Abstract
OBJECTIVE:
To assess efficacy and tolerability of ulipristal acetate, a selective progesterone receptor modulator, for treatment of symptomatic uterine leiomyomas.
METHODS:
This phase 3, double-blind, placebo-controlled study enrolled premenopausal women (aged 18–50 years) with abnormal uterine bleeding, one or more discrete leiomyomas, and uterine size 20 weeks of gestation or less. Patients were randomized 1:1:1 to 5 mg ulipristal, 10 mg ulipristal, or placebo once daily for 12 weeks followed by 12-week drug-free follow-up. Coprimary endpoints were rate of and time to amenorrhea, defined as no bleeding for the last 35 consecutive days of treatment. Secondary endpoints included rates of amenorrhea from day 11 and change from baseline to endpoint in the Revised Activities subscale of the Uterine Fibroid Symptom and Quality of Life questionnaire, which includes questions pertaining to physical and social activities. Safety assessments included adverse event monitoring and endometrial biopsies. A sample size of 150 was planned to compare separately each dose of ulipristal with placebo.
RESULTS:
From March 2014 to March 2016, 157 patients were randomized. Demographics were similar across treatment groups. Amenorrhea was achieved by 25 of 53 (47.2% [97.5% CI 31.6–63.2]) and 28 of 48 (58.3% [97.5% CI 41.2–74.1]) patients treated with 5 mg and 10 mg ulipristal, respectively, compared with 1 of 56 (1.8% [97.5% CI 0.0–10.9]) placebo-treated patients (both P<.001). Time to amenorrhea was shorter for both ulipristal doses compared with placebo (P<.001), and both doses of ulipristal resulted in improved quality of life compared with placebo (P<.001). Common adverse events (5% or greater in either ulipristal group during treatment) were hypertension, elevated blood creatinine phosphokinase, and hot flushes. Serious adverse events occurred in four patients, but none was considered related to treatment. Endometrial biopsies were benign.
CONCLUSION:
Ulipristal at 5 mg and 10 mg were well tolerated and superior to placebo in rate of and time to amenorrhea in women with symptomatic uterine leiomyomas.
CLINICAL TRIAL REGISTRATION:
Clinicaltrials.gov number, NCT02147197.
Uterine leiomyomas are the most common benign gynecologic condition, occurring in up to 70% of white women and exceeding 80% of black women of reproductive age in the United States.1,2 Approximately 25–50% of women with leiomyomas experience clinical symptoms,1 including excessive and prolonged uterine bleeding,3 that interfere with daily social and physical activities and negatively affect quality of life.4,5 Abnormal bleeding is often severe enough to require intervention,3 which is mostly surgical and invasive, leading to substantial direct costs of medical care and indirect costs such as time lost from work.6
Ulipristal acetate is an orally administered selective progesterone receptor modulator7 that at daily doses of 5–10 mg acts to decrease bleeding and reduce leiomyoma size.8–11 Although selective progesterone receptor modulator administration has been associated with histologic changes to endometrium,12 such changes have been shown to reverse spontaneously after treatment cessation.13,14
In trials conducted in Europe, ulipristal was found superior to placebo8 and noninferior to the gonadotropin-releasing hormone agonist leuprolide acetate9 in controlling bleeding and reducing leiomyoma size while maintaining estradiol at midfollicular levels.8,9 Maintenance of efficacy and safety of ulipristal have been demonstrated in trials of up to eight intermittent 12-week courses of treatment.10,11,15,16 These trials were conducted in a predominantly white population; however, the risk of developing leiomyomas is higher in black women, who tend to have earlier age of onset and greater disease severity than white women.2,5,17,18 The current study was designed to assess efficacy and safety of ulipristal in a diverse population more typical of U.S. patients.
MATERIALS AND METHODS
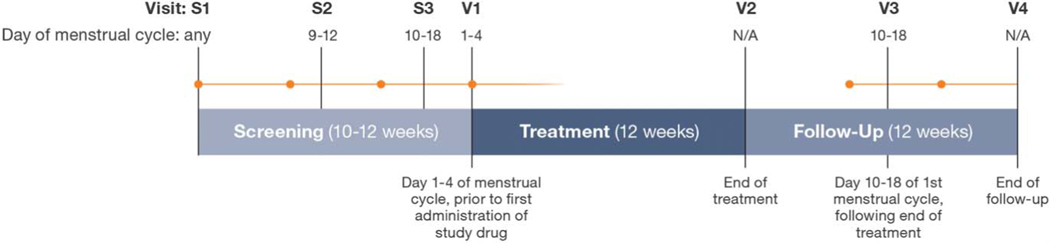
This was a phase 3, multicenter, randomized, double-blind, double-dummy, placebo-controlled, parallel-group study (UL1309; VENUS I; NCT02147197) conducted at 25 study centers across the United States. Patients were randomized 1:1:1 to once-daily oral 5 mg ulipristal, 10 mg ulipristal, or placebo. The randomization schedule was prepared by the sponsor using SAS PROC PLAN. The study comprised three screening visits, a 12-week treatment period, and a 12-week drug-free follow-up period (Fig. 1). The study was conducted in accordance with Good Clinical Practice guidelines as contained in the International Conference on Harmonization and U.S. Code of Federal Regulations and in accordance with the Declaration of Helsinki. The study protocol was approved by central or local institutional review boards before study initiation, and written informed consent was acquired from all study participants.
Fig. 1.
Study design. Orange dots and lines represent the commencement and continuation of menses, respectively. S, screening; V, visit.
Simon. Ulipristal Acetate for Uterine Leiomyomas. Obstet Gynecol 2018.
Premenopausal women (follicle-stimulating hormone 20 milli-international units/mL or less), 18–50 years of age, with symptomatic uterine leiomyomas were eligible for enrollment. Key inclusion criteria comprised cyclic (22–35 days) abnormal uterine bleeding (heavy or prolonged) in four or more of the previous six menstrual cycles; menstrual blood loss of 80 mL or greater measured by the alkaline hematin method over the first 8 days of menses; one or more leiomyomas of any size and location observable by transvaginal ultrasonography; and uterine size of 20 weeks of gestation or less by clinical examination.
Key exclusion criteria included a history of endometrial ablation or other uterine surgery within 2 months before screening or uterine artery embolization within 6 months before screening. Patients also were excluded if using progestin-containing contraceptives within 2 months before screening; were treated with a gonadotropin-releasing hormone agonist within 5 months before screening; or used a selective progesterone receptor modulator other than for emergency contraception at any time before screening. Women of childbearing potential were required to use nonhormonal method(s) of contraception throughout the study.
Coprimary efficacy endpoints were the proportion of patients who achieved amenorrhea (spotting permitted) during the last 35 consecutive days of treatment and time to amenorrhea during treatment. Secondary efficacy endpoints were the proportion of patients who achieved amenorrhea by day 11 and did not report bleeding (spotting permitted) for the duration of treatment and the change from baseline to end of treatment on the Revised Activities subscale of the Uterine Fibroid Symptom and Health-Related Quality of Life questionnaire19 that included questions pertaining to physical and social activities. Additional efficacy endpoints included mean change in Uterine Fibroid Symptom and Health-Related Quality of Life questionnaire subscale scores from baseline to end of treatment; mean change in total volume of the three largest leiomyomas from baseline to first menses after the end of treatment; and proportion of patients who achieved control of bleeding (post hoc endpoint), defined as 0 days of heavy bleeding and 8 days or less of bleeding (spotting permitted) within the last 56 days of treatment.
Bleeding assessments were based on daily participant e-diary entries, in which the heaviest bleeding level experienced over the previous 24 hours and daily presence or absence of bleeding were recorded using the terms “none,” “spotting,” “bleeding,” or “heavy bleeding.” Data from these assessments were analyzed to determine rate of amenorrhea, time to amenorrhea, and attainment of control of bleeding.
Analysis of quality of life was based on the Uterine Fibroid Symptom and Health-Related Quality of Life patient-administered questionnaire, which was developed and validated for detecting differences in symptom severity (eight items) and health-related quality of life among patients with uterine leiomyomas. The Health-Related Quality of Life questions included 29 items comprising six subscales (Concern, Control, Self-Consciousness, Sexual Function, Energy/Mood, Activities) and a Health-Related Quality of Life Total score. The Health-Related Quality of Life Total score included the Original Activities subscale.
Safety assessments included monitoring of adverse events, endometrial thickness measured by transvaginal ultrasonography (cycle days 10–18), endometrial biopsies, clinical laboratory measurements, and vital signs. Endometrial biopsies were taken at screening, between days 10 and 18 of the participant’s first menstrual cycle after the end of treatment, and at the end of the 12-week drug-free follow-up period or on early termination from the study. All endometrial biopsy specimens were evaluated and classified at a central laboratory by a panel of three independent pathologists blinded to study visit, treatment, and each other’s readings; diagnosis was based either on consensus of two of three pathologist evaluations or on the most severe diagnosis if there was no consensus. Biopsies also were assessed for progesterone receptor modulator-associated endometrial changes.12
Based on results from previous trials,8,9,20 it was calculated that a sample size of approximately 150 patients (50/treatment group) would be required to provide greater than 90% power to evaluate separately each ulipristal dose compared with placebo for the primary and secondary endpoints using sequential testing procedures (multiplicity-adjusted, α=0.025). Coprimary and secondary efficacy endpoints were analyzed for the intent-to-treat population, defined as all patients who were randomized to a treatment group. Dichotomous endpoints were analyzed using Cochran-Mantel-Haenszel tests controlling for (pooled) study site. Time to amenorrhea was analyzed using log-rank tests with events censored at day 50 if criteria for response were not met. Change from baseline in the Uterine Fibroid Symptom and Health-Related Quality of Life Revised Activities subscale score was analyzed by analysis of covariance controlling for baseline score and pooled study site. Evaluation of additional endpoints was performed using analysis of covariance unadjusted for multiplicity. Safety evaluations were descriptive and based on the safety population, defined as all patients who took one or more doses of the double-blind study drug.
RESULTS
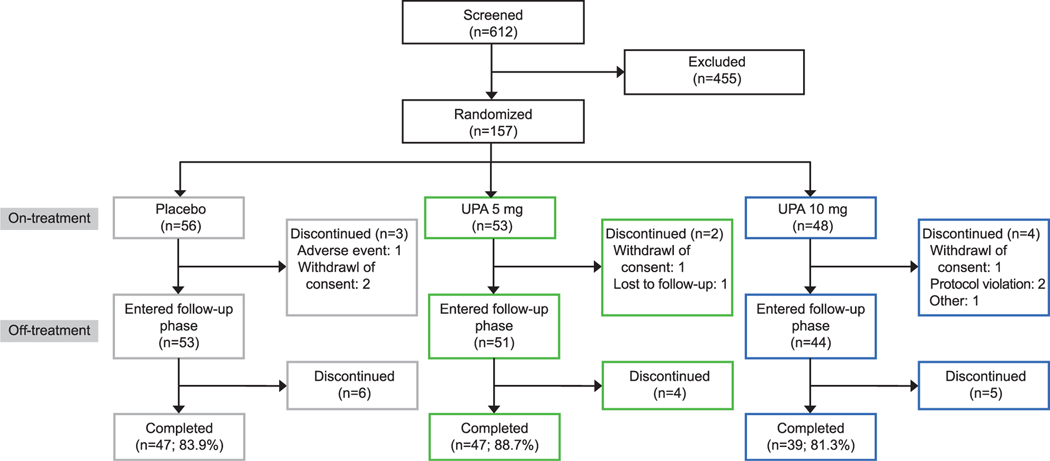
Of 612 screened patients, the intent-to-treat population included 157 patients, all of whom received one or more doses of the double-blind study drug (safety population). Of these, 148 patients completed the treatment period and 133 completed follow-up (Fig. 2). No patient discontinued as a result of lack of efficacy, and one patient in the placebo group discontinued as a result of an adverse event. Mean patient compliance (reported as range across treatment groups) to study drug was 94–96%; for e-diary reporting of bleeding, compliance was 89–91% for the treatment period and 83–90% for the follow-up period. Demographic and baseline clinical characteristics were similar across the three arms of the study (Table 1). For the first participant, the first visit date was March 31, 2014; for the last participant, the last visit date was March 29, 2016.
Fig. 2.
Patient disposition. Gray boxes indicate placebo, green boxes indicate ulipristal acetate (UPA) 5 mg, and blue boxes indicate UPA 10 mg.
Simon. Ulipristal Acetate for Uterine Leiomyomas. Obstet Gynecol 2018.
Table 1.
Demographic and Clinical Characteristics at Baseline (Safety Population)
| Characteristic | Placebo (n=56) | 5 mg Ulipristal (n=53) | 10 mg Ulipristal (n=48) | Total (n=157) |
|---|---|---|---|---|
| Age (y) | 41.6±5.1 | 40.5±5.9 | 41.3±5.1 | 41.1±5.4 |
| Age group (y) | ||||
| Younger than 20 | 0 | 0 | 0 | 0 |
| 20 to younger than 40 | 14 (25) | 21 (40) | 17 (35) | 52 (33) |
| 40 or older | 42 (75) | 32 (60) | 31 (65) | 105 (67) |
| Race | ||||
| Black | 35 (63) | 36 (68) | 37 (77) | 108 (69) |
| White | 19 (34) | 17 (32) | 10 (21) | 46 (29) |
| Asian | 2 (4) | 0 | 0 | 2 (1) |
| Native American or Alaska native | 0 | 0 | 1 (2) | 1 (1) |
| Ethnicity, Hispanic | 4 (7) | 6 (11) | 4 (8) | 14 (9) |
| Weight (kg) | 85.5±24.5 | 87.8±23.6 | 87.9±19.0 | 87.0±22.5 |
| BMI (kg/m2) | 31.3±8.5 | 31.8±8.7 | 32.1±6.6 | 31.7±8.0 |
| BMI 30 or greater | 26 (46) | 26 (49) | 27 (56) | 79 (50) |
| Menstrual blood loss (mL) | 195.6±82.1 | 253.2±234.0 | 208.9±147.6 | 219.1±166.8 |
| Median (IQR) | 187.3 (128.2) | 158.6 (171.6) | 167.5 (149.7) | 166.8 (143.1) |
| Leiomyoma total volume (cc) | 61.2±84.7 | 59.6±84.4 | 83.4±148.0 | 67.7±108.6 |
| Median (IQR) | 41.4 (58.6) | 25.1 (64.0) | 34.4 (65.8) | 34.5 (63.0) |
| Uterine volume (cc) | 317.0±193.9 | 305.6±240.6 | 340.4±271.3 | 321.0±235.9 |
| Median (IQR) | 259.3 (195.3) | 242.1 (202.5) | 271.7 (180.3) | 255.1 (185.2) |
| UFS-QOL* | ||||
| Revised Activities† | 33.6±26.3 | 30.1±23.8 | 27.9±24.3 | 30.7±24.8 |
| HRQOL Total score† | 37.8±22.7 | 35.5±21.3 | 32.5±19.9 | 35.4±21.4 |
| Concern | 26.0±25.1 | 24.8±23.1 | 17.1±17.9 | 22.9±22.6 |
| Control | 47.6±27.5 | 44.2±27.1 | 42.8±26.6 | 45.0±27.0 |
| Self-Consciousness | 34.8±31.6 | 37.4±29.0 | 33.0±25.8 | 35.1±28.9 |
| Sexual Function | 44.6±33.4 | 35.1±32.1 | 40.9±36.0 | 40.3±33.8 |
| Energy/Mood | 39.0±25.1 | 37.5±25.6 | 35.0±23.5 | 37.3±24.7 |
| Original Activities | 37.4±25.6 | 34.0±23.7 | 31.1±24.4 | 34.3±24.6 |
| Symptom Severity‡ | 59.4±20.8 | 62.3±18.4 | 64.8±19.9 | 62.0±19.7 |
BMI, body mass index; calculated as weight (kg)/[height (m)]2; IQR, interquartile range; UFS-QOL, Uterine Fibroid Symptom and Health-Related Quality of Life questionnaire; HRQOL, health-related quality of life.
Data are mean±SD or n (%) unless otherwise specified.
Intent-to-treat population.
HRQOL Total score and HRQOL subscale scores range from 0 to 100; higher scores indicate better health-related quality of life.
Symptom Severity subscale score ranges from 0 to 100; higher scores indicate greater symptom severity.
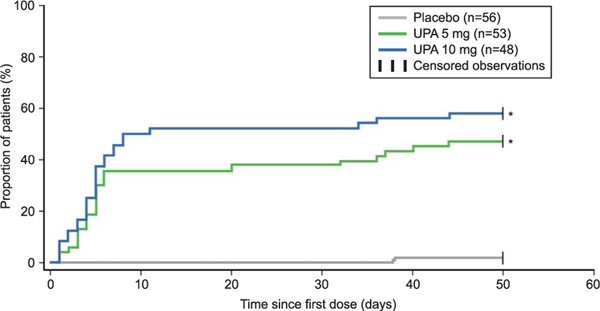
Results for coprimary, secondary, and other endpoints are shown in Table 2 and Figure 3. Greater efficacy was observed with both doses of ulipristal than with placebo for the coprimary efficacy endpoints. Responder rates for amenorrhea in the last 35 days of treatment were significantly greater for ulipristal-treated patients, 47.2% (97.5% CI 31.6–63.2) and 58.3% (97.5% CI 41.2–74.1) for the 5-mg and 10-mg groups, respectively, than placebo-treated patients, 1.8% (97.5% CI 0.0–10.9); P<.001 for both (Table 2). The majority of patients who achieved amenorrhea did so within the first 10 days of starting treatment with ulipristal (log-rank P<.001 for both doses vs placebo; Fig. 3).
Table 2.
Efficacy Endpoint Results (Intent-to-Treat Population)
| Placebo (n=56) | 5 mg Ulipristal (n=53) | 10 mg Ulipristal (n=48) | |
|---|---|---|---|
| Coprimary endpoint | |||
| Absence of bleeding responder on treatment* | |||
| Responder rate | 1 (1.8) | 25 (47.2) | 28 (58.3) |
| Responder rate difference | 45.4 (29.5–61.3) | 56.5 (40.1–73.0) | |
| P | <.001 | <.001 | |
| Secondary endpoints | |||
| Absence of bleeding responder by day 11† | |||
| Responder rate | 0 (0.0) | 23 (43.4) | 28 (58.3) |
| Responder rate difference | 43.4 (28.1–58.7) | 58.3 (42.4–74.3) | |
| P | <.001 | <.001 | |
| UFS-QOL Revised Activities subscale score‡ | |||
| LS change from baseline | 21.2±63.8 | 52.163.7 | 59.064.0 |
| LS mean difference | 30.9 (19.2–42.6) | 37.7 (25.5–49.9) | |
| P | <.001 | <.001 | |
| Additional endpoints | |||
| Volume of up to 3 largest uterine leiomyomas | |||
| LS mean % change from baseline to end of treatment§ | 7.2 | −9.6 | −16.3 |
| LS mean difference | −16.8 (−48.1 to 14.0) | −23.5 (−54.9 to 7.3) | |
| P | .218 | .086 | |
| LS mean % change from baseline to end of follow-up§ | 5.7 | −2.3 | −17.4 |
| LS mean difference | −8.0 (−43.7 to 27.3) | −23.1 (−57.8 to 10.8) | |
| P | .605 | .124 | |
| Control of bleeding∥ | |||
| n1 | 53 | 46 | 38 |
| Responder rate | 1 (1.9) | 27 (58.7) | 31 (81.6) |
| Responder rate difference | 56.8 (40.0–73.6) | 79.7 (65.0–94.4) | |
| P | <.001 | <.001 | |
UFS-QOL, Uterine Fibroid Symptom and Health-Related Quality of Life questionnaire; LS, least squares; n1, patients with 66 days or greater of treatment exposure.
Data are n (%), % (97.5% CI), or mean±standard error unless otherwise specified.
Cochran-Mantel-Haenszel tests controlling for study site were used for dichotomous endpoints; log-rank tests were used for time to amenorrhea; analysis of covariance controlling for baseline score and pooled study site was used for change from baseline in UFS-QOL Revised Activity subscale score.
Proportion of patients achieving amenorrhea for the last 35 consecutive days of treatment; results for coprimary endpoint of time to amenorrhea are shown in Figure 3.
Proportion of patients achieving amenorrhea by day 11 of treatment and did not report bleeding for the duration of treatment.
HRQOL Revised Activities subscale scores range from 0 to 100; higher scores indicate better health-related quality of life.
Represents log-transformed analyses of percent reduction in leiomyoma volume; positive values represent increase in volume and negative values represent reduction in volume.
Proportion of patients with 66 days of treatment or greater exposure achieving 0 days of heavy bleeding and 8 days of bleeding or less within the last 56 days of treatment.
Fig. 3.
Time to amenorrhea (intent-to-treat population). Hazard ratio (97.5% CI) for 5 mg ulipristal acetate (UPA) and 10 mg UPA, 35.5 (3.6–348.5) and 49.1 (5.0–480.9), respectively, *Log-rank P<.001 vs placebo. Time to amenorrhea was analyzed using log-rank tests.
Simon. Ulipristal Acetate for Uterine Leiomyomas. Obstet Gynecol 2018.
Secondary and additional bleeding outcomes favored ulipristal compared with placebo treatment. Significantly more patients achieved amenorrhea by day 11, reporting no bleeding (spotting permitted) for the duration of treatment, in the 5-mg ulipristal (43.4%) and 10-mg ulipristal (58.3%) groups compared with the placebo group (0%; P<.001 for both). Control of bleeding was also greater for 5-mg and 10-mg ulipristal-treated patients (58.7% and 81.6%, respectively) compared with placebo-treated patients (1.9%; P<.001 for both).
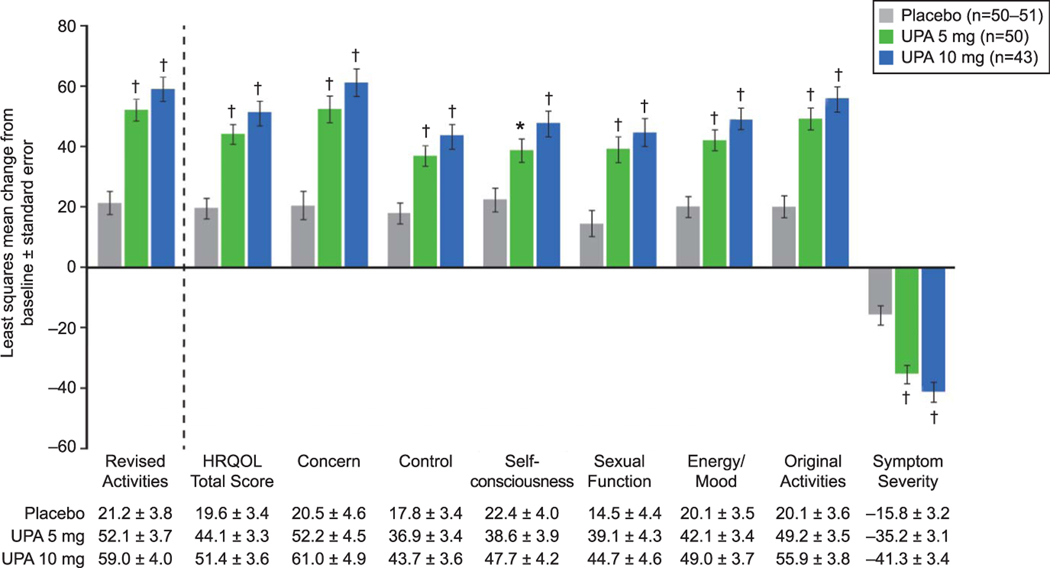
Improvement in physical and social activities as measured by change from baseline to end of treatment on the Uterine Fibroid Symptom and Health-Related Quality of Life Revised Activities subscale was significantly greater for both 5 mg and 10 mg ulipristal compared with placebo (P<.001 for both; Fig. 4). The Health-Related Quality of Life Total score and the Symptom Severity score were improved with 5 mg and 10 mg ulipristal compared with placebo (P<.001 for both; Fig. 4). All Health-Related Quality of Life subscale scores were similarly improved with 5 mg and 10 mg ulipristal compared with placebo (P<.01 for both; Fig. 4).
Fig. 4.
Change from baseline at end of treatment in Uterine Fibroid Symptom and Health-Related Quality of Life questionnaire (UFS-QOL) scores (intent-to-treat population). *P<.01, †P<.001 vs placebo. Error bars represent standard error. Analysis of covariance controlling for baseline score and pooled study site was used for change from baseline in UFS-QOL scores. Health-Related Quality of Life (HRQOL) Total score and HRQOL subscale scores range from 0 to 100; higher scores indicate better HRQOL. Symptom severity subscale score ranges from 0 to 100; higher scores indicate greater symptom severity. UPA, ulipristal acetate.
A post hoc log transformation analysis of percent change from baseline in leiomyoma volume was performed to control for data variability. A 7.2% increase in leiomyoma volume was observed in the placebo group (n=41) compared with a 9.6% reduction (P=.218) in the 5-mg ulipristal group (n=39) and a 16.3% reduction (P=.086) in the 10-mg ulipristal group (n=33) at the end of the treatment course. At the end of follow-up (12 weeks posttreatment), leiomyoma volume was increased from baseline by 5.7% in the placebo group (n=31) and decreased from baseline by 2.3% (P=.605) and 17.4% (P=.124) in the 5-mg (n=36) and 10-mg (n=30) ulipristal groups, respectively.
A summary of adverse events is presented in Table 3. No patients in either ulipristal group discontinued the study as a result of an adverse event; one patient in the placebo group discontinued the study as a result of an adverse event (treatment-emergent type 2 diabetes mellitus). Serious adverse events were reported by three patients during the treatment period and one patient during the off-treatment follow-up period—angina pectoris and anxiety (both in a single placebo-treated patient), cellulitis resulting from an allergic reaction to a spider bite (5 mg ulipristal-treated patient), hemorrhoids and influenza (both in a single 10 mg ulipristal-treated patient), and exacerbation of hypertension (10 mg ulipristal-treated patient)—but none was considered related to treatment. Treatment-emergent adverse events were reported by 28.6% of placebo-, 43.4% of 5 mg ulipristal-, and 54.2% of 10 mg ulipristal-treated patients during the on-treatment phase (Table 3). Most treatment-emergent adverse events were mild to moderate in intensity and considered unrelated to the study drug. The most common treatment-emergent adverse events reported by 5% or greater of patients in any ulipristal treatment arm during the treatment phase were hot flushes, blood creatine phosphokinase elevation, and hypertension. During the off-treatment follow-up phase, no single adverse event occurred in 5% or greater of patients in any treatment group.
Table 3.
Adverse Events (Safety Population)
| Placebo (n=56) | 5 mg Ulipristal (n=53) | 10 mg Ulipristal (n=48) | Ulipristal Total (n=101) | |
|---|---|---|---|---|
| Treatment phase | ||||
| AE leading to discontinuation | 1 (1.8)* | 0 | 0 | 0 |
| Serious AE† | 0 | 1 (1.9) | 2 (4.2) | 3 (3.0) |
| Death | 0 | 0 | 0 | 0 |
| TEAE | 16 (28.6) | 23 (43.4) | 26 (54.2) | 49 (48.5) |
| Most frequently reported TEAEs‡ | ||||
| Hot flush | 0 | 3 (5.7) | 2 (4.2) | 5 (5.0) |
| Blood CPK elevated | 0 | 1 (1.9) | 3 (6.3) | 4 (4.0) |
| Hypertension | 0 | 0 | 3 (6.3) | 3 (3.0) |
| Follow-up phase | ||||
| AE leading to discontinuation | 0 | 0 | 0 | 0 |
| Serious AE§ | 1 (1.8) | 0 | 0 | 0 |
| Death | 0 | 0 | 0 | 0 |
| TEAE | 10 (17.9) | 10 (18.9) | 12 (25.0) | 22 (21.8) |
AE, adverse event; TEAE, treatment-emergent AE; CPK, creatine phosphokinase.
Data are n (%).
Type 2 diabetes mellitus.
Includes cellulitis, hemorrhoids, hypertension, and influenza.
Five percent or greater in any group.
Includes angina pectoris and anxiety.
Hot flushes were reported as mild to moderate, and mean estradiol levels remained within the normal range (midcycle 63.9–356.7 pg/mL) throughout treatment and follow-up periods. Blood creatine phosphokinase elevation (normal range 33–211 units/L) was mostly mild and was present at baseline in four of five patients (Appendix 1, available online at http://links.lww.com/AOG/B62); no cardiac events were associated with these creatine phosphokinase changes. The patient who did not have elevated blood creatine phosphokinase at baseline had a single transient blood creatine phosphokinase elevation at the end of treatment (10 mg ulipristal) that resolved spontaneously by the next visit. Hypertension was reported as a worsening of pre-existing disease (ie, present at baseline) in five of six patients, and one participant was diagnosed with new hypertension during the 12-week follow-up period. None of the cases of hypertension was considered related to treatment. The majority of these patients was obese and not treated for hypertension at baseline.
No malignancy was found in any of the endometrial biopsies; one case of hyperplasia without atypia was observed at the end of treatment (10 mg ulipristal), diagnosed by one of three pathologists (ie, most severe diagnosis), which spontaneously resolved without treatment by the end of follow‐up. The proportion of patients with progesterone receptor modulator-associated endometrial changes, as assessed by at least two of three pathologists, was similar for all treatment groups at baseline and the end of treatment: 5 mg ulipristal (28.3 and 26.2%), 10 mg ulipristal (22.9 and 29.7%), and placebo (23.6 and 13.6%); and decreased by end of follow-up: 5 mg ulipristal (19.0%), 10 mg ulipristal (12.1%), and placebo (7.1%). Likewise, change from baseline in endometrial thickness was not different between the 5-mg ulipristal (least-squares mean change±standard error 0.11±0.76 mm), 10-mg ulipristal (0.61±0.77 mm), and placebo (0.16±0.74 mm) groups.
Improvement in anemia was seen at the end of treatment for patients treated with both 5 mg and 10 mg ulipristal. Hemoglobin levels improved from baseline (low; levels less than 115 g/L) to the end of treatment (normal; range 115–160 g/L) in 50.0% and 54.2% of 5 mg ulipristal- and 10 mg ulipristal-treated patients, respectively, compared with 10.3% of placebo-treated patients. Menstrual bleeding resumed in the off-treatment follow-up period (on average in approximately 4 weeks for the majority of patients) in all but one patient in each ulipristal group. Ulipristal treatment had no clinically relevant effects on other laboratory values (including adrenocorticotropin hormone, prolactin, and thyroid-stimulating hormone levels) or vital signs.
DISCUSSION
We report results from a 12-week trial of once-daily 5 mg and 10 mg ulipristal for the treatment of symptomatic uterine leiomyomas in a diverse (nearly 70% black and largely overweight) population that showed both ulipristal doses were superior to placebo in controlling abnormal uterine bleeding. For the coprimary endpoints of percentage of patients who achieved amenorrhea during the last 35 consecutive days of treatment, and time to amenorrhea, each ulipristal dose was superior to placebo (P<.001). Treatment with ulipristal also was shown to improve health-related quality of life, including physical and social activities. The efficacy of ulipristal observed in this study in a predominantly black and obese population is of clinical interest given the higher disease burden in these demographics.5
Results from the current study are consistent with those of the phase 3 studies conducted in Europe in a predominantly white, non-obese population. In the European studies of similar design to the current study,8,9 5 mg ulipristal and 10 mg ulipristal were superior to placebo8 and noninferior to leuprolide9 in achieving amenorrhea or controlled bleeding with median time to amenorrhea of 5–7 days. In the current study, almost all patients who achieved amenorrhea in both the 5-mg ulipristal and 10-mg ulipristal groups did so rapidly, within the first 10 days of treatment. Also consistent with the European studies is the improvement in quality of life observed in this study for patients treated with 5 mg ulipristal and 10 mg ulipristal as measured by the Health-Related Quality of Life Total score and all subscales of the Uterine Fibroid Symptom and Health-Related Quality of Life questionnaire.10,20 The 10-mg ulipristal dose consistently demonstrated numeric superiority over the 5-mg dose in the current study, but the trial was not powered to detect statistical differences between the two doses. Although the current study was one 12-week treatment course, studies of multiple intermittent treatment courses (up to eight repeated 12-week courses) with 5 mg and 10 mg ulipristal found maintenance of efficacy and improvements in quality of life.10,11,15,16
Ulipristal has been shown to reduce leiomyoma volume through mechanisms that include inhibition of leiomyoma cell proliferation, induction of apoptosis, and facilitation of extracellular matrix breakdown and reorganization.21 The leiomyoma volume data in this study were considered an additional efficacy endpoint and the sample size for the study was not powered for assessment of this endpoint. Despite the small sample size, reductions in leiomyoma volume of up to the three largest leiomyomas were observed in both ulipristal dose groups compared with an increase in leiomyoma volume in the placebo group, similar to reductions reported in other trials (ie, median decrease in leiomyoma volume of 12–21% with ulipristal and median increase of 3% with placebo),8 in which total leiomyoma volume was measured by magnetic resonance imaging as a coprimary efficacy endpoint. Studies consisting of multiple 12-week courses of treatment have shown additional decreases in leiomyoma volume with each subsequent treatment course for up to four courses.10,15
The current study, consistent with previous studies,8–11,15,16 showed that ulipristal was generally well tolerated. Although the small sample size and duration of treatment limit meaningful assessment of the most common adverse events, there were no treatment-related serious adverse events and no patient receiving ulipristal discontinued treatment because of an adverse event. The treatment-emergent adverse events of worsening hypertension and elevated creatine phosphokinase levels observed in this study were not common in other, larger studies of ulipristal11 or in studies of repeated, intermittent treatment with ulipristal.10,15 Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes in endometrium; all changes were benign, transient, and did not require any intervention.
Although hysterectomy continues to be the most common treatment for symptomatic uterine leiomyomas,6 the long-term health effect of hysterectomy remains poorly understood,22 and hysterectomy is not an option for women wishing to preserve fertility. Leuprolide is used for preoperative hematologic improvement of anemia; however, its use is limited by hypoestrogenic adverse effects.23 Pharmacologic treatments commonly used off-label include other gonadotropin-releasing hormone agonists and tranexamic acid, which also are prescribed for short-term use only. Chronic use of nonsteroidal antiinflammatories, levonorgestrel intrauterine devices, and oral and nonoral combination contraceptives is common, although data supporting their use for management of symptomatic uterine leiomyomas are limited.24 The findings from this and other studies suggest that ulipristal may be useful for the medical management of abnormal uterine bleeding associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment.
Supplementary Material
Acknowledgments
Sponsored by Allergan plc (Madison, New Jersey). Writing and editorial assistance were provided by Jennifer C. Jaworski, MS, and Wendy J. van der Spuy, PhD, of Prescott Medical Communications Group, Inc (Chicago, Illinois) with support from Allergan plc (Jersey City, New Jersey). Allergan (sponsor) played a role in the study design, conduct, analysis, interpretation, writing of the report, and the decision to publish this study.
Presented in part at the American Society for Reproductive Medicine Annual Meeting, October 16–20, 2016, Salt Lake City, Utah; the 106th Annual Meeting of the United States and Canadian Academy of Pathology, March 4–10, 2017, San Antonio, Texas; the 64th Annual Scientific Meeting of the Society for Reproductive Investigation, March 15–18, 2017, Orlando, Florida; and the Annual Clinical and Scientific Meeting of the American College of Obstetricians and Gynecologists, May 6–9, 2017, San Diego, California.
Financial Disclosure
Dr. Simon served (in the past year, or current) as a consultant/advisor to AbbVie, Inc, Allergan, Plc, AMAG Pharmaceuticals, Inc, Ascend Therapeutics, Azure Biotech, Inc, Millendo Therapeutics, Inc, Nuelle, Inc, Radius Health, Inc, Regeneron Pharmaceuticals, Inc, Roivant Sciences, Inc, Sanofi SA, Sebela Pharmaceuticals, Inc, Sermonix Pharmaceuticals, Inc, Shionogi Inc, Symbiotec Pharmalab, TherapeuticsMD, and Valeant Pharmaceuticals; on the speakers’ bureaus of Novo Nordisk, Shionogi Inc, and Valeant Pharmaceuticals; received grant/research support from AbbVie, Inc, Allergan, Plc, Agile Therapeutics, Bayer Healthcare LLC, New England Research Institute, Inc, ObsEva SA, Palatin Technologies, Symbio Research, Inc, and TherapeuticsMD; and is a stockholder by direct purchase in Sermonix Pharmaceuticals. Dr. Catherino is a consultant of AbbVie, Inc, Allergan plc, and Bayer. Dr. Segars served as a consultant for Bayer and has received grant/research support from Biospecifics, Inc. Drs. Blakesley, Chan, and Sniukiene are current full-time employees of Allergan plc. Dr. Al-Hendy is a consultant for AbbVie, Allergan plc, Bayer, Myovant, and Repros.
Footnotes
The views expressed in this article are those of the author(s) and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
Each author has indicated that he or she has met the journal’s requirements for authorship.
REFERENCES
- 1.Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers 2016;2:16043. [DOI] [PubMed] [Google Scholar]
- 2.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003; 188:100–7. [DOI] [PubMed] [Google Scholar]
- 3.Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health 2014;6:95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sparic R, Mirkovic L, Malvasi A, Tinelli A. Epidemiology of uterine myomas: a review. Int J Fertil Steril 2016;9:424–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart EA, Nicholson WK, Bradley L, Borah BJ. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health (Larchmt) 2013;22:807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 2012;206:211.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gainer EE, Ulmann A. Pharmacologic properties of CDB(VA)-2914. Steroids 2003;68:1005–11. [DOI] [PubMed] [Google Scholar]
- 8.Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 2012;366: 409–20. [DOI] [PubMed] [Google Scholar]
- 9.Donnez J, Tomaszewski J, Vazquez F, Bouchard P, Lemieszczu kB, Baró F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 2012;366:421–32. [DOI] [PubMed] [Google Scholar]
- 10.Donnez J, Vázquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BC, et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril 2014;101:1565–73.e1–18. [DOI] [PubMed] [Google Scholar]
- 11.Donnez J, Hudecek R, Donnez O, Matule D, Arhendt HJ, Zatik J, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril 2015;103:519–27.e3. [DOI] [PubMed] [Google Scholar]
- 12.Mutter GL, Bergeron C, Deligdisch L, Ferenczy A, Glant M, Merino M, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol 2008;21:591–8. [DOI] [PubMed] [Google Scholar]
- 13.Murji A, Whitaker L, Chow TL, Sobel ML. Selective progesterone receptor modulators (SPRMs) for uterine fibroids. The Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No.: CD010770. DOI: 10.1002/14651858.CD010770.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnez J, Donnez O, Dolmans MM. Safety of treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Expert Opin Drug Saf 2016;15: 1679–86. [DOI] [PubMed] [Google Scholar]
- 15.Donnez J, Donnez O, Matule D, Ahrendt HJ, Hudecek R, Zatik J, et al. Long-term medical management of uterine fibroids with ulipristal acetate. Fertil Steril 2016;105:165–73.e4. [DOI] [PubMed] [Google Scholar]
- 16.Fauser BC, Donnez J, Bouchard P, Barlow DH, Vázquez F, Arriagada P, et al. Safety after extended repeated use of ulipristal acetate for uterine fibroids. PLoS One 2017;12:e0173523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catherino WH, Eltoukhi HM, Al-Hendy A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin Reprod Med 2013;31:370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat VL, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci U S A 2008;105:19887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002;99:290–300. [DOI] [PubMed] [Google Scholar]
- 20.Nieman LK, Blocker W, Nansel T, Mahoney S, Reynolds J, Blithe D, et al. Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: a randomized, double-blind, placebo-controlled, phase IIb study. Fertil Steril 2011;95:767–72.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Courtoy GE, Donnez J, Marbaix E, Dolmans MM. In vivo mechanisms of uterine myoma volume reduction with ulipristal acetate treatment. Fertil Steril 2015;104:426–34.e1. [DOI] [PubMed] [Google Scholar]
- 22.Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol 2013;209:319.e1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stovall TG, Muneyyirci-Delale O, Summitt RL Jr, Scialli AR. GnRH agonist and iron versus placebo and iron in the anemic patient before surgery for leiomyomas: a randomized controlled trial. Leuprolide Acetate Study Group. Obstet Gynecol 1995;86:65–71. [DOI] [PubMed] [Google Scholar]
- 24.Sabry M, Al-Hendy A. Medical treatment of uterine leiomyoma. Reprod Sci 2012;19:339–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.