Abstract
Background:
Risk factors for post-operative conduction disturbances after cardiac valve surgery requiring a permanent pacemaker (PPM) are poorly characterized.
Objectives:
We sought to investigate the timing and risk factors for PPM implantation after mitral or aortic valve surgery.
Methods:
We reviewed all patients who underwent an open aortic or mitral valve surgery between January 1996 and December 2014 using New York’s State mandatory hospital discharge database. Patients with prior cardiac surgery or preexisting PPM were excluded. The primary endpoint was PPM implantation within 1 year.
Results:
Among 77,882 patients, 63.8% (n=49,706) had an aortic valve replacement (AVR), 18.9% (n=14,686) a mitral valve replacement (MVR), 10.5% (n=8,219) a mitral valve repair (MVr), 5.4% (n=4,202) an AVR plus MVR, and 1.4% (n=1,069) an AVR plus MVr. One-year PPM implantation was 4.5% after MVr, 6.6% after AVR, 9.3% after AVR plus MVr, 10.5% after MVR, and 13.3% after AVR plus MVR (p<0.001). Across all groups, the majority of PPM were implanted during index hospitalization (79.9%). MVr was associated with the lowest risk of PPM while AVR plus MVR with the highest risk. Older age, history of arrhythmias, pre-operative conduction disturbances and concomitant index procedures were associated with increased risk of PPM during the index hospitalization. Conversely, beyond 30 days, chronic comorbidities were associated with increased risk for PPM.
Conclusions:
Conduction disturbances requiring PPM remains a common adverse event after valve surgery. Identifying patients at risk for PPM will help facilitate perioperative planning and inform clinical decision making regarding post-operative rhythm surveillance.
Keywords: Aortic Valve, Mitral Valve, Permanent Pacemaker, Conduction disorders, Coronary artery by-pass grafting, Surgical ablation procedure
Condensed Abstract
Risk factors for conduction disturbances that will require a permanent pacemaker (PPM) after cardiac valve surgery are poorly characterized. We reviewed the experience of all open aortic or mitral valve procedures between January 1996 and December 2014 using New York’s State mandatory hospital discharge database (SPARCS). Older age, history of arrhythmias, pre-operative conduction disturbances and concomitant procedures with the index valve surgery were associated with increased risk of PPM during index hospitalization. Beyond 30 days, several chronic comorbidities were associated with increased risk. Identifying patients at risk for PPM will help facilitate perioperative planning and inform post-operative rhythm surveillance.
INTRODUCTION
Post-operative conduction disorders after cardiac surgery that require a permanent pacemaker (PPM) are associated with increased morbidity, in-hospital length of stay, and cost (1, 2). The risk of PPM implantation post-cardiac valve surgery stems from the proximity of the aortic and mitral valve apparatus to the sinoatrial node, atrioventricular node and His bundle, where trauma or ischemic injury of the conduction system may occur (2, 3). The frequency of PPM following cardiac surgery has been reported to occur in 0.8% to 34% of surgeries, and is greater after valve surgery than isolated coronary artery bypass graft (CABG) surgery (1–7). The variability in reported rates reflects the heterogeneity in populations: in particular, differences in surgical procedures (i.e., valve location, repair versus replacement and concomitant procedure) as well as comorbid conditions and preoperative conduction disorders. This heterogeneity in reporting has made it difficult to set standards for the optimal timing for implanting a PPM after cardiac surgery. The guidelines of the American College of Cardiology (ACC), American Heart Association (AHA) and Heart Rhythm Society (HRS) recommend PPM placement if the AV block is not expected to resolve after cardiac surgery [8]. The European Society of Cardiology recommends 7 days of observation prior to PPM placement for AV block following cardiac surgery and 5 days up to several weeks for sino-atrial dysfunction after cardiac surgery (8, 9). Additionally, bradycardia and cardiac conduction delay guidelines, provided by the ACC, AHA, and HRS, recommend implanting a PPM prior to discharge for patients who have new postoperative sinus node dysfunction or AV block associated with persistent symptoms or hemodynamic instability that does not resolve after either an aortic valve replacement or mitral valve repair or replacement (10). As a result, differences in operator experience and center-specific practices contribute to different rates of device implantation as well as to heterogeneity in timing of PPM implantation.
Preoperative patient characteristics, including comorbidities, presence of underlying conduction disturbances, and demographic information, can help delineate the risk of requiring a PPM following cardiac surgery. Both the type of valve surgery and additional concomitant procedures have already been identified as determinants for PPM implantation (1–7). As more data become available regarding the long-term morbidity associated with PPMs, a better understanding of the risk factors for postoperative PPM implantation may help guide perioperative management strategies and inform postoperative follow-up. This large-scale study sought to characterize the rates of PPM implantation following mitral and aortic valve surgery and to identify preoperative risk factors associated with increased risk for PPM implantation.
METHODS
Study Design.
We conducted a retrospective cohort study to determine the implantation rates and risk factors for PPM within 1 year after index hospitalization for mitral valve and aortic valve procedures, including aortic valve replacement (AVR), mitral valve replacement (MVR) and mitral valve repair (MVr), with any concomitant cardiac surgery procedure. Using New York’s Statewide Planning and Research Cooperative System (SPARCS), a database of hospital discharge data, mandated for all hospitalizations that occur in the state of NY and linked with New York state vital statistics death records, we identified individuals who had underwent mitral or an aortic valve surgery between January 1st, 1996, and December 31st, 2014. The date of last known date of death was December 31st, 2015. We included all hospitalizations for individuals who underwent mitral valve or aortic valve surgery as the primary procedure. For each type of primary valve procedure we then stratified by the concomitant cardiac procedure that was performed (n=120,375) (Supplemental Figure 1). Individuals were excluded for the following: prior open-heart surgery, prior pacemaker or defibrillator implantation, an implantation of a defibrillator during or post index hospitalization, ventricular septal defect repair, congenital heart disease, less than 18 years of age at the time of procedure, not a resident of NY state, and post-operative length of stay exceeding one year (Supplemental Table 1). The resultant study population includes 77,882 unique individuals.
Surgical procedures were identified using International Classification of Diseases, Ninth revision, Clinical Modification (ICD-9-CM) procedure codes. The index surgical procedures of interest were AVR, MVR and MVr. Concomitant surgical procedures that were analyzed included: CABG, surgical ablation procedures, and tricuspid valve surgery. We searched through inpatient and ambulatory visits for ICD-9 diagnosis and procedure codes, along with Healthcare Common Procedure Coding System codes (HCPCS), to identify whether a PPM or defibrellator was implanted prior to the index hospitalization (defined as hospitalization for valve surgery), at the time of the index hospitalization, or after discharge. Comorbid conditions were defined by looking at diagnosis or procedure codes in episodes of care prior to the index hospitalization. A minimum of two years of data prior to the index hospitalization were reviewed to ensure the capture of preoperative comorbid conditions. Stroke, defined as intracerebral hemorrhage and/or ischemic events, were identified by ICD-9-CM codes. We did not include transient ischemic attack in this definition. Each patient had a unique identifier that facilitated longitudinal analysis. ICD 9-CM codes used to define comorbidities and outcomes in this study are listed in Supplemental Table 2. Death was identified from: (1) the New York State’s vital death record that was linked to the SPARCS dataset by New York’s department of health, and (2) by the discharge disposition reported for the index procedure, subsequent hospitalizations or outpatient visit via SPARCS. This study was approved by the Program for Protection of Human Subjects of the Icahn School of Medicine at Mount Sinai and the New York State Department of Health data protection review board. These approvals included a waiver of informed consent.
Study Endpoints.
The primary endpoint of the study was PPM implantation within 1 year after valve surgery. Additionally the time of PPM implantation was analyzed during three-time intervals: index hospitalization, index hospitalization discharge to 30 days post-discharge, and 31 days to 1 year post-discharge. Rates of death and stroke were reported over 1 year by type of valve surgery.
Statistical Analysis.
Baseline characteristics are denoted as proportions for categorical variables and means with standard deviation for normally distributed continuous variables. Differences in baseline characteristics between treatment groups were evaluated using analysis of variance for continuous variables and Pearson’s χ² test for categorical variables. Device implantation within one year was analyzed using the Fine and Gray method and Cox proportional hazards regression modelling where death was treated as a competing event. Three multivariable models were created, one for each of the 3 time period (index hospitalization, index discharge to 30 days, and 31 days to 1 year), and each model was adjusted for age, sex, ethnicity, race, type of admission, type of insurance, length of stay (only for models of the 2nd and 3rd time periods), baseline comorbidities (endocarditis, hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, peripheral artery disease, cerebrovascular disease, pulmonary vascular disease, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease with and without dialysis, high degree heart block, fascicular block, atrial fibrillation and flutter, ventricular arrhythmia, sino-atrial node dysfunction, supraventricular tachycardia, history of other conduction disorder, cardiac arrest, cardioversion, and history of catheter ablation), and concomitant procedures, which included procedures to the aorta, during the index hospitalization. All adverse events that occurred during the index hospitalization were included as baseline covariates for the multivariable models for the latter 2 time periods (hospital discharge to 30 days, and 31 days to 1 year). The proportional hazard assumption was tested on all the variables in the models, if violated, a time interaction term was included. All tests were two-tailed and considered statistically significant when the α level was 0.05 or less. Per the data use agreement with SPARCS, variables with counts less than 11 are demarcated with the symbol * rather than with an actual value. All statistical analyses were performed on SAS version 9.4 (SAS Institute, Cary, NC). All trend analyses were performed using JoinPoint software, developed by the National Cancer Institute, which was used to analyze trends over time and to calculate annual percent change (APC).
RESULTS
Study Population.
The study population flow diagram is illustrated in Supplemental Figure 1. A total of 120,375 records of adult individuals were identified through SPARCS. Following exclusion of patients who met pre-specified exclusion criteria (N=42,493), a total of 77,882 patients who underwent mitral valve or aortic valve surgery in New York state between 1996 and 2014 were available for our analysis. Of these, 63.8% (n=49,706) had an AVR, 18.9% (n=14,686) a MVR, 10.5% (n=8,219) a MVr, 5.4% (n=4,202) an AVR plus MVR, and 1.4% (n=1,069) an AVR plus MVr.
Patient and Procedural Characteristics.
Baseline patient characteristics across study groups are reported in Table 1. The average age across groups ranged from 62.7±13.6 years in the MVr group to 70.6±12.4 years in the AVR group (p<0.001). Most individuals included in the study were male, with the exception of the MVR and AVR plus MVR groups where female constituted 60.2% and 57.0% of the groups respectively (p<0.001). Patients were predominantly white, ranging from 62.0% in the MVR population to 74.6% in the AVR population (p<0.001). Isolated valve procedures accounted for more than 50% of the procedures. CABG was the most common concomitant procedure, followed in frequency by surgical ablation, tricuspid valve surgery, and combined surgical ablation and CABG surgery. Concomitant aortic surgery was performed in 8.5% of AVR procedures.
Table 1.
Baseline clinical characteristics.
| Aortic Surgery | Mitral Surgery | Aortic and Mitral Surgery | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Replacement | Repair | Replacement | Both Replacement | Replace and or Repair | |||||||
| % | n | % | n | % | n | % | n | % | n | ||
| % of Total Population | 100 | 49706 | 100 | 8219 | 100 | 14686 | 100 | 4202 | 100 | 1069 | |
| Age (Mean (SD)) | 70.62(12.45) | 62.68(13.63) | 64.92(13.88) | 66.27(14.12) | 70.13(13.12) | <0.001 | |||||
| Sex | <0.001 | ||||||||||
| Female | 42.02 | 20884 | 41.74 | 3431 | 60.23 | 8845 | 57.04 | 2397 | 38.26 | 409 | |
| Male | 57.98 | 28822 | 58.26 | 4788 | 39.77 | 5841 | 42.96 | 1805 | 61.74 | 660 | |
| Race/Ethnicity | <0.001 | ||||||||||
| White Non-Hispanic | 74.61 | 37088 | 68.24 | 5609 | 62.04 | 9111 | 63.07 | 2650 | 69.50 | 743 | |
| Black Non-Hispanic | 4.51 | 2241 | 6.90 | 567 | 9.68 | 1422 | 9.33 | 392 | 7.02 | 75 | |
| Hispanic | 5.13 | 2552 | 5.55 | 456 | 7.12 | 1045 | 7.38 | 310 | 6.64 | 71 | |
| Other/Unknown | 15.74 | 7825 | 19.31 | 1587 | 21.16 | 3108 | 20.23 | 850 | 16.84 | 180 | |
| Type of Admission | |||||||||||
| Emergent/Urgent | 43.08 | 21411 | 39.77 | 3269 | 51.57 | 7574 | 50.26 | 2112 | 49.86 | 533 | <0.001 |
| Type of Insurance | <0.001 | ||||||||||
| Private | 20.66 | 10267 | 40.56 | 3334 | 25.09 | 3684 | 20.94 | 880 | 18.90 | 202 | |
| Medicaid | 11.92 | 5925 | 14.37 | 1181 | 22.54 | 3310 | 21.99 | 924 | 14.03 | 150 | |
| Medicare | 65.71 | 32664 | 42.34 | 3480 | 49.58 | 7281 | 54.02 | 2270 | 64.55 | 690 | |
| Other | 0.86 | 425 | 1.42 | 117 | 1.13 | 166 | 1.31 | 55 | 1.59 | 17 | |
| Uninsured | 0.86 | 425 | 1.30 | 107 | 1.67 | 245 | 1.74 | 73 | * | * | |
| Comorbidities | |||||||||||
| Endocarditis | 2.09 | 1040 | 3.95 | 275 | 6.07 | 891 | 6.54 | 275 | 5.99 | 64 | <0.001 |
| Hypertension | 76.63 | 38089 | 63.09 | 5185 | 63.50 | 9325 | 64.92 | 2728 | 73.9 | 790 | <0.001 |
| Diabetes Mellitus | 28.22 | 14027 | 17.70 | 1455 | 22.55 | 3312 | 23.32 | 980 | 23.95 | 256 | <0.001 |
| Dyslipidemia | 54.02 | 26851 | 43.20 | 3551 | 35.56 | 5222 | 37.96 | 1595 | 50.89 | 544 | <0.001 |
| Coronary Artery Disease | 68.45 | 34024 | 50.57 | 4156 | 55.63 | 8170 | 55.69 | 2340 | 65.67 | 702 | <0.001 |
| Peripheral Artery Disease | 27.19 | 13513 | 10.33 | 849 | 12.81 | 1881 | 16.83 | 707 | 24.6 | 263 | <0.001 |
| Cerebrovascular Disease | 7.30 | 3629 | 5.95 | 489 | 9.06 | 1331 | 9.45 | 397 | 9.54 | 102 | <0.001 |
| Pulmonary Vascular Disease | 2.72 | 1353 | 3.56 | 293 | 8.97 | 1318 | 8.61 | 362 | 4.49 | 48 | <0.001 |
| Congestive Heart Failure | 39.88 | 19821 | 45.09 | 3706 | 60.19 | 8840 | 64.54 | 2712 | 56.03 | 599 | <0.001 |
| Chronic Obstructive Pulmonary Disease | 23.84 | 11850 | 19.75 | 1623 | 24.98 | 3669 | 26.63 | 1119 | 23.29 | 249 | <0.001 |
| Chronic Kidney Disease (Non-dialysis-dependent) | 10.41 | 5173 | 7.97 | 655 | 10.36 | 1521 | 11.49 | 483 | 14.22 | 152 | <0.001 |
| Chronic Kidney Disease (Dialysis-dependent) | 0.62 | 309 | 0.66 | 54 | 0.79 | 116 | 1.26 | 53 | 1.22 | 13 | |
| High Degree Heart Block | 0.16 | 78 | 0.15 | 12 | 0.14 | 20 | * | * | * | * | 0.84 |
| Fascicular Block | 5.49 | 2730 | 3.03 | 249 | 3.68 | 540 | 5.07 | 213 | 6.36 | 68 | <0.001 |
| Atrial Fibrillation/Flutter | 23.26 | 11560 | 30.83 | 2534 | 46.24 | 6791 | 45.81 | 1925 | 34.52 | 369 | <0.001 |
| Supraventricular Tachycardia | 1.48 | 734 | 1.76 | 145 | 1.83 | 273 | 2.09 | 88 | 2.06 | 22 | <0.001 |
| SA Node Dysfunction | 1.22 | 604 | 1.40 | 115 | 1.89 | 278 | 1.83 | 77 | 2.71 | 29 | <0.001 |
| Ventricular Arrhythmia | 2.79 | 1385 | 3.94 | 324 | 4.00 | 587 | 4.07 | 171 | 4.68 | 50 | <0.001 |
| Other Conduction Disorders | 3.48 | 1728 | 3.16 | 260 | 3.83 | 563 | 4.90 | 206 | 4.68 | 50 | <0.001 |
| Cardiac Arrest | 0.41 | 202 | 0.44 | 36 | 0.56 | 82 | 0.43 | 18 | * | * | 0.13 |
| Prior Procedures | |||||||||||
| Cardioversion | 0.66 | 327 | 1.3 | 107 | 2.14 | 315 | 1.59 | 67 | 1.96 | 21 | <0.001 |
| Ablation | 0.44 | 220 | 0.77 | 63 | 0.55 | 81 | 0.57 | 24 | * | * | 0.002 |
| Concomitant Procedures | |||||||||||
| CABG | 41.23 | 20492 | 27.84 | 2288 | 31.59 | 4639 | 24.96 | 1049 | 33.49 | 358 | <0.001 |
| Surgical ablation | 1.94 | 965 | 10.71 | 880 | 8.74 | 1284 | 6.88 | 289 | 7.02 | 75 | <0.001 |
| TVS | 3.69 | 333 | 3.42 | 562 | 0.25 | ||||||
| Surgical ablation plus CABG | 2.55 | 230 | 1.95 | 321 | 0.002 | ||||||
Results reported as n (%) or mean ± standard deviation for categorical and continuous variables, respectively. SA: Sino-atrial.
High degree heart block is defined as patients with trifascicular block and mobitz type II atrioventricular block.
Other conductions disorder is defined as patients with AV block (1st or 2nd degree and unspecified), other heart block, other specified and unspecified conduction disorders
denotes redacted rates as per the data use agreement from SPARCS
Rates of PPM Implantation and Adverse Events.
Rates of PPM implantation were evaluated at index hospitalization, cumulatively at 30 days, and cumulatively at 1 year (Table 2 and Table 3; median and interquartile range for PPM implantation can be found in Supplemental Table 3). Overall, the majority of the PPM implantations occurred during the index hospitalization (80.0%, 4,684/5,856). Rates of PPM implantation by type of valve surgery are shown in Table 2. PPM implantation rates during the index hospitalization were 5.2% after AVR, 3.6% after MVr, 8.6% after MVR, 11.3% after AVR plus MVR and 7.7% after AVR plus MVr (p<0.001). At 1 year, rates of PPM implantation were 6.6% after AVR, 4.5% after MVr, 10.5% after MVR, 13.3% after AVR plus MVR, and 9.3% after AVR plus MVr (p<0.001). Table 3 and Figure 1 depict one year crude rates of PPM implantation stratified by index valve surgery and concomitant procedure. Concomitant CABG, surgical ablation and tricuspid valve procedures were associated with an increase in the risk of PPM implantation during aortic and mitral procedures alone and in combination. During index hospitalization, mortality rates were highest in the AVR plus MVR group (8.0%) and strokes rates were highest in the AVR plus MVr group (3.3%); while the lowest mortality and stroke rates were observed in the MVr group (2.4% and 1.5%, respectively). At 1 year, patients who underwent AVR plus MVR had the highest all-cause mortality (16.7, p<0.001), while patients who underwent MVr had the lowest rates of stroke (2.4, p<0.001). From 1996 to 2014, MVR and AVR plus MVR showed an increasing trend in PPM implantation during index hospitalization as well as from 31 days to 1 year post index hospitalization (APC=2.2, p<0.001, APC=2.7, p=0.002; and APC=2.3, p<0.001, APC=2.8, p<0.001; respectively). AVR showed an increasing trend only during 31 days to 1 year (APC=0.9, p=0.02). Trends in PPM implantation are represented in Supplemental Figures 2A to 2E and 3A to 3E.
Table 2.
Cumulative rates permanent pacemaker implantation, mortality and stroke within 1 year by type of valve surgery.
| Aortic Surgery | Mitral Surgery | Aortic and Mitral Surgery | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Replacement | Repair | Replacement | Both Replacement | Replace and Repair | |||||||
| % | n=52163 | % | n=9015 | % | n=16455 | % | n=4602 | % | n=1149 | ||
| Device implantation | |||||||||||
| Index Hospitalization | 5.2 | 2569 | 3.6 | 293 | 8.6 | 1266 | 11.3 | 474 | 7.7 | 82 | <0.001 |
| 30 Days | 5.6 | 2791 | 3.9 | 319 | 9.2 | 1350 | 11.9 | 499 | 8.1 | 87 | <0.001 |
| 1 Year | 6.6 | 3293 | 4.5 | 371 | 10.5 | 1534 | 13.3 | 559 | 9.3 | 99 | <0.001 |
| Mortality | |||||||||||
| Death at Index Hospitalization | 3.2 | 1591 | 2.4 | 196 | 6.0 | 887 | 8.0 | 335 | 6.2 | 66 | <0.001 |
| 30 Day | 4.3 | 2150 | 3.1 | 256 | 7.4 | 1087 | 9.7 | 408 | 7.9 | 84 | <0.001 |
| 1 Year | 8.3 | 4117 | 5.7 | 466 | 12.8 | 1877 | 16.7 | 703 | 14.4 | 154 | <0.001 |
| Stroke | |||||||||||
| Index Hospitalization | 1. 9 | 931 | 1. 5 | 125 | 2. 8 | 404 | 2. 9 | 123 | 3. 3 | 35 | <0.001 |
| 30 Day | 2.1 | 1059 | 1.7 | 143 | 3.0 | 439 | 3.1 | 129 | 3.6 | 38 | <0.001 |
| 1 Year | 2.7 | 1336 | 2.4 | 196 | 3.9 | 570 | 3.8 | 160 | 4.1 | 44 | <0.001 |
Table 3.
Cumulative rates of permanent pacemaker implantation by type of valve surgery and concomitant procedure.
| Procedure | n | Index Procedure % (n) | 30 Days % (n) | 1 Year % (n) | Percent of PPM Implanted During Index Hospitalization |
|---|---|---|---|---|---|
| Isolated aortic valve replacement | 28249 | 4.9(1372) | 5.2(1461) | 6.2(1756) | 77.8 |
| Aortic valve replacement plus CABG | 20492 | 5.3(1083) | 5.6(1148) | 6.8(1389) | 77.8 |
| Aortic valve replacement plus surgical ablation | 965 | 11.8(114) | 12.0(116) | 14.2(137) | 82.6 |
| Isolated mitral valve repair | 4952 | 2.8(139) | 3.0(148) | 3.5(174) | 79.4 |
| Mitral valve repair plus CABG | 2083 | 4.2(88) | 4.5(93) | 5.4(112) | 78.6 |
| Mitral valve repair plus surgical ablation | 675 | 4.7(32) | 5.6(38) | 6.2(42) | 76.2 |
| Mitral valve repair plus TVS | 304 | 7.2(22) | 7.2(22) | 7.9(24) | 91.7 |
| Mitral valve repair plus surgical ablation plus CABG | 205 | 5.9(12) | 6.8(14) | 8.8(18) | 66.7 |
| Isolated mitral valve replacement | 8535 | 7.2(614) | 7.6(645) | 8.9(756) | 81.0 |
| Mitral valve replacement plus CABG | 4359 | 9.3(405) | 9.5(415) | 11.3(492) | 82.3 |
| Mitral valve replacement plus surgical ablation | 1004 | 12.4(124) | 12.8(129) | 14.3(144) | 86.1 |
| Mitral valve replacement plus TVS | 508 | 16.9(86) | 17.7(90) | 19.7(100) | 86.0 |
| Mitral valve replacement plus surgical ablation plus CABG | 280 | 13.2(37) | 13.2(37) | 14.3(40) | 92.5 |
| Aortic valve replacement plus mitral valve replacement | 2864 | 9.9(285) | 10.3(295) | 11.9(342) | 83.1 |
| Aortic valve replacement plus mitral valve replacement plus CABG | 1049 | 13.7(144) | 14.4(151) | 15.8(166) | 86.7 |
| Aortic valve replacement plus mitral valve replacement plus surgical ablation | 289 | 15.6(45) | 15.9(46) | 17.3(50) | 90.0 |
| Aortic valve replacement plus isolated mitral valve repair | 636 | 6.0(38) | 6.1(39) | 7.4(47) | 80.9 |
| Aortic valve replacement plus mitral valve repair plus CABG | 358 | 8.7(31) | 8.9(32) | 10.6(38) | 81.6 |
| Aortic valve replacement plus mitral valve repair plus surgical ablation | 75 | 17.3(13) | 17.3(13) | 18.7(14) | 92.9 |
- Rates are reported as Kaplan-Meier estimates for the 30 days and 1 year time periods
Figure 1. One-year cumulative incidence of permanent pacemaker by valve surgery.
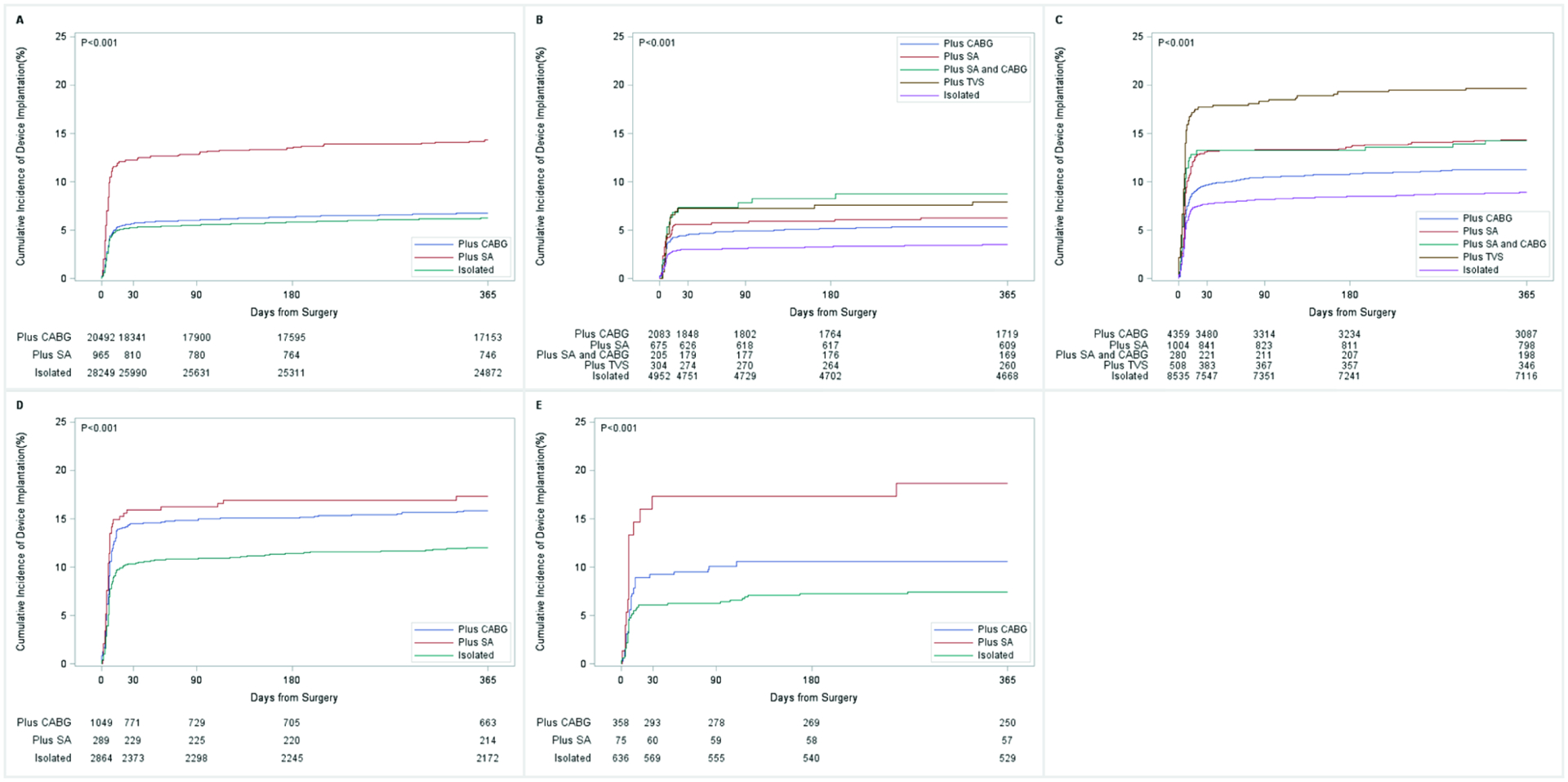
1A: aortic valve replacement; 1B: mitral valve repair; 1C: mitral valve replacement; 1D: aortic and mitral valve surgery; 1E: aortic valve replacement and mitral valve repair. These cumulative rates were derived using Fine and Gray’s competing risk model with time from index surgery to device placement as the time-dependent variable and death as the competing risk event. Concomitant CABG, surgical ablation and tricuspid valve procedures were associated with an increase in the risk of PPM implantation during aortic and mitral procedures alone and in combination. ISO: Isolated procedure; CABG: coronary artery bypass grafting; SA: Surgical Ablation procedure; SA+CABG: Surgical Ablation procedure and coronary artery bypass grafting; TVS: tricuspid valve surgery.
Risk Factors of PPM Implantation during the Index Hospitalization.
Figure 2 depicts the factors associated with an increased risk of PPM implantation in the overall population during the index hospitalization. Overall, older age, baseline conduction disturbances (including sino-atrial node dysfunction, fascicular block and/or high-degree heart block), and history of other conduction disorders (AV block (1st, 2nd, and unspecified), other heart block, other specified and unspecified conduction disorders) were associated with higher risk of PPM implantation. Of interest, in the risk adjusted analysis, endocarditis was not associated with risk of PPM implantation. Among the different index valve procedures, isolated AVR and MVr were associated with the lowest risk of PPM implantation, while AVR plus MVR was associated with the highest risk. Concomitant performance of surgical ablation or tricuspid valve surgery was associated with increased risk of PPM implantation. Supplemental Figures 4A through 4E depict the risk factors for PPM implantation during this time period stratified by the index valve procedure.
Figure 2. Risk factors associated with PPM implantation in the in-hospital period.

Hazard ratios were determined using the proportional hazard multivariable model with time to PPM from index surgery to index discharge as the time-dependent predictor and death as a competing risk. The model was adjusted for baseline comorbidities and patient demographics. Older age and presence of conduction disturbances at baseline were strongly associated with a higher risk of PPM implantation during the index hospitalization. CABG: coronary artery bypass grafting.
Risk Factors of PPM Implantation between Hospital Discharge and 30 Days.
Risk factors for PPM implantation in the period between hospital discharge and 30 days are illustrated in Figure 3. During this period, younger age (<40 years old), emergent and urgent admissions, and chronic comorbidities such as diabetes mellitus and chronic obstructive pulmonary disease were associated with increased risk of PPM.
Figure 3. Risk factors associated with PPM implantation between hospital discharge and 30 days.

Hazard ratios were determined using the proportional hazard multivariable model with time to PPM as the time-dependent predictor and death as a competing risk event. The model was adjusted for baseline comorbidities and patient demographics. Chronic conditions such as diabetes mellitus and chronic obstructive pulmonary disease are risk factors associated with PPM implantation. CABG: coronary artery bypass grafting.
Risk Factors of PPM Implantation between 31 Days and 1 Year.
Figure 4 depicts risk factors for PPM implantation during this period of time. Overall, comorbidities, such as peripheral artery disease, congestive heart failure, chronic kidney disease (non-dialysis-dependent), history of atrial fibrillation/flutter, and cardiac arrest were associated with increased risk of PPM during this time period. Supplemental Figures 5A to 5E depict risk factors for PPM implantation during this time period, stratified by the index valve surgery.
Figure 4. Risk factors associated with PPM implantation between 30 days and 1 year.

Hazard ratios were determined using the proportional hazard multivariable model with time to PPM from 31 days post index discharge to 1 year post index discharge as the time-dependent predictor and death as a competing risk event. The model was adjusted for baseline comorbidities and patient demographics. Chronic diseases are strongly associated with higher risks of PPM implantation after 31 days post index discharge. CABG: coronary artery bypass grafting.
DISCUSSION
Conduction disturbances requiring PPM implantation following mitral and aortic valve surgery have long been recognized as a common post-operative complication (1–7). With the growth of transcatheter valve therapies which are associated with high rates of pacemaker dependency, there is now renewed interest in characterizing the risk and determinants of post-surgical pacemaker dependency, given the long-term risks for infection, tricuspid regurgitation, thrombotic events and heart failure, associated with such devices.
Strengths of this study are its large population (77,882 patients who underwent mitral and or aortic valve surgery in New York State between 1996 and 2014) and the manner of which it incorporates preoperative risk factors and defines PPM risk across a range of cardiac valve surgeries during discrete periods of time in the 1st year. Prior reports were largely single-centered studies or based on small patient populations (5, 11–13). Key findings of this study are: (1) PPM implantation rates vary by type of valve surgery, and are highest in patients who undergo combined aortic and mitral procedures; (2) the risk of PPM implantation is greatest during index hospitalization; (3) patient factors associated with enhanced risk of PPM implantation varied by time interval post valve procedure; and (4) the characteristics of the operative procedure (valve position and concomitant procedures) no longer contributed to the risk of PPM implantation after the index hospitalization.
This study is important for several reasons; the results highlight the operative risks associated with valve surgery with and without concomitant procedures, and the patient factors that govern the risk of PPM implantation after discharge. This data should help to inform patients about the risk for PPM following valve surgery, as well as inform surveillance strategies for patients at risk for needing PPM post discharge, including those patients with known conduction disease, atrial fibrillation, vascular disease or renal dysfunction.
Surgical Risk Factors.
We observed that the need for PPM implantation following valve surgery depended on the type of procedure being performed as well as several pre-operative risk factors. Consistent with prior literature, combined aortic and mitral valve procedures were associated with greater risk of PPM implantation than isolated valve procedures. Among isolated valve procedures, MVR was associated with the highest risk of PPM implantation (14). Notably, the rates of PPM implantation for patients who underwent surgical AVR are similar to the rates seen among transcatheter AVR recipients in the PARTNER 3 Trial, which has reported its contemporary 30-day and 1 year rates of PPM implantation (baseline pacemaker excluded) for AVR to be 4.1% and 5.5% respectively (15). Moreover, concomitant procedures including: surgical ablation and tricuspid intervention significantly increased risk of PPM implantation during index hospitalization. Although, in the overall valve surgery population surgical ablation was not a significant risk factor for PPM implantation, it was significant when risk was analyzed by individual valve procedure (AVR, MVR, AVR plus MVR, and AVR plus MVr). These findings are consistent with prior reports showing an increased risk of PPM implantation in aortic and mitral valve surgeries with concomitant surgical ablation procedure (14). Notably, the rates of PPM implantation post AVR plus surgical ablation is nearly twofold higher than isolated AVR. This finding must be interpreted with caution as the underlying rhythm status and the indications for PPM implantation were not known. Concomitant tricuspid valve surgery was associated with a higher risk of PPM implantation. These findings are of particular importance given the growing interest in performing concomitant surgical ablations when valve surgeries are performed in patients with a history of atrial fibrillation, as well as tricuspid valve surgeries in patients with moderate tricuspid regurgitation who are undergoing mitral valve surgery (14, 16).
Understanding the additive risk of surgical ablation or tricuspid surgery informs perioperative planning because decisions to include epicardial leads for left ventricular pacing can be considered in patients with borderline ejection fractions who are determined to be at high risk for post-operative pacemaker dependency.
Clinical Risk Factors.
Consistent with prior reports, older age and presence of conduction disturbances at baseline were strongly associated with a higher risk of PPM implantation during the index hospitalization (1–7), irrespective of the type of valve surgery done. Chronic comorbidities including peripheral artery disease, endocarditis, congestive heart failure, and chronic kidney disease were observed to be associated with a higher risk of PPM implantation in later phases (beyond 30 days). The mechanisms by which such associations might produce advanced conduction disturbances over time can be several. Peripheral artery disease, for example, is a marker of systemic atherosclerotic burden and is highly associated with coronary artery disease, which can be associated with ischemic injury to the conduction system (17). Chronic kidney disease which is associated with systemic inflammation, endothelial dysfunction, atherosclersosis and coagulopathies was also strongly associated with PPM after mitral or aortic valve surgery (18, 19). In addition, chronic kidney disease is associated with pathological myocardial remodeling and fibrosis which enhances the risk of conduction disturbances and is associated with atrial fibrillation (20, 21). Congestive heart failure is also a risk factor for conduction disease; although patients with left ventricular dysfunction and left bundle branch block may also undergo device placement for cardiac resynchronization therapy in the absence of higher degrees of conduction disease (20, 21). Early identification of comorbidities associated with late conduction disturbances may inform post-discharge serial follow-up and guide the timing of implantation of PPM. Interestingly, endocarditis was not found to be associated with PPM placement during the index hospitalization, and, actually, those with endocarditis had lower rates of PPM placement during index hospitalization compared to those without endocarditis at time of surgery. This finding was likely due to the younger age of those receiving endocarditis relative valve surgery compared to those who underwent valve surgery for other indications. In fact, the majority of patients with endocarditis (52%, N=1,350/2,595) were under the age of 60 years, while the majority of patients without endocarditis (54%, N=40,629/75,287) were older than 70 years of age. Although our multivariable analysis adjusted for risk, this older patient population was likely to have both coronary disease and associated conduction disease and interactions between these comorbidities was not accounted for in the analysis.
In the current study we did not specifically investigate the long-term consequences of PPM implantation on patient outcomes. Prior evidence supports the negative effect of permanent right ventricular pacing on cardiac physiology and long-term outcomes, which has been shown to be associated with higher risk of mortality and hospitalization for heart failure (22–25). In addition, PPM are associated with increased risk of infections and tricuspid valve dysfunction (22–25). Although PPM implantation may be necessary to address the immediate risk of specific clinical events or symptoms (8, 9), the association of this form of therapy with other long-term morbidities that themselves may be life threatening, highlights the need to further understand the relationship between cardiac valve interventions and the risk of PPM implantation.
LIMITATIONS
This study is not without limitations. The data for this study comes from an administrative dataset. Consequently, it is subject to the limitations known to be associated with such datasets, including inaccurate coding, incomplete data, and no prescription drug information. Notably, we were unable to delineate and clinically characterize types of surgical ablation procedures. Also, since we excluded implantation of defibrillators from our analysis, we did not capture individuals who had a dual indication for both defibrillation (either for primary or secondary prevention) and pacing.
CONCLUSION
The risk of PPM implantation in patients undergoing cardiac valve surgery varied by type of valve surgery and by concomitant procedures; isolated AVR and MVr were associated with the lowest risk of PPM implantation and combined aortic and mitral valve replacement with the greatest. Patient factors associated with enhanced risk of PPM implantation differed based on device implantation time; older age and pre-operative conduction disturbances were associated with a greater risk of PPM during the index hospitalization and chronic comorbidities were associated with greater risk of PPM implantation in the first post-discharge year. Identification of patients at risk for requiring PPM will help with perioperative planning, setting patients expectations, as well as informing clinical decision making regarding appropriate post-operative follow up and monitoring.
Supplementary Material
Central Image. Incidence and Risk Factors for Permanent Pacemaker Implantation Following Mitral or Aortic Valve Surgery.

Older patient age and pre-operative conduction disturbances were associated with a greater risk of PPM during the index hospitalization and chronic comorbidities were associated with greater risk of PPM implantation in the first post-discharge year.
Perspectives.
Competency in Medical Knowledge:
Patient factors associated with enhanced risk of PPM implantation differed based on device implantation time; older age and pre-operative conduction disturbances were associated with a greater risk of PPM during the index hospitalization and chronic comorbidities were associated with greater risk of PPM implantation in the first post-discharge year.
Translational Outlook:
Although PPM implantation may be necessary to address the immediate risk of specific clinical events or symptoms the association of this form of therapy with other long-term morbidities that themselves may be life threatening, highlights the need to further understand the relationship between cardiac valve interventions and the risk of PPM implantation so that we can abate this risk.
Funding:
5U01HL088942 NIH/NHLBI grant: Network for Cardiothoracic Surgical Investigations in Cardiovascular Medicine. The funder did not play any role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript.
ABBREVIATIONS
- APC
Annual Percent Change
- AVR
Aortic Valve Replacement
- CABG
Coronary Artery By-pass Graft
- ICD-9-CM
International Classification of Diseases, Ninth revision, Clinical Modification
- MVR
Mitral Valve Replacement
- MVr
Mitral Valve Repair
- PPM
Permanent Pacemaker
- SA
Surgical Ablation
- SPARCS
Statewide Planning and Research Cooperative System
Footnotes
Disclosures: Dr Ailawadi reports being on consultant boards for Abbott, Edwards, Medtronic, and AtriCure. Dr Gillinov reports being a consultant to AtriCure, Medtronic, Abbott, CryoLife, Edwards LifeSciences, and ClearFlow. Cleveland Clinic has right to royalties from AtriCure.
The remaining authors have nothing to disclose.
REFERENCES
- 1.Schurr UP, Berli J, Berdajs D, et al. Incidence and risk factors for pacemaker implantation following aortic valve replacement. Interact Cardiovasc Thorac Surg. 2010;11(5):556–60. [DOI] [PubMed] [Google Scholar]
- 2.Leyva F, Qiu T, McNulty D, Evison F, Marshall H, Gasparini M. Long-term requirement for pacemaker implantation after cardiac valve replacement surgery. Heart Rhythm. 2017;14(4):529–34. [DOI] [PubMed] [Google Scholar]
- 3.Wiggins NB, Chong DT, Houghtaling PL, et al. Incidence, indications, risk factors, and survival of patients undergoing cardiac implantable electronic device implantation after open heart surgery. Europace. 2017;19(8):1335–42. [DOI] [PubMed] [Google Scholar]
- 4.Limongelli G, Ducceschi V, D’Andrea A, et al. Risk factors for pacemaker implantation following aortic valve replacement: a single centre experience. Heart. 2003;89(8):901–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Ghamdi B, Mallawi Y, Shafquat A, et al. Predictors of Permanent Pacemaker Implantation After Coronary Artery Bypass Grafting and Valve Surgery in Adult Patients in Current Surgical Era. Cardiol Res. 2016;7(4):123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erdogan HB, Kayalar N, Ardal H, et al. Risk factors for requirement of permanent pacemaker implantation after aortic valve replacement. J Card Surg. 2006;21(3):211–5; discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 7.Robich MP, Schiltz NK, Johnston DR, et al. Risk Factors and Outcomes of Patients Requiring a Permanent Pacemaker After Aortic Valve Replacement in the United States. J Card Surg. 2016;31(8):476–85. [DOI] [PubMed] [Google Scholar]
- 8.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51(21):e1–62. [DOI] [PubMed] [Google Scholar]
- 9.Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34(29):2281–329. [DOI] [PubMed] [Google Scholar]
- 10.Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018. [Google Scholar]
- 11.Dawkins S, Hobson AR, Kalra PR, Tang AT, Monro JL, Dawkins KD. Permanent pacemaker implantation after isolated aortic valve replacement: incidence, indications, and predictors. Ann Thorac Surg. 2008;85(1):108–12. [DOI] [PubMed] [Google Scholar]
- 12.Elahi MM, Lee D, Dhannapuneni RR. Predictors of permanent pacemaker implantation during the early postoperative period after valve surgery. Tex Heart Inst J. 2006;33(4):455–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Pecha S, Schafer T, Yildirim Y, et al. Predictors for permanent pacemaker implantation after concomitant surgical ablation for atrial fibrillation. J Thorac Cardiovasc Surg. 2014;147(3):984–8. [DOI] [PubMed] [Google Scholar]
- 14.Gillinov AM, Gelijns AC, Parides MK, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med. 2015;372(15):1399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380(18):1695–705. [DOI] [PubMed] [Google Scholar]
- 16.Chikwe J, Itagaki S, Anyanwu A, Adams DH. Impact of Concomitant Tricuspid Annuloplasty on Tricuspid Regurgitation, Right Ventricular Function, and Pulmonary Artery Hypertension After Repair of Mitral Valve Prolapse. J Am Coll Cardiol. 2015;65(18):1931–8. [DOI] [PubMed] [Google Scholar]
- 17.Park DS, Fishman GI. The cardiac conduction system. Circulation. 2011;123(8):904–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giustino G, Mehran R, Serruys PW, et al. Left Main Revascularization With PCI or CABG in Patients With Chronic Kidney Disease: EXCEL Trial. J Am Coll Cardiol. 2018;72(7):754–65. [DOI] [PubMed] [Google Scholar]
- 19.Baber U, Giustino G, Sartori S, et al. Effect of Chronic Kidney Disease in Women Undergoing Percutaneous Coronary Intervention With Drug-Eluting Stents: A Patient-Level Pooled Analysis of Randomized Controlled Trials. JACC Cardiovasc Interv. 2016;9(1):28–38. [DOI] [PubMed] [Google Scholar]
- 20.Kestenbaum B, Rudser KD, Shlipak MG, et al. Kidney function, electrocardiographic findings, and cardiovascular events among older adults. Clin J Am Soc Nephrol. 2007;2(3):501–8. [DOI] [PubMed] [Google Scholar]
- 21.Mathew J, Katz R, St John Sutton M, et al. Chronic kidney disease and cardiac remodelling in patients with mild heart failure: results from the REsynchronization reVErses Remodeling in Systolic Left vEntricular Dysfunction (REVERSE) study. Eur J Heart Fail. 2012;14(12):1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranasinghe I, Parzynski CS, Freeman JV, et al. Long-Term Risk for Device-Related Complications and Reoperations After Implantable Cardioverter-Defibrillator Implantation: An Observational Cohort Study. Ann Intern Med. 2016. [DOI] [PubMed] [Google Scholar]
- 23.Kipp R, Hsu JC, Freeman J, Curtis J, Bao H, Hoffmayer KS. Long-term morbidity and mortality after implantable cardioverter-defibrillator implantation with procedural complication: A report from the National Cardiovascular Data Registry. Heart Rhythm. 2018;15(6):847–54. [DOI] [PubMed] [Google Scholar]
- 24.Hawkins NM, Grubisic M, Andrade JG, et al. Long-term complications, reoperations and survival following cardioverter-defibrillator implant. Heart. 2018;104(3):237–43. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen JC, Andersen HR, Thomsen PE, et al. Heart failure and echocardiographic changes during long-term follow-up of patients with sick sinus syndrome randomized to single-chamber atrial or ventricular pacing. Circulation. 1998;97(10):987–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


