Abstract
Background
The burden of COVID-19 in patients with bullous pemphigoid (BP) and pemphigus is yet to be evaluated.
Objective
To assess the risks of COVID-19 and COVID-19-associated hospitalization and mortality in patients with BP and pemphigus and to delineate determinants of severe COVID-19 illness among these patients.
Methods
A population-based cohort study compared COVID-19 and its complications in patients with BP (n = 1845) and pemphigus (n = 1236) with age-, sex-, and ethnicity-matched control subjects.
Results
The risks of COVID-19 (hazard rate [HR], 1.12; 95% confidence interval [CI], 0.72-1.73; P = .691) and COVID-19-associated hospitalization (HR, 1.58; 95% CI, 0.84-2.98; P = .160) was comparable between patients with BP and controls. The risk of COVID-19-associated mortality was higher among patients with BP (HR, 2.82; 95% CI, 1.15-6.92; P = .023). The risk of COVID-19 (HR, 0.81; 95% CI, 0.44-1.49; P = .496), COVID-19-associated hospitalization (HR, 1.41; 95% CI, 0.53-3.76; P = .499), and COVID-19-associated mortality (HR, 1.33; 95% CI, 0.15-11.92; P = .789) was similar in patients with pemphigus and their controls. Systemic corticosteroids and immunosuppressants did not predispose COVID-19-positive BP and pemphigus patients to a more severe illness.
Limitations
Retrospective data collection.
Conclusions
Patients with BP experience increased COVID-19-associated mortality and should be monitored closely. Maintaining systemic corticosteroids and immunosuppressive adjuvant agents during the pandemic is not associated with worse outcomes.
Key words: bullous pemphigoid, coronavirus disease 2019, COVID-19, hospitalization, mortality, pemphigus
Capsule Summary.
-
•
Patients with bullous pemphigoid and pemphigus do not have an increased risk of COVID-19 infection relative to control individuals but COVID-19-associated mortality may be elevated.
-
•
COVID-19-positive bullous pemphigoid patients should be followed and monitored closely.
-
•
The use of systemic corticosteroids and immunosuppressive adjuvants did not predict worse COVID-19 outcomes and should not be ceased during the pandemic.
Introduction
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), started in China in late 2019 and has subsequently spread across the globe. Because severe COVID-19 is associated with a hyperinflammatory state,1 , 2 it is intriguing to investigate whether the presence of preexisting autoimmune diseases or past use of immunosuppressive agents affect the phenotype of COVID-19. Although certain studies demonstrated an increased risk and more aggressive course of SARS-CoV-2 infection in patients with autoimmune diseases,3, 4, 5 most others refuted this finding.6, 7, 8, 9, 10
Bullous pemphigoid (BP) and pemphigus are the most frequent autoimmune bullous diseases (AIBD) worldwide.11, 12, 13 Both diseases are associated with a life-threatening potential and impose an increased burden of mortality.13, 14, 15 The management of AIBD is challenging and often necessitates the administration of high-dose systemic corticosteroids and immunosuppressive agents.16 , 17 Treatment of these diseases represents an even greater challenge in light of the COVID-19 pandemic, given concerns about the vulnerability of pharmacologically immunosuppressed patients.18
The burden of COVID-19 and its complications for patients with AIBD has yet to be delineated, thus leaving the literature underpowered to formulate a treatment strategy for these patients during the pandemic. To optimize the treatment of AIBD, the predictors of worse outcomes in COVID-19-positive AIBD patients need to be identified and what influence AIBD-related medications may have on the prognosis of patients needs to be defined.
The aims of the current study are to evaluate the risk that patients with BP and pemphigus have for acquiring COVID-19 infection and developing its complications and to identify determinants predicting a severe COVID-19 course among these patients.
Methods
Study design and dataset
The current study was designed as a historical retrospective cohort study that followed patients with BP and pemphigus to estimate the incidence of COVID-19, COVID-associated hospitalization, and mortality. The current study received institutional review board approval before the start.
The computerized data set of Clalit Health Services (CHS) was the origin of the current study. CHS is the main health maintenance organization in Israel and provides private and public health care services; as of October 2018, CMS had more than 4,540,768 enrollees. CHS is renowned for its inclusiveness because it retrieves data from a multitude of sources and covers a range of settings, including general community clinics, primary care and referral centers, and ambulatory care centers and hospitals. The loss to follow-up is negligible and access to CHS services is free, rendering this data set highly compatible with the performance of reliable epidemiologic studies.19
Study population and definition of main variables
The computerized data set of CHS was systematically checked for incident cases with a diagnostic code of BP and pemphigus between the years 2002 and 2019. Patients were determined eligible if a documented diagnosis of BP or pemphigus was registered by a community-based board-certified dermatologist or if a diagnosis of BP or pemphigus was documented in discharge letters of patients admitted to dermatologic wards.
A control group encompassing 5 individuals per case of BP and pemphigus was originally recruited, with controls being matched based on sex, age, and ethnicity. The index date of matching was defined at the diagnosis of BP and pemphigus. The current analysis, however, included only participants who were alive at the beginning of the pandemic (Supplemental Fig 1 available via Mendeley at https://data.mendeley.com/datasets/f3rcw5rfjz/1.) Dates of death were ascertained by linking the study cohort with the National Registry of Deaths Database. All study participants were followed up from the onset of the pandemic in Israel (defined as the date of the first confirmed case on February 27, 2020) until September 11, 2020, or their death.
The diagnosis of COVID-19 relied on confirmation of cases by the molecular tests approved by the United States (US) Food and Drug Administration. COVID-19-associated hospitalization was defined as a COVID-19-confirmed patient who is admitted to an intensive care unit or an internal medicine or pulmonology inpatient ward. All hospitalized patients with COVID-19 were assigned 1 of the following severity degrees: mild (symptoms, such as cough, fever, fatigue, loss of smell, etc); moderate (clinical or radiologic diagnosis of COVID-19 pneumonia); severe (respiratory rate > 30, oxygen saturation <93% on room air, and PaO2/FiO2 < 300); and critical (severe systemic impairment, including septicemia or cardiac, hepatic, or renal insufficiency). The severity degree for non-hospitalized COVID-19-confirmed patients who were not managed in a health care facility was defined as subclinical.
Outcome measures were adjusted for underlying comorbidities as assessed by the Charlson comorbidity index, a validated epidemiologic method of quantifying comorbidities. This index is reliable in predicting mortality and is widely used in large-scale epidemiologic studies.20 Among others, Charlson comorbidity index encompasses diabetes mellitus and respiratory and cardiovascular diseases, for which there is evidence that they have the worse COVID-19 prognostic outcomes.21 COVID-19-associated hospitalization and mortality were adjusted for smoking owing to the association of the latter with worse outcomes for COVID-19.21 , 22 COVID-19-associated hospitalization and mortality were adjusted for systemic corticosteroids and immunosuppressive adjuvant drugs (azathioprine, mycophenolate mofetil, methotrexate, cyclophosphamide, rituximab), given the accumulation of evidence suggesting the vulnerability of pharmacologically immunosuppressed COVID-19 patients.23
Statistical analysis
Baseline characteristics were described by means and standard deviations (SD)s for continuous variables. Category values were indicated by percentages. Variables were compared using the chi-square test and t-test for category and continuous variables, respectively. Incidence rates of outcomes were calculated and expressed as the number of events per 1000 person-years. Hazard ratios (HRs) for the risk of incident outcomes were obtained using the Cox regression model. The cumulative survival of patients with COVID-19 was compared between the BP and pemphigus groups and their corresponding controls using a stratified log-rank test in the Kaplan-Meier method.
Results
Characteristics of the study population
The study population included 1845 patients with BP and 1236 patients with pemphigus. In all, 11,117 and 6574 control subjects were matched for the eligible patients with BP and pemphigus, respectively. Although the sex and ethnic composition was comparable between cases and controls, patients with BP and pemphigus were older than their matched controls at the onset of the pandemic (Supplementary Table I). Both patients with BP and pemphigus had higher mean Charlson comorbidity index scores, whereas patients with pemphigus had lower frequency of smoking (Supplementary Table II). The demographic and clinical features of the study participants are detailed in Supplementary Table I.
Descriptive data for COVID-19-positive BP and pemphigus patients
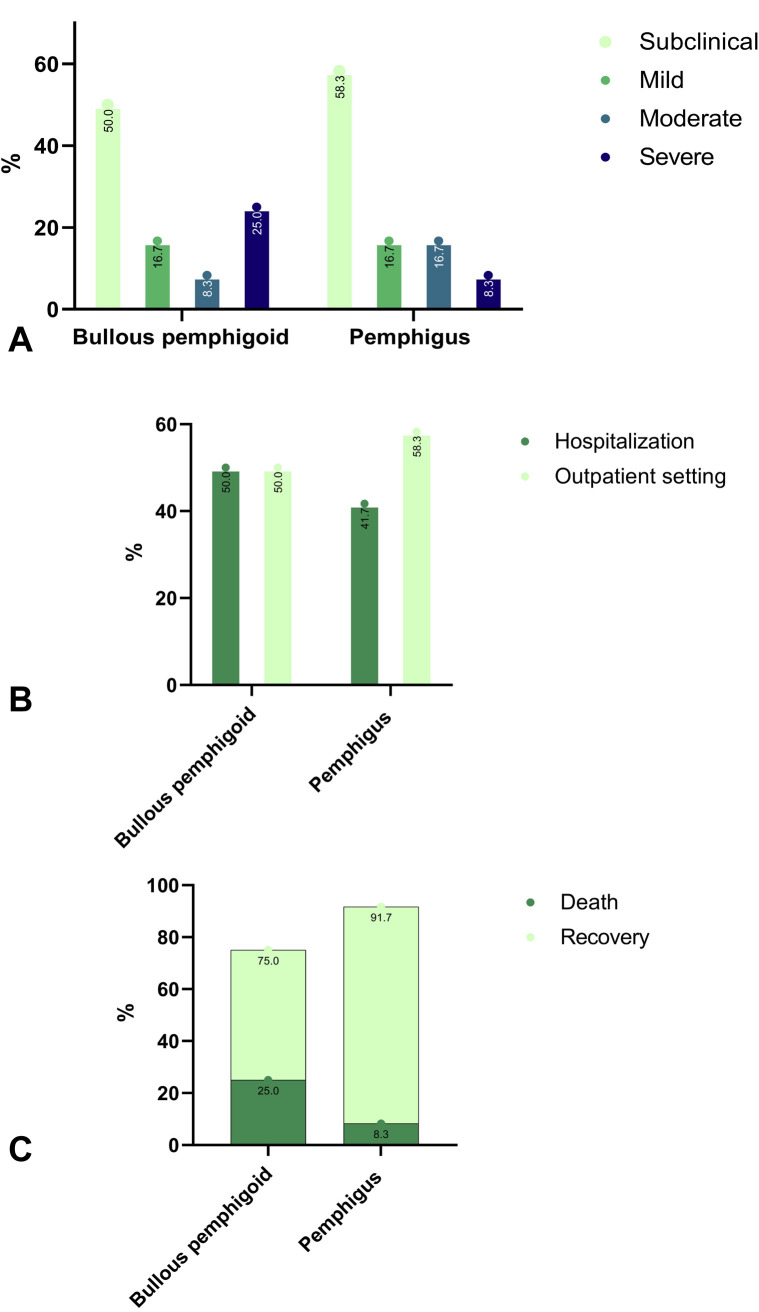
Overall, 24 (1.3%) patients with BP tested positive for COVID-19. The disease was subclinical in 12 (50.0%), mild in 4 (16.7%), moderate in 2 (8.3%), and severe in 6 (25.0%). Although 12 (50.0%) patients were hospitalized, not the same that had a subclinical disease, due to COVID-19 complications, none of them underwent mechanical ventilation. Six (25.0%) patients died following COVID-19 infection (Fig 1 ).
Fig 1.
The different features of COVID-19 among patients with bullous pemphigoid and pemphigus. A, severity; B, hospitalization; C, mortality.
Twelve (1.0%) patients with pemphigus had a COVID-19 infection. While 7 (58.3%) patients had a subclinical disease, 2 (16.7%) had a mild disease, 2 (16.7%) had moderate disease, and 1 (8.3%) had severe disease. Five (41.7%) patients were hospitalized, but none was mechanically ventilated. One patient (8.3%) died following COVID-19 infection (Fig 1).
Matched controls of BP presented with 130 (1.2%) cases of COVID-19, of whom 84 (64.6%), 11 (8.5%), 13 (10.0%), and 22 (16.9%) were subclinical, mild, moderate, and severe, respectively. Matched controls of pemphigus had 79 (1.2%) COVID-19 positive cases, with 60 (75.9%), 2 (2.5%), 5 (6.3%), and 12 (15.2%) individuals presenting with subclinical, mild, moderate, and severe disease, respectively.
The risk of COVID-19 among patients with bullous pemphigoid
The incidence rate of COVID-19 among patients with BP was estimated to be 24.4 (95% CI, 16.0-35.7) per 1,000 person-years. The incidence rates of hospitalization and mortality due to COVID-19 complications were 12.2 (95% CI, 6.6-20.7) and 7.1 (95% CI, 3.1-14.0) per 1000 person-years, respectively.
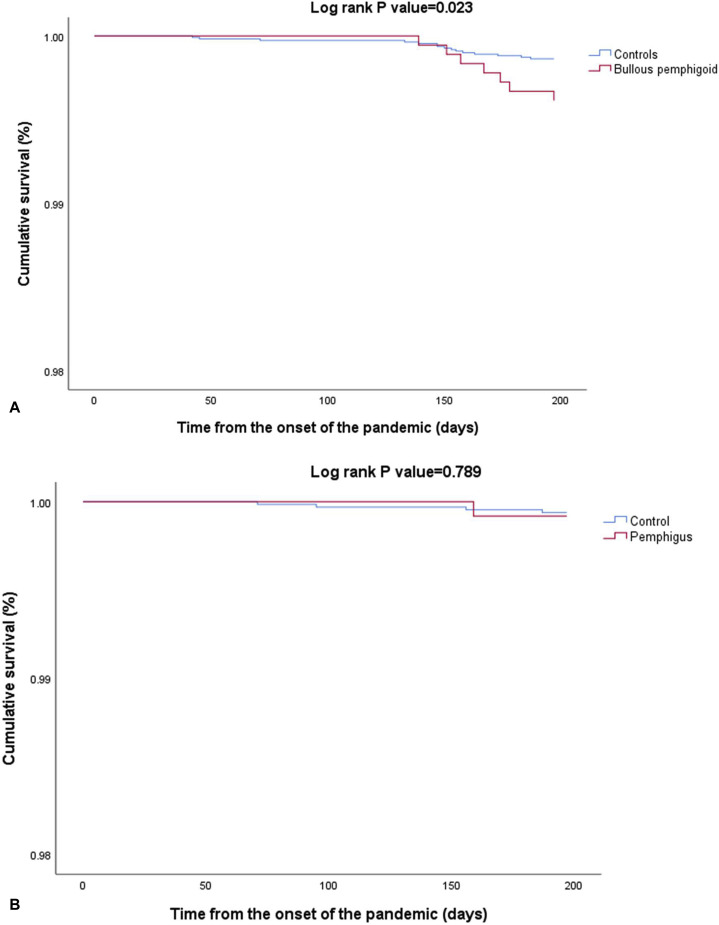
The unadjusted risk of acquiring the infection (HR, 1.12; 95% CI, 0.72-1.73; P = .691) and being hospitalized due to the infection (HR, 1.58; 95% CI, 0.84-2.98; P = .160) were comparable between patients with BP and their control subjects (Table I ). However, the risk of COVID-19-associated mortality was significantly higher among patients with BP as compared to their matched control subjects (HR, 2.82; 95% CI, 1.15-6.92; P = .023; Fig 2 , A). Increased mortality was more prominent among individuals older than 80.8 years of age (HR, 3.21; 95% CI, 1.21-8.56; P = .020) and persisted following adjustment for age, sex, ethnicity, comorbidities, exposure to systemic corticosteroids and immunosuppressants, and smoking (adjusted HR, 2.81; 95% CI, 1.14-6.94; P = .025).
Table I.
The risk of COVID-19 and its complications among patients with bullous pemphigoid
| COVID-19 infection |
COVID-19-associated hospitalization |
COVID-19-associated mortality |
||||
|---|---|---|---|---|---|---|
| BP | Controls | BP | Controls | BP | Controls | |
| Follow-up time, PY | 984.9 | 5,947.6 | 986.4 | 5,958.0 | 987.8 | 5,964.3 |
| Median follow-up time, years (range) | 0.5 (0.1-0.5) | 0.5 (0.1-0.5) | 0.5 (0.1-0.5) | 0.5 (0.1-0.5) | 0.5 (0.1-0.5) | 0.5 (0.1-0.5) |
| Number of events | 24 | 130 | 12 | 46 | 7 | 15 |
| Incidence rate/1000 PY (95% CI) | 24.4 (16.0-35.7) | 21.9 (18.3-25.9) | 12.2 (6.6-20.7) | 7.7 (5.7-10.2) | 7.1 (3.1-14.0) | 2.5 (1.5-4.1) |
| Unadjusted HR (95% CI) [P value] | 1.12 (0.72-1.73) [.619] | Reference | 1.58 (0.84-2.98) [.160] | Reference | 2.82 (1.15-6.92) [.023]‡ | Reference |
| Adjusted HR (95% CI) [P value] | 1.10 (0.71-1.71) [.665]∗ | Reference | 1.63 (0.86-3.09) [.133] † | Reference | 2.81 (1.14-6.94) [.025]†,‡ | Reference |
| Sex- and age-stratified analysis | ||||||
| Male-specific HR (95% CI) [P value] | 0.91 (0.43-1.90) [.796] | Reference | 1.13 (0.39-3.29) [.819] | Reference | 3.13 (0.78-12.50) [0.107] | Reference |
| Female-specific HR (95% CI) [P value] | 1.26 (0.73-2.17) [.395] | Reference | 1.98 (0.89-4.40) [.096] | Reference | 2.63 (0.81-8.53) [.108] | Reference |
| ≥80.8 year-specific HR (95% CI) [P value] | 1.51 (0.88-2.61) [.135] | Reference | 1.93 (0.92-4.06) [.084] | Reference | 3.21 (1.21-8.56) [.020]‡ | Reference |
| <80.8 year-specific HR (95% CI) [P value] | 0.73 (0.35-1.53) [.403] | Reference | 1.07 (0.31-3.65) [.921] | Reference | 1.89 (0.20-18.18) [.581] | Reference |
BP, Bullous pemphigoid; CI, confidence interval; HR, hazard ratio; N, Number; PY, person-year.
Multivariate logistic regression model adjusting for age, sex, ethnicity, and comorbidities (as estimated by Charlson comorbidity index).
Multivariate logistic regression model adjusting for age, sex, ethnicity, comorbidities (as estimated by Charlson comorbidity index), intake of systemic corticosteroids and immunosuppressant, and smoking.
denotes significant value.
Fig 2.
Survival of patients with bullous pemphigoid (A) and pemphigus (B) as compared to control subjects since the onset of the pandemic, as illustrated by Kaplan-Meier survival curves.
The risk of COVID-19 among patients with pemphigus
Among patients with pemphigus, the incidence rates of COVID-19 infection, COVID-19-associated hospitalization, and mortality were 18.1 (95% CI, 9.8-30.8), 7.5 (95% CI, 2.8-16.7), and 1.5 (95% CI, 0.1-7.4) per 1,000 person-years, respectively. Relative to control subjects, patients with pemphigus displayed a comparable risk of COVID-19 infection (HR, 0.81; 95% CI, 0.44-1.49; P = .496), COVID-19-associated hospitalization (HR, 1.41; 95% CI, 0.53-3.76; P = .499), and COVID-19-associated mortality (HR, 1.33; 95% CI, 0.15-11.92; P = .789; Fig 2, B). The comparable risk of the 3 outcomes persisted in age- and sex-stratified analyses as well as in multivariable analysis adjusting for putative confounders (Table II ).
Table II.
The risk of COVID-19 and its complications among patients with bullous pemphigus
| COVID-19 infection |
COVID-19-associated hospitalization |
COVID-19-associated mortality |
||||
|---|---|---|---|---|---|---|
| Pemphigus | Controls | Pemphigus | Controls | Pemphigus | Controls | |
| Follow-up time, PY | 662.4 | 3528.4 | 663.2 | 3535.8 | 664.3 | 3,537.9 |
| Median follow-up time, years (range) | 0.5 (0.1-0.5) | 0.5 (0.1-0.5) | 0.5 (0.1-0.5) | 0.5 (0.1-0.5) | 0.5 (0.1-0.5) | 0.5 (0.1-0.5) |
| Number of events | 12 | 79 | 5 | 19 | 1 | 4 |
| Incidence rate/1000 PY (95% CI) | 18.1 (9.8-30.8) | 22.4 (17.9-27.8) | 7.5 (2.8-16.7) | 5.4 (3.3-8.2) | 1.5 (0.1-7.4) | 1.1 (0.4-2.7) |
| Unadjusted HR (95% CI) [P value] | 0.81 (0.44-1.49) [.496] | Reference | 1.41 (0.53-3.76) [.499] | Reference | 1.33 (0.15-11.92) [.789] | Reference |
| Adjusted HR (95% CI)∗ [P value] | 0.79 (0.43-1.46) [.454]∗ | Reference | 1.36 (0.51-3.66) [.542] † | Reference | 1.15 (0.12-10.98) [.866] † | Reference |
| Sex- and age-stratified analysis | ||||||
| Male-specific HR (95% CI) [P value] | 1.21 (0.59-2.94) [.605] | Reference | 1.47 (0.41-5.25) [.558] | Reference | 1.79 (0.19-17.16) [.616] | Reference |
| Female-specific HR (95% CI) [P value] | 0.41 (0.13-1.32) [.134] | Reference | 1.33 (0.28-6.25) [.720] | Reference | NA | Reference |
| ≥66.6 year-specific HR (95% CI) [P value] | 0.78 (0.31-1.99) [.603] | Reference | 0.80 (0.18-3.54) [.773] | Reference | 1.41 (0.16-12.59) [.760] | Reference |
| <66.6 year-specific HR (95% CI) [P value] | 0.82 (0.37-1.82) [.627] | Reference | 3.03 (0.72-12.67) [.129] | Reference | NA | Reference |
CI, Confidence interval; HR, hazard ratio; n, number; PY, person-year.
Multivariate logistic regression model adjusting for age, sex, ethnicity, and comorbidities (as estimated by Charlson comorbidity index).
Multivariate logistic regression model adjusting for age, sex, ethnicity, comorbidities (as estimated by Charlson comorbidity index), intake of systemic corticosteroids and immunosuppressant, and smoking.
Determinants of moderate-to-severe COVID-19 infection among patients with BP and pemphigus
Table III demonstrates the demographic and clinical features of patients with BP and pemphigus who tested positive for COVID-19 and stratified by the severity of the infectious disease. Although smoking was less frequent among pemphigus patients with moderate-to-severe COVID-19, the remaining variables (including the frequency of systemic corticosteroids and immunosuppressive agents at the onset of the pandemic) distributed equally between BP and pemphigus patients with moderate-to-severe and subclinical-to-mild COVID-19 (Table III). When COVID-19-positive control subjects were both BP and pemphigus, stratified by severity, those with moderate-to-severe infection were older and had higher burden of comorbidities (Supplementary Table II).
Table III.
Determinants of COVID-19 severity among patients with bullous pemphigoid and pemphigus
| BP with subclinical-to-mild COVID-19 (n = 16) | BP with moderate-to-severe COVID-19 (n = 8) | P Value | Pemphigus with subclinical-to-mild COVID-19 (n = 9) | Pemphigus with moderate-to-severe COVID-19 (n = 3) | P Value | |
|---|---|---|---|---|---|---|
| Age at the onset of pandemic; years, mean (SD) | 73.2 (20.2) | 83.2 (9.8) | .119 | 56.1 (17.5) | 73.9 (13.0) | .139 |
| Duration of the disease; years, mean (SD) | 5.3 (3.9) | 3.0 (2.3) | .134 | 6.5 (4.5) | 12.2 (2.6) | .065 |
| Female sex; n (%) | 12 (75.0%) | 4 (50.0%) | .221 | 2 (22.2%) | 1 (33.3%) | .700 |
| Jewish ethnicity; n (%) | 11 (68.8%) | 8 (100.0%) | .076 | 8 (88.9%) | 3 (100.0%) | .546 |
| BMI, mg/kg2; mean (SD) | 30.2 (5.4) | 26.6 (4.0) | .118 | 27.7 (5.2) | 25.5 (NA)∗ | NA |
| Smoking; n (%) | 3 (18.8%) | 2 (25.0%) | .722 | 6 (66.7%) | 0 (0.0%) | .046 |
| CCI; mean (SD) | 2.5 (2.1) | 2.9 (1.1) | .643 | 0.7 (0.9) | 1.0 (1.0) | .588 |
| Systemic corticosteroids at the onset of the pandemic; n (%) | 3 (18.8%) | 4 (50.0%) | .112 | 6 (66.7%) | 1 (33.3%) | .310 |
| Adjuvant agents at the onset of the pandemic∗; n (%) | 0 (0.0%) | 0 (0.0%) | .999 | 1 (11.1%) | 0 (0.0%) | .546 |
| Systemic corticosteroids anytime during the course of the diseases; n (%) | 12 (75.0%) | 8 (100.0%) | .121 | 8 (88.9%) | 2 (66.7%) | .371 |
| Adjuvant agents anytime during the course of the diseases∗; n (%) | 0 (0.0%) | 1 (12.5%) | .149 | 2 (22.2%) | 2 (66.7%) | .157 |
BMI, Body mass index; BP, bullous pemphigoid; CCI, Charlson comorbidity index; n, number; SD, standard deviation.
Bold denotes significant value.
Patients managed by one of the following agents: azathioprine, mycophenolate mofetil, methotrexate, cyclophosphamide, dapsone, doxycycline, rituximab, plasmapheresis, intravenous immunoglobulins.
Discussion
The current population-based study revealed that although the risk of COVID-19 was comparable in patients with BP and pemphigus relative to their controls, COVID-19-associated mortality was significantly elevated among patients with BP. The duration of BP and pemphigus at the onset of the pandemic and exposure to systemic corticosteroids and immunosuppressive agents were not found to predict severe COVID-19 illness.
BP and pemphigus are among the life-threatening dermatoses posing a real therapeutic challenge and conferring a high inpatient burden.24 , 25 Patients with both conditions were found to experience an increased risk for respiratory, cutaneous, multiorgan, and systemic infections, which were associated with considerable inpatient mortality and costs.26 The susceptibility to bacterial and viral infectious conditions in AIBD is consistent with that of other autoimmune diseases27 and may be attributed to immune dysregulation, higher prevalence of predisposing comorbidities (such as diabetes mellitus and cardiovascular conditions), and the chronic exposure to immunomodulatory medications.6 , 28 Therefore, estimating the risk of COVID-19 among patients with AIBD was an urgent unmet need.
We did not find evidence for change in the overall risk of COVID-19 among patients with BP and pemphigus, compared to their controls, which is consistent with other autoimmune and rheumatic diseases.6, 7, 8, 9, 10 This increasing body of evidence indicates that the risk of catching the infection relies mainly on whether patients are exposed to the pathogen, adhere to social distancing, and follow safety instructions.
The more interesting question is whether patients with AIBD follow a more severe course of COVID-19 and are more predisposed to the infection's complications. We found that patients with BP had an elevated COVID-19-associated mortality. Although the severity of COVID-19 is yet to be elucidated in AIBD, some studies that followed patients with other autoimmune diseases disclosed a more aggressive course of COVID-19 in patients with preexisting connective tissue diseases,3 and a higher frequency of mechanical ventilation among those with rheumatic diseases.5 Other studies found a similar course and comparable frequency of complications among those with various systemic autoimmune diseases6 and inflammatory bowel disease (IBD).10 In the current study, smoking was less frequent among patients with pemphigus. Because smoking is associated with the worse outcomes of COVID-19,22 its lower prevalence in patients with pemphigus may account, at least in part, for the comparable risk of COVID-19 complications in pemphigus.
In the current study, exposure to systemic corticosteroids and immunosuppressive agents was not significantly associated with the severity of COVID-19. This finding may provide evidence-based advice on the importance of maintaining therapies during the pandemic. Discordant findings emerged from a global registry of patients with rheumatic diseases in which the use of systemic corticosteroids, but not other therapies, predicted an increased probability of COVID-19-associated hospitalization.4 Of note, exposure to tumor necrosis factor-α (TNF- α) antagonist was associated with a decreased risk of hospitalization in repository study.4 Systemic corticosteroids, but not biologic agents, were found to increase the risk of severe COVID-19 among patients with IBD.10 , 29 Solid organ transplant recipients, often placed on various immunosuppressive regimens, were found to be more susceptible to developing severe COVID-19.30 , 31
These conflicting findings may reflect the differential role exerted by immunosuppressive agents throughout different stages of the triphasic course of COVID-19.23 Although immunosuppressive drugs can be detrimental in the initial phase of the disease, when the host immune response is essential to constrain viral replication, these drugs may confer a protective role in the advanced severe phase of the diseases. In the latter, overshooting the host immune response (the “cytokine storm”), also referred to as secondary hemophagocytic lymphohistiocytosis, can result in acute respiratory distress syndrome, multi-organ failure, and mortality.23 Further research utilizing larger sample sizes is necessary to better delineate the precise role of immunosuppressive drugs in the course of COVID-19.
The observations of the current study, once verified by other research groups, indicate that percussions should be practiced by the health care system for elderly patients with BP. Patients should be notified in advance that they are at increased risk for mortality and BP patients should avoid contagion, even more so than other people. The health care system should organize an outreach program to ensure the well-being of BP patients. During hospitalization, close monitoring is highly recommended. At discharge, the hospital should notify community health care personnel about the excess risk for mortality in BP patients.
The current study throws light on an important and unexplored topic. The large sample size and the allocation of control groups enabled an assessment of the relative risk of COVID-19 in patients with BP and pemphigus. Given that the CHS data set facilitates comprehensive access to the whole array of health care services, it is highly compatible with detecting COVID-19 cases, even those occurring years following the initial diagnosis of BP and pemphigus. The latter cannot be fulfilled in hospital-based cohorts, where AIBD patients tend to be lost to follow-up as the time from the diagnosis increases. The study was performed in a country typified by a high incidence rate of COVID-19, thus allowing the detection and characterization of positive cases.
The study has several limitations. Because it was based on a computerized data set, the current study did not follow the acceptable immunopathologic criteria to define BP and pemphigus. However, eligibility was grounded on the diagnosis distributed by board-certified dermatologists or after admission to a dermatologic ward. In both settings, immunopathologic diagnostics are widely utilized,32 thus arguing in favor of the validity of the diagnosis. Because the index date dictating age matching was defined at the diagnosis of BP and pemphigus, the case and control groups displayed slightly different ages at the onset of the pandemic. However, multivariable analysis adjusting for age did not alter any of the outcome measures. An additional drawback stems from the small sample size of the subgroups compared to define predictors of COVID-19 severity among patients with BP and pemphigus. To overcome some of these shortcomings a registry for all AIBD patients that had a confirmed case of COVID-19 has been initiated by the AIBD task force of the European Academy of Dermatology and Venereology (https://recovab.umcg.nl).
The current population-based study provides a seminal report about the risk of COVID-19 in AIBD and defines the determinants of severe illness. Although the risk of acquiring COVID-19 infection was comparable in BP and pemphigus patients relative to their control subjects, patients with BP demonstrated an increased COVID-19-associated mortality. The administration of systemic corticosteroids and adjuvant immunosuppressive agents and the duration of BP and pemphigus did not seem to affect the severity of the infection or its complications. Consistent with current expert recommendations18 , 33 and using an evidence-based approach, the current study argues in favor of maintaining AIBD-related therapies during the pandemic. Given their greater vulnerability, patients with BP developing COVID-19 should be monitored closely.
Conflicts of interest
Dr. Cohen served as an advisor, investigator, or speaker for Abbvie, BI, Dexcel Pharma, Janssen, Novartis, Perrigo, Pfizer, and Rafa. Drs Kridin, Schonmann, Weinstein, Schmidt, and Ludwig have no conflicts of interest to declare.
Footnotes
Funding sources: None.
IRB approval status: The current study was approved by the Ben-Gurion University IRB in accordance with the declaration of Helsinki (approval code: 0212-17-COM).
References
- 1.Shi Y., Wang Y., Shao C., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vabret N., Britton G.J., Gruber C., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pablos J.L., Galindo M., Carmona L., et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. 2020;79(12):1544–1549. doi: 10.1136/annrheumdis-2020-218296. [DOI] [PubMed] [Google Scholar]
- 4.Gianfrancesco M., Hyrich K.L., Al-Adely S., et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Silva K.M., Serling-Boyd N., Wallwork R., et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’. Ann Rheum Dis. 2020;79(9):1156–1162. doi: 10.1136/annrheumdis-2020-217888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansarin K., Taghizadieh A., Safiri S., et al. COVID-19 outcomes in patients with systemic autoimmune diseases treated with immunomodulatory drugs. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218737. annrheumdis-2020-218737. [DOI] [PubMed] [Google Scholar]
- 7.Emmi G., Bettiol A., Mattioli I., et al. SARS-CoV-2 infection among patients with systemic autoimmune diseases. Autoimmun Rev. 2020;19(7):102575. doi: 10.1016/j.autrev.2020.102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu M., Gao Y., Zhang Y., Shi S., Chen Y., Tian J. The association between severe or dead COVID-19 and autoimmune diseases: a systematic review and meta-analysis. J Infect. 2020;81(3):e93–e95. doi: 10.1016/j.jinf.2020.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredi M., Cavazzana I., Moschetti L., et al. COVID-19 in patients with rheumatic diseases in northern Italy: a single-centre observational and case–control study. Lancet Rheumatol. 2020;2(9):e549–e556. doi: 10.1016/S2665-9913(20)30169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macaluso F.S., Orlando A. COVID-19 in patients with inflammatory bowel disease: a systematic review of clinical data. Dig Liver Dis. 2020;52(11):1222–1227. doi: 10.1016/j.dld.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kridin K. Pemphigus group: overview, epidemiology, mortality, and comorbidities. Immunol Res. 2018;66(2):255–270. doi: 10.1007/s12026-018-8986-7. [DOI] [PubMed] [Google Scholar]
- 12.Kridin K. Subepidermal autoimmune bullous diseases: overview, epidemiology, and associations. Immunol Res. 2018;66(1):6–17. doi: 10.1007/s12026-017-8975-2. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt E., Kasperkiewicz M., Joly P. Pemphigus. Lancet. 2019;394(10201):882–894. doi: 10.1016/S0140-6736(19)31778-7. [DOI] [PubMed] [Google Scholar]
- 14.Kridin K., Sagi S.Z., Bergman R. Mortality and cause of death in patients with pemphigus. Acta Derm Venereol. 2017;97(5):607–611. doi: 10.2340/00015555-2611. [DOI] [PubMed] [Google Scholar]
- 15.Kridin K., Shihade W., Bergman R. Mortality in patients with bullous pemphigoid: a retrospective cohort study, systematic review and meta-analysis. Acta Derm Venereol. 2018;99(1):72–77. doi: 10.2340/00015555-2930. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt E., Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- 17.Kridin K., Ahn C., Huang W.C., Ansari A., Sami N. Treatment update of autoimmune blistering diseases. Dermatol Clin. 2019;37(2):215–228. doi: 10.1016/j.det.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Kasperkiewicz M., Schmidt E., Fairley J.A., et al. Expert recommendations for the management of autoimmune bullous diseases during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2020;34(7):e302–e303. doi: 10.1111/jdv.16525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen A.D., Dreiher J., Regev-Rosenberg S., et al. The quality indigators program in Clalit Health Services: the first decade. Harefuah. 2010;149(4):204–265. [PubMed] [Google Scholar]
- 20.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Z., Peng F., Xu B., et al. Risk factors of critical and mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Q., Meng M., Kumar R., et al. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J Med Virol. 2020;92(10):1915–1921. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoot T.S., Kerckhoffs A.P.M., Hilbrands L.B., van Marum R.J. Immunosuppressive drugs and COVID-19: a review. Front Pharmacol. 2020;11:1333. doi: 10.3389/fphar.2020.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren Z., Hsu D.Y., Brieva J., Silverberg N.B., Langan S.M., Silverberg J.I. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol. 2017;176(1):87–99. doi: 10.1111/bjd.14821. [DOI] [PubMed] [Google Scholar]
- 25.Hsu D., Brieva J., Silverberg J.I. Costs of Care for hospitalization for pemphigus in the United States. JAMA Dermatol. 2016;152(6):645–654. doi: 10.1001/jamadermatol.2015.5240. [DOI] [PubMed] [Google Scholar]
- 26.Ren Z., Narla S., Hsu D.Y., Silverberg J.I. Association of serious infections with pemphigus and pemphigoid: analysis of the Nationwide Inpatient Sample. J Eur Acad Dermatol Venereol. 2018;32(10):1768–1776. doi: 10.1111/jdv.14961. [DOI] [PubMed] [Google Scholar]
- 27.Singh J.A., Cameron C., Noorbaloochi S., et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet. 2015;386(9990):258–265. doi: 10.1016/S0140-6736(14)61704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong S.J., Choi H., Lee H.S., et al. Incidence and risk factors of infection in a single cohort of 110 adults with systemic lupus erythematosus. Scand J Infect Dis. 2009;41(4):268–274. doi: 10.1080/00365540902744741. [DOI] [PubMed] [Google Scholar]
- 29.Brenner E.J., Ungaro R.C., Gearry R.B., et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159(2):481–491.e3. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernández-Ruiz M., Andrés A., Loinaz C., et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20(7):1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Travi G., Rossotti R., Merli M., et al. Clinical outcome in solid organ transplant recipients with COVID-19: a single-center experience. Am J Transplant. 2020;20(9):2628–2629. doi: 10.1111/ajt.16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kridin K., Zelber-Sagi S., Khamaisi M., Cohen A.D., Bergman R. Remarkable differences in the epidemiology of pemphigus among two ethnic populations in the same geographic region. J Am Acad Dermatol. 2016;75(5):925–930. doi: 10.1016/j.jaad.2016.06.055. [DOI] [PubMed] [Google Scholar]
- 33.Shakshouk H., Daneshpazhooh M., Murrell D.F., Lehman J.S. Treatment considerations for patients with pemphigus during the COVID-19 pandemic. J Am Acad Dermatol. 2020;82(6):e235–e236. doi: 10.1016/j.jaad.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]