Abstract
Nanotechnology is the fabrication, characterization, and potential application of various materials at the nanoscale. Over the past few decades, nanomaterials have attracted researchers from different fields because of their high surface-to-volume ratio and other unique and remarkable properties. Cobalt and cobalt oxide nanoparticles (NPs) have various biomedical applications because of their distinctive antioxidant, antimicrobial, antifungal, anticancer, larvicidal, antileishmanial, anticholinergic, wound healing, and antidiabetic properties. In addition to biomedical applications, cobalt and cobalt oxide NPs have been widely used in lithium-ion batteries, pigments and dyes, electronic thin film, capacitors, gas sensors, heterogeneous catalysis, and for environmental remediation purposes. Different chemical and physical approaches have been used to synthesize cobalt and cobalt oxide NPs; however, these methods could be associated with eco-toxicity, cost-effectiveness, high energy, and time consumption. Recently, an eco-friendly, safe, easy, and simple method has been developed by researchers, which uses biotic resources such as plant extract, microorganisms, algae, and other biomolecules such as starch and gelatin. Such biogenic cobalt and cobalt oxide NPs offer more advantages over other physicochemically synthesized methods. In this review, we have summarized the recent literature for the understanding of green synthesis of cobalt and cobalt oxide NPs, their characterization, and various biomedical applications.
Keywords: nanotechnology, green synthesis, cobalt nanoparticles, cobalt oxide nanoparticles, biomedical applications of nanoparticles
Graphical abstract
1. Introduction
Nanotechnology combines various chemical and physical processes to construct nanomaterials, which are less than 100 nm in at least one dimension, and have unique properties [1]. Nanotechnology has applications across different fields, such as nanomedicines, biomaterials, nanoelectronics, environment, imaging, industries, and agriculture [2,3]. In healthcare, it has been widely used for the diagnosis and treatment of diseases, drug delivery, and novel drug formulations [2,4].
Cobalt is a transition metal that has a beneficial effect on human health [5,6]. It constitutes a part of vitamin B12, which is useful in anemia treatment as it provokes the formation of red blood cells [6]. Cobalt has unique magnetic, optical, electrical, and catalytic characteristics that make it suitable for a wide range of applications in the field of nanoelectronics and nanosensors [7,8,9]. Cobalt can exhibit variable oxidation states (Co2+, Co3+, and Co4+), which makes it attractive to be used in several industries [10]. Because of this multivalent state, cobalt has the ability to be present in different spin states in its oxide forms, i.e., low, intermediate, and high [10,11].
Recently, cobalt nanoparticles (CoNPs) have attracted considerable attention because they are more economical than the noble metal nanoparticle (NP) and show different properties, such as electrical and magnetic, due to their large surface area [12,13]. CoNPs have been explored as a therapeutic agent for the treatment of diseases, such as microbial infection, which make them attractive for biomedical applications [14,15]. CoNPs are nontoxic in the body at lower levels, have strong activities against bacteria and fungi at lower concentrations, and have fewer side effects than antibiotics [16,17].
Different types of NPs and their various applications in the area of medicine, textiles, cosmetics, electronics, optics, energy generation, and environmental science have been reported [18,19,20,21,22]. These NPs include silver NPs (AgNPs), iron NPs, CoNPs, copper NPs, gold NPs, silica NPs, platinum NPs, palladium NPs, zinc oxide NPs, magnesium oxide NPs, cerium dioxide NPs, and titanium oxide NPs [18]. Among all NPs, cobalt and cobalt oxide (Co3O4) NPs have been exploited the most because of their unique and wide range of applications [5,6]. Co3O4 is an antiferromagnetic p-type semiconductor with a direct optical band gap of 1.48 and 2.19 eV [23,24]. Co3O4 is a multifunctional material and has many applications such as biomedical applications (antibacterial, antiviral, antifungal, antileishmanial, therapeutic agents, anticancer, and drug delivery), gas sensors, solar selective absorbers, anode materials in lithium-ion batteries, energy storage, pigments and dyes, field emission materials, capacitors, heterogeneous catalysis, magneto-resistive devices, and electronic thin films [25,26,27,28,29] as shown in Figure 1. The oxides of cobalt are abundant in nature, as only the Co3O4 and CoO are stable [30], with Co3O4 possessing the highest stability. In this review, we aim to focus on the biological synthesis, characterization, and biological activities of cobalt and cobalt oxide NPs.
Figure 1.
General applications of cobalt and cobalt oxide NPs.
2. Synthesis of NPs
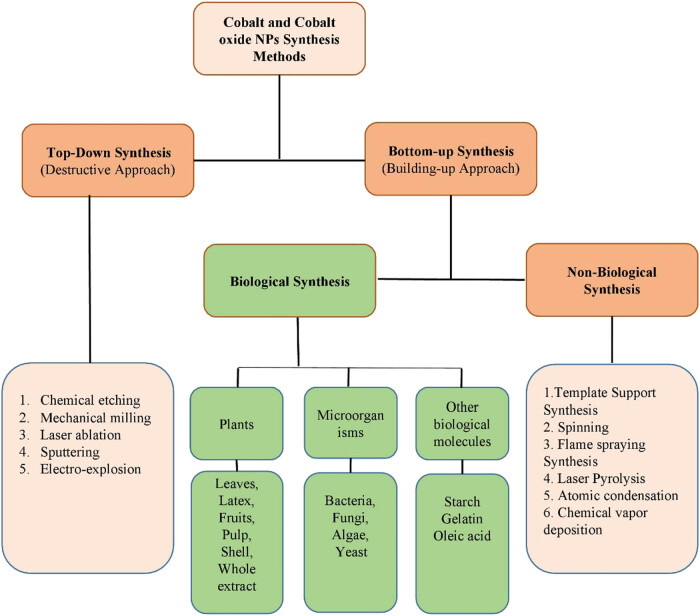
Different approaches can be used for the synthesis of NPs. These approaches are divided into two classes: top-down approach and bottom-up approach. A top-down approach is a destructive approach in which larger molecules are decomposed into smaller ones, and these smaller molecules are then converted into suitable NPs, whereas the bottom-up is a building approach that involves the assembly of atomic size to form nano-size particles [1]. Examples of the top-down approach include chemical etching, laser ablation, mechanical milling, electro-explosion, and sputtering. In these different methods, the bulk materials are first converted into powder form and then into particular NPs. Each synthesis method has its advantages and limitations. The top-down approach has many advantages, such as the production of the desired size and large quantity of nanoparticle, whereas the fabrication of NPs using this method leads to eco-toxicity, high energy consumption and on top of that is expensive and time-consuming [2,3]. The bottom-up approach is further divided into two classes: biological and non-biological approaches. Examples of non-biological approaches include template support synthesis, flame spraying, spinning, laser pyrolysis, atomic condensation, and deposition of chemical vapors. These methods also use toxic chemicals, are expensive and time-consuming, whereas the biological approach uses various biotic resources such as plants, algae, microorganisms, and other biological molecules like starch, egg albumin, and gelatin for the production of different types of NPs. This biological approach is also known as the green approach [2,3,4,5,6,7,8,9,10]. This method is eco-friendly, simple, reliable, biocompatible, and easy for the synthesis of NPs. The classification of various methods of fabrication of NPs is shown in Figure 2. However, in this review, we only focus on the green synthesis of cobalt and cobalt oxide NPs, and their biological applications.
Figure 2.
Various methods for the synthesis of cobalt and cobalt oxide NPs.
3. Green synthesis of cobalt and cobalt oxide NPs (biological methods)
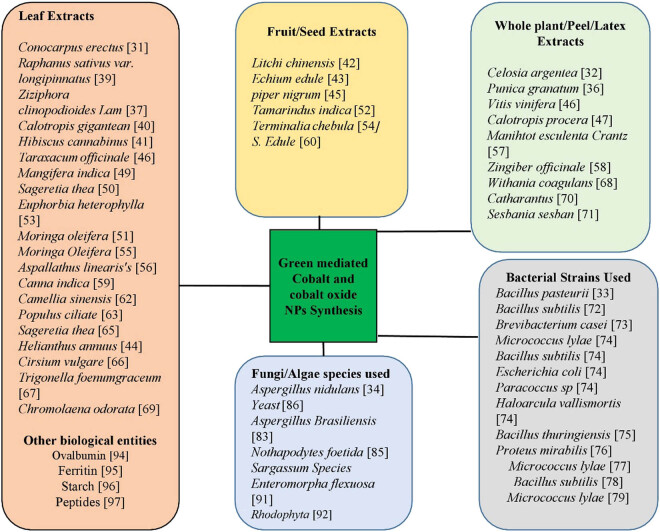
The synthesis of NPs by physical and chemical methods (traditional approaches) has some detrimental effects such as emission of highly expensive and toxic chemicals that carry many threats to the ecosystem, as well as require high energy consumptions, high cost, and time-consuming processes. To overcome these problems, green synthesis of NPs is being implemented. Green-mediated approach emerged as an eco-friendly, biocompatible, and most fascinating, offering more advantages as compared to other traditional approaches [31,32]. In the green-mediated synthesis of NPs, different plants/parts of plants, fruits, bacteria, algae, fungi, and other biological molecules such as starch and egg albumin have been used as a reducing/capping/oxidizing agent [33,34,35]. Different parts of plants, microorganisms, and other biological molecules have been exploited for the fabrication of cobalt and cobalt oxide NPs as shown in Figure 3. These biological resources contain different biomolecules and metabolites that are responsible for the oxidation/reduction, stabilization, and production of particular NPs. Cobalt and cobalt oxide NPs have been synthesized using different methods such as chemical, physical, and biological methods. However, biological approach resulted in less contaminated, safer, cost-effective, and large scale production of NPs [6,31,32].
Figure 3.
Use of different plant/parts of plant, microorganism, and other biological molecules for the synthesis of cobalt and cobalt oxide NPs.
3.1. Green synthesis using plant extracts
In contrast to bacteria, algae, and fungi, plants have been extensively used to synthesize cobalt and cobalt oxide NPs. This is because of their abundance, safe nature, and also the greater stabilization and reduction of plant phytochemicals. This method has been considered as an alternative to complex and costly physicochemical processes because of the following properties: viability, commercial feasibility, eco-friendliness, reliability, no waste generation, and simplicity [36,37]. Different parts of the plant such as leaves, roots, stem, fruits, seeds, latex, inner parts of the plant, shells, and peels have been used to synthesize Co and Co3O4 NPs as shown in Figure 3. The plant extracts have a variety of flavonoids, polysaccharides, amino acids, polyphenols, phenolic acids, ferulic acid, gentisic acid, terpenoids, thymol, tryptophan, and alkaloids, which act as a stabilizing, reducing, and chelating agent as presented in Table 1. These metabolites act as oxygen quenchers, reducing and stabilizing agents, metal chelating agents, and hydrogen donors. The reduction of metal ions by these substances within the plant extracts leads to the formation of respective nanomaterials [38]. Because of a rich source of metabolites, leaf extracts have been widely used for the synthesis of CoNPs. Plant-mediated synthesis of Co and Co3O4 NPs is a simple approach and there is no need for special requirements [6]. The metal salts have been mixed with the whole plant/part of plant extracts, and the reaction completes in a few hours under normal lab conditions [33,34,35,36,37,38].
Table 1.
Plant-mediated synthesis of Co and Co3O4 NPs
| Plant scientific name/common name | Part | NPs | Characterization | Phytoconstituents present in plant | Size (nm) | Shape | References |
|---|---|---|---|---|---|---|---|
| Conocarpus erectus/buttonwood or button mangrove | Leaf extract | Cobalt | SEM and XRD | Tannins, flavonoids, and phenolic acids | 20–60 | Spherical | [31] |
| Celosia argentea/plumed cockscomb or Silver cock’s comb | Whole plant extract | Cobalt | XRD, SEM, and EDX | Flavonoids, tannins, and phenolic acids | 27.42 | [32] | |
| Punica granatum/pomegranate | Peel extract | Cobalt oxide | XRD, SEM, EDX, AFM, FTIR, and UV | Gallic acid, puni87 calagins A and B, ellagic acid, and gallotannins | 40–80 | Spherical | [36] |
| Ziziphora clinopodioides Lam/kakuti-e kuhi | Leaf extract | Cobalt | UV-vis, XRD, EDS, SEM, TEM, and FTIR | Flavonoids, α and β pinen, terpenoids, thymol, piperitenone, sis-isopulegone, pulegone, and cineol | 28.19 | Crystal | [37] |
| Raphanus sativus var. longipinnatus/Radish | Leaf extract | Cobalt | UV-vis, FTIR, SEM, and EDX | Ferulic acid, gentisic acid, raphanusin, erucic acid, sinapate, raphanin, and sulforaphen | 80 | Spherical | [39] |
| Calotropis gigantea/giant milkweed, crown flower, giant calotrope, swallow-wort | Leaf extract | Cobalt oxide | XRD, UV-vis, SEM, TEM, and EDX | Triterpenoids, flavonoids (polyphenols), steroids, cardenolides, and alkaloids | 50 | Spherical | [40] |
| Hibiscus cannabinus/Deccan hemp and Java jute | Leaf extract | Cobalt | XUV-vis, XRD, SEM, TEM, and FTIR | Phytosterols, flavonoids, polyphenols, tannins, steroids, alkaloids, saponins, lignans, essential oils, and glucosides | 20.88 | Crystalline | [41] |
| Litchi chinensis/litchi fruits | Fruits extract | Cobalt oxide | XRD, SEM, TEM, and FTIR | Phenolic acid, flavonoids condensed tannins, luteolin, anthocyanin, and proanthocyanidins. | NA | Rod like | [42] |
| Sechium edule/perennial climber | Fruit extract | Cobalt oxide | XRD, FTIR, TEM, AFM, SEM, and VSM | Ascorbic acid | 3.79 | Irregular | [43] |
| Helianthus annuus/sunflower | Leaf extract | Cobalt oxide | XRD, TGA, SEM | NA | NA | Plate | [44] |
| Piper nigrum | Seeds | Cobalt oxide | UV-vis, AFM, FTIR | NA | 30–60 | Spongy triangular | [45] |
| Vitis vinifera/common grape vine | Whole plant extract | Cobalt oxide | XRD, FT-IR, Raman, TEM, SAED, EDX, DRS, PL, and VSM | Phenolic, stilbenoids, anthocyanin, and acetylated anthocyanin | 10–20 | Rod shape | [46] |
| Calotropis procera/Sodom apple | Latex | Cobalt oxide | XRD, DSC, TEM, EDX, FTIR, and UV-vis | Tryptophan, alkaloids, resins, tannins calotropin, calactin, and calotoxin | 10 | Spherical | [47] |
| Taraxacum officinale/common dandelion | Leaf extract | Cobalt oxide | UV-vis, FT-IR, SEM, and TEM | Flavonoids and phenolic | 50–100 | Spherical | [48] |
| Mangifera indica/mango | Leaf extract | Cobalt | UV-vis, XRD, FT-IR, and SEM | Polyphenolics, flavonoids, and triterpenoids | 25–40 | Irregular shape | [49] |
| Sageretia thea/Osbeck. | Leaf extracts | Cobalt oxide | XRD, ATR-FTIR, HR-SEM, HR-TEM, SAED, and EDS | Friedeline, syringic acid, beta-sitosterol, daucosterol, gluco-syringic acid, and taraxerol | 20.03 | [50] | |
| Moringa oleifera/drumstick tree, horseradish tree, and ben oil tree or benzolive tree | Leaf extract | Cobalt oxide | HR-TEM, EDS, FTIR, and AXS | α-Maltose, adenosine, catechin, chlorogenic acid, rutin, quercetin, kaempferol, caffeic acid, etc. | 20–50 | Symmetric | [51] |
| Tamarindus indica/tamarind fruit | Fruit pulp | Cobalt aluminate | FTIR, UV-vis, XRD, SEM, and RS | Tartaric acid, malic acid, and amino acids | 71 | Rectangular like | [52] |
| Euphorbia heterophylla/fire plant, painted euphorbia, Japanese poinsettia, desert poinsettia, and wild poinsettia | Leaf extract | Cobalt oxide | FTIR, XRD, Malvern Zetasizer Particle Size Analyzer, TEM, and UV-vis DRS | Alkaloid and saponin | 69.75 | Spherical | [53] |
| Terminalia chebula/black- or chebulic myrobalan | Fruit | Cobalt oxide | ATR-FTIR, XRD, TEM, SEM, and EDS | Hydrolysable tannins, gallic acid, chebulic acid, chebulic ellagitannins, and gallate esters | 15–25 | Spherical | [54] |
| M. oleifera/drumstick | Leaf extract | Cobalt | SEM, FTIR, UV-vis, and EDX | Oleic acid, ascorbic acid, dihexadecanoate, octadecenoic acid, methyl ester-hexadecanoic acid, and octadecenamide | 168–295 | Crystal | [55] |
| Aspalathus linearis/Rooibos | Leaf powder | Cobalt oxide | HR-TEM, EDS, Max solid-state Silicon drift detector, XRD, XPS, FTIR, and Raman spectroscopy | Phenolic compounds, aspalalinin, flavones, and flavonols | 3.6 | Quasi-spherical | [56] |
| Manihtot esculenta Crantz/cassava | Whole extract | Cobalt oxide | SEM, EDS, TEM, FT-IR, XRD, VSM, TGA, and EDX | Malonaldehyde, superoxide dismutase, reduced glutathione, and catalase | N/A | Prism like-anchored octahedron | [57] |
| Zingiber officinale/ginger | Whole plant | Cobalt | XRD, SEM, FTIR, VSM, and EDS | Gingerol, zingerone, shagaols, paradole, and starch | 20–50 | Crystal | [58] |
| Canna indica/Indian shot, African arrowroot, edible canna | Leaf extract | Bimetallic cobalt | UV-vis, TEM, EDX, and Thermo Electron LED | Glycosides, alkaloids, and terpenoids | 450–550 | Polydispersed | [59] |
| S. edule/chayote | Fruit extract | Bimetallic cobalt | AAS, SEM, SAED, TEM, XRD, and EPR | Trans-cinnamic acid, phenylacetic acid, trilinolenin, etc. | 47.3 | Prismoidal | [60] |
| Cinnamomum verum/true cinnamon tree or Ceylon cinnamon tree | Bark | Cobalt aluminate | MCM, XRD, SEM, TEM, XPS, IR, and UV-vis | Polysaccharides, polyphenols, flavonoids, and amino acids | 50–60 | Polyhedral | [61] |
| Camellia sinensis/tea plant, tea shrub, and tea tree | Leaf extract | Cobalt oxide | XRD, FESEM, EDX, HR-TEM, PL, FTIR, and UV-visible | Catechins, alkaloids, flavonoids, proteins, enzymes, vitamins, carbohydrates, polyphenols, lipids, and minerals | 39.13 | Quasi-rectangular | [62] |
| Populus ciliata/safaida | Leaf extract | Cobalt oxide | FTIR, TEM, SEM, and XRD | Alcohol-benzene, lignin, holocellulose, and alphacellulose | 15–35 | Square shape | [63] |
| Tamarindus indica/Indian tamarind | Fruit extract | Cobalt aluminate | FTIR, UV-vis, XRD, SEM, and TEM | Tartaric acid, malic and citric acid, potassium ditartrate, amino acids, and vitamin B | 71.3 | Cubic spinel | [64] |
| S. thea/Osbeck. | Leaf extract | Cobalt oxide | XRD, TEM, SEM, SAED, and EDS | Acids/base | 20.03 | Cubic | [65] |
| Cirsium vulgare | Leaf extract | Cobalt oxide | XRD, SEM, and TEM | NA | 20 | [66] | |
| Trigonella foenumgraceum/fenugreek | Leaf extract | Cobalt oxide | FTIR, XPS, XRD, EDS, UV-vis, and TEM | Flavonoids, polyphenols, and different glycosides | 13.2 | Quasi-spherical | [67] |
| Withania coagulans | Whole plant extract | Cobalt oxide | UV-vis and XRD | Withanolide, withaferin, and withacoagin | NA | Cube shape | [68] |
| Chromolaena odorata | Leaf extract | Cobalt | UV-vis, XRD, FT-IR, and SEM | NA | 20–49 | Irregular, cubic, and hexagonal shapes | [69] |
| Catharanthus roseus/periwinkle | Whole plant extract | Cobalt | XRD, FESEM, and EDX | Alkaloids, tannins, flavonoids, polyphenols, and carbohydrates | 27.08 | Spherical shape | [70] |
| Sesbania sesban | Whole extract | Cobalt oxide | HR-TEM, EDAX, and XRD | NA | 15–30 | Spherical | [71] |
Abbreviations: NPs, nanoparticles; NA, not available; SAED, selected area electron diffraction; UV-vis, ultraviolet visible spectroscopy; XPS, X-ray photoelectron spectroscopy; TEM, transmission electron microscopy; XRD, X-ray diffraction; AFM, atomic force microscopy; HR-SEM, high-resolution scanning electron microscopy; HR-TEM, high-resolution transmission electron microscopy; EDX, energy-dispersive X-ray(spectroscopy); FTIR, Fourier-transform infrared spectroscopy; NA, not available; UV-DRS, ultraviolet differential reflectance spectroscopy; TGA, thermo gravimetric analysis; VSM, vibrating sample magnetometer; PL, photoluminescence spectroscopy; PDXL, integrated X-ray powder diffraction software; EDS, energy-dispersive X-ray spectroscopy.
Raphanus sativus var. longipinnatus leaf extract was used to fabricate CoNPs with a spherical structure that represents the presence of ferulic acid, gentisic acid, raphanusin, erucic acid, sinapate, and sulforaphen. The green-synthesized NPs were characterized by UV-visible spectroscopy (UV-vis), X-ray diffraction (XRD), and scanning electron microscopy-energy-dispersive X-ray (spectroscopy) (SEM-EDX). These CoNPs showed potential antibacterial activities and also showed promising cytotoxic activities against the HeLa cancer cell lines in vitro [39]. Co3O4 NPs were synthesized having spherical morphologies with a diameter size of 50–60 nm, using Calotropis gigantea leaf extracts that indicate the presence of triterpenoids, flavonoids (polyphenols), steroids, cardenolides, and alkaloids [40]. Hibiscus cannabinus leaf extract was used to synthesize crystalline CoNPs having a diameter of 20.8 nm. These CoNPs were characterized by UV-vis, XRD, and Fourier-transform infrared spectroscopy (FTIR). Authors reported that the green-synthesized NPs have strong activities against Bacillus subtilis and Escherichia coli [41]. Onwudiwe et al. (2020) reported the bio-mediated synthesis of Co3O4 NPs using the fruit extract of Litchi chinensis. The characterizations of synthesized NPs were confirmed by XRD, SEM, transmission electron microscopy (TEM), and FTIR. SEM analysis revealed that the bioinspired NPs had elongate rod-like morphology. FTIR analysis was also carried out to detect the functional group of various phytochemicals that are involved in the oxidation/reduction and hence the formation of cobalt oxide NPs. The FTIR analysis confirmed the presence of phenolic and carboxylic functional groups, indicating that these groups are responsible for the formation of cobalt oxide NPs [42]. Sechium edule and Helianthus annuus fruits’ extracts [34,44] and Piper nigrum seeds [45] have also been reported for the synthesis of Co3O4 NPs. Vitis vinifera whole plant extracts were used for the synthesis of Co3O4 NPs having a pure single crystalline structure with a size of 10–20 nm in diameter. The TEM and FTIR analysis indicated that the plant extracts play an important role in the stabilization and reduction of nanorods via different types of organic compounds present in the plant extracts [46]. Latex derived from Calotropis procera was used to synthesize Co3O4 NPs with spherical morphologies and average size of 10 nm diameter. These NPs showed only a low toxicity at a very high concentration, proving that they are safe and may be used for different applications in various fields including the field of medicines [47]. Other than aforementioned, different parts of various plants, used for the synthesis of Co and Co3O4 NPs are shown in Table 1 and Figure 3.
3.2. Synthesis of Co and Co3O4 NPs using microorganisms
3.2.1. Bacteria-mediated synthesis
Similar to other synthesis routes such as those of plants and other microorganisms, bacteria also have an intrinsic ability to synthesize NPs of different sizes and morphologies. To date, different types of bacterial strains have been used to synthesize Co and Co3O4 NPs [33,72,74]. This approach also has some challenges compared to other methods that are yet to be solved, like the synthesis of complex materials with the desired phase, a full understanding of the synthesis mechanism at the molecular level to achieve better control over shape and size, and scaling up in order to obtain large amounts of nanomaterials [73]. Chemical methods are already advanced to provide greater control on the shape and size of nanomaterials, but this approach is eco-friendly and limits the drawbacks of the chemical synthesis [73]. However, lengthy procedures and contamination of cultures are the limitations of this approach. Different bacterial species have been used for the synthesis of cobalt and cobalt oxide NPs.
A gram-positive bacterium Bacillus thuringiensis was used to synthesize Co NPs having face-cubic morphology with an average size of 85.3 nm diameter. These green-synthesized NPs were investigated against malarial and dengue vectors (Aedes aegypti and Anopheles subpictus) and showed potential activities against these vectors [75]. Co3O4 NPs synthesized using bacterial strains Micrococcus lylae and B. subtilis have globular and rod shape morphology, respectively [74]. Gram-negative bacterium Proteus mirabilis was used to synthesize CoNPs with an average size of 57 nm and quasi-spherical morphology. The authors evaluated the antibacterial activity of these NPs, and the result showed that these NPs were promising antimicrobial potential against Salmonella typhi, E. coli, Clostridium perfringens, Staphylococcus aureus, and Bacillus cereus [76]. Table 2 provides some examples of bacterial-mediated synthesized Co and Co3O4 NPs.
Table 2.
Bacterial-mediated synthesis of Co and Co3O4 NPs
| Bacterial strain | Gram+/Gram− | NPs | Characterization | Size (nm) | Shape | References |
|---|---|---|---|---|---|---|
| Bacillus pasteurii | Gram+ | Cobalt oxide | XRD, SEM, FE SEM, TEM, and HR-TEM | 10–31 | Irregular | [33] |
| Bacillus subtilis | Gram+ | Cobalt oxide | XRD, SEM, FE SEM, TEM, and HR-TEM | 6.6 | Hollow rod | [72] |
| Brevibacterium casei | Gram+ | Cobalt oxide | XRD, SEM, FE SEM, TEM, and HR-TEM | 6 | Quasi-spherical | [73] |
| Micrococcus lylae | Gram+ | Cobalt oxide | XRD, SEM, FE SEM, TEM, and HR-TEM | 8 | Flower like | [77] |
| B. subtilis | Gram+ | Cobalt oxide | FESE, M ELS-Z2, FESEM, XPS, and EPMA | 3–5 | Rods | [78] |
| M. lylae | Gram+ | Cobalt oxide | XRD, TGA, FE-SEM, TEM, EDS, SAED, and HAADF-STEM | 40 | Spherical | [79] |
| M. lylae | Gram+ | Cobalt | Zetasizer Nano system and FESEM | 356 ± 55 | Globular | [74] |
| B. subtilis | Gram+ | Cobalt | Zetasizer Nano system and FESEM | NA | Rod shape | [74] |
| Escherichia coli | Gram− | Cobalt | Zetasizer Nano system and FESEM | 473 ± 54 | Rod shape | [74] |
| Paracoccus sp. | Gram− | Cobalt | Zetasizer Nano system and FESEM | NA | Biconcave | [74] |
| Haloarcula vallismortis | Gram− | Cobalt | Zetasizer Nano system and FESEM | NA | Globular | [74] |
| Proteus mirabilis | Gram− | Combine Cobalt | UV-vis, EDX, XRD, TEM, DLS, and PDI | 57 | Quasi-spherical | [76] |
| Bacillus thuringiensis | Gram+ | Cobalt | XRD, TEM, FESEM, FTIR, and EDX | 85.3 | Face-cubic | [75] |
Abbreviations: NPs, nanoparticles; NA, not available; SAED, selected area electron Diffraction; UV-vis, Ultraviolet visible spectroscopy; XPS, X-ray photoelectron spectroscopy; TEM, transmission electron microscopy; XRD, X-ray diffraction; HR-TEM, high-resolution transmission electron microscopy; EDX, energy-dispersive X-ray(spectroscopy); FTIR, Fourier-transform infrared spectroscopy; TGA, thermo gravimetric analysis; ATR-FTIR, attenuated total reflectance-Fourier transform infrared spectroscopy; FESEM, field emission scanning electron microscopy; EPMA, electron probe microanalyzer; HAAD-STEM, high-angle annular dark field scanning TEM; EDS, energy-dispersive X-ray spectroscopy.
3.2.2. Fungi-mediated synthesis of Co3O4 NPs
The fungi-mediated approach exhibits unique advantages, as the growth process of fungi is easy to handle and isolate, with the large amount of biomass and high yield of proteins [34]. The entophytic fungi also secrete large amounts of bioactive substances that are necessary for the synthesis of NPs in the presence of precursor substance [34,80]. During the fabrication of NPs from the precursor solution, biomass (fungi) along with the supernatant act as a reduction medium [34,81].
Using fungi, NPs can be synthesized by two pathways: intracellular and extracellular synthesis. The extracellular approach is more commonly used because of the facile separation, high mass harvesting, and easy culturing, which consequently leads to the scaling up of the process at the commercial level [82]. Fungi can also tolerate agitation, flow pressure, and other conditions in the bioreactor for commercial production. Filamentous fungi are highly resistant toward metals as compared to bacteria [83,84]. Different fungal and yeast strains have been used to synthesize cobalt oxide NPs. Aspergillus nidulans was used to synthesize spherical shape Co3O4 NPs with an average size of 20.29 nm diameter [34]. Some examples of yeast and fungi-mediated synthesis of Co3O4 NPs are presented in Table 3.
Table 3.
Fungal-mediated synthesis of Co and Co3O4 NPs
| Fungal species used | NPs | Characterization | Size (nm) | Shape | References |
|---|---|---|---|---|---|
| Aspergillus nidulans | Cobalt oxide | XRD, TEM, FTIR, and EDX | 20.29 | Spherical | [34] |
| Aspergillus brasiliensis | Cobalt oxide | DLS, EDX, XRD, FT-IR, HR-TEM, and FESEM | 20–27 | Quasi-spherical | [83] |
| Nothapodytes foetida | Cobalt oxide | UV-vis | N/A | H/A | [85] |
| Yeast | Cobalt oxide | SEM, XRD, and FT-IR | 24 | Hollow spheres | [86] |
Abbreviations: NPs, nanoparticles; NA, not available; UV-vis, ultraviolet visible spectroscopy; XPS, X-ray photoelectron spectroscopy; TEM, transmission electron microscopy; XRD, X-ray diffraction; HR-TEM, high-resolution transmission electron microscopy; EDX, energy-dispersive X-ray(spectroscopy); FTIR, Fourier-transform infrared spectroscopy; VSM, vibrating-sample magnetometer; FESEM, field emission scanning electron microscopy.
3.3. Algal-mediated approach for the synthesis
Algae are aquatic microorganisms that are used to a great extent for the synthesis of NPs. Algae are also called bionanofactories because they fabricate nanomaterials with high stability, are easy to handle, and do not need cell maintenance [87]. Algae are the key origin of bioactive metabolites that are involved in the fabrication of NPs [88,89]. They contain many bioactive metabolites such as proteins, polysaccharides, and various types of other phytochemicals that are comprised of amino, hydroxyl, and carboxyl functional groups, which are responsible for the fabrication of NPs [87,90]. Algae differ in size and can vary from microalgae to macroalgae [87,88,89,90]. A green-type macroalgae Enteromorpha flexuosa was used to synthesize cobalt–ferrite NPs with an average size of 5–15 nm. These bioinspired cobalt ferrite NPs were characterized by various advanced techniques such as XRD, SEM, TEM, energy-dispersive X-ray spectroscopy (EDS), and XPS [91]. Similarly, cobalt tungstate (CoWO4) NPs synthesized from red seaweeds (Rhodophyta) using agar-agar have also been reported [92].
3.4. Biological product-mediated synthesis of Co and Co3O4 NPs
The plants and microorganisms are widely used for the fabrication of NPs. Besides these, the biological derivatives are used to synthesize NPs as well. They also play a significant role in the stabilization and reduction of nanomaterials [93]. Co3O4 nanocrystals were synthesized from freshly extracted ovalbumin with 8–17 nm diameter confirmed by EPR [94]. Sun et al. (2019) also reported the synthesis of Co3O4 NPs using egg-albumin. Hosein et al. (2004) reported the synthesis of Co3O4 NPs with a uniform shape and size using ferritin [95]. Starch has also been exploited for the synthesis of carbon-encapsulated Co NPs with an average size of 20–35 nm diameter and confirmed by the nanostructure by SEM, TEM, and XRD [96]. Similarly, peptide TLVNN (threonine–leucine–valine–asparagine–asparagine) was also used as a capping agent for in situ syntheses of CoNPs for bioapplications [97].
4. Biological activities of Co and Co3O4 NPs
4.1. Antibacterial activity
Currently, throughout the globe, the emergence of bacterial resistance to the available antibiotics is a major health concern. Therefore, there is a need for the antibiotic agent that can kill pathogenic bacteria which show resistance to the available drugs [98]. The NPs have small size with high surface area compared to the bigger molecules and therefore possess strong antibacterial activities. The NPs have dose-dependent membrane permeation and inhibit the synthesis of bacterial proteins by disturbing the cell membrane [32,99]. Different metallic NPs such as gold, iron, silver, and metal oxide NPs such as iron oxide, cobalt oxide, and copper oxide showed significant antibacterial activities. The AgNPs are the main interest not only in biomedical industries but also in food industries because of its potential antimicrobial behavior [114]. Cobalt and cobalt oxide NPs also possess potential antibacterial activity. Varaprasad et al. (2017) measured the antibacterial activity of biogenic CoNPs against human pathogenic bacteria, and the results concluded that CoNPs have strong antibacterial activities compared to standard antibiotic drug ciprofloxacin [100]. Eltarahony et al. (2018) reported the green synthesis of combined CoNPs using gram-negative bacteria P. mirabilis. The characterization of NPs was confirmed by UV-vis, EDX, XRD, TEM, dynamic light scattering (DLS), and poly dispersity index (PDI). The antibacterial activity was observed using a well diffusion assay against S. typhi, E. coli, C. perfringens, S. aureus, and Enterococcus faecalis [76]. The result showed that the NPs have promising biocide efficiency against these bacteria [76]. Omran et al. (2019) synthesized quasi-spherical shape cobalt oxide NPs with an average size of 20–27 nm diameter using a fungal specie Aspergillus brasiliensis. The first-time mycosynthesized cobalt oxide NPs showed considerable activity against different bacteria [83]. Biogenic synthesis of CoNPs using Celosia argentea whole plant extract has been reported and studied for their antibacterial activities using the disk diffusion method. The synthesized NPs showed remarkable antibacterial activities against B. subtilis and E. coli [32]. Green-mediated cobalt oxide NPs have been synthesized using Hibiscus rosa-sinensis flower extract and their antibacterial activity was measured. These green-synthesized NPs showed promising activities against E. coli, Streptococcus mutans, S. aureus, and Klebsiella pneumonia [101]. Mainly two aspects have been suggested. First, in cobalt oxide NPs, the different positive states of cobalt ions, i.e., Co2+ and Co3+ interact with the parts of the bacterial cell that have a negative charge and cause the death of the bacterial cell. Second, there may be the excitement of electrons on the surface of cobalt oxide because of light irradiation in the conduction and valence band. In the conduction band, there is the formation of superoxide radical anion because of the reaction of excited electrons and oxygen molecules. Finally, the formation of hydrogen peroxide, which is a strong oxidant, occurs. On the surface of NPs, the reaction of water and superoxide radical anion destroys the bacterial cell. Therefore, Cobalt oxide nanoparticles can be a potent antibacterial agent at a minimal level of concentration [6,102]. The antibacterial activities of cobalt and cobalt oxide NPs synthesized from different green routes are presented in Table 4.
Table 4.
Antibacterial activities of green-synthesized cobalt and cobalt oxide NPs
| Biological entity | NPs | Test microorganisms | Method | References |
|---|---|---|---|---|
| Celosia argentea | Cobalt | Bacillus subtilis and Escherichia coli | Disk diffusion | [32] |
| Ziziphora clinopodioides Lam | Cobalt | Salmonella typhimurium, E. coli, Streptococcus pneumonia, Pseudomonas aeruginosa, Staphylococcus aureus, and B. subtilis | Disk diffusion | [37] |
| Raphanus sativus var. longipinnatus | Cobalt | Pseudomonas putida and Klebsiella pneumonia | Disk diffusion | [39] |
| Hibiscus cannabinus | Cobalt | B. subtilis and E. coli | Agar well diffusion | [41] |
| Vitis vinifera | Cobalt oxide | P. aeruginosa, E. coli, S. aureus, and B. subtilis | Disk diffusion | [46] |
| Calotropis procera | Cobalt oxide | E. coli, Pseudomonas sp., Alcaligenes sp., and Enterococcus sp. | Disc diffusion | [47] |
| Moringa oleifera | Cobalt | E. coli and S. aureus | Agar well diffusion | [51] |
| Sechium edule | Bimetallic cobalt | B. subtilis and E. coli | Disk diffusion | [60] |
| Populus ciliata | Cobalt oxide | Bacillus licheniformis, B. subtilis, K. pneumonia, and E. coli | Well diffusion | [63] |
| Sageretia thea | Cobalt oxide | P. aeruginosa, E. coli, K. pneumonia, Staphylococcus epidermis, S. aureus, and B. subtilis | Disc diffusion | [65] |
| Chromolaena odorata | Cobalt | E. coli, K. pneumonia, S. aureus, and Streptococcus pyogenes | Agar well diffusion | [69] |
| Catharanthus roseus/Periwinkle | Cobalt | B. subtilis and E. coli | Disc diffusion | [70] |
| Sesbania sesban | Cobalt oxide | S. aureus | Disk diffusion | [71] |
| Proteus mirabilis | Cobalt | P. aeruginosa, Salmonella typhi, E. coli, Clostridium perfringens, Enterococcus faecalis, Bacillus cereus, and S. aureus | Well diffusion | [76] |
| Aspergillus brasiliensis | Cobalt oxide | B. subtilis, S. aureus, P. aeruginosa, and E. coli | Agar well diffusion | [83] |
Abbreviation: NPs, nanoparticles.
4.2. Antifungal activity
The resistance of bacteria and fungi to the available antibiotics and drugs is at an alarming rate. Therefore, there is a need for strong antifungal agents that can destroy fungi that are resistant to the drugs available [19]. Cobalt and cobalt oxide NPs have various biomedical applications because of different properties including antifungal property. Hou et al. (2020) measured the antifungal properties of green-synthesized CoNPs, and the result showed that the CoNPs have strong antifungal activities against Candida krusei, Candida guilliermondii, Candida glabrata, and Candida albicans [37]. Similarly, cobalt oxide NPs synthesis using flower extract of H. rosa-sinensis and their antifungal activities has been reported. The result showed that the synthesized NPs have strong activities against Aspergillus flavus and A. niger [62].
4.3. Larvicidal activities of CoNPs
For tropical and subtropical countries, the vector and vector-borne diseases have become a big trouble for public health [103]. For the human vector-borne infectious disease, the microorganisms (parasite, bacterium, or virus) and the vectors (mosquito, fly, or tick) are the crucial elements [104]. Mosquitos are the vector of infectious diseases such as dengue, malaria, fever, and yellow fever [104]. The mosquitoes affect humans as well as domestic animals throughout the globe. The biocontrol method is being considered because of high resistance to the chemical insecticides and no new approaches to control the mosquitos. In this approach, different microbes, e.g., B. thuringiensis, have been used in regards to their toxicity to the target vector [75,105,106].
A. subpictus and A. aegypti are the vectors that cause malaria and dengue, respectively, and are the main species of medical interest. In more than 100 countries, about 2.5 billion people are at risk of infection with dengue. Fifty million people are affected worldwide with more than 24,000 deaths per year. There is a need for development of new agents to control these vectors [75]. CoNPs have also been tested against malarial A. subpictus and A. aegypti. Marimuthu et al. (2013) tested the bacterial-mediated synthesized CoNPs using B. thuringiensis against malarial and dengue vectors in vitro. The results showed that the green-synthesized CoNPs have promising activities that exceed those of biocontrol agent (B. thuringiensis) against these vectors [75]. Therefore, it may possible to use the CoNPs in drug formulation against these parasites.
4.4. Antileishmanial activity
The World Health Organization considered the leishmaniasis as one of the uncontrolled, emerging, and neglected diseases with the second-highest prevalence rate, with malaria placing itself at the topmost among the parasitic diseases [107]. Leishmaniasis is caused by leishmania parasites that exist in two forms, promastigote (motile) and amastigote (non-motile) [108]. Leishmaniasis occurs mainly in two clinical forms: visceral leishmaniasis (also called kala-azar), which is the most severe form and - if left untreated - may lead to death, and cutaneous leishmaniasis. It is predicted that about 0.5 million cases of visceral leishmaniasis and 1.5 million cases of cutaneous leishmaniasis occur throughout the globe per year [109]. This deadly disease is endemic in 98 countries of the world. Currently, no vaccines are available against leishmaniasis. The only option available are the treatment drugs and none of the available drugs is ideal because of their cost, duration of therapy, severe side effects, high toxicity, and - most importantly - the resistance of leishmania parasites to the available treatment [107]. Therefore, there is an immediate need to develop an effective antileishmanial approach to combat this deadly infection.
Talha et al. (2017) synthesized cobalt oxide NPs using the extract of Sageretia thea (Osbeck.), a medicinal plant. The characterizations were confirmed by XRD, FTIR, EDS, selected area electron diffraction, high-resolution scanning electron microscopy, and high-resolution transmission electron microscopy. For the first time the antileishmanial activity of the green-synthesized NPs was evaluated using MTT cytotoxic assay. The results showed that the antileishmanial response was dose dependent, and the amastigote parasites were most susceptible as compared to the promastigote. Therefore, cobalt oxide NPs may be one of the possible options in nanomedicine to treat leishmania at any stage of the life cycle [50].
4.5. Antioxidant activity
Oxidative metabolism is a key process for the survival of cells. However, this process has some side effects as they produce free radicals and reactive oxygen species. When these free radicals are produced in the body in the excess amount they can inundate the enzymes such as catalases, peroxidase, and superoxide dismutase and lead to lethal cellular effects by oxidizing cellular proteins, membrane lipids, DNA enzymes, and influence signaling pathways of the cell leading to termination of cellular respiration [110]. Oxidation affects food as well, which is one of the main causes of chemical spoilage that affects flavor, texture, nutritional value, and safety of food. Different types of natural and synthetic antioxidants are available to limit the side effects of oxidation [110,111].
NPs also have strong antioxidant activities. Plant-mediated cobalt oxide NPs having cubic shape morphologies with an average size of 20.03 nm diameter have been prepared using the leaf extract of S. thea. The antioxidant assays, free radical scavenging, total antioxidant capacity, and total reducing power have been evaluated. The biogenic cobalt oxide NPs showed superior radical scavenging potential and an average total antioxidant capacity and total reducing power [50]. Shahzadi et al. (2019) also observed radical scavenging activity of bioinspired CoNPs and reported that the scavenging power and antioxidant activity are dose dependent: the increase of activity leads to the increase in the concentration of CoNPs [32]. CoNPs using the leaf extract of Ziziphora clinopodioides Lam has been synthesized and the antioxidant activities were evaluated. The green-synthesized NPs showed impressive results and have good DPPH free radical scavenging activity [37]. Similarly, DPPH radical scavenging activity of cobalt oxide NPs synthesized from the Sesbania sesban extract has been reported to have minimum activities compared to silver and copper oxide NPs [71].
4.6. Cytotoxic activity
Cytotoxicity assay is a test for the characterization and evaluation of potentially harmful and toxic effects of biomolecules or any other materials on the living organisms/cell cultures. Various types of molecules, plant extracts, and NPs have been used to check the cytotoxic effect [112]. The green-synthesized CoNPs were used in different concentrations to investigate and determine the cytotoxicity of human umbilical vein endothelial cells (HUVECs) in vitro. The cells treated with various concentrations of CoNPs were examined by the MTT test for 48 h. The absorbance rate was determined at 570 nm, which indicated good viability on (HUVECs) cell lines even up to 1,000 mg/mL of CoNPs, and the absence of such type of toxicity of CoNPs has many safe applications in nanomedicines [37]. Padigya et al. (2016) observed the cytotoxic effect of the biogenic CoNPs on HeLa cell lines. They reported that CoNPs showed potential cytotoxic effects against HeLa cancer cell lines [39]. The green-synthesized cobalt oxide NPs were also investigated for cytotoxicity through brine shrimp cytotoxicity assay. Cytotoxicity of the Co3O4 NPs was confirmed by their dose-dependent response, whereas the median lethal concentration was calculated as 19.18 μg/mL [50].
4.7. Hemolytic activities
Hemolysis is the release of hemoglobin in the blood because of the disruption of erythrocyte membranes that may lead to jaundice or anemia. That is why it is very important to check the hemolytic activity of any newly synthesized preparate that can be used for pharmacological purposes [113]. Shahzadi et al. (2019) evaluated the hemolytic activity of green-synthesized CoNPs using the plant extract of C. argentea. The results showed that the biosynthesized CoNPs have less hemolytic activity (2.95%) compared to positive control triton-X-100 which has 95.25% toxicity, whereas the negative taken has 1.02% toxicity [32]. Zaib et al. (2019) fabricated CoNPs using the Catharanthus roseus extract and evaluated their hemolytic activities. They reported that the hemolysis rate of CoNPs is approximately 1.53%, with the negative and positive control rates of 1.02% and 95.28%, respectively [70]. Therefore, CoNPs can be used in drug formation because of their safety, cost-effectiveness, and nontoxic nature. Cobalt oxide NPs were prepared using S. thea extract and evaluated in regards to their biocompatibility and various biological applications. The result presented that the median lethal dose concentration was observed as >58.55 µg/mL and 200 µg/mL for macrophages and RBCs, respectively [50]. For that reason the biosynthesized cobalt oxide NPs might be used at low concentrations for drug formulations.
4.8. Other biological applications
Apart from antimicrobial, antioxidant, antileishmanial, cytotoxic, hemolytic, and larvicidal activities, Co and Co3O4 NPs have various biological and medical applications. After cardiovascular diseases, cancer is the second main cause of human dysphoria [19]. CoNPs showed promising anticancer activities. Kgosiemang et al. (2020) synthesized cobalt oxide NPs using the Euphorbia tirucalli extract and investigated the anti-proliferative activity using MTT assay against MCF-7 breast cancer cell lines. The result showed that the biosynthesized cobalt oxide NPs exhibit promising activities against MCF-7 breast cancer cell lines [53]. The cutaneous wound healing potential of green-synthesized CoNPs has been investigated and it was reported that the ointment of CoNPs has great potential in cutaneous wound healing [37]. The catalytic activity, enzyme inhibition, antidiabetic activity, and anticholinergic activity of biogenic cobalt and cobalt oxide NPs have been reported [37,50].
5. Conclusion
The green-synthesized cobalt and cobalt oxide NPs have various biological and biomedical applications. Traditionally, NPs are synthesized by either physical or chemical methods, which leads not only to environmental toxicity but also to costly and energy-intensive labor. Cobalt and cobalt oxide NPs are synthesized by a green route using the extracts of different plants/parts of plants, microorganisms, and other biological molecules such as gelatin, oleic acid, and starch. The biomediated cobalt and cobalt oxide NPs are environmentally friendly, facile in terms of synthesis, cost effective, and biocompatible.
Footnotes
Funding: The authors state no funding involved.
Author contributions: A. W. and M. A. – conceptualization and original draft preparation; A. W., A. A., A. B., M. D., S. A., and A. U. K. – review and editing. All the authors have read and agreed to the published version of the manuscript.
Conflict of interest: The authors state no conflict of interest.
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Contributor Information
Abdul Waris, Email: awaris@bs.qau.edu.pk.
Muhammad Ali, Email: muhammad.ali@qau.edu.pk.
References
- [1].Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arabian J Chem. 2019 Nov 1;12(7):908–31.; Khan I, Saeed K, Khan I.. Nanoparticles: properties, applications and toxicities. Arabian J Chem. 2019 Nov 1;12(7):908–31. [Google Scholar]
- [2].Nadeem M, Khan R, Afridi K, Nadhman A, Ullah S, Faisal S, et al. Green synthesis of cerium oxide nanoparticles (CeO2 NPs) and their antimicrobial applications: a review. Int J Nanomed. 2020;15:5951. [DOI] [PMC free article] [PubMed]; Nadeem M, Khan R, Afridi K, Nadhman A, Ullah S, Faisal S. et al. Green synthesis of cerium oxide nanoparticles (CeO2 NPs) and their antimicrobial applications: a review. Int J Nanomed. 2020;15:5951. doi: 10.2147/IJN.S255784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kubik T, Bogunia-Kubik K, Sugisaka M. Nanotechnology on duty in medical applications. Curr Pharm Biotechnol. 2005;6(1):17–33 [DOI] [PubMed]; Kubik T, Bogunia-Kubik K, Sugisaka M.. Nanotechnology on duty in medical applications. Curr Pharm Biotechnol. 2005;6(1):17–33. doi: 10.2174/1389201053167248. [DOI] [PubMed] [Google Scholar]
- [4].Smith DM, Simon JK, Baker Jr JR. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13(8):592–605. [DOI] [PMC free article] [PubMed]; Smith DM, Simon JK, Baker Jr JR. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13(8):592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Faucon MP, Pourret O, Lange B. Element case studies: cobalt and copper. In: Agromining: farming for metals. Cham: Springer; 2018. p. 233–9.; Faucon MP, Pourret O, Lange B. Agromining: farming for metals. Cham: Springer; 2018. Element case studies: cobalt and copper; pp. p. 233–9. [Google Scholar]
- [6].Iravani S, Varma RS. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 2020;22(9):2643–61.; Iravani S, Varma RS. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 2020;22(9):2643–61. [Google Scholar]
- [7].Egorova KS, Ananikov VP. Toxicity of metal compounds: knowledge and myths. Organometallics. 2017 Nov 13;36(21):4071–90.; Egorova KS, Ananikov VP.. Toxicity of metal compounds: knowledge and myths. Organometallics. 2017 Nov 13;36(21):4071–90. [Google Scholar]
- [8].Xu Q, Li W, Ding L, Yang W, Xiao H, Ong WJ. Function-driven engineering of 1D carbon nanotubes and 0D carbon dots: mechanism, properties and applications. Nanoscale. 2019;11(4):1475–504. [DOI] [PubMed]; Xu Q, Li W, Ding L, Yang W, Xiao H, Ong WJ.. Function-driven engineering of 1D carbon nanotubes and 0D carbon dots: mechanism, properties and applications. Nanoscale. 2019;11(4):1475–504. doi: 10.1039/c8nr08738e. [DOI] [PubMed] [Google Scholar]
- [9].Ansari SM, Bhor RD, Pai KR, Sen D, Mazumder S, Ghosh K, et al. Cobalt nanoparticles for biomedical applications: facile synthesis, physiochemical characterization, cytotoxicity behavior and biocompatibility. Appl Surf Sci. 2017 Aug 31;414:171–87.; Ansari SM, Bhor RD, Pai KR, Sen D, Mazumder S, Ghosh K. et al. Cobalt nanoparticles for biomedical applications: facile synthesis, physiochemical characterization, cytotoxicity behavior and biocompatibility. Appl Surf Sci. 2017 Aug 31;414:171–87. [Google Scholar]
- [10].Raveau B, Seikh MM. Charge ordering in cobalt oxides: impact on structure, magnetic and transport properties. Z Anorg Allg Chem. 2015 Jul;641(8–9):1385–94.; Raveau B, Seikh MM. Charge ordering in cobalt oxides: impact on structure, magnetic and transport properties. Z Anorg Allg Chem. 2015 Jul;641(8–9):1385–94. [Google Scholar]
- [11].Bersuker IB. Electronic structure and properties of transition metal compounds. New York: Wiley; 1996.; Bersuker IB. Electronic structure and properties of transition metal compounds. New York: Wiley; 1996. [Google Scholar]
- [12].Liu J, Wang Z, Yan X, Jian P. Metallic cobalt nanoparticles imbedded into ordered mesoporous carbon: a non-precious metal catalyst with excellent hydrogenation performance. J Colloid Interface Sci. 2017 Nov 1;505:789–95. [DOI] [PubMed]; Liu J, Wang Z, Yan X, Jian P.. Metallic cobalt nanoparticles imbedded into ordered mesoporous carbon: a non-precious metal catalyst with excellent hydrogenation performance. J Colloid Interface Sci. 2017 Nov 1;505:789–95. doi: 10.1016/j.jcis.2017.06.081. [DOI] [PubMed] [Google Scholar]
- [13].Su Y, Zhu Y, Jiang H, Shen J, Yang X, Zou W, et al. Cobalt nanoparticles embedded in N-doped carbon as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nanoscale. 2014;6(24):15080–9. [DOI] [PubMed]; Su Y, Zhu Y, Jiang H, Shen J, Yang X, Zou W. et al. Cobalt nanoparticles embedded in N-doped carbon as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nanoscale. 2014;6(24):15080–9. doi: 10.1039/c4nr04357j. [DOI] [PubMed] [Google Scholar]
- [14].Azharuddin M, Zhu GH, Das D, Ozgur E, Uzun L, Turner AP, et al. A repertoire of biomedical applications of noble metal nanoparticles. Chem Commun. 2019;55(49):6964–96. [DOI] [PubMed]; Azharuddin M, Zhu GH, Das D, Ozgur E, Uzun L, Turner AP. et al. A repertoire of biomedical applications of noble metal nanoparticles. Chem Commun. 2019;55(49):6964–96. doi: 10.1039/c9cc01741k. [DOI] [PubMed] [Google Scholar]
- [15].Cardoso VF, Francesko A, Ribeiro C, Bañobre‐López M, Martins P, Lanceros‐Mendez S. Advances in magnetic nanoparticles for biomedical applications. Adv Healthcare Mater. 2018 Mar;7(5):1700845. [DOI] [PubMed]; Cardoso VF, Francesko A, Ribeiro C, Bañobre‐López M, Martins P, Lanceros‐Mendez S.. Advances in magnetic nanoparticles for biomedical applications. Adv Healthcare Mater. 2018 Mar;7(5):1700845. doi: 10.1002/adhm.201700845. [DOI] [PubMed] [Google Scholar]
- [16].Liakos I, Grumezescu AM, Holban AM. Magnetite nanostructures as novel strategies for anti-infectious therapy. Molecules. 2014 Aug;19(8):12710–26. [DOI] [PMC free article] [PubMed]; Liakos I, Grumezescu AM, Holban AM.. Magnetite nanostructures as novel strategies for anti-infectious therapy. Molecules. 2014 Aug;19(8):12710–26. doi: 10.3390/molecules190812710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eleraky NE, Allam A, Hassan SB, Omar MM. Nanomedicine fight against antibacterial resistance: an overview of the recent pharmaceutical innovations. Pharmaceutics. 2020 Feb;12(2):142. [DOI] [PMC free article] [PubMed]; Eleraky NE, Allam A, Hassan SB, Omar MM.. Nanomedicine fight against antibacterial resistance: an overview of the recent pharmaceutical innovations. Pharmaceutics. 2020 Feb;12(2):142. doi: 10.3390/pharmaceutics12020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol. 2018;9(1):1050–74. [DOI] [PMC free article] [PubMed]; Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK.. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol. 2018;9(1):1050–74. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ahmad S, Munir S, Zeb N, Ullah A, Khan B, Ali J, et al. Green nanotechnology: a review on green synthesis of silver nanoparticles – an ecofriendly approach. Int J Nanomed. 2019;14:5087. [DOI] [PMC free article] [PubMed]; Ahmad S, Munir S, Zeb N, Ullah A, Khan B, Ali J. et al. Green nanotechnology: a review on green synthesis of silver nanoparticles – an ecofriendly approach. Int J Nanomed. 2019;14:5087. doi: 10.2147/IJN.S200254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pachapur V, Brar SK, Verma M, Surampalli RY. Nanomaterials in the environment. In: Nano-ecotoxicology of natural and engineered nanomaterials for animals and humans. American society of civil engineering library; 2015. p. 421–37.; Pachapur V, Brar SK, Verma M, Surampalli RY. Nanomaterials in the environment Nano-ecotoxicology of natural and engineered nanomaterials for animals and humans. American society of civil engineering library. 2015. pp. p. 421–37. . In:
- [21].Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arabian J Chem. 2017. 10.1016/j.arabjc.2017.05.011. [DOI]; Khan I, Saeed K, Khan I.. Nanoparticles: properties, applications and toxicities. Arabian J Chem. 2017 doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- [22].Ranjit K, Baquee AA. Nanoparticle: an overview of preparation, characterization and application. Int Res J Pharm. 2013;12:908–31.; Ranjit K, Baquee AA.. Nanoparticle: an overview of preparation, characterization and application. Int Res J Pharm. . 2013;12:908–31. [Google Scholar]
- [23].Anyaegbunam FN, Augustine C. A study of optical band gap and associated urbach energy tail of chemically deposited metal oxides binary thin films. Dig J Nanomater Biostruct. 2018 Jul 1;3(3):847–56.; Anyaegbunam FN, Augustine C.. A study of optical band gap and associated urbach energy tail of chemically deposited metal oxides binary thin films. Dig J Nanomater Biostruct. 2018 Jul 1;3(3):847–56. [Google Scholar]
- [24].Stella C, Soundararajan N, Ramachandran K. Structural, optical, and magnetic properties of Mn and Fe-doped Co3O4 nanoparticles. AIP Adv. 2015 Aug 4;5(8):087104.; Stella C, Soundararajan N, Ramachandran K.. Structural, optical, and magnetic properties of Mn and Fe-doped Co3O4 nanoparticles. AIP Adv. 2015 Aug 4;5(8):087104. [Google Scholar]
- [25].Korde P, Ghotekar S, Pagar T, Pansambal S, Oza R, Mane D. Plant extract assisted eco-benevolent synthesis of selenium nanoparticles – a review on plant parts involved, characterization and their recent applications. J Chem Rev. 2020 Apr 23;2:157–68.; Korde P, Ghotekar S, Pagar T, Pansambal S, Oza R, Mane D.. Plant extract assisted eco-benevolent synthesis of selenium nanoparticles – a review on plant parts involved, characterization and their recent applications. J Chem Rev. 2020 Apr 23;2:157–68. [Google Scholar]
- [26].Li W, Jung H, Hoa ND, Kim D, Hong SK, Kim H. Nanocomposite of cobalt oxide nanocrystals and single-walled carbon nanotubes for a gas sensor application. Sens Actuators B. 2010 Sep 21;150(1):160–6.; Li W, Jung H, Hoa ND, Kim D, Hong SK, Kim H.. Nanocomposite of cobalt oxide nanocrystals and single-walled carbon nanotubes for a gas sensor application. Sens Actuators B. 2010 Sep 21;150(1):160–6. [Google Scholar]
- [27].Li WY, Xu LN, Chen J. Co3O4 nanomaterials in lithium‐ion batteries and gas sensors. Adv Funct Mater. 2005 May;15(5):851–7.; Li WY, Xu LN, Chen J.. Co3O4 nanomaterials in lithium‐ion batteries and gas sensors. Adv Funct Mater. 2005 May;15(5):851–7. [Google Scholar]
- [28].Kumar R, Kim HJ, Park S, Srivastava A, Oh IK. Graphene-wrapped and cobalt oxide-intercalated hybrid for extremely durable super-capacitor with ultrahigh energy and power densities. Carbon. 2014 Nov 1;79:192–202.; Kumar R, Kim HJ, Park S, Srivastava A, Oh IK.. Graphene-wrapped and cobalt oxide-intercalated hybrid for extremely durable super-capacitor with ultrahigh energy and power densities. Carbon. 2014 Nov 1;79:192–202. [Google Scholar]
- [29].Adekunle AS, Oyekunle JA, Durosinmi LM, Oluwafemi OS, Olayanju DS, Akinola AS, et al. Potential of cobalt and cobalt oxide nanoparticles as nanocatalyst towards dyes degradation in wastewater. Nano-Struct Nano-Objects. 2020 Feb 1;21:100405.; Adekunle AS, Oyekunle JA, Durosinmi LM, Oluwafemi OS, Olayanju DS, Akinola AS. et al. Potential of cobalt and cobalt oxide nanoparticles as nanocatalyst towards dyes degradation in wastewater. Nano-Struct Nano-Objects. 2020 Feb 1;21:100405. [Google Scholar]
- [30].Hagelin-Weaver HA, Hoflund GB, Minahan DM, Salaita GN. Electron energy loss spectroscopic investigation of Co metal, CoO, and Co3O4 before and after Ar + bombardment. Applied Surface Science. 2004 Aug 31;235(4):420–48.; Hagelin-Weaver HA, Hoflund GB, Minahan DM, Salaita GN.. Electron energy loss spectroscopic investigation of Co metal, CoO, and Co3O4 before and after Ar + bombardment. Applied Surface Science. 2004 Aug 31;235(4):420–48. [Google Scholar]
- [31].Ahmed K, Tariq I, Siddiqui SU, Mudassir M. Green synthesis of cobalt nanoparticles by using methanol extract of plant leaf as reducing agent. Pure Appl Biol. 2016;5(3):453.; Ahmed K, Tariq I, Siddiqui SU, Mudassir M.. Green synthesis of cobalt nanoparticles by using methanol extract of plant leaf as reducing agent. Pure Appl Biol. 2016;5(3):453. [Google Scholar]
- [32].Shahzadi T, Zaib M, Riaz T, Shehzadi S, Abbasi MA, Shahid M. Synthesis of eco-friendly cobalt nanoparticles using Celosia argentea plant extract and their efficacy studies as antioxidant, antibacterial, hemolytic and catalytical agent. Arabian J Sci Eng. 2019;44(7):6435–44.; Shahzadi T, Zaib M, Riaz T, Shehzadi S, Abbasi MA, Shahid M.. Synthesis of eco-friendly cobalt nanoparticles using Celosia argentea plant extract and their efficacy studies as antioxidant, antibacterial, hemolytic and catalytical agent. Arabian J Sci Eng. 2019;44(7):6435–44. [Google Scholar]
- [33].Hsu CM, Huang YH, Chen HJ, Lee WC, Chiu HW, Maity JP, et al. Green synthesis of nano-Co3O4 by microbial induced precipitation (MIP) process using Bacillus pasteurii and its application as supercapacitor. Mater Today Commun. 2018;1(14):302–11.; Hsu CM, Huang YH, Chen HJ, Lee WC, Chiu HW, Maity JP. et al. Green synthesis of nano-Co3O4 by microbial induced precipitation (MIP) process using Bacillus pasteurii and its application as supercapacitor. Mater Today Commun. 2018;1(14):302–11. [Google Scholar]
- [34].Vijayanandan AS, Balakrishnan RM. Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans. J Environ Manage. 2018;15(218):442–50. [DOI] [PubMed]; Vijayanandan AS, Balakrishnan RM.. Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans . J Environ Manage. 2018;15(218):442–50. doi: 10.1016/j.jenvman.2018.04.032. [DOI] [PubMed] [Google Scholar]
- [35].Vaya D, Das BK. Green synthesis of cobalt oxide nanoparticles by a starch-assisted method. Nanosci Nanotechnol. 2019 Sep 1;9(3):362–70.; Vaya D, Das BK.. Green synthesis of cobalt oxide nanoparticles by a starch-assisted method. Nanosci Nanotechnol. 2019 Sep 1;9(3):362–70. [Google Scholar]
- [36].Bibi I, Nazar N, Iqbal M, Kamal S, Nawaz H, Nouren S, et al. Green and eco-friendly synthesis of cobalt-oxide nanoparticle: characterization and photo-catalytic activity. Adv Powder Technol. 2017;28(9):2035–43.; Bibi I, Nazar N, Iqbal M, Kamal S, Nawaz H, Nouren S. et al. Green and eco-friendly synthesis of cobalt-oxide nanoparticle: characterization and photo-catalytic activity. Adv Powder Technol. 2017;28(9):2035–43. [Google Scholar]
- [37].Hou H, Mahdavi B, Paydarfard S, Zangeneh MM, Zangeneh A, Sadeghian N, et al. Novel green synthesis and antioxidant, cytotoxicity, antimicrobial, antidiabetic, anticholinergics, and wound healing properties of cobalt nanoparticles containing Ziziphora clinopodioides Lam leaves extract. Sci Rep. 2020;10(1):1–9. [DOI] [PMC free article] [PubMed] [Retracted]; Hou H, Mahdavi B, Paydarfard S, Zangeneh MM, Zangeneh A, Sadeghian N. et al. Novel green synthesis and antioxidant, cytotoxicity, antimicrobial, antidiabetic, anticholinergics, and wound healing properties of cobalt nanoparticles containing Ziziphora clinopodioides Lam leaves extract. Sci Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-68951-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [38].Dwivedi AD, Gopal K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf A. 2010 Oct 20;369(1–3):27–33.; Dwivedi AD, Gopal K.. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf A. 2010 Oct 20;369(1–3):27–33. [Google Scholar]
- [39].Koyyati R, Kudle KR, Padigya PR. Evaluation of antibacterial and cytotoxic activity of green synthesized cobalt nanoparticles using Raphanus sativus var. longipinnatus leaf extract. Int J PharmTech Res. 2016;9(3):466–72.; Koyyati R, Kudle KR, Padigya PR.. Evaluation of antibacterial and cytotoxic activity of green synthesized cobalt nanoparticles using Raphanus sativus var. longipinnatus leaf extract. Int J PharmTech Res. 2016;9(3):466–72. [Google Scholar]
- [40].Sharma JK, Srivastava P, Singh G, Akhtar MS, Ameen S. Green synthesis of Co3O4 nanoparticles and their applications in thermal decomposition of ammonium perchlorate and dye-sensitized solar cells. Mater Sci Eng B. 2015;193:181–8.; Sharma JK, Srivastava P, Singh G, Akhtar MS, Ameen S.. Green synthesis of Co3O4 nanoparticles and their applications in thermal decomposition of ammonium perchlorate and dye-sensitized solar cells. Mater Sci Eng B. 2015;193:181–8. [Google Scholar]
- [41].Kharade Suvarta D, Nikam Gurunath H, Mane Gavade Shubhangi J, Patil Sachinkumar R, Gaikwad Kishor V. Biogenic synthesis of cobalt nanoparticles using Hibiscus cannabinus leaf extract and their antibacterial activity. Res J Chem Environ. 2020;24(5):9–13.; Kharade Suvarta D, Nikam Gurunath H, Mane Gavade Shubhangi J, Patil Sachinkumar R, Gaikwad Kishor V.. Biogenic synthesis of cobalt nanoparticles using Hibiscus cannabinus leaf extract and their antibacterial activity. Res J Chem Environ. 2020;24(5):9–13. [Google Scholar]
- [42].Onwudiwe DC, Ravele MP, Elemike EE. Eco-friendly synthesis, structural properties and morphology of cobalt hydroxide and cobalt oxide nanoparticles using extract of Litchi chinensis. Nano-Struct Nano-Objects. 2020;1(23):100470.; Onwudiwe DC, Ravele MP, Elemike EE.. Eco-friendly synthesis, structural properties and morphology of cobalt hydroxide and cobalt oxide nanoparticles using extract of Litchi chinensis . Nano-Struct Nano-Objects. 2020;1(23):100470. [Google Scholar]
- [43].Das RK, Golder AK. Co3O4 spinel nanoparticles decorated graphite electrode: bio-mediated synthesis and electrochemical H2O2 sensing. Electrochim Acta. 2017;251:415–26.; Das RK, Golder AK. Co3O4 spinel nanoparticles decorated graphite electrode: bio-mediated synthesis and electrochemical H2O2 sensing. Electrochim Acta. 2017;251:415–26. [Google Scholar]
- [44].Saeed M, Akram N, Naqvi SA, Usman M, Abbas MA, Adeel M, Nisar A. Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: characterization and photo-catalytic activity. Green Proc Synth. 2019;8(1):382–90.; Saeed M, Akram N, Naqvi SA, Usman M, Abbas MA, Adeel M, Nisar A.. Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: characterization and photo-catalytic activity. Green Proc Synth. 2019;8(1):382–90. [Google Scholar]
- [45].Saravanakumar P, Muthukumar M, Muthuchudarkodi RR, Ramkumar P. Piper nigrum mediated green synthesis, characterization of undoped cobalt oxide and cerium ion doped cobalt oxide nanoparticles. Int J Recent Res Aspects. 2018;918–23.; Saravanakumar P, Muthukumar M, Muthuchudarkodi RR, Ramkumar P. Piper nigrum mediated green synthesis, characterization of undoped cobalt oxide and cerium ion doped cobalt oxide nanoparticles. Int J Recent Res Aspects. 2018:918–23. [Google Scholar]
- [46].Kombaiah K, Vijaya JJ, Kennedy LJ, Kaviyarasu K, Ramalingam RJ, Al-Lohedan HA. Green synthesis of Co3O4 nanorods for highly efficient catalytic, photocatalytic, and antibacterial activities. J Nanosci Nanotechnol. 2019;19(5):2590–8. [DOI] [PubMed]; Kombaiah K, Vijaya JJ, Kennedy LJ, Kaviyarasu K, Ramalingam RJ, Al-Lohedan HA. Green synthesis of Co3O4 nanorods for highly efficient catalytic, photocatalytic, and antibacterial activities. J Nanosci Nanotechnol. 2019;19(5):2590–8. doi: 10.1166/jnn.2019.15826. [DOI] [PubMed] [Google Scholar]
- [47].Dubey S, Kumar J, Kumar A, Sharma YC. Facile and green synthesis of highly dispersed cobalt oxide (Co3O4) nano powder: characterization and screening of its eco-toxicity. Adv Powder Technol. 2018;29(11):2583–90.; Dubey S, Kumar J, Kumar A, Sharma YC.. Facile and green synthesis of highly dispersed cobalt oxide (Co3O4) nano powder: characterization and screening of its eco-toxicity. Adv Powder Technol. 2018;29(11):2583–90. [Google Scholar]
- [48].Rasheed T, Nabeel F, Bilal M, Iqbal HM. Biogenic synthesis and characterization of cobalt oxide nanoparticles for catalytic reduction of direct yellow-142 and methyl orange dyes. Biocatal Agric Biotechnol. 2019;19:101154.; Rasheed T, Nabeel F, Bilal M, Iqbal HM.. Biogenic synthesis and characterization of cobalt oxide nanoparticles for catalytic reduction of direct yellow-142 and methyl orange dyes. Biocatal Agric Biotechnol. 2019;19:101154. [Google Scholar]
- [49].Okwunodulu FU, Chukwuemeka-Okorie HO, Okorie FC. Biological synthesis of cobalt nanoparticles from Mangifera indica leaf extract and application by detection of manganese(ii) ions present in industrial wastewater. Chem Sci Int J. 2019;10:1–8.; Okwunodulu FU, Chukwuemeka-Okorie HO, Okorie FC.. Biological synthesis of cobalt nanoparticles from Mangifera indica leaf extract and application by detection of manganese(ii) ions present in industrial wastewater. Chem Sci Int J. 2019;10:1–8. [Google Scholar]
- [50].Khalil AT, Ovais M, Ullah I, Ali M, Shinwari ZK, Maaza M. Physical properties, biological applications and biocompatibility studies on biosynthesized single phase cobalt oxide (Co3O4) nanoparticles via Sageretia thea (Osbeck.). Arabian J Chem. 2020;13(1):606–19.; Khalil AT, Ovais M, Ullah I, Ali M, Shinwari ZK, Maaza M.. Physical properties, biological applications and biocompatibility studies on biosynthesized single phase cobalt oxide (Co3O4) nanoparticles via Sageretia thea (Osbeck.) Arabian J Chem. 2020;13(1):606–19. [Google Scholar]
- [51].Matinise N, Mayedwa N, Fuku XG, Mongwaketsi N, Maaza M. Green synthesis of cobalt(II, III) oxide nanoparticles using Moringa oleifera natural extract as high electrochemical electrode for supercapacitors. In AIP Conference Proceedings 2018 May 3, Vol. 1962, No. 1, p. 040005.; Matinise N, Mayedwa N, Fuku XG, Mongwaketsi N, Maaza M.. Green synthesis of cobalt(II, III) oxide nanoparticles using Moringa oleifera natural extract as high electrochemical electrode for supercapacitors. In AIP Conference Proceedings 2018 May 3. Vol. 1962(No. 1):p. 040005.. [Google Scholar]
- [52].Mindru I, Gingasu D, Patron L, Ianculescu A, Surdu VA, Culita DC, et al. A new approach: synthesis of cobalt aluminate nanoparticles using tamarind fruit extract. Mater Sci Eng B. 2019;246:42–8.; Mindru I, Gingasu D, Patron L, Ianculescu A, Surdu VA, Culita DC. et al. A new approach: synthesis of cobalt aluminate nanoparticles using tamarind fruit extract. Mater Sci Eng B. 2019;246:42–8. [Google Scholar]
- [53].Oktri Mulya Dewi N, Yulizar Y, Oky Bagus Apriandanu D. Green synthesis of Co3O4 nanoparticles using Euphorbia heterophylla L. leaves extract: characterization and photocatalytic activity. MS&E. 2019;509(1):012105.; Oktri Mulya Dewi N, Yulizar Y, Oky Bagus Apriandanu D.. Green synthesis of Co3O4 nanoparticles using Euphorbia heterophylla L. leaves extract: characterization and photocatalytic activity. MS&E. 2019;509(1):012105. [Google Scholar]
- [54].Edison TN, Atchudan R, Sethuraman MG, Lee YR. Supercapacitor performance of carbon supported Co3O4 nanoparticles synthesized using Terminalia chebula fruit. J Taiwan Inst Chem Eng. 2016;68:489–95.; Edison TN, Atchudan R, Sethuraman MG, Lee YR.. Supercapacitor performance of carbon supported Co3O4 nanoparticles synthesized using Terminalia chebula fruit. J Taiwan Inst Chem Eng. 2016;68:489–95. [Google Scholar]
- [55].Younis S, Ijaz I, Nazir A, Bukhari A, Rizwan A, Gilani E. Synthesis, characterization & bacterial evaluation of cobalt nanoparticles using drumstick leaf extract via green route. EasyChair; 2020. p. 15.; Younis S, Ijaz I, Nazir A, Bukhari A, Rizwan A, Gilani E. Synthesis, characterization & bacterial evaluation of cobalt nanoparticles using drumstick leaf extract via green route. EasyChair. 2020. p. p. 15.
- [56].Diallo A, Beye AC, Doyle TB, Park E, Maaza M. Green synthesis of Co3O4 nanoparticles via Aspalathus linearis: physical properties. Green Chem Lett Rev. 2015;8(3–4):30–6.; Diallo A, Beye AC, Doyle TB, Park E, Maaza M.. Green synthesis of Co3O4 nanoparticles via Aspalathus linearis: physical properties. Green Chem Lett Rev. 2015;8(3–4):30–6. [Google Scholar]
- [57].Ikhuoria EU, Omorogbe SO, Sone BT, Maaza M. Bioinspired shape controlled antiferromagnetic Co3O4 with prism like-anchored octahedron morphology: a facile green synthesis using Manihot esculenta Crantz extract. Sci Technol Mater. 2018;30(2):92–8.; Ikhuoria EU, Omorogbe SO, Sone BT, Maaza M.. Bioinspired shape controlled antiferromagnetic Co3O4 with prism like-anchored octahedron morphology: a facile green synthesis using Manihot esculenta Crantz extract. Sci Technol Mater. 2018;30(2):92–8. [Google Scholar]
- [58].Mianai RS, Ghasemzadeh MA, Monfared MR. Green fabrication of cobalt NPs using aqueous extract of antioxidant rich zingiber and their catalytic applications for the synthesis of pyrano[2,3-c]pyrazoles. Combin Chem High Throughput Screen. 2019;22(1):18–26. [DOI] [PubMed]; Mianai RS, Ghasemzadeh MA, Monfared MR.. Green fabrication of cobalt NPs using aqueous extract of antioxidant rich zingiber and their catalytic applications for the synthesis of pyrano[2,3-c]pyrazoles. Combin Chem High Throughput Screen. 2019;22(1):18–26. doi: 10.2174/1386207322666190307160354. [DOI] [PubMed] [Google Scholar]
- [59].Abimbola Akinsiku A, Olugbenga Dare E, Oyewale Ajani O, Ayo-Ajayi J, Ademosun OT, Oluwakayode Ajayi S. Room temperature phytosynthesis of Ag/Co bimetallic nanoparticles using aqueous leaf extract of Canna indica. E&ES. 2018;173(1):012019.; Abimbola Akinsiku A, Olugbenga Dare E, Oyewale Ajani O, Ayo-Ajayi J, Ademosun OT, Oluwakayode Ajayi S.. Room temperature phytosynthesis of Ag/Co bimetallic nanoparticles using aqueous leaf extract of Canna indica. E&ES. 2018;173(1):012019. [Google Scholar]
- [60].Chelli VR, Golder AK. One pot green synthesis of Pt, Co and Pt@Co core–shell nanoparticles using Sechium edule. J Chem Technol Biotechnol. 2019;94(3):911–8.; Chelli VR, Golder AK.. One pot green synthesis of Pt, Co and Pt@Co core–shell nanoparticles using Sechium edule . J Chem Technol Biotechnol. 2019;94(3):911–8. [Google Scholar]
- [61].Gingasu D, Mindru I, Patron L, Marinescu G, Ianculescu A, Surdu VA, et al. Synthesis of cobalt aluminate nanoparticles by combustion methods using cinnamon bark extract. Rev Roumaine Chim. 2018;63(5–6):459–66.; Gingasu D, Mindru I, Patron L, Marinescu G, Ianculescu A, Surdu VA. et al. Synthesis of cobalt aluminate nanoparticles by combustion methods using cinnamon bark extract. Rev Roumaine Chim. 2018;63(5–6):459–66. [Google Scholar]
- [62].Anuradha CT, Raji P. Facile synthesis and characterization of Co3O4 nanoparticles for high-performance supercapacitors using Camellia sinensis. Appl Phys A. 2020;126(3):164.; Anuradha CT, Raji P.. Facile synthesis and characterization of Co3O4 nanoparticles for high-performance supercapacitors using Camellia sinensis . Appl Phys A. 2020;126(3):164. [Google Scholar]
- [63].Hafeez M, Shaheen R, Akram B, Haq S, Mahsud S, Ali S, et al. Green synthesis of cobalt oxide nanoparticles for potential biological applications. Mater Res Exp. 2020;7(2):025019.; Hafeez M, Shaheen R, Akram B, Haq S, Mahsud S, Ali S. et al. Green synthesis of cobalt oxide nanoparticles for potential biological applications. Mater Res Exp. 2020;7(2):025019. [Google Scholar]
- [64].Mindru I, Gingasu D, Patron L, Ianculescu A, Surdu VA, Culita DC, et al. A new approach: synthesis of cobalt aluminate nanoparticles using tamarind fruit extract. Mater Sci Eng B. 2019;246:42–8.; Mindru I, Gingasu D, Patron L, Ianculescu A, Surdu VA, Culita DC. et al. A new approach: synthesis of cobalt aluminate nanoparticles using tamarind fruit extract. Mater Sci Eng B. 2019;246:42–8. [Google Scholar]
- [65].Khalil AT, Ovais M, Ullah I, Ali M, Shinwari ZK, Maaza M. Physical properties, biological applications and biocompatibility studies on biosynthesized single phase cobalt oxide (Co3O4) nanoparticles via Sageretia thea (Osbeck.). Arabian J Chem. 2020;13(1):606–19.; Khalil AT, Ovais M, Ullah I, Ali M, Shinwari ZK, Maaza M.. Physical properties, biological applications and biocompatibility studies on biosynthesized single phase cobalt oxide (Co3O4) nanoparticles via Sageretia thea (Osbeck.) Arabian J Chem. 2020;13(1):606–19. [Google Scholar]
- [66].Fallahi M, Norouzi B. Synthesis of cobalt oxide nanoparticles using Cirsium vulgare leaves extract and evaluation of electrocatalytic effects on oxidation of l-cysteine. Ionics. 2020;14:1–1.; Fallahi M, Norouzi B.. Synthesis of cobalt oxide nanoparticles using Cirsium vulgare leaves extract and evaluation of electrocatalytic effects on oxidation of l-cysteine. Ionics. 2020;14:1–1. [Google Scholar]
- [67].Akhlaghi N, Najafpour-Darzi G, Younesi H. Facile and green synthesis of cobalt oxide nanoparticles using ethanolic extract of Trigonella foenumgraceum (Fenugreek) leaves. Adv Powder Technol. 2020;24:3562–9.; Akhlaghi N, Najafpour-Darzi G, Younesi H.. Facile and green synthesis of cobalt oxide nanoparticles using ethanolic extract of Trigonella foenumgraceum (Fenugreek) leaves. Adv Powder Technol. 2020;24:3562–9. [Google Scholar]
- [68].Hasan M, Zafar A, Shahzadi I, Luo F, Hassan SG, Tariq T, et al. Fractionation of biomolecules in Withania coagulans extract for bioreductive nanoparticle synthesis, antifungal and biofilm activity. Molecules. 2020;25(15):3478. [DOI] [PMC free article] [PubMed]; Hasan M, Zafar A, Shahzadi I, Luo F, Hassan SG, Tariq T. et al. Fractionation of biomolecules in Withania coagulans extract for bioreductive nanoparticle synthesis, antifungal and biofilm activity. Molecules. 2020;25(15):3478. doi: 10.3390/molecules25153478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Igwe OU, Ekebo ES. Biofabrication of cobalt Nanoparticle odorata and their potential. Res J Chem. 2018;8(1):11–7.; Igwe OU, Ekebo ES.. Biofabrication of cobalt Nanoparticle odorata and their potential. Res J Chem. 2018;8(1):11–7. [Google Scholar]
- [70].Zaib M, Shahzadi T, Muzammal I, Farooq U. Catharanthus roseus extract mediated synthesis of cobalt nanoparticles: evaluation of antioxidant, antibacterial, hemolytic and catalytic activities. Inorg Nano-Met Chem. 2020;9:1–0.; Zaib M, Shahzadi T, Muzammal I, Farooq U.. Catharanthus roseus extract mediated synthesis of cobalt nanoparticles: evaluation of antioxidant, antibacterial, hemolytic and catalytic activities. Inorg Nano-Met Chem. 2020;9:1–0. [Google Scholar]
- [71].Ghadi FE, Ghara AR, Naeimi A. Phytochemical fabrication, characterization, and antioxidant application of copper and cobalt oxides nanoparticles using Sesbania sesban plant. Chem Pap. 2018;72(11):2859–69.; Ghadi FE, Ghara AR, Naeimi A. Phytochemical fabrication, characterization, and antioxidant application of copper and cobalt oxides nanoparticles using Sesbania sesban plant. Chem Pap. 2018;72(11):2859–69. [Google Scholar]
- [72].Shim HW, Jin YH, Seo SD, Lee SH, Kim DW. Highly reversible lithium storage in Bacillus subtilis-directed porous Co3O4 nanostructures. ACS Nano. 2011;5(1):443–9. [DOI] [PubMed]; Shim HW, Jin YH, Seo SD, Lee SH, Kim DW.. Highly reversible lithium storage in Bacillus subtilis-directed porous Co3O4 nanostructures. ACS Nano. 2011;5(1):443–9. doi: 10.1021/nn1021605. [DOI] [PubMed] [Google Scholar]
- [73].Kumar U, Shete A, Harle AS, Kasyutich O, Schwarzacher W, Pundle A, et al. Extracellular bacterial synthesis of protein-functionalized ferromagnetic Co3O4 nanocrystals and imaging of self-organization of bacterial cells under stress after exposure to metal ions. Chem Mater. 2008;20(4):1484–91.; Kumar U, Shete A, Harle AS, Kasyutich O, Schwarzacher W, Pundle A. et al. Extracellular bacterial synthesis of protein-functionalized ferromagnetic Co3O4 nanocrystals and imaging of self-organization of bacterial cells under stress after exposure to metal ions. Chem Mater. 2008;20(4):1484–91. [Google Scholar]
- [74].Jang E, Shim HW, Ryu BH, An DR, Yoo WK, Kim KK, et al. Preparation of cobalt nanoparticles from polymorphic bacterial templates: a novel platform for biocatalysis. Int J Biol Macromol. 2015;81:747–53. [DOI] [PubMed]; Jang E, Shim HW, Ryu BH, An DR, Yoo WK, Kim KK. et al. Preparation of cobalt nanoparticles from polymorphic bacterial templates: a novel platform for biocatalysis. Int J Biol Macromol. 2015;81:747–53. doi: 10.1016/j.ijbiomac.2015.09.009. [DOI] [PubMed] [Google Scholar]
- [75].Marimuthu S, Rahuman AA, Kirthi AV, Santhoshkumar T, Jayaseelan C, Rajakumar G. Eco-friendly microbial route to synthesize cobalt nanoparticles using Bacillus thuringiensis against malaria and dengue vectors. Parasitol Res. 2013;112(12):4105–12. [DOI] [PubMed]; Marimuthu S, Rahuman AA, Kirthi AV, Santhoshkumar T, Jayaseelan C, Rajakumar G.. Eco-friendly microbial route to synthesize cobalt nanoparticles using Bacillus thuringiensis against malaria and dengue vectors. Parasitol Res. 2013;112(12):4105–12. doi: 10.1007/s00436-013-3601-2. [DOI] [PubMed] [Google Scholar]
- [76].Eltarahony M, Zaki S, ElKady M, Abd-El-Haleem D. Biosynthesis, characterization of some combined nanoparticles, and its biocide potency against a broad spectrum of pathogens. J Nanomater. 2018;1:2018.; Eltarahony M, Zaki S, ElKady M, Abd-El-Haleem D. Biosynthesis, characterization of some combined nanoparticles, and its biocide potency against a broad spectrum of pathogens. J Nanomater. 2018;1:2018. [Google Scholar]
- [77].Iravani S, Varma RS. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 2020;22(9):2643–61.; Iravani S, Varma RS.. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 2020;22(9):2643–61. [Google Scholar]
- [78].Shim HW, Lee CS, Kim DW. Bacteria-mediated synthesis of free-standing cobalt oxide rods. J Nanosci Nanotechnol. 2010;10(2):1129–34. [DOI] [PubMed]; Shim HW, Lee CS, Kim DW.. Bacteria-mediated synthesis of free-standing cobalt oxide rods. J Nanosci Nanotechnol. 2010;10(2):1129–34. doi: 10.1166/jnn.2010.1846. [DOI] [PubMed] [Google Scholar]
- [79].Shim HW, Lim AH, Kim JC, Jang E, Seo SD, Lee GH, et al. Scalable one-pot bacteria-templating synthesis route toward hierarchical, porous-Co3O4 superstructures for supercapacitor electrodes. Sci Rep. 2013;31;2325. [DOI] [PMC free article] [PubMed]; Shim HW, Lim AH, Kim JC, Jang E, Seo SD, Lee GH. et al. Scalable one-pot bacteria-templating synthesis route toward hierarchical, porous-Co3O4 superstructures for supercapacitor electrodes. Sci Rep. 2013;31:2325. doi: 10.1038/srep02325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Baker S, Satish S. Endophytes: toward a vision in synthesis of nanoparticle for future therapeutic agents. Int J Bio-Inorg Hybd Nanomater. 2012;1(2):67–77.; Baker S, Satish S.. Endophytes: toward a vision in synthesis of nanoparticle for future therapeutic agents. Int J Bio-Inorg Hybd Nanomater. 2012;1(2):67–77. [Google Scholar]
- [81].Gupta S, Bector S. Biosynthesis of extracellular and intracellular gold nanoparticles by Aspergillus fumigatus and A. flavus. Antonie Van Leeuwenhoek. 2013 May 1;103(5):1113–23. [DOI] [PubMed]; Gupta S, Bector S.. Biosynthesis of extracellular and intracellular gold nanoparticles by Aspergillus fumigatus and A. flavus . Antonie Van Leeuwenhoek. 2013 May 1;103(5):1113–23. doi: 10.1007/s10482-013-9892-6. [DOI] [PubMed] [Google Scholar]
- [82].Ma L, Su W, Liu JX, Zeng XX, Huang Z, Li W, et al. Optimization for extracellular biosynthesis of silver nanoparticles by Penicillium aculeatum Su1 and their antimicrobial activity and cytotoxic effect compared with silver ions. Mater Sci Eng C. 2017 Aug 1;77:963–71. [DOI] [PubMed]; Ma L, Su W, Liu JX, Zeng XX, Huang Z, Li W. et al. Optimization for extracellular biosynthesis of silver nanoparticles by Penicillium aculeatum Su1 and their antimicrobial activity and cytotoxic effect compared with silver ions. Mater Sci Eng C. 2017 Aug 1;77:963–71. doi: 10.1016/j.msec.2017.03.294. [DOI] [PubMed] [Google Scholar]
- [83].Omran BA, Nassar HN, Younis SA, El‐Salamony RA, Fatthallah NA, Hamdy A, et al. Novel mycosynthesis of cobalt oxide nanoparticles using Aspergillus brasiliensis ATCC 16404 – optimization, characterization and antimicrobial activity. J Appl Microbiol. 2020;128(2):438–57. [DOI] [PubMed]; Omran BA, Nassar HN, Younis SA, El‐Salamony RA, Fatthallah NA, Hamdy A. et al. Novel mycosynthesis of cobalt oxide nanoparticles using Aspergillus brasiliensis ATCC 16404 – optimization, characterization and antimicrobial activity. J Appl Microbiol. 2020;128(2):438–57. doi: 10.1111/jam.14498. [DOI] [PubMed] [Google Scholar]
- [84].Chowdhury S, Basu A, Kundu S. Green synthesis of protein capped silver nanoparticles from phytopathogenic fungus Macrophomina phaseolina (Tassi) Goid with antimicrobial properties against multidrug-resistant bacteria. Nanoscale Res Lett. 2014 Dec;9(1):1–1. [DOI] [PMC free article] [PubMed]; Chowdhury S, Basu A, Kundu S.. Green synthesis of protein capped silver nanoparticles from phytopathogenic fungus Macrophomina phaseolina (Tassi) Goid with antimicrobial properties against multidrug-resistant bacteria. Nanoscale Res Lett. 2014 Dec;9(1):1–1. doi: 10.1186/1556-276X-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Valappil RS, Vijayanandan AS, Balakrishnan RM. Decolorization of Reactive Blue 220 aqueous solution using fungal synthesized Co3O4 nanoparticles. J Water Supply Res Technol—AQUA. 2019;68(8):675–86.; Valappil RS, Vijayanandan AS, Balakrishnan RM.. Decolorization of Reactive Blue 220 aqueous solution using fungal synthesized Co3O4 nanoparticles. J Water Supply Res Technol—AQUA. 2019;68(8):675–86. [Google Scholar]
- [86].Yang L, Guan W, Bai B, Xu Q, Xiang Y. Synthesis of yeast-assisted Co3O4 hollow microspheres – a novel biotemplating technique. J Alloys Compd. 2010;504(1):L10–3.; Yang L, Guan W, Bai B, Xu Q, Xiang Y.. Synthesis of yeast-assisted Co3O4 hollow microspheres – a novel biotemplating technique. J Alloys Compd. 2010;504(1):L10–3. [Google Scholar]
- [87].Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009 Jan 1;32(1):79. [DOI] [PubMed]; Song JY, Kim BS.. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009 Jan 1;32(1):79. doi: 10.1007/s00449-008-0224-6. [DOI] [PubMed] [Google Scholar]
- [88].Singaravelu G, Arockiamary JS, Kumar VG, Govindaraju K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf B. 2007 May 15;57(1):97–101. [DOI] [PubMed]; Singaravelu G, Arockiamary JS, Kumar VG, Govindaraju K.. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf B. 2007 May 15;57(1):97–101. doi: 10.1016/j.colsurfb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- [89].Rajeshkumar S, Malarkodi C, Gnanajobitha G, Paulkumar K, Vanaja M, Kannan C, et al. Seaweed-mediated synthesis of gold nanoparticles using Turbinaria conoides and its characterization. J Nanostruct Chem. 2013 Dec 1;3(1):44.; Rajeshkumar S, Malarkodi C, Gnanajobitha G, Paulkumar K, Vanaja M, Kannan C. et al. Seaweed-mediated synthesis of gold nanoparticles using Turbinaria conoides and its characterization. J Nanostruct Chem. 2013 Dec 1;3(1):44. [Google Scholar]
- [90].Salem DM, Ismail MM, Aly-Eldeen MA. Biogenic synthesis and antimicrobial potency of iron oxide (Fe3O4) nanoparticles using algae harvested from the Mediterranean Sea, Egypt. Egyptian J Aquat Res. 2019 Sep 1;45(3):197–204.; Salem DM, Ismail MM, Aly-Eldeen MA.. Biogenic synthesis and antimicrobial potency of iron oxide (Fe3O4) nanoparticles using algae harvested from the Mediterranean Sea, Egypt. Egyptian J Aquat Res. 2019 Sep 1;45(3):197–204. [Google Scholar]
- [91].Lu L, Jiao X, Fan J, Lei W, Ouyang Y, Xia X, et al. Cobalt ferrite on honeycomb-like algae-derived nitrogen-doped carbon for electrocatalytic oxygen reduction and ultra-cycle-stable lithium storage. Electrochim Acta. 2019;295:461–71.; Lu L, Jiao X, Fan J, Lei W, Ouyang Y, Xia X. et al. Cobalt ferrite on honeycomb-like algae-derived nitrogen-doped carbon for electrocatalytic oxygen reduction and ultra-cycle-stable lithium storage. Electrochim Acta. 2019;295:461–71. [Google Scholar]
- [92].Azevêdo HV, Raimundo RA, Ferreira LS, Silva MM, Morales MA, Macedo DA, et al. Green synthesis of CoWO4 powders using agar-agar from red seaweed (Rhodophyta): structure, magnetic properties and battery-like behavior. Mater Chem Phys. 2020 Feb 15;242:122544.; Azevêdo HV, Raimundo RA, Ferreira LS, Silva MM, Morales MA, Macedo DA. et al. Green synthesis of CoWO4 powders using agar-agar from red seaweed (Rhodophyta): structure, magnetic properties and battery-like behavior. Mater Chem Phys. 2020 Feb 15;242:122544. [Google Scholar]
- [93].Vaya D, Das BK. Green synthesis of cobalt oxide nanoparticles by a starch-assisted method. Nanosci Nanotechnol. 2019 Sep 1;9(3):362–70.; Vaya D, Das BK.. Green synthesis of cobalt oxide nanoparticles by a starch-assisted method. Nanosci Nanotechnol. 2019 Sep 1;9(3):362–70. [Google Scholar]
- [94].Ahmadov TO, Durmus Z, Baykal A, Kavas H. A simple approach for the synthesis of Co3O4 nanocrystals. Inorg Mater. 2011 Apr 1;47(4):426–30.; Ahmadov TO, Durmus Z, Baykal A, Kavas H.. A simple approach for the synthesis of Co3O4 nanocrystals. Inorg Mater. 2011 Apr 1;47(4):426–30. [Google Scholar]
- [95].Hosein HA, Strongin DR, Allen M, Douglas T. Iron and cobalt oxide and metallic nanoparticles prepared from ferritin. Langmuir. 2004 Nov 9;20(23):10283–7. [DOI] [PubMed]; Hosein HA, Strongin DR, Allen M, Douglas T.. Iron and cobalt oxide and metallic nanoparticles prepared from ferritin. Langmuir. 2004 Nov 9;20(23):10283–7. doi: 10.1021/la0491100. [DOI] [PubMed] [Google Scholar]
- [96].Yu C, Qiu JS. Preparation and magnetic behavior of carbon-encapsulated cobalt and nickel nanoparticles from starch. Chem Eng Res Des. 2008 Aug 1;86(8):904–8.; Yu C, Qiu JS.. Preparation and magnetic behavior of carbon-encapsulated cobalt and nickel nanoparticles from starch. Chem Eng Res Des. 2008 Aug 1;86(8):904–8. [Google Scholar]
- [97].Thanh NT, Puntes VF, Tung LD, Fernig DG. Peptides as capping ligands for in situ synthesis of water soluble Co nanoparticles for bioapplications. J Phys Conf Ser. 2005 Jan;17:70–6.; Thanh NT, Puntes VF, Tung LD, Fernig DG.. Peptides as capping ligands for in situ synthesis of water soluble Co nanoparticles for bioapplications. J Phys Conf Ser. 2005 Jan;17:70–6. [Google Scholar]
- [98].Kapil A. The challenge of antibiotic resistance: need to contemplate. Indian J Med Res. 2005 Feb 1;121(2):83–91. [PubMed]; Kapil A.. The challenge of antibiotic resistance: need to contemplate. Indian J Med Res. 2005 Feb 1;121(2):83–91. [PubMed] [Google Scholar]
- [99].Patil Shriniwas P. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem Biophys Rep. 2017 Jul;10:76. [DOI] [PMC free article] [PubMed]; Patil Shriniwas P.. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem Biophys Rep. 2017 Jul;10:76. doi: 10.1016/j.bbrep.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Varaprasad T, Govindh B, Rao BV. Green synthesized cobalt nanoparticles using Asparagus racemosus root extract & evaluation of antibacterial activity. Int J ChemTech Res. 2017;10:339–45.; Varaprasad T, Govindh B, Rao BV.. Green synthesized cobalt nanoparticles using Asparagus racemosus root extract & evaluation of antibacterial activity. Int J ChemTech Res. 2017;10:339–45. [Google Scholar]
- [101].Anuradha CT, Raji P. Effect of annealing temperature on antibacterial, antifungal and structural properties of bio-synthesized Co3O4 nanoparticles using Hibiscus Rosa-sinensis. Mater Res Exp. 2019 Jul 17;6(9):095063.; Anuradha CT, Raji P.. Effect of annealing temperature on antibacterial, antifungal and structural properties of bio-synthesized Co3O4 nanoparticles using Hibiscus Rosa-sinensis. Mater Res Exp. 2019 Jul 17;6(9):095063. [Google Scholar]
- [102].Iravani S, Varma RS. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 2020;22(9):2643–61.; Iravani S, Varma RS.. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 2020;22(9):2643–61. [Google Scholar]
- [103].Klempner MS, Unnasch TR, Hu LT. Taking a bite out of vector-transmitted infectious diseases. New Engl J Med. 2007 Jun 21;356(25):2567–9. [DOI] [PMC free article] [PubMed]; Klempner MS, Unnasch TR, Hu LT.. Taking a bite out of vector-transmitted infectious diseases. New Engl J Med. 2007 Jun 21;356(25):2567–9. doi: 10.1056/NEJMp078081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].James AA. Mosquito molecular genetics: the hands that feed bite back. Science. 1992 Jul 3;257(5066):37–9. [DOI] [PubMed]; James AA.. Mosquito molecular genetics: the hands that feed bite back. Science. 1992 Jul 3;257(5066):37–9. doi: 10.1126/science.1352413. [DOI] [PubMed] [Google Scholar]
- [105].Kaushik R, Saini P. Larvicidal activity of leaf extract of Millingtonia hortensis (Family: Bignoniaceae) against Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti. J Vector Borne Dis. 2008 Mar 1;45(1):66. [PubMed]; Kaushik R, Saini P.. Larvicidal activity of leaf extract of Millingtonia hortensis (Family: Bignoniaceae) against Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti. J Vector Borne Dis. 2008 Mar 1;45(1):66. [PubMed] [Google Scholar]
- [106].Barreto ML, Teixeira MG. Dengue no Brasil: situação epidemiológica e contribuições para uma agenda de pesquisa. Estudos Avançados. 2008 Dec;22(64):53–72.; Barreto ML, Teixeira MG.. Dengue no Brasil: situação epidemiológica e contribuições para uma agenda de pesquisa. Estudos Avançados. 2008 Dec;22(64):53–72. [Google Scholar]
- [107].Abamor ES. Antileishmanial activities of caffeic acid phenethyl ester loaded PLGA nanoparticles against Leishmania infantum promastigotes and amastigotes in vitro. Asian Pac J Tropic Med. 2017 Jan 1;10(1):25–34. [DOI] [PubMed]; Abamor ES.. Antileishmanial activities of caffeic acid phenethyl ester loaded PLGA nanoparticles against Leishmania infantum promastigotes and amastigotes in vitro . Asian Pac J Tropic Med. 2017 Jan 1;10(1):25–34. doi: 10.1016/j.apjtm.2016.12.006. [DOI] [PubMed] [Google Scholar]
- [108].Ogden GB, Melby PC. Encyclopedia of microbiology, 3rd edn. Elsevier Inc.; 2009.; Ogden GB, Melby PC. Encyclopedia of microbiology. 3rd edn. Elsevier Inc; 2009. [Google Scholar]
- [109].Cobo F. Imported infectious diseases: the impact in developed countries. Sawston, Cambridge: Woodhead Publishing; 2014 Oct 7.; Cobo F. Imported infectious diseases: the impact in developed countries. Sawston, Cambridge: Woodhead Publishing; 2014 Oct 7. [Google Scholar]
- [110].Bauer V, Sotnikova R, Machova J, Matyas S, Pucovský V, Štefek M. Reactive oxygen species induced smooth muscle responses in the intestine, vessels and airways and the effect of antioxidants. Life sciences. 1999 Oct 1;65(18–19):1909–17. [DOI] [PubMed]; Bauer V, Sotnikova R, Machova J, Matyas S, Pucovský V, Štefek M.. Reactive oxygen species induced smooth muscle responses in the intestine, vessels and airways and the effect of antioxidants. Life sciences. 1999 Oct 1;65(18–19):1909–17. doi: 10.1016/s0024-3205(99)00446-4. [DOI] [PubMed] [Google Scholar]
- [111].Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002;127(1):183–98. [DOI] [PubMed]; Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K.. Methods for testing antioxidant activity. Analyst. 2002;127(1):183–98. doi: 10.1039/b009171p. [DOI] [PubMed] [Google Scholar]
- [112].Ballantyne B. Local and systemic ophthalmic pharmacology and toxicology of organophosphate and carbamate anticholinesterases. In Toxicology of Organophosphate & Carbamate Compounds. USA: Academic Press. 2006 Jan 1. p. 423–45.; Ballantyne B. Local and systemic ophthalmic pharmacology and toxicology of organophosphate and carbamate anticholinesterases. In Toxicology of Organophosphate & Carbamate Compounds. USA: Academic Press; 2006 Jan 1. pp. p. 423–45. [Google Scholar]
- [113].Wilson DA. Gasterophilus in clinical veterinary advisor. The Horse. St. Louis, MO: Elsevier Saunders; 2012.; Wilson DA. Gasterophilus in clinical veterinary advisor. The Horse. St. Louis, MO: Elsevier Saunders; 2012. [Google Scholar]
- [114].Mikołajczuk-Szczyrba A, Kieliszek M, Giurgiulescu L, Sokołowska B. Characteristics and application of silver nanoparticles in the food industry-review. Carpathian J Food Sci Technol. 2019;11(4):153–60.; Mikołajczuk-Szczyrba A, Kieliszek M, Giurgiulescu L, Sokołowska B.. Characteristics and application of silver nanoparticles in the food industry-review. Carpathian J Food Sci Technol. 2019;11(4):153–60. [Google Scholar]