Abstract
Using data from rotavirus vaccine effectiveness (VE) studies, we assessed whether rotavirus season modifies rotavirus VE in infants. In the first year of life, adjusted VE was 72% for children born during rotavirus season and 84% for children born in other months (P = .01). Seasonal factors may interfere with vaccine performance.
Keywords: rotavirus disease, birth seasons, rotavirus vaccines, pooled analysis
Two safe and effective rotavirus vaccines (RotaTeq RV5, Merck Vaccines, and Rotarix RV1, GlaxoSmithKline Biologicals) are currently available; both are recommended for global use by the World Health Organization (WHO) [1]. In middle- and low-income countries where the burden of disease is greatest, there is a pattern of reduced rotavirus vaccine performance, demonstrated in both clinical trials and post-marketing studies, with efficacy ranging from 50% to 95% [2–4]. This is similar to previous experience with other enteric vaccines, including those for polio and cholera, which have reduced effectiveness in middle- and low-income countries [5–7]. A number of factors have been hypothesized to influence vaccine immunogenicity and protection, including nutritional status, preexisting maternal antibody levels, breastfeeding, concurrent use of oral polio vaccine (OPV), and coinfection with enteric pathogens [8, 9]. Nonetheless, hypotheses regarding which factors modify vaccine response remain speculative, since efficacy/effectiveness studies have been powered to estimate an overall vaccine effect, rather than differences within subgroups. Therefore, efforts to identify factors that could influence and, ultimately, improve the performance of vaccines are needed [10].
In temperate regions of the world, rotavirus diarrhea generally peaks during the cool, dryer months [11]. In some efficacy trials, rotavirus vaccine was given before the rotavirus season as a strategy to potentially maximize vaccine impact [12]. However, there is no clear evidence that protection from vaccine is associated with timing of birth in relation to rotavirus peak season. Here, our aim was to test the hypothesis that rotavirus vaccination confers differential protection depending on whether or not the child is born during the historic rotavirus season.
METHODS
The data for this analysis were combined from case-control studies conducted in 4 Latin American countries and an ongoing multisite, population-based surveillance network for new vaccines in the United States. These studies took place between 2007 and 2012, with study duration ranging from 1 to 5 years. The study population and methodology of the individual studies were described in detail in original study publications [13–17] (P. A. Gastanaduy, et al, unpublished data). Briefly, studies were conducted to assess the effectiveness of rotavirus vaccines for the prevention of an emergency department visit or hospital admission for rotavirus gastroenteritis among children. All studies were designed and conducted based on a generic protocol for monitoring the impact of rotavirus vaccination on the burden of gastroenteritis disease [18]. All studies assessed effectiveness using at least 1 of the following 3 control groups: children with rotavirus-negative diarrhea (enrolled in surveillance for rotavirus diarrhea, but tested negative for rotavirus), healthy controls (selected from neighborhood), or hospital-based controls (admitted to hospitals for conditions other than diarrhea). Controls were matched to case children by date of birth (±30 days). All control groups were combined and used in the current analysis.
Children were excluded from our pooled analysis if they (1) were aged <6 months, (2) had not completed the vaccination regimen (ie, full series of 2 doses of RV1 or 3 doses of RV5), and (3) had missing data on vaccination status or date of birth. As a result of these exclusions, the numbers reported in this analysis differ somewhat from those presented in original study-specific publications [13–17].
The primary exposure variables of interest were completion (yes/no) of vaccination regimen and month of birth. Other covariates included age and country of study. Our definition of a rotavirus season for each Latin American country was based on data from the WHO’s global surveillance network for rotavirus [19]. For the United States, the rotavirus season was defined based on data from the National Respiratory and Enteric Virus Surveillance System [20]. Months were considered as a part of the rotavirus season if more than 10% of stool specimens tested positive. Rotavirus season was defined as consecutive months between July and December in Bolivia, February and June in El Salvador, February and May in Guatemala, and January and April for Nicaragua and the United States. A child was considered to be born in rotavirus season if the month of birth was a month identified to be a part of rotavirus season as defined above.
All analyses were performed separately for children aged <12 months and ≥12 months. We broke the case-control matching and used ordinary logistic regression models to determine whether season of birth modified the effect of vaccination on rotavirus diarrhea. Independent variables in this model included age (in months), country of study, vaccination status, birth season, and an interaction term between vaccination status and birth season. The interaction term was examined for significance using the Wald test (the null hypothesis being that the interaction term in the regression model is not significantly different from zero). Additionally, separate models were fitted for each month of birth to explore the effect of individual months on vaccine effectiveness (VE). For this analysis, birth months were recoded in a running index from 1 to 12 representing July to June for Bolivia (Southern Hemisphere) and January to December for countries in the Northern Hemisphere.
RESULTS
The pooled data from the 5 studies resulted in a dataset of 10, 421 participants (1690 cases and 8731 controls). For each country, for infants aged <12 months, VE was lower for infants born in the rotavirus season, although the difference was not of statistical significance for any individual country.
In the pooled analysis, the effectiveness of rotavirus vaccine was significantly different based on a child’s birth season (Wald test, P = .01). Among infants aged <12 months, the adjusted VE was 72% (95% confidence interval [CI], 61, 80) for infants born in rotavirus season and 84% (95% CI, 78, 88) for infants born in other months (Table 1). However, in the second and subsequent years of age, there was no pattern of difference in VE between birth seasons (Wald test, P = .54). For children aged ≥12 months, the adjusted VE was 76% (95% CI, 70, 81) for children born in rotavirus season and 78% (95% CI, 73, 81) for those born in other months. When birth months were analyzed individually, we observed that VE was generally lower for children born from December to April (Figure 1). Exclusion of data from the United States resulted in a similar but statistically nonsignificant modification of the effect of vaccination by birth season in both age groups (data not shown).
Table 1.
Country-Specific and Pooled Rotavirus Vaccine Effectiveness by Season of Birth, Estimated From Multivariable Logistic Regression Models
| Countryc | Aged <12 mo | Aged ≥12 mo | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Born in Rotavirus Seasona | Number Vaccinated | VEb | [95%CI] | P Value | Number Vaccinated | VEb | [95%CI] | P Value | |||||||
| Cases | [%] | Controls | [%] | Cases | [%] | Controls | [%] | ||||||||
| Bolivia | Yes | 65/79 | 82 | 358/385 | 93 | 66 | [30,83] | .26 | 48/64 | 75 | 367/398 | 92 | 76 | [52,88] | .48 |
| No | 13/19 | 68 | 145/156 | 93 | 84 | [49,95] | 81/106 | 76 | 478/505 | 95 | 82 | [68,90] | |||
| El Salvador | Yes | 33/47 | 70 | 117/132 | 89 | 70 | [32,87] | .43 | 30/42 | 71 | 133/154 | 86 | 60 | [8,82] | .91 |
| No | 31/56 | 55 | 162/188 | 86 | 81 | [62,90] | 55/72 | 76 | 185/207 | 89 | 62 | [23,81] | |||
| Guatemala | Yes | 22/37 | 59 | 58/82 | 71 | 39 | [−38,73] | .09 | 16/38 | 42 | 113/196 | 58 | 53 | [4,77] | .74 |
| No | 9/33 | 27 | 101/162 | 62 | 77 | [48,90] | 33/92 | 36 | 180/345 | 52 | 59 | [33,75] | |||
| Nicaragua | Yes | 23/28 | 82 | 230/252 | 91 | 57 | [20, 81] | .88 | 40/45 | 89 | 275/307 | 90 | 10 | [−143,67] | .17 |
| No | 52/63 | 83 | 662/718 | 92 | 61 | [−25, 85] | 48/61 | 79 | 433/500 | 87 | 43 | [−12,71] | |||
| United States | Yes | 13/41 | 32 | 376/466 | 81 | 88 | [76,94] | .17 | 76/339 | 22 | 947/1547 | 60 | 84 | [77,89] | .71 |
| No | 13/75 | 17 | 434/566 | 77 | 94 | [88,97] | 73/353 | 21 | 886/1465 | 61 | 85 | [80,88] | |||
| Pooled | Yes | 156/232 | 67 | 1139/1317 | 86 | 72 | [61,80] | .01 | 210/528 | 40 | 1835/2602 | 71 | 76 | [70, 81] | .54 |
| No | 118/246 | 48 | 1504/1790 | 84 | 84 | [78,88] | 290/684 | 42 | 2162/3022 | 72 | 78 | [73, 81] | |||
Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
Months included in rotavirus season for each country. Bolivia, Jul–Dec; El Salvador, Feb–June; Guatemala, Feb–May; Nicaragua, Jan–Apr; United States, Jan–Apr.
VE was adjusted for age (as a continuous variable, in months) and country of study.
Vaccine type included in each country. Bolivia, RV1; El Salvador, RV1; Guatemala, RV1 or RV5; Nicaragua, RV5; United States, RV1 or RV5.
Figure 1.
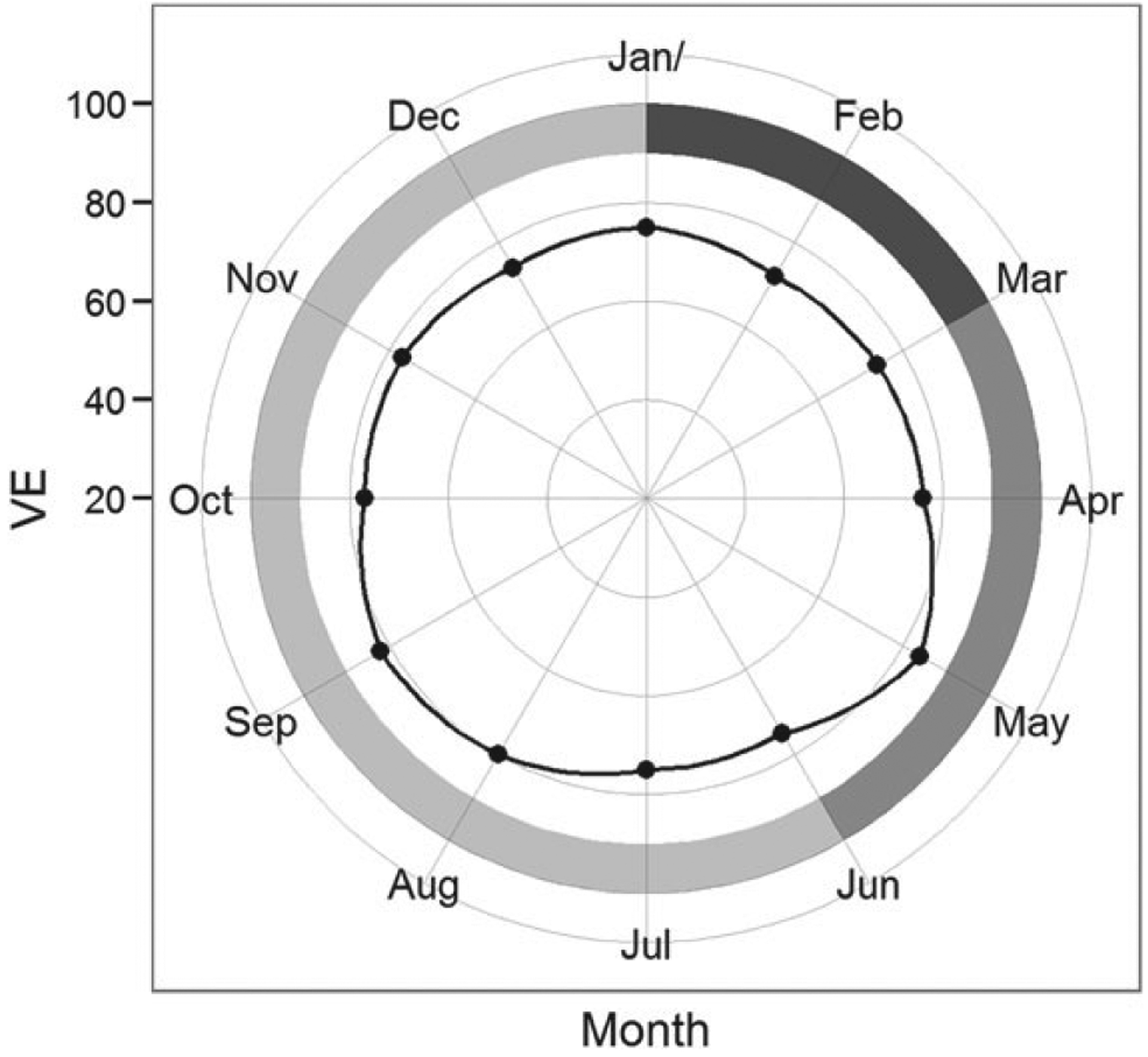
Effectiveness of rotavirus vaccines by month of birth for children born in Bolivia, El Salvador, Guatemala, Nicaragua, and the United States. For Bolivia, rotavirus season was defined as the period from July to June, so the calendar was shifted by 6 months to allow for comparison with other settings. Vaccine effectiveness (VE) was obtained from separate models for each month, adjusted for age (<12 months, ≥12 month) and country of study. VE (dots) was plotted with months arranged clockwise in a circle. The axis on the left shows the range of values for VEs. The shaded outer ring represents the averaged intensity of rotavirus seasonality (darker the shade, higher the intensity).
DISCUSSION
The findings of our pooled analysis suggest reduced protection from rotavirus vaccination for children born during the rotavirus season. Specifically, this effect of birth season was restricted to the first year of life, where we noted that VE was 12 percentage points lower for those born during months with greater rotavirus activity. The finding of a consistent pattern of lower VE for children born during the rotavirus season for each country supports the finding that birth season is a real effect. However, this effect was not significant in any single country, as none of the individual studies were powered to detect a smaller VE difference, which highlights the value of a pooled analysis to address this issue.
While we identified a seasonal variation in vaccine performance, our findings did not identify specific factors, of which there are a few that vary seasonally and that could underpin the observed patterns. First, infections with multiple enteric pathogens are very common in many developing settings [21–23]. Infection with other enteric pathogens at the time of vaccination could potentially interfere and impair response to vaccine [24]. If enteric pathogens do interfere with vaccine take, these data suggest that bacterial enteric pathogens, which have a higher prevalence during the nonrotavirus season, could be less likely to interfere with vaccine take than enteric viruses (eg, noroviruses), which tend to cocirculate during the rotavirus season. A second possibility relates to the effects of maternal antibodies. Maternal antibody levels are likely to be much higher during the rotavirus season; these passively acquired antibodies, which are transferred from mother to child either transplacentally or through breastfeeding, correlate with rotavirus vaccine immune response [25]. If a mother is exposed to natural rotavirus infection shortly prior to giving birth or while breastfeeding, she may transfer higher levels of antibodies that may, in turn, neutralize vaccine and decrease the effectiveness of rotavirus vaccine for a child born during months with higher rotavirus activity [26]. In theory, an analysis based on timing of vaccination, as opposed to season of birth, might differentiate between these 2 hypotheses. However, because these 2 events are so highly correlated, it was not possible to distinguish the 2 mechanisms in our data. Third, the time to first natural rotavirus exposure may vary depending on season of birth. Children born and vaccinated in or just after the rotavirus season may not have their first exposure to natural virus for nearly 1 year following vaccination, while children born during the “low season” may be exposed shortly after being vaccinated. If vaccine protection wanes, which some studies from developing countries suggest, children who are vaccinated closest to the rotavirus season may have the best protection. Also, most children will have been vaccinated and exposed to natural infection by their second year of life. Therefore, by that age, children who did not mount a robust response to vaccination may have had higher rates of rotavirus gastroenteritis (as suggested by our analysis) and therefore “caught up” immunologically with children who became protected from vaccine. Finally, simultaneous administration of OPV may interfere with response to the first dose of rotavirus vaccine but not the complete 2- or 3-dose course. However, OPV is administered routinely year round and so is unlikely to contribute to seasonal variation in rotavirus vaccine response.
A few limitations of our study must be acknowledged. First, we included only children aged ≥6 months and so did not know about their early life exposures. Therefore, we were unable to discriminate between a mechanism of waning immunity and differential natural exposure histories. Second, given the small number of studies included, we were unable to perform more detailed subgroup analyses, such as the role of specific strains and vaccine type in the association between VE and birth season. Finally, we combined datasets, accepting that there is heterogeneity between individual studies in terms of control recruitment, vaccine type, and local epidemiology. These issues should be considered when interpreting the results of pooled analyses.
In summary, our study adds to the evidence of variability of rotavirus vaccine performance and suggests that season of birth may influence VE. We did not, however, identify what specific causal factor associated with births during rotavirus season reduces VE. Future investigations should aim to determine what these factors are, as they could lead to strategies that would improve VE. Despite the apparent influence of birth season on effectiveness of rotavirus vaccine between age 6 months and 1 year, these and the overall body of data strongly support the public health importance of rotavirus vaccine to control severe pediatric gastroenteritis.
Footnotes
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rotavirus vaccines WHO position paper: January 2013—Recommendations. Vaccine 2013; 31:6170–1. [DOI] [PubMed] [Google Scholar]
- 2.Linhares AC, Velázquez FR, Pérez-Schael I, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet 2008; 371:1181–9. [DOI] [PubMed] [Google Scholar]
- 3.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606–14. [DOI] [PubMed] [Google Scholar]
- 4.Breiman RF, Zaman K, Armah G, et al. Analyses of health outcomes from the 5 sites participating in the Africa and Asia clinical efficacy trials of the oral pentavalent rotavirus vaccine. Vaccine 2012; 30(suppl 1):A24–9. [DOI] [PubMed] [Google Scholar]
- 5.John TJ. Problems with oral poliovaccine in India. Indian Pediatr 1972; 9:252–6. [PubMed] [Google Scholar]
- 6.Grassly NC, Jafari H, Bahl S, et al. Waning intestinal immunity after vaccination with oral poliovirus vaccines in India. J Infect Dis 2012; 205:1554–61. [DOI] [PubMed] [Google Scholar]
- 7.Lagos R, Fasano A, Wasserman SS, et al. Effect of small bowel bacterial overgrowth on the immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR. J Infect Dis 1999; 180:1709–12. [DOI] [PubMed] [Google Scholar]
- 8.Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis 2009; 200(suppl 1):S39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang V, Jiang B, Tate J, Parashar UD, Patel MM. Performance of rotavirus vaccines in developed and developing countries. Hum Vaccin 2010; 6:532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tate JE, Patel MM, Cortese MM, et al. Remaining issues and challenges for rotavirus vaccine in preventing global childhood diarrheal morbidity and mortality. Expert Rev Vaccines 2012; 11:211–20. [DOI] [PubMed] [Google Scholar]
- 11.Patel MM, Pitzer VE, Alonso WJ, et al. Global seasonality of rotavirus disease. Pediatr Infect Dis J 2013; 32:e134–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vesikari T, Joensuu J. Review of rotavirus vaccine trials in Finland. J Infect Dis 1996; 174(suppl 1):S81–7. [DOI] [PubMed] [Google Scholar]
- 13.Patel MM, Patzi M, Pastor D, et al. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ 2013; 346:f3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Palma O, Cruz L, Ramos H, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ 2010; 340:c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel M, Pedreira C, De Oliveira LH, et al. Duration of protection of pentavalent rotavirus vaccination in Nicaragua. Pediatrics 2012; 130: e365–72. [DOI] [PubMed] [Google Scholar]
- 16.Payne DC, Boom JA, Staat MA, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age, 2009–2011. Clin Infect Dis 2013; 57:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staat MA, Payne DC, Donauer S, et al. Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics 2011; 128:e267–75. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Generic Protocol for Monitoring Impact of Rotavirus Vaccination on Gastroenteritis Disease Burden and Viral Strains. Available at: http://whqlibdoc.who.int/hq/2008/WHO_IVB_08.16_eng.pdf. Accessed 10 January 2014.
- 19.World Health Organization. Global Rotavirus Information and Surveillance Bulletin Available at: www.who.int/immunization/diseases/rotavirus/rota_info_surv_bulletin/en/index.html. Accessed 10 January 2014.
- 20.Centers for Disease Control and Prevention. The National Respiratory and Enteric Virus Surveillance System (NREVSS). Available at: www.cdc.gov/surveillance/nrevss/rotavirus/default.html. Accessed 10 January 2014.
- 21.Albert MJ, Faruque ASG, Faruque SM, Sack RB, Mahalanabis D. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J Clin Microbiol 1999; 37:3458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodhidatta L, McDaniel P, Sornsakrin S, Srijan A, Serichantalergs O, Mason CJ. Case-control study of diarrheal disease etiology in a remote rural area in western Thailand. Am J Trop Med Hyg 2010; 83:1106–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urbina D, Arzuza O, Young G, Parra E, Castro R, Puello M. Rotavirus type A and other enteric pathogens in stool samples from children with acute diarrhea on the Colombian northern coast. Int Microbiol 2003; 6:27–32. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Moon S, Wang Y, Jiang B. Multiple virus infection alters rotavirus replication and expression of cytokines and Toll-like receptors in intestinal epithelial cells. Virus Res 2012; 167:48–55. [DOI] [PubMed] [Google Scholar]
- 25.McLean B, Holmes IH. Transfer of antirotaviral antibodies from mothers to their infants. J Clin Microbiol 1980; 12:320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan J, Nirwati H, Triasih R, et al. Maternal antibodies to rotavirus: could they interfere with live rotavirus vaccines in developing countries? Vaccine 2011; 29:1242–7. [DOI] [PubMed] [Google Scholar]


