Summary
Autoimmune diseases are caused by adaptive immune responses to self‐antigens. The development of antigen‐specific therapies that suppress disease‐related, but not unrelated immune responses in general, is an important goal of biomedical research. We have previously shown that delivery of myelin peptides to liver sinusoidal endothelial cells (LSECs) using LSEC‐targeting nanoparticles provides effective protection from CD4 T‐cell‐driven autoimmune encephalomyelitis. Here, we investigated whether this methodology might also serve antigen‐specific treatment of a CD8 T‐cell‐driven autoimmune disease. As a model for CD8 T‐cell‐mediated autoimmunity, we used OT‐1 T‐cell‐driven cholangitis in K14‐OVAp mice expressing the cognate MHC I‐restricted SIINFEKL peptide in cholangiocytes. To study whether peptide delivery to LSECs could modulate cholangitis, SIINFEKL peptide‐conjugated nanoparticles were administered intravenously one day before transfer of OT‐1 T cells; five days after cell transfer, liver pathology and hepatic infiltrates were analysed. SIINFEKL peptide‐conjugated nanoparticles were rapidly taken up by LSECs in vivo, which effectively cross‐presented the delivered peptide on MHC I molecules. Intriguingly, K14‐OVAp mice receiving SIINFEKL‐loaded nanoparticles manifested significantly reduced liver damage compared with vehicle‐treated K14‐OVAp mice. Mechanistically, treatment with LSEC‐targeting SIINFEKL‐loaded nanoparticles significantly reduced the number of liver‐infiltrating OT‐1 T cells, which up‐regulated expression of the co‐inhibitory receptor PD‐1 and down‐regulated cytotoxic effector function and inflammatory cytokine production. These findings show that tolerogenic LSECs can effectively internalize circulating nanoparticles and cross‐present nanoparticle‐bound peptides on MHC I molecules. Therefore, nanoparticle‐mediated autoantigen peptide delivery to LSECs might serve the antigen‐specific treatment of CD8 T‐cell‐driven autoimmune disease.
Keywords: antigen‐specific tolerance, autoimmune cholangitis, CD8 T cell, liver, nanomedicine
We show that CD8 T‐cell‐mediated bile duct damage can be prevented by targeting the specific epitope peptides to liver sinusoidal endothelial cells in vivo using nanoparticles. These cells effectively cross‐present the received peptides and induce CD8 T‐cell tolerance.
![]()
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- LSECs
liver sinusoidal endothelial cells
INTRODUCTION
Autoimmune diseases are chronic diseases that impose a substantial disease burden on patients and, collectively, also a major economic burden on healthcare systems. 1 These diseases are caused by adaptive immune responses to specific self‐antigens. Currently, the treatment of autoimmune diseases relies on unspecific and sometimes ineffective immunosuppressive agents, which can often have severe side effects. 2 In recent years, a large effort has been made to develop new treatment strategies specifically targeting the causative autoreactive T‐cell responses. 3 , 4 The common idea behind these approaches is the establishment of tolerogenic antigen presentation in vivo, in order to induce anergy 5 of autoreactive T cells or to facilitate the development of antigen‐specific regulatory T cells. 6 , 7 To that end, different methodologies have been developed, including antigen targeting to erythrocytes 8 or the application of ‘synthetic’ APCs lacking costimulatory activity. 9 We and others have developed small‐sized particle‐based strategies for directed antigen delivery to inherently tolerogenic APCs in vivo, 6 , 10 , 11 or, alternatively, codelivery of antigen peptide together with tolerogenic compounds to conventional APCs via nanoparticles. 12 , 13 Most of these approaches have been designed to target autoreactive CD4 T cells, which are presumably the main drivers of autoimmune diseases, and the susceptibility to develop these diseases is mainly affected by MHC II genes. 1 However, CD8 T cells also seem to be important contributors to various autoimmune diseases, 14 including not only the more common diseases such as type I diabetes 15 and alopecia areata, 16 but also rare conditions such as primary biliary cholangitis. 17 As autoreactive CD8 T cells are not targeted by most existing approaches, the development of suitable methods for antigen‐specific targeting of autoreactive CD8 T cells is highly desirable.
Our approach to target autoreactive CD4 T cells harnessed liver sinusoidal endothelial cells (LSECs) for the induction of autoantigen‐specific immune tolerance in vivo. 6 In our view, a number of reasons qualified LSECs as ideal target cells for nanoparticle‐based therapies. First, LSECs are inherently tolerogenic, liver‐resident APCs that have been extensively studied for their exceptional capability to induce T‐cell tolerance in CD4 T cells. 18 , 19 Second, LSECs have the capability to effectively induce antigen‐specific Foxp3+ Tregs. 20 Third, LSECs are potent scavenger cells involved in plasma ultrafiltration and uptake of small‐sized particles. 21 , 22 Fourth, we identified a nanoparticle type that is taken up by LSECs with very high selectivity reducing the risks of potential off‐target effects. 6 Utilizing these LSEC‐targeting nanoparticles, we were indeed able to facilitate effective antigen‐specific CD4 T‐cell tolerance and protection from CD4 T‐cell‐mediated autoimmune pathology in two independent models of experimental autoimmune encephalomyelitis, mimicking multiple sclerosis. 6
Importantly, LSECs can induce tolerance not only in CD4 T cells, but also potently in CD8 T cells, 23 , 24 , 25 owed at least in part to their exceptional capability to cross‐present extrinsic antigens. 23 , 26 We hence hypothesized that nanoparticle‐mediated delivery of MHC I‐restricted autoantigen peptides to LSECs might result in tolerogenic cross‐presentation and protection from CD8 T‐cell‐driven autoimmune disease. To test this hypothesis, we made use of the K14‐OVAp model of acute CD8 T‐cell‐driven cholangitis in which adoptively transferred antigen‐specific OT‐1 CD8 T cells recognize the MHC I‐restricted ovalbumin peptide SIINFEKL on cholangiocytes in the liver. 27 , 28
MATERIALS AND METHODS
Autoimmune cholangitis induction in K14‐OVAp mice
Inbred K14‐OVAp mice were bred and kept in the animal facility of the University Medical Centre Hamburg‐Eppendorf. Autoimmune cholangitis was induced as described previously 27 with slight modifications. Briefly, CD8 T cells specific for the MHC I‐restricted ovalbumin peptide SIINFEKL were isolated from CD45.1+ congenic OT‐1 mice, and 2 × 105 cells per mouse were transferred intravenously into female 8‐ to 12‐week‐old CD45.2+ K14‐OVAp recipients expressing the SIINFEKL peptide on cholangiocytes. Mice were monitored daily for signs of disease. Five days after OT‐1 cell transfer, K14‐OVAp mice were analysed for liver pathology and immune responses. We have used clinical and behavioural humane end‐points to reduce pain and distress. All animal experiments were carried out in accordance with the principles of the Basel Declaration, the European Directive 2010/63/EU and FELASA recommendations, and had been approved by the animal experimentation committee of the State of Hamburg.
Preparation of antigen peptide‐loaded nanoparticles
Oleic acid‐stabilized superparamagnetic iron oxide nanoparticles were encapsulated into an amphiphilic polymer (poly(maleic anhydride‐alt‐1‐octadecene)) as described. 29 , 30 Coupling of polymer‐coated nanoparticles to peptides was conducted in the presence of 1‐ethyl‐3‐(3‐dimethylaminopropyl)‐carbodiimide. A 150‐fold excess of the peptide (SIINFEKL) was added and incubated for 2·5 h. Free peptide was removed under centrifugal force by using a spin filter (100 kDa, 4200 rpm, 25°C).
Nanoparticle treatment
One day before disease induction by adoptive transfer of OT‐1 cells, K14‐OVAp mice were treated with SIINFEKL peptide‐loaded nanoparticles or unloaded control nanoparticles (equivalent to 0, 6·7, 13·3 or 20 µg peptide in 100 mM NaCl i.v.).
Assessment of disease severity
Body weight, appearance of fur and eyes, mobility and breathing were monitored and scored daily. For each category, 0–2 points (0 = unaffected, 1 = moderate impairment and 2 = severe impairment) were assigned and summed up to the final disease score with a maximum of 10 points.
Liver histology
Formalin‐fixed paraffin liver sections were stained with haematoxylin and eosin to visualize biliary inflammation. For evaluation of the liver‐infiltrating congenic OT‐1 cells (CD45.1+) and their interaction with cholangiocytes (CK19+), cryosections of liver tissue were stained with PE‐labelled antibody to CD45.1 (BioLegend) and primary antibody to CK19 (DSHB Hybridoma Product TROMA‐III) together with AF488‐labelled secondary antibody. Nuclei were stained with Hoechst 33342. To visualize MHC I‐restricted SIINFEKL presentation by cholangiocytes or cross‐presentation by LSECs, SIINFEKL‐H2Kb (eBioscience) was costained with CK19 antibody (DSHB Hybridoma Product TROMA‐III) or LYVE‐1 antibody (R&D systems). As indicated in the figure legends, AF488‐ or AF546‐labelled secondary antibodies have been used (Invitrogen).
Measurement of serum transaminases
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) serum levels were measured at the Institute of Experimental Immunology and Hepatology, University Medical Centre Hamburg‐Eppendorf, using a COBAS MIRA System (Roche Diagnostic, Mannheim, Germany).
Cell isolation
Non‐parenchymal liver cells were isolated by density gradient centrifugation as described previously. 27 LSECs were further separated by MACS as described before. 6 Spleen cells were isolated as described earlier. 20
Flow cytometry
Single cells were stained with PACO‐NHS (Life Technologies) for dead cell exclusion and for surface markers using antibodies to CD45.1, CD45.2, CD8, CD4, CD44, CD62L, Lag‐3, CD49b and PD‐1 (all from BioLegend). For granzyme B and Foxp3 staining, the Foxp3 staining buffer set (eBioscience) was used; for cytokine staining, cells were restimulated in vitro with PMA/ionomycin (Sigma‐Aldrich) for 4 h and concomitantly incubated with the Golgi inhibitor GolgiPlug (BD Biosciences). Afterwards, cells were fixed with 4% PFA and stained in saponin buffer (0·5% saponin, 2% BSA) for IFN‐γ, IL‐10, IL‐17 and TNF. All antibodies were purchased at BioLegend.
Cross‐presentation of SIINFEKL autoantigen
Isolated LSECs were incubated in Iscove's Modified Dulbecco’s Medium (Sigma) supplemented with 5% FCS (PAA Laboratories) with either SIINFEKL nanoparticle or free SIINFEKL peptide (each 10 μM or 100 μM) at 37°C in Iscove's Modified Dulbecco’s Medium supplemented with 5% FCS and 1%Pen/Strep for 2 h or 16 h and then stained with PE‐labelled anti‐CD146 antibody (clone ME‐9F1) and APC‐labelled antibody to H‐2Kb‐bound SIINFEKL peptide (clone 25‐D1.16) from BioLegend and analysed by flow cytometry.
Intravital microscopy
Mice were anaesthetized and prepared for intravital liver microscopy as described 6 , 18 , 30 with a confocal microscope equipped with a resonant scanner (Nikon A1R). Briefly, CdSe/CdS/ZnS core/shell/shell quantum dots, encapsulated with amphiphilic polymer (poly(maleic anhydride‐alt‐1‐octadecene)) and conjugated with SIINFEKL peptides, were injected intravenously and nanoparticle accumulation in the liver sinusoids was recorded for up to 30 min.
Statistics
For statistical analysis of differences between two groups, the Mann–Whitney test was applied; for comparison of more groups, the Kruskal–Wallis test was performed. Mean values ± SEM are shown. As indicated in the figure legends, *P < 0·05 was considered significant.
RESULTS
Cross‐presentation of nanoparticle‐bound peptides by LSECs
To obtain functional ovalbumin nanoconstructs, we covalently attached MHC I‐restricted SIINFEKL peptides to LSEC‐targeting nanoparticles, as schematically represented in Figure 1A. We then confirmed that the peptide‐conjugated nanoparticles retained their targeting selectivity, by assessing their internalization into LSECs in vivo by intravital microscopy of the liver in K14‐OVAp mice, taking advantage of nanoparticles with a fluorescent quantum dot core (Video S1). As shown in Figure 1B, fluorescent nanoparticles accumulated within 5 min after intravenous injection within the cells lining the hepatic sinusoids, that is LSECs, demonstrating that SIINFEKL‐conjugated nanoconstructs were effectively internalized by LSECs.
FIGURE 1.

LSECs internalize and cross‐present nanoparticle‐bound peptides on H2‐Kb molecules. (A) Schematic representation of SIINFEKL peptide‐loaded nanoparticle, consisting of a monodisperse iron oxide or quantum dot core of about 7 nm diameter, and an organic polymer coat to which SIINFEKL peptides were covalently conjugated. (B) Representative image of nanoparticle uptake in liver sinusoids (red fluorescent quantum dot core) before t = 0 min and 5‐min post‐tail vein injection, as assessed by intravital microscopy. (C) Immunofluorescence staining of SIINFEKL/H‐2Kb (red, AF546‐labelled secondary antibody) and LYVE‐1 + LSECs (green, AF488‐labelled secondary antibody). Nuclei are stained in blue. (D) Immunofluorescence staining of SIINFEKL/H‐2Kb (red, AF488‐labelled secondary antibody) and CK19 + cholangiocytes (green, AF546‐labelled secondary antibody). Nuclei are stained in blue. (E) Flow cytometric analysis of SIINFEKL cross‐presentation on H‐2Kb molecules by LSECs 2 or 16 h after incubation of LSECs with SIINFEKL‐loaded nanoparticles, or free peptide, or empty nanoparticles as controls. For quantitative analysis, the MFI of SIINFEKL/H‐2Kb ‐APC staining was analysed using the Kruskal–Wallis test. Results are shown as mean ± SEM; **P < 0·01; ***P < 0·001; ****P < 0·0001
Next, we assessed whether LSECs could utilize the covalently conjugated SIINFEKL peptides for cross‐presentation on H2‐Kb molecules. To that end, we injected SIINFEKL‐loaded nanoparticles into K14‐OVAp mice and stained liver sections that were sampled six days later with an antibody 31 that specifically recognizes SIINFEKL bound by H2‐Kb (Figure 1C). Indeed, we detected the complex of peptide bound to MHC I on cells lining the hepatic sinusoids (upper left); to confirm that these cells were LSECs, we counterstained with antibody to LYVE‐1 (upper right). These findings demonstrated that LSECs were able to utilize the nanoparticle‐bound SIINFEKL peptide for cross‐presentation. As control, we also stained the SIINFEKL/Kb complex in conjunction with CK19 (Figure 1D), marking the cholangiocytes, which expressed the SIINFEKL peptide as a transgene in K14‐OVAp mice. These results confirmed that the transgenic SIINFEKL peptide was presented by cholangiocytes of K14‐OVAp mice and that the nanoparticle‐delivered SIINFEKL peptide was cross‐presented by LSECs. To further confirm that LSECs were able to cross‐present nanoparticle‐bound peptide, we incubated isolated LSECs in vitro with nanoparticle‐bound SIINFEKL peptide in two concentrations or, as control, with the free, unconjugated peptide (Figure 1E). After two hours (upper panel), or after 16 h (lower panel), the LSECs were then stained with specific antibody to the SIINFEKL/Kb complex and assessed by flow cytometry. At both time‐points, as quantified by analysis of the mean fluorescence intensity (MFI), peptide presentation of LSECs incubated with nanoparticle‐bound peptide was clearly detectable and distinct from background, albeit less efficient than presentation of LSECs incubated with free peptide. Taken together, these findings demonstrated that LSECs have the capability to cross‐present peptides that were covalently bound to LSEC‐targeting nanoparticles.
Autoantigen peptide delivery to LSECs via nanoparticles prevents CD8 T‐cell‐driven autoimmune cholangitis
To address the question whether nanoparticle‐based delivery of MHC I‐restricted autoantigen peptides to LSECs might be protective in CD8 T‐cell‐driven autoimmunity, we tested SIINFEKL peptide‐conjugated nanoparticles in the K14‐OVAp model of autoimmune cholangitis. 27 K14‐OVAp mice were treated either with nanoparticles without peptide cargo (control) or with SIINFEKL nanoparticles; on the next day, mice received 2 × 105 autoreactive CD45.1 congenic OT‐1 cells by adoptive transfer. Intriguingly, the mice receiving SIINFEKL nanoparticles maintained a completely healthy appearance throughout the experiment, whereas the control mice manifested weight loss (Figure 2A) and an impaired general condition (Figure 2B). Five days after disease induction by OT‐1 transfer, a humane end‐point was reached due to obvious sickness symptoms in control mice; hence, autoimmune liver pathology and the fate of the congenic OT‐1 cells were analysed then. As expected, control mice receiving unloaded nanoparticles showed elevated serum levels of the liver enzymes ALT and AST; in contrast, the transaminase levels of those mice receiving SIINFEKL nanoparticles remained at the baseline (Figure 2C,D), suggesting that SIINFEKL nanoparticle treatment prevented OT‐1 cell‐mediated liver damage in K14‐OVAp mice. In line with these findings, histological analysis revealed smaller periportal infiltrates in the livers of SIINFEKL nanoparticle‐treated K14‐OVAp mice (Figure 2E) and greatly reduced numbers of CD45.1 + OT‐1 cells around CK19+ bile ducts, as compared to the control mice (Figure 2F). Taken together, the treatment of K14‐OVAp mice with SIINFEKL nanoparticles resulted in profound protection from OT‐1‐driven cholangitis, indicating that cross‐presentation of SIINFEKL peptides by LSECs induced antigen‐specific tolerance in the pathogenic OT‐1 cells.
FIGURE 2.

Autoantigen peptide delivery to LSECs prevents CD8 T‐cell‐driven autoimmune cholangitis. K14‐OVAp mice were treated with vehicle (black, n = 4) or SIINFEKL‐NP (blue, n = 5) one day before i.v. injection of OT‐1 T cells. At day 5 after cell transfer, the mice were analysed for clinical symptoms and liver pathology. (A) Change in body weight at day 5 relative to weight at the time of OT‐1 T‐cell transfer. (B) Disease severity at day 5 (P = 0·0079). (C, D) Serum levels of liver enzymes (ALT: P = 0·0317; AST: P = 0·0159). (E) H&E staining of liver samples. (F) Immunofluorescence staining of CD45.1+ liver‐infiltrating OT‐1 cells with PE‐labelled antibody (pink) and CK19+ cholangiocytes (green, AF488‐labelled secondary antibody); nuclei are stained in blue. Representative results of one out of three experiments are shown as mean ± SEM; Mann–Whitney test; *P < 0·05; **P < 0·01
In order to test the efficacy of SIINFEKL‐NP treatment at lower dosages, we performed a dose–response experiment in which K14OVAp mice received only 2/3 or 1/3 of the protective SIINFEKL‐NP dose (equivalent to 13·3 µg or 6·7 µg in comparison with 20 µg NP‐bound SIINFEKL peptide) one day before OT‐1 T‐cell transfer. Interestingly, even at suboptimal doses, SIINFEKL‐NP completely protected from weight loss and overt sickness symptoms (Figure S1A,B). However, upon treatment with reduced SIINFEKL‐NP dosages, elevation of serum transaminases as a measure of liver damage was similar to control mice receiving unloaded NPs (Figure S1C,D). Accordingly, the numbers of liver‐infiltrating lymphocytes and in particular of autoreactive OT‐1 CD8 T cells inversely correlated with escalating doses of SIINFEKL‐loaded NPs (Figure S1E,F).
Mechanisms underlying nanoparticle‐mediated tolerance induction in autoreactive CD8 T cells
To elucidate the mechanisms behind the observed SIINFEKL nanoparticle‐mediated protection from autoimmune liver disease, we analysed the liver‐infiltrating OT‐1 cells ex vivo by flow cytometry. As already shown by immunofluorescence staining (Figure 2F), flow cytometric analysis confirmed that upon SIINFEKL nanoparticle treatment, significantly fewer autoreactive CD45.1+ OT‐1 cells had infiltrated the liver (Figure 3A), as compared to control mice receiving nanoparticles without peptide cargo. Whereas almost all liver‐infiltrating OT‐1 cells showed an activated CD44hiCD62L‐ effector phenotype in control mice receiving unloaded nanoparticles, the number of CD44hiCD62L‐ cells among hepatic OT‐1 cells was significantly reduced following SIINFEKL nanoparticle treatment (Figure 3B). Moreover, we found a higher expression of the co‐inhibitory molecule PD‐1 on hepatic OT‐1 cells in SIINFEKL nanoparticle‐treated mice, as compared to the controls (Figure 3C). Furthermore, in contrast to the OT‐1 cells retrieved from control mice, OT‐1 cells from the livers of SIINFEKL nanoparticle‐treated mice produced significantly lower levels of effector molecules, such as granzyme B (Figure 3D), IFN‐γ (Figure 3E) and TNF (Figure 3F). These findings indicated that the treatment of K14‐OVAp mice with LSEC‐targeting SIINFEKL nanoparticles induced a tolerant phenotype in the SIINFEKL‐specific OT‐1 cells, resulting in the protection from OT‐1 cell‐driven autoimmune pathology.
FIGURE 3.
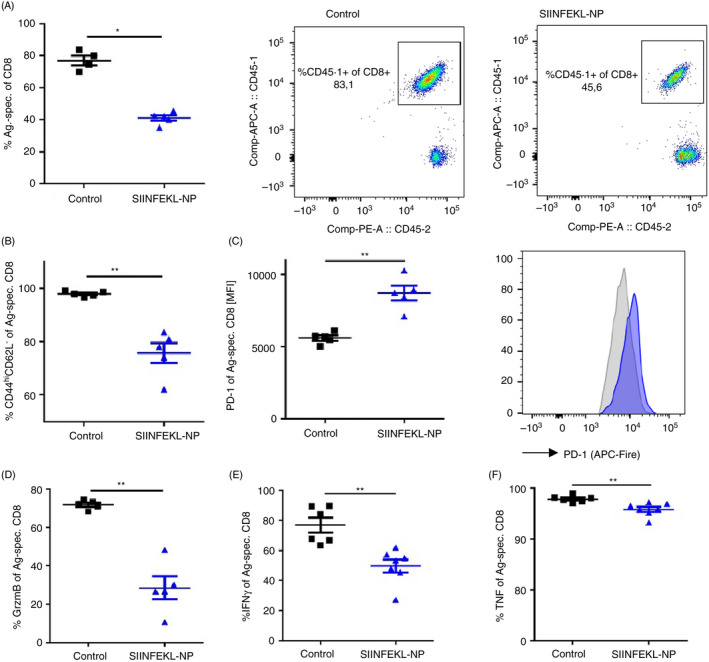
Nanoparticle‐mediated autoantigen peptide delivery to LSECs suppresses autoreactive CD8 T‐cell proliferation and effector function in liver. K14‐OVAp mice (n = 5–7) were treated with vehicle (black) or SIINFEKL‐NP (blue) one day before i.v. injection of OT‐1 T cells. At day 5 after cell transfer, liver‐infiltrating CD45.1+ OT‐1 cells were re‐isolated and analysed by flow cytometry with respect to frequency (A: P = 0·0159), expression of activation markers (B: P = 0·0079), co‐inhibitory receptor PD‐1 (C: P = 0·0079), granzyme B (D: P = 0·0079), and inflammatory cytokines IFN‐γ (E: P = 0·0012) and TNF (F: P = 0·0023). Representative results of one out of three experiments are shown as mean ± SEM; Mann–Whitney test; *P < 0·05; **P < 0·01
As SIINFEKL expression in K14‐OVAp mice is not entirely restricted to cholangiocytes, but also to epithelia of other tissues, 28 we also assessed potential systemic effects on OT‐1 T cells induced by SIINFEKL nanoparticle treatment. Therefore, we analysed splenic OT‐1 T cells and found reduced cell numbers following SIINFEKL nanoparticle treatment, as compared to controls (Figure 4A), possibly indicating reduced expansion of OT‐1 cells. As in the liver, the number of CD44hiCD62L‐ cells among the splenic OT‐1 cells was significantly reduced (Figure 4B), and expression of the co‐inhibitory molecule PD‐1 was significantly increased (Figure 4C) on splenic OT‐1 cells following SIINFEKL nanoparticle treatment, as compared to controls. Production of the effector molecules granzyme B (Figure 4D), IFN‐γ (Figure 4E) and TNF (Figure 4F) was also reduced in splenic OT‐1 T cells of SIINFEKL nanoparticle‐treated mice, as compared to controls. These findings indicated that CD8 tolerance induced by LSEC‐targeting nanoparticles was not restricted to the liver, but took effect systemically.
FIGURE 4.

Nanoparticle‐mediated autoantigen peptide delivery to LSECs affects autoreactive CD8 T cells in spleen. K14‐OVAp mice (n = 5–7) were treated with vehicle (black) or SIINFEKL‐NP (blue) one day before i.v. injection of OT‐1 T cells. At day 5 after cell transfer, systemic effects on CD45.1+ OT‐1 cells were analysed in the spleen by flow cytometry with respect to frequency (A: P = 0·0317), expression of activation markers (B: P = 0·0079), co‐inhibitory receptor PD‐1 (C: P = 0·0079), granzyme B (D: P = 0·0079) and inflammatory cytokines IFN‐γ (E: P = 0·0051) and TNF (F: P = 0·0025). Representative results of one out of three experiments are shown as mean ± SEM; Mann–Whitney test; *P < 0·05; **P < 0·01
Nanoparticle‐mediated autoantigen delivery to LSECs restricts activation of non‐specific T cells
Although driven by antigen‐specific CD8 T cells, the severity of autoimmune cholangitis in K14‐OVAp mice seems to critically depend on the additional bystander activity of non‐specific T cells. 27 Therefore, we analysed whether the antigen‐specific suppression of the OT‐1 cells in K14‐OVAp mice following SIINFEKL nanoparticle treatment was also indirectly affecting the activity of non‐specific liver‐infiltrating CD8 or CD4 T cells. Similar to the observations in the transferred CD45.1+ OT‐1 cells (Figure 3D), endogenous CD45.1‐CD8 T cells in the liver showed significantly reduced granzyme B levels (Figure 5A), whereas production of IFN‐γ (Figure 5B) and TNF (Figure 5C) following SIINFEKL nanoparticle treatment remained similar to endogenous CD8 T cells of the control group. Endogenous liver‐infiltrating CD4 T cells manifested significantly decreased production of IFN‐γ (Figure 5D) and TNF (Figure 5E) following SIINFEKL nanoparticle treatment, whereas the production of IL‐17 (Figure 5F), and the numbers of Foxp3+ regulatory T cells (Figure 5G) or IL10 + Lag‐3 + CD49b+ Tr1 cells (Figure 5H) was unaffected. Thus, SIINFEKL nanoparticle treatment seemed to not only directly regulate OT‐1 cell activity, but also indirectly reduce the inflammatory activation of non‐specifically recruited T cells.
FIGURE 5.
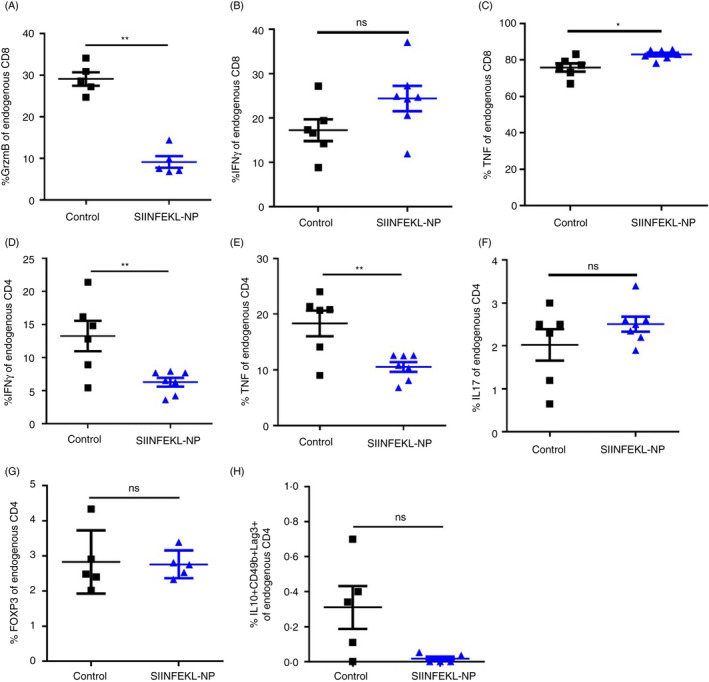
Nanoparticle‐mediated autoantigen delivery to LSECs restricts activation of endogenous T cells. K14‐OVAp mice (n = 5–7) were treated with vehicle (black) or SIINFEKL‐NP (blue) one day before i.v. injection of OT‐1 T cells. Endogenous liver‐infiltrating CD45.1‐negative CD8 T cells were analysed by flow cytometry for expression of granzyme B (A: P = 0·0079), IFN‐γ (B: P = 0·1014) and TNF (C: P = 0·0157). Non‐specific liver‐infiltrating CD4 T cells were analysed for IFN‐γ (D: P = 0·0192), TNF (E: P = 0·0192), IL‐17 (F: P = 0·4207), Foxp3 (G: P = 0·8413) and Tr1 cell markers Lag‐3, CD49b and IL‐10 (H: P = 0·0635). Representative results of one out of three experiments are shown as mean ± SEM; Mann–Whitney test; *P < 0·05; **P < 0·01
DISCUSSION
The lack of causative treatments for autoimmune diseases that specifically suppress the immune response to self‐antigens is a yet unmet biomedical challenge. 2 Our approach to this problem seeks to harness inherently tolerogenic LSECs for the induction of antigen‐specific T‐cell tolerance in vivo. 6 To that end, we made use of a small‐sized polymer‐coated nanoparticle, which we have found to be internalized by LSECs with high selectivity in vivo and to facilitate CD4 T‐cell tolerance. 6 Here, we tested whether these nanoparticles could also be harnessed for the antigen‐specific induction of CD8 T‐cell tolerance in vivo and the treatment of CD8 T‐cell‐mediated autoimmune disease. Indeed, we showed that SIINFEKL‐loaded nanoparticles were selectively internalized by LSECs, which could cross‐present the peptide on Kb molecules (Figure 1). Moreover, treatment of K14‐OVAp mice with SIINFEKL‐loaded nanoparticles resulted in profound protection from OT‐1‐driven cholangitis (Figure 2), indicating that the cross‐presentation of SIINFEKL peptides by LSECs induced antigen‐specific tolerance in the pathogenic OT‐1 cells. Mechanistically, antigen‐specific tolerance induction upon SIINFEKL nanoparticle treatment seemed to inhibit the activation of OT‐1 cells and their infiltration into the target tissue (Figure 3).
These results are in accordance with the findings of the group led by Percy Knolle that extensively studied the tolerogenic properties of LSECs regarding CD8 T‐cell tolerance induction. 22 Indeed, the increased expression of PD‐1 by OT‐1 cells in mice receiving SIINFEKL nanoparticle seems to recapitulate the key role of the PD‐L1/PD1 axis in tolerogenic LSEC‐CD8 T‐cell communication. 24 Of note, LSECs can not only confer a robust quiescent state to CD8 T cells, but also induce a memory‐like phenotype in CD8 T cells recognizing their cognate antigen on the LSEC surface. 32 This mechanism is of importance to effectively fight microbial or viral infections; however, such reactivation of LSEC‐primed CD8 T cells requires strong concomitant signals through the TCR, CD28 and the IL‐12 receptor, safeguarding the maintenance of immune tolerance in the absence of infection. 32 Thus, in the context of autoimmune responses, antigen delivery to LSECs via nanoparticles seems to be a safe and specific method for the effective induction of tolerance in autoreactive CD8 T cells.
Of note, it has been described that disease severity in the K14‐OVAp model can be influenced by endogenous T cells that are recruited to the lesions in a bystander fashion. 27 Interestingly, as a consequence of the attenuated OT‐1‐mediated inflammatory response in the liver of SIINFEKL‐NP‐treated mice, we also observed reduced inflammatory activity of non‐specifically recruited CD8 and CD4 T cells in the liver (Figure 5). Thus, SIINFEKL‐NP‐mediated tolerance induction in the disease‐driving OT1 cells concomitantly restricts the bystander activation of endogenous CD4 and CD8 T cells. Given that the SIINFEKL peptide expression in K14‐OVAp mice is not entirely restricted to cholangiocytes, but also to epithelia of other tissues, 28 we also monitored the systemic effects of OT‐1‐induced immunopathology. Interestingly, the SIINFEKL nanoparticle treatment did not only suppress autoimmune cholangitis locally in the liver, but also OT‐1 T cells homing to the spleen showed a comparable tolerant phenotype with low effector function and high expression of co‐inhibitory molecules as observed in the liver (Figure 4). Therefore, K14‐OVAp mice receiving SIINFEKL nanoparticles were completely protected from any general signs of overt disease, indicating that the profound suppressive effect was not only restricted to the liver but also affected other target tissues with potential epithelial autoantigen expression. Taken together, we find that our approach to induce antigen‐specific tolerance by utilizing LSEC‐targeting nanoparticles might not only be applicable in CD4 T‐cell‐driven autoimmune diseases, as shown before, 6 but also in autoimmune diseases with contribution of CD8 T cells. Therefore, LSEC‐targeting nanoparticles loaded with MHC I‐restricted autoantigen peptides should be further explored as potential antigen‐specific treatments for conditions in which autoreactive CD8 T cells are involved, such as type 1 diabetes 15 or alopecia areata. 16
CONFLICT OF INTEREST
Antonella Carambia, Ansgar W. Lohse, Joerg Heeren and Johannes Herkel are inventors of a patent related to this work (EP 2780036 (B1)). Johannes Herkel has consulted Topas Therapeutics GmbH. Reinaldo Digigow, Muharrem Şeleci and Disha Mungalpara are employees of Topas Therapeutics GmbH. All other authors declare no commercial or financial conflict of interest.
AUTHOR CONTRIBUTION
The authors contributed in the following ways: A Carambia, C Gottwick, M Heine, and FA Schuran and C Corban performed the experiments; D Schwinge, S Stein, R Digigow, M Şeleci, D Mungalpara and C Schramm provided essential material or methodologies; A Carambia, AW Lohse, C Schramm, J Heeren and J Herkel provided resources; A Carambia, J Heeren and J Herkel designed the study; A Carambia, J Heeren and J Herkel acquired funds; A Carambia and J Herkel wrote the paper; all authors reviewed and approved the manuscript.
Supporting information
Figure S1. Dose‐response of SIINFEKL‐NP administration. One day before disease induction by adoptive transfer of OT‐1 cells, K14‐OVAp mice (n = 6–7) were treated with SIINFEKL‐NP or empty NP (equivalent to 0, 6·7, 13·3, 20 µg peptide bound to NP). Mice were analysed on day 5 post T cell transfer. (A) Weight change, (B) disease severity, (C,D) transaminase levels, (E) liver‐infiltrating lymphocytes, and (F) hepatic infiltration of autoreactive OT‐1 cells were measured. For statistical analysis, the Kruskal‐Wallis test has been applied. Means ± SEM are shown; *P < 0·05; **P < 0·01.
Video S1. Intravital microscopy of SIINFEKL‐NP uptake in the liver. SIINFEKL peptides were covalently conjugated nanoparticles featuring a red fluorescent quantum dot core. Uptake of SIINFEKL peptide‐conjugated NPs by liver sinusoidal endothelial cells was assessed by intravital microscopy following intravenous injection into the tail vein and recorded as time‐lapse video.
ACKNOWLEDGEMENTS
The study was supported by the DFG – Deutsche Forschungsgemeinschaft (CRC 841), the German Federal Ministry of Research and Education (16GW0051, 13XP5079C) and the European Regional Development Fund (OpToPas). We thank Angelika Schmidt, Sabrina Kress, Sandra Ehret, Marko Hilken, Eva‐Marie Azizi and Carsten Rothkegel for excellent technical assistance. We also thank K. A. Hogquist for kindly providing K14‐OVAp mice.
Contributor Information
Antonella Carambia, Email: a.carambia@uke.de.
Johannes Herkel, Email: jherkel@uke.de.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001; 345:340–50. [DOI] [PubMed] [Google Scholar]
- 2. Garber K. Immunology: a tolerant approach. Nature. 2014; 507:418–20. [DOI] [PubMed] [Google Scholar]
- 3. Rosenblum MD, Gratz IK, Paw JS, Abbas AK. Treating human autoimmunity: current practice and future prospects. Sci Transl Med. 2012; 4:125sr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pearson RM, Podojil JR, Shea LD, King NJC, Miller SD, Getts DR. Overcoming challenges in treating autoimmunity: development of tolerogenic immune‐modifying nanoparticles. Nanomedicine. 2019; 18:282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prasad S, Xu D, Miller SD. Tolerance strategies employing antigen‐coupled apoptotic cells and carboxylated PLG nanoparticles for the treatment of type 1 diabetes. Rev Diabet Stud. 2012; 9:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carambia A, Freund B, Schwinge D, Bruns OT, Salmen SC, Ittrich H, et al. Nanoparticle‐based autoantigen delivery to Treg‐inducing liver sinusoidal endothelial cells enables control of autoimmunity in mice. J Hepatol. 2015; 62:1349–56. [DOI] [PubMed] [Google Scholar]
- 7. Prasad S, Neef T, Xu D, Podojil JR, Getts DR, Shea LD, et al. Tolerogenic Ag‐PLG nanoparticles induce tregs to suppress activated diabetogenic CD4 and CD8 T cells. J Autoimmun. 2018; 89:112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grimm AJ, Kontos S, Diaceri G, Quaglia‐Thermes X, Hubbell JA. Memory of tolerance and induction of regulatory T cells by erythrocyte‐targeted antigens. Sci Rep. 2015; 5:15907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsai S, Shameli A, Yamanouchi J, Clemente‐Casares X, Wang J, Serra P, et al. Reversal of autoimmunity by boosting memory‐like autoregulatory T cells. Immunity. 2010; 32:568–80. [DOI] [PubMed] [Google Scholar]
- 10. Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, et al. Microparticles bearing encephalitogenic peptides induce T‐cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012; 30:1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Y, Wu J, Wang J, Zhang W, Xu B, Xu X, et al. Targeted delivery of antigen to intestinal dendritic cells induces oral tolerance and prevents autoimmune diabetes in NOD mice. Diabetologia. 2018; 61:1384–96. [DOI] [PubMed] [Google Scholar]
- 12. Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle‐mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2012; 109:11270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kishimoto TK, Ferrari JD, LaMothe RA, Kolte PN, Griset AP, O'Neil C, et al. Improving the efficacy and safety of biologic drugs with tolerogenic nanoparticles. Nat Nanotechnol. 2016; 11:890–9. [DOI] [PubMed] [Google Scholar]
- 14. Liblau RS, Wong FS, Mars LT, Santamaria P. Autoreactive CD8 T cells in organ‐specific autoimmunity: emerging targets for therapeutic intervention. Immunity. 2002; 17:1–6. [DOI] [PubMed] [Google Scholar]
- 15. Tsai S, Shameli A, Santamaria P. CD8+ T cells in type 1 diabetes. Adv Immunol. 2008; 100:79–124. [DOI] [PubMed] [Google Scholar]
- 16. Simakou T, Butcher JP, Reid S, Henriquez FL. Alopecia areata: a multifactorial autoimmune condition. J Autoimmun. 2019; 98:74–85. [DOI] [PubMed] [Google Scholar]
- 17. Matsumura S, Kita H, He XS, Ansari AA, Lian ZX, van de Water J, et al. Comprehensive mapping of HLA‐A0201‐restricted CD8 T‐cell epitopes on PDC‐E2 in primary biliary cirrhosis. Hepatology. 2002; 36:1125–34. [DOI] [PubMed] [Google Scholar]
- 18. Carambia A, Frenzel C, Bruns OT, Schwinge D, Reimer R, Hohenberg H, et al. Inhibition of inflammatory CD4 T cell activity by murine liver sinusoidal endothelial cells. J Hepatol. 2013; 58:112–8. [DOI] [PubMed] [Google Scholar]
- 19. Neumann K, Rudolph C, Neumann C, Janke M, Amsen D, Scheffold A. Liver sinusoidal endothelial cells induce immunosuppressive IL‐10‐producing Th1 cells via the Notch pathway. Eur J Immunol. 2015; 45:2008–16. [DOI] [PubMed] [Google Scholar]
- 20. Carambia A, Freund B, Schwinge D, Heine M, Laschtowitz A, Huber S, et al. TGF‐β‐dependent induction of CD4⁺CD25⁺Foxp3⁺ Tregs by liver sinusoidal endothelial cells. J Hepatol. 2014; 61:594–9. [DOI] [PubMed] [Google Scholar]
- 21. Sørensen KK, Simon‐Santamaria J, McCuskey RS, Smedsrød B. Liver sinusoidal endothelial cells. Compr Physiol. 2015; 5:1751–74. [DOI] [PubMed] [Google Scholar]
- 22. Knolle PA, Wohlleber D. Immunological functions of liver sinusoidal endothelial cells. Cell Mol Immunol. 2016; 13:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen‐specific T‐cell tolerance. Nat Med. 2000; 6:1348–54. [DOI] [PubMed] [Google Scholar]
- 24. Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7‐homolog 1‐dependent CD8+ T cell tolerance. Hepatology. 2008; 47:296–305. [DOI] [PubMed] [Google Scholar]
- 25. Schildberg FA, Hegenbarth SI, Schumak B, Scholz K, Limmer A, Knolle PA. Liver sinusoidal endothelial cells veto CD8 T cell activation by antigen‐presenting dendritic cells. Eur J Immunol. 2008; 38:957–67. [DOI] [PubMed] [Google Scholar]
- 26. Schurich A, Böttcher JP, Burgdorf S, Penzler P, Hegenbarth S, Kern M, et al. Distinct kinetics and dynamics of cross‐presentation in liver sinusoidal endothelial cells compared to dendritic cells. Hepatology. 2009; 50:909–19. [DOI] [PubMed] [Google Scholar]
- 27. Schwinge D, Carambia A, Quaas A, Krech T, Wegscheid C, Tiegs G, et al. Testosterone suppresses hepatic inflammation by the downregulation of IL‐17, CXCL‐9, and CXCL‐10 in a mouse model of experimental acute cholangitis. J Immunol. 2015; 194:2522–30. [DOI] [PubMed] [Google Scholar]
- 28. McGargill MA, Mayerova D, Stefanski HE, Koehn B, Parke EA, Jameson SC, et al. A spontaneous CD8 T cell‐dependent autoimmune disease to an antigen expressed under the human keratin 14 promoter. J Immunol. 2002; 169:2141–7. [DOI] [PubMed] [Google Scholar]
- 29. Freund B, Tromsdorf UI, Bruns OT, Heine M, Giemsa A, Bartelt A, et al. A simple and widely applicable method to 59Fe‐radiolabel monodisperse superparamagnetic iron oxide nanoparticles for in vivo quantification studies. ACS Nano. 2012; 6:7318–25. [DOI] [PubMed] [Google Scholar]
- 30. Heine M, Bartelt A, Bruns OT, Bargheer D, Giemsa A, Freund B, et al. The cell‐type specific uptake of polymer‐coated or micelle‐embedded QDs and SPIOs does not provoke an acute pro‐inflammatory response in the liver. Beilstein J Nanotechnol. 2014; 5:1432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide‐MHC class I complexes using a monoclonal antibody. Immunity. 1997; 6:715–26. [DOI] [PubMed] [Google Scholar]
- 32. Böttcher JP, Schanz O, Wohlleber D, Abdullah Z, Debey‐Pascher S, Staratschek‐Jox A, et al. Liver‐primed memory T cells generated under noninflammatory conditions provide anti‐infectious immunity. Cell Rep. 2013; 3:779–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Dose‐response of SIINFEKL‐NP administration. One day before disease induction by adoptive transfer of OT‐1 cells, K14‐OVAp mice (n = 6–7) were treated with SIINFEKL‐NP or empty NP (equivalent to 0, 6·7, 13·3, 20 µg peptide bound to NP). Mice were analysed on day 5 post T cell transfer. (A) Weight change, (B) disease severity, (C,D) transaminase levels, (E) liver‐infiltrating lymphocytes, and (F) hepatic infiltration of autoreactive OT‐1 cells were measured. For statistical analysis, the Kruskal‐Wallis test has been applied. Means ± SEM are shown; *P < 0·05; **P < 0·01.
Video S1. Intravital microscopy of SIINFEKL‐NP uptake in the liver. SIINFEKL peptides were covalently conjugated nanoparticles featuring a red fluorescent quantum dot core. Uptake of SIINFEKL peptide‐conjugated NPs by liver sinusoidal endothelial cells was assessed by intravital microscopy following intravenous injection into the tail vein and recorded as time‐lapse video.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


