Summary
γδ T cells are found in highest numbers at barrier surfaces throughout the body, including the skin, intestine, lung, gingiva and uterus. Under homeostatic conditions, γδ T cells provide immune surveillance of the epidermis, intestinal and oral mucosa, whereas the presence of pathogenic microorganisms in the dermis or lungs elicits a robust γδ17 response to clear the infection. Although T cell migration is most frequently defined in the context of trafficking, analysis of specific migratory behaviors of lymphocytes within the tissue microenvironment can provide valuable insight into their function. Intravital imaging and computational analyses have been used to define ‘search’ behavior associated with conventional αβ T cells; however, based on the known role of γδ T cells as immune sentinels at barrier surfaces and their TCR-independent functions, we put forth the need to classify distinct migratory patterns that reflect the surveillance capacity of these unconventional lymphocytes. This review will focus on how γδ T cells traffic to various barrier surfaces and how recent investigation into their migratory behavior has provided unique insight into the contribution of γδ T cells to barrier immunity.
Keywords: γδ T cells, migration, immune surveillance, mucosal immunity
Barrier surfaces provide a first line of defense against foreign pathogens, delineate distinct microenvironments within the body, and perform functions that are essential to life. Therefore, maintaining the functional integrity of epithelial barriers is paramount to the health of the organism. Various immune cells provide surveillance of these host-microbe interfaces at steady-state in an effort to prevent microbial disruption of the epithelium and subsequent contamination of the underlying interstitium. In particular, lymphocytes migrate within barrier tissues and elicit a local immune response to facilitate repair of damaged epithelia, shape commensal bacteria populations, or promote the clearance of invading microorganisms. While many immune cell types are able to provide immunosurveillance, an elusive lineage of T cells expressing the γδ T cell receptor (TCR) is equipped to perform all of these functions and has been identified in major barrier sites in mice and humans. This review will focus on how murine γδ T cells traffic to various barrier surfaces and how recent investigation into their migratory behavior has provided unique insight into the contribution of γδ T cells to barrier immunity in mice.
Defining the physiological contribution of γδ T cells
Whereas phosphoantigens are known to activate human circulating γδ T cells, the means by which murine γδ T cells are activated is less clear1,2. Although it has been shown that some γδ TCRs recognize butyrophilin-like molecules, this interaction may be involved in defining tissue-specificity or function in a costimulatory manner, rather than initiating a canonical antigen-specific immune response2–4. Furthermore, γδ T cells are not major histocompatibility complex (MHC)-restricted1. Unlike conventional T cells, which are activated upon engagement of the TCRαβ with cognate antigen presented on the MHC of an antigen presenting cell (APC), γδ T cells may be activated following recognition of self-antigen by the TCR5 or distress signals by NK-like receptors6. This TCR-independence allows for greater versatility in γδ T cell effector function, and enables these cells to mount a rapid response to epithelial damage or infection6–8. In this sense, γδ T cells are widely considered to bridge innate and adaptive immunity, although the molecular mechanisms by which γδ T cells respond to local innate immune signals continues to be an area of ongoing investigation. Conversely, activated tissue-resident γδ T cells respond efficiently to pathogen re-challenge in a manner similar to memory αβ T cells9, thus demonstrating the capacity for dynamic responses to infection within this lymphocyte population.
The ability of γδ T cells to perform both innate and adaptive immune functions complements the necessity to respond to a variety of stimuli within host barrier surfaces. In individual tissues, this need is met by different subsets of γδ T cells, which are distinguished by Vγ gene expression in mice. Of the three different nomenclature systems, we will be using that proposed by Heilig and Tonegawa10, which names seven distinct Vγ subsets (Vγ1-7) that vary in localization, effector function, and contribution to homeostasis and disease. These subsets develop in sequential waves in the embryonic thymus, with the exception of Vγ7+ cells that can develop extrathymically1,11,12. After the initial differentiation of thymocytes into TCRγδ+ cells, the recognition of additional antigens in the thymus further delineates the functional phenotype into IL-17- or IFNγ-producing γδ T cells13,14.
The primary IL-17-producing γδ T cell subsets (γδ17) include Vγ4+ cells found in the dermis, lungs and gingiva, and Vγ6+ cells in the gingiva, tongue, lungs and female reproductive tract (FRT). Both Vγ4+ and Vγ6+ cells have been shown to function similarly to Th17 cells in that pathogen invasion into the tissue elicits an IL-17 response by γδ17 cells to help clear the infection15. However, excessive IL-17 production by these cells can promote inflammation and autoimmunity, thus supporting a pathogenic role for γδ17 cells in models of disease in the aforementioned tissues. Skin epidermal Vγ5+ and intestinal Vγ7+ cells both secrete IFNγ as part of a protective response against invading pathogens4,16, but can also produce growth factors and antimicrobial peptides to promote epithelial proliferation and repair following infection or injury4,17–19. Tight regulation of γδ T cell effector function helps prevent aberrant cytolytic activity, which is especially critical at the epidermal and intestinal barriers16. Extensive exposure of these tissues to commensal bacteria requires a tolerogenic phenotype among γδ T cells that provide immune surveillance under homeostatic conditions, whereas invasion of pathogenic microorganisms into the dermis or lungs warrants a more robust γδ17 response to clear the infection. However, the pressing question remains: with such diverse functions that cannot be easily explained by TCR signaling alone, how do γδ T cells respond with the appropriate effector function in their specific local microenvironments?
Uncovering novel roles for γδ T cells within epithelial barriers
With limited knowledge regarding the activating ligand for murine γδ T cells, the majority of our understanding of γδ T cells has come from the use of mice deficient in the T cell receptor delta locus (Tcrd). However, one disadvantage to this approach is that following germline depletion of γδ T cells, αβ T cells fill the empty niche within tissues and exert compensatory functions20. In spite of this, the Tcrd knockout (KO) mouse reinforced the importance of fetal and neonatal γδ T cell development in the thymus for normal γδ T cell distribution to tissues and circulation21,22. Antibody-mediated depletion was widely used to investigate the requirement for γδ T cells in response to infection and autoimmunity23,24,25 until generation of the TcrdH2BeGFP (TcrdEGFP) mouse revealed that internalization of these anti-TCRγδ antibodies rendered the cells “invisible” rather than inducing their depletion26. Thus, the reported effects on γδ T cell function in vivo from these prior studies likely highlight the importance of TCRγδ signaling in these specific contexts. More recently, the TcrdGDL mouse was generated27 to allow conditional depletion of γδ T cells and more reliable investigation into the requirement for γδ T cells in vivo.
γδ T cells are found in relatively low abundance in the secondary lymphoid organs in the absence of infection. Further, the isolation of γδ T cells from peripheral tissues is challenging as is maintaining cell viability ex vivo; therefore, additional methodology was needed to investigate the function of γδ T cells in the barrier tissues where these cells are most commonly found. Although other GFP reporter strains can be used to visualize γδ T cells in certain tissues28,29, the generation of the TcrdEGFP mouse further enhanced our ability to perform intravital imaging to assess γδ T cell localization and motility within barrier surfaces30–32.
The development of new tools to specifically evaluate γδ T cell localization and function has begun to elucidate novel roles for γδ T cells within healthy and diseased tissues. Moreover, the inherent complexity of the immune response at barrier surfaces has resulted in increased investigation of γδ T cell motility and how this influences host-microbe interactions within specific tissue microenvironments. Characterizing γδ T cell migratory behavior has also provided a means to evaluate intercellular interactions and define additional metrics of γδ T cell effector function. As this area of investigation continues to expand, it is conceivable that the identification of the molecular mechanisms underlying γδ T cell migratory behavior will not only help to illuminate the functions of γδ T cells in disease pathogenesis but also provide new targets for therapeutic development.
Defining T cell motility: trafficking, search and surveillance
First, it is necessary to clarify how we describe and distinguish different types of T cell motility or migratory behavior. In the literature, T cell migration is most frequently defined in the context of trafficking: this includes thymic egress, trafficking from the blood to secondary lymphoid organ to the tissue, or from the tissue back into circulation. All of these scenarios reflect lymphocyte migration from one place to another in the body. What is less frequently discussed is the specific motility or migratory behavior within the tissue microenvironment, especially in the context of γδ T cells. There have been several well-written reviews on interstitial T cell migration33–35, and therefore this review will only briefly highlight some of the main factors involved in this process and how it relates to questions that remain elusive in γδ T cell biology.
Search versus surveillance behaviors of lymphocytes
The first and most direct question is: how do we define γδ T cell migratory behavior? Krummel et al. define a “spectrum of motility” in which factors involved cell-intrinsic locomotion in combination with physical and chemical cues from the local microenvironment regulate migratory behavior within a given tissue36. T cell locomotion has been broadly characterized as amoeboid migration in which the cells exhibit a rounded shape with a dynamic leading edge and a stable uropod in the rear34,37. Much of the work analyzing T cell migration within a tissue has been performed in the context of conventional αβ T cells, using computational analysis to define T cell ‘search’ behavior. This search behavior has been used to explain the ability of naïve and antigen-specific lymphocytes to rapidly scan secondary lymphoid organs or peripheral tissues to find their cognate antigen, signaling partner, or target36,38. As a result, T cell search is defined as a random walk along trajectories composed of successive randomly-oriented steps39,40. This random walk continues until engagement of the TCR through interaction with an APC expressing its cognate antigen functions as a ‘stop signal’41. By incorporating data from intravital imaging, computational strategies have been optimized to reflect changes in T cell activation status and contribution of the structural or chemotactic environment in guiding T cell motility36.
Search behavior is predicated on lymphocytes seeking a specific target; however, many functions of γδ T cells are antigen-independent6–8. Thus, while these studies have been extremely informative in defining the kinetics and spatiotemporal dynamics of conventional T cell migration, these models fail to significantly advance our understanding of γδ T cell biology. Based on the known role of γδ T cells as immune sentinels at barrier surfaces, we put forth that distinct migratory patterns need to be defined to accurately classify and reflect the surveillance capacity of these unconventional lymphocytes. To this end, we will explore how γδ T cells in different tissue compartments provide continuous surveillance of these barrier interfaces.
Lymphocyte migration through complex tissue architecture
In addition to defining migratory patterns, we must also consider the molecular mechanisms by which surveillance behaviors are regulated. This leads to our second question: how do T cells navigate the complex microenvironment within a barrier surface? The architecture of a lymph node starkly contrasts to that in the epidermal or intestinal epithelial compartment, in which the degree of physical confinement is much higher due to (1) structural and spatial restrictions imposed by non-lymphoid cell types within the tissue, (2) the density of extracellular matrix (ECM), and/or (3) necessity for neighboring cells to remain anchored to the ECM (the latter is particularly relevant to enterocytes). The two main factors that contribute to T cell motility in these confined spaces are the porosity of the ECM and the ability of the cell to deform its nucleus42. It is widely thought that T cells do not require proteolytic cleavage of the matrix for their motility33, yet there are examples in which T cells have been shown to remodel the ECM for this purpose. For example, cytotoxic T cells secrete extracellular granzyme to degrade ECM proteins to facilitate their extravasation from the blood vessel43. γδ T cells express high constitutive levels of various granzymes44; therefore, it would be of interest to determine whether these granzyme stores are used to support migration within the tissue at steady-state or under pathological conditions. Alternatively, cytokines, pathogens, or other inflammatory mediators can also remodel the ECM, thus allowing T cells more freedom to migrate within inflamed tissues45.
In the event that these physical structures are not altered, the ellipsoid nucleus of the T cell will deform to fit into small pores or tight spaces within the tissue42. In fact, it has been shown that a lobe of the nucleus is incorporated into the protruding lamellipodia as a T cell begins to extravasate through the vascular wall46. Many of the intravital microscopy experiments visualizing γδ T cells in the barrier have been performed using nuclear GFP reporter mice (TcrdEGFP)30, which allows us to observe the extent to which the nucleus deforms. In the intestinal epithelium, the nucleus of γδ IELs constantly changes shape as the cells survey the basement membrane and intercalate between adjacent enterocytes31. Even tighter constriction of the nucleus is observed when γδ T cells cross the basement membrane between the lamina propria and epithelial compartment47. While imaging with a nuclear GFP reporter helps to resolve the overall migratory behavior of these cells, we still lack a clear view of the cell’s leading edge to observe changes in the polarization of a migrating lymphocyte. One way to address this limitation is to perform intravital imaging studies with the recently-developed TcrdGDL mouse27, in which γδ T cells can be identified by cytoplasmic GFP expression. Alternatively, fluorophore-labeled antibodies can be injected intravenously to mark cell surface proteins prior to imaging48. Visualizing the cytoplasm or membrane of γδ T cells will further define the spatiotemporal dynamics by which these cells extend membrane processes to reach between epithelial cells or make transient contacts with other leukocytes.
Interestingly, activated T cells exhibit increased nuclear stiffness42, which inhibits their ability to deform and fit through smaller spaces. Under these conditions it is possible that the composition and relative stiffness of the matrix could compensate for the lack of nuclear deformation. However, the question remains as to how γδ T cells with a “partially-activated” innate-like phenotype16 fit on the known spectrum of T activation as it relates to nuclear deformation, and whether this intermediate activation state contributes to the morphological changes needed to facilitate γδ T cell migratory behavior. Therefore, it is important to consider the architecture and situational microenvironment as we continue to define the migratory patterns of γδ T cells within barrier tissues.
Tissue localization of γδ T cells
As discussed earlier, γδ T cells are found in highest numbers at barrier surfaces throughout the body, including the skin, intestine, lung and other mucosal surfaces. In the subsequent sections we will highlight how γδ T cells traffic to specific locations within the body, their known migratory or surveillance behaviors in each tissue, and the impact of these behaviors on their effector function in the context of tissue homeostasis and disease.
Trafficking and seeding of epidermal γδ T cells
There are two main subsets of γδ T cells located in the skin; dendritic epidermal T cells (DETC) and dermal γδ T cells. Approximately 98% of DETCs express Vγ5 TCR, whereas dermal γδ T cells are a more heterogenous populations with up to 50% of these cells expressing Vγ4 TCR28,29,49. Within each location, these subsets exhibit differential effector functions and surveillance behaviors that ultimately contribute to wound healing, protection against microbial invasion, or upon aberrant activation can lead to disease pathology such as psoriasis.
Precursors to Vγ5+ cells appear in the first wave of fetal thymocyte development50. The expansion and maturation of Vγ5+ progenitor cells is dependent upon the expression of Skint1, which is a butyrophilin-like (Btnl) molecule expressed by thymic epithelial cells and keratinocytes51. In addition to the upregulation of sphingosine-1-phosphate receptor 1 (S1PR1), which is required for thymic egress of mature T cells52, Vγ5+ cells upregulate CCR10, the cognate receptor for CCL27 that is highly expressed in the skin52,53. Signaling through CCR10 is not involved in DETC development in the fetal thymus; however, trafficking to the epidermis is impaired in CCR10-deficient mice, resulting in an accumulation of Vγ5+ cells in the dermis of fetal mice54. This trafficking defect appears to be specific to the fetal period since CCR10 KO adult mice do not exhibit reduced DETC numbers. This may be attributed to a degree of functional redundancy between CCR10 and the expression of selectins in either targeting Vγ5+ cells to the skin55 or the local expansion of DETCs54. Similarly, loss of GPR15, an orphan G-protein coupled receptor that shares homology with other leukocyte chemokine receptors56,57, reduced the number of DETCs in neonates, but not adults58, indicating that an alternative pathway may contribute to the homeostatic regulation of these cells. Supporting this observation, Nakamura et al. found that as opposed to regulating the initial homing of these DETCs to the neonatal epidermis, CCR4 expression was essential for maintaining DETCs in postnatal and adult mice55,59. CD103 (αEβ7 integrin) is an important marker of tissue-resident leukocytes and is highly expressed by DETC thymic precursors, which also express its co-receptor, E-cadherin60. Although CD103-deficient mice exhibit a reduction in the total number of DETCs, it remains unclear whether this is due to a defect in thymocyte development, trafficking to the skin, or in maintenance of the tissue-resident population.
Surveillance behavior of DETCs
DETCs are thought to function as lymphoid stress sensors by extending their dendrites to interact with neighboring cells. These stable dendrites are oriented toward the apical epidermis and exhibit cytoplasm-filled swellings or projections61. Due to the detection of a phosphorylated tyrosine signal on the tips of these dendritic projections, these structures are referred to as ‘phosphorylated tyrosine-rich aggregates located on projections’, or PALPs. Moreover, Vγ5 TCR was found to be clustered and activated on PALPs, and TCR activation is thought to contribute PALP formation. These apical projections form synaptic structures in close proximity to squamous keratinocyte junctions, often at sites of tricellular interactions. Consistent with this, CD103 is found in these synapses and directly interacts with keratinocyte E-cadherin to facilitate anchoring of these long-lived apical dendrites. PALPs form at steady-state and are strategically located to recognize stressed or malignant keratinocytes, with one PALP allowing for efficient surveillance of at least three neighboring cells.
In response to injury, DETCs quickly retract their dendrites resulting in rounding of the cell8,62. Interestingly, basolateral dendrites are the first to be dissembled, with apical PALPs following soon after61. This may point to the relative importance of the apical PALP structures in maintaining surveillance or physical interaction with neighboring cells. Importantly, TCR activation alone was not sufficient to induce rounding, indicating that multiple signals are needed to initiate this response61. This morphological change is mediated in part through CD103 engagement with E-cadherin. In response to wounding, keratinocytes downregulate E-cadherin expression leading to DETC rounding63. Another ligand-receptor-mediated interaction involved in this rounding phenotype is the engagement of CD100 (semaphorin 4D) and plexin B, expressed by DETCs and keratinocytes, respectively64. Once bound, signaling through CD100 induces the activation of cofilin and ERK, resulting in cell rounding.
Following activation by either stress or TCR engagement, DETCs upregulate the expression of the tight junction protein, occludin65. Surprisingly, loss of occludin expression adversely affected rounding as DETCs in occludin-deficient mice still showed dendrite extension even after irradiation. CD100 levels were similar in the presence or absence of occludin, suggesting that occludin expression may be regulated downstream of CD10065.
The importance of PALP localization beneath the keratinocyte junctional complex was further demonstrated by a study showing that binding of junctional adhesion molecule-like protein (JAML), a cell surface molecule on DETCs, to Coxsackie and Adenovirus receptor (CAR) expressed in the junction, functions as a co-stimulatory second signal66. Blocking of JAML/CAR interactions results in delayed wound healing. The rapid detection of damage or distress by PALPs is thought to function as a stress sensor to promote a local release of effector molecules61.
DETCs are relatively sessile within the epidermis and migrate at speeds less than 1 μm/min. Even after rounding, DETCs do not appear to be motile; however, the continuous extension of dendrites, or probing of neighboring keratinocytes, could be classified as a unique form of surveillance behavior (Table 1). Intravital imaging of epidermis 72 hours after wounding showed that DETCs exhibited a more rapid probing behavior than was observed at steady-state61; however, whether this reflects an increased surveillance state or that the keratinocytes may not have returned to a baseline physiological state to support stable PALP formation is unknown. Likewise, the molecular mechanisms involved in the regulation of DETC probing behavior have yet to be described. Although it has been suggested that rounding of DETCs may facilitate intradermal migration or egress to the draining lymph node64,65, this is an area that requires further investigation.
Table 1.
Surveillance behaviors and host-microbe responses of tissue-specific Vγ subsets
| Tissue | Subset | Surveillance Behavior | Response to Commensal Bacteria & Pathogens |
|---|---|---|---|
| Skin (epidermis) | Vγ5 | • Sessile1 • Dendrites “probe” for antigen or stress signal from neighboring keratinocytes |
• Produce cytokines in response to Gram neg bacteria2 • DETC IL-17A induces AMP production by keratinocytes3 • Promote neutrophil recruitment and microbial clearance via IL-174 |
| Skin (dermis) | Vγ4 | • Patrolling behavior5,6 • Form stable interactions with APCs |
• Neutrophil recruitment and bacterial clearance by IL-17 response5,6 |
| Gingiva | Vγ6>Vγ4,1 | Motile, but migratory behavior remains uncharacterized7 | • Oral microbiome regulates Vγ subset composition and number7 • Vγ6+ cells most sensitive to changes in microbiota • γδ17 cells shape the oral microbiome |
| Lung (parenchyma) | Vγ4>Vγ1 | unknown | • APC interactions (MHCII+ macs, DCs)8 • IL-17 promotes neutrophil infiltration and microbial clearance9,10 |
| Lung (non-parenchyma) | Vγ6 | unknown | • IL-17 promotes neutrophil infiltration and microbial clearance11,12 • Commensal bacteria activate Vγ6+ cells to promote inflammation and tumor proliferation13 |
| Intestine (intraepithelial) | Vγ7>Vγ1 | • Flossing14–17 • Surveying • Probing18 |
• Absence of commensals reduces flossing in favor of surveying behavior (Edelblum, unpublished) • Increased flossing at “hotspots” near pathogen invasion14,16 • Epithelial MyD88 signaling promotes flossing response to Salmonella infection19 • γδ IELs can produce AMPs to limit bacterial invasion20,21 |
| Intestine (lamina propria) | Vγ1,4 | unknown | • Commensal bacteria influence γδ17 population22 • Memory Vγ4 response to secondary infection23,24 |
| Uterus | Vγ6 | unknown | • Protective against Candida albicans infection, thought to recruit neutrophils to FRT25 |
Chodaczek G, Papanna V, Zal MA, Zal T. Body-barrier surveillance by epidermal gammadelta TCRs. Nat Immunol. 2012;13(3):272-282.
Leclercq G, Plum J. Stimulation of TCR V gamma 3 cells by gram-negative bacteria. J Immunol. 1995;154(10):5313-5319.
MacLeod AS, Hemmers S, Garijo O, et al. Dendritic epidermal T cells regulate skin antimicrobial barrier function. J Clin Invest. 2013;123(10):4364-4374.
Cho JS, Pietras EM, Garcia NC, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120(5):1762-1773.
Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186(11):6091-6095.
Sumaria N, Roediger B, Ng LG, et al. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med. 2011;208(3):505-518.
Wilharm A, Tabib Y, Nassar M, et al. Mutual interplay between IL-17-producing gammadeltaT cells and microbiota orchestrates oral mucosal homeostasis. Proc Natl Acad Sci US A. 2019;116(7):2652-2661.
Wands JM, Roark CL, Aydintug MK, et al. Distribution and leukocyte contacts of gammadelta T cells in the lung. J Leukoc Biol. 2005;78(5):1086-1096.
Nakasone C, Yamamoto N, Nakamatsu M, et al. Accumulation of gamma/delta T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes Infect. 2007;9(3):251-258.
Cheng P, Liu T, Zhou WY, et al. Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol. 2012;13:38.
Murakami T, Hatano S, Yamada H, Iwakura Y, Yoshikai Y. Two Types of Interleukin 17A-Producing gammadelta T Cells in Protection Against Pulmonary Infection With Klebsiella pneumoniae. J Infect Dis. 2016;214(11): 1752-1761.
Simonian PL, Roark CL, Born WK, O’Brien RL, Fontenot AP. Gammadelta T cells and Th17 cytokines in hypersensitivity pneumonitis and lung fibrosis. Transl Res. 2009;154(5):222-227.
Jin C, Lagoudas GK, Zhao C, et al. Commensal Microbiota Promote Lung Cancer Development via gammadelta T Cells. Cell. 2019;176(5):998-1013 e1016.
Hoytema van Konijnenburg DP, Reis BS, Pedicord VA, Farache J, Victora GD, Mucida D. Intestinal Epithelial and Intraepithelial T Cell Crosstalk Mediates a Dynamic Response to Infection. Cell. 2017;171(4):783-794.
Edelblum KL, Shen L, Weber CR, et al. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc Natl Acad Sci USA. 2012;109(18):7097-7102.
Edelblum KL, Singh G, Odenwald MA, et al. gammadelta Intraepithelial Lymphocyte Migration Limits Transepithelial Pathogen Invasion and Systemic Disease in Mice. Gastroenterology. 2015;148(7):1417-1426.
Hu MD, Ethridge AD, Lipstein R, et al. Epithelial IL-15 Is a Critical Regulator of gammadelta Intraepithelial Lymphocyte Motility within the Intestinal Mucosa. J Immunol. 2018;201(2):747-756.
Sumida H, Lu E, Chen H, Yang Q, Mackie K, Cyster JG. GPR55 regulates intraepithelial lymphocyte migration dynamics and susceptibility to intestinal damage. Sci Immunol. 2017;2(18).
Ismail AS, Severson KM, Vaishnava S, et al. {gamma}{delta} intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci U S A. 2011;108(21):8743-8748.
Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol. 2009;182(5):3047-3054.
Walker CR, Hautefort I, Dalton JE, et al. Intestinal Intraepithelial Lymphocyte-Enterocyte Crosstalk Regulates Production of Bactericidal Angiogenin 4 by Paneth Cells upon Microbial Challenge. PLoS One. 2013;8(12):e84553.
Duan J, Chung H, Troy E, Kasper DL. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe. 2010;7(2):140-150.
Sheridan BS, Romagnoli PA, Pham QM, et al. gammadelta T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 2013;39(1):184-195.
Romagnoli PA, Brian S. Sheridan, Quynh-Mai Pham, Leo Lefrançois, and Kamal M. Khanna. IL-17A–producing resident memory γδ T cells orchestrate the innate immune response to secondary oral Listeria monocytogenes infection. PNAS. 2016.
Monin L, D. S. Ushakov, H. Arnesen, et al. γδ T cells compose a developmentally regulated intrauterine population and protect against vaginal candidiasis. Mucosal Immunology. 2020.
Trafficking and migratory behavior of dermal γδ T cells
Unlike DETCs, which make up a tissue-resident population that does not return to circulation, dermal γδ T cells express CCR6 and CCR2, which drive circulation of these cells to and from the periphery67. Consistent with its expression in polarized γδ17 cells, steady-state expression of CCR6 responds to CCL20 produced by keratinocytes to recruit IL-17-producing Vγ4+ and Vγ6+ cells to the dermis. Mice deficient in CCR7, which promotes migration from the tissue into the peripheral lymphatics, have no defect in DETC or dermal γδ T cell number indicating that dermal γδ T cells undergo local proliferation to maintain their presence in the tissue under homeostatic conditions29. However, in the context of inflammation, CCR6 is downregulated in favor of CCR2 to recruit dermal γδ T cells to inflamed regions67,68. Loss of CD69, a marker for tissue-resident cells, results in increased S1PR1 surface expression and γδ T cell migration from the dermis to the draining lymph node69–71. More recently, it was shown that expression of S1PR2 opposes this trafficking and functions to retain CD69+ γδ T cells in the dermis. It still remains unclear whether CD69 expression on these cells is required for their retention within the tissue69.
While these studies have begun to elucidate the mechanisms regulating dermal γδ T cell trafficking, two back-to-back intravital imaging studies using CXCR6-GFP knock-in mice revealed that these cells were more ameboid in shape compared to DETCs and exhibit a distinct patrolling behavior within the dermis28,29. One study reported an average migratory speed of 3-5 μm/min for dermal γδ T cells28, which is consistent with the reported average speed of T cells migrating within a barrier surface31. Dermal γδ T cell motility was described as a patrolling behavior in which migrating cells pause, turn at a 85° angle, and then continue to migrate28. This is similar to the flossing behavior of γδ IELs31,72 discussed later in this review. The complementary study reported an average speed of 2 μm/min for these cells, reflecting the presence of both an actively patrolling and a more stationary population of dermal γδ T cells29. The non-motile population of γδ T cells was attributed to formation of stable interactions between γδ T cells and MHCII+ cells within the dermis.
While there are a few reports of interactions between γδ T cells and APCs73, it would be of interest to determine whether there are two distinct migratory patterns for dermal γδ T cells; those that exhibit a surveillance behavior defined by continuous patrolling of the dermis, and a separate subset that exhibit a more traditional search behavior typical of αβ T cells. Alternatively, dermal-patrolling γδ17s could experience momentary arrest via transient interactions with MHCII+ cells. Dermal γδ T cells constitutively express 3-fold higher levels of occludin than DETCs, yet whether occludin contributes to γδ17 motility remains unknown65. Further evaluation of the dermal γδ T cell migratory behaviors and whether these lymphocytes make transient or sustained contacts with other leukocytes would increase our understanding of the kinetics and functional roles of γδ17 cells under homeostatic and inflammatory conditions. Moreover, identifying the molecular cues that regulate these patrolling behaviors and APC interactions may provide novel therapeutic strategies for inflammatory skin diseases.
The patrolling behavior of dermal γδ17 cells correlates well with the known effector function of these cells in providing an early response to microbial infection28,29. As sentinels, these γδ T cells may either respond to foreign antigen or directly recognize stressed keratinocytes to stimulate IL-17 release and facilitate clearance of an invading microorganism, such as Mycobacterium bovis29,74. Alternatively, dermal γδ T cells can be activated indirectly in the presence of IL-1β and IL-2328,49. IL-1β also stimulates keratinocytes to secrete CCL20 to promote the chemotaxis of CCR6+ γδ17 cells in vitro67. In line with their pro-inflammatory role, IL-17 produced by the dermal γδ T cell compartment can inhibit insulin-like growth factor 1 (IGF-1) production by DETCs, thus delaying wound healing and prolonging the inflammatory response75.
While it is clear that compartmentalization of γδ T cell subsets between the epidermis and dermis allows a division of labor based on specific effector functions, there are instances in which altering this spatial segregation negatively affects tissue homeostasis. In a model of psoriasis-like dermatitis, injection of IL-23 promoted the recruitment of CCR6+ dermal γδ17 cells into the epidermis76. Blocking CCL20/CCR6 signaling abrogated the migration of IL-22-producing dermal γδ T cells, leading the authors to conclude that infiltration of γδ17 cells into the epidermis may promote epidermal hyperplasia and dermal edema76.
Gingival γδ T cells
The oral mucosa is composed of stratified squamous epithelium, with architecture similar to the skin, and can be subdivided into the following specialized regions: the junctional epithelium that is attached to the tooth, the sulcular epithelium, and the oral epithelium on the external surface of the gingiva. Studies using TcrdEGFP mice showed that γδ T cells primarily reside within the junctional epithelium closest to the oral biofilm and are highly motile32. These gingival γδ T cells have a rounded morphology and appear to migrate at speeds similar to dermal γδ T cells28,29. Further characterization revealed that the majority of these gingival γδ T cells express the Vγ6 TCR and CD10332. Loss of IL-23R signaling under homeostatic conditions partially reduced the number of these IL-17-producing Vγ6+ cells and ablation of CCR6 signaling decreased the total number of gingival γδ T cells32, suggesting that CCR6 may facilitate their recruitment to the gingival epithelium. Moreover, mice deficient in γδ T cells exhibited increased periodontal pathology due to decreased production of amphiregulin77 and reduced IL-17 production in the gingiva, which led to alteration of the oral microbiome32. Conversely, gnotobiotic mice have a decreased frequency and total number of gingival γδ T cells32,77, indicating reciprocal interactions between the oral microbiome and this sentinel lymphocyte population. Together, these findings indicate that γδ17 cells provide surveillance of the gingival epithelium to shape the oral microbiome and promote repair following damage. More detailed investigation into the migratory behavior of these cells and the contribution of CD103 to gingival γδ T cell motility may uncover novel functional responses at steady-state and in response to inflammation.
γδ T cells in the lung
Within the lung, γδ T cells are primarily found in the lamina propria (LP) both in and around airway walls73. During the postnatal period, the majority of pulmonary γδ T cells express Vγ6 TCR. However, the numbers of Vγ4+ and Vγ1+ cells become more prominent in adult mice and comprise 5-10% of the total lymphocyte population in the lung73,78,79. These subpopulations are also differentially localized, with Vγ1+ and Vγ4+ cells found most frequently in the parenchyma while Vγ6+ cells are more broadly distributed in non-parenchymal locations73. Under homeostatic conditions, nearly half of pulmonary γδ T cells in the parenchyma were found to interact with macrophages and MHCII+ dendritic cells73. Moreover, TCRγδ staining was enhanced at sites of T cell/myeloid cell contact, suggesting that γδ T cells may be exerting a regulatory function to maintain mucosal homeostasis.
Studies in γδ T-cell-deficient mice have shown that these cells largely exhibit a protective function in response to pulmonary infection or injury80–83. This is consistent with the known functions for IL-17-producing Vγ4+ and Vγ6+ cells in promoting neutrophil infiltration and bacterial clearance84–87. While an expanded pulmonary γδ T cell population has been observed in response to lung infection79,88–91, the extent to which γδ T cells are trafficked into the lung from the periphery remains unclear. Neither CCR6 nor CCR4 were required for γδ17 infiltration into the lung following Mycobacterium bovis infection92,93. In fact, the local proliferation of Vγ1+ and Vγ4+ cells was shown to account for the increase in pulmonary γδ T cells following Streptococcus pneumoniae infection94. Thus, it remains to be determined whether a local expansion of γδ17 cells, rather than an influx of circulating lymphocytes, is consistent among the host response to various pulmonary infections.
Different γδ T cell subsets appear to have distinct roles in disease pathology in the lung. For example, Vγ1+ cells can promote, whereas Vγ4+ cells suppress, airway hyperresponsiveness95, and Vγ6+ contribute to lung fibrosis96. Moreover, commensal bacteria were shown to induce the expansion of pro-inflammatory Vγ6+ cells that promote tumor cell proliferation in the lung97. Therefore, further investigation into the mechanisms by which these specific subsets are recruited to or localized within the lung during infection or inflammation may provide additional insight into the effector functions of pulmonary γδ T cells. Along the same lines, deciphering which γδ T cells localize or directly interact with myeloid cells may reveal the contribution of individual Vγ subsets in the lung.
γδ T cells in the female reproductive tract
Recently, Monin et al. described a uterine population of γδ T cells that resides in the sub-epithelial stroma98, not in the epithelium as had been previously described99. γδ T cells in this compartment have rounded, lymphoid morphology and the vast majority express the Vγ6Vδ1 TCR, whereas others express Vγ4 TCR. Unlike Vγ6+ cells at other mucosal barriers, γδ T cells in the uterus are not dependent on local microbiota for development or function98. However, the signals that drive their thymic selection and trafficking to the uterus have yet to be defined.
Similar to other Vγ6 TCR+ populations, more than 90% of the uterine γδ T cell compartment elicits a robust IL-17A response following stimulation98. γδ T cell effector function in the uterus segregates with Vγ expression, as uterine Vγ6− cells produced IFNγ upon activation. Further, uterine Vγ6+ cells were found to be transcriptionally unique from the Vγ6+ population in the lung; most notable of the differences between the two populations is the expression of Pgr, the progesterone receptor gene, in uterine Vγ6+ cells. Interestingly, many co-stimulatory receptors were shared between lung and uterine Vγ6+ cells. These findings demonstrate that the local microenvironment may result in the adaptation of γδ T cells within individual barrier tissues.
Underlining the functional importance of γδ17 in the FRT, Tcrd KO mice were found to be more susceptible than WT mice to Candida albicans98. This was attributed to γδ T cell-mediated IL-17A production driving neutrophil recruitment in response to fungal infection. Yet, it remains unknown other regions of the FRT have distinct γδ T cell populations or if uterine γδ T cells can contribute to surveillance of the entire tissue. Therefore, studying the motility of this unique subset of γδ T cells will further elucidate the role of these cells in steady-state uterine function, including regulation of homeostatic turnover of the barrier epithelium or monitoring the FRT microbiome.
Intestinal γδ T cells
The intestinal mucosa consists of the epithelium and the underlying LP, which are separated by the basement membrane. Unlike the skin, which has a stratified epithelium that separates the host from the external environment, the intestine is lined by a single layer of columnar epithelial cells. The intestinal epithelium is organized into stem cell-containing crypts, and villi, which protrude into the lumen. Within the mucosa, γδ T cells are primarily found within the epithelium, comprising up to 60% of the total population of intraepithelial lymphocytes (IEL), and approximately 10% of LP lymphocytes100–102. Approximately 60% of γδ IELs express the Vγ7 TCR and 30% express Vγ1103,104. In contrast, the LP γδ T cell population is more heterogeneous and contains Vγ1+ and Vγ6+ cells, likely reflecting lymphocytes trafficking into the gut from the periphery.
The frequency of γδ IELs is highest in the duodenum and gradually decreases along the length of the intestine105, yet the proportion of Vγ subsets remains similar within each region. γδ IELs exhibit a largely protective response by dampening acute inflammation19,106 and promoting mucosal barrier integrity107–109. IELs are maintained in a state of partial activation, and are thought to be largely immunologically quiescent16; however, their ability to initiate a rapid response to enteric infection107–109 highlights the ability of γδ IELs to bridge innate and adaptive immunity110. In this section, we will explore how trafficking, surveillance behaviors, and ultimately, effector functions differ between γδ T cells in each compartment at steady-state or in response to infection or inflammation.
γδ IEL Trafficking and Development
γδ IELs begin to populate the gut of weanling mice between 2-3 weeks of age in a process that is independent of the presence of dietary antigen or microbiota111. The contribution of thymic vs extrathymic ontogeny of these γδ IELs remains a point of contention within the field11,16,112; however, Vγ7+ IELs have been shown to populate the small intestine in athymic mice111. Once γδ IELs establish residence in the epithelium, these cells do not re-enter into circulation103.
Unlike conventional antigen-specific IELs, γδ IELs are directly recruited to the intestine without the need for antigen exposure in secondary lymphoid organs16. This homing to the gut is facilitated by tissue-specific chemokines and their receptors. One of the earliest chemokine-chemokine receptor pairs identified in lymphocyte homing to the intestine is CCR9 and its ligand CCL25, which is constitutively expressed by intestinal epithelial cells (IEC)113. Mice deficient in CCR9 exhibit a 3-fold reduction in the number of small intestinal γδ IELs103,113,114, but this number is increased in the colon where only a small proportion of IELs express the chemokine receptor113,115. CCR9 primarily functions to induce trafficking of γδ T cells to the IEL compartment, which was shown when deletion of this receptor had no effect on LP lymphocyte number113, the Vγ subsets present in the IEL compartment, or the gradient of γδ IEL localization along the length of the small intestine103. IELs also highly express CXCR3, the ligand for which is CXCL10 expressed by IECs116–118. Interestingly, the CD8αα+ IEL population is increased in CXCR3-deficient mice suggesting that its expression is not required γδ IEL intestinal homing118.
In addition to chemokine receptors, several integrins have been implicated in the homing and retention of γδ T cells within the intestinal mucosa. The two most prominent integrins involved in trafficking of lymphocytes to the gut are α4β7 and αEβ7 (CD103). While β7 integrin-deficient mice have a substantial reduction in the number of IELs119, there is no appreciable defect in mice lacking α4 integrin120, indicating that CD103 is largely responsible for trafficking of γδ IELs to the intestine. Supporting this, CD103-deficient mice have a reduced γδ IEL population121; however, this may be dependent on individual animal facilities since we observe a less pronounced phenotype in our colony31. CD103 directly interacts with epithelial E-cadherin and is widely thought to facilitate the retention of leukocytes within the gut122,123. γδ IELs express other integrins such as β1 and β2124, but the role for these proteins in gut homing is less well understood. Mice with a partial deficit in the expression of β2 integrin (LFA-1/CD11a) or its ligand ICAM-1 exhibited a reduced γδ IEL population125. Similar findings were observed in mice deficient in α1 integrin, which pairs with the β1 subunit126. Taken together, these findings indicate that CCR9 and CD103 are key regulators involved in the trafficking and retention of γδ IELs to and within the intestine.
More recently, two orphan G-Protein Coupled Receptors (GPR) have been implicated in regulating γδ IEL localization within the gut. GPR18 is highly expressed on γδ IELs, and mice deficient in GPR18 exhibit a significant reduction in small intestinal γδ IELs48. Of the remaining γδ IELs present in GPR18 KO mice, there were strikingly fewer Vγ7+ cells. Interestingly, the generation of CCR9/GPR18 double KO mice showed that CCR9 was dominant over GPR18 in regard to regulating γδ IEL trafficking to the gut; however, the reduction in the frequency of Vγ7+ IELs was more specific to the lack of GPR18. Moreover, rescue experiments showed that restoring GPR18 expression in bone marrow led to an accumulation of γδ T cells specifically within the IEL compartment, suggesting that GPR18 may contribute to the epithelial retention of these lymphocytes. Although another report did not observe a defect in IEL number in GPR18 KO mice, GPR18 was required for repopulation of γδ IELs in the small intestine following bone marrow transplantation, supporting earlier findings127.
Whereas GPR18 promotes recruitment of γδ IELs to the gut, GPR55 inhibits the accumulation of γδ IELs in the small intestine128. Loss of GPR55 results in increased γδ T cell number in both the IEL and LP compartments. In response to indomethacin-induced injury, inhibition of GPR55 increased γδ T cell localization in the epithelial compartment presumably by enhancing S1PR1-dependent egress of γδ T cells from Peyer’s patches128. Another negative regulator of S1PR1 is CD69129, which is constitutively expressed at high levels in tissue resident cells, including γδ IELs130. These studies highlight some of the receptors involved in the positive and negative regulation of the initial trafficking of γδ IELs to the gut, yet it is clear that the many of the molecular mechanisms involved in this process have not been fully elucidated.
The ligand-receptor pairs described above reflect our current knowledge of how γδ IELs traffic to the intestine under homeostatic conditions. However, it is also important to consider that γδ IELs are self-renewing and proliferate within the epithelial compartment both at steady-state and in response to infection103,111,131,132. Several factors are involved in the maintenance of the γδ IEL compartment including IL-15/IL-15Rα, IL-7, and signaling through the aryl hydrocarbon receptor (AhR)133–137. Moreover, the maturation and expansion of Vγ7+ IELs is dependent upon IEC expression of Btnl1, which may in part promote the expression of IL-2Rβ, the receptor for IL-15111,138.
In the intestine, IL-15 is expressed by IECs and LP dendritic cells139. Membrane-bound epithelial IL-15/IL-15Rα is transpresented to its cognate receptor IL-2Rβ on T cells134, thus leading us to ask whether this direct interaction contributes to γδ T cell localization within the intestinal mucosa. Using two IL-15 transgenic mouse lines, one in which IL-15 is overexpressed by IECs and one with IL-15 overexpression in all cells except IECs, we found that γδ T cells localize to the compartment with the highest IL-15 expression47. Although the exchange of lymphocytes between the epithelial and LP compartment is presumed to be a rare event140, we were able to visualize several instances in which the nucleus of a γδ T cell was deformed as the lymphocyte straddled the basement membrane. Together, these findings indicate that local concentration of IL-15 and the necessity for direct epithelial interactions via IL-15 transpresentation may explain why the majority of γδ T cells remain within the epithelial compartment.
Surveillance behavior within the intestinal mucosa
The majority of our understanding regarding γδ T cell function in the intestine comes from investigating the mucosal immune response in Tcrd KO mice in response to local injury, enteric infection and models of inflammation7,19,106,108,109,141,142. These studies helped to identify many of the soluble factors that intestinal γδ T cells produce in response to alterations in the local microenvironment, both in the small intestine and the colon. However, much remained unknown regarding how these immune cells, γδ IELs in particular, were able to exert a largely protective role in intestinal homeostasis despite being outnumbered 10:1 by IECs. Most of the known physiological contributions of γδ IELs are attributed to their ability to secrete paracrine factors that act on epithelial cells or other leukocytes, which led us to ask: why is it necessary for these lymphocytes to be located within the epithelial compartment as opposed to the LP?
Previous dogma suggested that γδ IELs were sessile143, and that once inserted into the epithelium, these cells were passive travelers among the enterocytes that migrated up the crypt-villus axis. These observations were justified by initial intravital imaging studies of the small intestine using the TcrdEGFP reporter mouse that showed limited motility of intestinal γδ T cells compared to those in peripheral lymph nodes103. However, enhanced spatiotemporal resolution confirmed what many had suspected: γδ IELs are highly motile within the epithelial compartment and provide continuous surveillance of the epithelial barrier31. We found that γδ IELs largely migrate along the basement membrane and intermittently turn and migrate into the lateral intercellular space (LIS) between two adjacent enterocytes. This migratory behavior was later termed “flossing”72 (Fig. 1). At steady-state, γδ IELs are retained in the LIS for 4-6 minutes before exiting and resuming their surveillance behavior, often turning and reversing course to cover the same area. Consistent with the previous report made by Chennupati et al.103, we found that the average migratory speed of γδ T cells was 3.8 μm/min31; the slow speed likely reflecting the spatial constraints of the mucosal architecture.
Figure 1. Surveillance behaviors of γδ IELs.
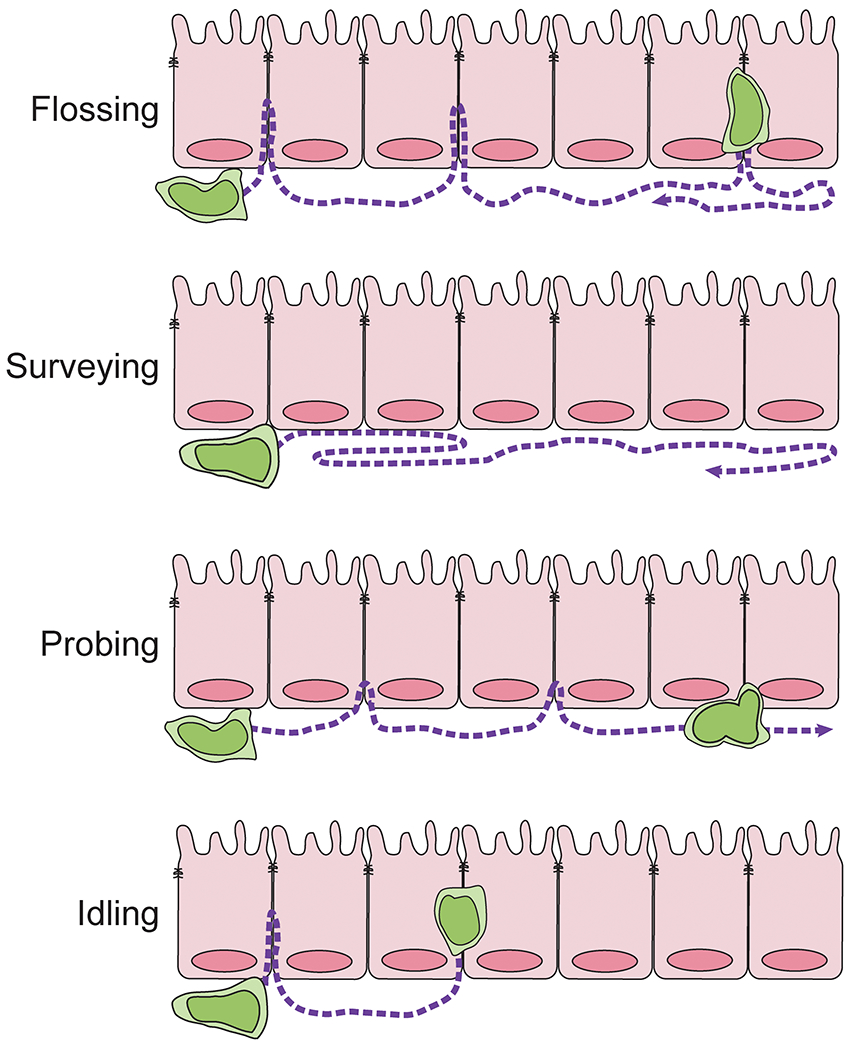
Under homeostatic conditions, γδ IELs exhibit a flossing behavior in which the cells migrate along the basement membrane and into the lateral intercellular space (LIS). In the absence of commensal bacteria, migration into the LIS is ablated resulting in continuous migration along the basolateral aspect of the epithelium (surveying). γδ IELs may not fully enter the LIS but instead extend projections between adjacent IECs (probing). Inhibiting IL-2Rβ/PI3K signaling results in an idling behavior characterized by an inability of γδ IELs to effectively polarize and migrate out of the LIS
This surveillance behavior is altered in the presence of an invading microorganism, such as Salmonella Typhimurium or Toxoplasma gondii72,144. In the context of Salmonella infection, γδ IELs migrate to enterocytes that are in direct contact with bacteria and remain in the LIS for 9-11 min. Although the γδ IELs are able to patrol the entire length of the villus, bacterial infection results in the generation of “hotspots” where γδ IELs migrate near sites of invasion72. This response was shown to be independent of TCR signaling and instead mediated via IEL/epithelial crosstalk downstream of epithelial MyD88 signaling. While the relationship between epithelial MyD88 and γδ IELs had been previously reported108, it was surprising that although the γδ TCR is constantly triggered in vivo145, TCR activation does not contribute to γδ IEL flossing behavior72. Consistent with the role for microbial recognition in regulating γδ IEL migratory behavior, visualization of γδ IELs in antibiotic-treated or germ-free mice revealed that the presence of commensals influenced the extent of γδ IEL-mediated surveillance, both by influencing their localization along the crypt-villus axis and the overall surveillance area72. In antibiotic-treated mice, we find that γδ IELs primarily migrate along the basement membrane and rarely enter the LIS (Edelblum, unpublished observations) indicating that signals from commensal bacteria are required to promote flossing behavior. We term this migratory pattern in which γδ IEL motility is restricted to the basolateral aspect of the epithelium, “surveying” behavior.
Further investigation into the molecular mechanisms regulating γδ IEL motility showed that the tight junction protein occludin, expressed by both γδ IELs and IECs, is required for γδ IEL surveillance behavior (Fig. 2)31. In the absence of γδ IEL occludin, the lymphocytes remain close to the basement membrane and are largely immobile. As a result of occludin deletion in γδ IELs, a substantial increase in pathogen translocation across the epithelium was observed within the first hour post-infection144. In contrast, CD103 interaction with epithelial E-cadherin regulates the duration of γδ IEL/epithelial contact31. Loss of CD103 resulted in enhanced surveillance behavior due to reduced retention of the γδ IEL in the LIS, thus allowing the cells to enter and exit the epithelial monolayer more rapidly. This more efficient surveillance behavior conferred additional protection against enteric pathogen invasion144. From these data, we concluded that the ability to migrate into the LIS permitted γδ IELs to initiate a localized effector response to limit pathogen invasion, and subsequently, the systemic spread of infection.
Figure 2. Ligand-binding interactions involved in regulating γδ IEL surveillance behavior.
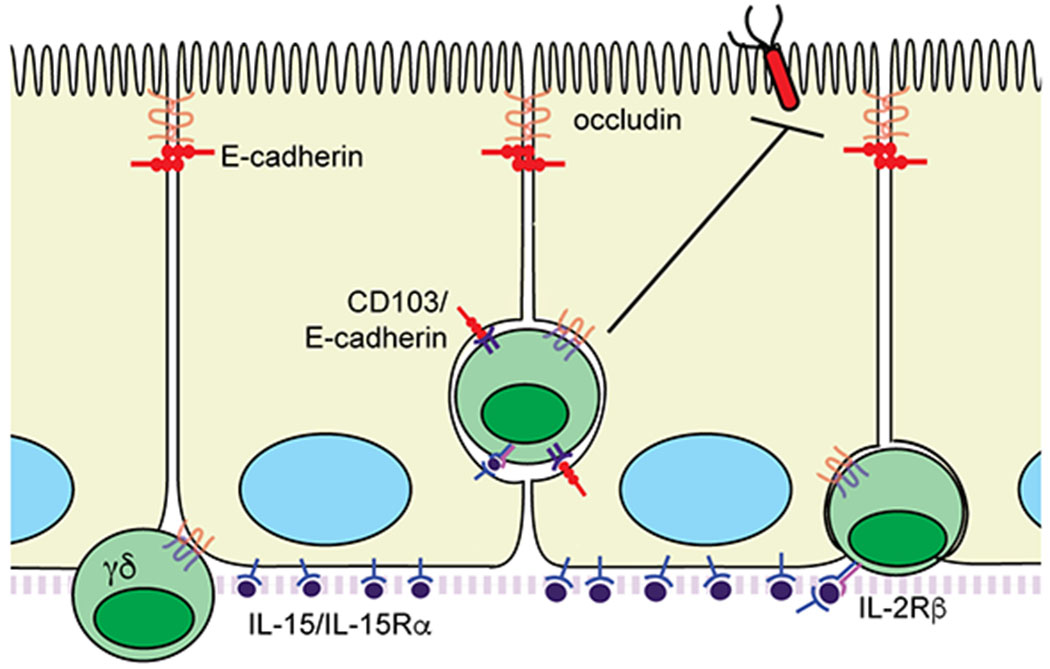
Homotypic interactions between epithelial and γδ IEL occludin are required for IEL motility. CD103 (αEβ7 integrin) binding to epithelial E-cadherin functions as a retention signal within the LIS. Activation of IL-2Rβ by epithelial IL-15/IL-15Rα complexes promotes γδ IEL motility and maintains γδ T cell localization within the epithelial compartment
We next investigated other potential ligand-receptor interactions between IELs and enterocytes that may regulate γδ IEL surveillance behavior. Previous reports had demonstrated that IL-15 induced the migration of activated peripheral blood T cells and NK cells in vitro146,147, but whether IL-15 promoted γδ T cell motility in addition to its role in γδ IEL proliferation and survival was unknown134,135,148. The requirement for transpresentation of IL-15/IL-15Rα by IECs to γδ T cells134 led us to investigate whether this direct ligand-receptor interaction contributes to γδ IEL migratory behavior. We found that IL-15 signaling promotes γδ IEL chemokinesis through activation of PI3K downstream of IL-2Rβ47. Further, blockade of IL-2Rβ signaling resulted in γδ IEL idling within the LIS due to the inability of the cell to appropriately polarize and initiate directional migration. This idling behavior in response to IL-2Rβ inhibition increased the frequency of Salmonella translocation, leading us to revise our previous model: γδ IEL migration into the LIS alone is not sufficient to limit microbial translocation, but precise localization of γδ IELs into the LIS near the site of invasion is required to confer protection against acute infection47.
Intravital imaging studies were also performed on mice deficient for GPR18 or GPR55 to evaluate IEL migratory behavior48,128. While loss of GPR18 had no apparent effect on γδ IEL motility48, the role of GPR55 is less clear103. Transplantation of GPR55-deficient γδ IELs into Tcrb-deficient mice showed that these cells migrated more rapidly yet showed no change in the dynamics of their flossing behavior. However, Sumida et al. reported changes in the association of γδ IELs with the epithelium in GPR55 KO mice103. Although the definition of this particular metric is unclear, γδ IELs were described to spend more time “probing” the epithelium, which could reflect the extension of a process, but not the cell body, into the LIS. We have also observed a similar migratory pattern under certain conditions; however, further characterization of this behavior is warranted. If a γδ IEL spends a significant time probing the barrier, this could lead to a reduction in the overall migratory speed of the lymphocyte, which is the opposite of the kinetics described in GPR55 KO mice. Alternatively, an increased track speed could be observed if γδ IELs were probing the epithelium instead of entering the LIS. It is apparent from these studies and visual inspection that γδ IEL motility patterns are heterogeneous, thus highlighting the need to develop an unbiased approach to identify and classify T cell surveillance behaviors within large sample sets.
Leveraging new models to pursue long-standing questions in γδ IEL biology
Unlike the classic example of lymphocyte/endothelial interactions during extravasation in which each aspect of adhesion and invasion are well-characterized, the paucity of studies investigating molecular interactions between γδ IELs and IECs contributes to our lack of knowledge regarding the regulation of γδ IEL migratory behavior. For additional insight, it may be helpful to turn to ligand-binding interactions involved in neutrophil transepithelial migration, since these are the only other leukocytes known to migrate into the LIS149. It is possible that many of the molecular interactions that facilitate neutrophil adherence to IECs, entry into, and retention within the LIS are conserved in γδ IELs. For example, γδ IELs express JAML66, along with various integrins and related proteins such as ICAM and CD47 that are involved in neutrophil transepithelial migration149. Therefore, these ligand-binding partners are prime targets to evaluate in the context of γδ IEL migratory behavior.
Recent findings that epithelial pattern recognition receptor signaling can promote γδ IEL surveillance behavior72 open the possibility that other innate immune signaling pathways may also regulate γδ IEL motility. Similarly, there is limited data on the extent to which γδ IEL motility is affected in a pro-inflammatory microenvironment. TNF exposure reduces overall γδ IEL migratory behavior by inducing the internalization of epithelial occludin31, yet besides IL-1547, the effect of inflammatory cytokines on γδ IEL motility remains unknown. Equally as important as elucidating the regulation of γδ IEL motility and their surveillance behaviors is determining whether inflammation leads to a dysregulation of IEL migratory patterns or if impaired γδ IEL surveillance contributes to the initiation of disease pathogenesis.
In an effort to address these questions, new tools and models have become available to study the kinetics of γδ IEL motility in recent years. Once limited to Transwell assays to study lymphocyte migration into 2D cultured epithelial monolayers, the use of enteroids has revolutionized the way we model IEL-IEC interactions ex vivo47,150. This advance comes at a particularly opportune time based on our evolving knowledge of the differences between cell migration in 2D and 3D cultures151. IEL/enteroid co-culture in 3D accurately recapitulates the dynamics of flossing behavior in vivo and is amenable to the use of pharmacological inhibitors and blocking/neutralizing antibodies. Lymphocytes are easily co-cultured with enteroids isolated from two different transgenic and/or knockout mice, and moreover, co-culturing γδ IELs with enteroids is one of the few ways to maintain the viability of these cells ex vivo. While IEL/enteroid co-cultures provide an opportunity to investigate signaling pathways or molecules involved in γδ IEL motility, migratory behavior, or crosstalk with IECs, there are two main limitations: (1) the lack of an intact LP compartment and (2) the technical challenge of adding microorganisms into the enteroid lumen to study host-microbe interactions. As a result, intravital imaging remains the gold standard for evaluating γδ T cell migratory behavior within a local microenvironment.
Intestinal lamina propria γδ T cells
Similar to the epidermis and dermis, different Vγ subsets populate the intestinal epithelial and LP compartments. Under homeostatic conditions, there are considerably fewer γδ T cells in the LP than in the epithelial compartment, and as a result, the Vγ subsets populating the LP are less well-defined. A heterogeneous population of Vγ1+, Vγ4+ and Vγ6+ cells likely enter the LP from the periphery. The composition of the LP γδ T cell compartment at steady-state is regulated in part by signals from commensal bacteria since the number of LP γδ17 cells is reduced in antibiotic-treated or gnotobiotic mice152. Whereas γδ IELs are widely considered to play a protective role against tissue injury and inflammation, LP γδ T cells have been described primarily in the context of inducing inflammation during colitis153,154 or providing a memory response to secondary infection following challenge with oral Listeria monocytogenes9,155.
The first evidence for a pathogenic role for γδ T cells in colitis was shown in Tcra-deficient mice, which develop spontaneous colitis between 16-20 weeks of age156. In these mice, the absence of αβ T cells is accompanied by an expansion of colonic LP γδ T cells157. Peripheral γδ T cells have also been shown to contribute to the development of T-cell-mediated colitis153,158. Although it was originally thought that the infiltration of γδ17 cells into the LP promoted a colitogenic CD4 Th1/Th17 effector response153,159, further investigation revealed that a subset of CD103+ α4β7hi γδ T cells from the mesenteric lymph node (MLN) were the primary contributors to disease pathogenesis154. Following transfer of inflammatory peripheral γδ T cells and naïve CD4 T cells to Tcrb- or Rag-deficient recipients, these cells expand in the blood and MLN and then traffic to the gut due their high expression of α4β7 and CCR9. Interestingly, these CD103+ α4β7hi γδ T cells were found both in the IEL and LP compartment only in colitic mice. Further investigation showed that the expansion of these IFNγ-producing, largely Vγ1+ population was not antigen-driven154.
LP γδ T cell populations also provide a protective memory against a secondary challenge with oral L. monocytogenes9,155. Primary infection with Listeria induces the expansion of a Vγ6+ cell population expressing α4β7, but not CD103, in the MLN. These Vγ6+ cells migrate to the LP and then contract to form a stable memory population with a multifunctional phenotype capable of producing IFNγ and/or IL-177. Upon re-challenge with oral Listeria, the memory Vγ6+ cells in the MLN rapidly produce IL-17 to recruit neutrophils in an effort to control and clear the infection13. Thus, depending on the context, pro-inflammatory γδ T cells are recruited from peripheral lymph nodes to the LP to elicit a robust response to enteric infection or promote mucosal inflammation. The migratory behavior of LP γδ T cells under inflammatory conditions has yet to be investigated but would likely provide useful insight regarding how these activated γδ T cells interact with other mucosal immune cells.
Conclusions
γδ T cells are ideally positioned at barrier interfaces to provide a rapid response to invading microorganisms and facilitate epithelial repair in response to injury. Moreover, the surveillance behavior of individual Vγ subsets may help maximize the potential of the cells’ programmed effector function within the given tissue architecture. In the stratified epidermis, Vγ5+ DETCs extend and retract their dendritic processes to probe neighboring keratinocytes. Although DETCs are considered to be sessile, just beneath the epidermis are highly motile IL-17 producing dermal Vγ4+ cells that provide surveillance to limit dissemination of pathogenic bacteria. In addition to responding to pathogens, tissue-resident γδ T cells also contribute to host-microbiota interactions. Vγ6+ cells migrate within the gingiva and produce IL-17 to shape the oral microbiota, whereas innate immune recognition of the intestinal microbiota promotes the flossing behavior of IFNγ-producing Vγ7+ IELs. Both DETCs and γδ IELs are in close proximity to commensal bacteria; however, γδ IELs actively migrate within the barrier and DETCs do not. Thus, the enhanced intraepithelial migratory behavior of γδ T cells in the gut may reflect a more permissive epithelial structure that is conducive to cell migration. While more careful study of γδ T cell surveillance behaviors is needed in all tissues, the current data indicate that compartmentalization of γδ T cells by effector phenotype may serve to provide optimal protection of the barrier tissue and/or influence the composition of the local microbiome. In spite of these functional similarities, increasing evidence suggests that each barrier microenvironment also uniquely shapes the resident γδ T cell population to conform with tissue-specific roles98.
Recent studies have uncovered novel γδ T cell effector functions at each barrier site and begun to elucidate how these tissue compartments select for specific Vγ clonotypes via the expression of butryophilin family members. To gain a clearer picture of γδ T cell biology at barrier interfaces, these findings should be integrated with a more detailed investigation of the following: (1) γδ T cell surveillance behaviors in different tissues to determine whether migratory patterns correlate with effector function, (2) the molecular mechanisms by which distinct Vγ subpopulations provide surveillance of various barrier surfaces, and (3) whether surveillance behaviors are dysregulated in disease. Addressing these fundamental questions would help inform the extent to which modulating γδ T cell migratory behavior could serve as additional therapeutic approach to limit the initiation or progression of inflammatory diseases that arise from the disruption of barrier integrity.
Acknowledgements
Research in the authors’ laboratory is supported by funding from the NIH (R01DK119349, R21AI143892, R21DK123488), New Jersey Health Foundation and the New Jersey Commission on Cancer Research (DCHS19CRF009). The authors would like to thank Sara Alonso and Tessa Bergsbaken for their critical reading of the manuscript. The authors declare no conflicts of interest.
References
- 1.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2(5):336–345. [DOI] [PubMed] [Google Scholar]
- 2.Hayday AC. gammadelta T Cell Update: Adaptate Orchestrators of Immune Surveillance. J Immunol. 2019;203(2):311–320. [DOI] [PubMed] [Google Scholar]
- 3.Hayday AC, Vantourout P. The Innate Biologies of Adaptive Antigen Receptors. Annu Rev Immunol. 2020;38:487–510. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen MM, Witherden DA, Havran WL. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spada FM, Grant Ethan P., Peters Peter J.. Self-Recognition of CD1 by g/d T Cells: Implications for Innate Immunity. Journal of Experimental Medicine. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–729. [DOI] [PubMed] [Google Scholar]
- 7.Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol. 2009;182(5):3047–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strid J, Roberts SJ, Filler RB, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9(2):146–154. [DOI] [PubMed] [Google Scholar]
- 9.Sheridan BS, Romagnoli PA, Pham QM, et al. gammadelta T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 2013;39(1):184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322(6082):836–840. [DOI] [PubMed] [Google Scholar]
- 11.Hayday A, Gibbons D. Brokering the peace: the origin of intestinal T cells. Mucosal Immunol. 2008;1(3):172–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naito T, Shiohara T, Hibi T, Suematsu M, Ishikawa H. ROR gamma t is dispensable for the development of intestinal mucosal T cells. Mucosal Immunol. 2008;1(3):198–207. [DOI] [PubMed] [Google Scholar]
- 13.Munoz-Ruiz M, Nital Sumaria, Pennington Daniel J, Silva-Santos Bruno. Thymic Determinants of γδ T Cell Differentiation. Trends in Immunology. 2017. [DOI] [PubMed] [Google Scholar]
- 14.Jensen KDC, Xiaoqin Su, Sunny Shin, et al. Thymic Selection Determines Gammadelta T Cell Effector Fate: Antigen-Naive Cells Make interleukin-17 and Antigen-Experienced Cells Make Interferon Gamma. Immunity. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papotto PH, Ribot JC, Silva-Santos B. IL-17(+) gammadelta T cells as kick-starters of inflammation. Nat Immunol. 2017;18(6):604–611. [DOI] [PubMed] [Google Scholar]
- 16.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11(7):445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida S, Mohamed Rania Hassan, Kajikawa Mizuho, et al. Involvement of an NKG2D Ligand H60c in Epidermal Dendritic T Cell-Mediated Wound Repair. The Journal of Immunology. 2012. [DOI] [PubMed] [Google Scholar]
- 18.Sharp LL, Jameson Julie M, Cauvi Gabrielle, Havran Wendy L. Dendritic Epidermal T Cells Regulate Skin Homeostasis Through Local Production of Insulin-Like Growth Factor 1. Nature Immunology. 2005. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 2002;99(22):14338–14343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jameson JM, Sharp Leslie L, Witherden Deborah A, Havran Wendy L. Regulation of Skin Cell Homeostasis by Gamma Delta T Cells. Frontiers in Bioscience. 2004. [DOI] [PubMed] [Google Scholar]
- 21.Itohara S, Mombaerts P, Lafaille J, et al. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993;72(3):337–348. [DOI] [PubMed] [Google Scholar]
- 22.Roberts SJ, Smith AL, West AB, et al. T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci U S A. 1996;93(21):11774–11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T, Yunfei Gao, Eileen Scully, et al. Gamma Delta T Cells Facilitate Adaptive Immunity Against West Nile Virus Infection in Mice. The Journal of Immunology. 2006. [DOI] [PubMed] [Google Scholar]
- 24.Maeda Y, Reddy P, Lowler KP, Liu C, Bishop DK, Ferrara JL. Critical role of host gammadelta T cells in experimental acute graft-versus-host disease. Blood. 2005;106(2):749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhl AA, Pawlowski NN, Grollich K, Loddenkemper C, Zeitz M, Hoffmann JC. Aggravation of intestinal inflammation by depletion/deficiency of gammadelta T cells in different types of IBD animal models. J Leukoc Biol. 2007;81(1):168–175. [DOI] [PubMed] [Google Scholar]
- 26.Koenecke C, Chennupati V, Schmitz S, Malissen B, Forster R, Prinz I. In vivo application of mAb directed against the gammadelta TCR does not deplete but generates “invisible” gammadelta T cells. Eur J Immunol. 2009;39(2):372–379. [DOI] [PubMed] [Google Scholar]
- 27.Sandrock I, Reinhardt A, Ravens S, et al. Genetic models reveal origin, persistence and non-redundant functions of IL-17-producing gammadelta T cells. J Exp Med. 2018;215(12):3006–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186(11):6091–6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumaria N, Roediger B, Ng LG, et al. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med. 2011;208(3):505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prinz I, Sansoni A, Kissenpfennig A, Ardouin L, Malissen M, Malissen B. Visualization of the earliest steps of gammadelta T cell development in the adult thymus. Nat Immunol. 2006;7(9):995–1003. [DOI] [PubMed] [Google Scholar]
- 31.Edelblum KL, Shen L, Weber CR, et al. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc Natl Acad Sci U S A. 2012;109(18):7097–7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilharm A, Tabib Y, Nassar M, et al. Mutual interplay between IL-17-producing gammadeltaT cells and microbiota orchestrates oral mucosal homeostasis. Proc Natl Acad Sci U S A. 2019;116(7):2652–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaylo A, Schrock DC, Fernandes NR, Fowell DJ. T Cell Interstitial Migration: Motility Cues from the Inflamed Tissue for Micro- and Macro-Positioning. Front Immunol. 2016;7:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weninger W, Biro M, Jain R. Leukocyte migration in the interstitial space of non-lymphoid organs. Nat Rev Immunol. 2014;14(4):232–246. [DOI] [PubMed] [Google Scholar]
- 35.Mrass P, Petravic J, Davenport MP, Weninger W. Cell-autonomous and environmental contributions to the interstitial migration of T cells. Semin Immunopathol. 2010;32(3):257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krummel MF, Bartumeus F, Gerard A. T cell migration, search strategies and mechanisms. Nat Rev Immunol. 2016;16(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lammermann T, Bader BL, Monkley SJ, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453(7191):51–55. [DOI] [PubMed] [Google Scholar]
- 38.Fricke GM, Letendre KA, Moses ME, Cannon JL. Persistence and Adaptation in Immunity: T Cells Balance the Extent and Thoroughness of Search. PLoS Comput Biol. 2016;12(3):e1004818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cahalan MD, Parker I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu Rev Immunol. 2008;26:585–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris TH, Banigan EJ, Christian DA, et al. Generalized Levy walks and the role of chemokines in migration of effector CD8+ T cells. Nature. 2012;486(7404):545–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mrass P, Takano H, Ng LG, et al. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med. 2006;203(12):2749–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Curr Opin Cell Biol. 2011;23(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prakash MD, Munoz MA, Jain R, et al. Granzyme B promotes cytotoxic lymphocyte transmigration via basement membrane remodeling. Immunity. 2014;41(6):960–972. [DOI] [PubMed] [Google Scholar]
- 44.Shires J, Theodoridis E, Hayday AC. Biological insights into TCRgammadelta+ and TCRalphabeta+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity. 2001;15(3):419–434. [DOI] [PubMed] [Google Scholar]
- 45.Sorokin L The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10(10):712–723. [DOI] [PubMed] [Google Scholar]
- 46.Barzilai S, Yadav SK, Morrell S, et al. Leukocytes Breach Endothelial Barriers by Insertion of Nuclear Lobes and Disassembly of Endothelial Actin Filaments. Cell Rep. 2017;18(3):685–699. [DOI] [PubMed] [Google Scholar]
- 47.Hu MD, Ethridge AD, Lipstein R, et al. Epithelial IL-15 Is a Critical Regulator of gammadelta Intraepithelial Lymphocyte Motility within the Intestinal Mucosa. J Immunol. 2018;201(2):747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Sumida H, Cyster JG. GPR18 is required for a normal CD8alphaalpha intestinal intraepithelial lymphocyte compartment. J Exp Med. 2014;211(12):2351–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai Y, Shen X, Ding C, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35(4):596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335(6189):443–445. [DOI] [PubMed] [Google Scholar]
- 51.Boyden LM, Lewis JM, Barbee SD, et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat Genet. 2008;40(5):656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong N, Kang C, Raulet DH. Positive selection of dendritic epidermal gammadelta T cell precursors in the fetal thymus determines expression of skin-homing receptors. Immunity. 2004;21(1):121–131. [DOI] [PubMed] [Google Scholar]
- 53.Morales J, Homey B, Vicari AP, et al. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci U S A. 1999;96(25):14470–14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin Y, Xia M, Sun A, Saylor CM, Xiong N. CCR10 is important for the development of skin-specific gammadeltaT cells by regulating their migration and location. J Immunol. 2010;185(10):5723–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang X, Campbell JJ, Kupper TS. Embryonic trafficking of gammadelta T cells to skin is dependent on E/P selectin ligands and CCR4. Proc Natl Acad Sci U S A. 2010;107(16):7443–7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farzan M, Choe H, Martin K, et al. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186(3):405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng HK, Unutmaz D, KewalRamani VN, Littman DR. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388(6639):296–300. [DOI] [PubMed] [Google Scholar]
- 58.Lahl K, Sweere J, Pan J, Butcher E. Orphan chemoattractant receptor GPR15 mediates dendritic epidermal T-cell recruitment to the skin. Eur J Immunol. 2014;44(9):2577–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura K, White AJ, Parnell SM, et al. Differential requirement for CCR4 in the maintenance but not establishment of the invariant Vgamma5(+) dendritic epidermal T-cell pool. PLoS One. 2013;8(9):e74019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schon MP, Schon M, Parker CM, Williams IR. Dendritic epidermal T cells (DETC) are diminished in integrin alphaE(CD103)-deficient mice. J Invest Dermatol. 2002;119(1):190–193. [DOI] [PubMed] [Google Scholar]
- 61.Chodaczek G, Papanna V, Zal MA, Zal T. Body-barrier surveillance by epidermal gammadelta TCRs. Nat Immunol. 2012;13(3):272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jameson J, Ugarte K, Chen N, et al. A role for skin gammadelta T cells in wound repair. Science. 2002;296(5568):747–749. [DOI] [PubMed] [Google Scholar]
- 63.Schlickum S, Sennefelder H, Friedrich M, et al. Integrin alpha E(CD103)beta 7 influences cellular shape and motility in a ligand-dependent fashion. Blood. 2008;112(3):619–625. [DOI] [PubMed] [Google Scholar]
- 64.Witherden DA, Watanabe M, Garijo O, et al. The CD100 Receptor Interacts with Its Plexin B2 Ligand to Regulate Epidermal gammadelta T Cell Function. Immunity. 2012;37(2):314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saito T, Yano M, Ohki Y, Tomura M, Nakano N. Occludin Expression in Epidermal gammadelta T Cells in Response to Epidermal Stress Causes Them To Migrate into Draining Lymph Nodes. J Immunol. 2017;199(1):62–71. [DOI] [PubMed] [Google Scholar]
- 66.Witherden DA, Verdino P, Rieder SE, et al. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science. 2010;329(5996):1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McKenzie DR, Kara EE, Bastow CR, et al. IL-17-producing gammadelta T cells switch migratory patterns between resting and activated states. Nat Commun. 2017;8:15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramirez-Valle F, Gray EE, Cyster JG. Inflammation induces dermal Vgamma4+ gammadeltaT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc Natl Acad Sci U S A. 2015;112(26):8046–8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laidlaw BJ, Gray EE, Zhang Y, Ramirez-Valle F, Cyster JG. Sphingosine-1-phosphate receptor 2 restrains egress of gammadelta T cells from the skin. J Exp Med. 2019;216(7):1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Debes GF, Arnold CN, Young AJ, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6(9):889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14(12):1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoytema van Konijnenburg DP, Reis BS, Pedicord VA, Farache J, Victora GD, Mucida D. Intestinal Epithelial and Intraepithelial T Cell Crosstalk Mediates a Dynamic Response to Infection. Cell. 2017;171(4):783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wands JM, Roark CL, Aydintug MK, et al. Distribution and leukocyte contacts of gammadelta T cells in the lung. J Leukoc Biol. 2005;78(5):1086–1096. [DOI] [PubMed] [Google Scholar]
- 74.Cho JS, Pietras EM, Garcia NC, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120(5):1762–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y, Wang Y, Zhou L, et al. Vgamma4 T Cells Inhibit the Pro-healing Functions of Dendritic Epidermal T Cells to Delay Skin Wound Closure Through IL-17A. Front Immunol. 2018;9:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mabuchi T, Singh TP, Takekoshi T, et al. CCR6 is required for epidermal trafficking of gammadelta-T cells in an IL-23-induced model of psoriasiform dermatitis. J Invest Dermatol. 2013;133(1):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krishnan S, Prise IE, Wemyss K, et al. Amphiregulin-producing gammadelta T cells are vital for safeguarding oral barrier immune homeostasis. Proc Natl Acad Sci U S A. 2018;115(42):10738–10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sim GK, Rajaserkar R, Dessing M, Augustin A. Homing and in situ differentiation of resident pulmonary lymphocytes. Int Immunol. 1994;6(9):1287–1295. [DOI] [PubMed] [Google Scholar]
- 79.Born WK, Lahn M, Takeda K, Kanehiro A, O’Brien RL, Gelfand EW. Role of gammadelta T cells in protecting normal airway function. Respir Res. 2000;1(3):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lahn M, Kanehiro A, Takeda K, et al. Negative regulation of airway responsiveness that is dependent on gammadelta T cells and independent of alphabeta T cells. Nat Med. 1999;5(10):1150–1156. [DOI] [PubMed] [Google Scholar]
- 81.Hahn YS, Taube C, Jin N, et al. V gamma 4+ gamma delta T cells regulate airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J Immunol. 2003;171(6):3170–3178. [DOI] [PubMed] [Google Scholar]
- 82.Anderson JM, Van Itallie CM. Tight junctions: closing in on the seal. Curr Biol. 1999;9(24):R922–924. [DOI] [PubMed] [Google Scholar]
- 83.Carding SR, Allan W, Kyes S, Hayday A, Bottomly K, Doherty PC. Late dominance of the inflammatory process in murine influenza by gamma/delta + T cells. J Exp Med. 1990;172(4):1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng P, Liu T, Zhou WY, et al. Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol. 2012;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakasone C, Yamamoto N, Nakamatsu M, et al. Accumulation of gamma/delta T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes Infect. 2007;9(3):251–258. [DOI] [PubMed] [Google Scholar]
- 86.Simonian PL, Roark CL, Born WK, O’Brien RL, Fontenot AP. Gammadelta T cells and Th17 cytokines in hypersensitivity pneumonitis and lung fibrosis. Transl Res. 2009;154(5):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murakami T, Hatano S, Yamada H, Iwakura Y, Yoshikai Y. Two Types of Interleukin 17A-Producing gammadelta T Cells in Protection Against Pulmonary Infection With Klebsiella pneumoniae. J Infect Dis. 2016;214(11):1752–1761. [DOI] [PubMed] [Google Scholar]
- 88.Uezu K, Kawakami K, Miyagi K, et al. Accumulation of gammadelta T cells in the lungs and their regulatory roles in Th1 response and host defense against pulmonary infection with Cryptococcus neoformans. J Immunol. 2004;172(12):7629–7634. [DOI] [PubMed] [Google Scholar]
- 89.Dodd J, Riffault S, Kodituwakku JS, Hayday AC, Openshaw PJ. Pulmonary V gamma 4+ gamma delta T cells have proinflammatory and antiviral effects in viral lung disease. J Immunol. 2009;182(2):1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moore TA, Moore BB, Newstead MW, Standiford TJ. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol. 2000;165(5):2643–2650. [DOI] [PubMed] [Google Scholar]
- 91.Tam S, King DP, Beaman BL. Increase of gammadelta T lymphocytes in murine lungs occurs during recovery from pulmonary infection by Nocardia asteroides. Infect Immun. 2001;69(10):6165–6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stolberg VR, Chiu BC, Martin BE, Shah SA, Sandor M, Chensue SW. Cysteine-cysteinyl chemokine receptor 6 mediates invariant natural killer T cell airway recruitment and innate stage resistance during mycobacterial infection. J Innate Immun. 2011;3(1):99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stolberg VR, Chiu BC, Schmidt BM, Kunkel SL, Sandor M, Chensue SW. CC chemokine receptor 4 contributes to innate NK and chronic stage T helper cell recall responses during Mycobacterium bovis infection. Am J Pathol. 2011;178(1):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kirby AC, Newton DJ, Carding SR, Kaye PM. Evidence for the involvement of lung-specific gammadelta T cell subsets in local responses to Streptococcus pneumoniae infection. Eur J Immunol. 2007;37(12):3404–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hahn YS, Taube C, Jin N, et al. Different potentials of gamma delta T cell subsets in regulating airway responsiveness: V gamma 1+ cells, but not V gamma 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol. 2004;172(5):2894–2902. [DOI] [PubMed] [Google Scholar]
- 96.Simonian PL, Roark CL, Diaz del Valle F, et al. Regulatory role of gammadelta T cells in the recruitment of CD4+ and CD8+ T cells to lung and subsequent pulmonary fibrosis. J Immunol. 2006;177(7):4436–4443. [DOI] [PubMed] [Google Scholar]
- 97.Jin C, Lagoudas GK, Zhao C, et al. Commensal Microbiota Promote Lung Cancer Development via gammadelta T Cells. Cell. 2019;176(5):998–1013 e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Monin L, Ushakov DS, Arnesen H, et al. . γδ T cells compose a developmentally regulated intrauterine population and protect against vaginal candidiasis. Mucosal Immunology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Itohara S, Farr AG, Lafaille JJ, et al. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343(6260):754–757. [DOI] [PubMed] [Google Scholar]
- 100.Goodman T, Lefrancois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988;333(6176):855–858. [DOI] [PubMed] [Google Scholar]
- 101.Boll G, Rudolphi A, Spiess S, Reimann J. Regional specialization of intraepithelial T cells in the murine small and large intestine. Scand J Immunol. 1995;41(2):103–113. [DOI] [PubMed] [Google Scholar]
- 102.Bonneville M, Janeway CA Jr., Ito K, et al. Intestinal intraepithelial lymphocytes are a distinct set of gamma delta T cells. Nature. 1988;336(6198):479–481. [DOI] [PubMed] [Google Scholar]
- 103.Chennupati V, Worbs T, Liu X, et al. Intra- and intercompartmental movement of gammadelta T cells: intestinal intraepithelial and peripheral gammadelta T cells represent exclusive nonoverlapping populations with distinct migration characteristics. J Immunol. 2010;185(9):5160–5168. [DOI] [PubMed] [Google Scholar]
- 104.Pereira P, Gerber D, Huang SY, Tonegawa S. Ontogenic development and tissue distribution of V gamma 1-expressing gamma/delta T lymphocytes in normal mice. J Exp Med. 1995;182(6):1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suzuki H, Jeong KI, Okutani T, Doi K. Regional variations in the number and subsets of intraepithelial lymphocytes in the mouse small intestine. Comp Med. 2000;50(1):39–42. [PubMed] [Google Scholar]
- 106.Komano H, Fujiura Y, Kawaguchi M, et al. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 1995;92(13):6147–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Swamy M, Abeler-Dorner L, Chettle J, et al. Intestinal intraepithelial lymphocyte activation promotes innate antiviral resistance. Nat Commun. 2015;6:7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ismail AS, Severson KM, Vaishnava S, et al. {gamma}{delta} intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci U S A. 2011;108(21):8743–8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dalton JE, Cruickshank SM, Egan CE, et al. Intraepithelial gammadelta+ lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterology. 2006;131(3):818–829. [DOI] [PubMed] [Google Scholar]
- 110.Hu MD, Jia L, Edelblum KL. Policing the intestinal epithelial barrier: Innate immune functions of intraepithelial lymphocytes. Curr Pathobiol Rep. 2018;6(1):35–46. [PMC free article] [PubMed] [Google Scholar]
- 111.Di Marco Barros R, Roberts NA, Dart RJ, et al. Epithelia Use Butyrophilin-like Molecules to Shape Organ-Specific gammadelta T Cell Compartments. Cell. 2016;167(1):203–218 e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rocha B The extrathymic T-cell differentiation in the murine gut. Immunol Rev. 2007;215:166–177. [DOI] [PubMed] [Google Scholar]
- 113.Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168(6):2811–2819. [DOI] [PubMed] [Google Scholar]
- 114.Wurbel MA, Malissen M, Guy-Grand D, et al. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood. 2001;98(9):2626–2632. [DOI] [PubMed] [Google Scholar]
- 115.Kunkel EJ, Campbell DJ, Butcher EC. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. 2003;10(3-4):313–323. [DOI] [PubMed] [Google Scholar]
- 116.Dwinell MB, Lugering N, Eckmann L, Kagnoff MF. Regulated production of interferon-inducible T-cell chemoattractants by human intestinal epithelial cells. Gastroenterology. 2001;120(1):49–59. [DOI] [PubMed] [Google Scholar]
- 117.Shibahara T, Wilcox JN, Couse T, Madara JL. Characterization of epithelial chemoattractants for human intestinal intraepithelial lymphocytes. Gastroenterology. 2001;120(1):60–70. [DOI] [PubMed] [Google Scholar]
- 118.Annunziato F, Cosmi L, Liotta F, et al. CXCR3 and alphaEbeta7 integrin identify a subset of CD8+ mature thymocytes that share phenotypic and functional properties with CD8+ gut intraepithelial lymphocytes. Gut. 2006;55(7):961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wagner N, Lohler J, Kunkel EJ, et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382(6589):366–370. [DOI] [PubMed] [Google Scholar]
- 120.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85(7):997–1008. [DOI] [PubMed] [Google Scholar]
- 121.Schon MP, Arya A, Murphy EA, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162(11):6641–6649. [PubMed] [Google Scholar]
- 122.Karecla PI, Bowden SJ, Green SJ, Kilshaw PJ. Recognition of E-cadherin on epithelial cells by the mucosal T cell integrin alpha M290 beta 7 (alpha E beta 7). Eur J Immunol. 1995;25(3):852–856. [DOI] [PubMed] [Google Scholar]
- 123.Cepek KL, Shaw SK, Parker CM, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372(6502):190–193. [DOI] [PubMed] [Google Scholar]
- 124.Chao CC, Sandor M, Dailey MO. Expression and regulation of adhesion molecules by gamma delta T cells from lymphoid tissues and intestinal epithelium. Eur J Immunol. 1994;24(12):3180–3187. [DOI] [PubMed] [Google Scholar]
- 125.Huleatt JW, Lefrancois L. Beta2 integrins and ICAM-1 are involved in establishment of the intestinal mucosal T cell compartment. Immunity. 1996;5(3):263–273. [DOI] [PubMed] [Google Scholar]
- 126.Meharra EJ, Schon M, Hassett D, Parker C, Havran W, Gardner H. Reduced gut intraepithelial lymphocytes in VLA1 null mice. Cell Immunol. 2000;201(1):1–5. [DOI] [PubMed] [Google Scholar]
- 127.Becker AM, Callahan DJ, Richner JM, et al. GPR18 Controls Reconstitution of Mouse Small Intestine Intraepithelial Lymphocytes following Bone Marrow Transplantation. PLoS One. 2015;10(7):e0133854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sumida H, Lu E, Chen H, Yang Q, Mackie K, Cyster JG. GPR55 regulates intraepithelial lymphocyte migration dynamics and susceptibility to intestinal damage. Sci Immunol. 2017;2(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mackay LK, Braun A, Macleod BL, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol. 2015;194(5):2059–2063. [DOI] [PubMed] [Google Scholar]
- 130.Fahrer AM, Konigshofer Y, Kerr EM, et al. Attributes of gammadelta intraepithelial lymphocytes as suggested by their transcriptional profile. Proc Natl Acad Sci U S A. 2001;98(18):10261–10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Z, Zhang C, Zhou Z, Zhang J, Zhang J, Tian Z. Small intestinal intraepithelial lymphocytes expressing CD8 and T cell receptor gammadelta are involved in bacterial clearance during Salmonella enterica serovar Typhimurium infection. Infect Immun. 2012;80(2):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hirose K, Suzuki H, Nishimura H, et al. Interleukin-15 may be responsible for early activation of intestinal intraepithelial lymphocytes after oral infection with Listeria monocytogenes in rats. Infect Immun. 1998;66(12):5677–5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li Y, Innocentin S, Withers DR, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629–640. [DOI] [PubMed] [Google Scholar]
- 134.Ma LJ, Acero LF, Zal T, Schluns KS. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8alphaalpha IELs. J Immunol. 2009;183(2):1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lodolce JP, Boone DL, Chai S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9(5):669–676. [DOI] [PubMed] [Google Scholar]
- 136.Laky K, Lefrancois L, Lingenheld EG, et al. Enterocyte expression of interleukin 7 induces development of gammadelta T cells and Peyer’s patches. J Exp Med. 2000;191(9):1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maki K, Sunaga S, Komagata Y, et al. Interleukin 7 receptor-deficient mice lack gammadelta T cells. Proc Natl Acad Sci U S A. 1996;93(14):7172–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lebrero-Fernandez C, Bergstrom JH, Pelaseyed T, Bas-Forsberg A. Murine Butyrophilin-Like 1 and Btnl6 Form Heteromeric Complexes in Small Intestinal Epithelial Cells and Promote Proliferation of Local T Lymphocytes. Front Immunol. 2016;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jabri B, Abadie V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat Rev Immunol. 2015;15(12):771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Marsh MN. Studies of intestinal lymphoid tissue. II. Aspects of proliferation and migration of epithelial lymphocytes in the small intestine of mice. Gut. 1975;16(9):674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266(5188):1253–1255. [DOI] [PubMed] [Google Scholar]
- 142.Inagaki-Ohara K, Dewi FN, Hisaeda H, et al. Intestinal intraepithelial lymphocytes sustain the epithelial barrier function against Eimeria vermiformis infection. Infect Immun. 2006;74(9):5292–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the ‘epimmunome’. Nat Immunol. 2010;11(8):656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Edelblum KL, Singh G, Odenwald MA, et al. gammadelta Intraepithelial Lymphocyte Migration Limits Transepithelial Pathogen Invasion and Systemic Disease in Mice. Gastroenterology. 2015;148(7):1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Malinarich FH, Grabski E, Worbs T, et al. Constant TCR triggering suggests that the TCR expressed on intestinal intraepithelial gammadelta T cells is functional in vivo. Eur J Immunol. 2010;40(12):3378–3388. [DOI] [PubMed] [Google Scholar]
- 146.Allavena P, Giardina G, Bianchi G, Mantovani A. IL-15 is chemotactic for natural killer cells and stimulates their adhesion to vascular endothelium. J Leukoc Biol. 1997;61(6):729–735. [DOI] [PubMed] [Google Scholar]
- 147.Wilkinson PC, Liew FY. Chemoattraction of human blood T lymphocytes by interleukin-15. J Exp Med. 1995;181(3):1255–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Suzuki H, Duncan GS, Takimoto H, Mak TW. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor beta chain. J Exp Med. 1997;185(3):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brazil JC, Parkos CA. Pathobiology of neutrophil-epithelial interactions. Immunol Rev. 2016;273(1):94–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nozaki K, Mochizuki W, Matsumoto Y, et al. Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J Gastroenterol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Doyle AD, Petrie RJ, Kutys ML, Yamada KM. Dimensions in cell migration. Curr Opin Cell Biol. 2013;25(5):642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Duan J, Chung H, Troy E, Kasper DL. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe. 2010;7(2):140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Do JS, Visperas A, Dong C, Baldwin WM 3rd, Min B. Cutting Edge: Generation of Colitogenic Th17 CD4 T Cells Is Enhanced by IL-17+ {gamma}{delta} T Cells. J Immunol. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Do JS, Kim S, Keslar K, et al. gammadelta T Cells Coexpressing Gut Homing alpha4beta7 and alphaE Integrins Define a Novel Subset Promoting Intestinal Inflammation. J Immunol. 2017;198(2):908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Romagnoli PA, Sheridan Brian S., Pham Quynh-Mai, Lefrançois Leo, and Khanna Kamal M.. IL-17A–producing resident memory γδ T cells orchestrate the innate immune response to secondary oral Listeria monocytogenes infection. PNAS. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75(2):274–282. [DOI] [PubMed] [Google Scholar]
- 157.Kawaguchi-Miyashita M, Shimada S, Kurosu H, et al. An accessory role of TCRgammadelta (+) cells in the exacerbation of inflammatory bowel disease in TCRalpha mutant mice. Eur J Immunol. 2001;31(4):980–988. [DOI] [PubMed] [Google Scholar]
- 158.Do JS, Visperas A, Freeman ML, Iwakura Y, Oukka M, Min B. Colitogenic effector T cells: roles of gut-homing integrin, gut antigen specificity and gammadelta T cells. Immunol Cell Biol. 2014;92(1):90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yurchenko E, Levings MK, Piccirillo CA. CD4+ Foxp3+ regulatory T cells suppress gammadelta T-cell effector functions in a model of T-cell-induced mucosal inflammation. Eur J Immunol. 2011;41(12):3455–3466. [DOI] [PubMed] [Google Scholar]


