Abstract
Background
In March 2020, the World Health Organization declared the coronavirus disease 2019 as a global pandemic. Though antiviral drugs and antimalarial drugs are considered treatment options for treating coronavirus disease 2019 (COVID-19), no specific antivirals are currently available for its treatment. Efficient use of drug discovery approaches including repurposing or repositioning of drugs used in the treatment of severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) is considered recently. The widespread application of corticosteroid therapy in COVID-19 should be backed with careful documented pragmatic research of its use in this context.
Main body
This article aims to analyze various trials registered across the globe providing an overall picture of the use of corticosteroids in the treatment of COVID-19. An extensive search was conducted on the clinical trial registries around the world to identify all the trials reporting information regarding the use of corticosteroids in COVID-19. Our initial search returned 231 trials, out of which 60 trials were finally included in the analysis. Fifty-six studies were interventional trials, and all the trials had clearly defined primary and secondary outcomes of interest, of which only 11 trials had evaluation of respiratory rate as one of their outcomes.
Conclusion
Few preliminary trial findings show promising results and recommend the use of methylprednisolone and dexamethasone in the severe form of the disease; however, there is insufficient data to prove its benefits over its risks. Routine use of corticosteroids should be favored only after a better insight is obtained, with the completion of these trials.
Keywords: Methylprednisolone; Hydrocortisone; Steroids, COVID-19; SARS-CoV2, Clinical trial registry
Background
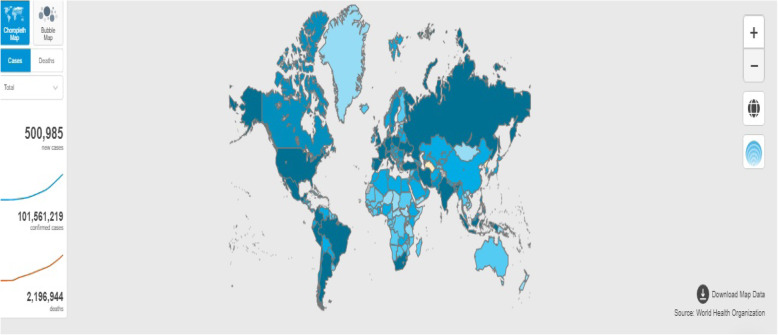
An outbreak of pneumonia caused by a new coronavirus spread in Wuhan province of China in December 2019. Sequencing of the sampling from patients with pneumonia revealed the viral genome phylogenetically closer to severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) [1]. The Coronavirus Study Group named the causative agent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease caused by this virus was named coronavirus disease 2019 (COVID-19 or 2019-nCoV) by the World Health Organization (WHO) [2, 3]. These viruses are enveloped, positive, single-stranded RNA viruses belonging to the family Coronaviridae, which can cause an array of symptoms including fever, dry cough, myalgia, fatigue, and dyspnea [4]. SARS-CoV-2 transmits from human-to-human by respiratory droplets caused by coughing or sneezing [5, 6]. The WHO declared COVID-19 as a Public Health Emergency of International Concern in January 2020 [7]. The infection has spread over to 216 countries (15,745,102 confirmed cases and 639,317 confirmed deaths) since its outbreak in November 2019 (as of 30 January 2021; Fig. 1).
Fig. 1.
Global COVID-19 spread showing number of confirmed cases as of 30 January 2021 (Source: https://covid19.who.int/)
Detection and diagnosis of this novel coronavirus mostly relied on molecular-based approaches such as nucleic acid testing, virus antigen, or serological antibody testing (against the N-protein of SARS-CoV) [8]. Treatment option includes antiviral drugs such as favipiravir, remdesivir, lopinavir, and ritonavir and antimalarial drugs such as chloroquine or hydroxychloroquine. Nevertheless, no vaccine or specific antiviral treatment recommended for COVID-19 is currently available [9]. The uncontrolled scenario of COVID-19 demands the use of effective drug discovery approaches for effective control of the disease [10–16]. Among these approaches, drug repurposing or drug repositioning is a time-effective way of treating a disease. One of the examples of successful application of drug discovery approach is drug repositioning of antivirals, and it has triggered a number of in vitro studies as well as clinical trials for a number of chemical molecules to evaluate their efficacy against COVID-19 [17–19]. Drug repurposing of corticosteroids has also been implemented recently as a part of a drug discovery approach. There are several studies reporting the use of corticosteroids in the treatment of severe coronavirus infections including COVID-19. The effectiveness of corticosteroids in some patients with SARS-CoV has resulted in a widespread application of this therapy in COVID-19, especially in patients in the ICU with severe infections, as these drugs prevent lung injury caused by severe community-acquired pneumonia (sCAP) due to their potential pharmacological effects on the suppression of exuberant and dysfunctional systematic inflammation [20].
Main body
Corticosteroids and their therapeutic role
The Infectious Diseases Society of America (IDSA) guidelines strongly recommends the use of dexamethasone in critically ill patients to treat acute respiratory distress syndrome (ARDS) and systemic inflammation, backed by moderate evidence. Dexamethasone at a total daily dose of 6 mg IV or PO for 10 days (or until discharge) or alternative glucocorticoids like methylprednisolone 32 mg and prednisone 40 mg are suggested. The level of recommendation decreases with decreasing severity of the disease. In non-severe COVID-19, the use of glucocorticoids is not recommended as there is a dearth of solid evidence. Additionally, experiences from SARS and MERS show risk of worsening clinical status, delayed viral clearance, and other adverse events [21]. Currently, available data on safety and effectiveness of corticosteroids in this setting is very few and inconclusive [20, 22, 23]. The value of corticosteroids as a treatment option in patients with severe COVID-19 infection needs careful documented pragmatic research in this context. In order to obtain strong clinical evidence, several studies have been launched that were registered on various clinical trial registries across the globe. The detailed analysis of these trials will give an overall picture of the use of corticosteroids in the treatment of COVID-19 around the world. This will help to identify the lacunae to be filled with definitive clinical evidence in order to reposition corticosteroid for COVID-19 treatment. Therefore, this study aims to analyze various trials registered across the globe providing an overall picture of the use of corticosteroids in the treatment of COVID-19.
Search strategy
An extensive search was conducted to identify all the trials reporting information regarding the use of corticosteroids in COVID-19. We searched the following clinical trial registries: Clinicaltrials.gov, Chinese Clinical Trial Registry (ChiCTR), Clinical Research Information Service (CRiS)–Republic of Korea, EU Clinical Trials Register, ISRCTN Registry, Iranian Registry of Clinical Trials (IRCT), German Clinical Trials Register (DRKS), Japan Primary Registries Network (JPRN), and Clinical Trial Registry–India. The search was run until 23 June 2020. In Clinicaltrials.gov, the following keywords were used for search: “(COVID-19 OR SARS-CoV-2 OR 2019-nCoV OR severe acute respiratory syndrome coronavirus 2 OR Wuhan coronavirus OR 2019 novel coronavirus OR novel coronavirus–infected Pneumonia) AND (“glucocorticoids” OR “steroids” OR “corticosteroids” OR “hydrocortisone” OR “prednisone” OR “methylprednisolone” OR “dexamethasone” OR “prednisolone”). A similar strategy was adapted for the other registries. We included the English language and interventional and non-interventional studies. No restrictions were placed on the dose or formulation of the intervention. All trials must have studied the safety and efficacy of steroids in COVID-19 care.
Recovery of trials
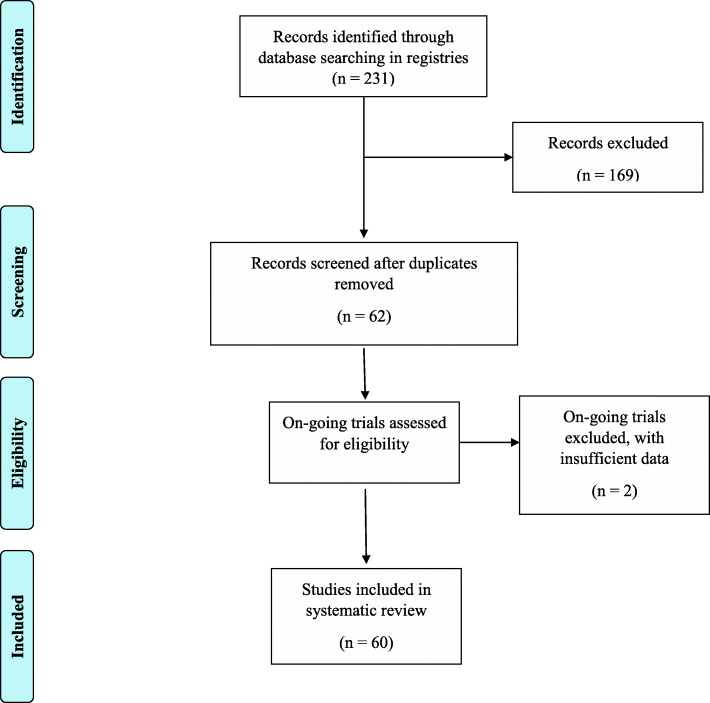
Our initial search returned 231 trials, out of which 62 potentially relevant trials were identified. Potentially eligible trials were identified by three authors by screening titles and study description. All eligible trials were then assessed independently by three authors, and potentially relevant trials were selected in accordance with the predefined inclusion criteria. Any disagreement was reviewed and resolved by a fourth independent reviewer. Authors of individual trials were contacted if necessary. After a careful review of the study description, out of 62 articles, 2 trials did not satisfy the inclusion criteria and were excluded from the analysis. Finally, data from 60 trials were included in the final review and synthesis of results. This is shown in Fig. 2.
Fig. 2.
PRISMA flow diagram of reporting search results
Data abstraction and study appraisal
We extracted the following general data from each study: trial number, title, origin (country) of study, intervention, treatment arms, doses, mean age of participants, stage of COVID–19, expected start and end date of trial, primary outcomes of the study, blinding, randomization, and study design.
Scrutiny of trials
Our initial search of the clinical trial registries resulted in 231 trials, of which 167 trials did not satisfy the inclusion criteria and three trials did not have complete data, and after removing the duplicates, 60 trials were included in the final analysis. Thus, 60 trials with 31,732 patients were included in this systematic review. The included trials were classified into trials that included only steroid therapy and those that included steroids in addition to other standard treatment as shown in Table 1.
Table 1.
General characteristics of the included trials
| Trial identifier | Country | Number of sites | Start date | Expected completion date | Stage of COVID-19 | Outcome measures |
|---|---|---|---|---|---|---|
| NCT04425863 | Argentina | Single | May 2020 | July 2020 | Severe acute respiratory syndrome |
1. Illness development 2. Reduction of ICU admission 3. Mortality rate |
| NCT04395105 | Argentina | Multi | May 2020 | January 2021 | Respiratory distress syndrome | Ventilator-free days at 28 days |
| NCT02735707 | Australia, Belgium, Canada, Croatia, Germany, Hungary, Ireland, Netherlands, New Zealand, Portugal, Romania, Spain, UK | Multi | April 2020 | December 2023 | Pneumonia |
1. All-cause mortality 2. Days alive and outside of ICU |
| NCT04343729 | Brazil | Single | April 2020 | September 2020 | Severe acute respiratory syndrome (SARS) | Mortality rate |
| NCT04327401 | Brazil | Multi | April 2020 | August 2020 | Moderate/severe ARDS | Ventilator-free days |
| NCT04377503 | Brazil | Not available | May 2020 | November 2020 | Cytokine release syndrome | Patient clinical status 15 days after randomization |
| NCT04374474 | Canada | Single | January 2021 | March 2022 | Not defined |
1. Change from Baseline Snap and Sniff Threshold Test at 3 months 2. Score from the Snap and Sniff Olfactory Test results 3. Change from baseline Smell Identification Test (SIT) at 3 months 4. Score from the Smell Identification test results. 5. Change from Baseline Snap and Sniff Threshold Test at 6 months 6. Score from the Snap and Sniff Olfactory Test results 7. Change from baseline Smell Identification Test (SIT) at 6 months |
| NCT04263402 | China | Not available | February 2020 | July 2020 | Severe pneumonia |
1. Rate of disease remission 2. Rate and time of entering the critical stage |
| NCT04244591 | China | Completed | January 2020 | April 2020 | Severe acute respiratory failure | Murray lung injury score |
| NCT04273321 | China | Completed | February 2020 | April 2020 | Pneumonia | The incidence of treatment failure in 14 days |
| ChiCTR2000029386 | China | Single | January 2020 | January 2021 | Pneumonia | SOFA score |
| ChiCTR2000029656 | China | Single | February 2020 | April 2020 | Pneumonia | ECG, chest imaging, complications, vital signs, and NEWS2 score |
| ChiCTR2000030481 | China | Multi | January 2020 | April 2020 | Pneumonia | The time of duration of COVID-19 nucleic acid RT-PCR test results of respiratory specimens (such as throat swabs) or blood specimens change to negative |
| NCT04348305 | Denmark | Multi | April 2020 | December 2021 | COVID-19 hypoxia | Days alive without life support at day 28 |
| 2020-001395-15 | Denmark | Multi | April 2020 | Not available | Severe hypoxia | 1. Days alive without life support (i.e., invasive mechanical ventilation, circulatory support or renal replacement therapy) from randomization to day 28). |
| NCT04331054 | France | Multi | April 2020 | July 2020 | COVID-19 infection | Time (in days) to clinical improvement within 30 days after randomization |
| NCT04361474 | France | Multi | May 2020 | May 2021 | Hyposmia | Patient with more than 2 points on the ODORATEST |
| NCT04359511 | France | Not available | June 2020 | December 2020 | Pneumonia | Clinical improvement defined by the improvement of 2 points on a 7-category ordinal scale, at 14 days |
| NCT04347980 | France | Multi | April 2020 | August 2020 | Acute respiratory distress syndrome (ARDS) | 28-day mortality |
| NCT04344730 | France | Single | April 2020 | December 2020 | Pneumonia |
1. Time-to-death from all causes within the first 60 days after randomization 2. Time to need for mechanical ventilation |
| NCT04344288 | France | Multi | April 2020 | November 2020 | Pneumonia | Respiratory indication for transfer to intensive care unit evaluated by a SpO2 < 90% |
| NCT04331470 | Iran | Single | April 2020 | May 2020 | Not defined | Clear chest CT scan and PCR test |
| IRCT20200204046369N1 | Iran | Multi | Not available | Not available | Not defined | PAO2/FiO2 through ABG method |
| IRCT20151227025726N17 | Iran | Single | Not available | Not available | ARDS |
1. Daily need for invasive mechanical ventilation 2. Death at the end of the study |
| IRCT20120225009124N4 | Iran | Single | Not available | Not available | Not defined | Improvement in SpO2 measured by pulse oximeter |
| IRCT20200406046963N1 | Iran | Single | Not available | Not available | ARDS( acute respiratory distress syndrome) |
1. Mortality rate after 60 days 2. Blood O2 saturation measurement 3. Need for oxygen therapy |
| IRCT20200404046947N1 | Iran | Multi | Not available | Not available | Not defined |
1. Findings on the CT scan 2. Mortality rate 3. O2 saturation levels 4. Need an oxygen therapy at day 3 and discharge time |
| IRCT20081027001411N3 | Iran | Multi | Not available | Not available | ARDS |
1. Findings on the CT scan 2. Mortality rate 3. O2 saturation levels 4. Need an oxygen therapy at day 3 and discharge time |
| IRCT20120215009014N354 | Iran | Single | Not available | Not available | Mild-to-moderate acute respiratory distress syndrome |
1. Need to mechanical ventilation 2. The patient’s clinical status 3. Mortality rate |
| IRCT20080901001165N52 | Iran | Single | Not available | Not available | Moderate to severe pneumonia | Need to receive ICU service |
| NCT04323592 | Italy | Single | March 2020 | May 2020 | Acute respiratory distress syndrome |
1. Admission to ICU and need for Invasive mechanical ventilation 2. In-hospital death within 28 days 3. Endotracheal intubation |
| KCT0005105 | Korea | Multi | April 2020 | September 2020 | Mild | Rate of SARS-CoV-2 eradication at day 14 from study enrollment |
| IRCT20200318046812N2 | Iran | Multi | Not available | Not available | Not defined | Admission to intensive care unit |
| NCT04345445 | Malaysia | Single | April 2020 | October 2020 | Pneumonia |
1. The proportion of patients requiring mechanical ventilation 2. Mean days in ventilation |
| NCT04360876 | Not available | Single | May 2020 | December 2020 | ARDS | Ventilator-free days (VFD) at day 28 |
| NCT04366115 | Not available | Not available | June 2020 | June 2023 | Not defined |
1. Dose-limiting toxicities 2. 28-day all-cause mortality for phases 1 and 2 |
| NCT04435795 | Not available | Not available | June 2020 | March 2021 | Not defined | Improvement in dyspnea at day 7 |
| NCT04355247 | Puerto Rico | Multi | April 2020 | April 2021 | High-risk COVID-19 |
1. Clinical complete response criteria 2. Need for ventilatory support 3. O2 Saturation of >/= 93% by day 14 of therapy 4. Mortality at day 28 5. Findings on CT chest on day 28 |
| NCT04438980 | Spain | Multi | May 2020 | February 2021 | Pneumonia |
1. Proportion of patients developing treatment failure 2. Need for mechanical ventilation 3. Decrease in SpO2 < 90% (in ambient air) or PaO2 < 60 mmHg (in ambient air) or PaO2FiO2 < 300 mmHg |
| NCT04394182 | Spain | Multi | April 2020 | April 2021 | Pneumonia | Oxygen saturation at day 2 |
| NCT04380818 | Spain | Multi | June 2020 | July 2021 | Pneumonia | Efficacy of low-dose pulmonary irradiation assessed by change in PAFiO2 by 20% |
| NCT04355637 | Spain | Multi | April 2020 | October 2020 | Pneumonia | Proportion of patients developing treatment failure |
| NCT04341038 | Spain | Single | April 2020 | June 2020 | Severe lung injury secondary to COVID-19 | Time to reach clinical stability |
| NCT04329650 | Spain | Multi | April 2020 | May 2020 | Pneumonia | Proportion of patients requiring ICU admission at any time within the study period |
| NCT04325061 | Spain | Multi | April 2020 | October 2020 | ARDS | 60-day mortality |
| 2020-001827-15 | Spain | Single | Not available | Not available | Pneumonia |
1. Proportion of patients with treatment failure up to 14 days after randomization 2. Mortality rate 3. ICU admission 4. Number of patients requiring mechanical ventilation 5. Clinical deterioration/worsening, defined as decrease in SpO2 below 90% or PaO2 below 60 mmHg in ambient air + radiological progression. |
| 2020-001622-64 | Spain | Single | April 2020 | Not available | Not defined |
1. Measurement of O2 saturation and/or blood gas, findings on chest x-ray, CBC, including inflammatory markers and blood biometrics, and ECG 2. 30-day ICU admission and hospital stay 3. Outbreaks of steroid-related psychosis |
| 2020-001934-37 | Spain | Multi | May 2020 | Not available | Not defined |
1. Mortality rate 2. Number of days of ICU stay 3. Number of patients requiring non-invasive ventilation (NIV) |
| 2020-001413-20 | Spain | Single | April 2020 | Not available | Pneumonia | Proportion of patients requiring ICU admission at any time within the study period |
| 2020-001445-39 | Spain | Single | March 2020 | Not available | Pneumonia | Time (days) to clinical stability after initiation of trial treatment for severe pneumonia secondary to COVID-19 and elevated inflammatory parameters |
| 2020-001307-16 | Spain | Single | April 2020 | Not available | ARDS | Death from any cause in the first 28 days after randomization |
| NCT04381364 | Sweden | Multi | May 2020 | December 2020 | Pneumonia | Duration of supplemental oxygen therapy |
| NCT04416399 | UK | Not available | June 2020 | December 2020 | Early infection | Emergency department visit related to COVID-19 |
| NCT04381936 | UK | Multi | March 2020 | June 2021 | SARS | All-cause mortality |
| NCT04411667 | USA | Multi | April 2020 | November 2020 | Not defined | Number of subjects requiring mechanical ventilation |
| NCT04377711 | USA | Multi | June 2020 | December 2020 | Symptomatic COVID-19 infection | Percentage hospital admission or death by day 30 |
| NCT04349410 | USA | Not available | April 2020 | November 2020 | Pneumonia |
1. Improvement in FMTVDM measurement with nuclear imaging 2. Ventilator status 3. Extubation status 4. Survival status in 30 days |
| NCT04193878 | USA | Multi | June 2020 | June 2024 | Pneumonia, acute respiratory failure | Number of patients with acute respiratory failure (ARF) within 10 days of randomization |
| NCT03852537 | USA | Single | December 2019 | July 2022 | Pneumonia |
1. Feasibility of the timely initiation of corticosteroids and implementation of biomarker-titrated corticosteroid dosing 2. Percentage of eligible patients adhered to the timely initiation within 30 days |
| NCT04374071 | USA | Completed | March 2020 | April 2020 | Pneumonia |
1. Number of patients transferred to ICU is each of the group 2. Number of patients requiring mechanical ventilation 3. Mortality rate |
Type of trials
Among the included trials, 57 trials were quantitative studies and the remaining three trials were qualitative studies, i.e., non-interventional studies, as shown in the Table 2.
Table 2.
Methodological quality of included trials
| Trial identifier | Estimated sample size | Allocation (randomized/non-randomized) | Blinding/masking | Study design |
|---|---|---|---|---|
| NCT04438980 | 72 | Randomized | Double | Interventional |
| NCT04435795 | 454 | Randomized | Triple | Interventional |
| NCT04425863 | 10 | Not available | Not available | Non-interventional, prospective cohort |
| NCT04416399 | 478 | Randomized | Open label | Interventional |
| NCT04411667 | 40 | Randomized | Open label | Interventional |
| NCT04395105 | 284 | Randomized | Open label | Interventional |
| NCT04394182 | 15 | Not available | Open label | Interventional |
| NCT04381936 | 12000 | Randomized | Open label | Interventional |
| NCT04348305 | 1000 | Randomized | Quadruple | Interventional |
| NCT04331054 | 436 | Randomized | Open label | Interventional |
| NCT04360876 | 90 | Randomized | Double | Interventional |
| NCT04355247 | 20 | Not available | Open label | Interventional |
| NCT04381364 | 446 | Randomized | Open label | Interventional |
| NCT04380818 | 106 | Non-randomized | Open label | Interventional |
| NCT04377711 | 400 | Randomized | Double | Interventional |
| NCT04377503 | 40 | Randomized | Open label | Interventional |
| NCT04374474 | 75 | Randomized | Open label | Interventional |
| NCT04366115 | 126 | Randomized | Open label | Interventional |
| NCT04361474 | 120 | Randomized | Single | Interventional |
| NCT04359511 | 210 | Randomized | Single | Interventional |
| NCT04355637 | 300 | Randomized | Open label | Interventional |
| NCT04349410 | 500 | Randomized | Single | Interventional |
| NCT04347980 | 122 | Randomized | Single | Interventional |
| NCT04263402 | 100 | Randomized | Single | Interventional |
| NCT04193878 | 600 | Randomized | Triple | Interventional |
| NCT03852537 | 90 | Randomized | Double | Interventional |
| NCT02735707 | 7100 | Randomized | Open label | Randomized, multifactorial trial |
| NCT04345445 | 310 | Randomized | Open label | Interventional |
| NCT04344730 | 550 | Randomized | Quadruple | Interventional |
| NCT04344288 | 304 | Randomized | Open label | Interventional |
| NCT04343729 | 425 | Randomized | Quadruple | Interventional |
| NCT04341038 | 84 | Randomized | Single | Interventional |
| NCT04331470 | 30 | Randomized | Double | Interventional |
| KCT0005105 | 141 | Randomized | Open label | Interventional |
| NCT04329650 | 200 | Randomized | Open label | Interventional |
| NCT04327401 | 350 | Randomized | Open label | Interventional |
| NCT04325061 | 200 | Randomized | Open label | Interventional |
| NCT04323592 | 173 | Non-randomized | Open label | Non-interventional, prospective cohort |
| NCT04374071 | 250 | Non-randomized | Not available | Non-interventional, retrospective cohort |
| NCT04244591 | 80 | Randomized | Open label | Interventional |
| NCT04273321 | 86 | Randomized | Open label | Interventional |
| IRCT20080901001165N52 | 50 | Randomized | Open label | Interventional |
| IRCT20200406046963N1 | 40 | Randomized | Open label | Interventional |
| IRCT20200404046947N1 | 68 | Randomized | Single | Interventional |
| IRCT20081027001411N3 | 60 | Randomized | Single | Interventional |
| IRCT20120215009014N354 | 81 | Randomized | Double | Interventional |
| IRCT20200204046369N1 | 48 | Non-randomized | Open label | Interventional |
| IRCT20200318046812N2 | 906 | Randomized | Open label | Interventional |
| IRCT20151227025726N17 | 48 | Randomized | Open label | Interventional |
| IRCT20120225009124N4 | 105 | Randomized | Open label | Interventional |
| ChiCTR2000029386 | 24 | Randomized | Not available | Interventional |
| ChiCTR2000029656 | 50 | Randomized | Open label | Interventional |
| ChiCTR2000030481 | 75 | Randomized (static) | Not available | Interventional |
| 2020-001827-15 | 72 | Randomized | Double | Interventional |
| 2020-001307-16 | 104 | Randomized | Open label | Interventional |
| 2020-001395-15 | 1000 | Randomized | Double | Interventional |
| 2020-001622-64 | 200 | Randomized | Open label | Interventional |
| 2020-001934-37 | 200 | Randomized | Open label | Interventional |
| 2020-001413-20 | 100 | Randomized | Open label | Interventional |
| 2020-001445-39 | 84 | Randomized | Open label | Interventional |
Heterogeneity of trials
All 60 trials included were heterogenous in that they had various inclusion and exclusion criteria and different treatment protocols for the treatment of various stages of COVID-19. The most common stage of COVID-19 among these trials is pneumonia, which is shown in Table 1.
Methodological quality of the trials
Among the 60 trials, 54 were randomized. It was unclear how randomization was carried out in three of the trials. Among 54 randomized trials, only 21 trials were blinded, of which 8 were single blinded, 8 were double blinded, 2 were triple blinded, and 3 were quadruple blinded, as shown in Table 2.
Steroid treatment
Regarding the steroid treatment, the most common steroid used is methylprednisolone (used in 28 trials) at various dosages depending on the age of the patients. Maximum loading dose of methylprednisolone used is 500 mg IV infusion over 1h in a trial (IRCT20080901001165N52). Steroids were given from a minimum of 3 days to a maximum of 21 days. Other steroids used are budesonide, ciclesonide, dexamethasone, formoterol, prednisolone, prednisone, and hydrocortisone. In 10 trials, the dose of the steroids used was unclear, and in one trial (ChiCTR2000030481), the treatment regimen was not mentioned. This is shown in Table 3.
Table 3.
Steroid treatment in patients with COVID-19
| Trial identifier | Title | Interventions | Dose | Age (in years) |
|---|---|---|---|---|
| NCT04425863 | Evaluation of ivermectin, aspirin, dexamethasone, and enoxaparin as treatment of Covid19 | Ivermectin; aspirin; dexamethasone; enoxaparin | Dexamethasone 4 mg/day IV | ≥ 5 |
| NCT04395105 | Dexamethasone versus usual care for the treatment of COVID-19 related ARDS: a multicenter and randomized open-label clinical trial | Dexamethasone | Dexamethasone 16 mg IV OD from days 1 to 5 and 8 mg from days 6 to 10 | ≥ 18 |
| NCT02735707 | Randomized, embedded, multifactorial adaptive platform trial for community-acquired pneumonia |
1. Fixed-duration Hydrocortisone 2. Shock-dependent hydrocortisone 3. Ceftriaxone 4. Moxifloxacin or Levofloxacin 5. Piperacillin-tazobactam 6. Ceftaroline 7. Amoxicillin-clavulanate 8. Macrolide administered for 3–5 days 9. Macrolide administered for up to 14 days 10. Five days of oseltamivir 11. Ten days of oseltamivir 12. Lopinavir/ritonavir 12. Hydroxychloroquine 13. Hydroxychloroquine + lopinavir/ritonavir 14. Interferon-β1a 15. Anakinra 16. Fixed-duration higher dose hydrocortisone 17. Tocilizumab 18.Sarilumab |
1. Fixed-duration hydrocortisone 50 mg IV q 6 h × 7 days 2. Shock-dependant hydrocortisone 50 mg IV q 6 h while in septic shock 3. Fixed-duration higher dose hydrocortisone—100 mg IV every 6 h × 7 days |
> 18 |
| NCT04377503 | Comparison of the efficacy and safety of tocilizumab versus methylprednisolone in the cytokine release syndrome of patients with COVID-19. A prospective randomized controlled phase II trial |
1. Tocilizumab 180 mg/ml 2. Methylprednisolone sodium succinate |
Methylprednisolone sodium succinate 1.5 mg/kg/day BD × 7 days followed by 1 mg/kg/day × 7 days, finally 0.5 mg/kg/day × 21 days | ≥ 18 |
| NCT04343729 | Methylprednisolone in the treatment of patients with signs of severe acute respiratory syndrome in Covid-19 (MetCOVID) | Methylprednisolone sodium succinate | Methylprednisolone sodium succinate 0.5 mg/kg | ≥ 18 |
| NCT04327401 | COVID-19-associated ARDS treated with dexamethasone: Alliance Covid-19 Brasil III (CoDEX) | Dexamethasone | Dexamethasone 20 mg IV 1/day × 5 days followed by 10 mg IV 1/day × 5 days | ≥ 18 |
| NCT04374474 | Olfactory retraining therapy and budesonide nasal rinse for anosmia treatment in patients post-CoVID 19. A randomized controlled trial |
1. Corticosteroid nasal irrigation 2. Smell household items; olfactory retraining |
Budesonide 240 ml nasal irrigation with Pulmicort Respules (0.5 mg) across both nose sides | ≥ 18 |
| NCT04263402 | An open, prospective/retrospective, randomized controlled cohort study to compare the efficacy of different hormone doses in the treatment of 2019-nCoV severe pneumonia | Methylprednisolone |
1. Methylprednisolone < 40 mg/day IV drip × 7 days 2. Methylprednisolone 40 to 80 mg/day IV drip × 7 days |
≥ 18 |
| NCT04244591 | Glucocorticoid therapy for critically ill patients with severe acute respiratory infections caused by COVID-19: a prospective, randomized controlled trial | Methylprednisolone therapy. Others: standard care | Methylprednisolone 40 mg q. 12 h × 5 days | ≥ 18 |
| NCT04273321 | Efficacy and safety of corticosteroids in COVID-19: a prospective randomized controlled trials | Methylprednisolone | Accord with the clinical diagnosis and/or etiological diagnosis diagnostic criteria | 18–75 |
| ChiCTR2000029386 | Effectiveness of glucocorticoid therapy in patients with severe novel coronavirus pneumonia: a randomized controlled trial | Methylprednisolone and intravenous injection | Methylprednisolone 1–2 mg/kg/day IV × 3 days | ≥ 18 |
| ChiCTR2000029656 | A randomized, open-label study to evaluate the efficacy and safety of low-dose corticosteroids in hospitalized patients with novel coronavirus pneumonia (COVID-19) | Methylprednisolone | Not available | ≥ 18 |
| ChiCTR2000030481 | The clinical value of corticosteroid therapy timing in the treatment of novel coronavirus pneumonia (COVID-19): a prospective randomized controlled trial | Not mentioned | Not available | ≥ 18 |
| NCT04348305 | Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia - the COVID STEROID Trial | Hydrocortisone | Hydrocortisone continuous infusion: 200 mg q 24 h bolus injections 50 mg (10 ml) every 6 h × 7 days | ≥ 18 |
| NCT04331054 | Protective role of inhaled steroids for Covid-19 infection |
1. Usual practice 2. Usual practice + Symbicort Rapihaler |
Symbicort (budesonide, formoterol) 200/6 μg, 2 puffs bid × 30 days | 18–75 |
| NCT04361474 | A randomized controlled trial evaluating the efficacy of local budesonide therapy in the management of hyposmia in COVID-19 patients without signs of severity |
1. Budesonide nasal spray 2. Physiological serum |
Budesonide 1 mg/2 ml diluted in 250 ml of physiological saline 3 syringes of 20 ml in each nasal cavity BD × 30 days | ≥ 18 |
| NCT04359511 | Efficacy and safety of corticosteroids in oxygen-dependent patients with COVID-19 pneumonia in Grand Ouest Interregion France |
1. Prednisone 2. Hydrocortisone |
Prednisone 0.7 mg/kg/day PO OD × 10 days or hydrocortisone hemisuccinate 3.5 mg/kg/day continuous infusion × 10 days | ≥ 18 |
| NCT04347980 | Dexamethasone combined with hydroxychloroquine compared to hydroxychloroquine alone for treatment of severe acute respiratory distress syndrome induced by coronavirus disease 19 (COVID-19): a multicentre, randomised controlled trial |
1. Dexamethasone and hydroxychloroquine 2. Hydroxychloroquine |
Dexamethasone 20 mg IV OD for 15 min × 5 days followed by 10 mg OD × 5 days | ≥ 18 |
| NCT04344730 | Dexamethasone and oxygen support strategies in ICU patients with Covid-19 pneumonia (COVIDICUS) | Dexamethasone injection + conventional oxygen | Dexamethasone 20 mg/5 ml IV | 18–80 |
| NCT04344288 | Corticosteroids during Covid-19 viral pneumonia related to SARS-Cov-2 infection (CORTI-Covid) | Prednisone | Prednisone 0.75 mg/kg/day × 5 days then 20 mg/day × 5 more days | ≥ 18 |
| NCT04331054 | Protective role of inhaled steroids for Covid-19 infection |
1. Usual practice 2. Usual practice + Symbicort Rapihaler |
Symbicort (budesonide, formoterol) 200/6 μg 2 puffs bid × 30 days | 18–75 |
| NCT04331470 | Evaluation of efficacy of levamisole and formoterol + budesonide in treatment of COVID-19 |
1. Levamisole pill + budesonide + formoterol inhaler/lopinavir/ritonavir + hydroxychloroquine 2. Lopinavir/ritonavir + hydoxychloroquine |
Budesonide + formoterol inhalation 1–2 puffs q 12 h | 15–100 |
| IRCT20080901001165N52 | Investigating the efficacy of high dose of glucocorticoid in patients with moderate to severe pneumonia related to COVID-19 | Methylprednisolone and prednisolone |
Day 1: Amp. methylprednisolone 500 mg IV infusion over 1 hour. At days 2 and 3: Amp. methylprednisolone 250 mg IV infusion over 1 h. At days 4 and 5: Amp. methylprednisolone 100 mg IV infusion over 1 h. Then, tab. prednisolone 25 mg PO daily until the day of discharge, then tab. prednisolone will gradually tapered off over 1 month |
18–85 |
| IRCT20200204046369N1 | Evaluation of methylprednisolone administration as a therapeutic option in the 2019 novel coronavirus (COVID-19): a non-randomized controlled study | Methylprednisolone | Methylprednisolone 20 mg/day | ≥ 18 |
| IRCT20200318046812N2 | Safety and efficacy of “Hydroxychloroquine + Azithromycin + naproxen + Prednisolone” and “Hydroxychloroquine + Azithromycin + naproxen” regimens in comparison with “Hydroxychloroquine + kaletra” on the need for intensive care unit treatment in patients with COVID-19; a randomized, multicenter, parallel | Hydroxychloroquine, azithromycin, naproxen , prednisolone | Prednisolone five 5 mg tablets a day × 5 days | 16–100 |
| IRCT20151227025726N17 | Evolution of the efficacy and safety of Dexamethasone administration in patients with mild to moderate COVID-19 acute respiratory disease syndrome | Dexamethasone | Dexamethasone 20 mg IV days 1–5, then 10 mg days 6–10 | ≥ 18 |
| IRCT20120225009124N4 | Efficacy of different methods of administration of combination regimen including dexamethasone, IV-IG and interferon beta for treatment of patients with severe COVID-19: a randomized controlled trial | Dexamethasone, IV-IG and interferon beta | Not available | 18–70 |
| IRCT20200406046963N1 | Evaluation of the efficacy and safety of methylprednisolone pulse therapy in treatment of COVID-19 patients with ARDS. | Methylprednisolone | Methylprednisolone 1000 mg for 3 days | 18–90 |
| IRCT20200404046947N1 | Study of methylprednisolone effects on treatment and clinical symptoms and laboratory signs of Iranian COVID-19 patients: a clinical trial study | Methylprednisolone | Methylprednisolone 250 mg for 3 days | ≥ 18 |
| IRCT20081027001411N3 | Study of prednisolone effects on treatment and clinical symptoms and laboratory signs of Iranian COVID-19 patients: a clinical trial study | Prednisolone | Prednisolone 0.5 mg/kg in three divided doses up to 30 mg per day for 5–7 days | ≥ 18 |
| IRCT20120215009014N354 | Evaluating the effect of intravenous hydrocortisone, methylprednisolone, and dexamethasone in treatment of patients with moderate to severe acute respiratory distress syndrome caused by COVID-19: a double blind randomized clinical trial | Hydrocortisone, methylprednisolone, and dexamethasone |
Group 1: Hydrocortisone 50 mg IV q. 6 h × 5 days Group 2: Methylprednisolone 40 mg IV q 12 h ×5 days Group 3: Dexamethasone IV 20 mg daily × 5 days |
18–70 |
| NCT04323592 | Methylprednisolone for patients with COVID-19 severe acute respiratory syndrome (MP-C19) | Methylprednisolone and other standard care | Methylprednisolone 80 mg/kg IV bolus | 18–80 |
| KCT0005105 | A trial of ciclesonide in adults with mild COVID-19 |
1. Ciclesonide (Alvesco®) 320 μg inhalation twice a day for 14 days 2. Ciclesonide (Alvesco®) 320 μg inhalation twice a day for 14 days + hydroxychloroquine 400 mg per day for 10 days |
Ciclesonide (Alvesco®) 320 μg inhalation BD × 14 days | 19–100 |
| NCT04345445 | Study to evaluate the efficacy and safety of tocilizumab versus corticosteroids in hospitalized COVID-19 patients with high risk of progression |
1. Tocilizumab IV 2. Methylprednisolone IV |
Methylprednisolone 120 mg/day for 3 days | 18–80 |
| NCT04435795 | Ciclesonide clinical trial for COVID-19 treatment | Ciclesonide | Ciclesonide 600 μg BID inhaled with aerochamber + Nasal ciclesonide 200 μg DIE | ≥ 18 |
| NCT04360876 | Targeted steroids for ARDS due to COVID-19 pneumonia: a pilot randomized clinical trial |
1. Dexamethasone injection 2. Placebo |
Dexamethasone 20 mg IV OD 5 days followed by 10 mg OD × 5 days | ≥ 18 |
| NCT04366115 | A randomized, double-blind, placebo-controlled, phase 1/2 study evaluating AVM0703 in patients with COVID-19 |
1. AVM0703 2. Placebo 3. Hydrocortisone |
1. AVM0703 (dexamethasone sodium phosphate) 10 mg/ml single IV infusion in NS over 1 hour 2. Hydrocortisone dose not available |
≥ 18 |
| NCT04355247 | Prophylactic corticosteroid to prevent COVID-19 cytokine storm | Methylprednisolone 80 mg/ml injectable suspension | Methylprednisolone 80 mg IV bolus injection OD × 5 days | ≥ 18 |
| NCT04438980 | Treatment of COVID-19 pneumonia with glucocorticoids. A randomized controlled trial |
1. Methylprednisolone 2. Placebo |
Methylprednisolone 120 mg/day IV infusion × 3 days | 18–80 |
| NCT04394182 | Low doses of lung radiation therapy in cases of COVID-19 pneumonia: prospective multicentric study in radiation oncology centers |
1. Ultra-low-dose radiotherapy 2. Ventilatory support with oxygen therapy 3. Lopinavir/ritonavir, hydroxychloroquine, azithromycin, piperacillin/tazobactam, Low molecular weight heparin, corticosteroid injection, tocilizumab |
Methylprednisolone 250 mg × 3 boluses | 18–120 |
| NCT04380818 | Low dose anti-inflammatory radiotherapy for the treatment of pneumonia by COVID-19: multi-central prospective study |
1. Low-dose radiotherapy; hydroxychloroquine Sulfate 2. Ritonavir/lopinavir 3. Tocilizumab Injection (Actemra) 4. Azithromycin 5. Corticosteroid 6. Low molecular weight heparin; oxygen supply |
Not available | 18–99 |
| NCT04355637 | Treatment with inhaled corticosteroids in patients hospitalized because of COVID19 pneumonia | Inhaled budesonide | Not available | 18–79 |
| NCT04341038 | Clinical trial to evaluate methylprednisolone pulses and tacrolimus in patients with COVID-19 lung injury (TACROVID) |
1. Tacrolimus 2. Methylprednisolone |
Methylprednisolone 120 mg daily × 3 days | Not available |
| NCT04329650 | Efficacy and safety of siltuximab vs. corticosteroids in hospitalized patients with COVID-19 pneumonia |
1. Siltuximab 2. Methylprednisolone |
Methylprednisolone 250 mg/24 h | ≥ 18 |
| NCT04325061 | Efficacy of dexamethasone treatment for patients with ARDS caused by COVID-19 (DEXA-COVID19) | Dexamethasone | Dexamethasone 20 mg/IV/daily × 5 days | > 18 |
| 2020-001827-15 | Early treatment of pneumonia Covid-19 with glucocorticoids. randomized controlled clinical trial | Methylprednisolone and hydroxychloroquine | Not available | ≥ 18 |
| 2020-001622-64 | Outpatient treatment of Covid-19 with early pulmonary corticosteroids as an opportunity to modify the course of the disease | Prednisone | Not available | 18–74 |
| 2020-001934-37 | Use of corticosteroids in patients with SARS-CoV2 coronavirus infection (glucocovid) pragmatic trial inserted in real practice during a pandemic covid-19 | Methylprednisolone | Not available | 18–85 |
| 2020-001413-20 | Phase 2, randomized, open-label study to compare the efficacy and safety of siltuximab vs. corticosteroids in hospitalized patients with COVID-19 pneumonia | Methylprednisolone and siltuximab | Not available | ≥ 18 |
| 2020-001445-39 | Pragmatic, controlled, open, single center, randomized, phase Ii clinical trial to evaluate methylprednisolone pulses and tacrolimus in hospitalized patients with severe pneumonia secondary to COVID-19. |
1. Methylprednisolone 2. Tacrolimus |
Not available | ≥ 18 |
| 2020-001307-16 | Efficacy and safety of corticoids in patients with adult respiratory distress syndrome (ARDS) secondary to COVID-19. | Methylprednisolone hemisuccinate | Not available | ≥ 18 |
| NCT04381364 | Inhalation of ciclesonide for patients with COVID-19: a randomised open treatment study (HALT COVID-19) | Ciclesonide inhalation | Ciclesonide inhalation 320 μg BD × 14 days | 18–84 |
| NCT04416399 | Use of high dose inhaled corticosteroids as treatment of early COVID-19 infection to prevent clinical deterioration and hospitalization | Budesonide dry powder inhaler | Budesonide 400 μg per inhalation, 2 inhalations twice a day × 28 days | > 18 |
| NCT04381936 | Randomized evaluation of COVID-19 therapy |
1. Lopinavir-ritonavir 2. Dexamethasone/prednisolone 3. Hydroxychloroquine 4. Azithromycin 5. Biological: convalescent plasma 6. Tocilizumab |
Dexamethasone 6 mg PO OD × 10 days | Child, adult, older adult |
| NCT04411667 | Randomized open label study of standard of care plus intravenous immunoglobulin (IVIG) compared to standard of care alone in the treatment of COVID-19 infection | IVIG (Octagam) premedication and methylprednisolone | Methylprednisolone 40 mg IV push × 1 30–50 min before each IVIG infusion | ≥ 18 |
| NCT04377711 | A phase 3, multicenter, randomized, double-blind, placebo-controlled study to assess the safety and efficacy of ciclesonide metered-dose inhaler in non-hospitalized patients 12 years of age and older with symptomatic COVID-19 infection |
1. Ciclesonide 2. Placebo |
Alvesco (ciclesonide) 320 μg b.i.d. × 30 days via pMDI | 12–100 |
| NCT04349410 | The fleming [FMTVDM] directed CoVid-19 treatment protocol |
1. Hydroxychloroquine, azithromycin 2. Hydroxychloroquine, doxycycline 3. Hydroxychloroquine, clindamycin 4. Hydroxychloroquine, clindamycin, primaquine—low dose 5. Hydroxychloroquine, clindamycin, primaquine—high dose 6. Remdesivir 7. Tocilizumab 8. Methylprednisolone 9. Interferon-Alpha2B 10: Losartan plus convalescent serum |
Methylprednisolone 80 mg IV over 30 min b.i.d. × 7 days, then taper off | Child, adult, older adult |
| NCT04193878 | Arrest respiratory failure from pneumonia (Arrest pneumonia) |
1. Inhaled budesonide and formoterol 2. Inhaled placebo |
Formoterol aerosolized—20 μg/2 ml) and budesonide—1.0 mg/2 ml q 12 h × 14 doses | ≥ 18 |
| NCT03852537 | SMART Trial: steroid dosing by biomarker guided titration in critically ill patients with pneumonia | Methylprednisolone. Other: usual care | Methylprednisolone—predetermined dosing table—discontinue if CR < 0.5 mg; 0.5 mg if CRP is 51–100 mmol/L or 0.75 mg/kg if CRP level is 101–150 mmol/L; 1 mg/kg if CRP 151–200 mmol/L or 1.5 mg/kg if CRP level > 200 mmol/L or dose equivalent of oral prednisone for the above | ≥ 18 |
| NCT04374071 | Early short course corticosteroids in hospitalized patients with COVID-19 | Methylprednisolone |
1. Methylprednisolone 0.5 to 1 mg/kg/day IV in two divided doses × 3 days 2. Hydroxychloroquine and IV methylprednisolone 0.5 to 1 mg/kg/day in 2 divided doses × 3–7 days |
≥ 18 |
Figure 3 depicts the number of trials studying different types of steroids, showing majority of the trials (N = 28) have decided to study the effectiveness of methylprednisolone in the treatment of COVID-19.
Fig. 3.
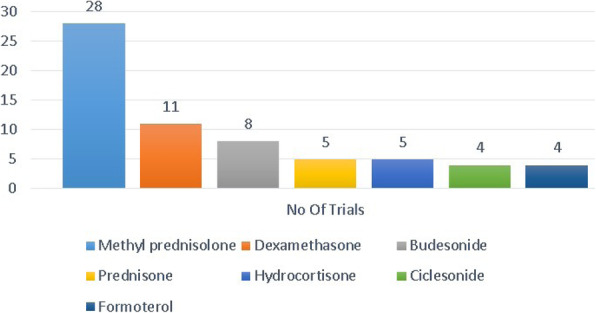
Number of trials using different kinds of steroids
Primary and secondary outcomes
Table 1 summarizes results from all 60 studies. All the trials had clearly defined primary and secondary outcomes of interest, in which only 11 trials had evaluation of respiratory rate as one of their outcomes. Common outcomes measured are respiratory rate, mortality rate, ventilation free days, days in ICU, patient Sequential Organ Failure Assessment (SOFA) score, Murray lung injury score, National Early Warning Score 2 (NEWS2) score, number of patients with treatment failure, rate of remission and progression, blood oxygen saturation, chest x-ray, steroid-related adverse effect, and toxicity monitoring. Table 4 summarizes the consolidation of completed trials with results. All the completed trials have used methylprednisone, dexamethasone, and hydrocortisone as drug of choices.
Table 4.
Characteristics of published completed trials
| Ref | Country | Year of publication | Steroid used | Primary outcomes | P value |
|---|---|---|---|---|---|
| [24] | China | 2020 | Methylprednisolone 396 of 409 [96.8%], dexamethasone 32 of 409 [7.8%] patients—hydrocortisone equivalent | Corticosteroid therapy had higher 28-day mortality rate. Delay in SARS-CoV RNA clearance (P = 0.00017) | < 0.05 |
| [25] | USA | 2020 | Hydrocortisone 200 mg/day and tapered till 50 mg/day | Treatment failure occurred in hydrocortisone patient is 42.1% compared to placebo 50.7% | < 0.045 |
| [26] | Netherlands | 2020 | Methylprednisolone 80 mg, 250 mg | There was a 79% higher likelihood of two stage improvement in respiratory status | < 0.025 |
| [27] | USA | 2020 | Hydrocortisone 50 mg, 100 mg | The in-hospital death in treatment group is 30% and 26% compared to no hydrocortisone, i.e. 33% | < 0.05 |
The data obtained from this review shows that steroids of different doses and types were included in numerous ongoing clinical trials. Their safety and efficacy in managing symptoms of COVID-19, especially in the pneumonia stage, were tested. The trials also included patients of different age groups at different stages of COVID-19. The COVID-19 infection goes through three stages from asymptomatic phase to ARDS (acute respiratory distress syndrome) phase. The 2019-nCoV, after entering the nasal cavity, adheres to the epithelial cells and binds to ACE2 receptor [24]. Owing to this reason, it may be evident that different corticosteroids act through different mechanisms to minimize the symptoms of COVID-19 infection. Table 3 represents the total number of population recruited in each trial, from which we estimate the total ARDS population recruited to be 3880 patients with disease stages ranging from moderate to severe respiratory distress of which methylprednisolone was the most commonly used corticosteroids. A study by H.P. Wiedemann et al. showed that methylprednisolone increased mortality rates by at least 14 days after the onset of ARDS, which gives an impression that the routine use of methylprednisolone is not effective in ARDS [25]. Another study by Nelson Lee et al. shows that SARS-CoV RNA concentrations in the second and third week of illness were significantly higher in patients who received early hydrocortisone treatment compared to placebo; thus, it is recommended to be avoided, but can be cautiously used in SARS [26]. The potential risks associated with high-dose corticosteroids in treating 2019-nCoV pneumonia include secondary infections, long-term complications, and prolonged virus shedding and escalating towards advanced stages [27]. Another study conducted by G.C. Khilnani and H. Vijay registered increased mortality rate (35.7%) with the use of corticosteroids [28–33]. Positively, the RECOVERY trial (Randomised Evaluation of COVID-19 therapy) concluded that in hospitalized patients with COVID-19, corticosteroid reduced 28-day mortality among those receiving invasive mechanical ventilation or oxygen at randomization, but not among patients not receiving respiratory support [34]. Moreover, excessive levels of glucocorticoids have shown to precipitate heart failure by aggravating fluid retention, triggering risk factors like glucose intolerance and dyslipidemia, and by worsening atheromatous vascular disease. Additionally, increased risk of mortality with high serum levels of cortisol have been reported, further establishing a link between use of corticosteroids and increased heart failure risk [35]. Thus, the usage of corticosteroids at various stages of COVID-19 is still questionable with higher mortality rates than the comparator. More information can be gained from results from the completed trials. Though four trials have completed its recruitment, results were not available in the registry. The completed four trials were registered in the Iranian clinical trial registry. The outcomes measured in these trials were mortality rate, need for ICU services, duration of stay in the hospital, assessment of side effects, readmission rate, need for oxygen therapy, blood O2, levels, chest x-ray, PAO2/fio2, and need for invasive mechanical ventilation and intubation.
Conclusion
Numerous interventional and non-interventional studies are being conducted to study the efficacy of corticosteroids in COVID-19. Corticosteroids can regulate immune-mediated lung injury and decrease the development to respiratory failure and death. Dexamethasone has been reported to reduce the duration of mechanical ventilation. Long-term glucocorticoid therapy has displayed significant improvement in indices of alveolar–capillary membrane permeability and mediators of inflammation and tissue repair. Few preliminary trial findings show promising results and recommend the use of methylprednisolone and dexamethasone in the severe form of the COVID-19. Few studies have reported that early administration of dexamethasone could reduce duration of mechanical ventilation and overall mortality in patients with established moderate to severe ARDS; however, there is insufficient data to prove its benefits over its risk. Routine use of corticosteroids should be favored only after a better insight is obtained, with the completion of these trials.
Acknowledgements
Not applicable
Abbreviations
- ACE
Angiotensin-converting enzyme
- ARDS
Acute respiratory distress syndrome
- ChiCTR
Chinese Clinical Trial Registry
- COVID-19
Coronavirus disease 2019
- CRiS
Clinical Research Information Service–Republic of Korea
- EU
European Union
- ICU
Intensive care unit
- IRCT
Iranian Registry of Clinical Trials
- MERS-CoV
Middle East respiratory syndrome coronavirus
- NEWS2
National Early Warning Score 2
- RNA
Ribonucleic acid
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- sCAP
Severe community-acquired pneumonia
- SOFA
Sequential Organ Failure Assessment
- WHO
World Health Organization
Authors’ contributions
RR and PSB contributed in the literature search, data collection, data analysis, and writing. PV did the data analysis, data interpretation, figures, and writing. SJUCJ is responsible for the concept, design, methods, data interpretation, writing, and proof reading. The authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets analyzed during the current study will be available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Reshma Raju, Email: reshmaraju602@gmail.com.
Prajith V., Email: prajithkanna@gmail.com.
Pratheeksha Sojan Biatris, Email: pratheekshasojan00417@gmail.com.
Sam Johnson Udaya Chander J., Email: mail2samjohnson@gmail.com.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, Penzar D, Perlman S, Poon LLM, Samborskiy D, Sidorov IA, Sola I, Ziebuhr J. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. Available from: https://www.who.int/zh/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Accessed 25 June 2020.
- 4.He F, Deng Y, Li W. Coronavirus disease 2019 (COVID-19): what we know? J Med Virol. 2020;92(7):719–725. doi: 10.1002/jmv.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan JF, Yuan S, Kok KH, To KK. Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (Accessed 29 June 2020)
- 8.Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, Myoung J, Kim BT, Kim SJ. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 10.Cava C, Bertoli G, Castiglioni I. In silico discovery of candidate drugs against Covid-19. Viruses. 2020;12(4):404. doi: 10.3390/v12040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleandrova VV, Speck-Planche A. The QSAR paradigm in fragment-based drug discovery: from the virtual generation of target inhibitors to multi-scale modeling. Mini reviews in medicinal chemistry. 2020;20(14):1357–1374. doi: 10.2174/1389557520666200204123156. [DOI] [PubMed] [Google Scholar]
- 12.Redkar S, Mondal S, Joseph A, Hareesha KS. A machine learning approach for drug-target interaction prediction using wrapper feature selection and class balancing. Molecular informatics. 2020;39(5):e1900062. doi: 10.1002/minf.201900062. [DOI] [PubMed] [Google Scholar]
- 13.Singh R, Bhardwaj V, Das P, Purohit R. Natural analogues inhibiting selective cyclin-dependent kinase protein isoforms: a computational perspective. J Biomol Struct Dyn. 2019;4:1–10. doi: 10.1080/07391102.2019.1696709. [DOI] [PubMed] [Google Scholar]
- 14.Liu B, He H, Luo H, Zhang T, Jiang J. Artificial intelligence and big data facilitated targeted drug discovery. Stroke Vasc Neurol. 2019;4(4):206–213. doi: 10.1136/svn-2019-000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Mayorga K, Madariaga-Mazon A, Medina-Franco JL, Maggiora G. The impact of chemoinformatics on drug discovery in the pharmaceutical industry. Expert Opin Drug Discov. 2020;15(3):293–306. doi: 10.1080/17460441.2020.1696307. [DOI] [PubMed] [Google Scholar]
- 16.Fenteany G, Gaur P, Sharma G, Pintér L, Kiss E, Haracska L. Robust high-throughput assays to assess discrete steps in ubiquitination and related cascades. BMC Mol Cell Biol. 2020;12(21):1–6. doi: 10.1186/s12860-020-00262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Serradilla M, Risco C, Pacheco B. Drug repurposing for new, efficient, broad spectrum antivirals. Virus Res. 2019;264:22–31. doi: 10.1016/j.virusres.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senanayake SL (2020) Drug repurposing strategies for COVID-19. Future Drug Discov 0(0) fdd-2020-0010. 10.4155/fdd-2020-0010
- 19.Shah B, Modi P, Sagar SR. In silico studies on therapeutic agents for COVID-19: drug repurposing approach. Life sciences. 2020;252:117652. doi: 10.1016/j.lfs.2020.117652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou W, Liu Y, Tian D, Wang C, Wang S, Cheng J, Hu M, Fang M, Gao Y. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther. 2020;5(1):18. doi: 10.1038/s41392-020-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Muller WJ, O'Horo JC, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y. Infectious Diseases Society of America Guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020;27:ciaa478. doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Zhang S, Dong X, Li Z, Xu Q, Feng H, Cai J, Huang S, Guo J, Zhang L, Chen Y, Zhu W, Du H, Liu Y, Wang T, Chen L, Wen Z, Annane D, Qu J, Chen D. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest. 2020;130(12):6417–6428. doi: 10.1172/JCI140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, François B, Aubron C, Ricard JD, Ehrmann S, Jouan Y, Guillon A, Leclerc M, Coffre C, Bourgoin H, Lengellé C, Caille-Fénérol C, Tavernier E, Zohar S, Giraudeau B, Annane D, Le Gouge A, CAPE COVID Trial Group. the CRICS-TriGGERSep Network Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(13):1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramiro S, Mostard RLM, Magro-Checa C, van Dongen CMP, Dormans T, Buijs J, Gronenschild M, de Kruif MD, van Haren EHJ, van Kraaij T, Leers MPG, Peeters R, Wong DR, Landewé RBM. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. 2020;79(9):1143–1151. doi: 10.1136/annrheumdis-2020-218479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55(4):2000607. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network (2006) Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354(16):1671–1684. 10.1056/NEJMoa051693 [DOI] [PubMed]
- 30.Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A, Chiu RW, Wong VW, Chan PK, Wong KT, Wong E, Cockram CS, Tam JS, Sung JJ, Lo YM. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khilnani GC, Hadda V. Corticosteroids and ARDS: a review of treatment and prevention evidence. Lung India. 2011;28(2):114. doi: 10.4103/0970-2113.80324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ (2020) (2020) Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med NEJMoa2021436. 10.1056/NEJMoa2021436
- 35.El Hadidi S, Rosano G, Tamargo J, Agewall S, Drexel H, Kaski JC, Niessner A, Lewis BS, Coats AJS (2020) Potentially inappropriate Prescriptions in Heart Failure with Reduced Ejection Fraction (PIP-HFrEF). Eur Heart J Cardiovasc Pharmacother:pvaa108. 10.1093/ehjcvp/pvaa108 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study will be available from the corresponding author on reasonable request.