Wright N, Morrow R, Englund JA. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics. 2007;119(1):e70-e76. [DOI] [PubMed] [Google Scholar]
- 31. Jevšnik M, Steyer A, Zrim T, et al. Detection of human coronaviruses in simultaneously collected stool samples and nasopharyngeal swabs from hospitalized children with acute gastroenteritis. Virol J. 2013;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rovida F, Campanini G, Piralla A, Adzasehoun KM, Sarasini A, Baldanti F. Molecular detection of gastrointestinal viral infections in hospitalized patients. Diagn Microbiol Infect Dis. 2013;77(3):231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bucher M, Meyer C, Grötzbach B, Wacheck S, Stolle A, Fredriksson-Ahomaa M. Epidemiological data on pathogenic Yersinia enterocolitica in Southern Germany during 2000-2006. Foodborne Pathog Dis. 2008;5(3):273-280. [DOI] [PubMed] [Google Scholar]
- 34. Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997-1001. [DOI] [PubMed] [Google Scholar]
- 35. Zhang H, Kang Z, Gong H, et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv. Published online Janurary 2020. doi: 10.1101/2020.01.30.927806 [DOI] [Google Scholar]
- 36. Zang R, Gomez Castro MF, McCune BT, et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5(47):eabc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- 38. Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159(1):81-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lo IL, Lio CF, Cheong HH, et al. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci. 2020;16(10):1698-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465-469. [DOI] [PubMed] [Google Scholar]
- 41. Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92(7):833-840. [DOI] [PubMed] [Google Scholar]
- 42. Danchin A, Ng TWP, Turinici G. A new transmission route for the propagation of the SARS-CoV-2 coronavirus. medRxiv. Published online February 2020. doi: 10.1101/2020.02.14.20022939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Medema G, Heijnen L, Elsinga G, Italiaander R, Brouwer A. Presence of SARS-coronavirus-2 in sewage. medRxiv. Published online July 2020. doi: 10.1101/2020.03.29.20045880 [DOI] [PubMed] [Google Scholar]
- 44. Metcalf TG, Melnick JL, Estes MK. Environmental virology: from detection of virus in sewage and water by isolation to identification by molecular biology—a trip of over 50 years. Annu Rev Microbiol. 1995;49:461-487. [DOI] [PubMed] [Google Scholar]
- 45. Food and Agriculture Organization of the United Nations and World Health Organization (eds.). Microbiological Hazards in Fresh Leafy Vegetables and Herbs: Meeting Report. World Health Organization; ; Food and Agriculture Organization of the United Nations; 2008. [Google Scholar]
- 46. Ritter L, Solomon K, Sibley P, et al. Sources, pathways, and relative risks of contaminants in surface water and groundwater: a perspective prepared for the Walkerton inquiry. J Toxicol Environ Health A. 2002;65(1):1-142. [DOI] [PubMed] [Google Scholar]
- 47. Kurdziel AS, Wilkinson N, Langton S, Cook N. Survival of poliovirus on soft fruit and salad vegetables. J Food Prot. 2001;64(5):706-709. [DOI] [PubMed] [Google Scholar]
- 48. D’Souza DH, Sair A, Williams K, et al. Persistence of caliciviruses on environmental surfaces and their transfer to food. Int J Food Microbiol. 2006;108(1):84-91. [DOI] [PubMed] [Google Scholar]
- 49. West PA. The human pathogenic vibrios—a public health update with environmental perspectives. Epidemiol Infect. 1989;103(1):1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pour une citoyenneté responsable de l’enseignement supérieur: enquêtes et propositions de PRELUDE - UNESCO Bibliothèque Numérique. Accessed May 05, 2020. https://unesdoc.unesco.org/ark:/48223/pf0000130342
- 51. Blot F. Discours et pratiques autour du “développement durable” et des “ressources en eau”. Une approche relationnelle appliquée aux bassins d’Adour-Garonne et du Segura. 2011:544. [Google Scholar]
- 52. Delmas G, Silva NJ, Pihier N, Weill F-X, Vaillant V, de Valk H. Les toxi-infections alimentaires collectives en France entre 2006 et 2008. 2010. [Google Scholar]
- 53. Ramia S. Transmission of viral infections by the water route: implications for developing countries. Rev Infect Dis. 1985;7(2):180-188. [DOI] [PubMed] [Google Scholar]
- 54. Usman M, Farooq M, Hanna K. Existence of SARS-CoV-2 in wastewater: implications for its environmental transmission in developing communities. Environ Sci Technol. 2020;54(13):7758-7759. [DOI] [PubMed] [Google Scholar]
- 55. Barnes MA, Turner CR, Jerde CL, Renshaw MA, Chadderton WL, Lodge DM. Environmental conditions influence eDNA persistence in aquatic systems. Environ Sci Technol. 2014;48(3):1819-1827. [DOI] [PubMed] [Google Scholar]
- 56. Sinton LW, Hall CH, Lynch PA, Davies-Colley RJ. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl Environ Microbiol. 2002;68(3):1122-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Geller C, Varbanov M, Duval RE. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses. 2012;4(11):3044-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roos YH. Water and pathogenic viruses inactivation—food engineering perspectives. Food Eng Rev. 2020;12:251-267. [Google Scholar]
- 59. Sánchez G, Bosch A. Survival of enteric viruses in the environment and food. Viruses Foods.:367-392. Published online August 2016. doi: 10.1007/978-3-319-30723-7_13 [DOI] [Google Scholar]
- 60. Kr M, Yy G, Tg L. Environmental transmission of SARS at Amoy Gardens. J Environ Health. 2006;68(9):26-30; quiz 51. [PubMed] [Google Scholar]
- 61. Ahmed W, Angel N, Edson J, et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci Total Environ. 2020;728:138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mauter MS, Zucker I, Perreault F, Werber JR, Kim J-H, Elimelech M. The role of nanotechnology in tackling global water challenges. Nat Sustain. 2018:1(4):166-175. [Google Scholar]
- 63. Greve P, Kahil T, Mochizuki J, et al. Global assessment of water challenges under uncertainty in water scarcity projections. Nat Sustain. 2018;1(9):486-494. [Google Scholar]
- 64. Water, sanitation, hygiene, and waste management for SARS-CoV-2, the virus that causes COVID-19. Accessed September 29, 2020. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-IPC-WASH-2020.4
- 65. Rimoldi SG, Stefani F, Gigantiello A, et al. Presence and vitality of SARS-CoV-2 virus in wastewaters and rivers. medRxiv. Published online May 2020. doi: 10.1101/2020.05.01.20086009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Randazzo W, Truchado P, Cuevas-Ferrando E, Simón P, Allende A, Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wu F, Zhang J, Xiao A, et al. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5(4):e00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Peccia J, Zulli A, Brackney DE, et al. SARS-CoV-2 RNA concentrations in primary municipal sewage sludge as a leading indicator of COVID-19 outbreak dynamics. medRxiv. Published online June 2020. doi: 10.1101/2020.05.19.20105999 [DOI] [Google Scholar]
- 69. Wurtzer S, Marechal V, Mouchel JM, et al. Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters. medRxiv. Published online May 2020. doi: 10.1101/2020.04.12.20062679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jiehao C, Jin X, Daojiong L, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020;71(6):1547-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sinclair RG, Choi CY, Riley MR, Gerba CP. Chapter 9 - Pathogen surveillance through monitoring of sewer systems. In: Laskin AI, Sariaslani S, Gadd GM. eds. Advances in Applied Microbiology. Vol. 65. Academic Press; 2008:249-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Asghar H, Diop OM, Weldegebriel G, et al. Environmental surveillance for polioviruses in the global polio eradication initiative. J Infect Dis. 2014;210(Suppl 1):S294-S303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hellmér M, Paxéus N, Magnius L, et al. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl Environ Microbiol. 2014;80(21):6771-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yang S, Paik K, McGrath GS, et al. Functional topology of evolving urban drainage networks. Water Resour Res. 2017;53(11):8966-8979. [Google Scholar]
- 77. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. La Rosa G, Bonadonna L, Lucentini L, Kenmoe S, Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;179:115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Adegoke AA, Amoah ID, Stenström TA, Verbyla ME, Mihelcic JR. Epidemiological evidence and health risks associated with agricultural reuse of partially treated and untreated wastewater: a review. Front Public Health. 2018;6:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Phanuwan C, Takizawa S, Oguma K, Katayama H, Yunika A, Ohgaki S. Monitoring of human enteric viruses and coliform bacteria in waters after urban flood in Jakarta, Indonesia. Water Sci Technol. 2006;54(3):203-210. [DOI] [PubMed] [Google Scholar]
- 81. Villar LM, de Paula VS, Diniz-Mendes L, et al. Molecular detection of hepatitis A virus in urban sewage in Rio de Janeiro, Brazil. Lett Appl Microbiol. 2007;45(2):168-173. [DOI] [PubMed] [Google Scholar]
- 82. Yépiz-Gómez MS, Gerba CP, Bright KR. Survival of respiratory viruses on fresh produce. Food Environ Virol. 2013;5(3):150-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mullis L, Saif LJ, Zhang Y, Zhang X, Azevedo MSP. Stability of bovine coronavirus on lettuce surfaces under household refrigeration conditions. Food Microbiol. 2012;30(1):180-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Teunis PFM, Rutjes SA, Westrell T, de Roda Husman AM. Characterization of drinking water treatment for virus risk assessment. Water Res. 2009;43(2):395-404. [DOI] [PubMed] [Google Scholar]
- 85. Wang XW, Li JS, Jin M, et al. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J Virol Methods. 2005;126(1):171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chin A, Chu JTS, Perera MRA, et al. Stability of SARS-CoV-2 in different environmental conditions. medRxiv. published online March 2020. doi: 10.1101/2020.03.15.20036673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. John DE, Rose JB. Review of factors affecting microbial survival in groundwater. Environ Sci Technol. 2005;39(19):7345-7356. [DOI] [PubMed] [Google Scholar]
- 88. Arslan Topal EI, Ünlü A, Topal M. Effect of aeration rate on elimination of coliforms during composting of vegetable–fruit wastes. Int J Recycl Org Waste Agric. 2016;5(3):243-249. [Google Scholar]
- 89. Christensen KK, Carlsbaek M, Kron E. Strategies for evaluating the sanitary quality of composting. J Appl Microbiol. 2002;92(6):1143-1158. [DOI] [PubMed] [Google Scholar]
- 90. Garrec N, Picard-Bonnaud F, Pourcher AM. Occurrence of Listeria sp and L monocytogenes in sewage sludge used for land application: effect of dewatering, liming and storage in tank on survival of Listeria species. FEMS Immunol Med Microbiol. 2003;35(3):275-283. [DOI] [PubMed] [Google Scholar]
- 91. Islam M, Morgan J, Doyle MP, Phatak SC, Millner P, Jiang X. Fate of Salmonella enterica serovar Typhimurium on carrots and radishes grown in fields treated with contaminated manure composts or irrigation water. Appl Environ Microbiol. 2004;70(4):2497-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Albrecht A, Witzenberger R, Bernzen U, Jäckel U. Detection of airborne microbes in a composting facility by cultivation based and cultivation-independent methods. Ann Agric Environ Med. 2007;14(1):81-85. [PubMed] [Google Scholar]
- 93. Ding Z, Qian H, Xu B, et al. Toilets dominate environmental detection of SARS-CoV-2 virus in a hospital. medRxiv. Published online April 2020. doi: 10.1101/2020.04.03.20052175 [DOI] [Google Scholar]
- 94. Yu ITS, Li Y, Wong TW, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350(17):1731-1739. [DOI] [PubMed] [Google Scholar]
- 95. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Barker J, Jones MV. The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. J Appl Microbiol. 2005;99(2):339-347. [DOI] [PubMed] [Google Scholar]
- 97. Ijaz MK, Brunner AH, Sattar SA, Nair RC, Johnson-Lussenburg CM. Survival characteristics of airborne human coronavirus 229E. J Gen Virol. 1985;66(12):2743-2748. [DOI] [PubMed] [Google Scholar]
- 98. Lin K, Marr LC. Aerosolization of ebola virus surrogates in wastewater systems. Environ Sci Technol. 2017;51(5):2669-2675. [DOI] [PubMed] [Google Scholar]
- 99. Brisebois E, Veillette M, Dion-Dupont V, et al. Human viral pathogens are pervasive in wastewater treatment center aerosols. J Environ Sci. 2018;67:45-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cheremisinoff NP. 4 - Groundwater contamination. In: Cheremisinoff NP. ed. Groundwater Remediation and Treatment Technologies. William Andrew Publishing; 1997:127-168. [Google Scholar]
- 101. Bhattacharjee S, Ryan JN, Elimelech M. Virus transport in physically and geochemically heterogeneous subsurface porous media. J Contam Hydrol. 2002;57(3):161-187. [DOI] [PubMed] [Google Scholar]
- 102. Weisbrod N, Meron H, Walker S, Gitis V. Virus transport in a discrete fracture. Water Res. 2013;47(5):1888-1898. [DOI] [PubMed] [Google Scholar]
- 103. Ye Y, Ellenberg RM, Graham KE, Wigginton KR. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ Sci Technol. 2016;50(10):5077-5085. [DOI] [PubMed] [Google Scholar]
- 104. Lv W, Zheng X, Yang M, Zhang Y, Liu Y, Liu J. Virus removal performance and mechanism of a submerged membrane bioreactor. Process Biochem. 2006;41(2):299-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wigginton KR, Ye Y, Ellenberg RM. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environ Sci Water Res Technol. 2015;1(6):735-746. [Google Scholar]
- 106. Martínez-Puchol S, Rusiñol M, Fernández-Cassi X, et al. Characterisation of the sewage virome: comparison of NGS tools and occurrence of significant pathogens. Sci Total Environ. 2020;713:136604. [DOI] [PubMed] [Google Scholar]
- 107. Callanan J, Stockdale SR, Shkoporov A, Draper LA, Ross RP, Hill C. Expansion of known ssRNA phage genomes: from tens to over a thousand. Sci Adv. 2020;6(6):eaay5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. OMS | Progrès en matière d’eau, d’assainissement et d’hygiène des ménages 2000-2017. WHO. Accessed September 30, 2020. http://www.who.int/water_sanitation_health/publications/jmp-report-2019/fr/
- 109. Shuval H. Estimating the global burden of thalassogenic diseases: human infectious diseases caused by wastewater pollution of the marine environment. J Water Health. 2003;1(2):53-64. [PubMed] [Google Scholar]
- 110. Croci L, Losio MN, Suffredini E, et al. Assessment of human enteric viruses in shellfish from the northern Adriatic sea. Int J Food Microbiol. 2006;114(2):252-257. [DOI] [PubMed] [Google Scholar]
- 111. Gabrieli R, Macaluso A, Lanni L, et al. Enteric viruses in molluscan shellfish. New Microbiol. 2007;30(4):471-475. [PubMed] [Google Scholar]
- 112. Ueki Y, Shoji M, Suto A, et al. Persistence of caliciviruses in artificially contaminated oysters during depuration. Appl Environ Microbiol. 2007;73(17):5698-5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bondad-Reantaso MG, Mackinnon B, Bin H, et al. Viewpoint: SARS-CoV-2 (the cause of COVID-19 in humans) is not known to infect aquatic food animals nor contaminate their products. Asian Fish Sci. Published online April 2020. doi: 10.33997/j.afs.2020.33.1.009 [DOI] [Google Scholar]
- 114. McLaughlin JB, DePaola A, Bopp CA, et al. Outbreak of vibrio parahaemolyticus gastroenteritis associated with Alaskan oysters. N Engl J Med. 2005;353(14):1463-1470. [DOI] [PubMed] [Google Scholar]
- 115. Robert-Pillot A, Baron S, Lesne J, Fournier JM, Quilici ML. Détection de Vibrio cholerae dans un écosystème marin par hybridation moléculaire après culture sur un milieu sélectif. Hydroécologie Appliquée. 2006;15:97-105. [Google Scholar]
- 116. Widdowson MA, Sulka A, Bulens SN, et al. Norovirus and foodborne disease, United States, 1991–2000. Emerg Infect Dis. 2005;11(1):95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Decaro N, Buonavoglia C. An update on canine coronaviruses: viral evolution and pathobiology. Vet Microbiol. 2008;132(3):221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Almeida JD, Tyrrell DA. The morphology of three previously uncharacterized human respiratory viruses that grow in organ culture. J Gen Virol. 1967;1(2):175-178. [DOI] [PubMed] [Google Scholar]
- 119. Hamre D, Procknow JJ. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N. 1966;121(1):190-193. [DOI] [PubMed] [Google Scholar]
- 120. McIntosh K, Dees JH, Becker WB, Kapikian AZ, Chanock RM. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci USA. 1967;57(4):933-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Abe K, Nomura N, Suzuki S. Biofilms: hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol Ecol. 2020;96(5):fiaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ni M, Chen C, Qian J, et al. Intra-host dynamics of Ebola virus during 2014. Nat Microbiol. 2016;1(11):16151. [DOI] [PubMed] [Google Scholar]
- 123. Domingo E, Sheldon J, Perales C. Viral quasispecies evolution. Microbiol Mol Biol Rev. 2012;76(2):159-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Berngruber TW, Froissart R, Choisy M, Gandon S. Evolution of virulence in emerging epidemics. PLoS Pathog. 2013;9(3):e1003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hamza IA, Jurzik L, Überla K, Wilhelm M. Methods to detect infectious human enteric viruses in environmental water samples. Int J Hyg Environ Health. 2011;214(6):424-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rodríguez RA, Pepper IL, Gerba CP. Application of PCR-based methods to assess the infectivity of enteric viruses in environmental samples. Appl Environ Microbiol. 2009;75(2):297-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Griffith F. The significance of pneumococcal types. J Hyg (Lond). 1928;27(2):113-159. [DOI] [PMC free article] [PubMed] [Google Scholar]