Abstract
The conclusions of the EFSA following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State, Spain, and co‐rapporteur Member State, Greece, for the pesticide active substance phosmet and the assessment of applications for maximum residue levels (MRLs) are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659. The conclusions were reached on the basis of the evaluation of the representative uses of phosmet as an insecticide on citrus fruits, pome fruits, peaches/nectarines and potatoes (field uses). The reliable end points, appropriate for use in regulatory risk assessment, are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are identified.
Keywords: phosmet, peer review, risk assessment, pesticide, insecticide
Summary
Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659, lays down the procedure for the renewal of the approval of active substances submitted under Article 14 of Regulation (EC) No 1107/2009. The list of those substances is established in Commission Implementing Regulation (EU) No 686/2012. Phosmet is one of the active substances listed in Regulation (EU) No 686/2012.
In accordance with Article 1 of Regulation (EU) No 844/2012, the rapporteur Member State (RMS), Spain, and co‐rapporteur Member State (co‐RMS), Greece, received an application from Gowan Comércio Internacional e Serviços, Limitada for the renewal of approval of the active substance phosmet. In addition, Gowan Comércio Internacional e Serviços, Limitada submitted applications for maximum residue levels (MRLs), as referred to in Article 7 of Regulation (EC) No 396/2005.
An initial evaluation of the dossier on phosmet was provided by the RMS in the renewal assessment report (RAR) and subsequently, a peer review of the pesticide risk assessment on the RMS evaluation was conducted by EFSA in accordance with Article 13 of Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659. The following conclusions are derived.
The uses of phosmet according to the representative uses as an insecticide on citrus fruits, pome fruits, peaches/nectarines and potatoes, as proposed at EU level result in a sufficient insecticidal efficacy against the target pests.
The assessment of the data package revealed no issues that could not be finalised or that need to be included as critical areas of concern with respect to identity, physical/chemical properties and analytical methods.
In the area of mammalian toxicology, the ■■■■■
■■■■■
The data available on environmental fate and behaviour are sufficient to carry out the required environmental exposure assessments at EU level for the representative uses.
In the area of ecotoxicology critical areas of concern have been identified for birds (reproductive risk), wild mammals (reproductive risk), aquatic invertebrates (acute and chronic risk), honey bees (acute risk) and non‐target arthropods (in‐field and off‐field).
Phosmet does not meet the criteria for endocrine disruption for humans and non‐target organisms.
Background
Commission Implementing Regulation (EU) No 844/20121, as amended by Commission Implementing Regulation (EU) No 2018/16592, (hereinafter referred to as ‘the Regulation’), lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/20093. This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States, the applicant and the public on the initial evaluation provided by the rapporteur Member State (RMS) and/or co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of an additional 3 months where additional information is required to be submitted by the applicant in accordance with Article 13(3). Furthermore, in accordance with Article 13(3a), where the information available in the dossier is not sufficient to conclude the assessment on whether the approval criteria for endocrine disruption are met, additional information can be requested to be submitted in a period of minimum 3 months, not exceeding 30 months, depending on the type of information requested.
In accordance with Article 1 of the Regulation, the RMS, Spain, and co‐RMS, Greece, received an application from Gowan Comércio Internacional e Serviços, Limitada for the renewal of approval of the active substance phosmet. In addition, Gowan Comércio Internacional e Serviços, Limitada submitted applications for maximum residue levels (MRLs) as referred to in Article 7 of Regulation (EC) No 396/20054. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (Greece), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier on phosmet in the RAR, which was received by EFSA on 29 September 2017 (Spain, 2017). The RAR included a proposal to set MRLs, submitted under Article 7 of Regulation (EC) No 396/2005.
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, Gowan Comércio Internacional e Serviços, Limitada, for consultation and comments on 7 December 2017. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 6 February 2018. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of a reporting table. The applicant was invited to respond to the comments in column 3 of the reporting table. The comments and the applicant's response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA, the RMS and co‐RMS on 14 November 2018. On the basis of the comments received, the applicant's response to the comments and the RMS's evaluation thereof, it was concluded that additional information should be requested from the applicant, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues, environmental fate and behaviour and ecotoxicology.
The outcome of the telephone conference, together with EFSA's further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
A final consultation on the conclusions arising from the peer review of the risk assessment and the MRL application took place with Member States via a written procedure in June 2020.
This conclusion report summarises the outcome of the peer review of the risk assessment of the active substance and the representative formulation, evaluated on the basis of the representative uses of phosmet as an insecticide on citrus fruits, pome fruits, peaches/nectarines and potatoes, as proposed by the applicant. In accordance with Article 12(2) of Regulation (EC) No 1107/2009, risk mitigation options identified in the RAR and considered during the peer review are presented in the conclusion. MRLs were assessed in peaches/nectarines.
A list of the relevant end points for the active substance and the formulation is provided in Appendix A.
A key supporting document to this conclusion is the peer review report (EFSA, 2020), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting table (15 November 2018);
the evaluation table (17 August 2020);
the report(s) of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion.
Given the importance of the RAR, including its revisions (Spain, 2020), and the peer review report, both documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Phosmet is the ISO common name for O,O‐dimethyl S‐phthalimidomethyl phosphorodithioate or N‐{[(dimethoxyphosphinothioyl)thio]methyl}phthalimide (IUPAC).
The representative formulated product for the evaluation was ‘Imidan 50 WP’, a wettable powder (WP) containing 500 g/kg phosmet.
The representative uses evaluated were foliar spray applications for the control of a wide variety of pests, of which Lepidoptera, Coleoptera, Thysanoptera and Hemiptera, and among these mostly scales, and Diptera, mostly fruit flies on citrus, pome fruits, peaches/nectarines and potatoes. Full details of the GAPs can be found in the list of end points in Appendix A.
Data were submitted to conclude that the use of phosmet according to the representative uses proposed at EU level results in a sufficient insecticidal efficacy against the target organisms, following the guidance document SANCO/2012/11251‐rev. 4 (European Commission, 2014b).
A data gap has been identified for a search of the scientific peer‐reviewed open literature on the active substance and its relevant metabolites, dealing with side effects on health, the environment and non‐target species and published within the 10 years before the date of submission of the dossier, to be conducted and reported in accordance with EFSA guidance on the submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009 (EFSA, 2011). RMS did not agree and considered the literature search submitted by applicant acceptable.
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion: European Commission, 2000a,b, 2010).
The proposed specification for phosmet is based on batch data from industrial plant production. The proposed minimum purity of the technical material is 950 g/kg. Toluene was considered relevant impurity with maximum content of 1 g/kg (see Section 2). The batches used in the (eco) toxicological assessment support the original reference specification but not the new proposal however the new technical specification can be considered acceptable from a toxicological point of view (See Sections 2 and 5). It should be noted that based on the data of the renewal procedure higher minimum purity of the active substance could be set while a new relevant impurity and some of the impurities should not be included in the specification and for others lower levels could be set. Overall, it is proposed to update the reference specification concerning the relevant impurities. It should be noted that the RMS disagreed with the proposal to update the reference specification, but agreed with toluene as the sole relevant impurity. There is no FAO specification available for phosmet; however, an evaluation report is published (FAO/WHO 318/2019) according to the new procedure, belonging to the material of Gowan Company. The technical concentrate (TC) is in compliance with the specification proposed in the FAO evaluation report.
The main data regarding the identity of phosmet and its physical and chemical properties are given in Appendix A. A data gap for information on self‐heating for the representative formulation was set.
Adequate methods are available for the generation of data required for the risk assessment. Methods of analysis are available for the determination of the active substance in the technical material and in the representative formulation and published CIPAC methods exist that are applicable for the determination of toluene in the technical material and in the representative formulation.
Phosmet in food and feed of plant origin can be monitored by high‐performance liquid chromatography with tandem mass spectrometry (HPLC–MS/MS) with a limit of quantification (LOQ) of 0.01 mg/kg in all commodity groups.
Phosmet residues in food of animal origin can be determined HPLC–MS/MS with LOQ of 0.01 mg/kg in all animal matrices; however, the residue definition is still open.
Phosmet residues in soil and water can be monitored by HPLC‐MS/MS with LOQs of 0.01 mg/kg and 0.05 μg/L, respectively. Phosmet residues in air can be monitored by GC‐NPD with an LOQ of 0.3 μg/m3.
HPLC‐MS/MS method can be used for monitoring phosmet residues in body fluids with LOQ 0.05 mg/L and phosmet–oxon residues in body fluids and tissues with LOQs of 0.01 mg/L and 0.1 mg/kg, respectively. Phosmet residues in tissues can be determined by using the monitoring method for residues in food of animal origin.
2. Mammalian toxicity
The toxicological profile of the active substance phosmet was discussed at the Pesticides Peer Review Experts’ Meetings PREV 07 (June 2019) and PREV 22 (January 2020) and based on the following guidance documents: European Commission (2003, 2012), EFSA PPR Panel, 2012, EFSA (2014a) and ECHA (2017).
The toxicological profile of phosmet relied upon batches which were not considered representative of the proposed renewal technical specification but support the original reference specification (see Section 1). The toxicological relevance of old and new impurities was evaluated by QSAR analysis and experimental data, enabling the new technical specification to be considered acceptable from a toxicological point of view. Toluene was identified as a relevant impurity in the technical specification; however, it is not considered of toxicological concern at the level found of 1 g/kg. The analytical methods used in the toxicity studies were overall, considered fit‐for‐purpose.
In the toxicokinetics studies in rats, phosmet was rapidly absorbed after oral administration, being mostly eliminated in urine (84% at 24 h after single dose) and to a lesser extent via faeces (5–10% at 24 h). The maximum concentration in plasma was attained at 0.5 h, with high affinity to red blood cells. Phosmet was widely distributed with highest levels of radioactivity detected in whole blood with no evidence of accumulation. The metabolic pathway is thiophosphoryl hydrolysis, S‐methylation, oxidation of the sulfur to sulfoxide and then to sulfone, and hydrolysis of the phthalimide ring to the respective phthalimide acid. Two major metabolites were identified in urine: N‐(methylsulfinylmethyl)‐phthalamic acid (PaAMS(O)M) and the corresponding sulfoxide N‐(methylsulfonylmethyl)‐phthalamic acid (PaAMS(O2)M). Unique human metabolites were not formed in an in vitro interspecies comparative metabolism study which showed a quite high level of consistency across species. The residue definition for body fluids should include phosmet and phosmet‐oxon for the purpose of human biomonitoring.
Based on the av ailable acute toxicity studies, the peer review considered that the criteria for classification as toxic if swallowed may be met for phosmet, while the harmonised classification is harmful if swallowed. Low acute toxicity was observed via the dermal route, while the harmonised classification is harmful in contact with skin. Phosmet was shown to be harmful if inhaled while there is no current harmonised classification regarding inhalation. There was no evidence neither of skin irritation nor of skin sensitisation; moderate irritation was observed in the unwashed eyes in rabbits. No phototoxic potential was observed.
In short‐term dietary studies, the most sensitive finding was the inhibition of cholinesterase (ChE) activity in plasma, red blood cells (RBC) and brain. The proposed overall short‐term no observed adverse effect level (NOAEL) is 1.88 mg/kg body weight (bw) per day from the 90‐day dog and 90‐day rat studies.
In long‐term dietary studies, the most relevant effect observed in rats and mice was reductions of RBC and brain AChE activity. For the 2‐year rat study, the agreed systemic NOAEL was 1.8 mg/kg bw per day based on RBC AChE inhibition; the carcinogenicity NOAEL was set at the highest dose, 9.4 mg/kg bw per day, based on the absence of carcinogenicity findings. For the 2‐year mouse study, the agreed systemic NOAEL was 4 mg/kg bw per day, based on increased incidence of convulsions in males, brain AChE inhibition in females and histopathological findings in the liver of the high‐dose males (cytoplasmic hepatocellular vacuolar degeneration); the carcinogenicity NOAEL was 4 mg/kg bw per day, based on the statistically significant increase in the incidence of liver cell adenomas5. Based on the available genotoxicity studies, phosmet is unlikely to be genotoxic in vivo.
In a two‐generation reproduction study in rats, the agreed NOAEL for parental and reproductive toxicity of phosmet was ■■■■■ bw per day based on decreased body weight and decreased RBC AChE activity at the mid dose and ■■■■■ per day onwards. Whereas the adverse effects on the offspring were only observed at the high dose (reduced mean number of live pups and pup weight per litter, reducing the pup survival index for the two generations) and the agreed NOAEL for offspring toxicity was 4.2 mg/kg bw per day. ■■■■■ In the rat and rabbit teratogenicity studies, phosmet did not show significant embryo/fetotoxicity in the absence of clear signs of maternal toxicity. A maternal NOAEL was set at 5 mg/kg bw per day based on decrease of body weight gain at the dose of ≥ 10 mg/kg bw per day in rats and rabbits and a developmental NOAEL at 10 mg/kg bw per day in rats based on decreased foetus weight at 15 mg/kg bw per day and at 5 mg/kg bw per day in rabbits based on incidence of variations at 15 mg/kg bw per day.
In regard to neurotoxicity, phosmet did not induce delayed neurotoxicity in two studies with adult domestic hens. The NOAEL for acute neurotoxicity was 4.5 mg/kg bw based on decreased ChE activity (RBC, plasma and brain), while the NOAEL for chronic neurotoxicity was set at 1.5 mg/kg bw per day based on decreased RBC AChE activity. ■■■■■ Phosmet showed no immunotoxic potential.
■■■■■ The previous toxicological reference values were: ADI 0.01 mg/kg bw per day, ARfD 0.045 mg/kg bw and AOEL 0.02 mg/kg bw per day European Commission, 2014c.
Based on the human skin in vitro dermal absorption study, dermal absorption values for the representative formulation Imidan 50 WP are 0.7% for the concentrate and 4% for the aqueous dilution (for use on pome, stone fruits and potatoes) with a pro rata correction for the application in citrus leading to a value of 8%. The non‐dietary exposure estimates (i.e. for operator, worker, bystander and resident) for all representative uses according to the GAP table ■■■■■.
The toxicological profile of metabolites, found as residues, was concluded during the experts’ meeting based on QSAR analysis. The majority of experts proposed that for phthalimide and phthalamic acid, the same reference values as for folpet be applicable (see Appendix A). ■■■■■
3. Residues
The assessment in the residue section is based on the following guidance documents: OECD (2009, 2011), European Commission (2011) and JMPR (2004, 2007).
Phosmet was discussed at the Pesticides Peer Review Meeting 9 in June 2019 and Peer Review Meeting 23 in February 2020.
The metabolism in plants of carbonyl‐labelled phosmet was investigated upon foliar applications in fruits (apples and cherries), root (potatoes) and cereals (maize). Phosmet was predominant in apples and maize fodder, mature forage and cobs but represented less than 10% total radioactive residues (TRRs) in cherries and was not recovered in potatoes. In potato, phthalic acid (25% TRR) and phthalamic acid (48% TRR) were the major residues. Phthalic acid was also a major residue in cherry and maize grains (16–20% TRR). Phosmet‐oxon was recovered in low amounts, however, due to limited storage stability and an unknown period of sample storage in the metabolism studies in cherries, potato and maize, the findings cannot be quantitatively relied on (data gap). Moreover, there is uncertainty regarding potential metabolites released from the phosphorodithioate side chain of phosmet because metabolism studies with a second radio‐label were not conducted, and therefore information to address the relevance of such metabolites for consumers is incomplete (data gap). Due to possible occurrence of phthalic acid in the environment from multiple sources, a comparison of background levels with residues resulting from treatment of crops by phosmet should be conducted to assess the contribution of phosmet uses to consumer dietary exposure to phthalic acid. The information is necessary to conclude on the relevance of this metabolite in primary crops and processed commodities, not least in view of the expected significant exposure potential from the pesticide use and the insufficient toxicological information on this compound (data gaps).
Rotational crops metabolism studies were not triggered since phosmet degrades rapidly in soil (DT90 < 100 days) and does not form major persistent soil metabolites.
Based on the overall data made available in the residues and mammalian toxicology sections, the plant residue definition for risk assessment is provisionally proposed as phosmet, phosmet‐oxon and phthalic acid. The residue definition is pending further information on residue occurrence and a ■■■■■. According to the unique marker concept, the residue definition for monitoring is proposed as phosmet alone.
Under standard hydrolysis conditions simulating food processing in two experiments, phosmet degraded little during pasteurisation while during baking/boiling/brewing and sterilisation significant degradation in multiple compounds (phthalimide, phthalamic acid and phthalic acid, N‐hydroxymethyl phthalamic acid and desmethyl‐phosmet) was observed. Although both studies were OECD guideline compliant tests, the identity and magnitude of some degradation products differed noticeably, and therefore, a scientific justification or confirmatory investigation is requested (data gap). Pending further clarity on residues that can be expected in commodities after food processing operations, a residue definition for processed commodities remains open.
The number of geographically independent, GAP compliant and validated residue trials was insufficient for the uses in pome fruit (SEU), citrus and peaches/nectarines (data gaps). In addition, information to conclude on the storage stability studies on phosmet‐oxon in extracts is necessary to further validate the residue trial results (data gaps). Information was also ■■■■■
The available metabolism studies in hens and goats have shortcomings in characterisation and identification of residues in all relevant edible commodities. A conclusion whether the studies could be nonetheless used for estimation of residue transfer in animal commodities and for risk assessment purposes is pending the availability of robust residue data in the pertinent feed commodities, a finalised dietary burden calculation, information on the relevance of potential metabolites released from the phosphorodithioate side chain of phosmet in animals and a ■■■■■ Therefore, a residue definition for animal commodities remains open.
A provisional chronic and acute consumer risk assessment was attempted with EFSA PRIMo rev.2.1 and rev.3, using available data that have been considered sufficiently reliable, the toxicological reference values derived by the peer review for phosmet (see Section 2) and provisionally assuming phosmet‐oxon as of similar toxicity to phosmet. The provisional dietary exposure estimates for consumers ■■■■■ (PRIMo rev.2.1 and rev.3, resp.). The provisional chronic and acute consumer risk assessment is likely to underestimate the actual consumer dietary exposure and risk since not all commodities and not all potentially relevant metabolites could be assessed due to the several data gaps identified, nonetheless this leads to a ■■■■■
As a consequence, MRLs ■■■■■ for several of the crops assessed in the peer review.
4. Environmental fate and behaviour
Phosmet was discussed at the Pesticides Peer Review Meeting TC 03 in June 2019.
The rates of dissipation and degradation in the environmental matrices investigated were estimated using FOCUS (2006) kinetics guidance. In soil laboratory incubations under aerobic conditions in the dark, phosmet exhibited low persistence. No major (> 10% applied radioactivity (AR)) metabolites triggering further assessment were formed. Mineralisation of the carbonyl ring 14C radiolabel and methylene moiety to carbon dioxide accounted for 52–77% AR and 14% AR after 120 days and 6 days, respectively. The formation of unextractable residues for these radiolabels accounted for 38% AR and 16% AR after 120 days and 6 days, respectively. As reliable soil degradation endpoints for phosmet were available for only three soils, a data gap was identified for an additional soil metabolism study to address the degradation rate of phosmet in soil in accordance with the data requirements of Commission Regulation (EU) No. 283/20136 (see Section 8). The metabolic pattern of phosmet in aerobic and anaerobic soils was identical, but there were differences in the amounts of degradation products formed: under anaerobic conditions, metabolite phthalamic acid was detected at max. 16.9% AR after 30 days, phthalic acid at max. 8.3% AR after 125 days and desmethyl‐phosmet at max. 10.8% AR after 5 days. Taking into consideration the low persistence of phosmet in soil and the representative uses under evaluation in this conclusion, the peer review concluded that the formation of these metabolites in soil is not relevant. However, the RMS is of the opinion that in Southern Europe, anaerobic conditions could occur and therefore these metabolites should be further assessed. Phosmet exhibited medium to low mobility in soil. It was concluded that the adsorption of phosmet was not pH dependent.
In laboratory incubations in dark aerobic natural sediment water systems, phosmet dissipated rapidly by partitioning to sediment forming the major metabolites phthalamic acid (max 76% AR at 6 h in water and 13% AR at 7 days in the sediment), phthalic acid (max. ca. 38% AR in water but only 4.6% max. in sediment) and N‐hydroxymethyl phthalimide (max. ca. 12% AR in water but only 2% max. in sediment). Mineralisation of the carbonyl 14C radiolabel, accounted for 80–92% AR at study end (100 days). Radioactive residues not extracted from sediment by acidified acetone represented 6–12% AR at the end of the study. Data from laboratory sterile aqueous photolysis experiments showed that aqueous photolysis may contribute to the degradation of phosmet in the environment, forming the relevant photolytic breakdown products phthalic acid (15.7% AR), phthalimide (62.5% AR), phthalamic acid (12.7% AR) and N‐hydroxymethyl phthalamic acid (19.5% AR). The necessary surface water and sediment exposure assessments (predicted environmental concentrations (PEC) calculations) were carried out for the metabolites phthalamic acid, phthalic acid, N‐hydroxymethyl‐phthalimide, N‐hydroxymethyl phthalamic acid and phthalimide using the FOCUS (FOCUS, 2001) step 1 and step 2 approach (version 3.2 of the Steps 1–2 in FOCUS calculator). For the active substance phosmet, appropriate step 3 (FOCUS, 2001) and step 4 calculations were available. The step 4 calculations appropriately followed the FOCUS (FOCUS, 2007) guidance, with no‐spray drift buffer zones of up to 30 m being implemented for the drainage scenarios (representing a 54.7–92.5% spray drift reduction), and combined no‐spray buffer zones with vegetative buffer strips of up to 20 m (reducing solute flux in run‐off by 80% and erosion run‐off of mass adsorbed to soil by 95%) being implemented for the run‐off scenarios. The SWAN tool (version 4.0.1) was appropriately used to implement these mitigation measures in the simulations. However, risk managers and others may wish to note that whilst run‐off mitigation is included in the step 4 calculations available, the FOCUS (FOCUS, 2007) report acknowledges that for substances with KFoc < 2,000 mL/g (i.e. phosmet), the general applicability and effectiveness of run‐off mitigation measures had been less clearly demonstrated in the available scientific literature, than for more strongly adsorbed compounds.
The necessary groundwater exposure assessments were appropriately carried out using FOCUS (European Commission, 2014a) scenarios and the models PEARL 4.4.4, PELMO 5.5.3 and MACRO 5.5.4.7 The potential for groundwater exposure from the representative uses by phosmet above the parametric drinking water limit of 0.1 μg/L was concluded to be low in geoclimatic situations that are represented by all the relevant FOCUS groundwater scenarios.
The applicant provided appropriate information to address the effect of water treatment processes on the nature of the residues that might be present in surface water, when surface water is abstracted for drinking water. The conclusion of this consideration was that neither phosmet nor any of its degradation products that trigger assessment (phthalamic acid, phthalic acid, N‐hydroxymethyl phthalimide, phthalimide and N‐hydroxymethyl phthalamic acid) would be expected to undergo any substantial transformation due to oxidation at the disinfection stage of usual water treatment processes.
The PEC in soil, surface water, sediment and groundwater covering the representative uses assessed can be found in Appendix A of this conclusion.
5. Ecotoxicology
The risk assessment was based on the following documents: European Commission (2002a,b), EFSA (2009), EFSA PPR Panel (2013) and EFSA (2013).
Some specific aspects related to the environmental risk assessment of phosmet were discussed at the Pesticide Peer Review Meetings PREV 06 and PREV 08 (June 2019).
The (eco)toxicological profile of phosmet relied upon batches that were not considered representative of the proposed renewal technical specification but support the original reference specification (see Section 1). Acute data with phosmet were available for two bird species (i.e. mallard duck and bobwhite quail). Hence, the geometric mean of the available LD50s was calculated. A low acute risk was concluded at the tier 1 and/or screening step for the uses on citrus, pome fruit (Central European Zone, hereafter CEZ), and potatoes. A high acute risk was instead identified for the ‘small insectivorous’ feeding guild for the uses of phosmet on peaches/nectarines and pome fruit (Southern European Zone, hereafter SEZ). Several refinements were proposed by the applicant. One proposal was to refine the MAF90 with residue trials measuring the dissipation rate of phosmet on arthropods and plants. These residue dissipation trials have been extensively discussed at the expert meeting.8 The experts at the meeting agreed that the ■■■■■ was ■■■■■ estimation of the dissipation rate on both food items, hence the refinement ■■■■■ in the final risk assessment. The applicant also identified great tit as focal species for the uses of phosmet in orchards (SEZ) and proposed a refinement based on the forager behaviour of this species. This refinement, accepted by the RMS, led to conclude a low acute risk for the uses in pome fruits (SEZ), but was not sufficient for demonstrating a low acute risk for the uses on peaches/nectarines. Therefore, a high acute risk to insectivorous birds was concluded for the uses of phosmet on peaches/nectarines.
The selection of the appropriate avian reproductive endpoint was discussed in the expert meeting.9 When using the endpoint selected, a high risk was identified at the tier 1 for the ‘small insectivorous’ (all uses) and for the ‘small granivorous’ (uses on peaches/nectarines, pome fruit CEZ, and pome fruits SEZ) feeding guilds. The main refinement proposed was again relying on the ■■■■■ As already discussed for the acute risk assessment, these were ■■■■■. A refinement of the PT for the selected focal insectivorous species was available and was accepted, ■■■■■ outcome of the risk assessment. Overall, a high reproductive risk to birds was concluded for all uses of phosmet assessed, leading to a critical area of concern.
Two acute studies with the active substance phosmet and rats were available in the dossier. The geometric mean of the relative LD50s has been used in the risk assessment for wild mammals. The tier 1 acute risk assessment identified high risk for the feeding guilds small herbivorous mammals (all uses) and frugivorous mammals (all uses except the one on potatoes, for which this guild is not relevant). Among the refinements proposed, a refined deposition value was accepted for grass, but not for fruits. As already discussed in the birds’ section, a refinement of MAF90 was not accepted following the agreement at the meeting regarding the available residue dissipation trials. Finally, a large number of residue trails for refining the initial RUD on fruits were available. This issue was discussed at the expert meeting,10 where the experts agreed that, due to the very large number of available RUDs (n = 110), the default value included in EFSA (2009) could be considered superseded, as this is based on a much smaller number of trials (n = 33). After applying the aforementioned refinements, a low risk was concluded for frugivorous mammals (all uses of phosmet) and for small herbivorous mammals (use on citrus and potatoes). However, a high acute risk to small herbivorous mammals was still identified for the uses on pome fruits (both CEZ and SEZ) and on peaches/nectarines.
The reproductive endpoint for wild mammals was discussed and agreed at the expert meeting.11 The tier 1 risk assessment identified a high reproductive risk for all focal species relevant for the uses of phosmet. A wide range of refinements were proposed. As already discussed, the refinement of the ■■■■■ based on the available ■■■■■ was ■■■■■ during the expert meeting. ■■■■■, the PT refinement for ‘lagomorph’ based on brown hare was not accepted during the expert meeting,12 due to failure of demonstrating that this is an appropriate specific focal species. Simulations with population models for wood mouse and common vole were also available. ■■■■■ the RMS ■■■■■ and the experts at the meeting considered that the available information ■■■■■ for an appropriate assessment.13 Hence, the outcome of the modelling simulations was ■■■■■ Other refinements were ■■■■■ accepted. These include: refinement of the crop interception factors, which were aligned with EFSA (2014b) (for herbivorous and omnivorous mammals), PD refinement for common vole (‘small herbivorous’), refinement of initial RUD on fruits (as explained for the acute assessment), identification of wood mouse as specific focal species for the ‘small omnivorous’ feeding guild and related PD refinement. ■■■■■ the refinements, ■■■■■ for all relevant feeding guilds was ■■■■■ for all uses of phosmet. Hence, ■■■■■ is concluded.
A low risk from the consumption of contaminated water and from secondary poisoning was concluded for both birds and mammals for all uses of phosmet.
Data were available for assessing the acute toxicity of the active substance phosmet to fish. For acute effects, data were available on four different species; therefore, the available endpoints were combined in a geomean for the risk assessment. Both the acute and the chronic risk assessment using PECsw calculated with FOCUS Step 3 resulted in a high risk for the majority of the scenarios from all the uses assessed. A modified exposure study with rainbow trout was available and considered acceptable. ■■■■■ the comparison of the exposure profile of the studies with the FOCUS profile as provided by the Applicant ■■■■■ Application of mitigation measures (20 metres non‐spray buffer + 20 m vegetated field strips) were considered for calculating FOCUS Step 4 PECsw. When using these PECsw, a low acute risk to fish could be concluded for all scenarios for the uses on potatoes and pome fruit (CEZ). A low risk was also concluded for one of two scenarios for the use on citrus. For the other uses (pome fruit SEZ and nectarines), a high acute risk to fish is concluded for four of seven scenarios. ■■■■■.
Toxicity data were available for daphnids, but reliable data for a second species of aquatic invertebrates (e.g. insects) were missing (data gap). However, this data gap is not considered essential, owing to the availability of mesocosm studies (see below). Both the acute and the chronic risk assessment using PECsw calculated with FOCUS Step 3 resulted in a high risk for all scenarios and all uses. Three mesocosms studies were available in the dossier, which were extensively discussed at the expert meeting.14 The experts agreed that one of the studies was not reliable, due to major deficiency on the exposure side. The other two studies were considered reliable. Nevertheless, in one of them ■■■■■ and ■■■■■ could be derived. The last study did provide a valid endpoint (NOEC = 2 μg/L), nevertheless, ■■■■■ in the test system ■■■■■ of the endpoint for the overall risk assessment. Hence, the risk assessment for aquatic invertebrates ■■■■■ When using FOCUS Step 4 PECsw (20 metres non‐spray buffer +20 m vegetated field strips), ■■■■■.
Acceptable toxicity data with algae were only available with the representative formulation Imidan 50WP. Using this endpoint and PECsw calculated with FOCUS Step 3, a low risk was concluded for the use on potatoes (all scenarios), while a high risk was identified for all other uses (all scenarios). Implementation of mitigation measures (20 metres non‐spray buffer +20 m vegetated field strips) would result in low risk for all uses and all scenarios.
A low risk to aquatic organisms was concluded for all metabolites of phosmet.
Data on the toxicity of phosmet to honey bees were available for acute contact and oral exposure. The assessment carried out both according to SANCO (European Commission 2002a) and to EFSA (2013) identified high acute risk for all uses and both routes of exposure.
Valid chronic data were available for both honey bee larvae and adults. When used in the risk assessment according to EFSA (2013), these data indicated a high risk for all uses of phosmet.
Four semi‐field and two field studies were available in the dossier. These have been discussed at the expert meeting.15 One semi‐field study was not considered reliable by the RMS. As the other three semi‐field studies were carried out with a very similar design, their results were combined in a common statistical re‐evaluation. The length of the period of observation for mortality and foraging activity was different in the different studies, but overall quite short. The combination of the studies showed high mortality after the application; however, effects on the colony cannot be fully assessed, as measurements of colony size and brood development were only performed 6–8 days after the start of the exposure and only roughly quantified (e.g. number of frames covered). It was also noted that the application rate used in these studies does not cover all the uses listed in the GAP. Overall, the experts agreed that the available semi‐field studies and their combination cannot be used to properly assess the risk. The RMS identified many shortcomings for the two available field studies, including the fact that mortality was only measured by means of bee traps placed only during the night. Even so, considerable and rather long‐lasting mortality effects were seen at both rates tested (500 and 750 g/ha) in both studies. Overall, the experts agreed that these studies cannot be used to properly assess risk, however they support, similarly to the semi‐field studies, the indication that exposure to phosmet increases the mortality of adult bees. Considering the inability of the available higher tier studies to address the risk, a high risk is concluded for all uses of phosmet, leading to a critical area of concern.
Based on the ■■■■■ A low risk was concluded from consumption of contaminated surface water.
■■■■■ Data for assessing the toxicity of phosmet to bumble bees and solitary bees were not available.
Data on the standard non‐target arthropods species were available. The tier 1 risk assessment suggested a low in‐field and off‐field risk to Typhlodromus pyri for most of the uses of phosmet; a high in‐field risk was only indicated for the uses on pome fruit (SEZ) and nectarines. Conversely, a high in‐field and off‐field risk to Aphidius rhopalosiphi was indicated for all uses of phosmet.
The tier 2 included several extended laboratory studies with Aphidius and two additional species. Among these two, Coccinella Septempunctata ■■■■■ A. rhopalosiphi ■■■■■. For these two species, a high in‐field and off‐field risk was indicated at the tier 2 for all uses of phosmet.
Three aged residue studies were also available, all carried out with Aphidius. These were discussed in the expert meeting.16 The experts considered that aged residues studies are not appropriate to identify recovery, but they could be used to demonstrate the potential for recolonisation. ■■■■■ the most sensitive organism among those tested, the available studies were ■■■■■ to conclude on the potential for recovery/recolonisation for all the other NTAs. ■■■■■
Four additional field studies were available for addressing the off‐field risk. These studies confirmed that Coccinellidae are the most vulnerable taxon of NTAs to phosmet. These studies were also discussed at the expert meeting16 The experts did not consider two of the studies to be relevant as they were not carried out in proper off‐field environments, i.e. considered not sufficiently representative. The other two studies were considered acceptable. For one of those, carried out in France (SEZ), an NOER of 5 g/ha was agreed; the other one was carried out in Germany (CEZ) and no suitable NOER could be established, as effects were seen at all tested rates. Overall, the majority of the experts concluded that the difference between the studies is not related to their geographical location, and that both should be considered when addressing the risk. As such, an appropriate Tier 3 off‐field NOER cannot be derived, and the off‐field risk assessment should be based on the tier 2 data.
It is noted that the RMS disagreed with this conclusion: in consideration of the different results obtained in these two tests, the RMS would use the Tier 3 off‐field NOER of 5 g/ha for all the SEZ uses, while they would agree on the lack of appropriate Tier 3 off‐field NOER for the CEZ uses. By using the approach suggested by the RMS, a low off‐field risk could be demonstrated for the uses on citrus using the NOER and a high level of drift reduction (≥ 93.6%). Nevertheless, such mitigation would ■■■■■ which, as discussed above, was ■■■■■ all representative uses.
Laboratory studies with phosmet were available for earthworms and the other standard soil macro‐organisms Hypoaspis aculeifer and Folsomia candida.
Based on the endpoints from these studies and the appropriate PECsoil, a low risk to earthworms and Hypoaspis was concluded for all uses of phosmet. For Folsomia, a low risk could only be concluded for the uses on citrus and on potatoes (1 × 500 g a.s./ha). A high risk was indicated for the remaining uses (pome fruit SEZ, pome fruit CEZ, nectarines).
A field study assessing the effects of the representative phosmet formulation on collembolan community in grasslands was available. The application of phosmet caused a clear and significant decrease on several collembolan species at both tested rates (2 × 500 and 2 × 750 g a.s./ha). The RMS concluded that, for some species, a recovery within one year had not been demonstrated. Hence, the high risk identified at the tier 1 was not addressed using the available higher tier study.
A low risk to soil micro‐organisms was concluded for all uses of phosmet.
Vegetative vigour and seedling emergence data were available to assess the toxicity of phosmet to non‐target terrestrial plants. The study on seedling emergence was not considered fully compliant with the relevant guideline, but overall acceptable for the risk assessment. A low risk to non‐target terrestrial plants and to sewage treatment plants was concluded for all uses of phosmet.
6. Endocrine disruption properties
With regard to the assessment of the endocrine disruption potential of phosmet for humans according to the ECHA/EFSA guidance (2018), for T‐modality ED criteria were considered not met and scenario 1a applied. The data set for the thyroids was considered sufficiently investigated and no thyroid mediated adversity was observed based on the lack of histopathological findings. However, the RMS considered that the T‐related endocrine adversity was not sufficiently investigated. The single increase in T4 level in a male pubertal assay was not associated with changes in thyroid‐stimulating hormone (TSH) and therefore not considered evidence of endocrine activity. Regarding the EAS‐modalities, the EAS parameters were considered not sufficiently investigated, since no study according to OECD TG 443 was provided. No EAS‐mediated adversity was observed. Overall, the endocrine activity was considered sufficiently investigated and it was negative; therefore, phosmet does not meet the ED criteria for EAS modalities.17
Based on the available data and assessment, it is concluded that phosmet does not meet the criteria for endocrine disruption for humans through the EATS‐modalities as set in point 3.6.5 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605.
The outcome of the assessment reported above for humans also applies to wild mammals as non‐target organisms.
For non‐target organisms other than mammals, for the T‐modality, a level 3 test according to OECD 231 (Amphibian Metamorphosis Assay) was available. Effects on amphibians’ development were only observed in the absence of histopathological changes in the thyroid. As a result, the endocrine activity through the T‐modality is considered negative and therefore T‐mediated adversity is unlikely.
For the E, A and S‐modalities, a fish short‐term reproduction assay according to OECD TG 229 was available. Effects on a number of parameters like fertilisation success, fecundity, vitellogenin level in males and nuptial tubercle score were observed. However, all those effects were observed at the middle tested dose (9.3 μg/L expressed as mean measured concentration) in the presence of signs of systemic toxicity (25% mortality in both sexes). It has to be noted that at the lowest tested concentration (1.0 μg/L), 25% mortality in females was also observed. Considering the available evidence, no ED‐mediated adversity and/or endocrine activity is expected in the absence of systemic toxicity.
Based on the available data and assessment, it is concluded that phosmet does not meet the criteria for endocrine disruption for non‐target organisms through EATS‐modalities as set out in point 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605.
7. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1, 2, 3, 4)
Table 1.
Soil
| Compound (name and/or code) | Persistence | Ecotoxicology |
|---|---|---|
| Phosmet | Low persistence Single first‐order and FOMC DT50 1.6–4.6 days (DT90 5.5–22.5 days, 20°C 44–50% MWHC soil moisture) | High risk to soil‐dwelling organisms (collembolans) (Uses on pome fruits and peaches/nectarines) |
Table 2.
Groundwater
| Compound (name and/or code) | Mobility in soil | > 0.1 μg/L at 1 m depth for the representative usesa | Pesticidal activity | Toxicological relevance |
|---|---|---|---|---|
| phosmet | Medium to low mobility KFoc 482–757 mL/g | No | Yes | Yes |
FOCUS scenarios or relevant lysimeter.
Table 3.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Phosmet | High risk to aquatic organisms living in the surface water |
| Phthalimide (aqueous photolysis) | Low risk to aquatic organisms living in the surface water |
| Phthalamic acid (aqueous photolysis, water and sediment) | Low risk to aquatic organisms living in the surface water |
| Phthalic acid (aqueous photolysis, water) | Low risk to aquatic organisms living in the surface water |
| N‐hydroxymethyl phthalimide (water) | Low risk to aquatic organisms living in the surface water |
| N‐hydroxymethyl phthalamic acid (aqueous photolysis) | Low risk to aquatic organisms living in the surface water |
Table 4.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| Phosmet | H332, harmful if inhaled |
8. Data gaps
This is a list of data gaps identified during the peer review process, including those areas in which a study may have been made available during the peer review process but not considered for procedural reasons (without prejudice to the provisions of Article 56 of Regulation (EC) No 1107/2009 concerning information on potentially harmful effects).
A search of the scientific peer‐reviewed open literature on the active substance and its relevant metabolites, dealing with side effects on health, the environment and non‐target species and published within the 10 years before the date of submission of the dossier, to be conducted and reported in accordance with EFSA guidance on the submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009 (EFSA, 2011; relevant for all representative uses evaluated).
Information on self‐heating for the representative formulation (relevant for all representative uses evaluated; see Section 1).
Data gap ■■■■■
Data gaps for ■■■■■
■■■■■
Additional information to conclude on the storage stability studies on phosmet‐oxon in extracts and subsequently on the validity of residue field trial results for phosmet‐oxon (relevant for all the representative uses; see section 3).
Additional information regarding sample storage time and conditions for the metabolism studies in cherry, potato and maize (relevant for all the representative uses; see Section 3).
Information to address the relevance for ■■■■■
At least three independent GAP compliant residue trials in apples or pears in SEU with analysis of phosmet and phosmet‐oxon, ensuring integrity of residues until analysis (relevant for the representative uses in pome fruit; see Section 3).
At least six independent GAP compliant residue trials in mandarins in SEU with analysis of phosmet and phosmet‐oxon, ensuring integrity of residues until analysis (relevant for the representative uses in citrus; see Section 3).
At least four independent GAP compliant residue trials in peaches in SEU with analysis of phosmet‐oxon, ensuring integrity of residues until analysis (relevant for the representative uses in peaches/nectarines; see Section 3).
Background ■■■■■
Scientific justification on the discrepancy between the two standard hydrolysis studies or confirmatory investigations regarding the occurrence of N‐hydroxymethyl phthalamic acid and desmethyl‐phosmet under processing conditions is required, including considerations on hydrolysis behaviour of phosmet‐oxon and potentially formed compounds (relevant for the representative uses citrus, pome fruit, peaches/nectarines; see Section 3).
A relevance assessment of ■■■■■
An assessment of the ■■■■■
Information against the data requirement on ■■■■■
Information on the aerobic degradation rate of phosmet in one additional soil (relevant for all the representative uses evaluated; see Section 4).
Reliable acute data for a second species of aquatic invertebrates (preferably insects) should be generated for phosmet (data gap not relevant to finalise the risk assessment for the representative uses, owing to the availability of mesocosm studies; see Section 5).
Suitable data for quantifying the exposure of ■■■■■ Data gap according to EFSA (2013) (relevant for all the representative uses; see Section 5).
Toxicity data for addressing ■■■■■ Data gap according to EFSA (2013) (relevant for all the representative uses; see Section 5).
9. Particular conditions proposed to be taken into account to manage the risk(s) identified
None.
10. Concerns
10.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/201118 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
-
■■■■■
■■■■■
-
■■■■■
■■■■■
10.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
10.3. Overview of the concerns identified for each representative use considered (Table 5)
Table 5.
Overview of concerns
| Representative use | Citrus | Pome fruits SEU/SEZ | Pome fruits CEU/CEZ | Peaches/Nectarines | Potatoes | |
|---|---|---|---|---|---|---|
| Operator risk | Risk identified | X3 | X3 | X3 | X3 | ■■■■■ |
| Assessment not finalised | ||||||
| Worker risk | Risk identified | X3 | X3 | X3 | X3 | X3 |
| Assessment not finalised | ||||||
| Resident/bystander risk | Risk identified | X3 | X3 | X3 | X3 | ■■■■■ |
| Assessment not finalised | ||||||
| Consumer risk | Risk identified | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ |
| Assessment not finalised | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | ■■■■■ | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | ■■■■■ | X5 | X5 | X5 | ■■■■■ |
| Assessment not finalised | ||||||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | ■■■■■ | X7,8 | X7,8 | X7,8 | ■■■■■ |
| Assessment not finalised | ||||||
| Risk to aquatic organisms | Risk identified | ■■■■■ | X6 | X6 | X6 | ■■■■■ |
| Assessment not finalised | ||||||
| Groundwater exposure to active substance | Legal parametric value breached | |||||
| Assessment not finalised | ||||||
| Groundwater exposure to metabolites | Legal parametric value breacheda | |||||
| Parametric value of 10 μg/Lb breached | ||||||
| Assessment not finalised | ||||||
The superscript numbers relate to the numbered points indicated in Sections 10.1 and 10.2. Where there is no superscript number, see Sections 2–7 for further information.
When the consideration for classification made in the context of this evaluation under Regulation (EC) No 1107/2009 is confirmed under Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission (2003).
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 5.)
Abbreviations
- 1/n
slope of Freundlich isotherm
- λ
wavelength
- ε
decadic molar extinction coefficient
- a.s.
active substance
- AAOEL
acute acceptable operator exposure level
- AChE
acetylcholinesterase
- ADE
actual dermal exposure
- ADI
acceptable daily intake
- AF
assessment factor
- AhR
aryl hydrocarbon receptor
- AOEL
acceptable operator exposure level
- AR
applied radioactivity
- AR
androgen receptor
- ARfD
acute reference dose
- AV
avoidance factor
- BUN
blood urea nitrogen
- bw
body weight
- CAS
Chemical Abstracts Service
- CEU/CEZ
Central Europe/Central European Zone
- ChE
cholinesterase
- CHO
Chinese hamster ovary cells
- CI
confidence interval
- CIPAC
Collaborative International Pesticides Analytical Council Limited
- CL
confidence limits
- DAR
draft assessment report
- DAT
days after treatment
- DM
dry matter
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- EEC
European Economic Community
- FAO
Food and Agriculture Organization of the United Nations
- FID
flame ionisation detector
- FIR
food intake rate
- FOB
functional observation battery
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- GC
gas chromatography
- GC‐NPD
gas chromatography with nitrogen phosphorus selective detector
- GM
geometric mean
- GS
growth stage
- HPLC
high‐pressure liquid chromatography or high‐performance liquid chromatography
- HPLC‐MS
high‐pressure liquid chromatography–mass spectrometry
- HQ
hazard quotient
- HR
hazard rate
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- iv
intravenous
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- KFoc
Freundlich organic carbon adsorption coefficient
- LC
liquid chromatography
- LC‐MS
liquid chromatography–mass spectrometry
- LC‐MS-MS
liquid chromatography with tandem mass spectrometry
- LOQ
limit of quantification
- M/L
mixing and loading
- mm
millimetre (also used for mean measured concentrations)
- MOA
mode of action
- MRL
maximum residue level
- MS
mass spectrometry
- MWHC
maximum water‐holding capacity
- NOAEL
no observed adverse effect level
- NOEC
no observed effect concentration
- NOEL
no observed effect level
- NPD
nitrogen–phosphorus detector
- OECD
Organisation for Economic Co‐operation and Development
- OM
organic matter content
- Pa
pascal
- PD
proportion of different food types
- PEC
predicted environmental concentration
- PECsoil
predicted environmental concentration in soil
- PECsw
predicted environmental concentration in surface water
- PHI
preharvest interval
- PIE
potential inhalation exposure
- PPE
personal protective equipment
- ppm
parts per million (10−6)
- PT
proportion of diet obtained in the treated area
- PTT
partial thromboplastin time
- QSAR
quantitative structure–activity relationship
- RAR
Renewal Assessment Report
- RBC
red blood cells
- REACH
Registration, Evaluation, Authorisation of Chemicals Regulation
- RUD
residue per unit dose
- SC
suspension concentrate
- SEU/SEZ
Southern Europe/Southern European Zone
- SMILES
simplified molecular‐input line‐entry system
- TK
technical concentrate
- TRR
total radioactive residue
- TSH
thyroid‐stimulating hormone (thyrotropin)
- TWA
time‐weighted average
- UF
uncertainty factor
- W/S
water/sediment
- w/v
weight per unit volume
- w/w
weight per unit weight
- WHO
World Health Organization
Appendix A – List of end points for the active substance and the representative formulation
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2021.6237
Appendix B – Used compound codes
1.
| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulab |
|---|---|---|
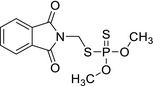
| phosmet | O,O‐dimethyl S‐phthalimidomethyl phosphorodithioate or N‐{[(dimethoxyphosphinothioyl)thio]methyl}phthalimide S=P(OC)(SCN1C(C2=CC=CC=C2C1=O)=O)OC LMNZTLDVJIUSHT‐UHFFFAOYSA‐N |
|
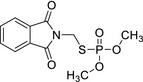
| Phosmet‐oxon | S‐((1,3‐dioxoisoindolin‐2‐yl)methyl) O,O‐dimethyl phosphorothioate O=P(OC)(SCN1C(C2=CC=CC=C2C1=O)=O)OC BEMXOWRVWRNPPL‐UHFFFAOYSA‐N |
|
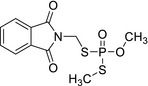
| Isophosmet | S‐((1,3‐dioxoisoindolin‐2‐yl)methyl) O,S‐dimethyl phosphorodithioate O=P(SCN1C(C2=CC=CC=C2C1=O)=O)(SC)OC JSDGNPMLVTZCBP‐UHFFFAOYSA‐N |
|
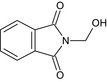
| N‐hydroxymethyl phthalimide | 2‐(hydroxymethyl)‐1H‐isoindole‐1,3(2H)‐dione O=C1c2ccccc2C(=O)N1CO MNSGOOCAMMSKGI‐UHFFFAOYSA‐N |
|
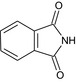
| phthalimide | 1H‐isoindole‐1,3(2H)‐dione O=C1NC(=O)c2ccccc12 XKJCHHZQLQNZHY‐UHFFFAOYSA‐N |
|
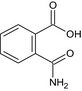
| phthalamic acid | 2‐carbamoylbenzoic acid OC(=O)c1ccccc1C(N)=O CYMRPDYINXWJFU‐UHFFFAOYSA‐N |
|
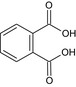
| phthalic acid | phthalic acid OC(=O)c1ccccc1C(=O)O XNGIFLGASWRNHJ‐UHFFFAOYSA‐N |
|
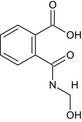
| N ‐hydroxymethyl phthalamic acid | 2‐[(hydroxymethyl)carbamoyl]benzoic acid OC(=O)c1ccccc1C(=O)NCO SAQWDZHRYAPCHZ‐UHFFFAOYSA‐N |
|
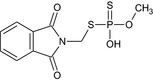
| desmethyl‐phosmet | S‐[(1,3‐dioxo‐1,3‐dihydro‐2H‐isoindol‐2‐yl)methyl] O‐methyl hydrogen phosphorodithioate COP(O)(=S)SCN1C(=O)c2ccccc2C1=O DLQUIOLJSSFFCY‐UHFFFAOYSA‐N |
|
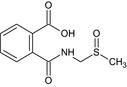
| N ‐(methylsulfinylmethyl)‐phthalamic acid (PaAMS(O)M) | 2‐{[(methylsulfinyl)methyl]carbamoyl}benzoic acid OC(=O)c1ccccc1C(=O)NCS(C)=O CYWOGIGLKVMHOJ‐UHFFFAOYSA‐N |
|
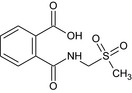
| N‐(methylsulfonylmethyl)‐phthalamic acid (PaAMS(O2)M) | 2‐{[(methylsulfonyl)methyl]carbamoyl}benzoic acid OC(=O)c1ccccc1C(=O)NCS(C)(=O)=O NJSYHTQSBLBUIQ‐UHFFFAOYSA‐N |
|
The metabolite name in bold is the name used in the conclusion.
ChemBioDraw v.13.0.2.3021.
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Anastassiadou M, Arena M, Auteri D, Brancato A, Bura L, Carrasco Cabrera L, Chaideftou E, Chiusolo A, Crivellente F, De Lentdecker C, Egsmose M, Fait G, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lostia A, Lythgo C, Magrans O, Mangas I, Miron I, Molnar T, Padovani L, Parra Morte JM, Pedersen R, Reich H, Santos M, Sharp R, Szentes C, Terron A, Tiramani M, Vagenende B and Villamar‐Bouza L, 2021. Conclusion on the peer review of the pesticide risk assessment of the active substance phosmet. EFSA Journal 2021;19(3):6237, 25 pp. 10.2903/j.efsa.2021.6237
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00293
Legal notice: This document has been sanitised by making unavailable information claimed confidential by the applicant. A revised version of the document will be made available as soon as a final decision on the confidentiality claims is issued by EFSA.
Acknowledgements: EFSA wishes to thank the rapporteur Member State, Spain, for the preparatory work on this scientific output.
Approved: 18 August 2020
Notes
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, p. 26–32.
Commission Implementing Regulation (EU) No 2018/1659 of 7 November 2018 amending Implementing Regulation (EU) No 844/2012 in view of the scientific criteria for the determination of endocrine disrupting properties introduced by Regulation (EU) 2018/605.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Refer to experts’ consultation 2.5 in the Report of Pesticides Peer Review Experts’ Meeting 07 (EFSA, 2020).
Commission Regulation (EU) No 283/2013 of 1 March 2013, setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, p. 1–84.
Simulations utilised the agreed Q10 of 2.58 (following EFSA, 2008) and Walker equation coefficient of 0.7.
Refer to experts’ consultation 5.1 (sub‐points 1–3) in the Report of Pesticides Peer Review Experts’ Meetings 06 and 08 (EFSA, 2020.
Refer to experts’ consultation 5.5 in the Report of Pesticides Peer Review Experts’ Meetings 06 and 08 (EFSA, 2020).
Refer to experts’ consultation 5.1 (sub‐point 4) in the Report of Pesticides Peer Review Experts’ Meetings 06 and 08 (EFSA, 2020).
Refer to experts’ consultation 5.2 in the Report of Pesticides Peer Review Experts’ Meetings 06 and 08 (EFSA, 2020).
Refer to experts’ consultation 5.4 in the Report of Pesticides Peer Review Experts’ Meetings 06 and 08 (EFSA, 2020).
Refer to experts’ consultation 5.3 in the Report of Pesticides Peer Review Experts’ Meetings 06 and 08 (EFSA, 2020).
Refer to experts’ consultation 5.6 in the Report of Pesticides Peer Review Experts’ Meetings 06 and 08 (EFSA, 2020).
Refer to experts’ consultation 5.7 in the Report of Pesticides Peer Review Experts’ Meetings 06 and 08 (EFSA, 2020).
Refer to experts’ consultation 5.8 in the Report of Pesticides Peer Review Experts’ Meetings 06 and 08 (EFSA, 2020).
Refer to experts’ consultation 2.6 in the Report of Pesticides Peer Review Experts’ Meetings 07 (EFSA, 2020).
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- ECHA (European Chemicals Agency), 2017. Guidance on the Application of the CLP Criteria; Guidance to Regulation (EC) No 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures. Version 5.0, July 2017. Reference: ECHA‐17-G-21‐EN; ISBN: 978‐92-9020‐050-5. Available online: https://echa.europa.eu/guidance-documents/guidance-on-clp
- ECHA and EFSA (European Chemicals Agency and European Food Safety Authority) with the technical support of the Joint Research Centre (JRC), Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311, 135 pp. 10.2903/j.efsa.2018.5311. ECHA‐18-G-01‐EN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Opinion on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil. EFSA Journal 2008;6(1):622, 32 pp. 10.2903/j.efsa.2008.622 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance on Risk Assessment for Birds and Mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009. EFSA Journal 2011;9(2):2092, 49 pp. 10.2903/j.efsa.2011.2092 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2014a. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products. EFSA Journal 2014;12(10):3874, 55 pp. 10.2903/j.efsa.2014.3874. Available online: www.efsa.europa.eu/efsajournal [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2014b. EFSA Guidance Document for evaluating laboratory and field dissipation studies to obtain DegT50 values of active substances of plant protection products and transformation products of these active substances in soil. EFSA Journal 2014;12(5):3662, 37 pp. 10.2903/j.efsa.2014.3662 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2020. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance phosmet. Available online: www.efsa.europa.eu
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2012. Guidance on Dermal Absorption. EFSA Journal 2012;10(4):2665, 30 pp. 10.2903/j.efsa.2012.2665 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290, 186 pp. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- European Commission , 2000a. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000.
- European Commission , 2000b. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000.
- European Commission , 2002a. Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002-rev. 2 final, 17 October 2002.
- European Commission , 2002b. Guidance Document on Aquatic Ecotoxicology Under Council Directive 91/414/EEC. SANCO/3268/2001‐rev. 4 final, 17 October 2002.
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2011. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. SANCO 7525/VI/95‐rev. 9. March 2011. p. 1–46.
- European Commission , 2012. Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC) No 1107/2009. SANCO/10597/2003-rev. 10.1, 13 July 2012.
- European Commission , 2014a. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010-v. 3, 613 pp., as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.2, May 2014.
- European Commission , 2014b. Guidance document on the renewal of approval of active substances to be assessed in compliance with Regulation (EU) No 844/2012. SANCO/2012/11251‐rev. 4, 12 December 2014.
- European Commission , 2014c. Review report for the active substance phosmet. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 24 November 2006 in view of the inclusion of phosmet in Annex I of Council Directive 91/414/EEC. SANCO/10050/2006-Final, 11 July 2014, 9 pp.
- FAO/WHO 318/2019 ,FAO specifications and evaluations for agricultural pesticides, Phosmet (O,O‐dimethyl S‐phthalimidomethylphosphorodithioate) Note: Evaluation Report only. Available online: http://www.fao.org/3/ca8627en/ca8627en.pdf
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2001. FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.1, March 2012.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2006. Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005-v. 2.0, 434 pp., as updated by the Generic guidance for Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration, v. 1.1, December 2014.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2007. Landscape and mitigation factors in aquatic risk assessment. Volume 1. Extended summary and recommendations. Report of the FOCUS Working Group on Landscape and Mitigation Factors in Ecological Risk Assessment. EC Document Reference SANCO/10422/2005 v. 2.0, 169 pp.
- JMPR (Joint Meeting on Pesticide Residues), 2004. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Rome, Italy, 20–29 September 2004, 383 pp.
- JMPR (Joint Meeting on Pesticide Residues), 2007. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Switzerland, 18–27 September 2007, 164 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2009. Guidance document on overview of residue chemistry studies. ENV/JM/MONO(2009)31, 28 July 2009.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: www.oecd.org
- Spain , 2017. Renewal Assessment Report (RAR) on the active substance phosmet prepared by the rapporteur Member State Spain, in the framework of Commission Implementing Regulation (EU) No 844/2012, September 2017. Available online: www.efsa.europa.eu
- Spain , 2020. Revised Renewal Assessment Report (RAR) on phosmet prepared by the rapporteur Member State Spain in the framework of Regulation (EC) No 844/2012, April 2020. Available online: www.efsa.europa.eu
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation


