Abstract
In the immune oncology era, the clinical efficacy of immune checkpoint inhibitors (ICIs) against most solid cancers is well known. In hepatocellular carcinoma, the recent success of combination therapy with targeting agents has accelerated the search for novel combination strategies. Radiotherapy (RT), an attractive modality, can be combined with ICIs, which act as strong modulators of the tumor immune microenvironment. Herein, we discuss immune modulation caused by radiation and the current trials of RT–ICI combination treatment as well as future perspectives.
Keywords: Hepatocellular carcinoma, Immune checkpoint inhibitor, Radiotherapy, Immune modulation, Tumor microenvironment, Immune oncology
Core Tip: Immune modulatory effect of radiation is highlighted as a combination strategy with immune checkpoint inhibitors. This strategy has been actively adopted in most solid cancers. Although it is in relatively early stage for hepatocellular carcinoma, accumulated evidence drives clinical trials on testing its efficacy. Still, there remain several challenges to overcome for the best oncologic outcome.
INTRODUCTION
As immune checkpoint inhibitors (ICIs) have demonstrated promising clinical outcomes, cancer immunotherapy has shifted the paradigm in cancer therapy. ICIs have shown efficacy against various types of cancers such as malignant melanoma and non-small cell lung cancer[1-3]. For hepatocellular carcinoma (HCC), ICI monotherapy has not been successful, with a response rate of no more than 20%, which suggests the need for a combination strategy. The recent IMbrave150 trial demonstrated superior progression-free survival (PFS) and better overall survival (OS) with combination treatment using atezolizumab [an anti-programmed death ligand-1 (PD-L1) agent] and bevacizumab (an anti-vascular endothelial growth factor-A agent) compared to those with sorafenib in HCC patients[4]. The IMbrave150 trial showed the potential of ICIs in combination with a tumor microenvironment (TME)-modulating agent for the treatment of HCC. Identification of the optimal combination treatment using ICIs as a novel therapy is gaining extensive attention.
Radiotherapy (RT), one of the major cancer treatments, promotes localized tumor cell killing and induces immune modulation in the TME[5,6]. Increasing evidence has demonstrated that radiation reinforces tumor-related immunity[7,8]. RT exerts synergistic effects with ICIs by increasing lymphocyte infiltration into tumors, inducing immunogenic cell death, and enhancing the performance of antigen-presenting cells (APCs)[9]. Herein, we discuss immune modulation by radiation, the rationale for RT–ICI combination treatment in preclinical settings, and future approaches to overcome the hurdles in combination therapy for HCC.
CHALLENGES IN CURRENT ICIs
Although ICIs show promising treatment outcomes, challenges remain in their application. One recent study reported that the tumor immune cell composition plays a key role in the response to immunotherapy[10-12]. Although the initial T-cell population mainly comprised effective “transitional” cells, a substantial number of infiltrating CD8 T-cells transformed gradually into dysfunctional T-cells. CD8 T-cells with cytotoxic functions were rare among intratumoral immune cells, while dysfunctional T-cells were the major immune cells in tumors. Furthermore, the proportion of dysfunctional T-cells was associated with tumor proliferation. These findings suggest that ICIs alone might be insufficient for achieving an adequate clinical response.
The infiltrating dysfunctional T cells by immunosuppressive mechanisms in TME is one of the reasons for failed ICI[13,14]. The exhausted T cells can explain the lack of response in ICI. To elevate the efficacy of ICI response, converting the dysfunctional T cell into functional T cell is important. The reinvigorating exhausted T cell is expected to improve the outcome of ICI. The successful reinvigoration of T cell function would recover the antitumor activity[15].
Currently, the reinvigoration of T-cells appears to be a key outcome in immune oncologic therapy. Huang et al[16] reported that the reinvigoration of T-cells is closely related to tumor burden, and this association was also correlated with the clinical response to ICIs[16-18]. The authors reported that the ratio of T-cell reinvigoration to the tumor burden was the key predictive factor of the clinical response to ICIs, which explains the heterogeneous and unsustainable clinical benefit in patients[16-18]. Therefore, reducing the tumor burden before administering ICIs seems important for improving clinical outcomes. In this regard, RT may be effective in reducing the tumor burden[19]. In addition, RT has been known for its modulation effect on the immune TME.
IMMUNE MODULATION EFFECT OF RADIATION
Besides cell killing, RT induces an immune-mediated antitumor response. Its effect in terms of immune modulation is summarized in Table 1. First, RT induces antigen release and immunogenic cell death. Radiation upregulates the expression of major histocompatibility complex (MHC) class I, thus enhancing the immune response and efficacy of ICIs[20,21]. Naturally, MHC expression is downregulated in tumors to allow immune evasion. Expression of MHC class I enables CD8 T-cells to recognize tumor cells and trigger a major cell-mediated cytotoxic response. Enhanced antigen presentation with upregulated MHC class I expression is one mechanism by which radiation induces immune-mediated cell death[20]. Radiation promotes the expression of not only MHC class I in tumor cells but also damage-associated molecular patterns, such as HMGB1, and the release of prophagocytic signals, such as calreticulin[20,22].
Table 1.
Four key steps of a radiation-induced immune response
|
Major steps
|
Ref.
|
| Induction of antigen release and immunogenic cell death | [20-22] |
| Induction of antigen-presenting cell maturation and antigen presentation | [23,24] |
| Induction of T-cell recruitment and infiltration | [21,29-32] |
| Induction of tumor cell sensitization to immune-mediated cell death | [27,28,33] |
Second, RT mediates the release of tumor antigens, which leads to the activation and transfer of dendritic cells to draining lymph nodes, resulting in tumor-specific T-cell activation and proliferation. After RT, antigens are released from dying tumor cells, and antigens are taken up by APCs such as macrophages, dendritic cells, and B-cells. Antigen uptake by APCs is an important step in priming adaptive immunity. Dendritic cells are activated after antigen uptake, and the activated dendritic cells migrate to lymph nodes. In tumor-draining lymph nodes, dendritic cells present antigens to either T-cells or B-cells[23]. HMGB1, a radiation-induced damage-associated molecular pattern, enhances dendritic cell maturation[24].
Several studies have demonstrated that RT increases the number of tumor-infiltrating lymphocytes (TILs), indicating that RT aids in overcoming the physical barriers of the tumor, which facilitates a substantial response by the adaptive immune system[25-28]. Two mechanisms have been proposed to explain the increased number of TILs after RT[21,29-31]. One mechanism involves the modification of the vascular endothelium, enabling the extravasation of immune cells. The expression of E-selectin and intercellular adhesion molecule (ICAM)-1, one of the cell adhesion molecules on the vascular endothelium, has been shown to increase after RT[32]. These molecules help the leukocytes migrate from vessels, which is a key step in enhancing the immune response against the tumor. Another mechanism involves the promotion of the expression of chemokines, increasing immune cell migration and invasion. Radiation increases the expression of CXCL16, the ligand for CXCR6[31]. CD8 T-cells expressing CXCR6 are recruited toward tumor cells as radiation exposure increases the expression of CXCL16. In summary, radiation promotes the migration of TILs into the TME, ultimately generating an immunogenic environment.
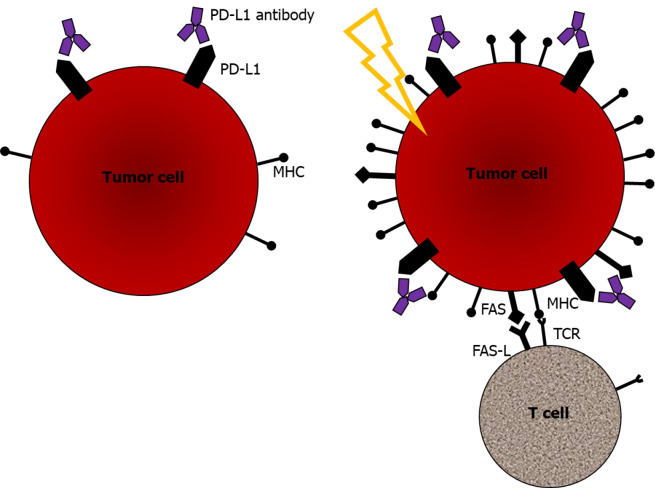
Lastly, RT induces the sensitization of tumor cells to immune-mediated cell death. As previously described, radiation increases MHC class I expression, together with the immunogenic release of damage-associated molecular patterns and prophagocytic signals. This mechanism induces immune-mediated cell death[28]. Along with the enhanced expression of MHC class I and HMGB1, radiation exposure induces FAS expression. FAS is a cell surface molecule that induces programmed cell death. FAS expression is upregulated in human tumor cell lines after radiation[33]. Upregulated FAS expression on tumor cells enhanced binding to nearby immune cells expressing the FAS ligand[27,33]. Radiation-induced upregulation of FAS expression is one of the important mechanisms by which the immune system can trigger tumor cell death. Taken together, RT can change the immunogenicity of tumors from low to high through these key mechanisms. The mechanisms that occurred when combining the ICI and radiation are summarized in Figure 1.
Figure 1.
Combination of immune checkpoint inhibitor and radiation enhances immune-mediated cell death. PD-L1: Programmed cell death ligand 1; MHC: Major histocompatibility complex; TCR: T cell receptor.
These immune-modifying mechanisms were also observed in a murine HCC model[34]. MHC class I expression was upregulated after RT in the HCC model. The expression level of MHC class I was significantly higher in the RT group than in the control group. Concordant with the expression of MHC class I, upregulated expression of HMGB1 and ICAM-1 was observed after RT. The upregulated expression of HMGB1 is expected to lead to dendritic cell maturation, and increased ICAM-1 expression is thought to induce leukocyte outflow. These molecules, induced by radiation, alter the TME to an immunogenic environment in HCC.
COMBINATION OF RADIATION WITH ICIS
The notion of combination treatment is now generally accepted with respect to the clinical application of ICIs. It is known that multiple co-stimulatory and inhibitory signals regulate T-cell activation[35,36]. These co-stimulatory and inhibitory signals play an important role in immune resistance. ICIs, which block these inhibitory signals, eliminate immune resistance mechanisms. Interestingly, these co-stimulatory and inhibitory signals are modulated by radiation[29,37]. Based on these findings, the combination of RT and ICIs is thought to have a synergistic effect, and some preclinical data support its use against HCC.
Our group demonstrated that RT induced PD-L1 expression in tumor cells and showed the potential antitumor effect of anti-PD-L1 agents against HCC[38]. The expression of PD-L1 is induced maximally between 24 and 48 h after RT. RT with up to 10 Gy was administered, and PD-L1 expression was upregulated in a dose-dependent manner. The antitumor effect was also examined for the anti-PD-L1 agent–RT combination in vivo. Tumor growth suppression and survival improvement were significantly superior in the combination group than in the anti-PD-L1 agent alone or RT alone group. Furthermore, the combination of an anti-PD-L1 agent and RT significantly increased cytotoxicity and the proliferation of CD8 T-cells compared to RT alone or the anti-PD-L1 agent alone.
T-cell immunoglobulin and mucin-domain-containing molecule-3 (TIM3) is an inhibitory molecule present on T-cells. TIM-3-expressing T-cells showed dysfunction or “exhaustion”[39]. It has been reported that TIM-3 expression is higher in HCC patients than in those with other liver diseases, such as chronic hepatitis and liver cirrhosis[40]. TIM-3-positive T-cells showed high expression in HCC cells, in contrast to that in normal cells present in adjacent tissues[41]. Although TIM-3 blockade can modulate the immune response via several cell types[42-44], there are limited studies regarding the effect of anti-TIM3 agents in HCC patients. RT upregulated TIM-3 expression in HCC cell lines, and the combination of an anti-TIM-3 agent and radiation promoted cytotoxicity and the proliferation of CD8 T-cells[45]. Furthermore, the combination of an anti-TIM-3 agent and radiation significantly suppressed tumor growth compared to radiation or anti-TIM-3 agent alone. Concordant with the results of tumor growth, the combination group demonstrated a significant improvement in survival.
Despite these promising preclinical data regarding the combination of RT and ICIs against HCC, clinical studies are severely limited. One study investigated the clinical implications of PD-L1 levels in patients with HCC undergoing RT[46]. The level of soluble PD-L1 (sPD-L1) was quantified in patients who underwent RT for HCC. The initial sPD-L1 level was significantly associated with tumor aggressiveness (tumor size and stage). A high initial sPD-L1 level was related to poorer OS than a low initial sPD-L1 level. The sPD-L1 levels increased significantly after both conventional RT and stereotactic body RT (SBRT), but the pattern of sPD-L1 change was different depending on the dose scheme. The sPD-L1 level increased immediately after RT but decreased at 1 mo after conventional RT, while a continuous increase was observed in those undergoing SBRT. In the SBRT group, the median sPD-L1 level at 1 mo increased to approximately 3-times the initial sPD-L1 level. Therefore, the combination of ICIs and RT may be a promising treatment in patients with HCC, and efficacy might be better with SBRT. This notion needs clinical validation to evaluate the efficacy of combined treatment with RT and ICIs for HCC. Several prospective trials registered at www.ClinicalTrials.gov are ongoing to investigate the combination of ICIs and RT (Table 2).
Table 2.
Ongoing clinical trials investigating the combination of radiotherapy and immune checkpoint inhibitors against hepatocellular carcinoma
|
NCT number
|
Institution
|
Disease
|
Estimated enrollment
|
Phase
|
Primary endpoint
|
Intervention
|
| NCT04167293 | China (Sun Yat-sen University Cancer Center) | HCC with portal vein invasion | 116 | II/III | 6-mo PFS | SBRT + sintilimab vs SBRT |
| NCT03817736 | Hong Kong (Queen Mary Hospital) | HCC | 33 | II | Number of patients eligible for curative surgical interventions | TACE/SBRT + ICI |
| NCT03203304 | United States (University of Chicago) | Unresectable HCC | 50 | I | Number of participants with adverse events | Nivolumab + SBRT vs nivolumab and ipilimumab + SBRT |
| NCT04611165 | South Korea (NCC) | HCC with major vascular invasion | 50 | II | PFS | Nivolumab + EBRT |
| NCT03482102 | United States (MGH) | Locally advanced/unresectable or metastatic HCC or biliary tract cancer | 70 | II | ORR | Tremelimumab + durvalumab + RT |
| NCT03316872 | Canada (UHN) | HCC progression after sorafenib | 30 | II | ORR | Pembrolizumab + SBRT |
| NCT04547452 | China (West China Hospital) | Metastatic HCC | 84 | II | PFS | SBRT + sintilimab vs sintilimab |
| NCT04193696 | China (Guangxi Medical University) | Advanced HCC | 39 | II | ORR | RT+ anti-PD-1 agent |
HCC: Hepatocellular carcinoma; PFS: Progression-free survival; SBRT: Stereotactic body radiation therapy; TACE: Transcatheter arterial chemoembolization; ICI: Immune checkpoint inhibitor; NCC: National Cancer Center; EBRT: External beam radiotherapy; MGH: Massachusetts General Hospital; ORR: Overall response rate; RT: Radiotherapy; UHN: University Health Network; PD-L1: Programmed cell death ligand 1.
CHALLENGES WITH THE COMBINATION OF RADIATION AND ICIS
Radiation fractionation
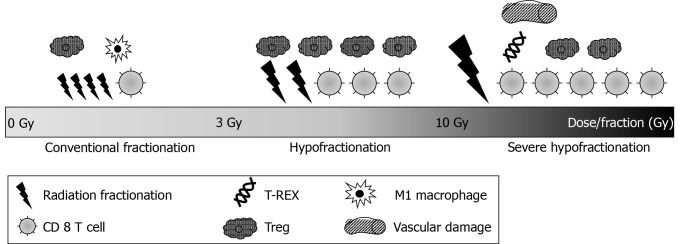
There is no established dose or fractionation regimen that optimizes the therapeutic effect of RT plus ICIs. It is clear that the immunologic response differs depending on the RT dose as per fractionation. With conventional fractionation, RT promotes the recovery of tumor vessels via the migration of immune cells through the endothelium[47] and induction of M1-type macrophages[47,48]. These actions have a positive effect on immunity. With hypofractionation, treatment-related lymphopenia occurs less frequently[49], and Tregs are activated, shifting the balance of T-cells toward the immunosuppressive state[50,51]. Additionally, preclinical data have shown that hypofractionation regimens favor an antitumor response and induce a strong lymphoid response[52,53].
In contrast, a high fractional dose led to a different immune response. As per preclinical data, fractional doses higher than 12 Gy induce the production of 3′ repair exonuclease 1 (TREX1), which degrades cytosolic DNA after RT[54]. Consequently, TREX1 inactivates the cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS)–stimulator of interferon genes (STING) pathway. With conventional fractionation, the cytosolic DNA after radiation binds to cGAS and activates STING[55,56]. Consequently, cGAS and STING induce the production of type I interferon and activate an antitumor immune response[57,58]. As a fractional dose of more than 12 Gy induces TREX1, the fractional dose of more than 12 Gy results in the inhibition of antitumor immune responses. Furthermore, doses greater than 10 Gy per fraction enhance vascular damage, leading to less effective T-cell recruitment because of reduced vascularity[59]. The different types of radiation-induced immune modulation by different fractionation scheme of radiation are summarized in Figure 2. For an optimal combination of RT and ICIs, it is important to gain an understanding of the immune response depending on different fractionations to improve therapy efficacy and administer personalized medicine[19].
Figure 2.
Types of radiation-induced immune modulation by different fractionation scheme of radiation.
Treatment sequence
The optimal timing of administering ICIs in combination with RT has not yet been defined. To determine the optimal timing of RT and ICI treatment, several preclinical studies have been performed. One report showed that administering ICIs 7 d after RT was less effective in enhancing OS than administering ICIs concurrently with RT[37]. In the PACIFIC trial, durvalumab started within 14 d of completing RT resulted in better PFS than durvalumab started after 14 d[2]. A recent study showed that OS was longer in patients who received ICIs concurrently with RT[60]. Among the patients who received concurrent treatment, induction immunotherapy administered more than 30 d before RT led to longer OS than that of administered within 30 d before RT. Scheduling of RT and immunotherapy must be considered with caution in the context of clinical trials.
CONCLUSION
ICIs have emerged as a promising therapy for various malignancies including HCC. T-cell reinvigoration by activating dysfunctional T-cells into cytotoxic T-cells is a key factor in the novel therapeutic effect of ICIs. However, ICI monotherapy has some limitations in circumstances such as T-cell dysfunction and high tumor burden. Meanwhile, RT has been shown to cause high immunogenicity in tumors through various mechanisms of immune modulation. The combination of ICIs and RT is being studied as a promising treatment for HCC to take advantage of the synergistic effect. Further studies are necessary to determine the appropriate treatment regimen for achieving optimal clinical benefit.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Peer-review started: January 5, 2021
First decision: January 17, 2021
Article in press: February 28, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guo K, Madian A, Mikulic D S-Editor: Fan JR L-Editor: A P-Editor: Wang LL
Contributor Information
Byung Min Lee, Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul 03722, South Korea.
Jinsil Seong, Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul 03722, South Korea. jsseong@yuhs.ac.
References
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M PACIFIC Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 3.Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I, Niemeijer AN, de Langen AJ, Monkhorst K, Baas P. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019 doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 5.Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4:522–526. doi: 10.1158/2159-8290.CD-13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 7.Chew V, Lee YH, Pan L, Nasir NJM, Lim CJ, Chua C, Lai L, Hazirah SN, Lim TKH, Goh BKP, Chung A, Lo RHG, Ng D, Filarca RLF, Albani S, Chow PKH. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut. 2019;68:335–346. doi: 10.1136/gutjnl-2017-315485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melero I, Berman DM, Aznar MA, Korman AJ, Pérez Gracia JL, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457–472. doi: 10.1038/nrc3973. [DOI] [PubMed] [Google Scholar]
- 10.Li H, van der Leun AM, Yofe I, Lubling Y, Gelbard-Solodkin D, van Akkooi ACJ, van den Braber M, Rozeman EA, Haanen JBAG, Blank CU, Horlings HM, David E, Baran Y, Bercovich A, Lifshitz A, Schumacher TN, Tanay A, Amit I. Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell 2019; 176: 775-789. :e18. doi: 10.1016/j.cell.2018.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V, Setty M, Choi K, Fromme RM, Dao P, McKenney PT, Wasti RC, Kadaveru K, Mazutis L, Rudensky AY, Pe'er D. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 2018; 174: 1293-1308. :e36. doi: 10.1016/j.cell.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo X, Zhang Y, Zheng L, Zheng C, Song J, Zhang Q, Kang B, Liu Z, Jin L, Xing R, Gao R, Zhang L, Dong M, Hu X, Ren X, Kirchhoff D, Roider HG, Yan T, Zhang Z. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med. 2018;24:978–985. doi: 10.1038/s41591-018-0045-3. [DOI] [PubMed] [Google Scholar]
- 13.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, Adamow M, Kuk D, Panageas KS, Carrera C, Wong P, Quagliarello F, Wubbenhorst B, D'Andrea K, Pauken KE, Herati RS, Staupe RP, Schenkel JM, McGettigan S, Kothari S, George SM, Vonderheide RH, Amaravadi RK, Karakousis GC, Schuchter LM, Xu X, Nathanson KL, Wolchok JD, Gangadhar TC, Wherry EJ. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph RW, Elassaiss-Schaap J, Kefford R, Hwu WJ, Wolchok JD, Joshua AM, Ribas A, Hodi FS, Hamid O, Robert C, Daud A, Dronca R, Hersey P, Weber JS, Patnaik A, de Alwis DP, Perrone A, Zhang J, Kang SP, Ebbinghaus S, Anderson KM, Gangadhar TC. Baseline Tumor Size Is an Independent Prognostic Factor for Overall Survival in Patients with Melanoma Treated with Pembrolizumab. Clin Cancer Res. 2018;24:4960–4967. doi: 10.1158/1078-0432.CCR-17-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki C, Kiyota N, Imamura Y, Rikitake J, Sai S, Koyama T, Hyogo Y, Nagatani Y, Funakoshi Y, Toyoda M, Otsuki N, Nibu KI, Minami H. Effect of tumor burden and growth rate on treatment outcomes of nivolumab in head and neck cancer. Int J Clin Oncol. 2020;25:1270–1277. doi: 10.1007/s10147-020-01669-y. [DOI] [PubMed] [Google Scholar]
- 19.Arnold KM, Flynn NJ, Raben A, Romak L, Yu Y, Dicker AP, Mourtada F, Sims-Mourtada J. The Impact of Radiation on the Tumor Microenvironment: Effect of Dose and Fractionation Schedules. Cancer Growth Metastasis. 2018;11:1179064418761639. doi: 10.1177/1179064418761639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A, Mesman E, Verreck FA, Spits H, Schlom J, van Veelen P, Neefjes JJ. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3:345–355. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–416. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, Schwendener R, Pruschy M, Knuth A, van den Broek M. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558–566. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 24.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E, Pradilla G, Ford E, Wong J, Hammers HJ, Mathios D, Tyler B, Brem H, Tran PT, Pardoll D, Drake CG, Lim M. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, Hodge JW. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 28.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 29.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, Demaria S. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallahan D, Kuchibhotla J, Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 1996;56:5150–5155. [PubMed] [Google Scholar]
- 33.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 34.Seong J, Kim KJ, Lim JH, Lee EJ. Radiation improves antitumor effect of immune checkpoint inhibitor, anti PD L-1 in murine hepatocarcinoma model. 10th International Liver Cancer Association 2016: 19-20. [Google Scholar]
- 35.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 36.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 37.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, Jones H, Wilkinson RW, Honeychurch J, Illidge TM. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 38.Kim KJ, Kim JH, Lee SJ, Lee EJ, Shin EC, Seong J. Radiation improves antitumor effect of immune checkpoint inhibitor in murine hepatocellular carcinoma model. Oncotarget. 2017;8:41242–41255. doi: 10.18632/oncotarget.17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu C, Anderson AC, Kuchroo VK. TIM-3 and its regulatory role in immune responses. Curr Top Microbiol Immunol. 2011;350:1–15. doi: 10.1007/82_2010_84. [DOI] [PubMed] [Google Scholar]
- 40.Li F, Li N, Sang J, Fan X, Deng H, Zhang X, Han Q, Lv Y, Liu Z. Highly elevated soluble Tim-3 levels correlate with increased hepatocellular carcinoma risk and poor survival of hepatocellular carcinoma patients in chronic hepatitis B virus infection. Cancer Manag Res. 2018;10:941–951. doi: 10.2147/CMAR.S162478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, Zou W. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 42.Liu JF, Ma SR, Mao L, Bu LL, Yu GT, Li YC, Huang CF, Deng WW, Kulkarni AB, Zhang WF, Sun ZJ. T-cell immunoglobulin mucin 3 blockade drives an antitumor immune response in head and neck cancer. Mol Oncol. 2017;11:235–247. doi: 10.1002/1878-0261.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Mingo Pulido Á, Gardner A, Hiebler S, Soliman H, Rugo HS, Krummel MF, Coussens LM, Ruffell B. TIM-3 Regulates CD103+ Dendritic Cell Function and Response to Chemotherapy in Breast Cancer. Cancer Cell 2018; 33: 60-74. :e6. doi: 10.1016/j.ccell.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim KJ, Lee HW, Seong J. Combination therapy with anti-T-cell immunoglobulin and mucin-domain containing molecule 3 and radiation improves antitumor efficacy in murine hepatocellular carcinoma. J Gastroenterol Hepatol. 2020 doi: 10.1111/jgh.15319. [DOI] [PubMed] [Google Scholar]
- 46.Kim HJ, Park S, Kim KJ, Seong J. Clinical significance of soluble programmed cell death ligand-1 (sPD-L1) in hepatocellular carcinoma patients treated with radiotherapy. Radiother Oncol. 2018;129:130–135. doi: 10.1016/j.radonc.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 47.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schäkel K, Garbi N, Jäger D, Weitz J, Schmitz-Winnenthal H, Hämmerling GJ, Beckhove P. Low-dose irradiation programs macrophage differentiation to an iNOS⁺/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 48.Prakash H, Klug F, Nadella V, Mazumdar V, Schmitz-Winnenthal H, Umansky L. Low doses of gamma irradiation potentially modifies immunosuppressive tumor microenvironment by retuning tumor-associated macrophages: lesson from insulinoma. Carcinogenesis. 2016;37:301–313. doi: 10.1093/carcin/bgw007. [DOI] [PubMed] [Google Scholar]
- 49.Wild AT, Herman JM, Dholakia AS, Moningi S, Lu Y, Rosati LM, Hacker-Prietz A, Assadi RK, Saeed AM, Pawlik TM, Jaffee EM, Laheru DA, Tran PT, Weiss MJ, Wolfgang CL, Ford E, Grossman SA, Ye X, Ellsworth SG. Lymphocyte-Sparing Effect of Stereotactic Body Radiation Therapy in Patients With Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2016;94:571–579. doi: 10.1016/j.ijrobp.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83:1306–1310. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei S, Egenti MU, Teitz-Tennenbaum S, Zou W, Chang AE. Effects of tumor irradiation on host T-regulatory cells and systemic immunity in the context of adoptive T-cell therapy in mice. J Immunother. 2013;36:124–132. doi: 10.1097/CJI.0b013e31828298e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poleszczuk J, Enderling H. The Optimal Radiation Dose to Induce Robust Systemic Anti-Tumor Immunity. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grapin M, Richard C, Limagne E, Boidot R, Morgand V, Bertaut A, Derangere V, Laurent PA, Thibaudin M, Fumet JD, Crehange G, Ghiringhelli F, Mirjolet C. Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: a promising new combination. J Immunother Cancer. 2019;7:160. doi: 10.1186/s40425-019-0634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–1149. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 59.Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS) Radiat Res. 2012;177:311–327. doi: 10.1667/rr2773.1. [DOI] [PubMed] [Google Scholar]
- 60.Samstein R, Rimner A, Barker CA, Yamada Y. Combined Immune Checkpoint Blockade and Radiation Therapy: Timing and Dose Fractionation Associated with Greatest Survival Duration Among Over 750 Treated Patients. Int J Radiat Oncol Biol Phys . 2017;99:S129–S130. [Google Scholar]