Abstract
Objective
The purpose of this study is to identify perioperative independent prognostic factors that are available to the consulting team to aid in determining prognosis in patients with acute invasive fungal sinusitis.
Study Design
Retrospective chart review of patients with biopsy-proven acute invasive fungal sinusitis from 2015 to 2018.
Setting
Academic tertiary care center.
Methods
Twenty-one patients were included from our single-center retrospective review. Kaplan-Meier graphs were created, and the Breslow test used to compare the curves to obtain P values. A univariate Cox regression analysis was performed on the data that were significant at 3 months from diagnosis.
Results
Twenty-one patients were included, and 17 (76%) had an underlying hematologic malignancy. Overall survival was 71% and 52% at 1 and 3 months, respectively, and 94% of patients with hematologic malignancy had an absolute neutrophil count ≤1 at diagnosis. Absolute neutrophil count values and fungal species were not associated with a difference in prognosis. Factors associated with decreased survival included current smoking and the absence of a rhinologist on the treatment team at the initial or subsequent debridement (hazard ratio, 3.03). Laboratory values such as beta-D-glucan and galactomannan were assessed in addition to disease extension at diagnosis.
Conclusion
This study presents a retrospective review of a single institution’s experience with acute invasive fungal sinusitis. Subspecialty level of care likely improves overall survival in these patients, whereas current smoking may imply a worse prognosis.
Keywords: AIFS, mortality, rhinology, prognosis
Acute invasive fungal sinusitis (AIFS) is an opportunistic infection of the nasal mucosa and sinuses that occurs predominantly in patients who are immunocompromised. These patients are typically immunocompromised due to diabetes, chronic steroid use, or autoimmune diseases or neutropenic due to hematologic malignancy, recent bone marrow transplant, or chemotherapy. Mortality in these patients who are immunosuppressed remains high, around 50% to 63% in literature published over the past decade.1
Despite improvement in endoscopy and imaging, the diagnosis of AIFS can be difficult, given the vague nature of the early signs and symptoms, as they can be relatively nonspecific. These may include fever with or without neutropenia, periorbital or facial swelling, rhinorrhea or epistaxis, or nasal crusting.1 As the disease invades deeper into the orbit or intracranially, cranial neuropathies or vision changes can occur.2 Imaging predominantly first with computed tomography scan shows most commonly unilateral nasal involvement.3 As the disease progresses, bone involvement and intracranial extension can be found.4
Although pathologic evidence of AIFS remains the standard for diagnosis, laboratory tests have been investigated for utility in invasive fungal infections. Testing for serum galactomannan Aspergillus antigen has been revealed to be a clinical tool with results that are complex to interpret and are insufficient as a sole diagnostic modality in the investigation of suspected AIFS.5 Likewise, beta-D-glucan has been shown to be a somewhat complex tool for the diagnosis of invasive fungal infection. The utility of this serologic study may be more pronounced when used as a serial assay correlated with clinically suspicious findings.6
Identified prognostic factors vary from the small case studies to the few meta-analyses that have been produced to date. Progression from limited disease or isolated nasal lateral wall involvement to involvement of the hard palate, septum, and orbit has been observed to portend a poorer prognosis.1,7 However, other studies have shown that only intracranial and cavernous sinus extension is a negative prognostic factor and that dissemination is a better indicator of morbidity.1,8,9 Elevated C-reactive protein and underlying hematologic malignancy have also been associated with a poor outcome.10 Factors associated with improvement in overall survival at this time are few, including prompt surgical resection, recovery of absolute neutrophil count (ANC), and use of immune-stimulating therapies.1,8 However, extent of disease may play a role in the ability to achieve complete surgical resection, and thus improve the likelihood of survival.11
The aim of this study is to better identify prognostic factors related to this patient population to help optimize diagnosis and treatment.
Methods
Following institutional review board approval (228077) from the University of Arkansas for Medical Sciences, a retrospective chart review was done of patients diagnosed with AIFS from June 2015 to June 2018. Pathology records were searched for the keywords “invasive fungal.” This group of patients was then cross-referenced with a search through EPIC of patients treated with Current Procedural Terminology code J32.9. The pathology and surgical reports were carefully examined to confirm patients with invasion of respiratory mucosa and angioinvasion on permanent pathology. Inclusion criteria were age >18 years and biopsy-proven AIFS. Patients with chronic invasive fungal sinusitis were excluded from analysis. From these patients, the following data were collected: demographics, smoking status, laboratory data (including microbiologic serologic findings), fungal cultures, radiographic/endoscopic findings on consultation, and subspecialty of the otolaryngologists involved. Specifically, the laboratory data collected were the ANC on the day of consultation, C-reactive protein, glucose on the day of consultation, albumin, beta-D-glucan, and galactomannan. Patients were placed into 3 imaging groups: disease confined to sinonasal cavity; involvement of orbit, palate, or nasopharynx; and intracranial extension, including cavernous sinus involvement. Survival follow-up was collected through the electronic medical record, and when survival status was not available, the families were contacted.
Data were analyzed with SPSS Statistics Premium version 24.0 (IBM Corp). The primary outcome was survival at 3 months. A Breslow-Wilcoxon test was run on the Kaplan-Meier curves, and the standard P < .05 was considered significant. Univariate survival analysis with Cox regression was run on the data that were significant per the Breslow-Wilcoxon test to return hazard ratios.
Results
Study Population
After exclusion criteria applied to 3 patients identified through the retrospective search, we were left with 21 patients who met all inclusion criteria. The population demographics and characteristics are presented in Table 1 . Our study had follow-up data available on 100% of patients at 1 and 3 months. Their underlying comorbidities included hematologic malignancies (n = 16) such as acute myeloid leukemia, follicular lymphoma, acute lymphocytic leukemia, and multiple myeloma; 6 of these patients also had diabetes mellitus. The remaining 5 patients, listed in the “other” column, had diabetes alone (n = 3) and chronic steroid use (n = 2).
Table 1.
Demographic Statistics for Overall Study Population and Subgroups.a
| Demographics | All patients (N = 21) | Hematologic malignancy (n = 16) | Diabetes + hematologic malignancy (n = 6) | Other (n = 5) |
|---|---|---|---|---|
| Age, y b | 51.2 (24-71) | 51.75 (24-71) | 58.3 (37-69) | 51 (32-60) |
| Sex | ||||
| Male | 81 (17) | 88 (14) | 100 (6) | 60 (3) |
| Female | 19 (4) | 12 (2) | 40 (2) | |
| Race/ethnicity | ||||
| White | 71.4 (15) | 75 (12) | 83 (5) | 80 (4) |
| Black | 14.3 (3) | 18.8 (3) | ||
| Hispanic | 9.5 (2) | 16.7 (1) | 20 (1) | |
| Asian | 4.8 (1) | 6.3 (1) | ||
| Current smoker | 33 (7) | 31 (5) | 33 (2) | 40 (2) |
Values are presented as % (No.) unless noted otherwise.
Mean (range).
Smoking Status
Smoking status was determined as patients who were currently consuming cigarettes at least on a weekly basis. Former smokers were excluded from this patient population. In univariate analysis, current smoking was associated with an increased risk of death at 3 months (P = .021; hazard ratio, 3.59; 95% CI, 1.01-12.8; Figure 1 ).
Figure 1.
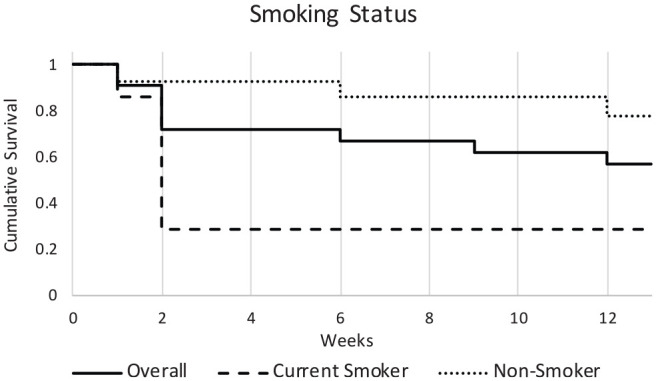
Effect of smoking of survival. Smoking is associated with an increased risk of death at 3 months (P = .021; hazard ratio, 3.59; 95% CI, 1.01-12.8).
Absolute Neutrophil Count
The average ANC in all patients was 1.7 (1000/mm3). When separated by underlying disease, patients with hematologic malignancies had an average ANC of 0.3, with 94% of patients having an ANC <1.0. This contrasts our group of patients with nonfunctional neutropenia, in whom 0% had an ANC <1.0 ( Table 2 ). Analysis of survival curves in patients with an ANC of 0 as compared with patients with an ANC >0 did not reveal any significant difference in survival (P = .876).
Table 2.
Speciation, Extent of Invasion, and Laboratory Workup.a
| All patients (N = 21) | Hematologic malignancy (n = 16) | Diabetes + hematologic malignancy (n = 6) | Other (n = 5) | |
|---|---|---|---|---|
| Organism | ||||
| Mucor/Rhizopus | 33 (7) | 25 (4) | 33 (2) | 60 (3) |
| Atypical | 33 (7) | 44 (7) | 50 (3) | |
| None identified | 5 (1) | 6 (1) | ||
| Maximum extension | ||||
| Nasal cavity | 48 (10) | 63 (10) | 33 (2) | 40 (2) |
| Orbit, palate, NP | 33 (7) | 31 (5) | 50 (3) | |
| Intracranial | 19 (4) | 6 (1) | 17 (1) | 60 (3) |
| Laboratory | ||||
| ANCb | 1.7 (0-14.21) | 0.3 (0-2) | 0.51 (0-2) | 6.18 (1.3-10.4) |
| <500 | 62 (13) | 81 (13) | 67 (4) | 0 |
| ≤1000 | 71 (15) | 94 (15) | 83 (5) | 0 |
| Beta-D-glucanc | 87.7 (19) | 92.1 (15) | 34.8 (5) | 66 (3) |
| + Beta-D-glucan >60 | 29 (6) | 31.3 (5) | 20 (1) | |
| +Aspergillus antigen | 14 (3) | 18.8 (3) | ||
| Albuminc | 2.26 (11) | 2.23 (9) | 2.83 (3) | 2.4 (2) |
Abbreviations: ANC, absolute neutrophil count; NP, nasopharynx.
Values are presented as % (No.) unless noted otherwise.
Mean (range).
Mean (No.).
Beta-D-Glucan/Galactomannan
Beta-D-glucan testing was available in 19 patients. Six of these patients had a positive result based on a reference value >59, which is standard with the University of Arkansas laboratory for at least an indeterminate test. The presence of an abnormal beta-D-glucan test result had a significant difference in survival curves (P = .048). The Cox regression run on the data, however, returned nonsignificant findings (hazard ratio, 3.842; 95% CI, 0.854-17.285; Figure 2 ). Galactomannan or Aspergillus antigen was available in 19 patients. Of these patients, only 3 tested positive. The sample size of positive cases was not large enough to provide significant statistical analysis of survival.
Figure 2.
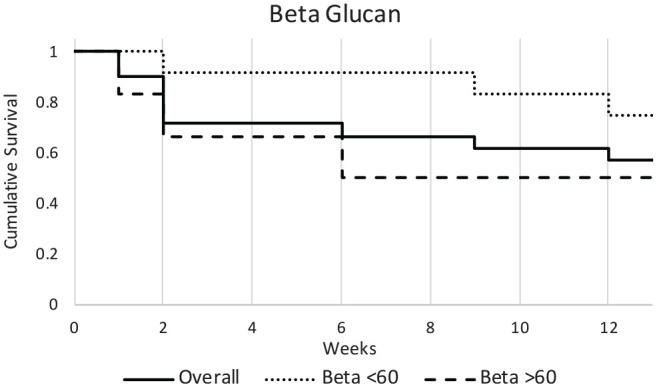
Beta-D-glucan level had no significant effect on survival. Cox regression (hazard ratio, 3.842; 95% CI, 0.854-17.285).
Fungal Speciation
Positive fungal cultures or polymerase chain reaction (PCR) data were available for 95% of patients (n = 20). Aspergillus and mucormycosis/Rhizopus infections were the most common. Atypical fungal infections were also noted, with organisms including Purpureocillium, Zygomycetes, Apophysomyces, Alternaria, and Curvularia. These pathogens accounted for 33% of patients (n = 7), and all these patients fell into the hematologic malignancy cohort. In our analysis, Mucor/Rhizopus species had the worst overall survival with 28% surviving ( Table 2 ). Despite this, no significant difference in survival was noted between the fungi groups (P = .177).
Disease Extension
Preoperative disease extension as determined by imaging was available for review in 100% of cases. Seven patients had orbital, palatal, or pharyngeal involvement. Four patients were identified preoperatively as having intracranial involvement, including cavernous sinus invasion ( Table 2 ). No significant differences in survival were identified among the 3 groups (P = .243)
Surgical Therapy and Subspecialty Involvement
Surgical therapy was a mainstay of treatment in our patient population, with 95% of patients (n = 20) undergoing surgical resection. The mean timing of consultation to surgical intervention was 33.8 hours ( Table 3 ). This delay is due to the lack of evidence of disease on initial scope examination and the development of more concerning signs and symptoms on follow-up examinations. Rhinologist involvement was present in 70% of surgically managed cases (n = 14), with 9 of these being rhinologist treated alone; in the other 5 cases, the rhinologist performed at least 1 debridement during care, following the initial debridement by the on-call otolaryngologist. The overall survival in our patient population was 71% (6 deaths) and 52% (10 deaths) at 1 and 3 months, respectively. The absence of rhinology subspecialty involvement in care portended an increased risk of death at 3 months (P < .001; hazard ratio, 3.03; 95% CI, 2.11-500; Figure 3 ). To account for possible differences in severity of cases between the groups, analysis of the disease extension was analyzed with a chi-square test and Cramer’s V, which showed no significant differences (P = .299). Further subgroup analysis of degree of rhinology involvement showed no difference in extent of disease regardless of whether the rhinologist was involved from the time of consult, following the initial intervention, or not at all (P = .593; Table 4 ). To determine a range of AIFS-specific mortality, mortality within 1 and 2 weeks following diagnosis was analyzed. Of the 21 patients in our study, 3 died within 1 week of diagnosis and 3 within 2 weeks of diagnosis. In these cases, the AIFS-specific mortality was between 14.3% and 28.6%.
Table 3.
Treatment Variables and Survival.
| All patients (N = 21) | Hematologic malignancy (n = 16) | Diabetes + hematologic malignancy (n = 6) | Other (n = 5) | |
|---|---|---|---|---|
| Treatment | ||||
| Surgery, % (No.) | 95 (20) | 94 (15) | 83 (5) | 100 (5) |
| Time from admit to consult, d, mean (range) | 9.23 (0-49) | 12 (0-49) | 8.5 (0-26) | 0.2 (0-1) |
| Time from consult to surgery, h, mean (median; range) | 33.8 (15; 3-264) | 33.5 (17; 3-264) | 18.4 (15; 3-56) | 35 (9; 6-137) |
| No. of debridements, mean (range) | 2.45 (1-5) | 2.5 (1-5) | 3.2 (2-5) | 2.2 (1-3) |
| AIFS-specific mortality, % (No.) | ||||
| 1 wk | 14.3 (3) | |||
| 2 wk | 28.6 (6) | |||
| Survival, % (No.) | ||||
| 1 mo | 71 (15) | 75 (12) | 67 (4) | 60 (3) |
| 3 mo | 52 (11) | 56 (9) | 50 (3) | 40 (2) |
Abbreviation: AIFS, acute invasive fungal sinusitis.
Figure 3.
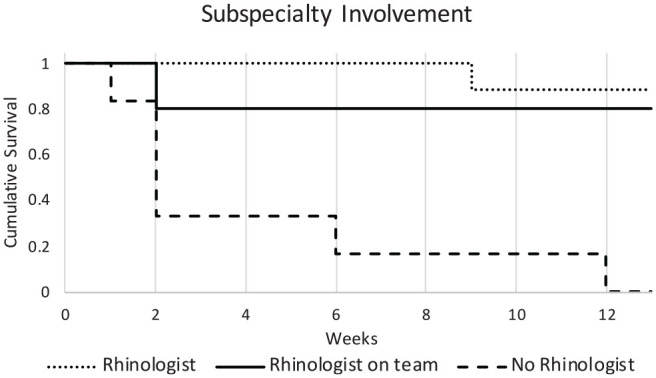
Effect of subspecialty involvement on survival. The absence of rhinology subspecialty involvement in care was associated an increased risk of death at 3 months (P < .001; hazard ratio, 3.03; 95% CI, 2.11-500).
Table 4.
Disease Extension at Consult With Regard to Level of Rhinologist Involvement. a
| Sinonasal | Nasopharynx, orbit, palate | Intracranial | |
|---|---|---|---|
| Rhinologist alone | 5 | 3 | 1 |
| Rhinologist on team | 3 | 2 | 0 |
| No rhinologist involvement | 3 | 1 | 2 |
P = .593.
Discussion
Our study aimed to identify perioperative independent prognostic factors that are available to the consulting team to aid in determining prognosis in an individual patient. Previous studies cite the survival rate to be variable depending on the studied follow-up period. Turner et al8 reported 46% overall survival, though surgical management improved survival to 53%. Wandell et al1 demonstrated 3-month survival as 63%, while Saedi et al12 reported 6-month survival at 60%. Our overall survival rate at 3 months was 52%, similar to these previous studies. Analysis of AIFS-specific mortality based on death within 1 to 2 weeks of diagnosis showed a rate of 14.3% to 28.6%. This range is similar to the AIFS-specific mortality (18%) reported by Parikh et al.13
Underlying immunocompromised states have been found to influence prognosis, with diabetes mellitus–associated AIFS implying an improved survival rate as compared with other etiologies of AIFS.1,8 This has been postulated to be due to a possible reversible cause of immunocompromising status as the blood glucose is normalized.1,2 The recovery of the ANC has been shown to be a reliable predictor of mortality.13 This may also help explain why patients who develop AIFS due to poorly controlled diabetes may have a survival advantage over patients with hematologic malignancy. However, no significant differences were found between these groups in our study population. The large overlap in the patient groups could be masking the survival differences, as out of the 9 patients with diabetes, 6 had coexisting hematologic malignancies as well. Additionally, the average blood glucose level for all patients with diabetes mellitus was 224, with only 4 patients having a blood glucose >200 and with just 1 of these patients not having diabetes mellitus with a hematologic malignancy. This indicates that only 1 patient is likely to have diabetic ketoacidosis as the key risk factor for development of AIFS.
At our institution, patients were generally placed on antifungal treatment prior to or as soon as a consult of the otolaryngology–head and neck surgery service was requested and AIFS was suspected. Additionally, some patients were already taking prophylactic antifungals because they were severely immunocompromised or had other foci of fungal disease. Since all our patients with AIFS were treated the same as far as antifungals are concerned, an analysis of the role of antifungal treatment in survival was not undertaken.
Our study did not find a significant difference in prognosis among fungal species. This mirrors the results that have been published previously. Interestingly, atypical fungal species that accounted for 33% of our patients were found exclusively in patients with hematologic malignancies.
Disease extension has been long thought to portend a prognostic role, and it routinely affects clinical decision making. Some published studies have noted that presence of orbital or intracranial extension is a negative prognostic factor.1,2 Surprisingly, our data did not support any difference between disease extension. This could be due to the small sample size, as we compared only 21 patients and larger studies and meta-analyses that showed a difference had much larger sample sizes.
Smoking status was thought to be an important factor in AIFS, as smoking was found to impair mucociliary clearance and imply decreased blood flow in capillaries in other organ systems, including skin.14,15 Our study found that current active smoking implied an increased risk of death at 3 months as compared with patients who did no smoke.
The literature to date reports conflicting findings with some aspects of this infection. Our data support the conclusion that rhinology subspecialty consultation for input and assistance on the management of patients suspected to have this infection improves survival. With the routine use of endoscopic examination and as high-volume endoscopic sinus surgeons, rhinologists bring a unique skill set to the treatment of AIFS as compared with other subspecialists. The ability to differentiate diseased versus nondiseased mucosa, as well as a high comfort level and experience with endoscopic approaches, may allow consistently full resections of diseased tissue in AIFS, which portends an improved prognosis.11 For these reasons, we believe that rhinology care increases the likelihood of survival for these patients as seen in our study.
There are some limitations to consider when interpreting our findings. The study is limited by the small sample size, although this is due to the rarity of this infection and to this being a single-center study. This limitation is notable in the ability to compare mortality between rhinologists and non–subspecialty trained otolaryngologists. Being a single-institution study, it may contain some selection bias with regard to the patient population that this care center serves. However, this was minimized by strict inclusion criteria of including patients diagnosed with AIFS prior to data collection. The average number of debridements (or surgical procedures) was 2.45 for the cohort, and those were mostly endoscopic, though some patients required open surgery following an endoscopic debridement.
Additional limitations exist in the ability to assess margin status. Despite the involvement of a single rhinologist in the care of the majority of these patients, there was no method to standardize the practice patterns of the 9 providers who performed a debridement, due to the urgent nature of these consultations and the retrospective nature of this study. Margins were not assessed routinely. We wanted to conduct an analysis of the effect of surgical approach on survival; however, given the varied surgical approach used, an analysis of its effect on survival was not feasible because of the small sample size.
Conclusion
AIFS continues to be a rare but feared complication associated with immunocompromised states. Many factors have been shown to have an effect on the mortality of patients with this infection. Our study indicates that rhinology subspecialty involvement in the care of a patient with AIFS decreases the mortality associated with this infection, whereas smoking status increases mortality at 3 months. Future multicenter studies are necessary, given the rarity of this infection, in the hope of elucidating factors that affect the high mortality associated with it.
Author Contributions
James Reed Gardner, design, conduct, analysis, drafting; Courtney J. Hunter, design, conduct, drafting; Donald Vickers, design, conduct, analysis, drafting; Deanne King, design, conduct, analysis, drafting); Alissa Kanaan, design, conduct, analysis, drafting.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
Footnotes
This article was presented at the American Rhinological Society Annual Meeting; September 12-15, 2019; New Orleans, Louisiana.
References
- 1. Wandell GM, Miller C, Rathor A, et al. A multi-institutional review of outcomes in biopsy-proven acute invasive fungal sinusitis. Int Forum Allergy Rhinol. 2018;8(12):1459-1468. doi: 10.1002/alr.22172 [DOI] [PubMed] [Google Scholar]
- 2. Craig JR. Updates in management of acute invasive fungal rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2019;27(1):29-36. doi: 10.1097/MOO.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 3. DelGaudio JM, Swain RE, Kingdom TT, Muller S, Hudgins PA. Computed tomographic findings in patients with invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 2003;129(2):236-240. doi: 10.1001/archotol.129.2.236 [DOI] [PubMed] [Google Scholar]
- 4. Middlebrooks EH, Frost CJ, De Jesus RO, Massini TC, Schmalfuss IM, Mancuso AA. Acute invasive fungal rhinosinusitis: a comprehensive update of CT findings and design of an effective diagnostic imaging model. AJNR Am J Neuroradiol. 2015;36(8):1529-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Melancon CC, Lindsey J, Russell GB, Clinger JD. The role of galactomannan Aspergillus antigen in diagnosing acute invasive fungal sinusitis. Int Forum Allergy Rhinol. 2019;9(1):60-66. doi: 10.1002/alr.22225 [DOI] [PubMed] [Google Scholar]
- 6. Theel ES, Doern CD. β-D-glucan testing is important for diagnosis of invasive fungal infections. J Clin Microbiol. 2013;51(11):3478-3483. doi: 10.1128/JCM.01737-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valera FC, do Lago T, Tamashiro E, Yassuda CC, Silveira F, Anselmo-Lima WT. Prognosis of acute invasive fungal rhinosinusitis related to underlying disease. Int J Infect Dis. 2011;15(12):e841-e844. doi: 10.1016/j.ijid.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 8. Turner JH, Soudry E, Nayak JV, Hwang PH. Survival outcomes in acute invasive fungal sinusitis: a systematic review and quantitative synthesis of published evidence. Laryngoscope. 2013;123(5):1112-1118. doi: 10.1002/lary.23912 [DOI] [PubMed] [Google Scholar]
- 9. DelGaudio JM, Clemson LA. An early detection protocol for invasive fungal sinusitis in neutropenic patients successfully reduces extent of disease at presentation and long term morbidity. Laryngoscope. 2009;119(1):180-183. doi: 10.1002/lary.20014 [DOI] [PubMed] [Google Scholar]
- 10. Cho HJ, Jang MS, Hong SD, Chung SK, Kim HY, Dhong HJ. Prognostic factors for survival in patients with acute invasive fungal rhinosinusitis. Am J Rhinol Allergy. 2015;29(1):48-53. doi: 10.2500/ajra.2015.29.4115 [DOI] [PubMed] [Google Scholar]
- 11. Roxbury CR, Smith DF, Higgins TS, et al. Complete surgical resection and short-term survival in acute invasive fungal rhinosinusitis. Am J Rhinol Allergy. 2017;31(2):109-116. doi: 10.2500/ajra.2017.31.4420 [DOI] [PubMed] [Google Scholar]
- 12. Saedi B, Sadeghi M, Seilani P. Endoscopic management of rhinocerebral mucormycosis with topical and intravenous amphotericin B. J Laryngol Otol. 2011;125(8):807-810. doi: 10.1017/S0022215111001289 [DOI] [PubMed] [Google Scholar]
- 13. Parikh SL, Venkatraman G, DelGaudio JM. Invasive fungal sinusitis: a 15-year review from a single institution. Am J Rhinol. 2004;18(2):75-81. [PubMed] [Google Scholar]
- 14. Utiyama DM, Yoshida CT, Goto DM, et al. The effects of smoking and smoking cessation on nasal mucociliary clearance, mucus properties and inflammation. Clinics (Sao Paulo). 2016;71(6):344-350. doi: 10.6061/clinics/2016(06)10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richardson D. Effects of tobacco smoke inhalation on capillary blood flow in human skin. Arch Environ Health. 1987;42(1):19-25. doi: 10.1080/00039896.1987.9935790 [DOI] [PubMed] [Google Scholar]


