Abstract
Background:
Cholinesterase inhibitor pesticides, especially organophosphates, are endocrine disruptors and a few existing studies have linked self-reports of exposure with increased depression and anxiety. Some evidence suggests that associations may be stronger in women, but the mechanism of this gender difference is unclear. We assessed whether acetylcholinesterase (AChE) inhibition between 2 time points (reflecting greater cholinesterase inhibitor exposure) during different agricultural seasons in the year was associated with anxiety/depression symptoms.
Methods:
We examined 300 adolescents (ages 11–17y, 51% female) living near agricultural settings in Ecuador (ESPINA study) twice in 2016: April and July-October. We assessed AChE activity (finger stick), estradiol, testosterone, dehydroepiandrosterone, cortisol (saliva) and anxiety and depression scales (CDI-2 and MASC-2).
Results:
The mean (SD) depression and anxiety scores were 52.8 (9.3) and 58.1 (9.6), respectively. The median (25th, 75th percentile) AChE change (July-October vs April) was −3.94% (−10.45%, 5.13%). For every 10% decrease in AChE activity, there was a 0.96 unit (95%CI: 0.01, 1.90) increase in depression symptoms and an OR of elevated depression score of 1.67 (1.04, 2.66). These associations were stronger in girls (OR=2.72 [1.23, 6.00]) than boys (1.18 [0.59, 2.37]). Adjustment for cortisol, testosterone and dehydroepiandrosterone reduced gender differences by 18–62%. No associations were observed with anxiety.
Discussion:
Inhibition of AChE activity at 2 points in time during different pesticide spray periods was associated with greater depression symptoms, affecting girls more than boys. Gender differences may be partly explained by endocrine disruption. These findings suggest that AChE inhibition may transiently affect the mood of adolescents.
Keywords: pesticide, cholinesterase inhibitors, organophosphates, depression, mental health
Introduction
Exposure to agricultural pesticides, such as organophosphate insecticides, has been associated with long-lasting negative health effects in children, including neurobehavioral alterations (Kofman et al. 2006; Rauh et al. 2006, 2011; Eskenazi et al. 2007; Marks et al. 2010; Bouchard et al. 2011; Horton et al. 2012), cognitive delays (Kofman et al. 2006; Rauh et al. 2006; Eskenazi et al. 2007, 2014; Marks et al. 2010; Bouchard et al. 2011; Rohlman et al. 2016), and internalizing symptoms such as anxiety and depression, although only a limited number of investigations have addressed the latter. Studies have documented associations of self-reported occupational pesticide usage (mostly organophosphates, and to a lesser extent, carbamates and organochlorine pesticides) with increased depression and anxiety symptoms, and suicidal thoughts and attempts in adult agricultural workers (London et al. 2005, 2012; Beseler et al. 2006, 2008; Beseler and Stallones 2008; Wesseling et al. 2010; Weisskopf et al. 2013; Harrison and Mackenzie Ross 2016; Koh et al. 2017). For instance, among adults with low depression scores at baseline in the Korean Farmers Cohort, it was found that self-reports of pesticide exposure, including frequency and intensity of pesticide use and history of pesticide poisoning was associated with greater scores of depression symptoms at the 3-year follow-up examination (Koh et al. 2017). In the Agricultural Health Study, which assessed agricultural workers in Iowa and North Carolina, in the United States of America, it was found that chronic occupational exposure to pesticides was positively associated with depression-related symptoms and depression diagnoses (Beseler et al. 2008). Additionally, acute pesticide exposure and poisoning also has been strongly associated with suicidal ideation and depression diagnoses (London et al. 2012). These studies found compelling associations between pesticide exposures and internalizing behaviors; however, none of these studies characterized these associations using biomarkers of pesticide exposure.
The main toxicity of organophosphate insecticides occurs through the inhibition of acetylcholinesterase (AChE) activity, which is an important regulator of the neurotransmitter acetylcholine (Qiao et al. 2003; Abou-Donia 2003; Slotkin 2004; Aldridge et al. 2005b). In the Secondary Exposures to Pesticides among Children and Adolescents (ESPINA) study, we found that lower AChE activity was associated with greater depression scores in adolescents, with stronger associations observed among younger adolescents and girls (Suarez-Lopez et al. 2019). In the same cohort study, we also found that lower AChE activity was associated with alterations in attention, inhibitory control and memory primarily among boys (Suarez-Lopez et al. 2013). The cholinergic system plays a key role in mood regulation (Janowsky et al. 1972; Risch et al. 1981; Furey and Drevets 2006; Mineur et al. 2013) and cholinergic alterations from cholinesterase inhibitor medications or pesticides have been found to be associated with increased depression and anxiety behaviors in experimental studies of both animals and humans (Gershon and Shaw 1961; Steinberg et al. 1997; Reynolds et al. 2011; Dagytė et al. 2011; Mineur et al. 2013; Siqueira et al. 2019).
While there is a growing body of research supporting the association between pesticide exposure and longer-term health effects, a limited number of studies have examined the effect of pesticide exposures on short-term alterations of various aspects of mental health. In a study exploring acute organophosphate poisoning in male rats, results suggest that this level of organophosphate poisoning can induce transient depressive-like behavior possibly related to hippocampal AChE inhibition (Siqueira et al. 2019). There has also been emerging evidence linking seasonal use of pesticides to increased pesticide exposures and decreased neurobehavioral performance of adolescent agricultural workers (Khan et al. 2014; Rohlman et al. 2016) and non-worker children (Suarez-Lopez et al. 2017b). While the half-lives of organophosphate pesticides are short, the normalization of erythrocytic AChE activity levels, after irreversible inhibition (enzymatic aging) by organophosphates, may take up to 3 months (Mason 2000). Furthermore, seasonal alterations of neurobehavioral performance may last for weeks or months after the end of pesticide applications (Rohlman et al. 2016; Suarez-Lopez et al. 2017b).
Like the findings of our previous studies (Suarez-Lopez et al. 2013, 2017b, 2019), other investigators have described effect modification by gender on the associations between pesticide exposures and various neurobehavioral outcomes. However, there is still uncertainty about what may be driving the gender differences in these associations. Pesticides, such as organophosphates, are endocrine disruptors and exposure could result in alterations in adrenal and sex hormones, as their regulation is partly mediated by the cholinergic system (Rhodes and Rubin 1999; Rubin et al. 2002; Balkan and Pogun 2018). It is plausible that gender differences in the pesticide-mental health associations may be explained by endocrine alterations related to pesticide exposures (Yang et al. 2019).
The aim of the present study was to assess the effect of inhibition of AChE activity between two agricultural seasons in the same year on internalizing symptoms among adolescents. Additionally, we aimed to assess whether sex and adrenal hormone levels may partly explain effect modification by gender that may be observed.
Methods
Participants
The study of Secondary Exposures to Pesticides among Children and Adolescents (ESPINA) is a prospective cohort of children growing up in the agricultural county of Pedro Moncayo, Pichincha, Ecuador, aimed at understanding the effects of pesticide exposures on child development. In 2008, a total of 313 children of 4–9 years of age were enrolled in the study. Inclusion criteria for children living with a flower plantation worker, included: cohabitation with a flower plantation worker for at least one year. Inclusion criteria for children, not living with any agricultural worker, included: 1) no cohabitation history with an agricultural worker; 2) never inhabiting a house where agricultural pesticides were stored; and 3) never having direct contact with pesticides. Further details of participant criteria have been published elsewhere (Suarez-Lopez et al. 2012).
Two follow-up examinations of ESPINA participants were conducted in 2016 (participants’ ages: 12–17 years). In April (April 16 - May 1), we examined 330 participants and in July-October (July 26 – October 23), we examined 535 participants; 95% of participants were examined between July 26 and August 26. A total of 311 participants examined in July-October were also examined in April. The total pooled sample size, for both examination periods, included 554 adolescent participants. The 554 participants included 238 who were examined in 2008 and 316 new volunteers. Of the 311 participants, examined at both time periods in 2016, 301 participants completed anxiety and depression assessments (see below) in July-October. We excluded one participant, examined in April, who had missing AChE levels. To maximize the study’s sample, we imputed missing family income information for nine children using 2008 family income information or parental education. Details of the imputation methods for this variable have been described previously (Suarez-Lopez et al. 2019). A total of 300 participants were included in the present analysis (Figure 1).
Figure 1.
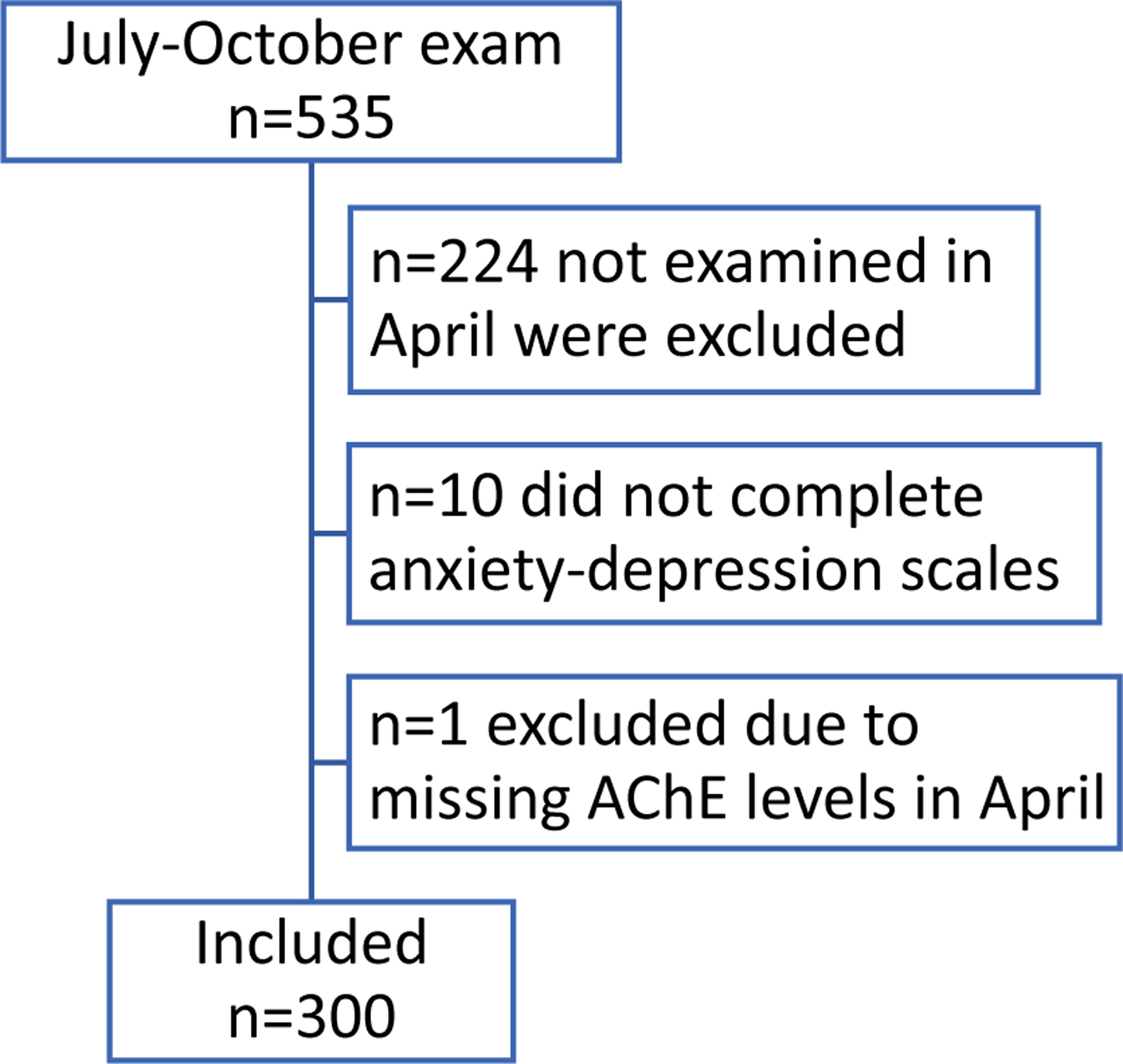
ESPINA study participant flow chart
As in 2008, the 2016 sample of new participants were recruited using the System of Local and Community Information (SILC) developed by Fundación Cimas del Ecuador, which includes data from the 2016 Pedro Moncayo County Community Survey. Of the 554 total participants, the analysis for this study included 300 participants who had AChE measurements at both the April and July-October exams and had completed either the Multidimensional Anxiety Scale for Children 2nd Edition (MASC-2, MHS Inc, North Tonawanda, NY) or the Children’s Depression Inventory 2nd Edition (CDI-2,MHS Inc, North Tonawanda, NY) during the July-October examination. Parental permission for participation and child assent were obtained for adolescent participants. This study was approved by the institutional review boards at the University of California: San Diego, Universidad San Francisco de Quito and the Ministry of Public Health of Ecuador.
Setting
Data for the ESPINA Study was collected from adolescents and parents living in the Pichincha province of Pedro Moncayo County, which is located on the Ecuadorian Andes. This county is known for its floriculture industry, which consists of 5.3% of the geographic area (1800 hectares) (Gobierno Municipal del Canton Pedro Moncayo, 2011) and employs 21% of all adults in the county (Suarez-Lopez et al., 2012). Flower plantations throughout Pedro Moncayo are treated with hand sprayers using more than 20 different insecticides, including organophosphates, carbamates, neonicotinoids and pyrethroids, as well as 50 different fungicides (Harari 2004; Grandjean et al. 2006; Suarez-Lopez et al. 2017a).
We conducted exams in schools across Pedro Moncayo County during weekends in April, September and October and during weekdays between July and August when school was closed for the summer. Surveys were conducted with the participants’ parents and other adult residents in their homes to obtain socioeconomic status, demographic and health characteristics, and pesticide exposure history of household members. All assessments were conducted by staff who were blinded to participants’ pesticide exposure status.
Measures
Weight and Height (Jul-Oct).
Participant height was measured to the nearest 1 mm in accordance with recommended procedures from the World Health Organization (World Health Organization 2006). Participant weight was measured using a digital scale (Tanita model 0108MC; Corporation of America, Arlington Heights, IL, USA).
Depression and Anxiety (Jul-Oct).
Symptoms of depression were examined using CDI-2 (MHS Inc) (Kovacs 2011), a self-report assessment that contains 12 items measuring the degree of ongoing depressive manifestations in adolescents (Bae 2012). The CDI-2 has demonstrated excellent psychometric properties, with scores comparable to those of the full-length version (Kovacs 2011; Bae 2012). Symptoms of anxiety were assessed using the 50-item MASC-2 Child survey. The MASC-2 Child survey has demonstrated good psychometric properties in identifying anxiety disorders in youth (van Gastel and Ferdinand 2008; Wei et al. 2013; Fraccaro et al. 2015). This tool has been successfully used among ethnically diverse and non-English speaking populations (Magiati et al. 2013), including Hispanic children (Isasi et al. 2014). With input from community members on appropriate terminology for Pedro Moncayo County, the ESPINA study team translated the MASC-2 survey into Spanish. The Spanish version was then reviewed and approved by MHS Inc. Completed questionnaires were scored using the MASC-2 Scoring Software (MHS Inc). Age and gender appropriate scaled scores (T-scores) for both the CDI-2 and MASC-2 were used in analyses and these were calculated using the CDI 2 Software (MHS Inc) and MASC-2 Software Scoring Software (MHS Inc), respectively. Elevated scores were defined as T-scores greater than 65 for both the MASC-2 and CDI-2 (Kovacs and MHS staff 2011; March 2012).
AChE activity (Apr, Jul-Oct).
Erythrocytic AChE activity and hemoglobin concentrations were measured from fresh finger-stick blood samples in both the April and July-October examinations, using the EQM Test-mate ChE Cholinesterase Test System 400 (EQM AChE Erythrocyte Cholinesterase Assay Kit 470; EQM Research, Inc, Cincinnati, OH). Percent change in AChE activity between April and July-October was calculated. We used the World Health Organization growth standards (World Health Organization Multicentre Growth Reference Study Group, 2006) to calculate the z-scores for height-for-age and BMI-for-age.
Sex and adrenal hormones (Jul-Oct).
Levels of 17-β estradiol, testosterone, cortisol, and dehydroepiandrosterone (DHEA) were measured using enzymatic assays (Salimetrics, Carlsbad, CA) in passive-drool morning saliva. Participants were asked to collect samples upon awakening to control for circadian differences. Levels of cortisol, testosterone, and DHEA were measured in both girls and boys. Estradiol was measured only in boys, as estradiol levels in women vary according to the stage of menstrual cycle.
Geospatial information.
Geographical coordinates of homes were obtained from portable global positioning system receivers when previously collected as part of the Local and Community Information System (SILC, Sistema de Información Local y Comunitario), which was developed by Fundación Cimas del Ecuador. Satellite imagery was used to determine the geospatial coordinates of greenhouse flower plantations. The distances of homes to the nearest flower plantation perimeter was calculated with ArcGIS (Esri, Redlands, CA).
Statistical analysis
We calculated the means and standard deviations (SD) for normally distributed variables and median (25th- 75th percentile) for skewed variables for all participants across 4 categories of AChE activity change between April and July-October. The categories selected included participants with AChE change % values ≥ 0.0% as category 4 and categories 1–3 corresponded to tertiles of AChE change that were < 0.0% for the overall group.
Considering effect modification by gender on the associations tested (see below), we present participant characteristics stratified by gender. We calculated the p-value for trend (p-trend) for participant characteristics across levels of AChE activity change, using linear regression and modeling AChE activity change as a continuous variable. The p-trends for skewed independent variables were modeled using log-transformed variables. We also calculated the crude mean differences for all participant characteristics between boys and girls using t-test.
We used multiple linear regression to estimate associations of percent inhibition in AChE activity (1-AChE change % between April and July-October) with depression and anxiety scores. Statistical significance was defined using an alpha of 0.05. We adjusted models for confounders defined a-priori including age, gender, z-score for height-for-age, z-score for BMI-for-age, parental years of education and family income. To improve model parsimony (considering the limited sample size of our study), we removed parental years of education due to the negligible effect it had on the associations (≤4%) after its addition to the model. We also adjusted for hemoglobin concentration (baseline and follow-up) because erythrocytic AChE activity is a function of hemoglobin concentration. Due to our recently reported effect modification by age and gender on the associations between AChE activity and depression scores (Suarez-Lopez et al. 2019), we tested interaction terms using a product term (AChE inhibition %*covariate) using a linear regression model and stratified those associations by gender or by tertiles of age, as appropriate. We then plotted the associations between AChE change % and mental health outcomes as adjusted means (least square means) across four categories of AChE change, defined above, for the overall group and by gender. We tested for curvilinear associations by testing the significance of quadratic terms of the independent variable (AChE inhibition %*AChE inhibition %).
Using logistic regression, we calculated odds ratios (OR) for elevated scores of depression or anxiety associated with AChE inhibition %, modeled as per 10% inhibition and as four categories as described above. We also calculated the ORs stratified by gender and tertiles of age.
Considering the observed effect modification by gender on the associations between AChE inhibition and depression, we assessed whether sex or adrenal hormone levels could be mediating this effect modification. The statistical model used in the main analyses (above) was further adjusted for testosterone, cortisol, DHEA and estradiol stratified by gender. To account for circadian variability, these models were further adjusted for time of saliva sample collection and time of awakening.
Sensitivity Analyses
Girls had a greater inhibition of AChE activity than boys and this was evident in the greater number of female participants in the −34% to −12% category and the fewer participants in the 0% to 70% category compared to males. To assess whether the different AChE inhibition amounts by gender could explain the observed effect modification by gender, we conducted sensitivity analyses among a subgroup of male and female participants who had a similar distribution of participants across the four categories of AChE change. We randomly selected and removed 24 female participants from the −12.5% to −34% AChE change category in order to frequency-match females to male participants in this category. We also randomly selected and removed 10 male participants, in the 0% to 70.3% category, in order to frequency-match males to females in the same category. Males and females had a similar frequency of participants in the −12.4% to −6.5% and −6.4 to <0.0% categories, so no participants were removed from these groups. This selection was made considering that there were 24 more females in the −12.5% to −34% AChE change category and 10 more males in the 0% to 70.3% category. In this subgroup we conducted linear regression analysis as described above.
Results
Participant Characteristics
The mean (SD) age of the participants was 14.5 years (1.51), 51% were female and the mean (SD) z-scores for height-for-age and body mass index (BMI)-for-age were −1.53 SD (0.93) and 0.44 SD (0.83), respectively. The median (25th, 75th percentile) monthly family income was $500 USD (365, 710) and the mean (SD) scores of depression and anxiety were 52.8 (9.3) and 58.1 (9.6), respectively. Nine percent of participants had elevated depression scores, whereas 25% had elevated anxiety scores. The median (25th, 75th) AChE activity change between April and July-October was −3.9% (−10.5%, 5.1%).
Participant characteristics across AChE change categories between April and July- October, separate for boys and girls, are described in Table 1. Only hemoglobin was associated with percent change of AChE in both boys and girls, whereas testosterone and cortisol levels in girls showed inverse associations with percent change of AChE activity. Table 2 presents participant characteristics by gender. Girls, compared to boys, had greater AChE activity inhibition, BMI-for-age z-score, age family household income, and lower mean values in April for hemoglobin concentration and AChE activity at both exam periods. Additionally, girls had higher anxiety scores and were more likely than boys to have elevated anxiety scores (27% vs. 23%). Girls and boys had similar depression scores and prevalence of elevated depression scores (9% vs. 10%). Girls had lower testosterone but higher DHEA concentrations compared to boys.
Table 1.
Participant characteristics across categories of change in AChE activity by gender.
| Female (n = 153) | Male (n = 147) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Categories of AChE change % (Jul-Oct vs Apr) | Categories of AChE change % (Jul-Oct vs Apr) | ||||||||
| −34 to −12.5 | −12.4 to −6.5 | −6.4 to <0.0 | 0.0 to 70.3 | −34 to −12.5 | −12.4 to −6.5 | −6.4 to <0.0 | 0.0 to 70.3 | ||
| N | 42 | 28 | 28 | 55 | 18 | 33 | 32 | 64 | |
| Age, y | 14.7 (1.9) | 14.4 (1.7) | 15.0 (1.9) | 14.6 (1.7) | 14.1 (1.9) | 14.2 (1.9) | 14.4 (1.9) | 14.4 (1.7) | |
| Monthly family income, USD | 600 (380, 740) | 450 (365, 710) | 500 (388, 795) | 430 (360, 720) | 400 (358, 700) | 500 (350, 720) | 500 (365, 700) | 445 (365, 700) | |
| Z-score height-for-age, SD | −1.44 (0.97) | −1.42 (0.74) | −1.82 (0.71) | −1.57 (0.9) | −1.43 (0.84) | −1.25 (1.19) | −1.77 (0.9) | −1.53 (0.93) | |
| Z-score BMI-for-age, SD | 0.40 (0.68) | 0.55 (0.94) | 0.71 (0.9) | 0.68 (0.77) | 0.03 (0.8) | 0.46 (0.93) | 0.3 (0.84) | 0.29 (0.79) | |
| Hemoglobin (Apr), g/dL | 13.2 (1.7) | 13.1 (0.9) | 12.7 (0.8) | 12.5 (1.5) | 13.6 (1.0) | 13.6 (1.3) | 13.3 (1.2) | 12.8 (1.1) | |
| Hemoglobin (Jul-Oct), mg/dL | 12.1 (0.8) | 12.2 (0.8) | 12.8 (0.8) | 13.1 (0.9) | 12.6 (1.1) | 12.9 (1.3) | 13.1 (1.4) | 13.6 (1.1) | |
| Awakening time, hour | 6.99 (0.85) | 6.80 (0.80) | 6.69 (0.80) | 6.85 (0.89) | 6.53 (0.51) | 6.88 (1.09) | 6.86 (0.96) | 6.71 (0.8) | |
| Saliva collection time, hour | 7.47 (1.34) | 7.13 (1.07) | 7.01 (0.87) | 7.41 (1.4) | 6.75 (0.64) | 7.34 (1.09) | 7.27 (1.28) | 7.21 (0.89) | |
| Testosterone, pg/mL | 32.2 (25.1, 44.2) | 32.3 (20.6, 41.5) | 33.0 (19.9, 41.3) | 26.8 (19.6, 39.3) | 55.7 (26.3, 99.3) | 58.5 (28.4, 93.8) | 51.9 (24.9, 91.9) | 59.6 (28.6, 89.3) | |
| DHEA, pg/mL | 85.9 (45.9, 147.2) | 58.8 (43.8, 118.9) | 84.7 (44.9, 172.7) | 78.9 (38.9,125.5) | 21.7 (12.1, 69.1) | 44.3 (17.6, 97.3) | 28.5 (19.1, 54.1) | 46.2 (18.6, 74.9) | |
| Cortisol, μg/dL | 0.21 (0.17, 0.29) | 0.19 (0.10, 0.24) | 0.20 (0.13, 0.32) | 0.17 (0.12, 0.24) | 0.23 (0.15, 0.32) | 0.20 (0.12, 0.30) | 0.26 (0.14, 0.37) | 0.22 (0.11, 0.30) | |
| Estradiol, pg/mL | - | - | - | - | 0.44 (0.25, 0.47) | 0.39 (0.35, 0.50) | 0.42 (0.28, 0.57) | 0.43 (0.32, 0.58) | |
Values are mean (SD) or median (25th-75th percentile)
Table 2.
Participant characteristics by gender.
| Girls (n=153) | Boys (n=147) | Pdifference | |
|---|---|---|---|
| Age, y | 14.7 (1.8) | 14.3 (1.8) | 0.05 |
| Monthly family income, USD | 593 (398) | 523 (258) | 0.07 |
| Z–score height–for–age, SD | −1.55 (0.87) | −1.50 (0.98) | 0.67 |
| Z–score BMI–for–age, SD | 0.58 (0.81) | 0.30 (0.84) | <0.01 |
| Hemoglobin (Apr), g/dL | 12.9 (1.4) | 13.2 (1.2) | 0.10 |
| Hemoglobin (Jul–Oct), mg/dL | 12.6 (0.9) | 13.2 (1.3) | <0.01 |
| AChE difference (Apr vs Jul–Oct), % | −4.01 (17.23) | −0.81 (12.99) | 0.07 |
| AChE (Apr), U/mL | 3.72 (0.53) | 3.88 (0.50) | 0.01 |
| AChE (Jul–Oct), U/mL | 3.53 (0.50) | 3.82 (0.54) | <0.01 |
| Anxiety, score | 59.3 (8.9) | 56.8 (10.1) | 0.03 |
| Depression, score | 53.3 (8.6) | 52.2 (10.1) | 0.31 |
| Awakening time, hour | 6.9 (0.8) | 6.8 (0.9) | 0.37 |
| Saliva collection time, hour | 7.3 (1.2) | 7.2 (1.0) | 0.45 |
| Testosterone, pg/mL | 30.5 (20.7, 41.2) | 56.0 (27.34, 91.8) | <0.01 |
| DHEA, pg/mL | 78.9 (43.4, 139.7) | 39.8 (17.6, 75.0) | <0.01 |
| Cortisol, μg/dL | 0.19 (0.13, 0.28) | 0.22 (0.13, 0.31) | 0.39 |
| Estradiol, pg/mL | 0.42 (0.29, 0.55) | − |
Values are mean (SD) or median (25th−75th percentile)
The degree of cholinesterase inhibition was highest among participants in the youngest age tertile (11.0–13.49y: mean of AChE change: −4.0%, SD= 10.7), followed by participants in the oldest age tertile (15.3–17.9y: −3.0%, SD=12.6) and participants of the middle tertile (13.5–15.2y: −1.6%, SD= 15.5).
Cholinesterase inhibition and depression symptoms
Overall, for every 10% inhibition of AChE activity there was an increase in depression scores by 0.96 units (95% CI: 0.01, 1.90). These associations differed by participant age: older adolescents had a stronger association between AChE inhibition and depression scores (Table 3) than younger adolescents (pinteraction=0.04). The associations between AChE inhibition and depression scores also differed by gender, being stronger in females (depression score per 10% AChE inhibition (β)=1.00, 95% CI: −0.07, 2.08): compared to males (β=0.61, 95% CI: −1.26, 2.48, Figure 2). We did not observe evidence of curvilinear associations on any of the outcomes.
Table 3.
Associations between change in AChE activity between two agricultural seasons with depression and anxiety symptoms (n=300).
| Mental health score (β) per 10% inhibition in AChE activity (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Depression | Anxiety | |||||
| Total | Obsession - compulsion | Generalized Anxiety Disorder | Social Anxiety | |||
| All | 0.96 (0.01, 1.90)* | −0.47 (−1.48, 0.53) | 0.06 (−0.96, 1.08) | 0.22 (−0.79, 1.22) | −0.31 (−1.31, 0.69) | |
| Age (tertiles) | pinteraction= 0.04 | pinteraction= 0.39 | pinteraction= 0.56 | pinteraction= 0.37 | pinteraction= 0.17 | |
| 11.0–13.49y (n=99) | 0.74 (−1.11, 2.59) | −0.56 (−1.38, 2.51) | −0.49 (−1.47, 2.44) | 0.68 (−1.32, 2.67) | 0.83 (−1.11, 2.77) | |
| 13.5–15.2y (n=97) | 0.53 (−1.23, 2.28) | −1.05 (−3.22, 1.12) | −0.29 (−2.34, 1.75) | 0.15 (−1.91, 2.21) | −1.43 (−3.44, 0.59) | |
| 15.3–17.9y (n=105) | 2.79 (0.48, 5.09)* | −1.29 (−3.25, 0.66) | −0.19 (−2.43, 2.05) | −0.20 (−2.33, 1.93) | −0.50 (−2.70, 1.69) | |
| Gender | pinteraction= 0.91 | pinteraction= 0.57 | pinteraction= 0.80 | pinteraction= 0.69 | pinteraction= 0.36 | |
| Female (n=153) | 1.00 (−0.07, 2.08)† | −0.38 (−1.53, 0.78) | −0.36 (−0.81, 1.54) | 0.55 (−0.58, 1.69) | −0.10 (−1.25, 1.06) | |
| Male (n=148) | 0.61 (−1.26, 2.48) | −0.77 (−2.76, 1.22) | −0.34 (−2.36, 1.66) | −0.46 (−2.48, 1.56) | −0.75 (−2.73, 1.22) | |
Adjustments: age, gender, AChE activity in April, hemoglobin concentration (baseline and follow-up), z-score for height-for-age, z-score for BMI-for-age, and family income.
p<0.01
p<0.1
Figure 2.
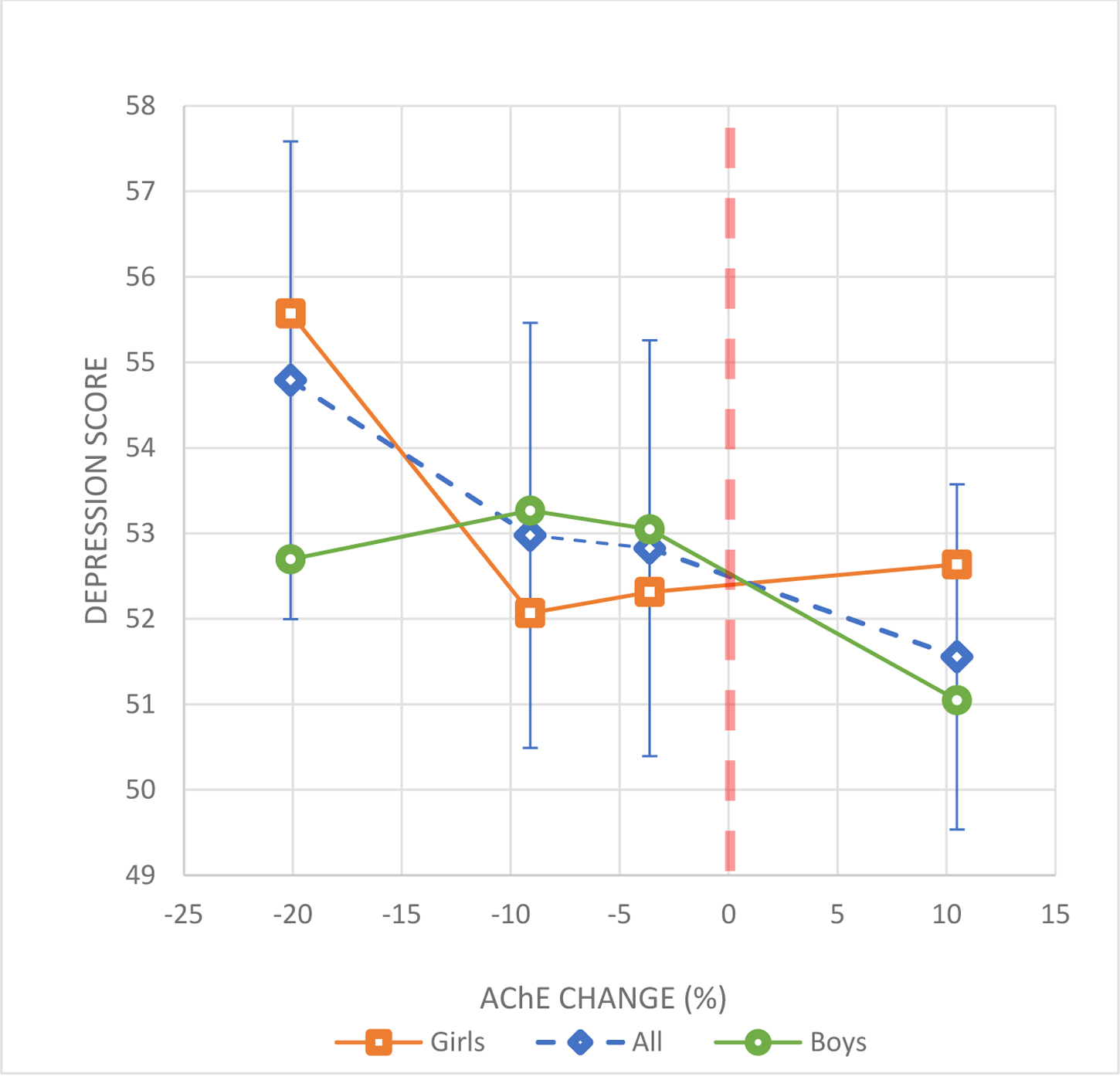
Change of AChE activity between 2 agricultural seasons and depression scores in adolescents.
Markers (squares, diamonds or circles) correspond to the mean depression scores for the following categories of AChE change: 1) −34% to −12.5%, 2) −12.4 to −6.5%, 3) −6.4% to <0.0% and 4) ≥0.0%. Error bars correspond to the 95% CI for the means for all participants. Error bars for boys and girls are not shown to improve visualization.
Lines between AChE change categories were plotted to improve visualization of the associations.
Adjustments: age, gender, AChE concentration at baseline, hemoglobin concentration (baseline and follow-up), z-score for height-for-age, z-score for BMI-for-age, and family income.
These associations were also observed in logistic regression analyses. Overall, for every 10% inhibition of AChE activity (Table 4), the odds of having elevated depressive symptoms increased by 67% (OR=1.67; 95% CI: 1.04, 2.66). Participants with AChE inhibition between −34% to −12.5% (the lowest tertile of negative AChE change values) had nearly 8 times the odds (OR=7.84; 95% CI: 1.84, 33.4) of having elevated depressive symptoms compared to those who did not have AChE inhibition (AChE change ≥0.0%). Even with lower levels of AChE inhibition, there were noticeably elevated ORs, although these associations were not statistically significant. As we had found in linear regression analyses, AChE inhibition and depression associations differed by participant age and gender; the strongest ORs were observed among participants in the oldest tertile (OR: 3.7) and the associations were roughly twice as strong in females than males (OR: 2.7 vs 1.2). Table S1 presents the associations between AChE inhibition and depression scores stratified by both age and gender, in which the associations are strongest among the oldest age groups in both genders.
Table 4,
Odds ratios for elevated anxiety and depression symptoms and AChE inhibition.
| OR (95% CI) | ||
|---|---|---|
| Depression | Anxiety | |
| Categories of AChE inhibition | ||
| −34% to −12.5% (n=60) | 7.84 (1.84, 33.4) | 0.80 (0.36, 1.76) |
| −12.4% to −6.5% (n=61) | 2.35 (0.57, 9.81) | 1.00 (0.43, 2.32) |
| −6.4% to <0.0% (n=60) | 3.37 (1.00, 11.43) | 0.64 (0.24, 1.70) |
| 0.0% to 70.3% (n=119) | Ref. | Ref. |
| OR per 10% inhibition in AChE activity (95% CI) | ||
| Depression | Anxiety | |
| All | 1.67 (1.04, 2.66) | 0.97 (0.75, 1.25) |
| Age (tertiles)a | ||
| 11.0–13.4y (n=99) | 0.70 (0.24, 2.09) | 1.22 (0.65, 2.27) |
| 13.5–15.2y (n=97) | 2.09 (0.31, 14.11) | 3.01 (0.93, 9.78) |
| 15.3–17.9y (n=105) | 3.74 (1.29, 10.88) | 0.87 (0.49, 1.56) |
| Gender | ||
| Female (n=153) | 2.72 (1.23, 6.00) | 0.99 (0.73, 1.34) |
| Male (n=147) | 1.18 (0.59, 2.37) | 0.93 (0.59, 1.47) |
Adjustments: age, gender, AChE activity in April, hemoglobin concentration (baseline and follow-up), z-score for height-for-age, z-score for BMI-for-age, and family income.
Not adjusted for age
Cholinesterase inhibition and anxiety symptoms
Neither the total anxiety score nor scores for any anxiety subtypes were associated with AChE inhibition in either the linear regression or logistic regression models (Tables 2–3). We also did not observe evidence of effect modification by gender or age. However, we did observe stronger inverse associations between AChE inhibition and obsessive-compulsive symptoms with older age, although none of the associations were statistically significant. Additionally, we did not observe any associations with harm avoidance, physical symptoms of anxiety, tense or restless, panic, and humiliation rejection scores (Table S2).
Sensitivity analysis of effect modification by gender
Since girls had a greater mean inhibition of AChE activity than boys, we conducted sensitivity analyses in a subsample that was frequency-matched across categories of AChE change % between the genders to see if the gender differences in the AChE-depression associations would be removed by homogenizing the amount of inhibition between boys and girls. This subgroup had similar means of % AChE inhibition between females and males (−2.1% vs. −2.4%, respectively). The effect modification by gender not only persisted but was strengthened in this subsample. The linear associations between % AChE inhibition and depression scores were unchanged for boys (βper 10% inhibition: 0.62, 95% CI: −1.47, 2.71) and stronger for girls (βper 10% inhibition: 1.55, 95% CI: 0.34, 2.76) compared to the full sample (Table 3).
Hormones and effect modification by gender
After adjustment for saliva sample collection time, the difference between boys and girls in β coefficients of the association between AChE change and depression scores was reduced from 0.39 to 0.21 units in the full sample (Table 4 and 5) and from 0.93 to 0.73 units in the sensitivity subset. The difference between genders in the β coefficients of the association between AChE change and depression scores was reduced by 0.08 and 0.05 units in the full and sensitivity samples, respectively. Adjustment for DHEA or testosterone resulted in increased differences in the β coefficients between genders, primarily as a result of a weakening in the associations among boys in both the full and sensitivity samples. Adjustment for estradiol resulted in a substantial strengthening of the association among boys in the full sample (β= 0.90) and in the sensitivity subsample (β= 0.87). Since estradiol was only measured in boys, we are unable to assess whether the effect modification by gender was modified after adjustment for estradiol. After adjusting for all hormones (excluding estradiol for comparison purposes), there was a noticeable decrease in the differences in β coefficients by gender in both the full and sensitivity samples by 62% and 18%, respectively. The correlations between hormones and change in AChE activity by gender are listed in Table S2. The associations of constructs of pesticide exposure markers (including AChE activity) with sex and adrenal hormones are topic for a separate manuscript.
Table 5.
Associations between AChE inhibition and depression by gender, adjusted for adrenal and sex hormones.
| Depression score difference per 10% AChE inhibition (β (95% CI)) | |||||||
|---|---|---|---|---|---|---|---|
| Adjustments: | No hormones | Cortisol only | DHEA only | Testosterone only | Estradiol only | All hormones excluding estradiol | All hormones |
| A) Full Sample | |||||||
| Female (n=153) | 0.91 (−0.20, 2.03) | 0.72 (−0.40, 1.84) | 0.95 (−0.18, 2.07) | 0.98 (−0.14, 2.09) | − | 0.71 (−0.40, 1.81) | - |
| Male (n=148) | 0.70 (−1.25, 2.65) | 0.59 (−1.34, 2.53) | 0.63 (−1.37, 2.63) | 0.59 (−1.34, 2.52) | 0.90 (−1.02, 2.81) | 0.63 (−1.38, 2.65) | 0.91 (−1.07, 2.88) |
| Difference in β between genders | 0.21 | 0.13 | 0.32 | 0.39 | - | 0.08 | - |
| B) AChE inhibition-matched sample by gender (sensitivity) | |||||||
| Female (n=125) | 1.50 (−0.08, 3.07) | 1.31 (−0.26, 2.88) | 1.55 (−0.04, 3.14) | 1.51 (−0.07, 3.09) | - | 1.22 (−0.34, 2.77) | - |
| Male (n=134) | 0.77 (−1.49, 3.03) | 0.63 (−1.60, 2.87) | 0.64 (−1.68, 2.96) | 0.63 (−1.60, 2.87) | 0.87 (−1.34, 3.09) | 0.62 (−1.72, 2.96) | 0.74 (−1.55, 3.02) |
| Difference in β between genders | 0.73 | 0.68 | 0.91 | 0.88 | - | 0.60 | - |
Adjustments: age, AChE activity at baseline, hemoglobin concentration (baseline and follow-up), z-score for height-for-age, z-score for BMI-for-age, family income, time of saliva sample collection and time of awakening and concentrations of hormones listed
Discussion
We observed that exposures to cholinesterase inhibitors, characterized as inhibition of AChE, between two agricultural seasons in the same year was associated with increased depression symptoms in adolescents. Inhibition of AChE activity seemed to have a stronger association with depression scores among females compared to men and among older compared to younger adolescents. However, we observed consistent positive associations in all age groups and genders (greater inhibition associated with greater depression scores) which indicates internal consistency of the findings across these groups. Additionally, we did not observe associations with anxiety symptoms. These findings parallel earlier cross-sectional analyses in the full sample of our cohort study (n=529), in which lower AChE activity was also associated with increased depression scores (but not anxiety), with stronger associations among girls compared to boys (Suarez-Lopez et al. 2019). The present study provides stronger evidence that cholinesterase inhibitors can affect mood regulation, as the degree of cholinesterase inhibition between two agricultural seasons was strongly related to depression symptoms. The findings of both studies, based on the ESPINA cohort, are among the first to characterize associations of depression and anxiety with a biomarker of pesticide exposure.
Our study findings concur with studies among adult agricultural workers that have reported positive associations between self-reports of exposure to organophosphates with symptoms of depression (London et al. 2005, 2012; Beseler et al. 2008; Meyer et al. 2010; Malekirad et al. 2013; Freire and Koifman 2013; Weisskopf et al. 2013; Beard et al. 2014; Koh et al. 2017), anxiety (Malekirad et al. 2013), and suicide attempts (London et al. 2005, 2012; Pearce et al. 2007; Freire and Koifman 2013). Depression is an important risk factor of suicidality, and suicide rates are notoriously high in farming populations exposed to pesticides. Case series and ecological studies support a significant association between organophosphate use and suicide (London et al. 2005). Although we did not assessed suicidality in our study, community members in Pedro Moncayo County expressed concern about perceived increases in adolescent depression and suicide attempts, which prompted the present analyses.
The findings of this study also suggest that cholinesterase inhibition can result in subacute alterations in the mood of adolescents, adding to data from earlier studies in children that have reported transient alterations in neurobehavioral performance associated with pesticide spray seasons (Khan et al. 2014; Rohlman et al. 2016; Suarez-Lopez et al. 2017b). In Egypt, adolescent pesticide applicators were evaluated for neurobehavioral performance via survey related to pesticide exposure, by assessing via pesticide levels in urine before, during and after seasons of pesticide application. Researchers found that deficits in neurobehavioral performance were markedly increased in those with more exposure to pesticides, and that these alterations remained for months after exposure (Khan et al. 2014; Rohlman et al. 2016). In our own ESPINA cohort, we found that children examined closer to the end of the Mother’s day flower harvest, marking the end of a peak pesticide spray season, had lower neurobehavioral performance in 3 of 5 domains assessed compared to children examined later during a period of lower floricultural production and pesticide use. (Suarez-Lopez et al. 2017b). Research assessing the short- and medium- term effects of pesticide exposures on children’s health is complex and the present study contributes to this understudied field of research. Beyond transient effects on mental health processes, it is plausible that repeated exposures throughout childhood may lead to long-lasting developmental alterations.
Reduced acetylcholine hydrolysis, as a result of cholinesterase-inhibitors, such as carbamate and organophosphate pesticides, results in overstimulation of nicotinic and muscarinic receptors (Taylor 2011). The cholinergic system has a key role in the central nervous system and has modulatory effects on mood and behavior. Both clinical and animal studies have demonstrated that blocking the activation of nicotinic and muscarinic receptors result in antidepressant signs and/or symptoms (Furey and Drevets 2006; Mineur et al. 2013). In mice, exposure to physostigmine, a cholinesterase inhibitor, led to increased depression and anxiety behaviors which were reversed once nicotinic or muscarinic acetylcholine receptor antagonists were administered (Mineur et al. 2013). Likewise, physostigmine was found to induce depressive symptoms and exacerbate mood disorders in normal and manic subjects (Janowsky et al. 1973, 1974; Davis et al. 1976; Steinberg et al. 1997). Another study similarly found that rats who received the organophosphate insecticide chlorpyrifos had increased depressive behaviors and that such behaviors subsided upon receiving atropine, an anticholinergic agent (Siqueira et al. 2019). In addition, rats exposed to cholinesterase inhibitor pesticides, had a reduction in hippocampal, striatal and prefrontal cortical AChE activity and plasma butyrylcholinesterase activity (Siqueira et al. 2019). These studies corroborate past reports of human exposure to cholinesterase inhibitors that resulted in the cholinergic balance hypothesis of mania and depression (Janowsky et al. 1972; Risch et al. 1981). The hypothesis was based upon reports and case studies of both the emergence of depressive symptoms among psychotic patients who were administered a cholinergic inhibitor (Rowntree et al. 1950), and by reports of cholinesterase inhibitor insecticide poisoning leading to symptoms of depression and parasympathetic toxicity (Gershon and Shaw 1961; Dagytė et al. 2011). Considering the role of the cholinergic system on mood modulation, anticholinergic drugs have been investigated as potential treatments for depression, but inconsistent antidepressant effects have been found (Goldman and Erickson 1983; Shytle et al. 2002; Howland 2009). Parallel to the cholinergic system, serotonin is a neurotransmitter that has been directly linked with mood regulation and there is evidence that organophosphate pesticides can affect serotonergic mechanisms and serotonin-related emotional behaviors in animal models (Aldridge et al. 2004, 2005b, a; Chen et al. 2011). The associations observed in these experimental and observational studies, including our own, may be explained in part by the interplay of the cholinergic and serotonergic systems in the regulation of behavior and neurobehavioral processes (Steckler and Sahgal 1995; Jeltsch-David et al. 2008; Sparks et al. 2018). The cholinergic system is one of many factors involved in mood regulation.
In the present study, we observed stronger associations between AChE inhibition and depression symptoms among girls compared to boys, and these findings are in agreement with our cross-sectional findings in the full cohort of participants assessed in July-October 2016 (n=529) (Suarez-Lopez et al. 2019). While the biological mechanism explaining why AChE inhibition affected girls more than boys is unclear, it is known that adolescent girls have higher rates of depression compared to boys (Perou et al. 2013; Whiteford et al. 2013) and this increased susceptibility to internalizing behaviors may be a contributing factor to the stronger associations observed in the present study. Initially we hypothesized that an important explanation of this effect modification by gender was that girls had a greater amount of AChE activity inhibition compared to boys. However, this was not the case; in our sensitivity analysis using the frequency-matched sample across the four categories of AChE inhibition between the genders, we still observed that the associations were substantially stronger in girls than boys. In fact, the difference between genders was strengthened in this subgroup.
Further assessments into understanding the reasons for the differing associations by gender yielded important findings about the role of sex and adrenal hormones. We observed that the gender difference on the AChE-depression symptom association was reduced by 18% to 62% after adjustment for cortisol, testosterone and DHEA in the sensitivity and full samples, respectively. These notable reductions suggest that these hormones may explain some of the effect modification by gender on the pesticide-depression associations observed within our study. Our findings also suggest that estradiol may have an even larger role on this effect modification than the other hormones measured since the estimates among boys strengthened the most (more similar to the association strengths of girls) after adjusting for estradiol. However, since estradiol was not measured in girls, we are not able to adequately assess this hypothesis. Acetylcholine receptors in the hypothalamus are one of the primary regulators of hypothalamic-pituitary-adrenal axis and acetylcholine stimulates the synthesis and secretion of corticotropin-releasing hormone (CRH) which then stimulates the secretion of adrenocorticotropic hormone, with subsequent cortisol and DHEA increases (Rhodes and Rubin 1999; Rubin et al. 2002; Hall 2010; Balkan and Pogun 2018). Triazophos, an organophosphate insecticide, has been found to yield metabolites that bind to glucocorticoid receptors and affect the binding of estrogen, progesterone and androgen hormones with their receptors, in addition to disrupting steroid hormones synthesis (Yang et al. 2019). Our crude analyses (Table 1) indicate that AChE inhibition is inversely associated with both testosterone and estradiol levels in girls but not boys, which strengthens the plausibility of our findings that hormones may mediate in part the effect modification by gender. A more exhaustive characterization of pesticide exposure markers, including AChE activity, with sex and adrenal hormones in this study population is topic for a separate manuscript.
We also observed effect modification by age, in which the associations were strongest in the oldest adolescents (15.3–17.9 y) compared to the youngest adolescents (11–15.2 y). This contrasts with the cross-sectional findings of the full cohort of participants of this same study population (Suarez-Lopez et al. 2019), in which the associations between AChE activity and depression scores were stronger in younger rather than older adolescents. We do not have an explanation for these discrepant findings. The fact that AChE inhibition was greatest among participants in the youngest tertile (see participant characteristics) discards the explanation that differing amounts of AChE inhibition across age categories may be explaining this effect modification.
As in our prior publication based on this study population (Suarez-Lopez et al. 2019), we did not observe associations between AChE inhibition and any of the anxiety subcomponents or overall anxiety score in adolescents. The findings of both of these studies within the ESPINA cohort contrast with the few studies that have observed positive associations between self-reports of exposure to organophosphates and anxiety among agricultural workers (Malekirad et al. 2013; Harrison and Mackenzie Ross 2016).
Children and adolescents living in agricultural communities are subject to a variety of pesticide exposure pathways including para-occupational sources such as off-target drift of pesticides from agricultural crops onto nearby homes or schools and agricultural worker “take-home” pathways, or agricultural field work, residential use, produce and contaminated water sources. Participants in our study did not work in agriculture, other than a few providing occasional assistance in family crops, but many will join this labor force in years to come as they transition into adulthood. Our findings, in conjunction with previous research, emphasize the importance for improved measures to reduce para-occupational exposures for adolescent pesticide exposure, particularly as critical neurodevelopment occurs within this age range.
Although our study findings suggest that cholinesterase inhibition can result in subacute alterations in mood, we are unable to fully test this hypothesis since depression symptoms were assessed at only one point in time in 2016. Future studies should include serial assessments of mental health status and AChE activity across multiple points in time to adequately assess whether cholinesterase inhibition may transiently affect depressive symptoms. Another limitation is the sample size of our study. Despite ESPINA having the largest number of participants among studies assessing agricultural seasonal effects on health, the statistical power of our study is insufficient to assess 2-level interactions (gender and age). For this reason, we are cautious to not give much weight to the age and gender stratified analyses presented in Table S1 considering that we have small number of participants (n~49) in each of the sex*age categories. However, we had enough power to detect effect modification by age and to some extent gender. Conducting studies assessing both exposure and outcome measurements between agricultural seasons during the same year is logistically complex and this is an important limiting factor to the size and number of existing studies assessing cyclical health effects on populations living near agriculture. A strength of our study are the short examination windows during both the April exam (3 weeks) and July-October exam (4 weeks for 95% of participants). This allowed us to reduce seasonal effects that may occur within each of the 2 exam periods, but most importantly during the second exam period which is when the depression and anxiety tests were conducted (July-October).
Conclusions:
We observed that inhibition of AChE activity between pesticide spray seasons, characterizing greater exposure to cholinesterase inhibitor pesticides, was associated with greater depression symptoms in adolescents, and these associations were stronger in girls than boys and among older than younger adolescents. The observed association differences between genders were substantially attenuated after adjusting for sex and adrenal hormones. This provides evidence that pesticide-related sex and adrenal level disruption may explain, in part, the gender differences observed in the pesticide-mental health associations. Replication of these findings is needed. This is the first study to describe associations between change of a biomarker of pesticide exposure and depression symptoms in children and is among the largest studies to assess agricultural seasonal effects on health. These findings bring attention to the role of the cholinergic system on mood regulation and concurs with prior experimental and cross-sectional findings on the association of AChE activity and cholinesterase inhibitor exposures with depression symptoms.
Supplementary Material
Acknowledgments:
The ESPINA study received funding from the National Institute of Occupational Safety and Health (1R36OH009402) and the National Institute of Environmental Health Sciences (R01ES025792, R21ES026084). We thank Fundación Cimas del Ecuador, the Parish Governments of Pedro Moncayo County, community members of Pedro Moncayo and the Education District of Pichincha-Cayambe-Pedro Moncayo counties for their support on this project. We also thank Dr. José Suárez-Torres, Dr. David R Jacobs Jr., Daria Malangone, Franklin de la Cruz, Danilo Martinez, Janeth Barros and Cecilia Cardenas for their contributions to our project and manuscript.
Footnotes
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of Institutional Review Boards of the University of California San Diego, Universidad San Francisco de Quito and the Ministry of Public Health of Ecuador, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Competing Interests: The authors declare they have no actual or potential competing financial interests.
References
- Abou-Donia MB (2003) Organophosphorus ester-induced chronic neurotoxicity. Arch Environ Health 58:484–97. doi: 10.3200/AEOH.58.8.484-497 [DOI] [PubMed] [Google Scholar]
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA (2005a) Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ Health Perspect 113:527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA (2005b) Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect 113:1027–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Slotkin TA (2004) Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect 112:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae Y (2012) Test Review: Children’s Depression Inventory 2 (CDI 2)KovacsM.Children’s Depression Inventory 2 (CDI 2) (2nd ed.). North Tonawanda, NY: Multi-Health Systems Inc, 2011. J Psychoeduc Assess 30:304–308. doi: 10.1177/0734282911426407 [DOI] [Google Scholar]
- Balkan B, Pogun S (2018) Nicotinic Cholinergic System in the Hypothalamus Modulates the Activity of the Hypothalamic Neuropeptides During the Stress Response. Curr Neuropharmacol 16:371–387. doi: 10.2174/1570159X15666170720092442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard JD, Umbach DM, Hoppin JA, et al. (2014) Pesticide exposure and depression among male private pesticide applicators in the agricultural health study. Environ Health Perspect 122:984–91. doi: 10.1289/ehp.1307450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beseler CL, Stallones L (2008) A Cohort Study of Pesticide Poisoning and Depression in Colorado Farm Residents. Ann Epidemiol 18:768–774. [DOI] [PubMed] [Google Scholar]
- Beseler CL, Stallones L, Hoppin JA, et al. (2006) Depression and pesticide exposures in female spouses of licensed pesticide applicators in the agricultural health study cohort. J Occup Environ Med 48:1005–13. doi: 10.1097/01.jom.0000235938.70212.dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beseler CL, Stallones L, Hoppin JA, et al. (2008) Depression and pesticide exposures among private pesticide applicators enrolled in the Agricultural Health Study. Environ Health Perspect 116:1713–9. doi: 10.1289/ehp.11091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, et al. (2011) Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect 119:1189–95. doi: 10.1289/ehp.1003185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-Q, Yuan L, Xue R, et al. (2011) Repeated exposure to chlorpyrifos alters the performance of adolescent male rats in animal models of depression and anxiety. Neurotoxicology 32:355–361. doi: 10.1016/j.neuro.2011.03.008 [DOI] [PubMed] [Google Scholar]
- Dagytė G, Den Boer JA, Trentani A (2011) The cholinergic system and depression. Behav Brain Res 221:574–582. doi: 10.1016/j.bbr.2010.02.023 [DOI] [PubMed] [Google Scholar]
- Davis KL, Hollister LE, Overall J, et al. (1976) Physostigmine: effects on cognition and affect in normal subjects. Psychopharmacology (Berl) 51:23–7. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Kogut K, Huen K, et al. (2014) Organophosphate pesticide exposure, PON1, and neurodevelopment in school-age children from the CHAMACOS study. Environ Res 134C:149–157. doi: 10.1016/j.envres.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, et al. (2007) Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect 115:792–8. doi: 10.1289/ehp.9828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraccaro RL, Stelnicki AM, Nordstokke DW (2015) Test Review: Multidimensional Anxiety Scale for Children by MarchMarch JS. (2013). Multidimensional Anxiety Scale for Children (2nd ed.). Toronto, Ontario, Canada: Multi-Health Systems. Can J Sch Psychol 30:70–77. doi: 10.1177/0829573514542924 [DOI] [Google Scholar]
- Freire C, Koifman S (2013) Pesticides, depression and suicide: A systematic review of the epidemiological evidence. Int J Hyg Environ Health 216:445–460. doi: 10.1016/J.IJHEH.2012.12.003 [DOI] [PubMed] [Google Scholar]
- Furey ML, Drevets WC (2006) Antidepressant Efficacy of the Antimuscarinic Drug Scopolamine. Arch Gen Psychiatry 63:1121. doi: 10.1001/archpsyc.63.10.1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon S, Shaw FH (1961) Psychiatric sequelae of chronic exposure to organophosphorus insecticides. Lancet (London, England) 1:1371–4. [DOI] [PubMed] [Google Scholar]
- Goldman ME, Erickson CK (1983) Effects of acute and chronic administration of antidepressant drugs on the central cholinergic nervous system. Comparison with anticholinergic drugs. Neuropharmacology 22:1215–22. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Harari R, Barr DB, Debes F (2006) Pesticide exposure and stunting as independent predictors of neurobehavioral deficits in Ecuadorian school children. Pediatrics 117:e546–56. doi: 10.1542/peds.2005-1781 [DOI] [PubMed] [Google Scholar]
- Hall JE (2010) Guyton and Hall Textbook of Medical Physiology. Elsevier Health Sciences [Google Scholar]
- Harari R (2004) Seguridad, salud y ambiente en la floricultura. IFA, PROMSA, Quito [Google Scholar]
- Harrison V, Mackenzie Ross S (2016) Anxiety and depression following cumulative low-level exposure to organophosphate pesticides. Environ Res 151:528–536. doi: 10.1016/j.envres.2016.08.020 [DOI] [PubMed] [Google Scholar]
- Horton MK, Kahn LG, Perera F, et al. (2012) Does the home environment and the sex of the child modify the adverse effects of prenatal exposure to chlorpyrifos on child working memory? Neurotoxicol Teratol 34:534–41. doi: 10.1016/j.ntt.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland RH (2009) The antidepressant effects of anticholinergic drugs. J Psychosoc Nurs Ment Health Serv 47:17–20. [DOI] [PubMed] [Google Scholar]
- Isasi CR, Carnethon MR, Ayala GX, et al. (2014) The Hispanic Community Children’s Health Study/Study of Latino Youth (SOL Youth): design, objectives, and procedures. Ann Epidemiol 24:29–35. doi: 10.1016/j.annepidem.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef K, Davis JM, Sekerke HJ (1973) Parasympathetic suppression of manic symptoms by physostigmine. Arch Gen Psychiatry 28:542–7. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ (1972) A cholinergic-adrenergic hypothesis of mania and depression. Lancet (London, England) 2:632–5. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Khaled El Yousef M, Davis JM (1974) Acetylcholine and depression. Psychosom Med 36:248–257. doi: 10.1097/00006842-197405000-00008 [DOI] [PubMed] [Google Scholar]
- Jeltsch-David H, Koenig J, Cassel J-C (2008) Modulation of cholinergic functions by serotonin and possible implications in memory: General data and focus on 5-HT1A receptors of the medial septum. Behav Brain Res 195:86–97. doi: 10.1016/j.bbr.2008.02.037 [DOI] [PubMed] [Google Scholar]
- Khan K, Ismail AA, Abdel Rasoul G, et al. (2014) Longitudinal assessment of chlorpyrifos exposure and self-reported neurological symptoms in adolescent pesticide applicators. BMJ Open 4:e004177. doi: 10.1136/bmjopen-2013-004177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofman O, Berger A, Massarwa A, et al. (2006) Motor inhibition and learning impairments in school-aged children following exposure to organophosphate pesticides in infancy. Pediatr Res 60:88–92. doi: 10.1203/01.pdr.0000219467.47013.35 [DOI] [PubMed] [Google Scholar]
- Koh S-B, Kim TH, Min S, et al. (2017) Exposure to pesticide as a risk factor for depression: A population-based longitudinal study in Korea. Neurotoxicology 62:181–185. doi: 10.1016/j.neuro.2017.07.005 [DOI] [PubMed] [Google Scholar]
- Kovacs M (2011) CDI 2: Children’s Depression Inventory 2nd Edition. North Tonawanda, NY [Google Scholar]
- Kovacs MMHS staff (2011) CDI 2: Children’s Depression Inventory 2nd Edition, Technical Manual, 2nd edn. MHS Inc, North Tonawanda, NY [Google Scholar]
- London L, Beseler C, Bouchard MF, et al. (2012) Neurobehavioral and neurodevelopmental effects of pesticide exposures. Neurotoxicology 33:887–96. doi: 10.1016/j.neuro.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London L, Flisher AJ, Wesseling C, et al. (2005) Suicide and exposure to organophosphate insecticides: Cause or effect? Am J Ind Med 47:308–321. doi: 10.1002/ajim.20147 [DOI] [PubMed] [Google Scholar]
- Magiati I, Ponniah K, Ooi YP, et al. (2013) Self-reported depression and anxiety symptoms in school-aged Singaporean children. Asia Pac Psychiatry. doi: 10.1111/appy.12099 [DOI] [PubMed] [Google Scholar]
- Malekirad AA, Faghih M, Mirabdollahi M, et al. (2013) Neurocognitive, Mental Health, and Glucose Disorders in Farmers Exposed to Organophosphorus Pesticides. Arch Ind Hyg Toxicol 64:1–8. doi: 10.2478/10004-1254-64-2013-2296 [DOI] [PubMed] [Google Scholar]
- March J (2012) Multidimensional Anxiety Scale for Children 2 Manual, 2nd edn. MHS Inc, North Tonawanda, NY [Google Scholar]
- Marks AR, Harley K, Bradman A, et al. (2010) Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ Health Perspect 118:1768–74. doi: 10.1289/ehp.1002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason HJ (2000) The recovery of plasma cholinesterase and erythrocyte acetylcholinesterase activity in workers after over-exposure to dichlorvos. Occup Med (Lond) 50:343–7. [DOI] [PubMed] [Google Scholar]
- Meyer A, Koifman S, Koifman RJ, et al. (2010) Mood Disorders Hospitalizations, Suicide Attempts, and Suicide Mortality Among Agricultural Workers and Residents in an Area With Intensive Use of Pesticides in Brazil. J Toxicol Environ Heal Part A 73:866–877. doi: 10.1080/15287391003744781 [DOI] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, et al. (2013) Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A 110:3573–8. doi: 10.1073/pnas.1219731110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce J, Barnett R, Jones I (2007) Have urban/rural inequalities in suicide in New Zealand grown during the period 1980–2001? Soc Sci Med 65:1807–1819. doi: 10.1016/J.SOCSCIMED.2007.05.044 [DOI] [PubMed] [Google Scholar]
- Perou R, Bitsko RH, Blumberg SJ, et al. (2013) Mental health surveillance among children--United States, 2005–2011. MMWR Surveill Summ 62 Suppl 2:1–35. [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Tate CA, et al. (2003) Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Env Heal Perspect 111:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, et al. (2011) Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect 119:1196–201. doi: 10.1289/ehp.1003160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera F, et al. (2006) Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 118:e1845–59. doi: 10.1542/peds.2006-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CF, Butters MA, Lopez O, et al. (2011) Maintenance Treatment of Depression in Old Age. Arch Gen Psychiatry 68:51. doi: 10.1001/archgenpsychiatry.2010.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes ME, Rubin RT (1999) Functional sex differences (‘sexual diergism’) of central nervous system cholinergic systems, vasopressin, and hypothalamic-pituitary-adrenal. Brain Res Rev 30:135–152. doi: 10.1016/S0165-0173(99)00011-9 [DOI] [PubMed] [Google Scholar]
- Risch SC, Cohen RM, Janowsky DS, et al. (1981) Physostigmine induction of depressive symptomatology in normal human subjects. Psychiatry Res 4:89–94. [DOI] [PubMed] [Google Scholar]
- Rohlman DS, Ismail AA, Rasoul GA, et al. (2016) A 10-month prospective study of organophosphorus pesticide exposure and neurobehavioral performance among adolescents in Egypt. Cortex 74:383–395. doi: 10.1016/j.cortex.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowntree DW, Nevin S, Wilson A (1950) The effects of diisopropylfluorophosphonate in schizophrenia and manic depressive psychosis. J Neurol Neurosurg Psychiatry 13:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RT, Rhodes ME, O’Toole S, Czambel RK (2002) Sexual diergism of hypothalamo-pituitary-adrenal cortical responses to low-dose physotigmine in elderly vs. young women and men. Neuropsychopharmacology 26:672–81. doi: 10.1016/S0893-133X(01)00376-1 [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Lukas RJ, et al. (2002) Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry 7:525–35. doi: 10.1038/sj.mp.4001035 [DOI] [PubMed] [Google Scholar]
- Siqueira AA, Cunha AF, Marques GLM, et al. (2019) Atropine counteracts the depressive-like behaviour elicited by acute exposure to commercial chlorpyrifos in rats. Neurotoxicol Teratol 71:6–15. doi: 10.1016/j.ntt.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Slotkin T (2004) Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol 198:132–51. doi: 10.1016/j.taap.2003.06.001 [DOI] [PubMed] [Google Scholar]
- Sparks DW, Tian MK, Sargin D, et al. (2018) Opposing Cholinergic and Serotonergic Modulation of Layer 6 in Prefrontal Cortex. Front Neural Circuits 11:107. doi: 10.3389/fncir.2017.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, Sahgal A (1995) The role of serotonergic-cholinergic interactions in the mediation of cognitive behaviour. Behav Brain Res 67:165–99. doi: 10.1016/0166-4328(94)00157-b [DOI] [PubMed] [Google Scholar]
- Steinberg B, Trestman R, Mitropoulou V, et al. (1997) Depressive Response to Physostigmine Challenge in Borderline Personality Disorder Patients. Neuropsychopharmacology 17:264–273. doi: 10.1016/S0893-133X(97)00051-1 [DOI] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Butcher CR, Gahagan S, et al. (2017a) Acetylcholinesterase activity and time after a peak pesticide-use period among Ecuadorian children. Int Arch Occup Environ Health. doi: 10.1007/s00420-017-1265-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Checkoway H, Jacobs DR, et al. (2017b) Potential short-term neurobehavioral alterations in children associated with a peak pesticide spray season: The Mother’s Day flower harvest in Ecuador. Neurotoxicology. doi: 10.1016/j.neuro.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Himes JH, Jacobs DR, et al. (2013) Acetylcholinesterase activity and neurodevelopment in boys and girls. Pediatrics 132:e1649–e1658. doi: 10.1542/peds.2013-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Hood N, Suárez-Torres J, et al. (2019) Associations of acetylcholinesterase activity with depression and anxiety symptoms among adolescents growing up near pesticide spray sites. Int J Hyg Environ Health. doi: 10.1016/j.ijheh.2019.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Jacobs DR, Himes JH, et al. (2012) Lower acetylcholinesterase activity among children living with flower plantation workers. Environ Res 114:53–9. doi: 10.1016/j.envres.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gastel W, Ferdinand RF (2008) Screening capacity of the Multidimensional Anxiety Scale for Children (MASC) for DSM-IV anxiety disorders. Depress Anxiety 25:1046–52. doi: 10.1002/da.20452 [DOI] [PubMed] [Google Scholar]
- Wei C, Hoff A, Villabø MA, et al. (2013) Assessing Anxiety in Youth with the Multidimensional Anxiety Scale for Children. J Clin Child Adolesc Psychol. doi: 10.1080/15374416.2013.814541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Moisan F, Tzourio C, et al. (2013) Pesticide exposure and depression among agricultural workers in France. Am J Epidemiol 178:1051–8. doi: 10.1093/aje/kwt089 [DOI] [PubMed] [Google Scholar]
- Wesseling C, van Wendel de Joode B, Keifer M, et al. (2010) Symptoms of psychological distress and suicidal ideation among banana workers with a history of poisoning by organophosphate or n-methyl carbamate pesticides. Occup Environ Med 67:778–84. doi: 10.1136/oem.2009.047266 [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, et al. (2013) Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet (London, England) 382:1575–86. doi: 10.1016/S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2006) WHO Multicentre Growth Reference Study: 2. Methodology. [Google Scholar]
- Yang F-W, Li Y-X, Ren F-Z, et al. (2019) Assessment of the endocrine-disrupting effects of organophosphorus pesticide triazophos and its metabolites on endocrine hormones biosynthesis, transport and receptor binding in silico. 133:110759. doi: 10.1016/j.fct.2019.110759 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


