Abstract
Dance is a culturally salient form of physical activity (PA) for older Latinos. Resting-state functional connectivity (FC) is a putative biomarker for age-related cognitive decline. We aimed to investigate the impact of the BAILAMOS™ dance program on FC in three brain functional networks (Default Mode [DMN], Frontoparietal [FPN], and Salience [SAL] networks), and cognition. Ten cognitively healthy older Latinos participated in the four-month BAILAMOS™ dance program. We assessed PA levels (self-reported and device-assessed) and estimated cardiorespiratory fitness, cognition, and resting-state FC via functional magnetic resonance imaging at baseline and post-intervention. We performed paired t-tests and Pearson correlations. Given the pilot nature of the study, significance levels were set at p < 0.05 and effect sizes are reported. We observed a significant increase in self-reported moderate leisure-time PA from pre- to post-intervention (t(9) = 3.16, p = 0.011, d = 0.66). FC within-FPN regions of interest (ROIs) significantly increased pre- to post-intervention (t(9) = 2.35, p = 0.043, d = 0.70). DMN ROIs showed an increase, with a moderate effect size, in the integration with other networks’ ROIs (t(9) = 1.96, p = 0.081, d = 0.64) post-intervention. Increases in moderate leisure-time PA at post-intervention were associated with increases in the FC within-FPN (R = 0.79, p = 0.006). Our results suggest that dance might be a promising approach for improving age-related disruption of FC within- and between-networks commonly associated with cognitive decline.
Keywords: Older Latino adults, Dance, Physical activity, Brain functional connectivity, Cognition
Introduction
Increasing physical activity (PA) levels is paramount to decreasing the risk of age-related chronic diseases (Booth, Roberts, and Laye 2012). Older Latinos have a higher prevalence of obesity, type 2 diabetes, and metabolic syndrome compared to non-Latino whites (Benjamin et al. 2017), and these conditions increase the risk for age-related cognitive decline (González et al. 2018). Older Latinos also engage in less leisure-time PA than other racial/ethnic groups (Keadle et al. 2016). Therefore, increasing engagement in PA might be an effective approach for reducing numerous risk factors for chronic conditions, including cognitive impairment, among higher-risk groups such as Latinos.
Age-related cognitive decline tends to manifest in the domains of attention, memory, and executive function earlier and more acutely than other cognitive domains (Hedden and Gabrieli 2004). These cognitive changes are preceded by a heterogeneous array of changes to brain morphology and function (Lin et al. 2018). Within brain function, functional connectivity (FC) is a valuable measure of brain health because it correlates both with normal age-related decline in brain function and decline due to pathologies (Lin, Xing, & Han 2018). Notably, dysfunctions due to cognitive decline are more accentuated in specific brain networks such as DMN (Ferreira & Busatto 2013; Jagust 2013; Voss et al. 2016), FPN (Ferreira & Busatto 2013; Jagust 2013; Voss et al. 2016), and SAL (Onoda, Ishihara, & Yamaguchi 2012; Voss et al. 2016).
Researchers have used resting-state FC to identify brain regions that are both negatively affected by aging and modifiable through participation in PA. A review of neuroimaging studies on PA in healthy older adults and those with mild cognitive impairment suggested that the default-mode-network (DMN), fronto-executive network (FEN), and frontoparietal network (FPN) were resting networks potentially affected by PA interventions (Huang et al. 2016). Moreover, another critical review summarized studies regarding the relationships between FC, exercise, fitness, and PA in older adults (Stillman, Donofry, and Erickson 2019), and results suggested that PA and exercise might be effective for preserving or strengthening FC within and between large-scale brain networks (e.g., DMN, FPN, and salience [SAL]) disrupted with both normal and pathological aging.
Interventions utilizing dance as a form of PA offers a combination of physical, cognitive, and social activities potentially effective for maintaining or improving cognition because it includes components that influence adherence such as social activity, enjoyment, and a variety of movements (Esmail et al. 2020; Predovan, Julien, Esmail, & Bherer 2019). Although dance is considered a socially and cognitively enriching type of PA (Kattenstroth et al. 2013), the impact of dance on cognition and brain network FC in healthy older adults is poorly understood.
Studies that have examined the effect of a dance intervention on cognition have reported improved overall cognition (concentration, attention, and non-verbal learning) (Kattenstroth et al. 2013), as well as improvements within specific domains of cognitive flexibility (i.e., ability to adapt behaviors in response to changes in the environment) (Coubard et al. 2011), visuospatial learning (Merom et al. 2016), motor-cognitive dual-task performance while walking (Hamacher et al. 2015), and verbal short-term memory, long-term free recall and recognition (Rehfeld et al. 2018) compared to other forms of exercise or control conditions. Nonetheless, these studies proposed a range of different dance styles (e.g., Agilando™, contemporary dance, Rock and Roll, Foxtrot, Waltz, Rumba, Line Dance).
Studies investigating the impact of dance training on resting FC are scarce. Voss et al. (2019) found no changes in the DMN and SAL after a 24-week dance intervention compared to two groups who engaged in aerobic exercise and one control group. Conversely, participation in a 24–week traditional Greek dance program led to increased FC in the executive-control network, DMN, and FPN (Zilidou et al. 2018).
No studies have explored the impact of Latin dance styles on FC and cognition in older Latinos. It is possible that benefits derived from dance are moderated by dance styles, participant’s racial/ethnic background, and the synergy between dance style and cultural background. This is an important gap in the literature because older Latinos are at higher risk of developing chronic diseases associated with cognitive impairment, engage in less leisure-time PA, and consider dance as a culturally relevant form of PA (Mier, Medina, and Ory 2007; Wilbur et al. 2003; Marquez et al. 2016; Melillo et al. 2001).
The present pilot study examined the impact of Latin dance training on FC and cognition in an older, Spanish-speaking Latino sample. Given the small sample size and exploratory nature of the study, we focused on examining effect size in addition to statistically significant changes. We aimed to (a) investigate the impact of a four-month Latin dance program (BAILAMOS™) on FC in DMN, FPN, SAL, and cognitive performance; and (b) investigate whether changes in PA and estimated CRF as a result of the BAILAMOS™ dance program were associated with changes in FC in DMN, FPN, and SAL. We hypothesized that (a) FC within and between the DMN, FPN, and SAL regions would be significantly greater and/or present moderate effect sizes after participation in the BAILAMOS™ program; (b) changes in self-reported and device-assessed PA and estimated CRF would be associated with changes in cognition and FC within and between the DMN, FPN, SAL areas; and (c) any improvements in cognitive performance would be associated with changes in FC within and between the DMN, FPN, SAL areas.
Materials and Methods
Study design
This study was an exploratory single-group pre-post design. This is a secondary analysis of a pilot randomized-controlled open trial examining the impact of the BAILAMOS™ dance program on cognitive function, brain structure, and brain FC in older Latino adults. Participants were randomized to either BAILAMOS™ (n = 12) or a wait-list control group (n = 10). However, large attrition in the control group (60%), and high rates of differential attrition (44%) rendered the comparison group data unusable. Hence, we likely would not be powered to detect meaningful differences and infer causality. Therefore, for the purposes of this study, we only include data from participants from the BAILAMOS™ dance group. The study was approved by the University of Illinois at Chicago Institutional Review Board and conducted in accordance with the Declaration of Helsinki with written informed consent obtained from all participants.
Participants
Participants were recruited using established relationships of the University of Illinois at Chicago with the Latino community in Chicago (e.g., senior centers, churches). Research staff conducted presentations at the senior center in which the study took place, and at Roman Catholic churches in the vicinity of the senior center site. The majority of residents in the chosen neighborhood self-identify as Latinos. Additionally, recruitment included flyers in senior housing facilities, presentations at health centers and clinics, health fairs, and word of mouth. Participants in the current study were those in the BAILAMOS™ dance group with MRI scans at both pre- and post-intervention (n = 10) from the pilot randomized-controlled open trial mentioned above.
Inclusion criteria were: (a) aged 60 years or more; (b) self-identification as Latino/Hispanic; (c) self-reported ability to understand Spanish; (d) self-reported participation in less than two days per week of aerobic exercise; (e) at risk for disability (see below for a definition); (f) cognitively healthy (i.e., scored >14) as assessed by a modified version of the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh 1975) for telephone administration (21-point; Wilbur et al. 2012). Wilbur and colleagues mentioned that past studies using the 30-point MMSE with low-educational attainment older adults identified one-third incorrect as a cut-point for impaired/poor cognition (Raji et al. 2010), therefore, above 14 was the cut-point for normal cognition in the modified version; (g) danced less than two times/month over the past 12 months; (h) willingness to be randomly assigned to treatment or wait-list control group; and (i) no current plans to leave the country for more than two consecutive weeks over the next year.
We defined being at risk for disability as one of the following: (a) self-reported diagnosis of diabetes (Al Snih et al. 2007); (b) self-reported underweight (body mass index [BMI] lower than 18.5), overweight or obesity (BMI greater than 25.0) (Al Snih et al. 2007); or (d) difficulty or change with any one of the following four tasks: (1) walking a long distance (four blocks or half-mile), (2) climbing ten steps, (3) transferring from a bed or chair, (4) walking a short distance on a flat surface. Participants answered two questions for each task: “Have you had difficulty completing (task)” and “Have you changed the way you complete (task) or how often you do this, due to a health or physical condition?”
Self-reported exclusion criteria included: (a) uncontrolled cardiovascular disease; (b) pacemaker or metallic implants (infusion pumps, metal prostheses, metallic-backed transdermal patches or metallic shrapnel); (c) claustrophobia that precludes MRI; (d) stroke within the past year; (e) healing or unhealed fracture(s); (f) hip or knee replacement within the past six months; (g) heart failure; (h) recurrent falls within the past year; (i) regular use of a walker or wheelchair; and (j) weigh more than 300 pounds (unable to fit into the MRI). We used the Exercise Assessment and Screening for You (EASY) questionnaire to detect conditions that could prevent exercise participation (Resnick et al. 2008) and to determine whether physician evaluation and clearance was necessary before engaging in PA (Chodzko-Zajko, Resnick, and Ory 2012).
Measures
At baseline and post-intervention, we collected demographic information, overall physical health, cognitive function, self-reported and device-assessed PA, estimated CRF, and functional resting-state MRI. Data collectors were blinded to study condition. Baseline data were collected on average, nine weeks prior to the intervention start date. The period between data collection and the intervention start date was longer than we planned because we had to replace the dance instructor who was going to lead the classes. Moreover, this change was followed by the holiday season, which also delayed the beginning of the intervention. Post-intervention data were collected during the last week of the intervention and up to one week after the end of the intervention. Functional resting-state MRI data were collected on average, three weeks prior to the intervention, and two days after the intervention. At each class, we collected data on participants’ attendance, rate of perceived exertion, and class enjoyment.
Demographics, overall health, rate of perceived exertion, and enjoyment
Demographic information included age, sex, education, income, marital status, country of origin, race, ethnicity, preferred language, years lived in the U.S., and number of children. Physical health measures included measurements of weight (Tronix 5002 Stand-on Scale or Seca 803 Flat Scale), height (Seca 216 Mechanical Stadiometer or Seca 213 Portable Stadiometer), and body mass index (BMI)(kg/m2). At each class, we asked participants to grade their perceived exertion from 6 (no exertion) to 20 (maximal exertion) with the Borg Rating of Perceived Exertion (RPE) (Borg 1982) and to respond to a question about their perceived enjoyment on a Likert scale from 1 to 7 (strongly disagree to strongly agree).
Cognitive function
We utilized a set of neuropsychological tests that have been shown to be valid across ethnic and socioeconomic backgrounds and have been validated in Spanish (Marquine et al. 2012; Wilson et al. 2016). We administered all neuropsychological tests at baseline and post-intervention. We measured cognitive performance in three cognitive domains (i.e., executive function, working memory, and episodic memory) and one global cognition domain. These domains and respective tests have been previously proposed (Wilson et al. 2002; Wilson et al. 2005; Acevedo et al. 2009)
Four neuropsychological tests were utilized as measures of executive function: (1) Trail Making Test (TMT) parts A and B (Adjutant General’s Office 1944); (2) Stroop C (color task of the short form; Wilson et al. 2005) of the Stroop Neuropsychological Screening Test (Trenerry et al. 1989), and the Stroop C-W (color-word task); (3) Word fluency test (Welsh et al. 1994), and (4) Symbol Digit Modalities Test (Smith 1982). Three tests were used as measures of working memory, the two parts (forward and backward) of the Digit Span test (Wechsler 1987), and the Digit Ordering test (Cooper, Sagar, Jordan, Harvey, & Sullivan 1991; Wilson et al. 2005). Two tests were used to measure episodic memory, the Logical Memory I (Immediate) and II (Delayed) (Wechsler 1987).
We first converted the raw scores of the above mentioned neuropsychological tests to z-scores utilizing baseline means and standard deviations (Wilson et al. 2002). Then we combined the z-scores into composite scores of executive function, episodic memory, working memory, and global cognition by averaging the z-scores from each test (Wilson et al. 2002).
Physical Activity and cardiorespiratory fitness
Participants responded to the Community Healthy Activities Model Program for Seniors (CHAMPS) Physical Activity Questionnaire for Older Adults (Stewart et al. 2001). It assesses weekly frequency and duration of PA in four different lifestyle domains (leisure-time, household, occupational, and transportation) typically undertaken by older adults. The Spanish version of CHAMPS has been validated and employed with older Latinos (Rosario et al. 2008).
Device-assessed PA was acquired with a triaxial GT3X+ accelerometer (Actigraph, Pensacola, Florida). Participants wore it for one week at baseline and post-intervention, respectively, on their non-dominant wrist for seven consecutive days, removing it for showering and swimming. The research staff members instructed participants about the use of the accelerometer and provided a handout with additional instructions and pictures in Spanish or English. Participants also received an accelerometer log to record the times they removed and put back on the accelerometer. Data were included in the analysis if the participant wore the accelerometer for at least four days for more than 10 hours/day (Hart et al. 2011). Data were processed with ActiLife version 6.13.3 software after being converted to 60 seconds epochs. We defined non-wear time as at least 60 consecutive minutes of 0 activity counts. Average counts per minute (CPM) and steps are reported as the main outcome for device-assessed PA.
We estimated CRF with Jurca’s et al. (2005) regression equation, which estimates CRF in metabolic equivalents (METs) without exercise testing. The equation takes into account participants’ sex, age, BMI, resting heart rate, and PA levels on a scale from 1 to 5 (see Jurca et al. 2005 for detailed scale). We used the following equation: sex × (2.77) – age × (0.10) – BMI × (0.17) − resting heart rate × (0.03) + PA score + 18.07.
Neuroimaging data acquisition and processing
Participants underwent neuroimaging at University of Illinois at Chicago Advanced Imaging Center. Whole-brain images were acquired on a GE MR 750 Discovery 3T scanner (General Electric Health Care, Waukesha, WI) using an 8-channel head coil. Participants were instructed to remain still in a supine position on the scanner table. We provided earplugs to improve their comfort and positioned foam pads to minimize head movement. Resting-state functional MR images were acquired with a fast echo-planar imaging (EPI) sequence with the following parameters: repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; flip angle = 90°; FOV = 24 mm × 24 mm; acquisition matrix size 64 X 64 X 256; slice thickness = 4 mm; gap = 0 mm; 256 axial slices.
Functional connectomes were generated using the resting-state fMRI toolbox, CONN (http://www.nitrc.org/projects/conn) (Whitfield-Gabrieli and Nieto-Castanon 2012). In brief, raw EPI images were realigned, co-registered, normalized, and smoothed before analyses. Confound effects from motion artifact, white matter, and cerebrospinal fluid were regressed out of the signal. Using the “networks.nii” (with ROIs defined from CONN’s ICA analyses of HCP dataset / 497 subjects), functional brain networks (e.g., DMN, FPN, SAL, and Language) were derived using pairwise BOLD signal correlations, which were then converted to z-scores using Fisher’s r-to-z transformation. The ROIs selected for the present study are displayed in Table 1. The DMN, FPN, and SAL were selected as networks of interest due to evidence of the effects of aging and PA on these networks. The Language network was selected as a control network because most of language processes are relatively robust to brain aging (Shafto and Tyler 2014) and the lack of consistent evidence showing effects of PA on the Language network.
Table 1.
Networks regions of interest derived from functional connectomes generated from the resting-state fMRI toolbox (CONN)
| Network | Region of interest label (ROI) | Description of the anatomical region | MNI coordinates (x, y, z) |
|---|---|---|---|
| DMN | MPFC | Medial prefrontal cortex | 1, 55, −3 |
| DMN | LP_L | Left lateral parietal lobule | −39, −77, 33 |
| DMN | LP_R | Right lateral parietal lobule | 47, −67, 39 |
| DMN | PCC | Precuneus | 1, −61, 38 |
| SAL | ACC | Anterior cingulate cortex | 0, 22, 35 |
| SAL | AInsula_L | Left anterior insula | −44, 13, 1 |
| SAL | AInsula_R | Right anterior insula | 47, 14, 0 |
| SAL | RPFC_L | Left rostral prefrontal cortex | −32, 45, 27 |
| SAL | RPFC_R | Right rostral prefrontal cortex | 32, 46, 27 |
| SAL | SMG_L | Left supramarginal gyrus | −60, −39, 31 |
| SAL | SMG_R | Right supramarginal gyrus | 62, −35, 32 |
| FPN | LPFC_L | Left lateral prefrontal cortex | −43, 33, 28 |
| FPN | LPFC_R | Right lateral prefrontal cortex | 41, 38, 30 |
| FPN | PPC_L | Left posterior parietal cortex | −46, −58, 49 |
| FPN | PPC_R | Right posterior parietal cortex | 41, 38, 30 |
| LAN | IFG_L | Left inferior frontal gyrus | −51,26,2 |
| LAN | IFG_R | Right inferior frontal gyrus | 54,28,1 |
| LAN | pSTG_L | Left posterior superior temporal gyrus | −57,−47,15 |
| LAN | pSTG_R | Right posterior superior temporal gyrus | 59,−42,13 |
Procedures
Individuals interested in participating provided consent to be called by research staff to perform phone screening. Those individuals that learned about the study via word of mouth or flyers called to schedule a time for the screening. Bilingual and bicultural research staff members conducted the screening.
Eligible participants were scheduled for baseline testing, which was performed by bilingual research staff. Data collection occurred over two different sessions. In the first visit, a research staff member administered the informed consent, questionnaires (demographic information and PA), and neuropsychological tests in Spanish or English, as requested by the participant. Prior to the pilot, research staff members received training on how to conduct the select neuropsychological tests. The training was led by a research trainer from the Rush Alzheimer’s Disease Center. Participants also received the accelerometer to wear for the next seven days. The research staff provided all information related to proper accelerometer use. Also, at this session, the staff member showed images of the MRI machine to make sure that participants understood the details about the MRI exam and agreed to participate in the study. This session took place at the Pilsen satellite senior center. Participants attended a second in-person session for MRI data acquisition at the University of Illinois at Chicago Advanced Imaging Center. Participants received a $50 gift card compensation after the data collection. The same procedures (except informed consent and demographic questionnaires) were repeated for post-testing.
Intervention
Participants took part in the BAILAMOS™ dance program. Details about the program have been previously reported (Marquez et al. 2014). Briefly, the program was a four-month, twice a week for 60-min dance program with four Latin dance styles (Merengue, Cha Cha Cha, Bachata, and Salsa) ordered by difficulty level and aiming to offer light to moderate intensity PA. Participants learned a new style of dance every four weeks. Within those four weeks, participants learned new steps when the instructor felt participants were ready to proceed according to the dance BAILAMOS™ dance program manual. All music used in the program was Latin, initially chosen by the instructor. Participants were also invited to give their input, and songs were included in the instructor’s selection.
Dance sessions included warm-up and stretching, instructions of the respective dance style with steps for singles and couples, and cool down. Couples learned steps of both leaders and followers, and continually rotated partners. All participants learned the steps without a partner; then, all participants paired up, learned with a partner, and changed partners after that (e.g., for each new song). Steps were first taught without music. Once participants were comfortable with the fundamental steps of a style of dance, music was incorporated moving forward.
Twice a month, participants attended fiestas de baile (dance parties) in which they had time to practice the learned steps. Attendance at each session and fiestas de baile were recorded. Adherence was calculated by the number of classes attended, divided by the number of classes conducted (32 total). Initially, the dance program was led by a white female instructor who was a proficient Spanish speaker with about five years of experience teaching Latin dance. Due to unforeseen reasons, the instructor could no longer teach the classes; and the co-creator of the BAILAMOS™ dance program, a Latino male with more than 20 years of teaching experience took over the dance instruction.
Statistical Analysis
All analyses were conducted in RStudio version 3.5.14 (RStudio Team 2019). We assessed distributions with the Shapiro-Wilk normality test. Due to the small sample size and the skewed distribution of the self-reported PA data, we first opted to conduct the Wilcoxon Signed Ranked test for paired samples to examine changes in the outcome variables pre- and post-intervention. For sensitivity analysis, we carried out paired t-tests. Since results were the same using either approach, we chose to present data from the paired t-tests, means and standard deviations. We conducted Spearman correlations to investigate whether changes in PA levels were correlated with changes in cognitive function and FC within and between ROIs in the DMN, FPN, SAL, and a network utilized as control, the language network. Significance levels were set at p < 0.05.
We computed Cohen’s d effect size with effsize package (Torchiano 2019). Effect sizes were classified as small (d = 0.2), moderate (d = 0.5), and large (d ≥ 0.8) (Sullivan and Feinn 2012). We utilized the BrainNet Viewer (https://www.nitrc.org/projects/bnv/) (Xia, Wang, and He 2013) to display figures of brain FC between ROIs.
We conducted four different analyses comparing the FC pre- and post- BAILAMOS™ dance program. First, we compared the FC between each networks’ ROIs and seeds across the whole brain. We displayed results for those ROIs with moderate effect sizes (0.5 ≥ d ≤ 0.8). Second, we compared the average FC within-ROIs for each network and display results for the FPN since FC within-FPN presented moderate effect size changes. Third, we compared the average FC within-ROIs between each network. Fourth, we created a ratio of integration/segregation. This ratio was calculated with the average FC between-networks divided by the average FC from within-networks ROIs. We compared the ratio for each network of interest pre- and post-BAILAMOS™. The ratio was created based on previous research suggesting that understanding both network segregation and network integration is essential to grasp how they relate to different aspects of cognition (Cohen and D’Esposito 2016).
As the study was an exploratory pilot, we did not conduct a priori sample size calculations. However, a post-hoc power calculation showed that it would be necessary to achieve an N = 34 to detect a moderate effect size change with 80% statistical power. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Demographic information, attendance, perceived exertion, and enjoyment
Participants (N = 10) were 67.1±6.2 years old, a majority were females (n = 7), overweight (28.3±5.3 kg/m2), immigrated to the US from Mexico (n = 9) or Guatemala (n = 1) when they were 25.1±12.4 years old, and had been living in the U.S. for 39.5±12.8 years at the time of data collection. They spent from 4 to 15 years in school (8.7±4.2), reported low (n=6) or medium (n=4) income, and most had never participated in structured PA before (n=7). The average attendance was 77±26.9% of classes and dance parties. Self-reported RPE ranged from 8.5 to 11 (10.0±2.6) over the 32 classes of the BAILAMOS™ dance program, reflecting a primarily light intensity range. The average perception of enjoyment during classes ranged from 5.6 to 7.0 (6.6±0.7).
BAILAMOS™ effects on PA levels, estimated CRF, and cognition
Participants had high adherence to the accelerometer with an average of 6.8 ± 0.7 valid days for 1251.1 ± 238.8 min/day at baseline and 6.44 ± 0.88 for 1241.2 ± 255.6 min/day at post-intervention. There were no significant increases in device-assessed PA or estimated CRF (Table 2) after the four-month BAILAMOS™ dance program. In contrast, there was a significant increase in self-reported moderate LTPA (t(9) = 3.16, p = 0.011, d = 0.66). Participants increased more than two hours of time spent in moderate leisure-time PA (Mdiff = 126.52 min/week, [95% CI = 36.03; 217.02]). Although statistically non-significant, there were small and moderate effects sizes for changes in self-reported light LTPA (d = 0.23), moderate PA (d = 0.31), leisure-time MVPA (d = 0.32), and total LTPA (d = 0.31).
Table 2.
Self-reported and device-assessed physical activity pre- and post-BAILAMOS™
| Physical activity | Pre M (SD) | Post M (SD) | Mean difference (95%CI) | p | d |
|---|---|---|---|---|---|
| Device-assessed | |||||
| Steps | 11304.92 (4228.32) | 12614.85 (7053.13) | 1309.93 (−1331.47; 3951.33) | 0.291 | 0.11 |
| CPM | 13564.14 (3274.16) | 13765.39 (3078.331) | 203.65 (−1867.69; 2274.99) | 0.829 | 0.06 |
| Self-reported (min/week) | |||||
| Light PA | 433.50 (238.26) | 403.33 (147.52) | −16.80 (−198.48; 164.88) | 0.692 | −0.14 |
| Light leisure PA | 184.50 (207.61) | 228.00 (163.83) | 43.50 (−112.69; 199.69) | 0.544 | 0.23 |
| Moderate PA | 192.00 (258.82) | 270.00 (196.85) | 78.00 (−27.71; 183.71) | 0.129 | 0.31 |
| Moderate leisure PA | 143.47 (186.01) | 270.00 (196.85) | 126.52 (36.03; 217.02) | 0.011* | 0.66 |
| MVPA | 238.50 (320.73) | 292.50 (223.86) | 54.00 (−70.23; 178.23) | 0.351 | 0.17 |
| Leisure MVPA | 205.50 (208.98) | 292.50 (223.85) | 87.00 (−21.70; 135.70) | 0.103 | 0.32 |
| Total PA | 672.00 (501.29) | 701.32 (313.18) | 29.32 (−224.24; 282.89) | 0.799 | 0.06 |
| Total leisure PA | 390.00 (398.18) | 507.00 (398.18) | 117.00 (−63.67; 297.67) | 0.177 | 0.31 |
| CRF | 5.33 (2.79) | 5.51 (2.72) | 0.18 (−0.35; 0.71) | 0.465 | 0.06 |
Statistically significant for p<0.05.
Participation in BAILAMOS™ did not lead to statistically significant, nor meaningful effect sizes changes, in cognitive performance in global cognition nor any cognitive domain.
BAILAMOS™ effects on functional connectivity
We first compared the FC between each networks’ ROIs and ROIs across the whole brain (Table 3). We chose to display results for those ROIs with moderate effect sizes (0.5 ≥ d ≤ 0.8) (Fig. 1, 2, and 3). We also compared the average FC within-ROIs for each network (Table 4) and display results for the FPN (Fig. 4) since FC within-FPN presented moderate effect size changes. Third, we compared the average FC within-ROIs between each network (results not shown due to non-significant and negligible effect sizes). Fourth, we compared the ratio for each network of interest pre- and post-BAILAMOS™ (Table 5).
Table 3.
Functional connectivity of networks’ regions of interest x whole-brain pre- and post-BAILAMOS™
| ROIs (Network) FC x Whole-brain | Pre M (SD) | Post M (SD) | Mean Diff (95% CI) | p | d |
|---|---|---|---|---|---|
| MPFC (DMN) | 0.0015 (0.0931) | 0.0198 (0.0610) | 0.0183 (−0.0428; 0.0794) | 0.515 | 0.22 |
| LP_L (DMN) | 0.0758 (0.0486) | 0.0852 (0.0959) | 0.0093 (−0.0542; 0.0729) | 0.746 | 0.11 |
| LP_R (DMN) | 0.0906 (0.0662) | 0.1153 (0.0778) | 0.0247 (−0.0125; 0.0619) | 0.167 | 0.33 |
| PCC (DMN) | 0.0621 (0.1054) | 0.1061 (0.0931) | 0.0439 (−0.0220; 0.1100) | 0.166 | 0.44 |
| ACC (SAL) | 0.1646 (0.1486) | 0.0585 (0.0767) | −0.0159 (−0.0723; 0.0404) | 0.538 | −0.23 |
| AInsula_L (SAL) | 0.2242 (0.0478) | 0.1943 (0.0981) | −0.0299 (−0.0919; 0.0320) | 0.302 | −0.36 |
| AInsula_R (SAL) | 0.2103 (0.0570) | 0.1653 (0.1001) | −0.0450 (−0.1201; 0.0301) | 0.208 | −0.54 |
| RPFC_L (SAL) | 0.2165 (0.0651) | 0.1869 (0.0679) | −0.0296 (−0.0760; 0.0168) | 0.183 | −0.44 |
| RPFC_R (SAL) | 0.2101 (0.0775) | 0.1817 (0.0950) | −0.0283 (−0.0856; 0.0289) | 0.291 | −0.32 |
| SMG_L (SAL) | 0.2206 (0.0666) | 0.1771 (0.0854) | −0.0435 (−0.1035; 0.0165) | 0.135 | −0.56 |
| SMG_R (SAL) | 0.2159 (0.0546) | 0.1774 (0.0969) | −0.0384 (−0.0985; 0.0216) | 0.185 | −0.45 |
| LPFC_L (FPN) | 0.1272 (0.1030) | 0.1732 (0.0660) | 0.0459 (−0.0324; 0.1243) | 0.217 | 0.52 |
| LPFC_R (FPN) | 0.1616 (0.0700) | 0.1500 (0.0674) | −0.0116 (−0.0529; 0.0297) | 0.542 | −0.16 |
| PPC_L (FPN) | 0.1189 (0.0962) | 0.1395 (0.0553) | 0.0206 (−0.0386; 0.0798) | 0.452 | 0.24 |
| PPC_R (FPN) | 0.1711 (0.0642) | 0.1682 (0.0761) | −0.0029 (−0.0642; 0.0584) | 0.917 | −0.04 |
Fig. 1.
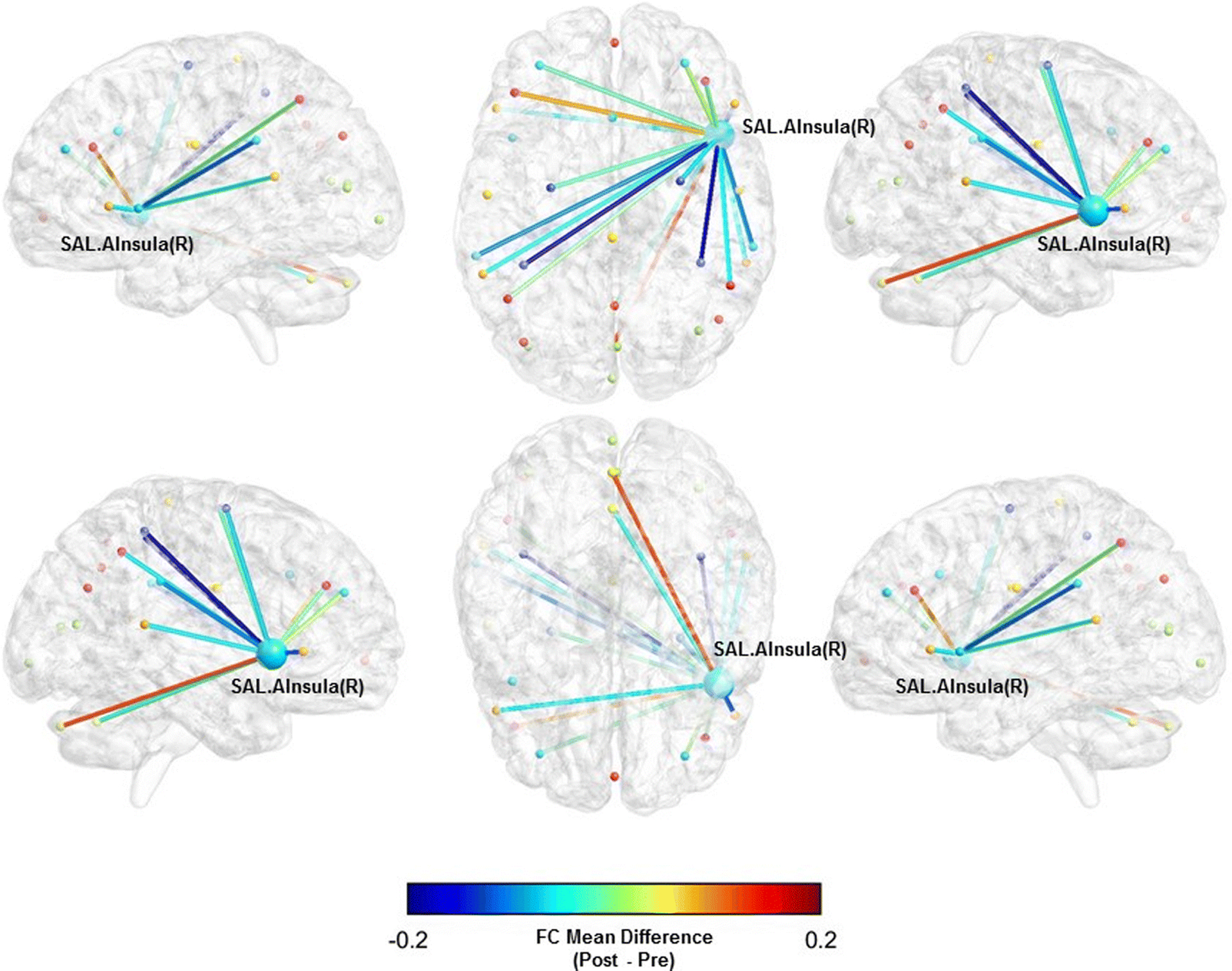
Functional connectivity mean difference between the right anterior insula (SAL) and whole-brain pre- and post-BAILAMOS™
Fig. 2.
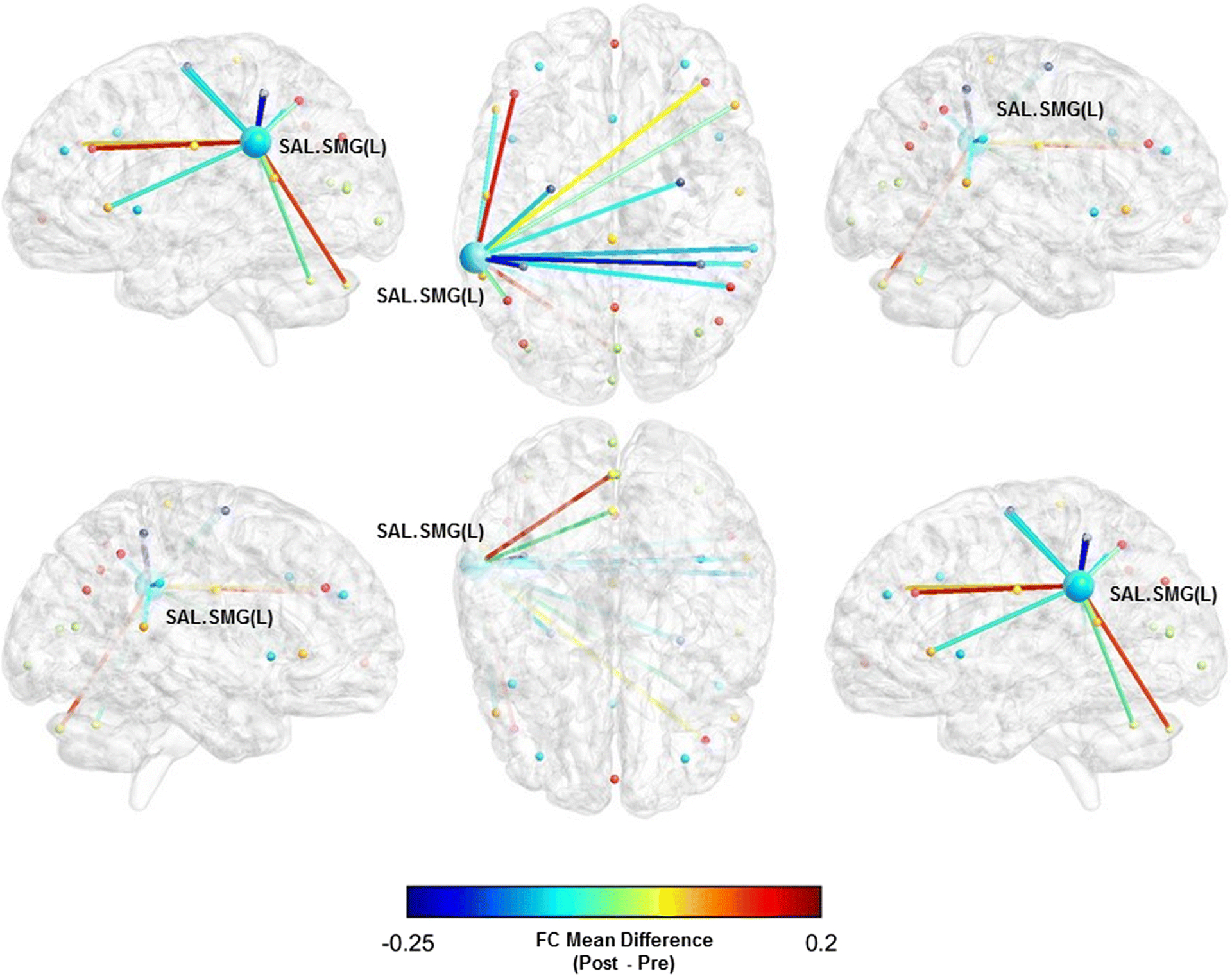
Functional connectivity mean difference between the left supramarginal gyrus (SAL) and whole-brain pre- and post-BAILAMOS™
Fig. 3.
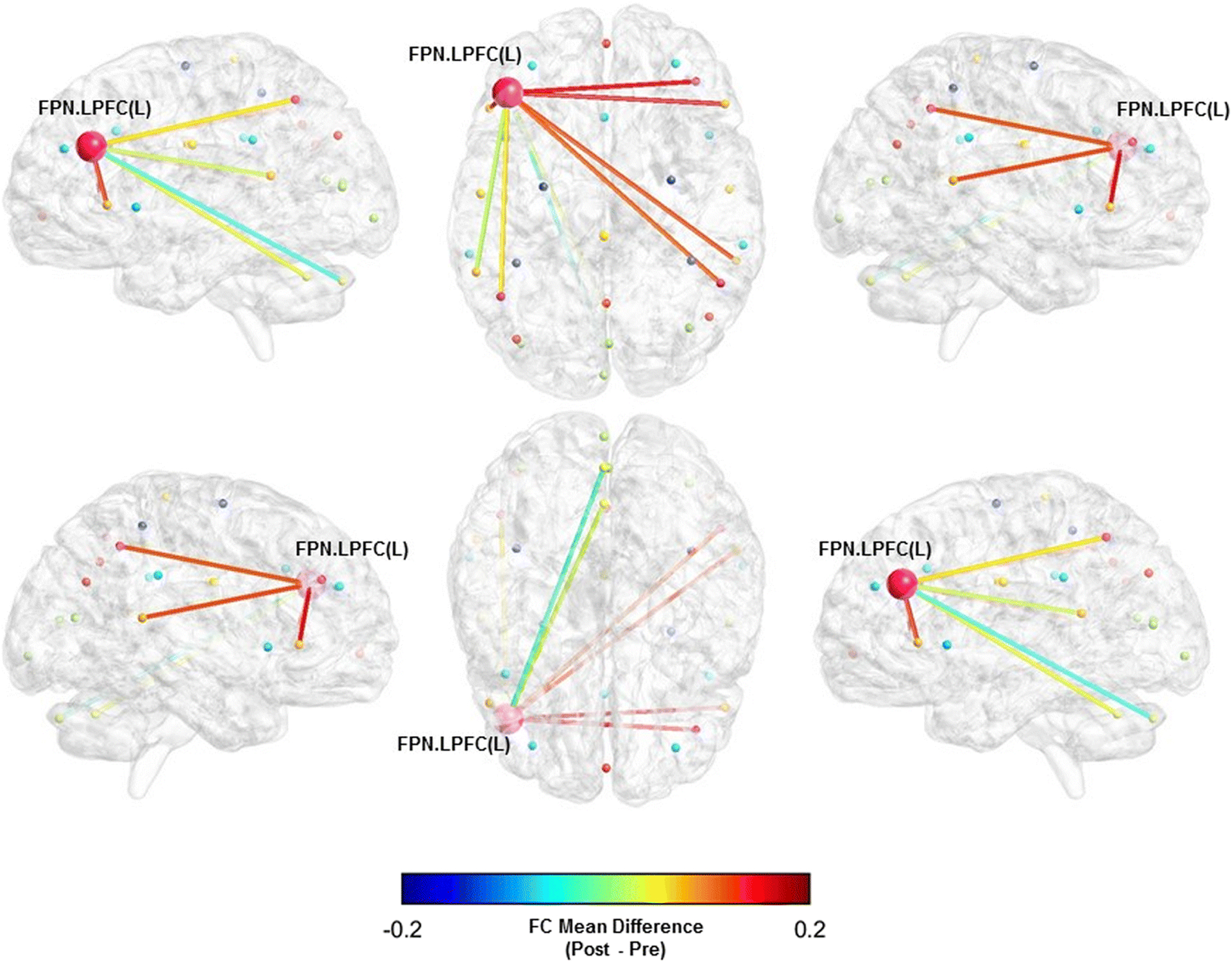
Functional connectivity mean difference between the left lateral prefrontal cortex (FPN) and whole-brain pre- and post-BAILAMOS™
Table 4.
Average functional connectivity of regions of interest within-networks pre- and post-BAILAMOS™
| FC of ROIs within-networks | Pre M (SD) | Post M (SD) | Mean Diff (95% CI) | p | d |
|---|---|---|---|---|---|
| DMN | 0.5719 (0.2069) | 0.5100 (0.1924) | −0.0618 (−0.1953; 0.0716) | 0.321 | −0.31 |
| FPN | 0.4923 (0.1530) | 0.5955 (0.1341) | 0.1013 (0.0037; 0.1988) | 0.043* | 0.70 |
| SAL | 0.5582 (0.1426) | 0.5160 (0.1469) | −0.0422 (−0.1515; 0.0671) | 0.405 | −0.29 |
| LAN | 0.4886 (0.1139) | 0.5280 (0.1437) | 0.0394 (−0.0448; 0.1237) | 0.318 | 0.29 |
Statistically significant for p<0.05.
Fig. 4.
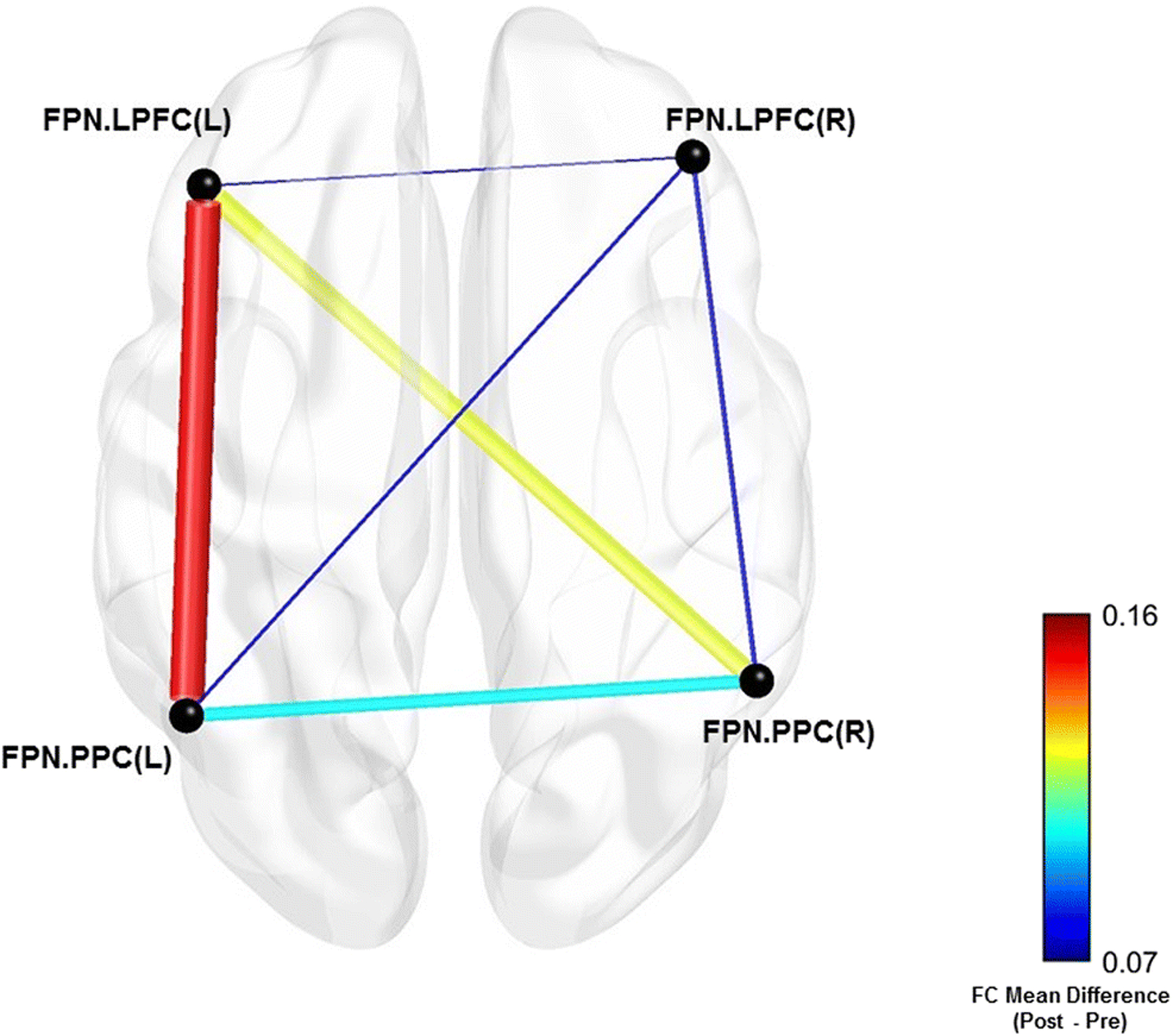
Mean difference between FPN regions of interest pairs pre- and post-BAILAMOS™
Table 5.
Ratio integration/segregation pre- and post-BAILAMOS™
| Ratio integration/s egregation | Pre M (SD) | Post M (SD) | Mean Diff (95% CI) | p | d |
|---|---|---|---|---|---|
| DMN | 0.1435 (0.1625) | 0.2398 (0.1337) | 0.0962 (−0.0145; 0.2070) | 0.081 | 0.64 |
| FPN | 0.4150 (0.2682) | 0.3477 (0.1645) | −0.0672 (−0.2360; 0.1016) | 0.391 | −0.28 |
| SAL | 0.1040 (0.1447) | 0.1090 (0.2205) | 0.0049 (−0.1073; 0.1172) | 0.923 | 0.02 |
| LAN | 0.4276 (0.1260) | 0.4163 (0.1950) | −0.0113 (−0.1511; 0.1285) | 0.860 | −0.06 |
We did not observe statistically significant differences in the FC between the networks’ ROIs and the whole-brain (Table 3). However, there was a moderate sized effect of the BAILAMOS™ dance program in three ROIs associated with two of our resting state networks: the right anterior insula (SAL) (Mdiff = −0.0450, [95% CI = −0.1201; 0.0301], d = −0.54), left supramarginal gyrus (SAL) (Mdiff = −0.0435, [95% CI = −0.1035; 0.0165], d = −0.56), and left lateral prefrontal cortex (FPN) (Mdiff = 0.0459, [95% CI = −0.0324; 0.1243], d = 0.52).
We display the three ROIs x Whole-brain FC with moderate effect sizes (Table 3) in Fig. 1, 2, and 3.
Averaging the FC within the ROIs of each network of interest, we found that within-network FC of the FPN increased significantly after participation in the BAILAMOS™ dance program with a moderate effect size (t(9) = 2.35, p = 0.043, d = 0.70) (Table 4).
To explore which ROI was driving this significant increase, we explored the FC within the FPN ROIs. We noticed that although non-significant (t(9) = 1.82, p = 0.101, d = 0.77), a moderate effect size increase in FC between the left lateral prefrontal cortex and the left posterior parietal cortex was driving the average increase in the FPN (Fig. 4). The other ROI-ROI FCs did not show significant changes and presented negligible effect sizes.
Taking the average FC within the ROIs of each network (i.e., DMN, FPN, and SAL), we calculated the FC of each network as a whole. With the FC of each network, we compared the FC changes between each network. We observed negligible non-significant effects in FC between networks pre- and post-BAILAMOS™. Next, we calculated a ratio for integration/segregation of the three networks of interest and the network we used as a control (Language). This ratio was calculated to demonstrate whether each network showed a trend to have stronger connectivity with other networks (i.e., integration) or to stronger connectivity within its own network (i.e., segregation) (Table 5). The results did not show statistically significant differences over the course of the intervention. However, the DMN ROIs showed moderate effect size changes towards greater integration with other networks’ ROIs (t(9) = 1.96, p = 0.081, d = 0.64) after participation in the BAILAMOS™ dance program. Moreover, the negative small effect size in the FPN ratio integration/segregation (d = −0.28) aligns with increased FC within FPN ROIs previously observed in Table 4.
Correlation between changes in PA, cognition, and FC
We aimed to investigate whether changes in PA levels were correlated with changes in cognition and FC, and tested whether changes in cognition were correlated with changes in FC. Nonetheless, as we did not observe changes in cognitive performance, this analysis was performed only for the self-reported PA (moderate leisure PA) and FCs that demonstrated a statistically significant change or a moderate effect size in the previously reported results. We observed that increases in moderate leisure-time PA post-intervention were associated with increases in the FC within-FPN (R = 0.79, p = 0.006).
Discussion
The present study aimed to investigate the impact of the BAILAMOS™ dance program on resting FC in DMN, FPN, SAL networks, and performance across multiple cognitive domains; and investigate whether changes in PA as a result of the BAILAMOS™ dance program were associated with changes in resting FC in DMN, FPN, and SAL networks, and cognitive performance. Although our study was not adequately powered, results partially supported the first hypothesis. We observed a significant increase, with moderate effect size, in the FC within the FPN ROIs, but not within the DMN and SAL ROIs. We also detected moderate effect size FC reductions in the right anterior insula (SAL) and supramarginal gyrus (SAL), and a moderate effect size FC increase in the lateral prefrontal cortex (FPN) using a whole-brain approach. Moreover, the DMN showed a moderate-sized increase in FC between its ROIs and other networks’ ROIs, suggesting a trend of increased integration with other networks. Partially supporting the second hypothesis, we observed significant associations between increases in moderate leisure-time PA and increases in the FC within-FPN. Nonetheless, inconsistent with the third hypothesis, we observed neither significant nor moderate effect size improvements in cognitive performance and its associations with changes in PA and FC. Given our small sample size, testing these hypotheses do not provide definitive answers, instead, inform us and other researchers for future research regarding the impact of dance on FC and cognition in older Latinos.
Poorer cognition among older Latinos is linked to the higher prevalence of obesity, type 2 diabetes, and metabolic syndrome compared to non-Latino whites (Benjamin et al. 2017). These risk factors can be attenuated with increased levels of PA (Booth, Roberts, and Laye 2012). We did find a moderate effect size increase on self-reported moderate leisure PA. Nonetheless, changes in estimated CRF and device-assessed PA were not found, nor were any associations with changes in cognitive performance. Although increased estimated CRF resulting from exercise programs has been found to be related to reducing aging-related impairment in the FC of associative networks (e.g., DMN, SAL) in resting-state fMRI (Porto et al. 2018; Voss et al. 2019, 2016), our study did not replicate these results. A possible explanation is that we utilized an estimated measure of CRF instead of a direct assessment of CRF. Also, the light-to-moderate PA intensity nature of the BAILAMOS™ program might not have been sufficiently intense to result in device-assessed PA and CRF changes.
After participation in BAILAMOS™, older Latinos showed increased FC within-FPN, which was associated with increases in self-reported moderate leisure PA. This result concurred with previous reviews showing the association of increased PA levels and improved FC in the FPN (Huang et al. 2016; Stillman, Donofry, and Erickson 2019). Nonetheless, this result should be interpreted with caution since our small sample size might have generated spurious associations.
The FPN seems to have some functional overlap with dance features. Dosenbach et al. (2007) suggested the FPN activation profile supports control initiation and provides flexibility by adjusting control in response to feedback in a top-down fashion. This profile aligns with tasks that are essential to individuals learning how to dance. While dancing, the participant initiates control of the dance steps based on the beats of the music and instructions from the instructor, which is followed by constant adjustments in response to feedback from the music, dance partner, and instructor. Similar to our findings, Zilidou et al. (2018) found that a four-month traditional Greek dance program increased FC within-FPN ROIs. Taken together, it seems that dance is a potential PA type to alter the FC in a major brain network
Consistent with previously reported patterns of brain activation studies (Dosenbach et al. 2006; Fincham et al. 2002), we observed that the FC between the left lateral prefrontal cortex and the left precuneus was driving the increase in the FC within-FPN ROIs. We also observed that two ROIs from the SAL network demonstrated FC reduction after participation in the BAILAMOS™ dance program. Interestingly, the FPN and SAL, which are cognitive-control networks, demonstrated opposite responses to the BAILAMOS™ dance program. This result suggests some alignment with the dual-network hypothesis between the FPN and SAL (Dosenbach et al. 2007). Based on differences in FC and activation profiles, Dosenbach et al. (2007) argue that the FPN and SAL support distinct functions. They propose that the SAL network exerts more stable cognitive-control and “contributes to the flexible control of human goal-directed behavior through the stable, across-trial implementation of task sets” (Dosenbach et al. 2007, p.11076). Meanwhile, the FPN is more adaptative, supports control initiation, and provides flexibility by adjusting control in response to feedback. They found that the FPN and SAL were separated from each other as for their FCs, suggesting that they carry out dissociable control functions.
The role of PA in increasing the FC within-DMN is more prominent in the literature (Boraxbekk et al. 2016; Li et al. 2014; Voss et al. 2010a; Voss et al., 2010b; Voss et al. 2016, Stillman et al. 2019). Similarly, our results we did show moderate effect size changes in the DMN ratio integration/segregation, signaling to increased integration with ROIs of other networks. Importantly, evidence suggests that FC between-networks is, in general, reduced with aging (Onoda, Ishihara, and Yamaguchi 2012; Tomasi and Volkow 2012). Previous exercise interventions demonstrated enhanced FC between the DMN and other networks (McGregor et al. 2018; Chirles et al. 2019; Prehn et al. 2019), but none of these interventions utilized dance.
Our results present dance as a potential form of avoiding loss of FC between-networks that are associated with cognitive decline, specifically the integration of the DMN and other resting-state networks. However, it remains to be determined whether increased integration is a beneficial compensatory mechanism response to the usual age-related lowering of within-network FC or represents an interference to within-network FC (Goldstone et al. 2016). Taken together, results suggest that future research should further investigate the role of dance in promoting greater integration between resting-state networks.
Noticeably, we detected moderate effect size increases in FC of two major brain networks associated with cognitive decline (i.e., DMN and FPN). These results contribute to past research showing that dance has previously shown improvements on FC and brain plasticity in brain networks (Kattenstroth et al. 2013; Rehfeld et al. 2018; 2017; Müller et al. 2017). Specifically, older Latinos present poorer cognitive performance and brain health than non-Latino whites (Sloan and Wang 2005; Brewster et al. 2014; Díaz-Venegas et al. 2019; Díaz-Venegas et al. 2016; Brickman et al. 2008), which makes the results of the present study a valuable contribution for the field, also considering the lack of studies focusing on the effects of dance interventions on FC and cognition in older Latinos. Our exploratory results signal the need for a better understanding of how a culturally relevant PA (i.e., dance) can increase PA levels, FC in major brain networks, and how it can be translated to improved cognitive performance.
The null results on cognitive performance are not consistent with changes in cognitive performance found in previous studies utilizing dance interventions (Coubard et al. 2011; Hamacher et al. 2015; Kattenstroth et al. 2013; Rehfeld et al. 2017). This may have been partly due to an interaction of several factors such as the small sample size, the light-to-moderate PA intensity of our dance program, the duration of the intervention, and the relatively young age and cognitively healthy sample.
Our results and interpretations must be considered in the context of several limitations, many of which have been highlighted throughout the text. In addition to those previously mentioned, we adopted single-group pre-post analysis without a control group. Although this type of design is acceptable for pilot stages, results must be interpreted with caution as without a comparison group, we cannot disentangle the extent to which benefits derive from the program rather than secular trends. Second, only participants with complete observations at pre- and post-intervention data collection were included in the study; with that, potential non-random effects might be playing a role in our results. Third, we did not assess participant’s perceived cognitive load during dance classes. Therefore, we do not have information on whether participants perceived the dance classes as cognitively challenging. Fourth, we did not evaluate participants’ prior formal or informal experience with dance, which led participants with different levels of experience to learn the same steps. Thus, participants’ perception of classes’ cognitive challenge potentially varied as a function of their prior experience with dance.
Despite these limitations, this is the first study investigating the effects of dance on cognition and FC in brain networks associated with aging in older Latino adults. Our results provide initial insights on the effects of a culturally relevant and enjoyable PA type, Latin dance, on the brain health of an ethnic minority group at risk for dementia. Future studies should consider larger sample sizes, increasing the PA frequency and intensity throughout the dance program, better monitor PA intensity, introduce an assessment of perceived cognitive load of the classes and propose a longer intervention period.
Supplementary Material
Acknowledgments
We would like to thank all participants, the research staff that assisted with recruitment, data collection, and data management. A special thanks to Priscilla Vasquez, Isabela G. Marques, Jacqueline Guzman, and Zhi Yang.
Funding: The study was funded by the Alzheimer’s Association (NIRGD-11-205469) and National Institute on Aging (P30AG022849) to the University of Illinois at Chicago Midwest Roybal Center for Health Promotion and Translation grants.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest/Competing interests: OAA is the co-founder of KeyWise, Inc. OAA serves on the advisory board of Blueprint Health and Embodied Labs. The authors declare that they have no conflict of interest.
Ethics approval: The study was approved by the University of Illinois at Chicago Institutional Review Board Protocol #2015–0497 and was performed in line with the principles of the Declaration of Helsinki.
Consent to participate: All participants signed the informed consent form as approved by the University of Illinois at Chicago Institutional Review Board Protocol #2015–0497.
Consent to publication: Not applicable
Availability of data and material: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability: Statistical analyses were conducted in RStudio version 3.5.14. The codes generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Guilherme M. Balbim, University of Illinois at Chicago, Department of Kinesiology and Nutrition, Chicago, Illinois, United States..
Olusola A. Ajilore, University of Illinois at Chicago, Department of Psychiatry, Chicago, Illinois, United States.
Kirk I. Erickson, University of Pittsburgh, Department of Psychology, Pittsburgh, Pennsylvania, United States..
Melissa Lamar, Rush University, Division of Behavioral Sciences, Chicago, Illinois, United States..
Susan Aguiñaga, University of Illinois at Urbana-Champaign, Department of Kinesiology and Community Health, Champaign, Illinois, United States..
Eduardo E. Bustamante, University of Illinois at Chicago, Department of Kinesiology and Nutrition, Chicago, Illinois, United States..
David X. Marquez, University of Illinois at Chicago, Department of Kinesiology and Nutrition, Chicago, Illinois, United States.
References
- Adjutant General’s Office. (1944). Army Individual Test Battery: Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General’s Office. [Google Scholar]
- Al Snih S, Ottenbacher KJ, Markides KS, Kuo Y-F, Escbach K, & Goodwin JS (2007). The Effect of Obesity on Disability vs Mortality in Older American. Archives of Internal Medicine, 167(22), 774–780. 10.1001/archinte.167.22.2528-b [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Rajat Deo MD, M., … A., G. (2017). Heart Disease and Stroke Statistics — 2017 Update A Report From the American Heart Association. Circulation, 135(10), e146–e603. 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth FW, Roberts CK, & Laye MJ (2012). Lack of exercise is a major cause of chronic diseases. Comprehensive Physiology, 2(2), 1143–1211. https://doi.org/0.1002/cphy.c110025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraxbekk CJ, Salami A, Wåhlin A, & Nyberg L (2016). Physical activity over a decade modifies age-related decline in perfusion, gray matter volume, and functional connectivity of the posterior default-mode network-A multimodal approach. NeuroImage, 131, 133–141. 10.1016/j.neuroimage.2015.12.010 [DOI] [PubMed] [Google Scholar]
- Borg GAV (1982). Psychophysical bases of perceived exertion. Medicine & Science in Sports & Exercise, 14(5), 377–381. 10.1249/00005768-198205000-00012 [DOI] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, … Ances BM (2012). Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression. Journal of Neuroscience, 32(26), 8890–8899. 10.1523/JNEUROSCI.5698-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb EJ, Kinnavane L, & Aggleton JP (2017). Hippocampal–diencephalic–cingulate networks for memory and emotion: An anatomical guide. Brain and Neuroscience Advances, 1, 239821281772344. 10.1177/2398212817723443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL (2004). Memory and executive function in aging and ad: Multiple factors that cause decline and reserve factors that compensate. Neuron, 44(1), 195–208. 10.1016/j.neuron.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Cabeza R (2002). Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging, 17(1), 85–100. 10.1037/0882-7974.17.1.85 [DOI] [PubMed] [Google Scholar]
- Chen H, Bermúdez OI, & Tucker KL (2002). Waist Circumference and Weight Change Are Associated With Disability Among Elderly Hispanics. Journal of Gerontology: Medical Sciences, 57(1), 19–25. [DOI] [PubMed] [Google Scholar]
- Chodzko-Zajko WJ, Resnick B, & Ory MG (2012). Beyond screening: Tailoring physical activity options with the EASY tool. Translational Behavioral Medicine, 2(2), 244–248. 10.1007/s13142-012-0134-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang Y, Eldreth D, Erickson KI, Varma V, Harris G, Fried LP, … Carlson MC (2014). Neurobiology of Aging Cardiovascular risks and brain function : a functional magnetic resonance imaging study of executive function in older adults. Neurobiology of Aging, 35(6), 1396–1403. 10.1016/j.neurobiolaging.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, … Elavsky S (2004). Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences. 10.1073/pnas.0400266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J, Sagar HJ, Jordan N, Harvey NS, & Sullivan EV (1991). Cognitive impairment in early, untreated Parkinson’s Disease and its relationship to motor disability. Brain, 114(5), 2095–2122. 10.1093/brain/114.5.2095 [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Cornier M, Melanson EL, Salzberg AK, Bechtell JL, & Tregellas JR (2012). Physiology & Behavior The effects of exercise on the neuronal response to food cues. Physiology & Behavior, 105(4), 1028–1034. 10.1016/j.physbeh.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coubard OA, Duretz S, Lefebvre V, Lapalus P, Ferrufino L, & Heath JE (2011). Practice of contemporary dance improves cognitive flexibility in aging. Frontiers in Aging Neuroscience, 3(September), 1–12. 10.3389/fnagi.2011.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince S, Madden DJ, Huettel SA, & Cabeza R (2008). Effects of aging on the neural correlates of successful item and source. Journal of Experimental Psychology: Learning, Memory, and Cognition, 34(4), 791–808. 10.1037/0278-7393.34.4.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux-Fitzgerald A, Powell R, Dewhurst A, & French DP (2016). The acceptability of physical activity interventions to older adults: A systematic review and meta-synthesis. Social Science and Medicine, 158, 14–23. 10.1016/j.socscimed.2016.04.006 [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, & Petersen SE (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12(3), 99–105. 10.1016/j.tics.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, … Petersen SE (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–11078. 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, … Petersen SE (2006). A core system for the implementation of task sets. Neuron, 50(5), 799–812. 10.1016/j.neuron.2006.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenberger P, Wolf M, Schumann M, & de Bruin ED (2016). Exergame and balance training modulate prefrontal brain activity during walking and enhance executive function in older adults. Frontiers in Aging Neuroscience, 8(APR), 1–16. 10.3389/fnagi.2016.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmail A, Vrinceanu T, Lussier M, Predovan D, Berryman N, Houle J, … Bherer L (2020). Effects of movement training vs. aerobic exercise training on cognition, physical fitness and quality of life in older adults: a randomized controlled trial. Journal of Bodywork & Movement Therapies, 24(1), 212–220. 10.1016/j.jbmt.2019.05.004 [DOI] [PubMed] [Google Scholar]
- Evero N, Hackett LC, Clark RD, Phelan S, & Hagobian TA (2012). Aerobic exercise reduces neuronal responses in food reward brain regions. Journal of Applied Physiology, 112, 1612–1619. 10.1152/japplphysiol.01365.2011 [DOI] [PubMed] [Google Scholar]
- Ferreira LK, & Busatto GF (2013). Resting-state functional connectivity in normal brain aging. Neuroscience and Biobehavioral Reviews, 37(3), 384–400. 10.1016/j.neubiorev.2013.01.017 [DOI] [PubMed] [Google Scholar]
- Fincham JM, Carter CS, Van Veen V, Stenger VA, & Anderson JR (2002). Neural mechanisms of planning: A computational analysis using event-related fMRI. Proceedings of the National Academy of Sciences of the United States of America, 99(5), 3346–3351. 10.1073/pnas.052703399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 1–10. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Goldstone A, Mayhew SD, Przezdzik I, Wilson RS, Hale JR, & Bagshaw AP (2016). Gender specific re-organization of resting-state networks in older age. Frontiers in Aging Neuroscience, 8(NOV), 1–15. 10.3389/fnagi.2016.00285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamacher D, Hamacher D, Rehfeld K, Hökelmann A, & Schega L (2015). The effect of a six-month dancing program on motor-cognitive dual-task the effect of a six-month dancing program on motor-cognitive dual-task performance in older adults. Journal of Aging and Physical Activity, 23(4), 647–652. 10.1123/japa.2014-0067 [DOI] [PubMed] [Google Scholar]
- Hart TL, Swartz AM, Cashin SE, & Strath SJ (2011). How many days of monitoring predict physical activity and sedentary behaviour in older adults? International Journal of Behavioral Nutrition and Physical Activity, 8(1), 62. 10.1186/1479-5868-8-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Qin W, Liu Y, Zhang X, Duan Y, Song J, … Yu C (2013). Age-related decrease in functional connectivity of the right fronto-insular cortex with the central executive and default-mode networks in adults from young to middle age. Neuroscience Letters, 544, 74–79. 10.1016/j.neulet.2013.03.044 [DOI] [PubMed] [Google Scholar]
- Hedden T, & Gabrieli JDE (2004). Insights into the ageing mind: A view from cognitive neuroscience. Nature Reviews Neuroscience, 5(2), 87–96. 10.1038/nrn1323 [DOI] [PubMed] [Google Scholar]
- Hsu CL, Best JR, Wang S, Voss MW, Hsiung RGY, Munkacsy M, … Liu-Ambrose T (2017). The impact of aerobic exercise on fronto-parietal network connectivity and its relation to mobility: An exploratory analysis of a 6-month randomized controlled trial. Frontiers in Human Neuroscience, 11(June), 12. 10.3389/fnhum.2017.00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Fang R, Li BY, & Chen S. Di. (2016). Exercise-related changes of networks in aging and mild cognitive impairment brain. Frontiers in Aging Neuroscience, 8(MAR). 10.3389/fnagi.2016.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang PWN, & Braun KL (2017). The effectiveness of dance interventions to improve older adults’ health: A systematic literature review. Alternative Therapies in Health and Medicine, 21(5), 64–70. [PMC free article] [PubMed] [Google Scholar]
- Jagust W (2013). Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron, 77(2), 219–234. 10.1016/j.neuron.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L, Pearlson GD, Zhang X, Steffens DC, Ji X, Guo H, & Wang L (2018). Physical exercise increases involvement of motor networks as a compensatory mechanism during a cognitively challenging task. International Journal of Geriatric Psychiatry, 33(8), 1153–1159. 10.1002/gps.4909 [DOI] [PubMed] [Google Scholar]
- Jurca R, Jackson AS, LaMonte MJ, Morrow JR, Blair SN, Wareham NJ, … Laukkanen R (2005). Assessing cardiorespiratory fitness without performing exercise testing. American Journal of Preventive Medicine, 29(3), 185–193. 10.1016/j.amepre.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Kattenstroth JC, Kalisch T, Holt S, Tegenthoff M, & Dinse HR (2013). Six months of dance intervention enhances postural, sensorimotor, and cognitive performance in elderly without affecting cardio-respiratory functions. Frontiers in Aging Neuroscience, 5(February), 1–16. 10.3389/fnagi.2013.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keadle SK, McKinnon R, Graubard BI, & Troiano RP (2016). Prevalence and trends in physical activity among older adults in the United States: A comparison across three national surveys. Preventive Medicine, 89, 37–43. 10.1016/j.ypmed.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch LP, Diersch N, Sumanapala DK, & Cross ES (2018). Dance training shapes action perception and its neural implementation within the young and older adult brain. Neural Plasticity, 2018. 10.1155/2018/5459106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, & Colcombe S (2018). Fitness effects on the cognitive function of older adults: a meta-analytic study. Perspectives on Psychological Science, 13(2), 213–217. 10.1177/1745691617707316 [DOI] [PubMed] [Google Scholar]
- Li R, Zhu X, Yin S, Niu Y, Zheng Z, Huang X, … Li J (2014). Multimodal intervention in older adults improves resting-state functional connectivity between the medial prefrontal cortex and medial temporal lobe. Frontiers in Aging Neuroscience. 10.3389/fnagi.2014.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Xing G, & Han Y (2018). Advances in resting state neuroimaging of mild cognitive impairment. Frontiers in Psychiatry, 9(December), 1–11. 10.3389/fpsyt.2018.00671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Rosenberg MD, Yoo K, Hsu TW, O’Connell TP, & Chun MM (2018). Resting-state functional connectivity predicts cognitive impairment related to Alzheimer’s disease. Frontiers in Aging Neuroscience, 10(APR), 1–10. 10.3389/fnagi.2018.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, & Dosenbach NU (2018). The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues in Clinical Neuroscience, 20(2), 133–140. 10.1126/science.130.3367.126-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez DX, Hoyem R, Fogg L, Bustamante EE, Staffileno B, & Wilbur J (2016). Physical activity of urban community-dwelling older Latino adults. Journal of Physical Activity and Health, 8(s2), S161–S170. 10.1123/jpah.8.s2.s161 [DOI] [PubMed] [Google Scholar]
- Marquez DX, Wilbur J, Hughes S, Berbaum ML, Wilson R, Buchner DM, & McAuley E (2014). B.A.I.L.A. - A Latin dance randomized controlled trial for older Spanish-speaking Latinos: Rationale, design, and methods. Contemporary Clinical Trials, 38(2), 397–408. 10.1016/j.cct.2014.06.012. B.A.I.L.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcfadden KL, Cornier M, Melanson EL, Bechtell JL, & Tregellas JR (2013). Effects of exercise on resting-state default mode and salience network activity in overweight/obese adults. Neuro Report, 24, 866–871. 10.1097/WNR.0000000000000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo KD, Williamson E, Houde SC, Futrell M, Read CY, & Campasano M (2001). Perceptions of older Latino adults regarding physical fitness, physical activity, and exercise. Journal of Gerontological Nursing, 27(9), 38–46. [DOI] [PubMed] [Google Scholar]
- Menon V (2015). Salience network. (Toga AW, Ed.), Brain Mapping: An Encyclopedic Reference (Vol. 2). Elsevier Inc. 10.1016/B978-0-12-397025-1.00052-X [DOI] [Google Scholar]
- Merom D, Grunseit A, Eramudugolla R, Jefferis B, Mcneill J, & Anstey KJ (2016). Cognitive benefits of social dancing and walking in old age: the Dancing Mind randomized controlled trial. Frontiers in Aging Neuroscience, 8(February), 26. 10.3389/fnagi.2016.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mier N, Medina AA, & Ory MG (2007). Mexican-Americans with type 2 diabetes: perspectives on definitions, motivators, and programs of physical activity. Preventing Chronic Disease, 4(2), A24. [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R (2012). The insular cortex: a review. Progress in Brain Research (1st ed., Vol. 195). Elsevier B.V. 10.1016/B978-0-444-53860-4.00007-6 [DOI] [PubMed] [Google Scholar]
- Nir Y, Hasson U, Levy I, Yeshurun Y, & Malach R (2006). Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. NeuroImage, 30(4), 1313–1324. 10.1016/j.neuroimage.2005.11.018 [DOI] [PubMed] [Google Scholar]
- Northey JM, Cherbuin N, Pumpa KL, Smee DJ, & Rattray B (2018). Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. British Journal of Sports Medicine, 52(3), 154–160. 10.1136/bjsports-2016-096587 [DOI] [PubMed] [Google Scholar]
- Onoda K, Ishihara M, & Yamaguchi S (2012a). Decreased functional connectivity by aging is associated with cognitive decline. Journal of Cognitive Neuroscience, 24(11), 2186–2198. 10.1162/jocn_a_00269 [DOI] [PubMed] [Google Scholar]
- Onoda K, Ishihara M, & Yamaguchi S (2012b). Decreased functional connectivity by aging is associated with cognitive decline. Journal of Cognitive Neuroscience, 24, 2186–2198. [DOI] [PubMed] [Google Scholar]
- Park DC, & Reuter-Lorenz P (2009). The adaptive brain: aging and neurocognitive scaffolding. Annual Review of Psychology, 60(1), 173–196. 10.1146/annurev.psych.59.103006.093656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto FHG, Coutinho AM, de Souza Duran FL, de Sá Pinto AL, Gualano B, Buchpiguel CA, … Brucki SMD (2018). Aerobic training modulates salience network and default mode network metabolism in subjects with mild cognitive impairment. NeuroImage: Clinical, 19(June 2017), 616–624. 10.1016/j.nicl.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predovan D, Julien A, Esmail A, & Bherer L (2019). Effects of dancing on cognition in healthy older adults: a systematic review. Journal of Cognitive Enhancement, 3, 161–167. 10.1007/s41465-018-0103-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld K, Lu A, Ho A, Kaufmann J, Brigadski T, & Mu P (2018). Dance training is superior to repetitive physical exercise in inducing brain plasticity in the elderly. PloS One, 13(7), e0196636. 10.1371/journal.pone.0196636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld K, Müller P, Aye N, Schmicker M, Dordevic M, Kaufmann J, … Müller NG (2017). Dancing or Fitness Sport? The Effects of Two Training Programs on Hippocampal Plasticity and Balance Abilities in Healthy Seniors. Frontiers in Human Neuroscience. 10.3389/fnhum.2017.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineberg AE, & Banich MT (2018). Functional connectivity at rest is sensitive to individual differences in executive function: A network analysis. Human Brain Mapping, 37(8), 2959–2975. 10.1002/hbm.23219.Functional [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick B, Ory MG, Hora K, Rogers ME, Page P, Chodzko-Zajko W, & Bazzarre TL (2008). The Exercise Assessment and Screening for You (EASY) Tool: Application in the Oldest Old Population. American Journal of Lifestyle Medicine, 2(5), 432–440. 10.1177/1559827608320229 [DOI] [Google Scholar]
- Rosario M, Vázquez J, Cruz W, & Ortiz A (2008). Internal consistency of the CHAMPS physical activity questionnaire for Spanish speaking older adults. Puerto Rico Health Sciences Journal, 27(3), 224–228. [PubMed] [Google Scholar]
- RStudio Team. (2019). RStudio: Integrated Development for R. Boston, MA: RStudio, Inc. Retrieved from http://www.rstudio.com/ [Google Scholar]
- Smith A (1982). Symbol Digit Modalities Test manual-revised. Los Angels, LA: Western Psychological Services. 10.1007/978-0-387-79948-3_6121 [DOI] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, & Ritter PL (2001). CHAMPS Physical Activity Questionnaire for Older Adults: Outcomes for intervention. Medicine and Science in Sports and Exercise, 33(7), 1126–1141. 10.1109/VSMM.2001.969701 [DOI] [PubMed] [Google Scholar]
- Suo C, Singh MF, Gates N, Wen W, Sachdev P, Brodaty H, … Valenzuela MJ (2016). Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Molecular Psychiatry, 21(11), 1633–1642. 10.1038/mp.2016.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, & Volkow ND (2012). Aging and functional brain networks. Molecular Psychiatry, 17(5), 549–558. 10.1038/mp.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchiano M (2019). _effsize: Efficient Effect Size Computation_. 10.5281/zenodo.1480624 [DOI] [Google Scholar]
- Trenerry MR, Crosson B, DeBoe J, & Leber WR (1989). Stroop neuropsychological screening test. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Van Den Heuvel MP, & Pol HEH (2010). Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology, 20(3), 519–534. 10.1016/j.psiq.2011.05.001 [DOI] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, … Kramer AF (2010a). Functional connectivity: A source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia, 48(5), 1394–1406. 10.1016/j.neuropsychologia.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, … Kramer AF (2010b). Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Frontiers in Aging Neuroscience. 10.3389/fnagi.2010.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Sutterer M, Weng TB, Burzynska AZ, Fanning J, Salerno E, … Kramer AF (2019). Nutritional supplementation boosts aerobic exercise effects on functional brain systems. Journal of Applied Physiology, 126(1), 77–87. 10.1152/japplphysiol.00917.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Weng TB, Burzynska AZ, Wong CN, Cooke GE, Clark R, … Kramer AF (2016). Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. NeuroImage, 131, 113–125. 10.1016/j.neuroimage.2015.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1987). The Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbam G, & Heyman A (1994). The Consortium to Establish a Registry for Alzheimer’s Disease (CRAD). Part V. A normative study of the neuropsychological battery. Neurology, 44(4), 609–614. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn : A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wickham H (2009). ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer. [Google Scholar]
- Wilbur J, Marquez DX, Fogg L, Wilson RS, Staffileno BA, Hoyem RL, … & Manning AF (2012). The relationship between physical activity and cognition in older Latinos. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 67(5), 525–534. 10.1093/geronb/gbr137 [DOI] [PubMed] [Google Scholar]
- Wilbur J, Chandler PJ, Dancy B, & Lee H (2003). Correlates of physical activity in urban Midwestern Latinas. American Journal of Preventive Medicine, 25(3), 69–76. 10.1016/S0749-3797(03)00167-3 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, & Bennett DA (2005). Early and late life cognitive activity and cognitive systems in old age. Journal of the International Neuropsychological Society, 11(4), 400–407. [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, & Bennett DA (2005). Early and late life cognitive activity and cognitive systems in old age. Journal of the International Neuropsychological Society, 11(4), 400–407. 10.1017/S1355617705050459 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, & Bennett DA (2002). Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging, 17(2), 179–193. 10.1037/0882-7974.17.2.179 [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, & He Y (2013). BrainNet viewer: A network visualization tool for human brain connectomics. PLoS ONE, 8(7), e68910. 10.1371/journal.pone.0068910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, … Seeley WW (2010). Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain, 133(5), 1352–1367. 10.1093/brain/awq075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilidou VI, Frantzidis CA, Romanopoulou ED, Paraskevopoulos E, Douka S, & Bamidis PD (2018). Functional re-organization of cortical networks of senior citizens after a 24-week traditional dance program. Frontiers in Aging Neuroscience, 10(December), 1–14. 10.3389/fnagi.2018.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


