Abstract
Phosphylation of the pivotal enzyme acetylcholinesterase (AChE) by nerve agents (NAs) leads to irreversible inhibition of the enzyme and accumulation of neurotransmitter acetylcholine, which induces cholinergic crisis, that is, overstimulation of muscarinic and nicotinic membrane receptors in the central and peripheral nervous system. In severe cases, subsequent desensitisation of the receptors results in hypoxia, vasodepression, and respiratory arrest, followed by death. Prompt action is therefore critical to improve the chances of victim’s survival and recovery. Standard therapy of NA poisoning generally involves administration of anticholinergic atropine and an oxime reactivator of phosphylated AChE. Anticholinesterase compounds or NA bioscavengers can also be applied to preserve native AChE from inhibition. With this review of 70 years of research we aim to present current and potential approaches to counteracting NA poisoning.
Key words: bioscavenger, cholinesterases, cyclosarin, Novichoks, organophosphate, sarin, tabun, VX
Abstract
Fosfilacijom esencijalnog enzima acetilkolinesteraze (AChE) živčanim bojnim otrovom, enzim postaje ireverzibilno inhibiran, što dovodi do nakupljanja neurotransmitera acetilkolina i kolinergičke krize zbog prekomjerne stimulacije muskarinskih i nikotinskih membranskih receptora u središnjem i perifernom živčanom sustavu. U teškim slučajevima desenzibilizacija receptora rezultira hipoksijom, nesvjesticom i zastojem disanja, nakon čega slijedi smrt. Stoga je brzo djelovanje presudno za preživljavanje osobe izložene živčanom bojnom otrovu. Standardna terapija u slučaju otrovanja uključuje antikolinergik atropin i oksimski reaktivator fosfilirane AChE. Kako bi se očuvala aktivnost nativne AChE u slučaju izloženosti živčanom bojnom otrovu, istražuju se i drugačiji pristupi terapiji, kao što su spojevi koji kratkotrajno i reverzibilno inhibiraju AChE te egzogeni enzimi koji djeluju kao biološka čistila živčanih bojnih otrova. U ovom preglednom radu cilj nam je predstaviti trenutačne i potencijalne pristupe u terapiji i detoksikaciji živčanih bojnih otrova.
Ključne riječi: biološka čistila, ciklosarin, kolinesteraze, Novichok, organofosfati, sarin, tabun, VX
Organophosphates (OPs) are ester, amide, or thiol derivatives of phosphorous, phosphonic, or phosphinic acids. They occur in important biomolecules like DNA and RNA, some cofactors and coenzymes, phosphoproteins, and phospholipids, but can also be synthesised. The first OP compounds were synthesised in the early 19th century (1, 2). Since then, OPs were developed on a large scale and have been used as industrial catalysts, emulsifiers, oil additives, polymer resin modifiers, plasticisers, solvents, and flame retardants. The most important benefit of OPs was the development and production of pesticides, as they were found to have a lethal effect on insects. Although OP pesticides are mostly banned now, they account for more than three million accidental or deliberate cases of poisoning a year worldwide (3, 4).
Shortly before World War II (WWII), OPs were developed as chemical warfare nerve agents (NAs) and still pose a great threat in terrorist attacks, as recently witnessed in Syria, Malaysia, and the UK (5, 6). NAs have fatal effects in the acute phase of poisoning and can cause considerable long-term complications in survivors due to irreversible inhibition of a pivotal enzyme acetylcholinesterase (AChE). Exposure to NAs leads to overstimulation of the cholinergic pathway and consequently to the desensitising of the nicotinic and muscarinic cholinergic receptors, which is manifested with severe symptoms of poisoning and can even lead to death (7, 8). Current standard treatment with atropine and an oxime still leaves much to be desired, as it does not warrant recovery. This review looks into options for improvement through recent developments in NA poisoning treatment and prophylaxis/pretreatment.
Nerve agents
History
The first known AChE inhibitor, tetraetylpyrophosphate (TEPP), was discovered by a French chemist Philippe de Clermont in 1854 (1). Yet, neither the toxicity nor the mode of action of TEPP was known at the time. It was not until 1932, when Willy Lange synthesised some compounds containing the P-F bound, that the toxic effects of exposure to the vapours of OP compounds were observed (9). Although Lange seemed to be aware of the potential pesticidal activity of OP compounds, he never pursued it. A German chemist Gerhard Schrader later developed a new simple method to synthesise TEPP, which then became the first commercial OP insecticide. In 1936, while working for a chemical corporation IG Farben in search for new potential insecticides, Schrader synthesised (R/S)-ethyl N,N-dimethylphosphoramido cyanidate (tabun), for which he found to be too toxic for application in agriculture (10). IG Farben reported tabun discovery to the German Ministry of War, which lead to a chemical weapons programme intended to develop highly toxic and volatile NAs for military purposes. This is how sarin (GB), soman (GD), and cyclosarin (GF) were developed besides tabun (GA) between 1936 and 1949 (11). Fortunately, this so-called “G” (Germany) series of NAs was never used in WWII.
NAs were also synthesised during the Cold War by both the Western and Eastern bloc. The United Kingdom synthesised VX, the representative of “V” agents, in homage to the victory of the Allied forces in WWII, and the United States developed it further for military purposes (12). The Soviet Union generated compounds similar to the V agents, such as VR, also known as Russian VX, and a new generation of compounds known as “intermediate volatility agents” (IVA). One variant of IVA, known as GV, was also developed in the United States (13, 14).
The end of the Cold War saw the development of yet another class of NAs in the Soviet Union, called Novichok agents. In the early 1990s, a Soviet defector Vil Mirzayanov exposed a chemical weapons programme known as Foliant, and revealed the chemical structures of several Novichok series compounds: A-230, A232, A-234, A240 (Novichok-5), and A-262 (Novichok-7) (15, 16). Novichok agents were developed as binary agents and are more potent than VX, the most hazardous among NAs (15). The main advantage of binary agents is the reduced risk of accidental dispersion and poisoning, since chemical precursors are separated and less toxic than the final product (14, 17). The structures of the above mentioned NAs are presented in Figure 1.
Figure 1.
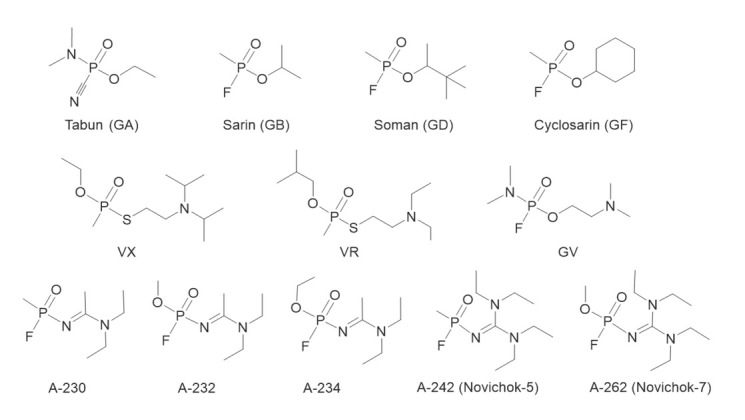
Representatives of the G-series (tabun, sarin, soman, cyclosarin), V-series (VX, VR), IVA agents (GV), and Novichok series (16) of nerve agents (A-230, A-232, A234, A242, A-262)
Although NAs, tabun in particular, were produced and stockpiled by the German army in WWII, tabun and sarin were first used by the Iraq military against Iranian troops and civilians in the Iran-Iraq War of 1983–1988 (11, 18). Considering their relatively undemanding synthesis, NAs were also used in terrorist attacks, such as the one in Matsumoto, Japan in 1994, and six months later in the Tokyo subway, when thousands of people were poisoned and 19 died (11). In 1995, many countries, including the USA, signed the Chemical Weapons Convention, agreeing to destroy their stockpiled chemical warfare agents, including NAs, by 2012. The Convention entered into force in 1997, and 193 states have signed it since then (19).
Despite vigorous and worldwide control of chemical warfare agent threat by the Organization for Prohibition of Chemical Weapons (OPCW), between 2013 and 2017, sarin was used in a series of chemical attacks in the Syrian civil war in Damascus and the surrounding East Ghouta region, Aleppo and the nearby Idlib region, and possibly the Hama region (5, 17, 20). VX was used for the assassination of Kim Jong Nam in Malaysia in 2017, and Novichoks in an attempted assassination of the former Russian spy Sergey Skripak and his daughter in Salisbury UK, 2018 (6, 16). More recently (August 2020), Novichoks were used on a Russian politician A. Navalny, as confirmed by several Europian research laboratories and OPCW (21, 22). Apparently, the threat posed by NAs is still very real.
Chemistry
The NAs of the G-series are volatile liquids that evaporate spontaneously at room temperature, unlike VX, which is oily and evaporates very slowly (8, 11, 12). VX is therefore more persistent and will contaminate the environment longer than the G agents. Moreover, VX is lipophilic and will more easily penetrate the skin than the hydrophilic G agents, which are mostly inhaled and penetrate the lungs (8, 23).
NAs are mainly odourless and colourless but can appear darker if they contain impurities. Their toxicity is expressed by the median lethal dose (LD50) or median lethal concentration and time (LCt50). The first is used for the percutaneous route of exposure and the second for exposure through inhalation. The most toxic among the G agents are cyclosarin (LD50 30 mg/70 kg man) and soman (LD50 350 mg/70 kg man; LCt50 25–70 mg/min/m3) (8). However, of all NAs the most toxic is VX, regardless of exposure route (LD50 10 mg/70 kg man; LCt50 5–50 mg/min/m3) (8).
All NAs are chiral compounds. Tabun, sarin, cyclosarin, and VX have one chiral centre at phosphorus atom and therefore two isomers [P(+) and P(−)], while soman has an additional stereocentre at a carbon atom of the pinacolyl group, so it has two diastereoisomers, that is, four isomers [C(+)P(+); C(+)P(−) and C(−)P(−); C(−)P(+)] (24, 25).
Cholinesterases
NAs and other OP compounds interact with serine esterases: AChE, butyrylcholinesterase (BChE), neuropathy target esterase, carboxylesterase, trypsin, chymotrypsin, and phosphorus triester hydrolases (aryldialkylphosphatases, paraoxonases, and diisopropyl fluorophosphatase) (11, 26). Being the structural analogues of the transition state in acetylcholine (ACh) hydrolysis, NAs act as potent irreversible inhibitors of serine esterases and are the substrates of phosphorus triester hydrolases (26).
This irreversible inhibition of AChE is what makes NAs so toxic. The physiological function of AChE is to break down (metabolise) the neurotransmitter ACh in the central and peripheral nervous system (CNS and PNS, respectively). ACh binds to and activates nicotinic receptors (a family of voltage-gated ion channels which mediate the faster, ionotropic component of cholinergic signalling) and muscarinic receptors (a family of G protein-coupled receptors which mediate the slower, metabotropic component of cholinergic signalling). The hydrolysis of ACh is a two-step process (Figure 2A). In the acylation step, the acetyl group of a substrate (ACh) is cleaved from the choline moiety to form a covalent bond to a serine residue of AChE. In the deacylation step, the acetyl group is hydrolysed from the serine residue. High rate of ACh hydrolysis, limited almost only by its diffusion to the synaptic cleft, singles out AChE as one of the most efficient enzymes known today (27). Both ChEs (AChE and BChE) belong to the family of serine hydrolases and to the superfamily of the α/β hydrolase fold enzymes. The active site of AChE is a 20 Å deep and 5 Å wide gorge divided into five structural subsites: an esteratic site containing a catalytic triad (His-Ser-Glu), oxyanion hole, a choline-binding subsite, an acyl pocket, and a peripheral anionic subsite on the edge of the active site (28). The active site of AChE is lined with 14 highly conserved aromatic amino acid residues, which interact with the cationic substrate and put it in the position for hydrolysis (28, 29). Moreover, a strong electrostatic dipole (produced by seven acidic amino acid residues located near the entrance of the gorge) is oriented along the axis of the active site at the gorge’s bottom and accounts for attracting positively charged substrate and other ligands toward the active site (30). One crystallographic study has shown that the active site may have a back door through which the products of ACh hydrolysis are released (31). Unlike AChE, the BChE active site is lined with eight aromatic and six aliphatic amino acid residues, which enables it to hydrolyse substrates larger than ACh (32, 33).
Figure 2.
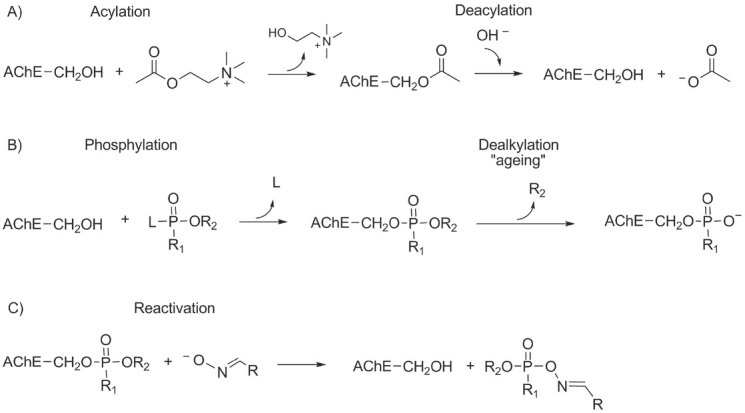
Hydrolysis of acetylcholine (A), phosphylation and aging (B), and reactivation of nerve agent-inhibited acetylcholinesterase (C)
AChE is present in the CNS and PNS, neuromuscular junctions, and red blood cells. BChE is more widespread, and is found in the CNS, PNS, pancreas, liver, intestines, heart, kidneys, lungs, and plasma/serum (34, 35). The primary and essential role of AChE is to break down the nerve impulse mediated by ACh, but it also has non-cholinergic functions such as in neuritogenesis, cell-cell interactions, proliferation, apoptosis, synaptogenesis, activation of dopamine neurons, and amyloid fibre formation (36, 37). Although BChE is not as essential as AChE, it has a detoxifying role, as it scavenges tissue AChE from OPs, hydrolyses cocaine, aspirin, succinylcholine, and other xenobiotics, and metabolises some pro-drugs into their active forms (e.g. bambuterol to terbutaline) (32, 35, 38). As it also hydrolyses ACh, it is a co-regulator of cholinergic neurotransmission (39).
Inhibition of more than 50 % of synaptic AChE activity will trigger the symptoms of poisoning, and death will occur mainly as a result of respiratory distress, when over 90 % of synaptic AChE is phosphylated by NA (11). As nicotinic and muscarinic receptors are localised in most organs, NA poisoning affects many systems in the body, and symptom severity depends on the dose, route, and duration of exposure (Table 1) (8, 23, 40, 41).
Table 1.
Symptoms of nerve agent poisoning arising from acetylcholine build-up at muscarinic and nicotinic membrane receptors (8, 23, 40, 41)
| System | Symptoms |
|---|---|
| Brain | Restlessness, headache, dizziness, convulsions, inhibition of central respiratory centres, loss of consciousness, coma |
| Eyes | Blurred vision, conjunctivitis, myosis |
| Respiratory | Rhinorrhoea, bronchoconstriction, bronchorrhea, pulmonary oedema |
| Cardiovascular | Either tachycardia or bradycardia, and either hypotension or hypertension |
| Gastro-intestinal | Cramping, abdominal pain, nausea, salivation, vomiting, defecation, urinary incontinence |
| Muscle | Twitching, fasciculation, tremors, muscle cramps, paralysis |
| Skin | Increased sweating |
The nucleophilic attack of catalytic serine’s hydroxyl group on the phosphorus moiety of the NA leads to the phosphylation of AChE (Figure 2B) and consequently to the loss of its catalytic function. Unlike acetylated AChE, which is quickly regenerated by water, the hydrolysis of phosphylated AChE is extremely slow (42, 43).
Cholinesterases have different affinity for NA isomers due to the asymmetry of the ChE active sites gorge. AChE and BChE exhibit enantioselectivity, that is, preference for one enantiomer binding over another (11, 25, 44, 45, 46). For example, the P(−) isomers of sarin and VX are the most potent AChE inhibitors, and the C(+/−) P(−) isomers of soman are up to 50 times more toxic than the C(+/−) P(+) isomers (25).
Phosphylated AChE undergoes dealkylation (also known as aging, Figure 2B), and the substituent on the phosphorus atom of NA becomes negatively charged, impeding AChE reactivation (47, 48, 49, 50). The half-time of aging of AChE conjugates varies with the NA and depends on pH and temperature. It can range from around 2 min with soman, 3 and 7 h with sarin and cyclosarin, respectively to more than 19 h with tabun and VX (26, 51, 52). Nevertheless, sarin- and VX-BChE conjugate will age slower than the corresponding NA-AChE conjugate, but it is the opposite with soman, cyclosarin, and tabun, whose OPs will form faster ageing conjugates with BChE than with AChE (26).
Another reaction is also possible, that is, reactivation of phosphylated AChE by nucleophiles stronger than water. Compounds with an oxime moiety (CH=NOH) can restore the activity of phosphylated AChE before it ages (42) through the nucleophilic attack of the oximate anion on the phosphorus atom of the phosphylated catalytic serine of AChE. The covalent bond between the NA and AChE adduct breaks, a phosphylated oxime is generated, and AChE is again catalytically active (Figure 2C) (42). This has been exploited for the development of potential NA poisoning antidotes. However, the reactivation of aged AChE is still an insurmountable challenge for scientists, even though some progress has been made in 2018, when Zhuang et al. (53) reported some re-alkylation of aged AChE with a member of the library of quinone methide precursors.
Treatment of nerve agent poisoning
NA poisoning is treated with an antimuscarinic drug, oxime reactivator of phosphylated AChE, and anticonvulsant if necessary (Figure 3).
Figure 3.
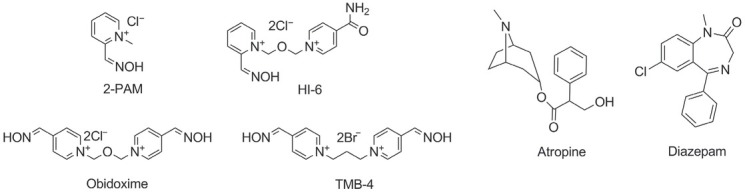
Standard pyridinium aldoxime reactivators of phosphylated acetylcholinesterase, antimuscarinic atropine, and anticonvulsant diazepam, currently approved for nerve agent poisoning therapy
Antimuscarinic drugs
When the scientists started to look into NA poisoning therapy in the 1930s, symptoms of the exposed victims pointed to atropine as potential remedy. Being a competitive muscarinic receptor antagonist, atropine blocks the effects of ACh on muscarinic receptors but has no effect on nicotinic receptors. Although it does not cross the blood-brain barrier (BBB) readily, it has beneficial effects in the CNS and PNS, against central apnoea, oversecretion, and even convulsions and cardiac toxicity (54, 55). To this day, atropine has remained the first drug of choice against symptoms of NA poisoning, because muscarinic effects it counters are the most life-threatening. In the meantime, alternative, more lipophilic anticholinergic drugs such as benactyzine have been investigated to improve therapy (56).
Reactivators
Since atropine is ineffective against nicotinic effects and does not restore AChE activity, further research focused on finding compounds that could complement atropine, such as nucleophilic agents like hydroxylamine, hydroxamic acid, and oximes, which can restore AChE activity (57). The intent was to synthesise a compound whose nucleophilic potential would enable displacement of the phosphorus moiety conjugated at the catalytic serine of AChE and reactivate the enzyme. In the early 1950s, Irwin B. Wilson and Sara Ginsburg set out to design such a compound. The idea was to use neurotransmitter ACh (because of permanent positive charge on the quaternary nitrogen group) as a template, and their efforts resulted in the synthesis of pyridine-2-aldoxime methiodide, known as pralidoxime or 2-PAM (58). Although synthesised without the knowledge of the AChE active site structure, 2-PAM was the first effective oxime reactivator of phosphylated AChE and remained in clinical use to this day.
The efficacy of 2-PAM varied with NA conjugated to AChE, and scientists directed their efforts toward designing more effective oximes that would cover a broader spectrum of OPs. Soon after 2-PAM, between 1958 and 1960, they synthesised trimedoxime bromide (TMB-4), methoxime (MMB-4), and obidoxime (23, 42).
In the late 1980s, Hagedorn and co-workers synthesised hundreds of oximes of the so-called H-series, among which HI-6 and Hlö-7 stood out (59, 60, 61). 2-PAM, obidoxime, HI-6, and TMB-4 became known as the standard oximes (Figure 3) and have been the only oximes approved for clinical and/or military use so far. Even so, their efficacy is not universal (61, 62, 63). 2-PAM, obidoxime, and HI-6 showed clinically relevant AChE reactivation in cases of sarin poisoning, obidoxime and HI-6 in cases of VX poisoning, and HI-6 in cases of cyclosarin poisoning (64). But none of the standard oximes is efficient against tabun poisoning at clinically relevant parameters (reactivation half-time of approximately 5 min and oxime concentration of <50 μmol/L) (64, 65, 66). The same is the case with soman poisoning due to the fast ageing of the soman-AChE conjugate (24).
Besides not being equally effective for all NAs, standard pyridinium oximes are hydrophilic and cannot pass the BBB due to the charged nitrogen atom. Therefore, only 1–10 % of the oximes’ plasma concentration is present in the brain, and their action is mostly limited to the PNS (67). Moreover, oximes cannot reactivate aged AChE and can also be toxic in doses needed for reactivation of non-aged AChE. This is why many still search for oxime reactivators that could overcome these limitations.
Over the last 70 years, there were many attempts to design and synthesise better AChE reactivators resulted in thousands of candidate compounds. Although some showed more potency in reactivating inhibited AChE than the standard oximes, a universal reactivator is unlikely to be found. Here is why. The accommodation and orientation of an oxime is a key property for successful reactivation (68, 69, 70, 71). Kinetically, the reactivation efficiency of an oxime is primarily attributed to the nucleophilic displacement rate of NA and to the affinity of the phosphylated ChE for the oxime (47, 48, 61, 68, 72, 73, 74, 75). To improve oxime kinetics, scientists have designed and synthesised double- or triple-binding mode reactivators. These compounds interact with multiple subsites of the active centre, including the peripheral anionic subsite of AChE, with the aim to increase enzyme’s affinity for the oxime (74, 76, 77, 78, 79).
The search for potentially more effective reactivators also involved a variety of structural modifications in the number or type of the ring, number and position of oxime moiety, structure and position of connecting linkers, and structure and position of side-chain ligands (74, 80, 81, 82, 83, 84). Some of the research investigated Alzheimer’s drugs like tacrine or donepezil as precursors of new reactivators (85, 86, 87). In addition, a number of studies highlighted piperidine derivatives, tetrahydroacridine and tryptoline moiety-containing compounds, and oximes containing tetrahydroisoquinoline and phenyltetrahydroisoquinoline groups as potent reactivators (79, 86, 88, 89, 90, 91, 92, 93). Several research groups focused on designing centrally acting oximes, compounds with no permanent positive charge but amenable to protonation, which would be able to cross the BBB. Upon establishing the pH-dependent equilibrium in the CNS, the protonated form would reactivate synaptic AChE (76, 91–101). In that respect, recent studies identified the RS 194B oxime as capable of rapidly reversing symptoms caused by a lethal dose of inhaled sarin vapour and paraoxon aerosol in macaques (102, 103).
A number of strategies have also been investigated for better delivery of charged reactivators to the brain like targeting nano-particles (104, 105), using pro-drugs (106), enhancing the compound’s lipophilicity by adding fluoride or chloride to the oxime structure (98, 107, 108), administering efflux transporter P-glycoprotein inhibitors (like tariquidar) (109), or adding a sugar moiety (110, 111).
The tabun-AChE conjugate is especially hard to reactivate (52, 62, 112, 113, 114, 115, 116, 117). One reason is the amino group of tabun, which sterically obscures oxime group access to the phosphorus moiety of tabun bound to the catalytic serine (52). Another is that resonance structures of tabun can be formed due to the free electron pair of the amino group of nitrogen, so the nucleophilicity of the oxime group is probably not high enough to reactivate such conjugate structures (112). Among the standard oximes, TMB-4 showed some potential in reactivating the tabun-AChE conjugate, but the dose required for in vivo application is too toxic (62, 116, 117). So far, studies have shown that only pyridinium oximes with the para-positioned oxime group like K203, K048, K074, and K075 have sufficient potency, superior to TMB-4, to restore AChE activity after tabun inhibition (69, 116, 118). A more recent study with triazole containing oxime library pointed out several oximes that were better reactivators of the tabun-AChE conjugate than obidoxime and 2-PAM (74). As the ambition of finding a unique reactivator is unrealistic, a solution may be in the combination of multiple oximes with complementary reactivity which would cover poisoning with a broad spectrum of NAs (119).
Non-oxime treatment alternatives have also been studied such as non-competitive antagonists and allosteric modulators selectively targeting nicotinic receptors (nAChR). Bispyridinium compound MB327 [ 1,1’-(propane-1,3-diyl)bis(4-tert-butylpyridinium) diiodide] (Figure 4) was found to block the open ion channel of the human muscle-type nAChR (120). Its non-competitive antagonism, as well as in vitro reversal of the neuromuscular blocking action of tabun, soman, and sarin in guinea pigs showed that, unlike oximes, it can protect against several NAs when used in combination with other drugs (121, 122). Recently, a reactivation potential was also discovered with 4-amino-2-[(diethylamino)methyl]phenol (ADOC) (Figure 4) (123). Several structural derivatives of ADOC were synthesised by De Koning’s group, and one of them (named “3l”) proved to be the most potent non-oxime reactivator so far reported (124, 125).
Figure 4.
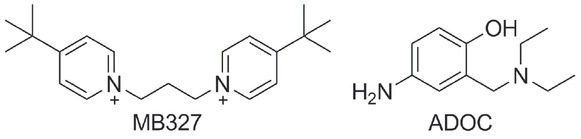
Non-oxime compounds investigated as potential treatment in case of nerve agent poisoning (120, 123)
Despite many attempts and an outstanding number of synthesised oxime libraries, however, only a small number of compounds gets to be tested in vivo due to adverse pharmacokinetic and pharmacodynamics properties and/or low reactivation efficacy, especially against tabun or soman.
Anticonvulsant drugs
Another feature of NA poisoning is electrographic seizures, followed by motor convulsions (126). These can quickly progress to status epilepticus (SE) or more severe conditions, all contributing to mortality or neuronal damage in survivors. It is believed that the overstimulation of cholinergic pathways and hypoxia are the main causes of seizure onset (127). Seizures activate neuronal inflammation and induce signalling in astrocytes, which leads to higher expression of the glial fibrillary acidic protein (GFAP) (128). High levels of GFAP, in turn, cause astrogliosis and glial scarring (129). Seizures also activate microglia, which results in the release of both pro-inflammatory (IL-1β, IL-1α, IL-6, IFN-γ) and anti-inflammatory (IL-10, IL-4, TGF-β, arginase) cytokines and reactive oxygen species (ROS) causing oxidative stress (128, 130–132). Therefore, OP-induced seizures require effective treatment to minimise brain damage such as treatment with benzodiazepine anticonvulsants diazepam, lorazepam, and midazolam (133, 134). Other drugs are also being studied to that effect, including agonists of the inhibitory neurotransmitter system or antagonists of the excitatory neurotransmitter system (127) and compounds with neuroprotective properties like anti-glutamatergic drugs, including NMDA antagonists ketamine and gacyclidine or AMPA/GluK1 receptor antagonist tezampanel (135, 136).
Protection of native AChE from inhibition
Fast ageing of phosphonylated AChE and poor efficacy of therapy have prompted the scientists to look for other means of counteracting NA poisoning like sheltering native AChE from phosphylation. Pre-exposure application (pretreatment), which enhances the efficacy of post-exposure therapy, however, is to be distinguished from prophylaxis, which omits the application of post-exposure therapy (137–140). Both protect native AChE from irreversible inhibition by NAs, but all NA exposure is highly likely to require subsequent therapy. Pretreatment is therefore desirable when exposure to NAs is expected, especially in the military. Protection can be achieved with anticholinesterase compounds, which allow a fraction of AChE to remain active in the presence of a phosphylating agent (141, 142). It can also be achieved with bioscavengers that degrade NAs in the bloodstream before reaching their physiological targets (143, 144).
Anticholinesterase compounds
Pseudo-irreversible inhibitors like carbamates form a short-living covalent complex with the catalytic serin of AChE. Unlike dephosphylation, decarbamylation is relatively fast and AChE is soon spontaneously reactivated (40, 145, 146). One such natural carbamate, physostigmine (from the plant Physostigma venenosum), has been used in medicine to treat Myastheniae gravis for a while now, and so has the synthetic carbamate neostigmine. These compounds are effective against poisoning with several OPs, but only another carbamate, pyridostigmine, has also been proved effective against soman (141, 142, 147, 148). Pyridostigmine was introduced in military practice for pretreatment of NA poisoning in the 1980s (11, 141, 147), but application at effective doses has shown undesirable side effects (mostly gastrointestinal) and was associated with the Gulf War illness (11, 141, 147, 149). This problem was partly addressed by combining pyridostigmine with anticholinergics trihexyfinidil and benactyzine. The product is called PANPAL and is approved for use in the Czech army (148). Pretreatment with PANPAL and standard post-exposure treatment has been proven effective against the G-series and VX (142, 148). However, pyridostigmine does not cross the BBB, so its prophylactic activity is limited to the PNS (142, 147). Carbamates that penetrate the BBB, like pyridostigmine-aprophen prodrugs (pyridophens) or physostigmine-scopolamine combinations, unfortunately, exhibited unwanted neurobehavioral effects (150, 151).
Research into reversible inhibitors that form noncovalent interactions with AChE (145) has yielded some promising options such as donepezil, huperzine A, and galantamine, which are usually used to treat neurodegenerative disorders like Alzheimer’s disease (152, 153). The most interesting is a plant alkaloid galantamine (found in amaryllis, daffodil, and snowdrop) (152, 153). It crosses the blood-brain barrier, exhibits the neuroprotective properties (nicotinic allosteric ligand), and is not toxic at therapeutic doses (153, 154). Moreover, the half-life of galantamine in circulation is quite long, up to 7 h, and can be administered orally (152, 154).
Oximes are also investigated as reversible AChE inhibitors (23, 142, 155), and a transdermal patch containing HI-6 (TRANSANT) has been approved for use in the Czech and Slovakian military (11, 156). Generally, short circulation half-life, toxicity, and the inability of oximes to cross the BBB are shortcomings that need to be addressed when considering oximes as pretreatment.
Bioscavengers
Since the late 1980s, research has been looking into enzymes of human or other origin which neutralise NAs in the bloodstream, before they reach target organs or get stored in the adipose tissue. There are such endogenous NA scavengers produced by the organism [e.g. human BChE, paraoxonase (PON1), albumin, animal carboxylesterase] (157–159), but they can only protect against low doses of NAs and either react too slowly with them or prefer the less toxic NA isomer. This is why research has been focused on exogenous bioscavengers that act rapidly against a broad spectrum of NAs, have prolonged circulation life (ideally more than 10 days), have no immunogenic or toxic properties, and are available in sufficient amounts to achieve effective concentrations at a reasonable cost (143, 160, 161). Some of these issues can be addressed by PEGylation or by encapsulating recombinant human or non-human enzymes in nanoparticles (119, 162, 163).
Bioscavengers can be divided in three categories: stoichiometric, oxime-assisted catalytic (also sometimes referred to as pseudo-catalytic), and catalytic (Figure 5).
Figure 5.
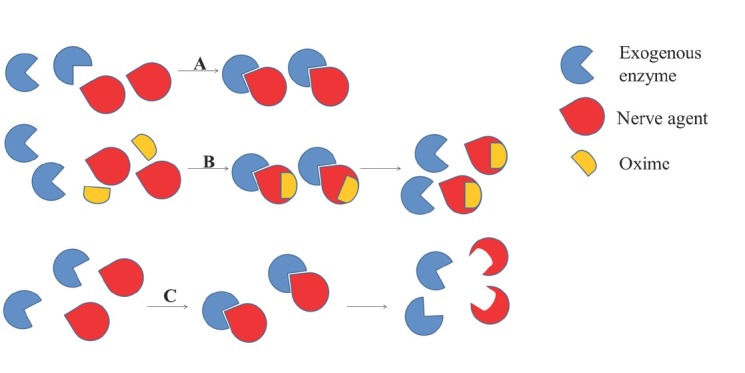
Bioscavenging of native acetylcholinesterase from inhibition with nerve agents. A) stoichiometric bioscavenging; mole-to-mole reaction between exogenous enzyme scavenger and nerve agent, B) oxime-assisted catalytic scavenging; exogenous enzyme turned into catalytic bioscavenger in the presence of an oxime enabling nerve agent degradation by cycles of inhibition and reactivation of exogenous enzyme scavenger, C) catalytic bioscavenging; low amount of exogenous enzyme scavenger rapidly hydrolyses nerve agent with a turnover
Stoichiometric bioscavengers
Stoichiometric bioscavengers react with an NA in a 1:1 ratio, i.e. one molecule of the enzyme is phosphylated by one molecule of NA. This way NA concentration in the bloodstream decreases, but the enzyme remains permanently inhibited. Stoichiometric bioscavengers are considered effective if NA is removed from the bloodstream within one circulation period, which is about 7 min in humans (143).
Human BChE is the most studied stoichiometric bioscavenger (159), as it rapidly reacts with NAs and other OPs. However, its concentration in human plasma is about 50 nmol/L, which is not enough to counteract NA poisoning. In a 70-kg human this concentration should reach ~2400 nmol/L (corresponding to a dose of 200 mg) to protect native AChE against 2-fold soman LD50 (164), and higher concentrations have been shown to protect against as high as 5.5-fold soman and even 8-fold VX LD50 in animal studies (165). These prophylactic concentrations can only be achieved with exogenous BChE.
The most convenient source of human BChE is outdated plasma (166). BChE from this source is a tetrameric protein with a circulatory half-life of about 12 to 15 days and it does not have any toxic or immunogenic effects on people (160, 167).
Considering that the isolation and purification of human BChE from plasma are quite expensive, and the availability of plasma depends on donors, alternative production of recombinant human BChE (168) has been investigated in expression systems such as Chinese hamster ovary cells (169, 170), wine fly cells (171), silkworm larvae (172), tobacco (173), maize (174), rice (175), transgenic goats (176), yeast (177), bacteria (178), and, lately, adenovirus-mediated human BChE delivery (179). The major challenges with these alternatives are that high BChE expression is not easy to achieve and the enzyme has to be purified from toxic contaminants. In this respect, the development of a new affinity method on huprine gel was a ground-breaking achievement, as it reduced the cost and time of purification (171, 180). Another achievement was tetramerisation of monomeric recombinant BChE by adding polyproline peptides (181) or polyethylene glycol polymers (182) or by co-expression of the proline-rich end terminal attachment domain (PRAD) to prolong its circulation life (183). Biological half-life in the bloodstream can also be prolonged by encapsulation, polysialylation, PEGylation, and fusion of recombinant human BChE with albumin (170, 176, 183, 184, 185).
Human recombinant AChE has also been investigated as a potential stoichiometric bioscavenger. Bloodstream-stable nanoparticles containing AChE or plant-derived human AChE have been shown effective in scavenging OP molecules (186, 187). In addition, PEGylated AChE has demonstrated greater scavenging efficiency and higher stereoselectivity for the toxic isomers of soman, tabun, and VX than BChE (188, 189).
Oxime-assisted catalytic bioscavengers
Oxime-assisted catalytic bioscavengers are enzyme scavengers combined with an efficient reactivator, which allows them to degrade NAs by continuous cycles of inhibition and reactivation. In these two-component systems, the most important is the rate of dephosphylation, as it has to quicker than aging (116, 190). The most logical approach is to combine human plasma BChE with an oxime to enhance NA degradation. However, combining BChE with standard pyridinium oximes proved to be ineffective, as these oximes are mainly AChE reactivators (191). Research has therefore looked into several promising newly synthesised oximes that reactivate phosphylated BChE (70, 191, 192, 193, 194, 195, 196, 197), and some progress has been achieved with new classes of BChE reactivators like quaternary benzaldoximes (192) imidazole aldoximes (193, 195), cinchona derivatives oximes (196), and hydroxypyridine aldoximes (87, 90). Furthermore, members of the oxime libraries that can cross the BBB were found to effectively reactivate sarin-, cyclosarin-, VX-, and paraoxon-inhibited BChE and AChE (91, 108, 193).
Several studies reported that site-directed mutagenesis of the AChE active site resulted in enzyme variants with increased phosphylation and/or reactivation rate, reduced aging rate, and a combination of these features (68, 161, 190, 198, 199). Combined with an oxime, such enzyme exhibits different in vitro kinetics, which further implies which oxime-enzyme pair could be converted into in vivo scavengers of different OPs (115, 200, 201, 202, 203). Certain AChE mutants have been shown to slow down the aging of their conjugates with soman (188, 190, 204). The most effective among them is the AChE mutant F338A, in which the phenylalanine of the choline binding site at position 338 is mutated to alanine (204, 205). The F338A mutation can readily be combined with the mutation of tyrosine to alanine at position 337 (Y337A) to enlarge the enzyme’s active site gorge dimensions, which should enable the positioning of the oxime in the vicinity of the phosphylated catalytic serine to ensure reactivation (68, 161, 190). The idea of the Y337A/F338A mutant is therefore to slow down aging and speed up the reactivation rate, which was demonstrated in vitro and ex vivo, when soman was quickly degraded in cycles of Y337A/F338A inhibition and reactivation by HI-6 (71, 190). Effective oxime-assisted catalytic soman and VX bioscavenging has also been reported in vivo with sub-stoichiometric amounts of the Y337A/F338A AChE mutant and an oxime reactivator (71, 206).
Other recent ex vivo studies showed some progress with a combination of Y337A and novel pyridinium aldoxime (analogous to 2-PAM) in tabun degradation in whole human blood (78, 161).
Finding an oxime that would effectively reactivate native ChEs in whole blood would be an improvement over stoichiometric or oxime-assisted catalytic scavenging in terms of cost, immunity, or circulation time challenges. Recent studies reported such potential with 3-hydroxy-2-pyridine aldoxime and chlorinated double-charged mono-oxime, which were successful in ex vivo degradation of VX, sarin, cyclosarin, and paraoxon (91, 108).
Catalytic bioscavengers
Catalytic bioscavengers are enzymes that can hydrolyse NAs into non-toxic products. The advantage of catalytic over stoichiometric bioscavengers is that they need lower doses to provide superior protection of native AChE from phosphylation, because one enzyme degrades multiple NAs molecules (207, 208). Several natural occurring human enzymes whose substrates are OPs are being investigated as potential catalytic bioscavengers, such as PON1 from plasma, erythrocyte and liver prolidase, carboxylesterase, platelet activating factor acetylhydrolase, liver senescence marker, and cytosolic aminopeptidases (142, 144, 209, 210, 211, 212).
The greatest interes is for PON1 which hydrolyses several OP compounds at a high rate, it is enantioselective, prefers the more toxic S(–) enantiomer of tabun, but, unfortunately, shows greater enantioselectivity to the less toxic isomers of soman (213, 214). Increasing PON1 catalytic activity up to 100 times would be sufficient to effectively scavenge various nerve agents, which is why scientists have been looking into mutations that would increase its catalytic activity (142, 208, 209). PON1 can be isolated from plasma but is complexed with HDL cholesterol, which renders isolation and purification expensive and complicated, and the isolated enzyme unstable (142, 209). Directed evolution of a chimeric PON1 via gene shuffling, combined with high-throughput screening, has resulted in PON1 that can quickly hydrolyse the most toxic enantiomers of the G-agents and effectively protect against cyclosarin toxicity in vivo (208, 215). However, no PON1 mutant has been found so far that can quickly hydrolyse V-agents.
Bacterial phosphotriesterases (PT E ) , diisopropylfluorophosphatase (DFPase) isolated from the squid, bacterial prolidase, fungal laccase, and haem chloroperoxidase also hydrolyse OPs (144, 216). These enzymes are used for destroying nerve agent supplies, decontamination of soil, clothing, and water. Encapsulation with nanoparticles gives them potential to counteract NAs (216). The most potent is the PTE obtained from Brevundimonas (Pseudomonas) diminuta. Its enantioselectivity has been redesigned by direct evolution, and the evolved mutants successfully and quickly hydrolyse the most toxic isomers of the V- and G-series and are effective in pre- and post-exposure treatments (217, 218).
There were attempts to transform BChE into a catalytic scavenger by introducing mutations. The first studied variant was that of human BChE with glycine at position 117 replaced by histidine (G117H), which introduced a second nucleophile. This BChE variant can slowly hydrolyse paraoxon, sarin, VX, and some other OPs (189, 216). Other mutants were also tested but were even less efficient than G117H.
Research also looked into biological systems such as monoclonal catalytic antibodies or artificial enzyme systems based on functionalised cyclodextrins with nucleophile groups (219, 220). Yet the greatest potential lies in nano-encapsulated cocktails of enzymes that could be used in injectable formulations and have broad-spectrum activity (144, 216).
Casualty treatment
Symptoms of cholinergic crisis are common indications for antidote administration on the battlefield, but there are also ready-to-use kits (ChE check mobile or Test mate) for determining erythrocyte AChE activity on the spot (221, 222). Inhalation of G-agents results with a very fast onset of symptoms requiring immediate therapy to enable survival. V-agent poisoning, in addition, requires continuous and prolonged treatment with oximes (64).
Immediate post-exposure treatment of NA casualties involves decontamination and antidote administration, usually provided by the closest soldier and/or first responder (combat medic). It is important that first responders are adequately protected (charcoal masks, gloves, protective clothing) from contamination from casualties or the environment. Decontamination has to be as quick as possible to limit NA absorption through skin and prevent contamination of the rescuers. Contaminated items of clothing are to be removed and skin scrubbed and rinsed profusely with water (unless there is none) and soap (if available). Alternatively, there are skin decontamination kits available for military personnel, such as M291, which is a mixture of reactive adsorbents (Ambersorb 348F carbonaceous adsorbent) and charcoal or RSDL, which contains Dekon 139 and a small amount of 2,3 butadiene monoxime (DAM) dissolved in a solvent composed of polyethylene glycol monomethyl ether (MPEG) and water (135).
Emergency treatment starts with intramuscular injection of atropine and an oxime reactivator which is self-administered (autoinjectors) or administered by the closest soldier or a first responder (26), especially if the casualty is a civilian. Autoinjectors contain atropine (2 mg) or a mixture of atropine and oxime and/or anticonvulsant (diazepam or its water-soluble formulation avizafone) (223, 224). Oximes approved for use include 2-PAM (USA, France, UK), obidoxime (Germany, Norway, the Netherlands), HI-6 (Canada, Croatia, Czech Republic, Sweden), and TMB-4 (Israel) (64, 225, 226). Depending on the country, each soldier is typically equipped with one to three kits and one diazepam auto-injector. One autoinjector is indicated for a casualty with only myosis and severe rhinorrhoea. The administration of the second autoinjector depends on the severity of symptoms and respiratory distress. The recommended interval between kits is about 5–15 min. The third kit and diazepam are recommended if the casualty shows signs of apnoea, muscle fasciculation or twitching, seizure or loses consciousness (223, 224). If the casualty shows signs of atropinisation (dry mouth and skin, increased heart rate, dilated pupils) after the application of atropine-autoinjector, it is likely that NA poisoning did not take place (55, 227).
If a victim manifests breathing difficulties, emergency responders should provide supportive treatment, such as intubation and oxygen ventilation, before evacuation from the hot zone and transfer to the hospital. If the symptoms of poisoning persist on hospital admission, post-exposure treatment should be continued promptly. Otherwise, if symptoms subside, erythrocyte cholinesterase activity is first to be determined to confirm poisoning. If indicated, atropine (2 mg for adults or 20 μg/kg for children) is to be applied intravenously every 5–10 min until the signs of atropinisation appear (227). Symptomatic patients should additionally receive an oxime intravenously, e.g. 30 mg/kg bodyweight of pralidoxime (2-PAM) chloride or mesylate, every 4–6 h (227). The duration of oxime treatment will depend on clinical response and AChE activity measurements, but it usually lasts as long as atropine treatment, which is up to 48 h but could be prolonged in case of VX poisoning due to the depot of VX.
As AChE/BChE activity tests can just confirm the inhibition, NA can only be identified with techniques like gas or liquid chromatography/mass spectrometry (GC-MS or LC-MS) in body fluids (e.g. plasma or urine) to ensure administration of adequate oxime (if available) and best chances of survival and recovery (228).
Conclusion
Numerous oxime libraries and approaches have been studied over the last 70 years in order to improve NA poisoning therapy or protect native AChE from inhibition. It is still an ongoing effort and further research is needed to optimise the pharmacokinetics and pharmacodynamics of new potential reactivators and to enhance the half-life of exogenous bioscavengers and diminish their immunogenicity.
Acknowledgements
This work was supported by the Croatian Science Foundation grants no. IP-2013-11-4307 and IP-2018-01-7683.
Footnotes
Conflict of interests
None to declare.
References
- 1.Holmstedt B. Koelle GB. Cholinesterases and anticholinesterase agents. Berlin: Springer-Verlag; 1963. Structure-activity relationships of the organophosphorus anticholinesterase agents; pp. 428–85. editor. p. [Google Scholar]
- 2.Chambers HW. Chambers JE, Levi PE. Organophosphates: chemistry, fate, and effects. San Diego: Academic Press; 1992. Organophosphorus compounds: an overview; pp. 3–17. p. [Google Scholar]
- 3.Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371:597–607. doi: 10.1016/S0140-6736(07)61202-1. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur HG, Garg H. Larramendy ML, Soloneski S. Pesticides - toxic aspects. London: IntechOpen; 2014. Pesticides: environmental impacts and management strategies; pp. 187–229. p. doi. [DOI] [Google Scholar]
- 5.Dolgin E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat Med. 2013;19:1194–5. doi: 10.1038/nm1013-1194. doi. [DOI] [PubMed] [Google Scholar]
- 6.Stone R. U.K. attack puts nerve agent in the spotlight. Science. 2018;359:1314–5. doi: 10.1126/science.359.6382.1314. doi. [DOI] [PubMed] [Google Scholar]
- 7.Bakry NM, el-Rashidy AH, Eldefrawi AT, Eldefrawi ME. Direct actions of organophosphate anticholinesterases on nicotinic and muscarinic acetylcholine receptors. J Biochem Toxicol. 1988;3:235–59. doi: 10.1002/jbt.2570030404. doi. [DOI] [PubMed] [Google Scholar]
- 8.Wiener SW, Hoffman RS. Nerve agents: a comprehensive review. J Intensive Care Med. 2004;19:22–37. doi: 10.1177/0885066603258659. doi. [DOI] [PubMed] [Google Scholar]
- 9.Costa LG. Costa LG, Galli CL, Murphy SD. Toxicology of pesticides: experimental, clinical, and regulatory perspectives, NATO ASI Series. Vol. 13. Berlin-Heidelberg: Springer; 1987. Toxicology of pesticides: a brief history; pp. 1–10. p. [Google Scholar]
- 10.Tucker JB. War of Nerves: Chemical Warfare from World War I to Al-Qaeda. New York: Anchor; 2007. [Google Scholar]
- 11.Delfino RT, Ribeiro TS, Figueroa-Villar JD. Organophosphorus compounds as chemical warfare agents: a review. J Braz Chem Soc. 2009;20:407–28. doi: 10.1590/S0103-50532009000300003. doi. [DOI] [Google Scholar]
- 12.Sidell FR. Sidell FR, Takafuji ET, Franz DR. Medical aspects of chemical and biological warfare. Textbook of military medicine. Washington (DC): Office of the Surgeon General, Department of the Army USA; 1997. Nerve agents; pp. 129–79. p. [Google Scholar]
- 13.Hoenig SL. Compendium of chemical warfare agents. New York (NY): Springer; 2007. Nerve agents; pp. 77–128. p. doi. [DOI] [Google Scholar]
- 14.Pitschmann V. Overall view of chemical and biochemical weapons. Toxins (Basel) 2014;6:1761–84. doi: 10.3390/toxins6061761. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vásárhelyi G, Földi L. History of Russia’s chemical weapons. AARMS. 2007;6:135–46. [Google Scholar]
- 16.Carlsen L. After Salisbury nerve agents revised. Mol Inform. 2019;38(8–9):e1800106. doi: 10.1002/minf.201800106. doi. [DOI] [PubMed] [Google Scholar]
- 17.Pita R, Domingo J. The use of chemical weapons in the Syrian conflict. Toxics. 2014;2:391–402. doi: 10.3390/toxics2030391. doi. [DOI] [Google Scholar]
- 18.Balali-Mood M, Saber H. Recent advances in the treatment of organophosphorous poisonings. Iran J Med Sci. 2012;37:74–91. PMCID: PMC3470074. [PMC free article] [PubMed] [Google Scholar]
- 19.Timperley C, Forman J, Aas P, Abdollahi M, Benachour D, Al-Amri A, Baulig A, Becker-Arnold R, Borrett V, Carino FA, Curty C, Gonzalez D, Geist M, Kane W, Kovarik Z, Martínez-Alvarez R, Mikulak R, Mourao N, Neffe S, Izzati F. Advice from the Scientific Advisory Board of the Organisation for the Prohibition of Chemical Weapons on riot control agents in connection to the Chemical Weapons Convention. RSC Advances. 2018;8:41731–9. doi: 10.1039/C8RA08273A. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John H, van der Schans MJ, Koller M, Spruit HET, Worek F, Thiermann H, Noort D. Fatal sarin poisoning in Syria 2013: forensic verification within an international laboratory network. Forensic Toxicol. 2018;36:61–71. doi: 10.1007/s11419-017-0376-7. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shwirtz M. Nerve agent was used to poison Navalny, chemical weapons body confirms. The New York Times. https://www.nytimes.com/2020/10/06/world/europe/navalny-opcw-russia-novichok.html online [displayed 22 November 2020]. Available at. [Google Scholar]
- 22.Organisation for the Prohibition of Chemical Weapons (OPCW) OPCW issues report on technical assistance requested by Germany. https://www.opcw.org/media-centre/news/2020/10/opcw-issues-report-technical-assistance-requested-germany [displayed 22 November 2020]. Available at. [Google Scholar]
- 23.Jokanović M, Stojiljković MP. Current understanding of the application of pyridinium oximes as cholinesterase reactivators in treatment of organophosphate poisoning. Eur J Pharmacol. 2006;553:10–17. doi: 10.1016/j.ejphar.2006.09.054. doi. [DOI] [PubMed] [Google Scholar]
- 24.Bucht G, Puu G. Aging and reactivatability of plaice cholinesterase inhibited by soman and its stereoisomers. Biochem Pharmacol. 1984;33:3573–7. doi: 10.1016/0006-2952(84)90139-4. doi. [DOI] [PubMed] [Google Scholar]
- 25.Benschop HP, De Jong LPA. Nerve agent stereoisomers: analysis isolation and toxicology. Acc Chem Res. 1988;21:368–74. doi: 10.1021/ar00154a003. doi. [DOI] [Google Scholar]
- 26.Worek F, Koller M, Thiermann H, Szinicz L. Diagnostic aspects of organophosphate poisoning. Toxicology. 2005;214:182–9. doi: 10.1016/j.tox.2005.06.012. doi. [DOI] [PubMed] [Google Scholar]
- 27.Quin DM. Acetylcholinesterase: enzyme structure, reaction dynamics, and virtual transitions states. Chem Rev. 1987;78:955–79. doi: 10.1021/cr00081a005. doi. [DOI] [Google Scholar]
- 28.Taylor P, Radić Z. The cholinesterases: from genes to proteins. Annu Rev Pharmacol Toxicol. 1994;34:281–320. doi: 10.1146/annurev.pa.34.040194.001433. doi. [DOI] [PubMed] [Google Scholar]
- 29.Bourne Y, Taylor P, Bougis PE, Marchot P. Crystal structure of mouse acetylcholinesterase. A peripheral site-occluding loop in a tetrameric assembly. J Biol Chem. 1999;274:2963–70. doi: 10.1074/jbc.274.5.2963. doi. [DOI] [PubMed] [Google Scholar]
- 30.Silman I, Sussman JL. Acetylcholinesterase: how is structure related to function? Chem Biol Interact. 2008;175:3–10. doi: 10.1016/j.cbi.2008.05.035. doi. [DOI] [PubMed] [Google Scholar]
- 31.Sanson B, Colletier JP, Xu Y, Lang PT, Jiang H, Silman I, Sussman JL, Weik M. Backdoor opening mechanism in acetylcholinesterase based on X-ray crystallography and molecular dynamics simulations. Protein Sci. 2011;20:1114–8. doi: 10.1002/pro.661. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Çokuğras AN. Butyrylcholinesterase: structure and physiological importance. Turk J Biochem. 2003;28:54–61. [Google Scholar]
- 33.Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J Biol Chem. 2003;278:41141–7. doi: 10.1074/jbc.M210241200. doi. [DOI] [PubMed] [Google Scholar]
- 34.Chatonnet A, Lockridge O. Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem J. 1989;260:625–34. doi: 10.1042/bj2600625. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci. 2003;4:131–8. doi: 10.1038/nrn1035. doi. [DOI] [PubMed] [Google Scholar]
- 36.Grisaru D, Sternfeld M, Eldor A, Glick D, Soreq H. Structural roles of acetylcholinesterase variants in biology and pathology. Eur J Biochem. 1999;264:672–86. doi: 10.1046/j.1432-1327.1999.00693.x. doi. [DOI] [PubMed] [Google Scholar]
- 37.Soreq H, Seidman S. Acetylcholinesterase-new roles for an old actor. Nat Rev Neurosci. 2001;2:294–302. doi: 10.1038/35067589. doi. [DOI] [PubMed] [Google Scholar]
- 38.Bosak A, Gazić I, Vinković V, Kovarik Z. Stereoselective inhibition of human, mouse, and horse cholinesterases by bambuterol enantiomers. Chem Biol Interact. 2008;175:192–5. doi: 10.1016/j.cbi.2008.04.050. doi. [DOI] [PubMed] [Google Scholar]
- 39.Xie W, Stribley JA, Chatonnet A, Wilder PJ, Rizzino A, McComb RD, Taylor P, Hinrichs SH, Lockridge O. Postnatal developmental delay and supersensitivity to organophosphate in gene-targeted mice lacking acetylcholinesterase. J Pharmacol Exp Ther. 2000;293:896–902. PMID: 10869390. [PubMed] [Google Scholar]
- 40.Bajgar J. Prophylaxis against organophosphorus poisoning. J Med Chem Def. 2004;1:1–16. [Google Scholar]
- 41.Newmark J. Therapy for nerve agent poisoning. Arch Neurol. 2004;61:649–52. doi: 10.1001/archneur.61.5.649. doi. [DOI] [PubMed] [Google Scholar]
- 42.Hobbiger F. Koelle GB. Cholinesterases and anticholinesterase agents. Handbook of experimental pharmacology. Vol. 15. Berlin, Heidelberg: Springer; 1963. Reactivation of phosphorylated acetylcholinesterase; pp. 921–88. editor. p. [Google Scholar]
- 43.Holmstedt B. Giacobini E. Cholinesterases and cholinesterase inhibitors. London: Martin Dunitz Ltd; 2000. Cholinesterase inhibitors: an introduction; pp. 1–8. editor. p. [Google Scholar]
- 44.Benschop HP, Konings CA, Van Genderen J, De Jong LP. Isolation, anticholinesterase properties, and acute toxicity in mice of the four stereoisomers of the nerve agent soman. Toxicol Appl Pharmacol. 1984;72:61–74. doi: 10.1016/0041-008x(84)90249-7. doi. [DOI] [PubMed] [Google Scholar]
- 45.Reiner E, Radić Z. Giacobini E. Cholinesterases and cholinesterase inhibitors. London: Martin Dunitz Ltd; 2000. Mechanism of action of cholinesterase inhibitor; pp. 103–19. editor. p. [Google Scholar]
- 46.Bosak A, Katalinić M, Kovarik Z. Kolinesteraze: struktura, uloga, inhibicija [Cholinesterases: structure, role, and inhibition, in Croatian] Arh Hig Rada Toksikol. 2011;62:175–90. doi: 10.2478/10004-1254-62-2011-2107. doi. [DOI] [PubMed] [Google Scholar]
- 47.Millard CB, Koellner G, Ordentlich A, Shafferman A, Silman I, Sussman JL. Reaction products of acetylcholinesterase and VX reveal a mobile histidine in the catalytic triad. J Am Chem Soc. 1999;121:9883–4. doi: 10.1021/ja992704i. doi. [DOI] [Google Scholar]
- 48.Ekström F, Akfur C, Tunemalm AK, Lundberg S. Structural changes of phenylalanine 338 and histidine 447 revealed by the crystal structures of tabun-inhibited murine acetylcholinesterase. Biochemistry. 2006;45:74–81. doi: 10.1021/bi051286t. doi. [DOI] [PubMed] [Google Scholar]
- 49.Masson P, Nachon F, Lockridge O. Structural approach to the aging of phosphylated cholinesterases. Chem Biol Interact. 2010;187:157–62. doi: 10.1016/j.cbi.2010.03.027. doi. [DOI] [PubMed] [Google Scholar]
- 50.Chandar NB, Ganguly B. A first principles investigation of aging processes in soman conjugated AChE. Chem Biol Interact. 2013;204:185–90. doi: 10.1016/j.cbi.2013.05.013. doi. [DOI] [PubMed] [Google Scholar]
- 51.de Jong LP, Wolring GZ. Stereospecific reactivation by some Hagedorn-oximes of acetylcholinesterases from various species including man, inhibited by soman. Biochem Pharmacol. 1984;33:1119–25. doi: 10.1016/0006-2952(84)90523-9. doi. [DOI] [PubMed] [Google Scholar]
- 52.Worek F, Thiermann H, Szinicz L, Eyer P. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem Pharmacol. 2004;68:2237–48. doi: 10.1016/j.bcp.2004.07.038. doi. [DOI] [PubMed] [Google Scholar]
- 53.Zhuang Q, Franjesevic AJ, Corrigan TS, Coldren WH, Dicken R, Sillart S, DeYong A, Yoshino N, Smith J, Fabry S, Fitzpatrick K, Blanton TG, Joseph J, Yoder RJ, McElroy CA, Ekici ÖD, Callam CS, Hadad CM. Demonstration of in vitro resurrection of aged acetylcholinesterase after exposure to organophosphorus chemical nerve agents. J Med Chem. 2018;61:7034–42. doi: 10.1021/acs.jmedchem.7b01620. doi. [DOI] [PubMed] [Google Scholar]
- 54.Shih TM, Rowland TC, McDonough JH. Anticonvulsants for nerve agentinduced seizures: the influence of the therapeutic dose of atropine. J Pharmacol Exp Ther. 2007;320:154–61. doi: 10.1124/jpet.106.111252. doi. [DOI] [PubMed] [Google Scholar]
- 55.Thiermann H, Steinritz D, Worek F, Radtke B, Eyer P, Eyer F, Felgenhauer N, Zilker T. Atropine maintenance dosage in patients with severe organophosphate pesticide poisoning. Toxicol Lett. 2011;206:77–83. doi: 10.1016/j.toxlet.2011.07.006. doi. [DOI] [PubMed] [Google Scholar]
- 56.Sidell FR, Newmark J, McDonough JH. Lenhart MK, Tuorinsky SD. Textbooks of military medicine, medical aspects of chemical warfare. Washington (DC): Department of the Army USA; 2008. Nerve agents; pp. 155–219. p. [Google Scholar]
- 57.Childs AF, Davies DR, Green AL, Rutland JP. The reactivation by oximes and hydroxamic acids of cholinesterase inhibited by organo-phosphorus compounds. Br J Pharmacol Chemother. 1955;10:462–5. doi: 10.1111/j.1476-5381.1955.tb00106.x. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson IB, Ginsburg S. Reactivation of acetylcholinesterase inhibited by alkylphosphates. Arch Biochem Biophys. 1955;54:569–71. doi: 10.1016/0003-9861(55)90075-8. doi. [DOI] [PubMed] [Google Scholar]
- 59.Rousseaux CG, Dua AK. Pharmacology of HI-6, an H-series oxime. Can J Physiol Pharmacol. 1989;67:1183–9. doi: 10.1139/y89-188. doi. [DOI] [PubMed] [Google Scholar]
- 60.Eyer P. In memory of Ilse Hagedorn. Toxicology. 2007;233:3–7. doi: 10.1016/j.tox.2006.09.014. doi. [DOI] [PubMed] [Google Scholar]
- 61.Worek F, Thiermann H, Wille T. Oximes in organophosphate poisoning: 60 years of hope and despair. Chem Biol Interact. 2016;259(Pt B):93–8. doi: 10.1016/j.cbi.2016.04.032. doi. [DOI] [PubMed] [Google Scholar]
- 62.Dawson RM. Review of oximes available for treatment of nerve agent poisoning. J Appl Toxicol. 1994;14:317–31. doi: 10.1002/jat.2550140502. doi. [DOI] [PubMed] [Google Scholar]
- 63.Antonijević B, Stojiljković MP. Unequal efficacy of pyridinium oximes in acute organophosphate poisoning. Clin Med Res. 2007;5:71–82. doi: 10.3121/cmr.2007.701. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thiermann H, Worek F, Kehe K. Limitations and challenges in treatment of acute chemical warfare agent poisoning. Chem Biol Interact. 2013;206:435–43. doi: 10.1016/j.cbi.2013.09.015. doi. [DOI] [PubMed] [Google Scholar]
- 65.Thiermann H, Szinicz L, Eyer F, Worek F, Eyer P, Felgenhauer N, Zilker T. Modern strategies in therapy of organophosphate poisoning. Toxicol Lett. 1999;107:233–9. doi: 10.1016/s0378-4274(99)00052-1. doi. [DOI] [PubMed] [Google Scholar]
- 66.Worek F, Szinicz L, Eyer P, Thiermann H. Evaluation of oxime efficacy in nerve agent poisoning: development of a kinetic-based dynamic model. Toxicol Appl Pharmacol. 2005;209:193–202. doi: 10.1016/j.taap.2005.04.006. doi. [DOI] [PubMed] [Google Scholar]
- 67.Lorke DE, Kalasz H, Petroianu GA, Tekes K. Entry of oximes into the brain: a review. Curr Med Chem. 2008;15:743–53. doi: 10.2174/092986708783955563. doi. [DOI] [PubMed] [Google Scholar]
- 68.Kovarik Z, Radić Z, Berman HA, Simeon-Rudolf V, Reiner E, Taylor P. Mutant cholinesterases possessing enhanced capacity for reactivation of their phosphonylated conjugates. Biochemistry. 2004;43:3222–9. doi: 10.1021/bi036191a. doi. [DOI] [PubMed] [Google Scholar]
- 69.Kovarik Z, Čalić M, Šinko G, Bosak A, Berend S, Lucić Vrdoljak A, Radić B. Oximes: Reactivators of phosphorylated acetylcholinesterase and antidotes in therapy against tabun poisoning. Chem Biol Interact. 2008;175:173–9. doi: 10.1016/j.cbi.2008.04.011. doi. [DOI] [PubMed] [Google Scholar]
- 70.Kovarik Z, Katalinić M, Šinko G, Binder J, Holas O, Jung YS, Musilova L, Jun D, Kuča K. Pseudo-catalytic scavenging: Searching for a suitable reactivator of phosphorylated butyrylcholinesterase. Chem Biol Interact. 2010;187:167–71. doi: 10.1016/j.cbi.2010.02.023. doi. [DOI] [PubMed] [Google Scholar]
- 71.Kovarik Z, Maček Hrvat N, Katalinić M, Sit RK, Paradyse A, Žunec S, Musilek K, Fokin VV, Taylor P, Radić Z. Catalytic soman scavenging by the Y337A/F338A acetylcholinesterase mutant assisted with novel site-directed aldoximes. Chem Res Toxicol. 2015;28:1036–44. doi: 10.1021/acs.chemrestox.5b00060. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Worek F, Reiter G, Eyer P, Szinicz L. Reactivation kinetics of acetylcholinesterase from different species inhibited by highly toxic organophosphates. Arch Toxicol. 2002;76:523–9. doi: 10.1007/s00204-002-0375-1. doi. [DOI] [PubMed] [Google Scholar]
- 73.Ekström F, Pang YP, Boman M, Artursson E, Akfur C, Börjegren S. Crystal structures of acetylcholinesterase in complex with HI-6, Ortho-7 and obidoxime: structural basis for differences in the ability to reactivate tabun conjugates. Biochem Pharmacol. 2006;72:597–607. doi: 10.1016/j.bcp.2006.05.027. doi. [DOI] [PubMed] [Google Scholar]
- 74.Kovarik Z, Kalisiak J, Maček Hrvat N, Katalinić M, Zorbaz T, Žunec S, Green C, Radić Z, Fokin VV, Sharpless KB, Taylor P. Reversal of tabun toxicity enabled by a triazole annulated oxime library-reactivators of acetylcholinesterase. Chem Eur J. 2019;25:4100–14. doi: 10.1002/chem.201805051. doi. [DOI] [PubMed] [Google Scholar]
- 75.Kovarik Z, Ciban N, Radić Z, Simeon-Rudolf V, Taylor P. Active site mutant acetylcholinesterase interactions with 2-PAM, HI-6, and DDVP. Biochem Biophys Res Commun. 2006;342(3):973–8. doi: 10.1016/j.bbrc.2006.02.056. doi. [DOI] [PubMed] [Google Scholar]
- 76.de Koning MC, Joosen MJ, Noort D, van Zuylen A, Tromp MC. Peripheral site ligand-oxime conjugates: A novel concept towards reactivation of nerve agent-inhibited human acetylcholinesterase. Bioorg Med Chem. 2011;19:588–94. doi: 10.1016/j.bmc.2010.10.059. doi. [DOI] [PubMed] [Google Scholar]
- 77.Maraković N, Knežević A, Vinković V, Kovarik Z, Šinko G. Design and synthesis of N -substituted-2-hydroxyiminoacetamides and interactions with cholinesterases. Chem Biol Interact. 2016;259:122–32. doi: 10.1016/j.cbi.2016.05.035. doi. [DOI] [PubMed] [Google Scholar]
- 78.Kovarik Z, Maček Hrvat N, Kalisiak J, Katalinić M, Sit RK, Zorbaz T, Radić Z, Fokin VV, Sharpless KB, Taylor P. Counteracting tabun inhibition by reactivation by pyridinium aldoximes interacting with active center gorge mutations of acetylcholinesterase. Toxicol Appl Pharmacol. 2019;372:40–6. doi: 10.1016/j.taap.2019.04.007. doi. [DOI] [PubMed] [Google Scholar]
- 79.Maček Hrvat N, Kalisiak J, Šinko G, Radić Z, Sharpless KB, Taylor P, Kovarik Z. Evaluation of high-affinity phenyltetrahydroisoquinoline aldoximes, linked through anti-triazoles, as reactivators of phosphylated cholinesterases. Toxicol Lett. 2020;321:83–9. doi: 10.1016/j.toxlet.2019.12.016. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Primožič I, Odžak R, Tomić S, Simeon-Rudolf V, Reiner E. Pyridinium, imidazolium, and quinucludinium oximes: synthesis, interaction with native and phosphylated cholinesterases, and antidotes against organophosphorus compounds. J Med Chem Def. 2004;2:1–30. [Google Scholar]
- 81.Oh KA, Yang GY, Jun D, Kuca K, Jung YS. Bispyridiumaldoxime reactivators connected with CH2O(CH2 n OCH2 linkers between pyridinium rings and their reactivity against VX. Bioorg Med Chem Lett. 2006;16:4852–5. doi: 10.1016/j.bmcl.2006.06.063. doi. [DOI] [PubMed] [Google Scholar]
- 82.Musilek K, Holas O, Jun D, Dohnal V, Gunn-Moore F, Opletalova V, Dolezal M, Kuca K. Monooxime reactivators of acetylcholinesterase with E-but-2-ene linker: preparation and reactivation of tabun- and paraoxon-inhibited acetylcholinesterase. Bioorg Med Chem. 2007;15:6733–41. doi: 10.1016/j.bmc.2007.08.002. doi. [DOI] [PubMed] [Google Scholar]
- 83.Acharya J, Dubey DK, Srivastava AK, Raza SK.. In vitro reactivation of sarin-inhibited human acetylcholinesterase (AChE) by bis-pyridinium oximes connected by xylene linkers. Toxicol In Vitro. 2011;25:251–6. doi: 10.1016/j.tiv.2010.07.024. doi. [DOI] [PubMed] [Google Scholar]
- 84.Čalić M, Bosak A, Šinko G, Jelić D. In vitro evaluation of aldoxime interactions with human acetylcholinesterase. Croat Chem Acta. 2008;81:47–57. [Google Scholar]
- 85.Kryger G, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with E2020 (Aricept): implications for the design of new anti-Alzheimer drugs. Structure. 1999;7:297–307. doi: 10.1016/s0969-2126(99)80040-9. doi. [DOI] [PubMed] [Google Scholar]
- 86.McHardy SF, Bohmann JA, Corbett MR, Campos B, Tidwell MW, Thompson PM, Bemben CJ, Menchaca TA, Reeves TE, Cantrell WRJr, Bauta WE, Lopez A, Maxwell DM, Brecht KM, Sweeney RE, McDonough J. Design, synthesis, and characterization of novel, nonquaternary reactivators of GF-inhibited human acetylcholinesterase. Bioorg Med Chem Lett. 2014;24:1711–4. doi: 10.1016/j.bmcl.2014.02.049. doi. [DOI] [PubMed] [Google Scholar]
- 87.Renou J, Dias J, Mercey G, Verdelet T, Rousseau C, Gastellier A-J, Arboleas M, Touvrey-Loiodice M, Baati R, Jean L, Nachon F, Renard P-Y. Synthesis and in vitro evaluation of donepezil- based reactivators and analogues for nerve agent-inhibited human acetylcholinesterase. RSC Adv. 2016;6:17929–40. doi: 10.1039/C5RA25477A. doi. [DOI] [Google Scholar]
- 88.de Koning MC, van Grol M, Noort D. Peripheral site ligand conjugation to a non-quaternary oxime enhances reactivation of nerve agent-inhibited human acetylcholinesterase. Toxicol Lett. 2011;206:54–9. doi: 10.1016/j.toxlet.2011.04.004. doi. [DOI] [PubMed] [Google Scholar]
- 89.Kliachyna M, Santoni G, Nussbaum V, Renou J, Sanson B, Colletier J-P, Arboléas M, Loiodice M, Weik M, Jean L, Renard P-Y, Nachon F, Baati R. Design, synthesis and biological evaluation of novel tetrahydroacridine pyridinealdoxime and -amidoxime hybrids as efficient uncharged reactivators of nerve agent-inhibited human acetylcholinesterase. Eur J Med Chem. 2014;78:455–67. doi: 10.1016/j.ejmech.2014.03.044. doi. [DOI] [PubMed] [Google Scholar]
- 90.Renou J, Loiodice M, Arboléas M, Baati R, Jean L, Nachon F, Renard P-Y. Tryptoline-3-hydroxypyridinaldoxime conjugates as efficient reactivators of phosphylated human acetyl and butyrylcholinesterases. Chem Commun. 2014;50:3947–50. doi: 10.1039/C4CC00561A. doi. [DOI] [PubMed] [Google Scholar]
- 91.Zorbaz T, Braïki A, Maraković N, Renou J, de la Mora E, Maček Hrvat N, Katalinić M, Silman I, Sussman JL, Mercey G, Gomez C, Mougeot R, Pérez B, Baati R, Nachon F, Weik M, Jean L, Kovarik Z, Renard P-Y. Potent 3-hydroxy-2-pyridine aldoxime reactivators of organophosphate-inhibited cholinesterases with predicted blood-brain barrier penetration. Chem Eur J. 2018;24:9675–91. doi: 10.1002/chem.201801394. doi. [DOI] [PubMed] [Google Scholar]
- 92.Mercey G, Verdelet T, Saint-André G, Gillon E, Wagner A, Baati R, Jean L, Nachon F, Renard P-Y. First efficient uncharged reactivators for the dephosphylation of poisoned human acetylcholinesterase. Chem Commun. 2011;47:5295–7. doi: 10.1039/C1CC10787A. doi. [DOI] [PubMed] [Google Scholar]
- 93.Mercey G, Renou J, Verdelet T, Kliachyna M, Baati R, Gillon E, Arboléas M, Loiodice M, Nachon F, Jean L, Renard P-Y. Phenyltetrahydroisoquinoline-pyridinaldoxime conjugates as efficient uncharged reactivators for the dephosphylation of inhibited human acetylcholinesterase. J Med Chem. 2012;55:10791–5. doi: 10.1021/jm3015519. doi. [DOI] [PubMed] [Google Scholar]
- 94.Kalisiak J, Ralph EC, Zhang J, Cashman JR. Amidineoximes: reactivators for organophosphate exposure. J Med Chem. 2011;54:3319–30. doi: 10.1021/jm200054r. doi. [DOI] [PubMed] [Google Scholar]
- 95.Sit RK, Radić Z, Gerardi V, Zhang L, Garcia E, Katalinić M, Amitai G, Kovarik Z, Fokin VV, Sharpless KB, Taylor P. New structural scaffolds for centrally acting oxime reactivators of phosphylated cholinesterases. J Biol Chem. 2011;286:19422–30. doi: 10.1074/jbc.M111.230656. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kalisiak J, Ralph EC, Cashman JR. Nonquaternary reactivators for organophosphate-inhibited cholinesterases. J Med Chem. 2012;55:465–74. doi: 10.1021/jm201364d. doi. [DOI] [PubMed] [Google Scholar]
- 97.Radić Z, Sit RK, Kovarik Z, Berend S, Garcia E, Zhang L, Amitai G, Green C, Radić B, Fokin VV, Sharpless KB, Taylor P. Refinement of structural leads for centrally acting oxime reactivators of phosphylated cholinesterases. J Biol Chem. 2012;287:11798–809. doi: 10.1074/jbc.M111.333732. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chambers JE, Chambers HW, Meek EC, Pringle RB. Testing of novel brain-penetrating oxime reactivators of acetylcholinesterase inhibited by nerve agent surrogates. Chem Biol Interact. 2013;203:135–8. doi: 10.1016/j.cbi.2012.10.017. doi. [DOI] [PubMed] [Google Scholar]
- 99.Kovarik Z, Maček N, Sit RK, Radić Z, Fokin VV, Barry Sharpless K, Taylor P. Centrally acting oximes in reactivation of tabun-phosphoramidated AChE. Chem Biol Interact. 2013;203:77–80. doi: 10.1016/j.cbi.2012.08.019. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sit RK, Kovarik Z, Maček Hrvat N, Žunec S, Green C, Fokin VV, Sharpless KB, Radić Z, Taylor P. Pharmacology, pharmacokinetics, and tissue disposition of zwitterionic hydroxyiminoacetamido alkylamines as reactivating antidotes for organophosphate exposure. J Pharmacol Exp Ther. 2018;367:363–72. doi: 10.1124/jpet.118.249383. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taylor P, Yan-Jye S, Momper J, Hou W, Camacho-Hernandez GA, Radić Z, Rosenberg Y, Kovarik Z, Sit R, Sharpless KB. Assessment of ionizable, zwitterionic oximes as reactivating antidotal agents for organophosphate exposure. Chem Biol Interact. 2019;308:194–7. doi: 10.1016/j.cbi.2019.05.015. doi. [DOI] [PubMed] [Google Scholar]
- 102.Rosenberg YJ, Mao L, Jiang X, Lees J, Zhang L, Radić Z, Taylor P. Post-exposure treatment with the oxime RS194B rapidly reverses early and advanced symptoms in macaques exposed to sarin vapor. Chem Biol Interact. 2017;274:50–7. doi: 10.1016/j.cbi.2017.07.003. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosenberg YJ, Wang J, Ooms T, Rajendran N, Mao L, Jiang X, Lees J, Urban L, Momper JD, Sepulveda Y, Shyong YJ, Taylor P. Post-exposure treatment with the oxime RS194B rapidly reactivates and reverses advanced symptoms of lethal inhaled paraoxon in macaques. Toxicol Lett. 2018;293:229–34. doi: 10.1016/j.toxlet.2017.10.025. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles) Brain Res. 1995;674:171–4. doi: 10.1016/0006-8993(95)00023-j. doi. [DOI] [PubMed] [Google Scholar]
- 105.Wagner S, Kufleitner J, Zensi A, Dadparvar M, Wien S, Bungert J, Vogel T, Worek F, Kreuter J, Briesen HV. Nanoparticulate transport of oximes over an in vitro blood-brain barrier model. PLoS One. 2010;5(12):e14213. doi: 10.1371/journal.pone.0014213. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Demar JC, Clarkson ED, Ratcliffe RH, Campbell AJ, Thangavelu SG, Herdman CA, Leader H, Schulz SM, Marek E, Medynets MA, Ku TC, Evans SA, Khan FA, Owens RR, Nambiar MP, Gordon RK. Pro-2-PAM therapy for central and peripheral cholinesterases. Chem Biol Interact. 2010;187:191–8. doi: 10.1016/j.cbi.2010.02.015. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jeong HC, Park NJ, Chae CH, Musilek K, Kassa J, Kuča K, Jung Y-S. Fluorinated pyridinium oximes as potential reactivators for acetylcholinesterases inhibited by paraoxon organophosphorus agent. Bioorg Med Chem. 2009;17:6213–7. doi: 10.1016/j.bmc.2009.07.043. doi. [DOI] [PubMed] [Google Scholar]
- 108.Zorbaz T, Malinak D, Maraković N, Maček Hrvat N, Zandona A, Novotny M, Skarka A, Andrys R, Benkova M, Soukup O, Katalinić M, Kuca K, Kovarik Z, Musilek K. Pyridinium oximes with ortho-positioned chlorine moiety exhibit improved physicochemical properties and efficient reactivation of human acetylcholinesterase inhibited by several nerve agents. J Med Chem. 2018;61:10753–66. doi: 10.1021/acs.jmedchem.8b01398. doi. [DOI] [PubMed] [Google Scholar]
- 109.Joosen MJ, van der Schans MJ, van Dijk CG, Kuijpers WC, Wortelboer HM, van Helden HP. Increasing oxime efficacy by blood-brain barrier modulation. Toxicol Lett. 2011;206:67–71. doi: 10.1016/j.toxlet.2011.05.231. doi. [DOI] [PubMed] [Google Scholar]
- 110.Heldman E, Ashani Y, Raveh L, Rachaman ES. Sugar conjugates of pyridinium aldoximes as antidotes against organophosphate poisoning. Carbohydr Res. 1986;151:337–47. doi: 10.1016/s0008-6215(00)90353-7. doi. [DOI] [PubMed] [Google Scholar]
- 111.Garcia GE, Campbell AJ, Olson J, Moorad-Doctor D, Morthole VI. Novel oximes as blood-brain barrier penetrating cholinesterase reactivators. Chem Biol Interact. 2010;187:199–206. doi: 10.1016/j.cbi.2010.02.033. doi. [DOI] [PubMed] [Google Scholar]
- 112.Eto M. Zweig G. The organophosphorus pesticides. Cleveland: CRC Press Inc; 1976. Organic and biological chemistry; p. 142. editor. p. [Google Scholar]
- 113.Cabal J, Kuca K, Kassa J. Specification of the structure of oximes able to reactivate tabun-inhibited acetylcholinesterase. Basic Clin Pharmacol Toxicol. 2004;95:81–6. doi: 10.1111/j.1742-7843.2004.950207.x. doi. [DOI] [PubMed] [Google Scholar]
- 114.Čalić M, Bosak A, Kuca K, Kovarik Z. Interactions of butane, but-2-ene or xylene-like linked bispyridinium paraaldoximes with native and tabun-inhibited human cholinesterases. Chem Biol Interact. 2008;175:305–8. doi: 10.1016/j.cbi.2008.04.010. doi. [DOI] [PubMed] [Google Scholar]
- 115.Artursson E, Akfur C, Hörnberg A, Worek F, Ekström F. Reactivation of tabun-hAChE investigated by structurally analogous oximes and mutagenesis. Toxicology. 2009;265:108–14. doi: 10.1016/j.tox.2009.09.002. doi. [DOI] [PubMed] [Google Scholar]
- 116.Kovarik Z, Čalić M, Šinko G, Bosak A. Structure-activity approach in the reactivation of tabun-phosphorylated human acetylcholinesterase with bispyridinium para-aldoximes. Arh Hig Rada Toksikol. 2007;58:201–9. doi: 10.2478/v10004-007-0013-7. doi. [DOI] [PubMed] [Google Scholar]
- 117.Berend S, Katalinić M, Lucić Vrdoljak A, Kovarik Z, Kuca K, Radić B.. In vivo experimental approach to treatment against tabun poisoning. J Enzyme Inhib Med Chem. 2010;25:531–6. doi: 10.3109/14756360903357593. doi. [DOI] [PubMed] [Google Scholar]
- 118.Kovarik Z, Lucić Vrdoljak A, Berend S, Katalinić M, Kuča K, Musilek K, Radić B. Evaluation of oxime K203 as antidote in tabun poisoning. Arh Hig Rada Toksikol. 2009;60:19–26. doi: 10.2478/10004-1254-60-2009-1890. doi. [DOI] [PubMed] [Google Scholar]
- 119.Elsinghorst PW, Worek F, Thiermann H, Wille T. Drug development for the management of organophosphorus poisoning. Expert Opin Drug Discov. 2013;8:1467–77. doi: 10.1517/17460441.2013.847920. doi. [DOI] [PubMed] [Google Scholar]
- 120.Seeger T, Eichhorn M, Lindner M, Niessen KV, Tattersall JEH, Timperley CM, Bird M, Green AC, Thiermann H, Worek F. Restoration of soman-blocked neuromuscular transmission in human and rat muscle by the bispyridinium non-oxime MB327 in vitro. Toxicology. 2012;294:80–4. doi: 10.1016/j.tox.2012.02.002. doi. [DOI] [PubMed] [Google Scholar]
- 121.Turner SR, Chad JE, Price M, Timperley CM, Bird M, Green AC, Tattersall JEH. Protection against nerve agent poisoning by a noncompetitive nicotinic antagonist. Toxicol Lett. 2011;206:105–11. doi: 10.1016/j.toxlet.2011.05.1035. doi. [DOI] [PubMed] [Google Scholar]
- 122.Price ME, Whitmore CL, Tattersall JEH, Green AC, Rice H. Efficacy of the antinicotinic compound MB327 against soman poisoning - Importance of experimental end point. Toxicol Lett. 2018;293:167–71. doi: 10.1016/j.toxlet.2017.11.006. doi. [DOI] [PubMed] [Google Scholar]
- 123.Katz FS, Pecic S, Tran TH, Trakht I, Schneider L, Zhu Z, Ton-That L, Luzac M, Zlatanic V, Damera S, Macdonald J, Landry DW, Tong L, Stojanovic MN. Discovery of new classes of compounds that reactivate acetylcholinesterase inhibited by organophosphates. Chembiochem. 2015;16:2205–15. doi: 10.1002/cbic.201500348. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Koning MC, Horn G, Worek F, van Grol M. Discovery of a potent non-oxime reactivator of nerve agent inhibited human acetylcholinesterase. Eur J Med Chem. 2018;157:151–60. doi: 10.1016/j.ejmech.2018.08.016. doi. [DOI] [PubMed] [Google Scholar]
- 125.Horn G, de Koning MC, van Grol M, Thiermann H, Worek F. Interactions between acetylcholinesterase, toxic organophosphorus compounds and a short series of structurally related non-oxime reactivators: Analysis of reactivation and inhibition kinetics in vitro. Toxicol Lett. 2018;299:218–25. doi: 10.1016/j.toxlet.2018.10.004. doi. [DOI] [PubMed] [Google Scholar]
- 126.McDonough JH Jr, Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–79. doi: 10.1016/s0149-7634(96)00050-4. doi. [DOI] [PubMed] [Google Scholar]
- 127.Rump S. Sohns T, Voicu A, Szinicz L, Finke E-J, Mircioiu C, Lundy P, Brain KR, Kempf H. NBC risks current capabilities and future perspectives for protection. NATO science series (Series 1: disarmament technologies) Vol 25. Dordrecht: Springer; 1999. Convulsions in organophosphate intoxications: their mechanism and treatment; pp. 189–95. p. [Google Scholar]
- 128.Guignet M, Lein PJ. Aschner M, Costa LG. Advances in neurotoxicology. Vol 3. Massachusetts: Academic Press; 2019. Neuroinflammation in organophosphate-induced neurotoxicity; pp. 35–79. p. [Google Scholar]
- 129.Chen Z, Duan RS, Quezada HC, Mix E, Nennesmo I, Adem A, Winblad B, Zhu J. Increased microglial activation and astrogliosis after intranasal administration of kainic acid in C57BL/6 mice. J Neurobiol. 2005;62:207–18. doi: 10.1002/neu.20099. doi. [DOI] [PubMed] [Google Scholar]
- 130.Banks CN, Lein PJ. A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology. 2012;33:575–84. doi: 10.1016/j.neuro.2012.02.002. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Turtzo LC, Lescher J, Janes L, Dean DD, Budde MD, Frank JA. Macrophagic and microglial responses after focal traumatic brain injury in the female rat. J Neuroinflammation. 2014;11:82. doi: 10.1186/1742-2094-11-82. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Becher B, Spath S, Goverman J. Cytokine networks in neuroinflammation. Nat Rev Immunol. 2017;17:49–59. doi: 10.1038/nri.2016.123. doi. [DOI] [PubMed] [Google Scholar]
- 133.Marrs TC. Diazepam in the treatment of organophosphorus ester pesticide poisoning. Toxicol Rev. 2003;22:75–81. doi: 10.2165/00139709-200322020-00002. doi. [DOI] [PubMed] [Google Scholar]
- 134.Tattersall J. Seizure activity post organophosphate exposure. Front Biosci (Landmark Ed) 2009;14:3688–711. doi: 10.2741/3481. doi. [DOI] [PubMed] [Google Scholar]
- 135.Timperley CM, Abdollahi M, Al-Amri AS, Baulig A, Benachour D, Borrett V, Cariño FA, Geist M, Gonzalez D, Kane W, Kovarik Z, Martínez-Álvarez R, Fusaro Mourão NM, Neffe S, Raza SK, Rubaylo V, Suárez AG, Takeuchi K, Tang C, Trifirò F, van Straten FM, Vanninen PS, Vučinić S, Zaitsev V, Zafar-Uz-Zaman M, Zina MS, Holen S, Forman JE, Alwan WS, Suri V. Advice on assistance and protection from the Scientific Advisory Board of the Organisation for the Prohibition of Chemical Weapons: Part 2. On preventing and treating health effects from acute, prolonged, and repeated nerve agent exposure, and the identification of medical countermeasures able to reduce or eliminate the longer term health effects of nerve agents. Toxicology. 2019;413:13–23. doi: 10.1016/j.tox.2018.11.009. doi. [DOI] [PubMed] [Google Scholar]
- 136.Aroniadou-Anderjaska V, Apland JP, Figueiredo TH, De Araujo Furtado M, Braga MF. Acetylcholinesterase inhibitors (nerve agents) as weapons of mass destruction: History, mechanisms of action, and medical countermeasures. Neuropharmacology. 2020;181:108298. doi: 10.1016/j.neuropharm.2020.108298. doi. [DOI] [PubMed] [Google Scholar]
- 137.North Atlantic Treaty Organization (NATO) Handbook on the medical aspects of NBC defensive operations. https://fas.org/nuke/guide/usa/doctrine/dod/fm8-9/3ch2.htm [displayed 22 November 2020]. Available at. [Google Scholar]
- 138.Myhrer T, Aas P. Pretreatment and prophylaxis against nerve agent poisoning: Are undesirable behavioral side effects unavoidable? Neurosci Biobehav Rev. 2016;71:657–70. doi: 10.1016/j.neubiorev.2016.10.017. doi. [DOI] [PubMed] [Google Scholar]
- 139.Layish I, Krivoy A, Rotman E, Finkelstein A, Tashma Z, Yehezkelli Y.. Pharmacologic prophylaxis against nerve agent poisoning. Isr Med Assoc J. 2005;7:182–7. PMID: 15792266. [PubMed] [Google Scholar]
- 140.Bajgar J, Fusek J, Kassa J, Kuca K, Jun D. Gupta RC. Handbook of toxicology of chemical warfare agents. 2nd ed. Cambridge, MA: Academic Press; 2015. Pharmacological prophylaxis against nerve agent poisoning: experimental studies and practical implications; pp. 979–87. editor. p. doi. [DOI] [Google Scholar]
- 141.Bajgar J, Fusek J, Kassa J, Kuca K, Jun D. Chemical aspects of pharmacological prophylaxis against nerve agent poisoning. Curr Med Chem. 2009;16:2977–86. doi: 10.2174/092986709788803088. doi. [DOI] [PubMed] [Google Scholar]
- 142.Masson P. Evolution of and perspectives on therapeutic approaches to nerve agent poisoning. Toxicol Lett. 2011;206:5–13. doi: 10.1016/j.toxlet.2011.04.006. doi. [DOI] [PubMed] [Google Scholar]
- 143.Lenz DE, Yeung D, Smith JR, Sweeney RE, Lumley LA, Cerasoli DM. Stoichiometric and catalytic scavengers as protection against nerve agent toxicity: a mini review. Toxicology. 2007;233:31–9. doi: 10.1016/j.tox.2006.11.066. doi. [DOI] [PubMed] [Google Scholar]
- 144.Nachon F, Brazzolotto X, Trovaslet M, Masson P. Progress in the development of enzyme-based nerve agent bioscavengers. Chem Biol Interact. 2013;206:536–44. doi: 10.1016/j.cbi.2013.06.012. doi. [DOI] [PubMed] [Google Scholar]
- 145.Eckert S, Eyer P, Mückter H, Worek F. Kinetic analysis of the protection afforded by reversible inhibitors against irreversible inhibition of acetylcholinesterase by highly toxic organophosphorus compounds. Biochem Pharmacol. 2006;72:344–57. doi: 10.1016/j.bcp.2006.04.015. doi. [DOI] [PubMed] [Google Scholar]
- 146.Gordon JJ, Leadbeater L, Maidment MP. The protection of animals against organophosphate poisoning by pretreatment with a carbamate. Toxicol Appl Pharmacol. 1978;43:207–16. doi: 10.1016/s0041-008x(78)80045-3. doi. [DOI] [PubMed] [Google Scholar]
- 147.Keeler JR, Hurst CG, Dunn MA. Pyridostigmine used as a nerve agent pretreatment under wartime conditions. JAMA. 1991;266:693–5. doi: 10.1001/jama.1991.03470050093029. doi. [DOI] [PubMed] [Google Scholar]
- 148.Kassa J. Therapeutic and neuroprotective efficacy of pharmacological pretreatment and antidotal treatment of acute tabun or soman poisoning with the emphasis on pretreatment drug PANPAL. Arh Hig Rada Toksikol. 2006;57:427–34. [PubMed] [Google Scholar]
- 149.Golomb BA. Acetylcholinesterase inhibitors and Gulf War illnesses. Proc Natl Acad Sci U S A. 2008;105:4295–300. doi: 10.1073/pnas.0711986105. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Leader H, Wolfe AD, Chiang PK, Gordon RK. Pyridophens: binary pyridostigmine-aprophen prodrugs with differential inhibition of acetylcholinesterase, butyrylcholinesterase, and muscarinic receptors. J Med Chem. 2002;45:902–10. doi: 10.1021/jm010196t. doi. [DOI] [PubMed] [Google Scholar]
- 151.Philippens IH, Joosen MJ, Vanwersch RA. Stress adversely affects efficacy of physostigmine-scopolamine pretreatment against soman in guinea pigs. Pharmacol Biochem Behav. 2005;82:125–32. doi: 10.1016/j.pbb.2005.07.018. doi. [DOI] [PubMed] [Google Scholar]
- 152.Aracava Y, Pereira EF, Akkerman M, Adler M, Albuquerque EX. Effectiveness of donepezil, rivastigmine, and (+/-) huperzine A in counteracting the acute toxicity of organophosphorus nerve agents: comparison with galantamine. J Pharmacol Exp Ther. 2009;331:1014–24. doi: 10.1124/jpet.109.160028. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pereira EF, Aracava Y, Alkondon M, Akkerman M, Merchenthaler I, Albuquerque EX. Molecular and cellular actions of galantamine: clinical implications for treatment of organophosphorus poisoning. J Mol Neurosci. 2010;40:196–203. doi: 10.1007/s12031-009-9234-3. doi. [DOI] [PubMed] [Google Scholar]
- 154.Alexandrova EA, Aracava Y, Pereira EF, Albuquerque EX. Pretreatment of Guinea pigs with galantamine prevents immediate and delayed effects of soman on inhibitory synaptic transmission in the hippocampus. J Pharmacol Exp Ther. 2010;334:1051–8. doi: 10.1124/jpet.110.167700. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lucić Vrdoljak A, Čalić M, Radić B, Berend S, Jun D, Kuča K, Kovarik Z. Pretreatment with pyridinium oximes improves antidotal therapy against tabun poisoning. Toxicology. 2006;228:41–50. doi: 10.1016/j.tox.2006.08.012. doi. [DOI] [PubMed] [Google Scholar]
- 156.Bajgar J. Complex view on poisoning with nerve agents and organophosphates. Acta Medica (Hradec Kralove) 2005;48:3–21. doi: 10.14712/18059694.2018.23. doi. [DOI] [PubMed] [Google Scholar]
- 157.Maxwell DM, Brecht KM. Carboxylesterase: specificity and spontaneous reactivation of an endogenous scavenger for organophosphorus compounds. J Appl Toxicol. 2001;21(Suppl 1):S103–7. doi: 10.1002/jat.833. doi. [DOI] [PubMed] [Google Scholar]
- 158.Sogorb MA, Vilanova E. Serum albumins and detoxication of anti-cholinesterase agents. Chem Biol Interact. 2010;187:325–9. doi: 10.1016/j.cbi.2010.03.001. doi. [DOI] [PubMed] [Google Scholar]
- 159.Lockridge O. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol Ther. 2015;148:34–46. doi: 10.1016/j.pharmthera.2014.11.011. doi. [DOI] [PubMed] [Google Scholar]
- 160.Doctor BP, Saxena A. Bioscavengers for the protection of humans against organophosphate toxicity. Chem Biol Interact. 2005;157–158:167–71. doi: 10.1016/j.cbi.2005.10.024. doi. [DOI] [PubMed] [Google Scholar]
- 161.Kovarik Z, Maček Hrvat N. Efficient detoxification of nerve agents by oxime-assisted reactivation of acetylcholinesterase mutants. Neuropharmacology. 2020;171:108111. doi: 10.1016/j.neuropharm.2020.108111. doi. [DOI] [PubMed] [Google Scholar]
- 162.Liu Y, Li J, Lu Y. Enzyme therapeutics for systemic detoxification. Adv Drug Deliv Rev. 2015;90:24–39. doi: 10.1016/j.addr.2015.05.005. doi. [DOI] [PubMed] [Google Scholar]
- 163.Zhang P, Jain P, Tsao C, Sinclair A, Sun F, Hung H-C, Bai T, Wu K, Jiang S. Butyrylcholinesterase nanocapsule as a long circulating bioscavenger with reduced immune response. J Control Release. 2016;230:73–8. doi: 10.1016/j.jconrel.2016.04.008. doi. [DOI] [PubMed] [Google Scholar]
- 164.Ashani Y, Pistinner S. Estimation of the upper limit of human butyrylcholinesterase dose required for protection against organophosphates toxicity: a mathematically based toxicokinetic model. Toxicol Sci. 2004;77:358–67. doi: 10.1093/toxsci/kfh012. doi. [DOI] [PubMed] [Google Scholar]
- 165.Saxena A, Sun W, Fedorko JM, Koplovitz I, Doctor BP. Prophylaxis with human serum butyrylcholinesterase protects guinea pigs exposed to multiple lethal doses of soman or VX. Biochem Pharmacol. 2011;81:164–9. doi: 10.1016/j.bcp.2010.09.007. doi. [DOI] [PubMed] [Google Scholar]
- 166.Saxena A, Tipparaju P, Luo C, Doctor BP. Pilot-scale production of human serum butyrylcholinesterase suitable for use as a bioscavenger against nerve agent toxicity. Process Biochem. 2010;45:1313–8. doi: 10.1016/j.procbio.2010.04.021. doi. [DOI] [Google Scholar]
- 167.Genovese RF, Sun W, Johnson CC, Ditargiani RC, Doctor BP, Saxena A. Safety of administration of human butyrylcholinesterase and its conjugates with soman or VX in rats. Basic Clin Pharmacol Toxicol. 2010;106:428–34. doi: 10.1111/j.1742-7843.2009.00508.x. doi. [DOI] [PubMed] [Google Scholar]
- 168.Čadež T, Kovarik Z. Advancements in recombinant technology for production of butyrylcholinesterase, a bioscavenger of nerve agents. Period Biol. 2020;121–122:55–63. doi: 10.18054/pb.v121i1-2.10867. doi. [DOI] [Google Scholar]
- 169.Nachon F, Nicolet Y, Viguiea N, Masson P, Fontecilla-Camps JC, Lockridge O. Engineering of a monomeric and lowglycosylated form of human butyrylcholinesterase: expression, purifcation, characterization and crystallization. Eur J Biochem. 2002;269:630–7. doi: 10.1046/j.0014-2956.2001.02692.x. doi. [DOI] [PubMed] [Google Scholar]
- 170.Terekhov S, Smirnov I, Bobik T, Shamborant O, Zenkova M, Chernolovskaya E, Gladkikh D, Murashev A, Dyachenko I, Paliko V, Palikova Y, Knorre V, Belogurov A Jr, Ponomarenko N, Blackburn GM, Masson P, Gabibov A. A novel expression cassette delivers efficient production of exclusively tetrameric human butyrylcholinesterase with improved pharmacokinetics for protection against organophosphate poisoning. Biochimie. 2015;118:51–9. doi: 10.1016/j.biochi.2015.07.028. doi. [DOI] [PubMed] [Google Scholar]
- 171.Brazzolotto X, Wandhammer M, Ronco C, Trovaslet M, Jean L, Lockridge O, Renard PY, Nachon F. Human butyrylcholinesterase produced in insect cells: huprine-based affinity purification and crystal structure. FEBS J. 2012;279:2905–16. doi: 10.1111/j.1742-4658.2012.08672.x. doi. [DOI] [PubMed] [Google Scholar]
- 172.Li S, Ip DT, Lin HQ, Liu JM, Miao YG, Ke LJ, Wan DC. High-level expression of functional recombinant human butyrylcholinesterase in silkworm larvae by Bac-to-Bac system. Chem Biol Interact. 2010;187:101–5. doi: 10.1016/j.cbi.2010.03.055. doi. [DOI] [PubMed] [Google Scholar]
- 173.Geyer BC, Kannan L, Garnaud PE, Broomfield CA, Cadieux CL, Cherni I, Hodgins SM, Kasten SA, Kelley K, Kilbourne J, Oliver ZP, Otto TC, Puffenberger I, Reeves TE, Robbins N. Woods RR, Soreq H, Lenz DE, Cerasoli DM, Mor TS. Plant-derived human butyrylcholinesterase, but not an organophosphorous-compound hydrolyzing variant thereof, protects rodents against nerve agents. Proc Natl Acad Sci U S A. 2010;107:20251–6. doi: 10.1073/pnas.1009021107. 2nd. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Egelkrout E, Hayden CA, Wales M, Walker J, Novikov B, Grimsley J, Howard J. Production of the bioscavenger butyrylcholinesterase in maize. Mol Breeding. 2017;37:136. doi: 10.1007/s11032-017-0731-8. doi. [DOI] [Google Scholar]
- 175.Corbin JM, Hashimoto BI, Karuppanan K, Kyser ZR, Wu L, Roberts BA, Noe AR, Rodriguez RL, McDonald KA, Nandi S. Semicontinuous bioreactor production of recombinant butyrylcholinesterase in transgenic rice cell suspension cultures. Front Plant Sci. 2016;7:412. doi: 10.3389/fpls.2016.00412. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huang YJ, Huang Y, Baldassarre H, Wang B, Lazaris A, Leduc M, Bilodeau AS, Bellemare A, Côté M, Herskovits P, Touati M, Turcotte C, Valeanu L, Lemée N, Wilgus H, Bégin I, Bhatia B, Rao K, Neveu N, Brochu E, Pierson J, Hockley DK, Cerasoli DM, Lenz DE, Karatzas CN, Langermann S. Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning. Proc Natl Acad Sci U S A. 2007;104:13603–8. doi: 10.1073/pnas.0702756104. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Terekhov SS, Smirnov IV, Stepanova AV, Bobik TV, Mokrushina YA, Ponomarenko NA, Belogurov AA Jr, Rubtsova MP, Kartseva OV, Gomzikova MO, Moskovtsev AA, Bukatin AS, Dubina MV, Kostryukova ES, Babenko VV, Vakhitova MT, Manolov AI, Malakhova MV, Kornienko MA, Tyakht AV, Vanyushkina AA, Ilina EN, Masson P, Gabibov AG, Altman S. Microfluidic droplet platform for ultrahigh-throughput single-cell screening of biodiversity. Proc Natl Acad Sci U S A. 2017;114:2550–5. doi: 10.1073/pnas.1621226114. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Brazzolotto X, Igert A, Guillon V, Santoni G, Nachon F. Bacterial expression of human butyrylcholinesterase as a tool for nerve agent bioscavengers development. Molecules. 2017;22:1828. doi: 10.3390/molecules22111828. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gupta V, Cadieux LC, Mcmenamin D, Medina-Jaszek CA, Arif M, Ahonkhai O, Wielechowski E, Taheri M, Che Y, Goode T, Limberis MP, Li M, Cerasoli DM, Tretiakova AP, Wilson JM. Adeno-associated virus-mediated expression of human butyrylcholinesterase to treat organophosphate poisoning. PLoS One. 2019;14(11):e0225188. doi: 10.1371/journal.pone.0225188. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lockridge O, David E, Schopfer LM, Masson P, Brazzolotto X, Nachon F. Purification of recombinant human butyrylcholinesterase on Hupresin®. J Chromatogr B. 2018;1102–1103:109–15. doi: 10.1016/j.jchromb.2018.10.026. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Larson MA, Lockridge O, Hinrichs SH. Polyproline promotes tetramerization of recombinant human butyrylcholinesterase. Biochem J. 2014;462:329–35. doi: 10.1042/BJ20140421. doi. [DOI] [PubMed] [Google Scholar]
- 182.Rosenberg YJ, Saxena A, Sun W, Jiang X, Chilukuri N, Luo C, Doctor BP, Lee KD. Demonstration of in vivo stability and lack of immunogenicity of a polyethyleneglycolconjugated recombinant CHO-derived butyrylcholinesterase bioscavenger using a homologous macaque model. Chem Biol Interact. 2010;187:279–86. doi: 10.1016/j.cbi.2010.02.042. doi. [DOI] [PubMed] [Google Scholar]
- 183.Terekhov SS, Smirnov IV, Shamborant OG, Bobik TV, Ilyushin DG, Murashev AN, Dyachenko IA, Palikov VA, Knorre VD, Belogurov AA, Ponomarenko NA, Kuzina ES, Genkin DD, Masson P, Gabibov AG. Chemical polysialylation and. in vivo tetramerization improve pharmacokinetic characteristics of recombinant human butyrylcholinesterase-based bioscavengers. Acta Naturae. 2015;7:136–41. doi: 10.32607/20758251-2015-7-4-136-141. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huang YJ, Lundy PM, Lazaris A, Huang Y, Baldassarre H, Wang B, Turcotte C, Côté M, Bellemare A, Bilodeau AS, Brouillard S, Touati M, Herskovits P, Bégin I, Neveu N, Brochu E, Pierson J, Hockley DK, Cerasoli DM, Lenz DE, Wilgus H, Karatzas CN, Langermann S. Substantially improved pharmacokinetics of recombinant human butyrylcholinesterase by fusion to human serum albumin. BMC Biotechnol. 2008;8:50. doi: 10.1186/1472-6750-8-50. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ilyushin DG, Smirnov IV, Belogurov AA Jr, Dyachenko IA, Zharmukhamedova TIu, Novozhilova TI, Bychikhin EA, Serebryakova MV, Kharybin ON, Murashev AN, Anikienko KA, Nikolaev EN, Ponomarenko NA, Genkin DD, Blackburn GM, Masson P, Gabibov AG. Chemical polysialylation of human recombinant butyrylcholinesterase delivers a long-acting bioscavenger for nerve agents in vivo. Proc Natl Acad Sci U S A. 2013;110:1243–8. doi: 10.1073/pnas.1211118110. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Atsmon J, Brill-Almon E, Nadri-Shay C, Chertkoff R, Alon S, Shaikevich D, Volokhov I, Haim KY, Bartfeld D, Shulman A, Ruderfer I, Ben-Moshe T, Shilovitzky O, Soreq H, Shaaltiel Y. Preclinical and first-in-human evaluation of PRX-105, a PEGylated, plant-derived, recombinant human acetylcholinesterase-R. Toxicol Appl Pharmacol. 2015;287:202–9. doi: 10.1016/j.taap.2015.06.004. doi. [DOI] [PubMed] [Google Scholar]
- 187.Pascual L, Sayed SE, Martínez-Máñez R, Costero AM, Gil S, Gavina P, Sancenon F. Acetylcholinesterase-capped mesoporous silica nanoparticles that open in the presence of Maček Hrvat N, Kovarik Z. Counteracting poisoning with chemical warfare nerve agents Arh Hig Rada Toksikol. 2020;71:266–284. doi: 10.1021/acs.orglett.6b02793. diisopropylfluorophosphate (a sarin or soman simulant). Org Lett 2016;18:5548–51. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cohen O, Kronman C, Raveh L, Mazor O, Ordentlich A, Shafferman A. Comparison of polyethylene glycolconjugated recombinant human acetylcholinesterase and serum human butyrylcholinesterase as bioscavengers of organophosphate compounds. Mol Pharmacol. 2006;70:1121–31. doi: 10.1124/mol.106.026179. doi. [DOI] [PubMed] [Google Scholar]
- 189.Masson P, Nachon F, Broomfield CA, Lenz DE, Verdier L, Schopfer LM, Lockridge O. A collaborative endeavor to design cholinesterase-based catalytic scavengers against toxic organophosphorus esters. Chem Biol Interact. 2008;175:273–80. doi: 10.1016/j.cbi.2008.04.005. doi. [DOI] [PubMed] [Google Scholar]
- 190.Cochran R, Kalisiak J, Küçükkilinç T, Radić Z, Garcia E, Zhang L, Ho KY, Amitai G, Kovarik Z, Fokin VV, Sharpless KB, Taylor P. Oxime-assisted acetylcholinesterase catalytic scavengers of organophosphates that resist aging. J Biol Chem. 2011;286:29718–24. doi: 10.1074/jbc.M111.264739. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Horn G, Wille T, Musilek K, Kuca K, Thiermann H, Worek F. Reactivation kinetics of 31 structurally different bispyridinium oximes with organophosphate-inhibited human butyrylcholinesterase. Arch Toxicol. 2015;89:405–14. doi: 10.1007/s00204-014-1288-5. doi. [DOI] [PubMed] [Google Scholar]
- 192.Radić Z, Dale T, Kovarik Z, Berend S, Garcia E, Zhang L, Amitai G, Green C, Radić B, Duggan BM, Ajami D, Rebek J, Taylor P. Catalytic detoxification of nerve agent and pesticide organophosphates by butyrylcholinesterase assisted with non-pyridinium oximes. Biochem J. 2013;450:231–42. doi: 10.1042/BJ20121612. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sit RK, Fokin VV, Amitai G, Sharpless KB, Taylor P, Radić Z. Imidazole aldoximes effective in assisting butyrylcholinesterase catalysis of organophosphate detoxification. J Med Chem. 2014;57:1378–89. doi: 10.1021/jm401650z. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bušić V, Katalinić M, Šinko G, Kovarik Z, Gašo-Sokač D. Pyridoxal oxime derivative potency to reactivate cholinesterases inhibited by organophosphorus compounds. Toxicol Lett. 2016;262:114–22. doi: 10.1016/j.toxlet.2016.09.015. doi. [DOI] [PubMed] [Google Scholar]
- 195.Katalinić M, Maček Hrvat N, Baumann K, Morasi Piperčić S, Makarić S, Tomić S, Jović O, Hrenar T, Miličević A, Jelić D, Žunec S, Primožič I, Kovarik Z. A comprehensive evaluation of novel oximes in creation of butyrylcholinesterase-based nerve agent bioscavengers. Toxicol Appl Pharmacol. 2016;310:195–204. doi: 10.1016/j.taap.2016.09.015. doi. [DOI] [PubMed] [Google Scholar]
- 196.Katalinić M, Zandona A, Ramić A, Zorbaz T, Primožič I, Kovarik Z. New cinchona oximes evaluated as reactivators of acetylcholinesterase and butyrylcholinesterase inhibited by organophosphorus compounds. Molecules. 2017;22(7):1234. doi: 10.3390/molecules22071234. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zorbaz T, Malinak D, Kuca K, Musilek K, Kovarik Z. Butyrylcholinesterase inhibited by nerve agents is efficiently reactivated with chlorinated pyridinium oximes. Chem Biol Interact. 2019;307:16–20. doi: 10.1016/j.cbi.2019.04.020. doi. [DOI] [PubMed] [Google Scholar]
- 198.Kovarik Z, Radić Z, Berman HA, Simeon-Rudolf V, Reiner E, Taylor P. Acetylcholinesterase active centre and gorge conformations analysed by combinatorial mutations and enantiomeric phosphonates. Biochem J. 2003;373:33–40. doi: 10.1042/BJ20021862. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Katalinić M, Šinko G, Maček Hrvat N, Zorbaz T, Bosak A, Kovarik Z. Oxime-assisted reactivation of tabun-inhibited acetylcholinesterase analysed by active site mutations. Toxicology. 2018;406–407:104–13. doi: 10.1016/j.tox.2018.05.008. doi. [DOI] [PubMed] [Google Scholar]
- 200.Kovarik Z, Radić Z, Berman HA, Taylor P. Mutation of acetylcholinesterase to enhance oxime-assisted catalytic turnover of methylphosphonates. Toxicology. 2007;233:79–84. doi: 10.1016/j.tox.2006.08.032. doi. [DOI] [PubMed] [Google Scholar]
- 201.Taylor P, Kovarik Z, Reiner E, Radić Z. Acetylcholinesterase: converting a vulnerable target to a template for antidotes and detection of inhibitor exposure. Toxicology. 2007;233:70–8. doi: 10.1016/j.tox.2006.11.061. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Katalinić M, Kovarik Z. Reactivation of tabun-inhibited acetylcholinesterase investigated by two oximes and mutagenesis. Croat Chem Acta. 2012;85:209–12. doi: 10.5562/cca1815. doi. [DOI] [Google Scholar]
- 203.Kovarik Z, Maček Hrvat N, Žunec S, Katalinić M. Detoxification of tabun-exposed mice by an acetylcholinesterase mutant assisted with a novel pyridinium aldoxime. BiIol Serb. 2019;41:4–8. doi: 10.5281/zenodo.3532038. doi. [DOI] [Google Scholar]
- 204.Mazor O, Cohen O, Kronman C, Raveh L, Stein D, Ordentlich A, Shafferman A. Aging-resistant organophosphate bioscavenger based on polyethylene glycol-conjugated F338A human acetylcholinesterase. Mol Pharmacol. 2008;74:755–63. doi: 10.1124/mol.108.047449. doi. [DOI] [PubMed] [Google Scholar]
- 205.Kronman C, Cohen O, Mazor O, Ordentlich A, Raveh L, Velan B, Shafferman A. Next generation OP-bioscavengers: a circulatory long-lived 4-PEG hypolysine mutant of F338AHuAChE with optimal pharmacokinetics and pseudo-catalytic characteristics. Chem Biol Interact. 2010;187:253–8. doi: 10.1016/j.cbi.2009.12.004. doi. [DOI] [PubMed] [Google Scholar]
- 206.Maček Hrvat N, Žunec S, Taylor P, Radić Z, Kovarik Z. HI-6 assisted catalytic scavenging of VX by acetylcholinesterase choline binding site mutants. Chem Biol Interact. 2016;259:148–53. doi: 10.1016/j.cbi.2016.04.023. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sweeney RE, Maxwell DM. A theoretical expression for the protection associated with stoichiometric and catalytic scavengers in a single compartment model of organophosphorus poisoning. Math Biosci. 2003;181:133–43. doi: 10.1016/s0025-5564(02)00154-2. doi. [DOI] [PubMed] [Google Scholar]
- 208.Ashani Y, Leader H, Aggarwal N, Silman I, Worek F, Sussman JL, Goldsmith M.. In vitro evaluation of the catalytic activity of paraoxonases and phosphotriesterases predicts the enzyme circulatory levels required for in vivo protection against organophosphate intoxications. Chem Biol Interact. 2016;259:252–6. doi: 10.1016/j.cbi.2016.04.039. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rochu D, Chabrière E, Masson P. Human paraoxonase: a promising approach for pre-treatment and therapy of organophosphorus poisoning. Toxicology. 2007;233:47–59. doi: 10.1016/j.tox.2006.08.037. doi. [DOI] [PubMed] [Google Scholar]
- 210.Bosak A, Bavec A, Konte T, Šinko G, Kovarik Z, Goličnik M. Interactions of paraoxonase-1 with pharmacologically relevant carbamates. Molecules. 2020;25:1–15. doi: 10.3390/molecules25010211. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kirby SD, Norris J, Sweeney R, Bahnson BJ, Cerasoli DM. A rationally designed mutant of plasma platelet-activating factor acetylhydrolase hydrolyzes the organophosphorus nerve agent soman. Biochim Biophys Acta. 2015;1854:1809–15. doi: 10.1016/j.bbapap.2015.09.001. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hemmert AC, Otto TC, Wierdl M, Edwards CC, Fleming CD, MacDonald M, Cashman JR, Potter PM, Cerasoli DM, Redinbo MR. Human carboxylesterase 1 stereoselectively binds the nerve agent cyclosarin and spontaneously hydrolyzes the nerve agent sarin. Mol Pharmacol. 2010;77:508–16. doi: 10.1124/mol.109.062356. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yeung DT, Smith JR, Sweeney RE, Lenz DE, Cerasoli DM. A gas chromatographic-mass spectrometric approach to examining stereoselective interaction of human plasma proteins with soman. J Anal Toxicol. 2008;32:86–91. doi: 10.1093/jat/32.1.86. doi. [DOI] [PubMed] [Google Scholar]
- 214.Valiyaveettil M, Alamneh Y, Biggemann L, Soojhawon I, Doctor BP, Nambiar MP. Efficient hydrolysis of the chemical warfare nerve agent tabun by recombinant and purified human and rabbit serum paraoxonase 1. Biochem Biophys Res Commun. 2010;403:97–102. doi: 10.1016/j.bbrc.2010.10.125. doi. [DOI] [PubMed] [Google Scholar]
- 215.Worek F, Seeger T, Goldsmith M, Ashani Y, Leader H, Sussman JS, Tawfik D, Thiermann H, Wille T. Efficacy of the rePON1 mutant IIG1 to prevent cyclosarin toxicity in vivo and to detoxify structurally different nerve agents in vitro. Arch Toxicol. 2014;88:1257–66. doi: 10.1007/s00204-014-1204-z. doi. [DOI] [PubMed] [Google Scholar]
- 216.Masson P, Rochu D. Catalytic bioscavengers against toxic esters, an alternative approach for prophylaxis and treatments of poisonings. Acta Naturae. 2009;1:68–79. PMCID: PMC3347506. [PMC free article] [PubMed] [Google Scholar]
- 217.Bigley AN, Xu C, Henderson TJ, Harvey SP, Raushel FM. Enzymatic neutralization of the chemical warfare agent VX: evolution of phosphotriesterase for phosphorothiolate hydrolysis. J Am Chem Soc. 2013;135:10426–32. doi: 10.1021/ja402832z. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Goldsmith M, Eckstein S, Ashani Y, Greisen P Jr, Leader H, Sussman JL, Aggarwal N, Ovchinnikov S, Tawfik DS, Baker D, Thiermann H, Worek F. Catalytic efficiencies of directly evolved phosphotriesterase variants with structurally different organophosphorus compounds in vitro. Arch Toxicol. 2016;90:2711–24. doi: 10.1007/s00204-015-1626-2. doi. [DOI] [PubMed] [Google Scholar]
- 219.Smirnov I, Belogurov A Jr, Friboulet A, Masson P, Gabibov A, Renard PY. Strategies for the selection of catalytic antibodies against organophosphorus nerve agents. Chem Biol Interact. 2013;203:196–201. doi: 10.1016/j.cbi.2012.10.011. doi. [DOI] [PubMed] [Google Scholar]
- 220.Letort S, Balieu S, Erb W, Gouhier G, Estour F. Interactions of cyclodextrins and their derivatives with toxic organophosphorus compounds. Beilstein J Org Chem. 2016;12:204–28. doi: 10.3762/bjoc.12.23. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Taylor PW, Lukey BJ, Clark CR, Lee RB, Roussel RR. Field verification of Test-mate ChE. Mil Med. 2003;168:314–9. PMID: 12733677. [PubMed] [Google Scholar]
- 222.Worek F, Baumann M, Pfeiffer B, Aurbek N, Balszuweit F, Thiermann H. Cholinesterase kit for field diagnosis of organophosphate exposure. CBRN Medical Defense International. 2013 https://www.securetec.net/wp-content/uploads/2018/08/Challenge_ChE_check_mobile_Special_2013.pdf [displayed 13 November 2020]. Available at. [Google Scholar]
- 223.Moshiri M, Darchini-Maragheh E, Balali-Mood M. Advances in toxicology and medical treatment of chemical warfare nerve agents. Daru. 2012;20(1):81. doi: 10.1186/2008-2231-20-81. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Timperley CM, Forman JE, Abdollahi M, Al-Amri AS, Baulig A, Benachour D, Borrett V, Cariño FA, Geist M, Gonzalez D, Kane W, Kovarik Z, Martínez-Álvarez R, Mourão NMF, Neffe S, Raza SK, Rubaylo V, Suárez AG, Takeuchi K, Tang C, Trifirò F, van Straten FM, Vanninen PS, Vučinić S, Zaitsev V, Zafar-Uz-Zaman M, Zina MS, Holen S. Advice on assistance and protection provided by the Scientific Advisory Board of the Organisation for the Prohibition of Chemical Weapons: Part 1. On medical care and treatment of injuries from nerve agents. Toxicology. 2019;415:56–69. doi: 10.1016/j.tox.2019.01.004. doi. [DOI] [PubMed] [Google Scholar]
- 225.Thiermann H, Aurbek N, Worek F. Worek F, Jenner J, Thiermann H. Chemical warfare toxicology: Volume 2: Management of poisoning. London: The Royal Society of Chemistry; 2016. Treatment of nerve agent poisoning; pp. 1–42. p. [Google Scholar]
- 226.Bentur Y, Layish I, Krivoy A, Berkovitch M, Rotman E, Bar Haim S, Yehezkelli Y, Kozer E. Civilian adult self injections of atropine-trimedoxime (TMB4) auto-injectors. Clin Toxicol (Phila) 2006;44:301–6. doi: 10.1080/15563650600584519. doi. [DOI] [PubMed] [Google Scholar]
- 227.Marrs TC, Rice P, Vale JA. The role of oximes in the treatment of nerve agent poisoning in civilian casualties. Toxicol Rev. 2006;25:297–323. doi: 10.2165/00139709-200625040-00009. doi. [DOI] [PubMed] [Google Scholar]
- 228.Koller M, Becker C, Thiermann H, Worek F. GC-MS and LC-MS analysis of nerve agents in body fluids: intra-laboratory verification test using spiked plasma and urine samples. J Chromatogr B. 2010;878:1226–33. doi: 10.1016/j.jchromb.2009.12.023. doi. [DOI] [PubMed] [Google Scholar]


