Abstract
There is a growing demand for vegetal food having health benefits such as improving the immune system. This is due in particular to the presence of polyphenols present in small amounts in many fruits, vegetables and functional foods. Extracting polyphenols is challenging because extraction techniques should not alter food quality. Here, we review technologies for extracting polyphenolic compounds from foods. Conventional techniques include percolation, decoction, heat reflux extraction, Soxhlet extraction and maceration, whereas advanced techniques are ultrasound-assisted extraction, microwave-assisted extraction, supercritical fluid extraction, high-voltage electric discharge, pulse electric field extraction and enzyme-assisted extraction. Advanced techniques are 32–36% more efficient with approximately 15 times less energy consumption and producing higher-quality extracts. Membrane separation and encapsulation appear promising to improve the sustainability of separating polyphenolic compounds. We present kinetic models and their influence on process parameters such as solvent type, solid and solvent ratio, temperature and particle size.
Keywords: Polyphenols, Foods, Process techniques, Extraction, Kinetics, Modeling
Introduction
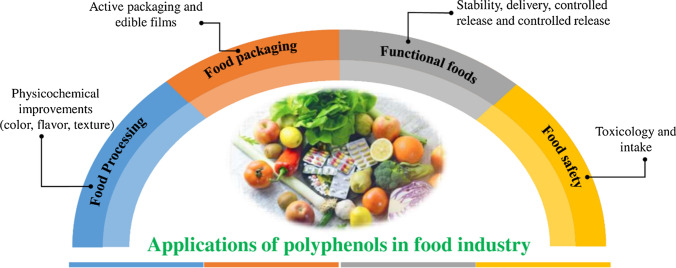
The continuous demand for nutrient-rich diet in contemporary health conscious society has led to an increasing consumption of polyphenol-rich foods (Iriondo-Dehond et al. 2018). Polyphenol compounds form an important part of the bioactive group. These compounds are a diverse family having more than one hydroxyl groups with a structure ranging from a simple molecule to a complex polymer. The compounds are naturally present in small amounts in various foods, shrubs and medicinal plants like tea, grapes, vegetables, wine, cereals, coffee, eucalyptus and neem. Polyphenols possess antioxidant, anticarcinogenic, anti-inflammatory and antimicrobial properties (Rowland 1999; Treutter 2006; Barakat et al. 2010; Sanchez et al. 2013; Xiao et al. 2013; Frontuto et al. 2019; Román et al. 2019). Figure 1 illustrates the role of polyphenols in the food industry.
Fig. 1.
Role of polyphenols in the food industry. The dietary characteristics of polyphenols improve the physicochemical properties of foods, giving several health benefits
The production and development of such novel foods is a challenge, as it has to fulfill the consumer expectations for the final product to be healthy, nutritious and palatable. The polyphenolic family has played an integral role in pharmaceutical sector, food as well as nutraceutical industry. They have a potential in tackling health-related diseases and pandemics like COVID-19 (Paraiso et al. 2020). At a molecular level, they show a promising potential to inhibit viral proteases involved in replication with low risk of toxicity. However, the research on natural approaches against viral diseases is still in its infant stages. Studies on secondary metabolites with health benefits have identified the widespread use of polyphenol giving positive dietary effect on humans (Theodorou et al. 2006; Piccolella et al. 2019). Apart from contributing to the color and sensory characteristics of foods, they have also shown applications in food preservation and giving protection against bacteria and other pathogens (Kuai et al. 2020; Ribeiro et al. 2020; Sridhar et al. 2020). In the recent years, special sensors have been fabricated for the detection of biomolecules from foods (Govindarajalu et al. 2019; Verma and Rani 2020). However, the performance of such sensors still remains a huge concern (Saravanan et al. 2020). For this reason, great efforts have been made to understand the evaluation, characteristics and quantification of polyphenols. The concept of circular economy has led to the introduction of “zero waste strategy” for the recovery of such special compounds (Fu et al. 2015; Romani et al. 2020). It not only acts as a solution to tackle problems immediately in case of crisis but also provides a long-term sustenance in the food sector. However, feasible, cost-effective, energy-efficient, environmentally friendly methods as well as suitable parameter optimizations still need to be explored for a controlled intake.
Apart from widely used extraction techniques of Soxhlet and maceration, ultrasound-assisted extraction (UAE) (Zhou et al. 2019), microwave-assisted extraction (Rodsamran and Sothornvit 2019) and supercritical fluid extraction (Sánchez-Camargo et al. 2020) have been analyzed extensively in food and bioprocessing sector for its merits and economic values (Osorio-Tobón 2020). Investigation is still being carried out to find an isolation method, which has the least experimental set-up, low cost with environmental and user-friendly characteristics (Kothari et al. 2012). One of the methods gaining interest in recent years is encapsulation technologies (Wang et al. 2020). The enhanced extraction of polyphenols was also carried out using low-transition temperature mixtures (e.g., glycerol and sodium acetate) at a specified proportion (Karageorgou et al. 2017).
However, investigation on emerging developments and kinetics for the optimization of parameters still remains a concern. Kinetic studies are one of the ways that help in understanding the extraction rate, factors, energy consumption and scaleup (Natolino and Da Porto 2020). The need for mathematical modeling is essential to optimize the extraction parameters and control the process to get the best output.
Rapid exploration has been carried out on different types, characteristics and health effects of polyphenolic compounds (Tresserra-Rimbau et al. 2018; Sanches Silva et al. 2020). In a period of 116 years (1900–2016), 85% of the total published works on polyphenols was in the form of peer-reviewed research articles, while the remaining included book chapters, conference proceedings and review articles. The USA published the highest number of research articles (6713) followed by China (4959) and Spain (3677). According to Web of Science, the total number of articles published in the area of polyphenols increased by more than two and half times in the last 10 years from 2006 to 2016 with majority of studies done on grape seeds (Adebooye et al. 2018). The global polyphenol market was valued at USD 873.7 million in 2018 and is expected to achieve a growth rate of 6.1% owing to increasing demands and market size (Ameer et al. 2017). However, an exhaustive understanding of the developments of polyphenols from different foods still needs to be achieved. Thus, this article brings focus on the technologies developed for the isolation of polyphenolic compounds from different foods. Techniques mainly percolation, decoction, heat reflux extraction, Soxhlet extraction, maceration, ultrasound-assisted extraction, microwave-assisted extraction, supercritical fluid extraction, high-voltage electric discharge, pulse electric field and enzyme-assisted extraction have been given adequate importance. Additionally, future advancements in extraction technology, mainly membrane separation and encapsulation techniques with their usage in concentrating polyphenolic compounds, have been given enough focus. Key parameters like nature of solvent, solid–solvent ratio, temperature, particle size and their influence on food have been thoroughly discussed. Lastly, mathematical models for optimizing the overall extraction have been highlighted for controlling the process. This article could provide a promising platform for understanding the scope of polyphenols in the food industry addressing major issues like quality, adaptability and cost.
Extraction methods of polyphenols
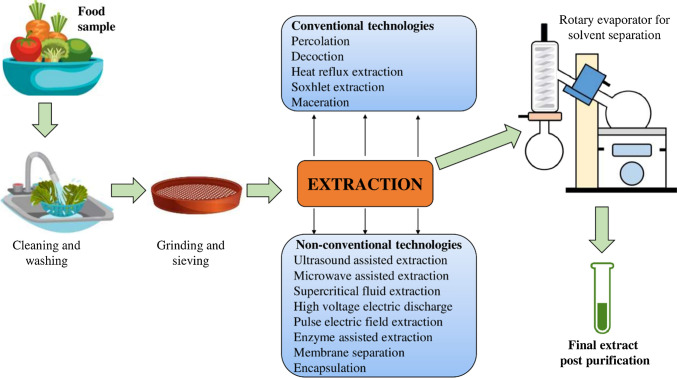
Extraction plays an important role for isolation and purification of many bioactive components from food material. In order to obtain the extract from the food sample, steps like size reduction, extraction, filtration, concentration and drying should be noted (Azmir.J et al. 2013; Živković et al. 2018). Figure 2 illustrates the general flow diagram for the isolation method of polyphenols from foods. Various extraction techniques have been analyzed to estimate the polyphenol recovery from foods ranging from traditional methods to modern methods. The most extensively used techniques for extraction include Soxhlet extraction, maceration, ultrasound-assisted extraction, microwave-assisted extraction, supercritical fluid extraction, high-voltage electric discharge, pulse electric field extraction and enzyme-assisted extraction (Pasrija and Anandharamakrishnan 2015; Luo et al. 2018). Apart from these technologies, membrane separation and encapsulation methods have also shown their potential for better extraction of polyphenols (de Santana Magalhães et al. 2019; El-Messery et al. 2019).
Fig. 2.
Isolation of polyphenols from food materials. Different extraction technologies have been investigated for the separation of bioactive compounds from foods, ranging from conventional to non-conventional methods. Extraction efficiency is dependent on factors such as the nature of solvent, solvent–solid ratio, temperature and particle size
Conventional technologies
As polyphenolic compounds are usually obtained inside different foods, beverages and plant matrices in small quantities, extraction methods become necessary. Pretreatment techniques like drying, crushing and grinding may be required depending on the samples. After extraction, isolation and purification are done to obtain the active compounds (Chuo et al. 2020). Conventional extraction methods mainly include percolation, decoction, heat reflux extraction, Soxhlet extraction and maceration. These are varied depending on the composition and characteristics of food samples. Although conventional methods are easy to use, they pose negative effects like high extraction time, huge energy consumption and solvent wastage (Zwingelstein et al. 2020).
Percolation
Percolation is a traditional procedure used for separation of active compounds from fluid extract. It consists of a narrow percolator (generally cone-shaped). The food sample is mixed thoroughly with water, and the solution is added from the top through the column into a closed container. The mixture seeps down with time (24–48 h depending on the sample), thus obtaining the pure extract. The enriched wet extract is concentrated in evaporators to get the desired concentration. The technique has been used for isolating a variety of polyphenolics from food matrices (Hansen and Møller 1975; Rathore et al. 2012).
Decoction
Decoction involves boiling the crude aqueous extract to a certain volume for a specific time to get the heat-stable materials. The liquid settles down and is cooled, strained or filtered. The technique can be used for extracting water-soluble constituents. However, it should be noted that this procedure holds inefficient for heat and light-sensitive compounds. Additionally, mass transfer and kinetic effects are needed to be considered. Keeping negatives aside, this technique has found applications in various aromatic (Fuleky and Czinkota 1993) and medicinal plants (Rijo et al. 2014; Hashemi et al. 2019).
Heat reflux extraction
Heat reflux extraction is a much preferred technique as compared to percolation and decoction as it requires less extraction time and solvent (Zhang et al. 2018b). The technology involves heating the matrix for a particular time, leading to a complex chemical reaction. As the process uses reflux extractor as the main reactor, better mass transfer and contact efficiency are achieved between solvent and treated matrix (Tian et al. 2016). The vapor trickles down to the flask, thus controlling the temperature of the reaction. The technology has been preferred due to its simplicity and easy operation (Zhang et al. 2018a). The technology has found application in extracting many natural, phytochemical compounds and essential oils (Gao and Liu 2005; Aliboudhar and Tigrine-Kordjani 2014).
Soxhlet extraction
Soxhlet extraction has always been the most extensively used process for extraction purposes involving concentration of analyte leading to separation of bioactive constituents from natural products. Sample preparation is the most critical and can be done using variety of techniques (Luque de Castro and Priego-Capote 2010). Leaching is considered as one of the traditionally and practically used methods for solid pretreatment. When it comes to environmentally friendly methods, Soxhlet extraction is one of the relevant techniques. In conventional Soxhlet, sample is added inside a thimble and fresh solvent is added in the round-bottomed flask. The fresh solvent is passed through the thimble during the extraction process and then used as recovery. When the liquid reaches the top, the siphon drops the solvent back into the round-bottomed flask through the thimble holder. This operation is repeated until the process reaches saturation. The extraction of polyphenols is carried out for about 24–50 h, and more than half of the solvent is used for extraction studies (Sen et al. 2017).
Due to its increased simplicity, the Soxhlet extraction method is still considered. However, there are some drawbacks to this technique. It uses large amounts of samples (10–30 g), long extraction times (18–24 h depending on the sample), large amounts of solvent usage (300–500 mL per sample) and excessive loss of heat energy (Hawthorne et al. 2000).
On the other hand, maceration is considered a more suitable extraction technique as it uses lower temperatures, lesser time duration and gave higher yield of polyphenolic content. For instance, a study was done for determining the phenolic and flavonoid content of Syzygium cumini. L seed kernel. Soxhlet extraction gave a total phenolic content (TPC) of 30.05 mg GAE/g at 100 °C in 6 h, while batch extraction gave a phenolic content of 79.87 mg GAE/g at 50 °C in 105 min (Mahindrakar and Rathod 2020). Another investigation on extraction of curcumin was analyzed using process intensification methods. It was concluded that batch extraction gave higher yield (7.89 mg/g) at lower temperatures (30 °C) as compared to Soxhlet, which took almost the same yield at increased temperatures (Shirsath et al. 2017). Thus, it can be concluded that maceration is a more promising and affordable technique.
Maceration
Maceration is one of the go-to methods for determination of polyphenolic compounds (Ćujić et al. 2016). This is due to its simplicity, least experimental set-up, low cost and environmentally friendly characteristics.
The speed of agitation and time are the two most important factors to be considered in this technique. The speed of the magnetic stirrer may lead to a vortex formation, which leads to a turbulence when the speed of the stirrer is varied. Due to these parameters, an increase in mass transfer rate may also be possible. Thus, the speed of the stirrer should be maintained between 180 and 240 rpm. If the speed is increased, there is high variations in equilibrium concentration and hence the diffusion coefficient (Shewale and Rathod 2018). Thus, the full study is undergone until all the compounds are extracted and process reaches equilibrium (Amita Pandey and Tripathi 2014).
Apart from the advantages and simplicity in design, maceration technique poses few disadvantages. There may be batch-to-batch variations leading to error. Additionally, with the upcoming technology and fast-growing world, time could play a key role in this extraction. A huge amount of time is required for reaching equilibrium. Table 1 shows the comparison between Soxhlet extraction and maceration technique (Ozel and Kaymaz 2004; Azwanida 2015; Shukla et al. 2016) as they have extensively used for extraction studies.
Table 1.
Comparison between Soxhlet extraction and maceration technique
| Criteria | Soxhlet extraction | Maceration |
|---|---|---|
| Terminology |
Finely ground sample is placed in a thimble Solvent in the flask is heated, it vaporizes into the thimble containing sample and condenses back into the flask. When the liquid reaches the top, the contents get emptied and the extraction continues |
Soaking of coarse food materials into a container with a solvent Frequent agitation and mixing take place due to which cell walls break releasing bioactive compounds The solvent is recovered, and the extract is obtained using filtration process |
| Sample type | Dry and finely divided solids | Coarse or powdered form |
| Extraction time span | Low | High |
| Solvent generally used | Petroleum ether, hexane | Ethanol/methanol–water mixtures |
| Selectivity of solvent | Solvent should be carefully selected. Exposure to hazardous or flammable organic solvents can have negative effects on the overall extraction depending on the food sample chosen | Depends on the compounds extracted from the food or plant sample |
| Cost | High cost as solvents chosen need to be highly pure for taking part in the extraction | The choice of solvent enhances the extraction process. Solvents used in the soaking process play a critical role |
| Purity and efficiency | Lower yield of polyphenolic content and flavonoids | High yield with maximum phytochemicals |
| Energy consumption | High | Low |
Non-conventional technologies
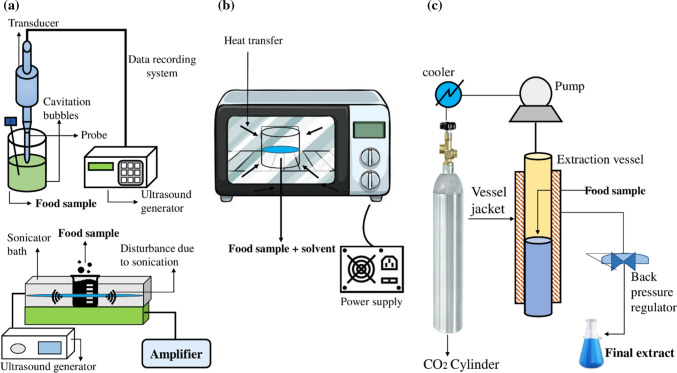
Various studies have shown the potential of conventional extraction methods like Soxhlet extraction and maceration technique with promising results. However, such methods require huge usage of solvent, time and energy. The commercially used techniques for the extraction include ultrasound-assisted extraction, microwave-assisted extraction and supercritical fluid extraction (Luo et al. 2018). These techniques have shown a promising potential for improvements in polyphenolic content by 32–36% with about 17.6-fold lower energy consumption as compared to thermal treatments (Maza et al. 2019). Table 2 shows the unique characteristics of the above technologies (Azwanida 2015; Llompart et al. 2019; Fayaz et al. 2020; Wen et al. 2020). Emerging techniques like high-voltage electric discharge, pulse electric field extraction and enzyme-assisted extraction have also gained interest and have been investigated with different food and plant matrices. These techniques show high quality of extract with the least consumption of raw material and energy. Figure 3 shows the emerging technologies required for the isolation of polyphenols. Figure 3a shows ultrasound-assisted extraction systems (probe and bath sonicator) illustrating the cavitation phenomenon due to application of frequency on food matrix. Figure 3b gives a schematic of the heat exchange occurring between the food material and environment on application of microwave energy. Figure 3c depicts the mechanism of supercritical fluid extraction with CO2 as critical solvent.
Table 2.
Characteristics of extraction techniques including ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE) and supercritical fluid extraction (SFE) commercially used in the industry
| Criteria | Ultrasound-assisted extraction (UAE) | Microwave-assisted extraction (MAE) | Supercritical fluid extraction (SFE) |
|---|---|---|---|
| Terminology | Involves the application of ultrasound energy on the food material leading to an increase in surface area between solvent and sample owing to increased yields |
Application of microwave energy for the separation of materials using a solvent. The microwave radiation interacts with the sample causing heat transfer by conduction |
Involves separation of one food (a bioactive compound) component from the sample using supercritical fluids as an extracting solvent. CO2 is the most extensively used supercritical solvent |
| Unique characteristics |
Alterations in the physical and chemical properties of the sample. Enhancement of mass transfer of solvent into the plant material |
Improved sample recoveries with high efficiencies observed | Easy alterations can be done by changing temperature, pressure or adding a solvent with least effect on final sample |
| Optimum extraction parameters | 20–2000 kHz, 10–30 min | 400–600 W, 10–30 min, with appropriate temperature depending on samples | 31 °C, 7380 kPa |
| Selectivity | High | Method prefers to interact with polar molecules and solvents with high di-electric constant | High |
| Benefits and ease of use | Simple set-up, low solvent usage and least extraction time | Least extraction time and solvent usage as compared to conventional | No cost of buying solvent and least sample usage |
| Disadvantages | Cavitation issues may arise and should be taken care of | Thermal degradation of compounds may occur and proper conditions should be maintained | Can only be used for extracting bioactive compounds with high yields |
| Cost | Low cost technology with least energy usage | Low set-up cost | High initial equipment cost |
| Scalability | Used for small and large-scale polyphenolic extractions | High power consumption if working on a large scale | High generally used for optimization studies |
Fig. 3.
a Ultrasound-assisted extraction (UAE) assembly with probe and bath extraction illustrating mechanism of bubble cavitation. Application of ultrasound energy leads to rupturing of cell wall of food material resulting in increased yields. b Schematic of a microwave-assisted extraction (MAE). The mechanism involves application of microwave energy to the food matrix leading heat transfer. Solvents with high dielectric constants are generally preferred for the process. c Mechanism of supercritical fluid extraction (SFE). The technique involves the usage of supercritical fluids as extracting solvent
Ultrasound-assisted extraction
Ultrasound-assisted extraction (UAE) deals with the application of high-intensity ultrasonic waves into the treated food sample. The technology is known for its simplicity and is comparatively cheaper as compared to other conventional extraction techniques (Dai and Mumper 2010). The introduction of high-frequency waves leads to a disturbance in solute–solvent mixture, resulting in breakage of cell walls and solvent diffusion (Cares et al. 2010). Criteria such as swelling rate, disruption and particle size post-treatment need to be considered for obtaining a higher efficiency (Xu et al. 2007). An increase in intensity during the process leads to intramolecular forces breaking the particle–particle bond. This leads to bond breakage and excessive penetration of solvent into the compounds resulting in cavitation.
The reason why ultrasound extraction holds an edge with respect to technology is due to shorter residence time between particles to solvent, the usage of small amounts of material, the least amount of solvents needed for use (100 ml minimum) and increased yields in overall polyphenolic extraction (Chmelová et al. 2020; Oroian et al. 2020). It holds useful in the isolation of bioactive elements within a very small period of time. One disadvantage is the requirement of a surplus amount of constant ultrasound energy for extraction of phenolic content (Savic and Savic Gajic 2020).
Microwave-assisted extraction
Microwave-assisted extraction (MAE) is another advanced technique used for isolation of polyphenolic compounds. The introduction of electric and magnetic field leads to heat transfer and conduction, resulting in a dipole moment between solvent and sample. The rotation also leads to successive collisions because of which thermal energy is produced in a closed environment. The phenomenon takes place very fast as the heating takes place in a closed medium. In this process, it heats the whole sample thoroughly by convection (Wen et al. 2020). Microwave extraction has smaller extraction time, minimal solvent requirement, increased extract purity, cost-effective, and better phenolic extraction yield in comparison with traditional methods. However, a tremendous amount of heat and energy loss occurs while conducting the process (Périno-Issartier et al. 2011).
Supercritical fluid extraction
In recent years, supercritical fluid extraction (SFE) has gained a lot of interest to extract bio-actives from plants at atmospheric temperatures preventing thermal denaturation. Supercritical fluid extraction is considered an efficient technique for separation studies due to its design and simplicity of construction. Transport phenomena studies allow better understanding of flow behavior and boundary conditions making it a faster extraction method than conventional techniques. Important criterions such as temperature, pressure, sample volume, solvent additions, flow rate controlling are to be strictly considered. Many solvents were tried due to the special conditions desired for carrying out the extraction procedures. Solvents like hexane (Lee et al. 2019), pentane (Lanças et al. 1994), toluene (Pripakhaylo et al. 2019), nitrous oxide and sulfur hexafluoride (Sakaki et al. 1990) were considered to be the most suitable for studies. However, carbon dioxide (CO2) has been the most extensively used solvent due to its ease of solvent removal and least cost (Pasrija and Anandharamakrishnan 2015). It is considered as a promising solvent because of its supercritical existence. Additionally, the gas is non-corrosive, inexpensive, colorless and odorless making it one of the ideal choices for isolation and purification in food industry (Sánchez-Vicente et al. 2009; Campalani et al. 2020).
However, there may also be few disadvantages when considering the following extraction studies. The phase equilibrium plays an essential role during the designing of a highly sensitive process with too many operating conditions to be followed. A large amount of pressure and environmental conditions also need to be met for the initiation of separation studies (Chaves et al. 2020).
Keeping the negatives aside, supercritical fluid extraction has shown positive signs as compared to other conventional techniques for extraction of polyphenolic compounds.
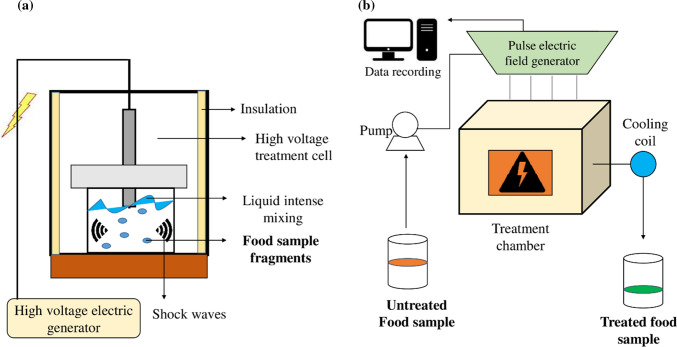
High-voltage electrical discharge
High-voltage electric discharge (HVED) technique is a sustainable technology involving the application of high voltages into an aqueous solution. It can be a good alternative as compared to thermal and conventional treatments. Figure 4a shows the mechanism of high-voltage electric discharge treatment. The two submersed electrodes having high voltage release energy through a plasma channel into the mixture causing the disturbance (Roselló-Soto et al. 2015). The principle of high-voltage electric discharge can be described in two phases: the first phase (prebreakdown phase) and the second phase (breakdown phase). The first phase involves the introduction of light shock waves to the food sample, thus forming small bubbles. Adjusting the electric field intensity leads to the initiation of breakdown phase, resulting in release of active ingredients from the sample. However, the modification of electric field should be performed carefully as transition from prebreakdown phase to breakdown phase (electrohydraulic phase) could lead to several effects such as strong shock waves, structural damage to the sample, plasma bubbles inside the sample and strong liquid turbulence (Li et al. 2019). Energy usage forms an important for better recovery of active compounds and determining the efficiency of the system. The energy of the treatment (Rajha et al. 2014; Barba et al. 2015) can be shown in Eq. (1) and Eq. (2):
| 1 |
| 2 |
where E is the specific energy (kJ/kg), m the product mass, WHVED is pulse energy (kJ/pulse), V is the voltage (V) and I is the current applied (A).
Fig. 4.
a Mechanism of high-voltage electric discharge treatment (HVED) and b pulse electric field extraction (PEF) treatment
In recent years, the technology has been extensively used for extracting polyphenols from various foods like grapes (Brianceau et al. 2016), pomegranate peel (Xi et al. 2017), peanut shells (Yan et al. 2018) and papaya (Lončarić et al. 2020). The technology thus poses as a sustainable method for the extraction of polyphenols.
Pulse electric field extraction
Pulse electric field extraction (PEF) is a green technology used for extraction of phytochemicals from many food materials in the absence of heat. The method involves the usage of electric pulses of moderate intensity leading to rupturing of cell membranes. The sample is placed between two electrodes, and electric field is varied depending on the sample. The release of compounds is due to the intermittent pulses of electricity produced during the process. (Puértolas et al. 2010). Figure 4b shows the mechanism of pulse electric field extraction.
Electric field strength (E = V/d), pulse duration and number of pulses play an integral role in determining the efficiency of the whole sample. The intensities range from low (< 100–200 V/cm) to high (> 1500 V/cm). However, it is noted that short pulses are most effective for extraction of polyphenols in pulse electric field.
The energy consumption for this treatment (El Darra et al. 2013) can be calculated as per Eq. (3):
| 3 |
where V is the electric field voltage (V), I the current (A), t is time (s) and m is the mass of the food sample (g).
In recent years, the technique has been in demand for analyzing many bioactive compounds in fruits and vegetables like strawberry (Stübler et al. 2019), orange (Luengo et al. 2013), red beet (Loginova et al. 2011), grapes (Delsart et al. 2012; Brianceau et al. 2015), tea (Liu et al. 2019) and onion (Liu et al. 2018).
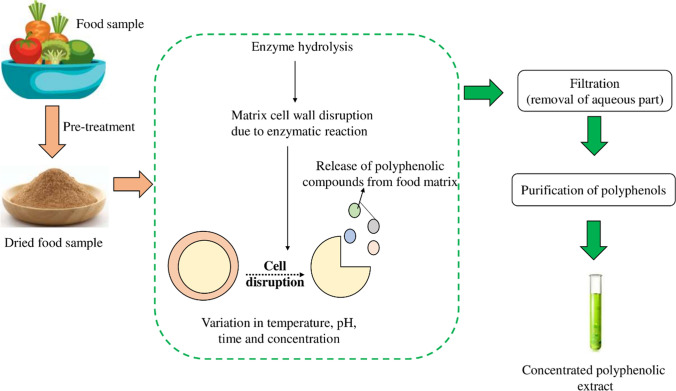
Enzyme-assisted extraction
Enzyme-assisted extraction (EAE) is a sustainable technology dealing with introduction of enzymes into a mixture enhancing overall efficiency. Figure 5 shows the mechanism of enzyme-assisted extraction. The basic mechanism involves disruption of cell wall of food material by hydrolyzing it using an enzyme as a catalyst under optimum extraction conditions for release of bioactive components (Nadar et al. 2018). Addition of an enzyme softens the cell wall of the sample giving it easier access to the solvent medium. Since bioactive compounds, polyphenols and other phytochemicals exist inside the cells and are difficult to extract, this technique helps to release such compounds. The enzymes mainly used for extraction are cellulose (Yuliarti et al. 2015; Wikiera et al. 2016), protease (Oliveira et al. 2020) and pectinase (Marić et al. 2018; Domínguez-Rodríguez et al. 2021). Table 3 shows the estimation of total phenolic content extracted from food samples using different enzymes. Particle size and enzyme ratios play an integral role in moderating the yield of polyphenols.
Fig. 5.
Extraction of phenolics using enzyme-assisted technology (EAE). The technique is a green technology involving the addition of a suitable enzyme for increasing the overall efficiency. Addition of an enzyme like cellulase or xylanase leads to enzymatic hydrolysis, resulting in faster cell wall breakage. Variation in process conditions leads to release of polyphenolic compounds from the food matrix
Table 3.
Estimation of total phenolic content from food samples using enzyme-assisted extraction (EAE)
| Food sample | Enzyme used | Conditions | Total phenolic content (in terms of gallic acid equivalent (GAE)) | Reference |
|---|---|---|---|---|
| Chokeberry pomace | Viscozyme L and CeluStar XL | Enzyme to solute ratio: 6% v/w, 40 °C, pH: 3.5, 7 h |
With enzyme: 15 mg GAE/g Without enzyme: 11.6 mg GAE/g |
Kitrytė et al. (2017) |
| Grape pomace | Cellulase, tannase | Acetate buffer, pH: 5, 45 °C, 2 h | 0.74–0.76 mg GAE/g | Meini et al. (2019) |
| Guava leaves | Cellulase, xylanase |
Dried powdered guava leaf (5 g) with water pH: 5, 12 h Enzyme dosage: 0.5 g |
With cellulase enzyme: 27.2 No significant influence on xylanase enzyme |
Wang et al. (2017) |
| Citrus peel | Viscozyme L | Citrus peel (0.5 g), acetate buffer, pH: 4.8, 60 °C, 0.8% concentration of enzyme usage, 1 h |
With enzyme: 1590 Without enzyme: 1169.23 |
Nishad et al. (2019) |
The technology is an environmentally friendly method majorly using water as solvent medium. Additionally, the extraction is carried out at low temperatures and requires low energy, thus preventing polyphenols from degradation. However, not many enzymes have investigated specifically for isolation in polyphenols from foods. The technology has extensively been used for extraction of polyphenols from vegetables and beverages like cabbage (Huynh et al. 2014) and wine (De Camargo et al. 2016).
Table 4 shows the estimation of polyphenolic content using conventional and non-conventional technologies as discussed above for different foods. The method selection depends on factors like raw material, concentration, bioactivity, target molecule, process yields, cost, energy consumption and impact on the environment (Maroun and Chacar 2018).
Table 4.
Estimation of total phenolic content using various non-conventional extraction methods like ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), supercritical fluid extraction (SFE), high-voltage electric discharge (HVED) and pulse electric field extraction (PEF) for different foods
| Food sample | Technique | Conditions | Total phenolic content (mg gallic acid equivalent (GAE)/g) |
Conclusions | Reference |
|---|---|---|---|---|---|
| Cantaloupe melon peel and seeds | Ethanol extraction using water |
200 mg powder of peels and seeds. Solvent usage: 10 ml, 6 h, 50 °C |
Peel: 25.48 Seed: 1.50 |
Improvement in radical stability between hydrogen and phenoxyl radicals. Melon extracts could be used in food and cosmetic products |
Vella et al. (2019) |
| Citrus peels | Maceration | 0.3 g sample, 50 ml ethanol–water (20:80 v/v), 15 min, 90 °C | 280–673 |
Highest polyphenol content was found in the flavanone hesperidin. Evaluated citrus peel by-products could be transformed into value-added products |
Gómez-Mejía et al. (2019) |
| Mango peel | Maceration and ultrasound-assisted extraction (UAE) |
Maceration: 5 g peel powder, 40 °C, 5000 rpm, 10 min Ultrasound-assisted extraction: Frequency: 35 kHz@Temperature: 35, 45, 55 °C@Solvent analyzed: Methanol and ethanol |
Maceration: 18.66 UAE: 67.58 |
UAE proved as a better extraction technique. Mango peel has an adequate amount of phenolics, making it a suitable ingredient for preparation of functional foods | Safdar et al. (2017) |
| Grape seeds | Maceration, ultrasound-assisted extraction and Soxhlet extraction |
Soxhlet extraction Seed sample: 25 g, 50 °C, 300 ml n-hexane solvent usage for 6 h UAE with maceration: Seed sample: 25 g, 20 kHz, 150 W, 30 min at 30 °C-50 °C. Maceration time: 12 h |
105.20 | Better oil recovery observed when grape seeds were subjected to UAE as compared to traditional Soxhlet | Da Porto et al. (2013) |
| Apple | Ultrasound-assisted extraction (UAE) vs ultrasound-assisted extraction with hydrostatic treatment | 25 kHz, 70% amplitude, 20 °C, 60 min | 748 | High extraction efficiency and inactivation of enzymes observed during ultrasound extraction combined with hydrostatic treatment | Abid et al. (2014) |
| Cantaloupe melon | Ultrasound-assisted extraction | 376 W/cm2, 10 min | – | Juice homogeneity improvements during treatment | Fonteles et al. (2012) |
| Rosemary and thyme extracts | Conventional vs ultrasound-assisted extraction |
Conventional: 1200 rpm Ultrasound-assisted extraction: 28.7 W/cm2 400 W, 40 °C |
Thyme: 158 Rosemary: 15.4 |
Ultrasound stimulated activity of Bifidobacterium. Polyphenolic compounds noticed in both thyme and rosemary.@Growth rate improvement of salmonella enterica in thyme. Carotenoids improvements from thyme and rosemary |
Munekata et al. (2020) |
| Citrus peels | Maceration and ultrasound-assisted extraction | Acetone (1:3), 30 min, 37 °C | 21.99 | Ultrasound-assisted extraction turned out to be an ideal technique in terms of yield, total phenols, flavonoids and antioxidant activity | Saini et al. (2019) |
| Green tea | Microwave-assisted extraction (MAE) |
120,360,600 W 1,3,5 min |
116.58 | Optimum conditions were 350.65 W power, 5 min irradiation time | Taşkın and Aksoylu Özbek (2020) |
| Sea buckthorn bush | Conventional extraction vs microwave-assisted extraction |
Microwave extraction (MAE): 400 W, 20–100 °C, 15 min Conventional: 8000 rpm, 5 min |
Microwave-assisted extraction (MAE): 1147 Conventional: 741 |
MAE turned out to be more preferred extraction method as compared to conventional for determining polyphenolic content antioxidant activity of the bush food | Périno-Issartier et al. (2011) |
| Carrot juice | Microwave-assisted extraction | 165 W, 9.39 min extraction time, 8.06:1 g/g oil to waste ratio | 215 | Enriched flaxseed oil used in carrot juice was in good quality, high in phenolic content and antioxidant activity (70% inhibition) | Elik et al. (2020) |
| Propolis | Maceration, microwave-assisted extraction, ultrasound-assisted extraction |
Maceration: 24 h, 250 rpm, room temperature ultrasound extraction: 20 kHz, 15 min microwave extraction: 140 W, 1 min |
185–504 |
Ultrasound-assisted extraction proved as a much efficient technique for isolation of polyphenolics extraction efficiency and flavonol content. Sample preparation of propolis played a critical role in obtaining the results |
Oroian et al. (2019) |
| Carob bark | Microwave-assisted extraction | 80 °C, 35% ethanol, 29.5 min | 33.6 | High amount of gallic acid was seen. Microwave extraction was found to be a suitable technique for revalorization of agro-food waste | Quiles-Carrillo et al. (2019) |
| Lamiaceae herbs | Supercritical fluid extraction (SFE) | 35 MPa, 100 °C | – |
Highest amount phenolic diterpenes detected in thyme and sage. Improved antioxidant activity as compared to commercial antioxidants |
Babovic et al. (2010) |
| Spearmint leaves | Supercritical fluid extraction and solvent extraction | Supercritical extraction: 100–300 bar, 40–60 °C, 60–90 min | – |
Greater flavonoid content with higher yields. Best yield was achieved at 200 bar, 60 °C and 60 min. Solvent extraction: 257.67 mg/g Supercritical fluid extraction: 60.57 mg/g |
Bimakr et al. (2011) |
| Apple seeds | Supercritical fluid extraction and Soxhlet extraction | 24 MPa, 40 °C, 1 L/h of CO2, 140 min |
Supercritical fluid extraction: 2.96 μg Soxhlet: 1.56 μg |
SFE gave higher oxidative stability than Soxhlet extraction. The final product post-supercritical extractionwas rich in linoleic acid (63.76 g/ 100 g of oil) |
Ferrentino et al. (2020) |
| Strawberry leaves | Supercritical fluid extraction | 308 K, 20 MPa, ethanol: CO2: 1:10 | 1709.1 μg | Solvent density, solubility of organic compounds and vapor pressure played an important role influencing phenolic content and antioxidant activity | Sato et al. (2019) |
| Sesame cake | High-voltage electric discharge (HVED) | Energy input: 83 kJ/kg, 10% ethanol, 0.5 Hz, pulse duration: 10 μs | 54.3–440.3 | Technique showed least usage of organic solvents with higher diffusion giving increased efficiency | Sarkis et al. (2015) |
| Orange peels | High-voltage electric discharge and enzyme-assisted extraction | Energy input: 222 kJ/kg, 80 min | 700 | Intense extraction of biomolecules with high polyphenols and reducing sugar yields from defatted orange peels was found during the combination of high-voltage electric discharge and enzyme-assisted extraction | El Kantar et al. (2018b) |
| Grape seeds | High-voltage electric discharge | Number of discharges: 300, electric field strength: 40 kV/cm, electrode diameter: 25 mm | 8300 | Peleg’s model showed the best extraction kinetics (R2 > 0.995) | Liu et al. (2011) |
| Pomegranate peels | High-voltage electric discharge | Voltage: 40 kV, electrode diameter: 35 mm, electric field strength: 10 kV/cm, Time taken: 7 min | 46 | High-voltage electric discharge improved the recovery of polyphenols by 3 for ultrasound extraction and by 1.3 times for pulse electric field extraction | Rajha et al. (2019) |
| Grape fruit peels | High-voltage electric discharge | Energy: 7.27 kJ/kg to 218 kJ/kg, 20% aqueous glycerol | 86 |
Addition of glycerol reduced pretreatments by 6 times. Same diffusivity of polyphenols was obtained in water from high-voltage electric discharge at 218 kJ/kg and in aqueous glycerol at 36 kJ/kg |
El Kantar et al. (2019) |
| Onion | Pulse electric field extraction (PEF) | 2.5 kV/cm, 90 pulses, 45 °C | 102.86 | Technique proved an environmentally friendly method with greater extraction yields and least sample consumption as compared to Soxhlet extraction | Liu et al. (2018) |
| Tea | Pulse electric field extraction | 1.25 kV/cm, 100 pulses, energy: 22 kJ/kg, 2 h | 398 |
77% of total polyphenols were extracted on application of electric field. Significant increase in extraction recovery implied improvement in cell membrane permeability post-treatment |
Liu et al. (2019) |
| Lemon residues | Pulse electric field extraction |
0,3.5, 7 kV/cm, 0, 2.5, 5 bars pressure, extraction time: 45 min |
292 | Huge variations in phenolic content after pressing the sample and increasing the field strength | Peiró et al. (2019) |
| Orange, pomelo, lemon | Pulse electric field extraction | 3 kV/cm and 10 kV/cm | – | Efficiency increased post-pressing by: 25% for orange, 37% for pomelo and 59% for lemon | El Kantar et al. (2018a) |
The emerging novel food processing technology for isolation of polyphenols has a promising potential to produce safe products with high quality. These techniques could minimize adverse losses taking place during conventional processing of bioactive compounds from fruits, vegetables and other products. Advanced technologies like membrane separation and encapsulation have also paved their way at a commercial level. These are discussed below.
Membrane separation
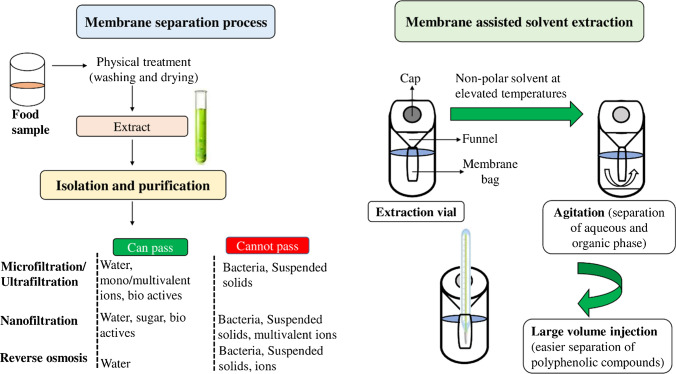
The use of membrane separation technology has been gaining lot of interest for separating as well as concentrating phenolic compounds and purifying them. The technology offers far better replacement as compared to traditional technologies like Soxhlet extraction as they possess low operating costs, easy scaleup and give higher product quality (Castro-Muñoz et al. 2019). In recent years, pressure-driven membrane process mainly ultrafiltration, microfiltration and nanofiltration have been largely studied in the agro-food sector. Figure 6 shows the membrane process technology used for isolating polyphenolic compounds from a food sample. Studies done for polyphenol recovery from artichoke (Conidi et al. 2014), wine lees (Giacobbo et al. 2017) and other natural compounds (Cañadas et al. 2020) have shown variation in improved recoveries with greater efficiencies. The processing of juices and liquid foods has also yielded useful health benefits and given higher recoveries when treated with membrane technology (Avram et al. 2017). Table 5 shows membrane separation as a technology for isolation of polyphenolic compounds from different foods.
Fig. 6.
Membrane process used for isolating polyphenolic compounds from different foods. Membrane separation technology has various benefits like easy operation, environment friendliness, energy savings and high quality of products
Table 5.
Application of membrane separation technology for isolation of polyphenolics from different foods
| Food sample | Technique | Conditions | Phenolic content–(mg gallic acid equivalent (GAE)/g) | Conclusions | References |
|---|---|---|---|---|---|
| Pistachio hull | Membrane separation with ultrasound-assisted extraction (UAE) |
2 stage membrane process: 1 kDa cellulose membrane, 4 bar pressure, 250 rpm |
120.31 |
Highest amount of phenolic compound and antioxidant activity in the retentate part. 34 compounds were found. Most abundant were gallic acid, galloylshikimic acid, pyrogallol and quercetin |
Seifzadeh et al. (2019) |
| Red wine lees | Microwave-assisted extraction (MAE) and membrane separation |
3 stage membrane process MAE: 356 Wh, 0.5–3 min, 1:10 wine: solvent used Membrane area: 13.85 cm2, 68.9 bar Pore size: 0.15 μm |
933–1939 | Usage of membrane separation technology gave more importance to membrane material used and pore sizes. Aliphatic polyamide membrane gave the highest retention toward polyphenolic compounds as compared to polyvinylidene fluoride and cross-linked membranes | Arboleda Meija et al. (2019) |
| Pomegranate juice | Membrane separation using polyvinylidene fluoride and polysulfone membrane | Absorbance: 765 nm |
Polyvinylidene fluoride membrane: 1934.3 Polysulfone membrane: 1888.1 |
Lower retention of polyvinylidene fluoride membranes as compared to polysulfone membranes | Galiano et al. (2016) |
| Roselle extract | Ultrafiltration and nanofiltration membranes | Membrane area: 0.0155 m2, thermal bath temperature (35 °C) |
Ultrafiltration: 29.1 Nanofiltration:28.4 |
Nanofiltration membranes gave higher (95%) permeate fluxes and retention values for total soluble solids, acidity and bioactive components. No damages in quality of the extract |
Cissé et al. (2012) |
| Lyciumbarbarum. L extracts | Membrane separation with aqueous extraction | Polyethersulphone membrane (0.3–0.4 kDa) | 1870.7 | 73–80% retention values of total polyphenols were observed | Conidi et al. (2020) |
Membrane-assisted solvent extraction has shown to be an efficient alternatives compared to traditional extraction techniques in terms of toxicity and quality of extract. This is mainly because of the least solvent usage (approximately 800 µL) for extraction. The analyte passes through the membrane to the acceptor phase according to the partition coefficient in the sample-solvent mixture (Vincelet et al. 2010). Additionally, the entire extraction is carried out in a vial on a flat membrane separating the aqueous phase with organic phase, thus not requiring any space to perform (Barbara Hauser 2002). Nonpolar solvents are generally preferable to inhibit loss of solvent through membrane. The technology with direct coupling to large-volume injection and gas chromatography detection has shown to be a quick and economic procedure for estimation of bioactive compounds from different foods and wastewaters (Schellin and Popp 2005; Rodil et al. 2007; Antónia Nunes et al. 2019).
However, one of the major challenges faced during separation and isolation of compounds is membrane fouling. Membrane fouling is the most influential factor that restricts the performance of membranes in its long-term operations. Factors such as origin of foulants, pore size and materials used play a vital role during characterization and control aspects (Chang et al. 2019). There have been several studies done to reduce the blockage of membranes and fabrication improvements (Dickhout et al. 2019; Li et al. 2020; Xu et al. 2020). Due to this reason, many membranes are used more as a purification process than as an extraction technology. The general method to evaluate membrane fouling is to compare the water flux through original and used membranes keeping same parameters. It can be determined using the formula (Seifzadeh et al. 2019) as given by Eq. (4):
| 4 |
where Fw is pure water flux (L/m2 h), is feed pressure (Pa), is water viscosity (Pa s) and R is membrane resistance.
Thus, it can be concluded that although the technology has progressed through the years, advancements still need to be carried out for better scaleup purposes.
Encapsulation techniques
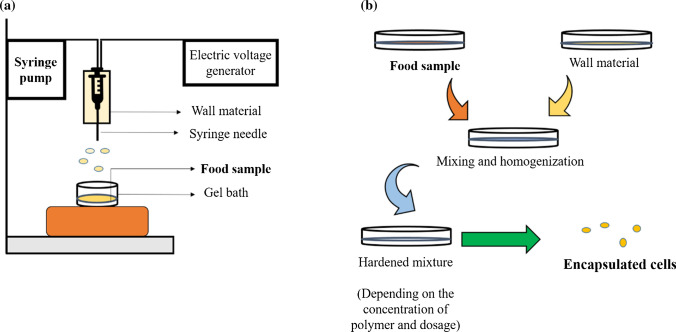
Encapsulation technology has been considered as a highly advanced method for enabling modification of physical properties or isolation of food materials. In recent years, various researchers have recommended carrier agents and phenolic compounds, which can be used for producing micro- or nanocapsules (Lohith Kumar and Sarkar 2018; Saini et al. 2020). The encapsulation technique gives polyphenols and other micronutrients protection from the environment (Ezhilarasi et al. 2013). Certain factors need to be considered for determining the performance of encapsulation technology. Figure 7a shows the experimental procedure involved in encapsulation. Figure 7b illustrates the step-by-step mechanism for extraction of polyphenols using encapsulation technique using suitable wall material. Selection of technique and wall material holds a key factor to encapsulate phenolic compounds. The carrier or wall material is important as it controls the release action and protects the efficiency. Several techniques and wall materials have been evaluated for encapsulation of polyphenolic compounds as discussed in Table 6.
Fig. 7.
Illustration of a experimental procedure and b mechanism for encapsulating polyphenols from food samples using wall materials. The main goal of encapsulation is to resist the core material from external conditions and effects like light, moisture, temperature and oxygen resulting in shelf-life increase. Proper selection of wall material is necessary for increased efficiency of final product
Table 6.
Encapsulation techniques for separation of polyphenols from food samples
| Encapsulating technique | Process technology | Polyphenols extracted | Wall material | Food sample | Inferences | Reference |
|---|---|---|---|---|---|---|
| Liposomes |
Flexible system which can entrap both oil and water functional compounds. Generally used for entrapping aqueous solution within a lipid membrane |
Catechin, epicatechin, quercetin, vanillin | Chitosan | Coca hull via drinking yoghurt | Reduction in phenolic degradation by protecting them from interacting with other proteins in yoghurt | Altin et al. (2018) |
| Electro-spinning |
Rapid technique involving the application of electric field to stretch the ultra-thin filaments using a syringe needle. Generally carried out at room temperature to stop the degradation of polyphenol compounds |
Phenolic acids and anthocyanins | Gelatin | Sour cherry | Eight times better protection of glucoside molecules as compared to non-encapsulated sour cherry concentrate | Isik et al. (2018) |
| Electro-spraying |
Single-step process where the solution is subjected to electric field and is broken into droplets due to high electric potential The technology is a slight modification of electrospinning process |
Curcumin | Water-soluble protein | Turmeric |
Elimination of interactions between curcumin and muscle proteins Reduction of antioxidant activity observed |
Gómez-Estaca et al. (2015) |
| Spray drying |
Technology involves dispersion of phenolic compounds into the carrier material follower by atomization in a hot chamber. The solid particles formed from liquid droplets offer increased stability and solubility |
Polyphenolic compounds | Sodium alginate | Olive leaf | Protection and controlled release of oleuropein under gastric conditions observed | González et al. (2019) |
| Freeze-drying | Process involves pressure reduction with removal of water from frozen food materials.@Involves a phase change from solid to gaseous phase. Generally used for encapsulating water-soluble bioactive compounds | Flavonoids | Whey proteins, pectin | Yellow onion | Results showed that the encapsulated polyphenols can be used as a functional food ingredient and had improvement on consumer’s health | Milea et al. (2019) |
| Emulsification | Encapsulation technique involves dispersion of two or more immiscible liquids where one liquid gets dispersed in the form of droplets.@Technique offers better stability and controlled release of polyphenolic compounds | Resveratrol molecules | Chitosan | Nutraceuticals | Slowing down of diffusion rate and release kinetics were studied using encapsulation techniques | Sanna et al. (2015) |
However, there remains a huge gap in finding a universally applicable technique for polyphenol encapsulation due to their complex structure. Another challenge lies in keeping up to the consumer standards in terms of nutritional value, product quality, safety and cost. In the last few years, several other innovative green technologies have also been analyzed for improving the characteristics and yield of polyphenols in foods. Table 7 gives an overview of such green technologies, which are in their early stages and have a potential for scaleup.
Table 7.
Green technologies that have a potential at commercial level
| Technology involved | Process methodology | Food analyzed | References |
|---|---|---|---|
| Cloud point extraction |
One-step procedure involving the extraction of polyphenolic and bioactive compounds using nonionic surfactants. The surfactants tend to separate out from the main solution yielding a cloud formation when heated. Simple and rapid process with reduced extraction time, less toxic and yields negligible environmental pollution as compared to conventional techniques |
Olive oil | Kiai et al. (2018) |
| Ultrasound-assisted extraction (UAE) using glycerol-based natural eutectic mixtures |
Technology involving mixing of two solid materials with high melting points which do not interact to form a new chemical compound. Hydrogen bonding interactions and phase behaviors play a key role in studying this process |
Agri-food wastes | Mouratoglou et al. (2016) |
| Infrared irradiation technology |
One of clean energy sources for improved extraction of natural products and bioactive compounds using a ceramic infrared emitter. Entire extraction requires low energy, easy to use, economical and has a great potential for scaleup |
Pomegranate, olive, apricot pomace | Abi-Khattar et al. (2019); Rajha et al. (2019) |
| Rapid solid–liquid dynamic extraction |
An innovative solid–liquid cyclic pressurization process involving the rapid extraction of polyphenols from their organic or inorganic solvent mixtures. The technique uses liquid pressure and takes place at room temperature (or slightly lower) in order to avoid thermal stress on phenolic compounds. The technique is environmentally friendly and requires less energy as compared to conventional extraction process |
Wine | Gallo et al. (2019) |
| Vacuum-based solvent-free microwave extraction |
Green extraction method which does not require solvent usage. The food matrix is exposed to microwave radiation leading to expansion of cells resulting in extraction of solutes. The application of a vacuum condition allows the boiling point of solvent (water) to become lower than ambient pressure. Thus, the water can continuously boil at a reduced pressure and temperature allowing much efficient mixing preventing polyphenols from degradation |
Medicinal herb (C. nutans) | Othman et al. (2020) |
Process parameters for optimization
Influence of solvent
It has been found that solvents are one of the main factors influencing the reaction kinetics. Solvents play a major role in the reactions between radicals and antioxidants (Tavasi et al. 2009). Phenolic compounds generally show a higher yield and greater solubility characteristic toward organic solvents (Hameed et al. 2020). When the food power is added, a cell wall breakage occurs. Due to this, diffusion reaction takes place bringing out phenolic compounds in the solution. It should be noted that solution should not be kept for a long time as the compounds are highly volatile. Due to excessive reaction temperature, the molecules can also form polyphenol oxidase leading to mutual equilibrium between the free and blocked groups in the structure of food material(Olszowy 2019). Literature has also reported that a shortage of solvent can also lead to multiple clusters and bond formation in the measuring system (Dawidowicz and Olszowy 2013; Dawidowicz et al. 2015). Solvent toxicity is another issue which needs to be carefully considered. For instance, a recent study analyzed the effects of toxicity during polyphenolic compounds extraction from red grapes (Makris et al. 2016). After optimization using response surface methodology and carefully comparing the two solvents, it was found that mixing 20% w/v glycerol was a more preferred option as compared to 2% w/v tartaric acid, which gave a negative effect on polyphenol recovery. Thus, choosing the right solvent for food sample needs to be given adequate importance irrespective of the extraction technique conducted.
Influence of solid–solvent ratio
The selection of solvent-to-solid ratios plays a vital role in process optimization and can thus have an impact on the yield. The main objective is to pick the best ratio for intensification studies. The amount of polyphenolic compounds depends on polarity of solvent, and it generally increases in the order of methanol > ethanol > ethyl acetate > acetone > n-butanol > water (Vetal et al. 2013). The initial extraction rate is not impacted in the whole extraction process. The extraction can be explained in the following phases: the solvent begins to mix with the solute and disruption of sample cell causes release of bioactive compounds which are extracted accordingly (Herodež et al. 2003; Qu et al. 2010). However, an increase in the solvent ratio can affect the whole extraction process leading to solvent wastage. This is due to the hydroxyl groups and hydrophilic compounds present in the solution. The hydroxyl groups mix with the polyphenols and hydrophilic compounds forming an enzyme polyphenol oxidase. This leads to difficulty in isolation of polyphenolic compounds due to improved enzyme activity (Shewale and Rathod 2018). For instance, a study was conducted to understand the polyphenol recovery in snake grass medicinal plant using microwave-assisted extraction. The results after mathematical modeling indicated a water–ethanol solvent mixture of 50% vol of ethanol and solid-feed ratio of 14 mL/g gave optimum results and increased the extraction rate by twofold–fivefold (Mustapa et al. 2015). On the contrary, it should be noted that a higher water/solvent ratio infers more water and less solvent being utilized during extraction leading to greater energy consumption for performing the study. Also, secondary factors like cost and vapor pressure may affect the extraction yield. Thus, a correct consistency in solute to solvent needs to be maintained for better extraction of bioactive compounds.
Influence of temperature
Beside the solvent type, the temperature is another variable that affects overall efficiency. It has been analyzed that extraction of thermally stable compounds offers increased yields at greater temperatures and lesser extraction times (Cissé et al. 2012). This may be due to the barrier properties when the solute diffuses into the solvent. For instance, an optimization study was conducted on Jabuticaba leaves (a Brazilian native plant) using pressurized liquid extraction. The variables chosen were temperature of 313–393 K, pressure 5–10 MPa and extraction time 3–15 min. The findings showed an 8% rise in phenolic content and 13% in anthocyanin content with optimized conditions of 553 K, 5 MPa with 9 min time (Santos et al. 2012). Additionally, an increase in temperature of approximately 200 K had a significant impact on the overall yield. On the other hand, for thermally sensitive compounds, increased temperatures have negative effects on the overall extraction process, resulting in degradation or loss of volatile phenolic/flavonoid compounds (Xiao et al. 2008). With increase in temperature, there may be better interaction and improved analyte contact with solvent. Thus, temperature is a critical component, which needs to be monitored carefully according to the amount of sample used.
Influence of particle size
Modifications in particle size can greatly affect the extraction yields of plant samples. Size reduction offers greater surface area for better interaction between food matrices. Miniscule variations in particle size can lead to significant changes in overall yield. For extraction of polyphenolic compounds, a smaller particle size involves greater diffusivity and thus an improved mass transfer. As the particle size is small, cell rupturing is easier. For instance, a study done on black chokeberry showed a variation in phenolic content among different plant parts (pulp, seeds and pellicle) (Galvan D’Alessandro et al. 2012). Similar studies were conducted on spruce bark (Piceaabies), which also showed positive results. The results revealed lowest size gives the highest amount of phenolic acids and tannins (Pătrăuţanu et al. 2019). Another research was done to investigate the particle size variation on soybean meal using supercritical fluid extraction. The study showed an optimum particle size for soybean meal lies in the range of 20–30 mesh. Additionally, it was concluded that a small variation from this particle size (lesser or greater than 20 mesh) could result in a significant reduction (10–15% decrease) in recovery of bioactive compounds (Zuo et al. 2008). Thus, the parameter can play a vital role in determining the extraction kinetics.
Kinetic modeling
The kinetic study is generally conducted to for better reaction control and understanding degradation rate of the extraction. Various mathematical models have been explored to evaluate their feasibility. An extensive research has being undertaken to estimate the degradation of total phenolic compounds from different foods (Karacabey et al. 2013; Shewale and Rathod 2018). Table 8 shows a list of various kinetic models applied to food samples. However, main challenge lies in understanding the mechanism of initial period of extraction. The kinetic extraction takes place in two stages (Sturzoiu and Stroescu 2011): the first stage involves washing stage where there is an initial mixing of solute with solvent. The second stage involves a much slower solute transfer with a diffusion process. The concentration of compounds (Cs), extraction order (n), activation energy (Ea) and rate constant (k) plays a vital role in understanding kinetics of the extraction. Mathematics models and statistical evaluation using regression coefficient (R2), root-mean-square deviation (RMSD), normal root-mean-square deviation (NRMSD) have been proposed for making a strong agreement between theoretical data and experimental data.
Table 8.
Kinetic models widely explored for different food materials
| Model name | Formula | Food sample | Process technology | Mathematical inferences | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peleg’s model | Spruce bark (Piceaabies) | Ultrasound-assisted extraction | R2 = 0.98 | Charpe and Rathod (2016) | ||||||
| Chickpea, bean | Batch extraction in distilled water |
Ea for chickpea = 301.28 kJ/mol Ea for bean = 86.77 kJ/mol |
Shafaei et al. (2016) | |||||||
| First-order model | Pomegranate peel | Ultrasound-assisted extraction |
R2 = 0.964 RMSE = 0.034 Duty cycle = 90% |
Rakshit et al. (2020) | ||||||
|
Jamun (Syzygiumcumini. L) |
Maceration (stirring) vs membrane separation |
Ea = 9.5 kJ/mol 0.45 μm membrane was suitable for best polyphenol recovery and purity |
Balyan et al. (2019) | |||||||
| Second-order model | Saffron floral bio-residues | Microwave-assisted extraction |
R2 > 0.99 RMSD: 0.65–3.35% |
Da Porto and Natolino (2018) | ||||||
| Apple pomace | Maceration method (stirring) | R2 = 0.9873 | Skrypnik and Novikova (2020) | |||||||
| Page model | Soybeans | Solvent extraction |
R2 = 0.961 RMSD = 0.108 |
S. et al. (2010) | ||||||
| Red rice | Convective drying |
R2 = 0.99 Chi-square < 0.014665 Temperature = 80 °C |
(Santos et al. (2020) | |||||||
| Power law model | Craft beer | Ultrasound-assisted extraction with water as solvent |
B = 0.073 n = 0.342 RMS = 5.35 Temperature = 47 °C |
Alonso-Riaño et al. (2020) | ||||||
| Logarithmic model | Soybeans | Solvent extraction |
R2 = 0.989 RMSD = 0.082 |
S. et al. (2010) | ||||||
| Patricelli’s model | Green tea | Pressure-assisted extraction and solvent extraction |
R2 = 0.996 Yield obtained at 300 MPa: 17.9% 500 MPa: 29.5% Yield obtained during solvent extraction: 1.81% |
Xi et al. (2015) | ||||||
| Two site kinetic model | Grape marc | Ultrasound-assisted extraction | Highest yield of 24.42 mg/g total phenolic content was obtained for 80 min at 47.4 W/L | Tao et al. (2014) | ||||||
| Weibull distribution model | Sugarcane juice | Ohmic heating (moderate electric field) and conventional extraction |
Presence of electric field influenced the overall extraction at 60 °C and 80 °C. Amount of total phenolic content degradation = 23% |
Brochier et al. (2016) | ||||||
| Rosemary | Electrospinning | 88% of rosemary polyphenols were released in food simulants | Estevez-Areco et al. (2018) | |||||||
Peleg’s model
This model is generally used to determine the quality or decay rate of food materials. The hyperbolic model equation describes the determination of several compounds from plant extracts. It is given by Charpe and Rathod 2016 as per Eq. (5):
| 5 |
where Ct is the concentration during the extraction study at time t (mg/g powder), Kp1 Peleg’s rate constant (min g/mg) and Kp2 Peleg’s capacity constant (g/mg). As the initial concentration of solute is zero, C0 term is neglected. The extraction takes place in two stages: at the start it is first order and then moves down to zero order. Peleg’s equation with time can be given as per Eq. (6):
| 6 |
The value of Ct is calculated by Eq. (6) by plotting a graph between 1/Ct vs 1/t for determining values of Kp1 and Kp2 (Vetal et al. 2013).
First-order model
The differential form of first-order model equation (Harouna-Oumarou et al. 2007) can be written as Eq. (7),
| 7 |
where K1 is the first-order rate constant and t is the time at that instant.
Applying the boundary conditions Ct = 0 at t = 0 and Ct = t at t = t, we get the integral form as Eq. (8),
| 8 |
Rearranging to get the linear form as Eq. (9),
| 9 |
The log (Cs − Ct) vs time (t) graph was plotted for different parameters. By plotting the graph, calculations were done by KF1 as slope and Cs (concentration of the solution obtained during equilibrium) as the intercept value.
Second-order model
Another suitable kinetic model applied for evaluating the rate kinetics is the second-order model. This model was first used to understand the extraction process of polyphenolic content activated from saffron residues. It concluded enough data regarding the rate kinetics of solid–liquid extraction process (Da Porto and Natolino 2018).
The rate of the solid–liquid extraction can be mathematically described by Eq. (10):
| 10 |
where Ct is the concentration of polyphenolic compounds during extraction at time (t), Cs the concentration of polyphenolics when the extraction reaches equilibrium and k is the second-order reaction rate constant.
After applying boundary condition, Ct = 0 to Ct and t = 0 to t Eq. (11)
| 11 |
Solving Eq. (11) to get Eq. (12),
| 12 |
Re-arranging Eq. (12), we get the final equation for calculating total polyphenolic concentration to get Eq. (13):
| 13 |
where h is the initial extraction rate, kS is second-order rate constant, which is graphically calculated using slope and intercept by plotting the t/Ct vs t graph (Qu et al. 2010).
Page model
The page model has been extensively used for calculating the diffusion rate for different grains and seeds (Roberts et al. 2008). The model can be mathematically described as Eq. (14):
| 14 |
where Ct is the experimental concentration at time t and k refers to drying rate constant.
The normalized page equation is Eq. (15):
| 15 |
The values of k and n are determined by plotting the ln (− ln (Ct)) vs ln (t) curve. The graph will determine k and n as the intercept and slope of the curve.
Power law model
Power law model is generally applied to understand the diffusion process of a solvent or a chemical agent through non-swelling devices. The formula can be defined as Eq. (16) and Eq. (17):
| 16 |
| 17 |
where y is the yield of the food material or final concentration, B is the constant of the carrier agent applied during the extraction, t is the time (min) and n refers to the diffusional exponent required for transport mechanisms (Kiew and Mat Don 2013).
Logarithmic model
Logarithmic model was proved the best fit for extraction of bioactive compounds with various medicinal plants possessing antioxidant properties (Ali et al. 2018). The mathematical equation can be written as Eq. (18):
| 18 |
where a and b are logarithmic model constants, Ct refers to the concentration of phenolic compounds at time t.
Patricelli’s model
The Patricelli’s model was applied to explain the active ingredients and polyphenolic extraction in various food materials. The model is based on two simultaneous processes: (1) washing stage, where the active ingredients are washed within the solvent, and (2) diffusion stage, where the polyphenolic compounds are broken within the cell matrix leading to decrease in particle size. The mathematical equation can be written as Eq. (19):
| 19 |
where C1 is the active ingredient yields at equilibrium for washing step (% w/w), C2 the active ingredient yield at equilibrium for the diffusion step (% w/w), kPA1 the mass transfer coefficient for washing step (min−1), while kPA2 is for the diffusion step (min−1). It is assumed that kPA1 is greater than kPA2. The derivative form of Patricelli’s equation can also be written as Eqs. (20) and (21):
| 20 |
The rate during the start of the reaction (v0) at t = 0 is:
| 21 |
Two-site kinetic model
The two-site kinetic model is applicable to two different solute fractions and can take place in fast and slow extraction periods. The desorption rate of fast extraction of polyphenols (F) is given by kT1 and that of slow extraction (1-F) is given by kT2. The mathematical equation can be described as follows: Eq. (22) (Duba et al. 2015):
| 22 |
where C is the mass of polyphenols extracted per mass of substrate, C0 is the initial mass of total polyphenols per mass of substrate and t is the time.
Weibull distribution model
The Weibull distribution model is based on assumption that thermal sensitivity to heat depends on transient heating intensity and residual activity. The distribution is generally used for analyzing enzyme activity at different processing conditions. The mathematical equation for this model can be as follows (Eq. (23)):
| 23 |
where C is the concentration of enzyme at time t and C0 is the initial concentration of enzymes. The value of n determines the shape of the distribution curve, while b is the thermal reaction rate. A distribution of n > 1 indicates a semi-logarithmic curve (Brochier et al. 2016).
Factors influencing extraction kinetics
Moisture ratio and drying rate
Moisture ratio (MR) and drying rate is one of the critical parameters for understanding the kinetics of foods. The reduced drying time with variation in drying conditions has been reported for different food stuffs such as apricot (Toǧrul and Pehlivan 2003), eggplant (Doymaz and Göl 2011), birch (He and Wang 2020), rice starch (Ding et al. 2019), apple pomace (Zlatanović et al. 2019) and beef (Holman et al. 2019). The moisture ratio and drying rate can be calculated according to Eq. (24) and Eq. (25):
| 24 |
| 25 |
where Mt, Me and Mt + dt are moisture contents at time t, equilibrium moisture and moisture content at time (t + dt), respectively. The plot of MR vs time curves can be widely used in most food, agriculture and biological materials. Table 9 shows the different mathematical model applied for calculating moisture content and drying.
Table 9.
Moisture absorption models for calculating moisture content and drying
| Mathematical model | Model equation | Application in foods | References |
|---|---|---|---|
| Newton | Red chili | Hossain and Bala (2007) | |
| Modified page | Mango slices | Akoy (2014) | |
| Wang and Singh | Smith apples | Blanco-Cano et al. (2016) | |
| Two-term model | Plum | Jazini and Hatamipour (2010) | |
| Logarithmic | Basil leaves, stone apple | Kadam et al. (2011; Rayguru and Routray (2012) | |
| Henderson and Pabis | Pumpkin | Hashim et al. (2014) | |
| Othmer and Jaatinen | Spices (S.aromaticumand C. Cassia) | Radha Krishnan et al. (2013) |
Influence of temperature
The thermal processing of foods requires heating between 50 and 150°C depending upon various factors like pH, shelf life, quality and enzymatic reactions. The effect of thermal stability depends on the sample preparations, rate constant (k) and activation energy (Ea). The value of activation energy (Vishwasrao et al. 2017) can be obtained using the formula given in Eq. (26):
| 26 |
where Ea is the activation energy (kJ/mol), R is the gas constant (8.314 J mol−1 K−1) T and Tref are temperatures.
Z-value is defined as the temperature increase to have tenfold decrease in decimal reduction time (Wilińska et al. 2008) as stated in Eq. (27):
| 27 |
where D1 and D2 are decimal reduction times at T1 and T2, respectively.
Considering major parameters like moisture ratio, drying rate and temperature, Table 10 gives a review of the kinetic studies performed for different food materials with the parameters optimized and their kinetic models.
Table 10.
Optimization of kinetic studies of different plant and food materials with their respective models
| Food material | Treatment medium | Kinetic model used | Kinetic parameters | Reference | ||
|---|---|---|---|---|---|---|
| Temperature | K (min−1) | Activation energy (Ea) (kJ mol−1) | ||||
| Beef muscle | Water bath | First-order model | 100 | 1.2 × 10–3 | 81 | Goñi and Salvadori (2011) |
| Garlic | Water bath | Biphasic model | 80–100 | 0.098–2.044 | 202.81 | Fante and Noreña (2012) |
| Red beet | Water bath attached with pobel tubes | First-order | 90 | 3.2 × 10–3 | 35.37 | Fernández-López et al. (2013) |
| Litchi | Water bath | Weibull model | 90 | 2.7 × 10–2 | 79.7 | Yu et al. (2011) |
| Soybean lecithin | Vortex stirrer | Higuich model | 37 | 0.011 | – | Zhang and Wang (2019) |
| Carrot | Blanching in boiling water bath | Jean and Das model | 70 | – | 35.987 | Maleki et al. (2020) |
Statistical modeling and evaluation
The goodness of fit is evaluated for each model based on statistical evaluation. This is done after the kinetic extraction to understand the deviation between the models. Statistical error functions are calculated to find the errors for the extraction models. The mathematical expressions (Da Porto and Natolino 2018) of the error functions are given in Eqs. (28,29,30)
| 28 |
| 29 |
| 30 |
where n and m are the number of observations, Ccalculated and Cexperimental denote the model and experimental concentration with time, respectively. Closer prediction to the experimental data signifies the RMSD value is near zero.
Chi-square value (χ2) measures the accuracy of models and determines the acceptability of data with the expected distribution data. It can be calculated using the formula (Bhushan and Girirajsinh 2019) in Eq. (31):
| 31 |
where Cexperimental and Cpredicted refer to the experimental and predicted values of the concentration responses, respectively, and n refers to the sample points taken. The significance levels generally range between 0 and 1. Smaller Chi-square values denote an acceptable fit between the model and experimental values..
Sensitivity analysis
Sensitivity analysis is a promising tool for optimizing a set of parameters generally by regression analysis. It is done to assure the kinetic modeling correspond to the best fit values. The analysis is applied to each of the parameters by keeping the other parameters in their constant (perturbations). These are generally done in the range of ± 20% perturbations (Alcázar and Ancheyta 2007). A zero percent perturbation implies nonlinearity and poor estimation.
The technique can be carried out in three steps as described by Félix et al. 2019. The first step is the initialization of parameters by plugging in random numbers to obtain the guesses. The second step involves nonlinear regression using least square methods by applying the objective function based on sum of squared errors (SSE) for getting an optimal solution using Eq. (32):
| 32 |
The final step involves plotting the values against the number of experimental observations to understand the fit of the proposed model and calculated parameters.
Economic analysis
Economic feasibility study plays a major role during selection of appropriate technology for extraction. Major factors like capital investment cost (CAPEX) and operating cost (OPEX) should be calculated for estimating initial set-up. Apart from this, external factors like battery limits (if electrical equipment), engineering cost, location, installation cost, pipeline fitments, maintenance cost, raw materials and financial reserves should be considered. The cost indexing (Kratky and Zamazal 2020) is generally done using the formula in Eq. (33)
| 33 |
where Ce is the cost of equipment, a and b are coefficients, n is the index and S is the size parameter.
The operating cost is generally described as the sum of direct operating cost, indirect operating cost and distribution costs. The cash cost is the cost of making products. It can be estimated using the formula in Eq. (34):
| 34 |
It should be noted that profit made by producing the food material is generally subjected to taxes depending on country and location.
The simple payback time (SPT) is the capital investment given to the particular equipment (CAPEX) over the annual cash flows. It can be described using the formula in Eq. (35):
| 35 |
where CAPEX is the capital expenditure, AI is the annual income and PC is the production cost which is inclusive of tax and depreciation (Towler and Sinnott 2013).
Challenges and limitations
The extraction of polyphenolic compounds and other bioactive compounds can be a challenge due to its complex properties. Polyphenolic compounds often suffer from degradation with small changes in extraction methods. For example, technologies like high pressure and temperature processing involved about 22% loss in phenolic content with a small change in color while studying strawberries (Terefe et al. 2009). Another study aquatic plant treatment showed an increase in phenolic content may lead to contamination and change in odor, thus harming the aquatic ecosystem (Zhang et al. 2020). Factors like light, temperature change and air result in spontaneous and rapid variation in polyphenolic and bioactive content facilitating losses. Thus, it becomes crucial to ensure the conditions for the stability of contents before and after extraction (Hoe et al. 2020). When polyphenols are added to food in the form of an extract, choosing the right method and parameter optimization is critical in maintaining the properties. The performance of extraction primarily depends on solvent, food matrix phase, concentration and its extraction process. Choosing the right solvent post-consideration of its polarity may be optimal for carrying out overall extraction (Brewer 2011). Figure 8 shows the challenges and limitations faced during the isolation of polyphenols from different foods.
Fig. 8.
Challenges and limitations faced during the isolation of polyphenols from foods. Although polyphenols offer several benefits, suitable conditions, cost, proper extraction methods and safety standards still need to be set for commercial scaleup
When it comes to industry implementation, these compounds are known to be added mainly in the cosmetic and food industry (Albuquerque et al. 2021). Although they are in small amounts, extensive research on polyphenolic compounds has shown their applications in different foods imparting color, flavor, odor and bitterness. However, more investigation needs to be done on analyzing the right dosage and their toxicological information. Additionally, cost, safety and sustainability aspects of the whole extraction technology need to be monitored for a potential scaleup for the intake of these compounds (Olszewska et al. 2020).
Conclusion
Polyphenols are a class of compounds which can be found in various foods, beverages and plants. These compounds are essential as they have a huge potential in maintaining immunity and their characteristics show promising effects in preventing various diseases. Considering crisis situations like COVID-19, such foods could play a role in protection from diseases and pandemics. This review involved emerging developments taking place for the isolation of polyphenolic compounds followed by kinetic studies being carried out for scaleup purposes. Conventional technologies like percolation, decoction, heat reflux, Soxhlet extraction and maceration as well as non-conventional technologies mainly ultrasound extraction, microwave extraction, supercritical extraction, enzyme-assisted extraction, pulse electric field, high-voltage electric discharge and their applications in extracting polyphenolic compounds were discussed. Further, emphasis was given to advanced and green techniques, mainly membrane separation, encapsulation, cloud point extraction, infrared irradiation technology, ultrasound-assisted extraction using eutectic mixtures, rapid solid–liquid dynamic extraction and vacuum-based microwave extraction as they could be the possible future in isolating polyphenolic compounds with more precision and accuracy. Mathematical equations and kinetic models were reviewed so as to optimize extraction parameters and control the process, energy, time and solvent usage. Future outlooks can be noticed in scaling-up Hurdle technology as they involve a combination of processes for estimating different parameters like pH and temperature for isolation of bioactive compounds. Emphasis can also be given by using computational and molecular modeling for determining the characteristics and scavenging potential of polyphenols. Additionally, the use of simulations for optimizing conditions can assist in choosing the best extraction medium. Greater possibilities also lie in the field of nanoencapsulated food intake due to its precise measurements, portability and quality. However, factors like manufacturing, biological influence and cost need to be considered in advance in order to satisfy the consumer taste and food standards.
Abbreviations
- UAE
Ultrasound-assisted extraction
- GAE
Gallic acid equivalent
- HVED
High-voltage electric discharge
- PEF
Pulse electric field extraction
- EAE
Enzyme-assisted extraction
- MAE
Microwave-assisted extraction
- SFE
Supercritical fluid extraction
- MR
Moisture ratio
- RMSD
Root-mean-square deviation
- NRMSD
Normal root-mean-square deviation
- CAPEX
Capital expenditure cost
- OPEX
Operating cost
- SPT
Simple payback time
- SSE
Sum of squared errors
Funding
None.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ponnusamy Senthil Kumar, Email: senthilkumarp@ssn.edu.in.
Ashish Kapoor, Email: ashishko@srmist.edu.in.
References
- Abid M, Jabbar S, Hu B, et al. Synergistic impact of sonication and high hydrostatic pressure on microbial and enzymatic inactivation of apple juice. LWT–Food Sci Technol. 2014;59:70–76. doi: 10.1016/j.lwt.2014.04.039. [DOI] [Google Scholar]
- Abi-Khattar AM, Rajha HN, Abdel-Massih RM, et al. Intensification of polyphenol extraction from olive leaves using ired-irrad®, an environmentally-friendly innovative technology. Antioxid. 2019;8:1–16. doi: 10.3390/antiox8070227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebooye OC, Alashi AM, Aluko RE. A brief review on emerging trends in global polyphenol research. J Food Biochem. 2018 doi: 10.1111/jfbc.12519. [DOI] [Google Scholar]
- Akoy EOM. Experimental characterization and modeling of thin-layer drying of mango slices. Int Food Res J. 2014;21:1911–1917. [Google Scholar]
- Albuquerque BR, Heleno SA, Oliveira MBPP, et al. Phenolic compounds: current industrial applications, limitations and future challenges. Food Funct. 2021 doi: 10.1039/d0fo02324h. [DOI] [PubMed] [Google Scholar]
- Alcázar LA, Ancheyta J. Sensitivity analysis based methodology to estimate the best set of parameters for heterogeneous kinetic models. Chem Eng J. 2007;128:85–93. doi: 10.1016/j.cej.2006.10.012. [DOI] [Google Scholar]
- Aliboudhar H, Tigrine-Kordjani N. Effect of extraction technique on the content and antioxidant activity of crude extract of Anacyclus clavatus flowers and their essential oil composition. Nat Prod Res. 2014;28:2140–2149. doi: 10.1080/14786419.2014.927872. [DOI] [PubMed] [Google Scholar]
- Ali A, Chua BL, Ashok GA. Effective extraction of natural antioxidants from piper betle with the aid of ultrasound: Drying and extraction kinetics. J Eng Sci Technol. 2018;13:1–16. [Google Scholar]
- Alonso-Riaño P, Diez MTS, Blanco B, et al. Water ultrasound-assisted extraction of polyphenol compounds from brewer’s spent grain: Kinetic study, extract characterization, and concentration. Antioxidants. 2020;9:1–18. doi: 10.3390/antiox9030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin G, Gültekin-Özgüven M, Ozcelik B. Liposomal dispersion and powder systems for delivery of cocoa hull waste phenolics via Ayran (drinking yoghurt): comparative studies on in-vitro bioaccessibility and antioxidant capacity. Food Hydrocoll. 2018;81:364–370. doi: 10.1016/j.foodhyd.2018.02.051. [DOI] [Google Scholar]
- Ameer K, Shahbaz HM, Kwon JH. Green extraction methods for polyphenols from plant matrices and their byproducts: a review. Compr Rev Food Sci Food Saf. 2017;16:295–315. doi: 10.1111/1541-4337.12253. [DOI] [PubMed] [Google Scholar]
- Antónia Nunes M, Pawlowski S, Costa ASG, et al. Valorization of olive pomace by a green integrated approach applying sustainable extraction and membrane-assisted concentration. Sci Total Environ. 2019;652:40–47. doi: 10.1016/j.scitotenv.2018.10.204. [DOI] [PubMed] [Google Scholar]
- Arboleda Meija JA, Parpinello GP, Versari A, et al. Microwave-assisted extraction and membrane-based separation of biophenols from red wine lees. Food Bioprod Process. 2019;117:74–83. doi: 10.1016/j.fbp.2019.06.020. [DOI] [Google Scholar]
- Avram AM, Morin P, Brownmiller C, et al. Concentrations of polyphenols from blueberry pomace extract using nanofiltration. Food Bioprod Process. 2017;106:91–101. doi: 10.1016/j.fbp.2017.07.006. [DOI] [Google Scholar]
- Azmir J, Zaidul ISM, Rahman MM, et al. Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng. 2013;117:426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- Azwanida, A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants. 2015;04:3–8. doi: 10.4172/2167-0412.1000196. [DOI] [Google Scholar]
- Babovic N, Djilas S, Jadranin M, et al. Supercritical carbon dioxide extraction of antioxidant fractions from selected Lamiaceae herbs and their antioxidant capacity. Innov Food Sci Emerg Technol. 2010;11:98–107. doi: 10.1016/j.ifset.2009.08.013. [DOI] [Google Scholar]
- Balyan U, Verma SP, Sarkar B. Phenolic compounds from Syzygium cumini (L.) Skeels leaves: Extraction and membrane purification. J Appl Res Med Aromat Plants. 2019;12:43–58. doi: 10.1016/j.jarmap.2018.12.002. [DOI] [Google Scholar]
- Barakat N, Makris DP, Kefalas P, Psillakis E. Removal of olive mill waste water phenolics using a crude peroxidase extract from onion by-products. Environ Chem Lett. 2010;8:271–275. doi: 10.1007/s10311-009-0216-z. [DOI] [Google Scholar]
- Barba FJ, Galanakis CM, Esteve MJ, et al. Potential use of pulsed electric technologies and ultrasounds to improve the recovery of high-added value compounds from blackberries. J Food Eng. 2015;167:38–44. doi: 10.1016/j.jfoodeng.2015.02.001. [DOI] [Google Scholar]
- Bhushan C, Girirajsinh TP. Microwave-assisted deep eutectic solvent extraction of phenolic antioxidants from onion (Allium cepa L.) peel: a Box – Behnken design approach for optimization. J Food Sci Technol. 2019 doi: 10.1007/s13197-019-03891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimakr M, Rahman RA, Taip FS, et al. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod Process. 2011;89:67–72. doi: 10.1016/j.fbp.2010.03.002. [DOI] [Google Scholar]
- Blanco-Cano L, Soria-Verdugo A, Garcia-Gutierrez LM, Ruiz-Rivas U. Modeling the thin-layer drying process of Granny Smith apples: application in an indirect solar dryer. Appl Therm Eng. 2016;108:1086–1094. doi: 10.1016/j.applthermaleng.2016.08.001. [DOI] [Google Scholar]
- Brewer MS. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf. 2011;10:221–247. doi: 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- Brianceau S, Turk M, Vitrac X, Vorobiev E. Combined densification and pulsed electric field treatment for selective polyphenols recovery from fermented grape pomace. Innov Food Sci Emerg Technol. 2015;29:2–8. doi: 10.1016/j.ifset.2014.07.010. [DOI] [Google Scholar]
- Brianceau S, Turk M, Vitrac X, Vorobiev E. High voltage electric discharges assisted extraction of phenolic compounds from grape stems: effect of processing parameters on flavan-3-ols, flavonols and stilbenes recovery. Innov Food Sci Emerg Technol. 2016;35:67–74. doi: 10.1016/j.ifset.2016.04.006. [DOI] [Google Scholar]
- Brochier B, Mercali GD, Marczak LDF. Influence of moderate electric field on inactivation kinetics of peroxidase and polyphenol oxidase and on phenolic compounds of sugarcane juice treated by ohmic heating. LWT - Food Sci Technol. 2016;74:396–403. doi: 10.1016/j.lwt.2016.08.001. [DOI] [Google Scholar]
- Campalani C, Amadio E, Zanini S, et al. Supercritical CO2 as a green solvent for the circular economy: extraction of fatty acids from fruit pomace. J CO2 Util. 2020;41:101259. doi: 10.1016/j.jcou.2020.101259. [DOI] [Google Scholar]
- Cañadas R, González-Miquel M, González EJ, et al. Overview of neoteric solvents as extractants in food industry: A focus on phenolic compounds separation from liquid streams. Food Res Int. 2020;136:109558. doi: 10.1016/j.foodres.2020.109558. [DOI] [PubMed] [Google Scholar]
- Cares MG, Vargas Y, Gaete L, et al. Ultrasonically assisted extraction of bioactive principles from quillaja saponaria molina. Phys Procedia. 2010;3:169–178. doi: 10.1016/j.phpro.2010.01.024. [DOI] [Google Scholar]
- Castro-Muñoz R, Conidi C, Cassano A. Membrane-based technologies for meeting the recovery of biologically active compounds from foods and their by-products. Crit Rev Food Sci Nutr. 2019;59:2927–2948. doi: 10.1080/10408398.2018.1478796. [DOI] [PubMed] [Google Scholar]
- Chang YR, Lee YJ, Lee DJ. Membrane fouling during water or wastewater treatments: current research updated. J Taiwan Inst Chem Eng. 2019;94:88–96. doi: 10.1016/j.jtice.2017.12.019. [DOI] [Google Scholar]
- Charpe TW, Rathod VK. Kinetics of ultrasound assisted extraction of wedelolactone from eclipta alba. Brazilian J Chem Eng. 2016;33:1003–1010. doi: 10.1590/0104-6632.20160334s20140234. [DOI] [Google Scholar]
- Chaves JO, de Souza MC, da Silva LC, et al. Extraction of flavonoids from natural sources using modern techniques. Front Chem. 2020;8:1–26. doi: 10.3389/fchem.2020.507887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmelová D, Škulcová D, Legerská B, et al. Ultrasonic-assisted extraction of polyphenols and antioxidants from Picea abies bark. J Biotechnol. 2020;314–315:25–33. doi: 10.1016/j.jbiotec.2020.04.003. [DOI] [PubMed] [Google Scholar]
- Chuo SC, Nasir HM, Mohd-Setapar SH, et al. A Glimpse into the extraction methods of active compounds from plants. Crit Rev Anal Chem. 2020 doi: 10.1080/10408347.2020.1820851. [DOI] [PubMed] [Google Scholar]
- Cissé M, Bohuon P, Sambe F, et al. Aqueous extraction of anthocyanins from Hibiscus sabdariffa: experimental kinetics and modeling. J Food Eng. 2012;109:16–21. doi: 10.1016/j.jfoodeng.2011.10.012. [DOI] [Google Scholar]
- Conidi C, Cassano A, Garcia-Castello E. Valorization of artichoke wastewaters by integrated membrane process. Water Res. 2014;48:363–374. doi: 10.1016/j.watres.2013.09.047. [DOI] [PubMed] [Google Scholar]
- Conidi C, Drioli E, Cassano A. Biologically active compounds from goji (Lycium barbarum l.) leaves aqueous extracts: Purification and concentration by membrane processes. Biomol. 2020;10:1–18. doi: 10.3390/biom10060935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ćujić N, Šavikin K, Janković T, et al. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016;194:135–142. doi: 10.1016/j.foodchem.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Dai J, Mumper RJ. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Mol. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Porto C, Natolino A. Extraction kinetic modelling of total polyphenols and total anthocyanins from saffron floral bio-residues: Comparison of extraction methods. Food Chem. 2018;258:137–143. doi: 10.1016/j.foodchem.2018.03.059. [DOI] [PubMed] [Google Scholar]
- Da Porto C, Porretto E, Decorti D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason Sonochem. 2013;20:1076–1080. doi: 10.1016/j.ultsonch.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Dawidowicz AL, Olszowy M. The importance of solvent type in estimating antioxidant properties of phenolic compounds by ABTS assay. Eur Food Res Technol. 2013;236:1099–1105. doi: 10.1007/s00217-013-1982-1. [DOI] [Google Scholar]
- Dawidowicz AL, Olszowy M, Jóźwik-Dolęba M. Importance of solvent association in the estimation of antioxidant properties of phenolic compounds by DPPH method. J Food Sci Technol. 2015;52:4523–4529. doi: 10.1007/s13197-014-1451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camargo AC, Regitano-D’Arce MAB, Biasoto ACT, Shahidi F. Enzyme-assisted extraction of phenolics from winemaking by-products: antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem. 2016;212:395–402. doi: 10.1016/j.foodchem.2016.05.047. [DOI] [PubMed] [Google Scholar]
- del Sánchez-Camargo A, P, Ballesteros-Vivas D, Buelvas-Puello LM, , et al. Microwave-assisted extraction of phenolic compounds with antioxidant and anti-proliferative activities from supercritical CO2 pre-extracted mango peel as valorization strategy. Lwt. 2020 doi: 10.1016/j.lwt.2020.110414. [DOI] [Google Scholar]
- Delsart C, Ghidossi R, Poupot C, et al. Enhanced extraction of phenolic compounds from merlot grapes by pulsed electric field treatment. Am J Enol Vitic. 2012;63:205–211. doi: 10.5344/ajev.2012.11088. [DOI] [Google Scholar]
- de Santana Magalhães F, de Souza Martins M, Sá VL, Cardoso MH, Reis M. Recovery of phenolic compounds from pequi (Caryocar brasiliense Camb.) fruit extract by membrane filtrations: comparison of direct and sequential processes. J Food Eng. 2019;257:26–33. doi: 10.1016/j.jfoodeng.2019.03.025. [DOI] [Google Scholar]
- Dickhout JM, Virga E, Lammertink RGH, de Vos WM. Surfactant specific ionic strength effects on membrane fouling during produced water treatment. J Colloid Interface Sci. 2019;556:12–23. doi: 10.1016/j.jcis.2019.07.068. [DOI] [PubMed] [Google Scholar]
- Ding L, Zhang B, Tan CP, et al. Effects of limited moisture content and storing temperature on retrogradation of rice starch. Int J Biol Macromol. 2019;137:1068–1075. doi: 10.1016/j.ijbiomac.2019.06.226. [DOI] [PubMed] [Google Scholar]
- Domínguez-Rodríguez G, Marina ML, Plaza M. Enzyme-assisted extraction of bioactive non-extractable polyphenols from sweet cherry (Prunus avium L) pomace. Food Chem. 2021;339:128086. doi: 10.1016/j.foodchem.2020.128086. [DOI] [PubMed] [Google Scholar]
- Doymaz I, Göl E. Convective drying characteristics of eggplant slices. J Food Process Eng. 2011;34:1234–1252. doi: 10.1111/j.1745-4530.2009.00426.x. [DOI] [Google Scholar]
- Duba KS, Casazza AA, Ben MH, et al. Extraction of polyphenols from grape skins and defatted grape seeds using subcritical water: Experiments and modeling. Food Bioprod Process. 2015;94:29–38. doi: 10.1016/j.fbp.2015.01.001. [DOI] [Google Scholar]
- El Darra N, Grimi N, Maroun RG, et al. Pulsed electric field, ultrasound, and thermal pretreatments for better phenolic extraction during red fermentation. Eur Food Res Technol. 2013;236:47–56. doi: 10.1007/s00217-012-1858-9. [DOI] [Google Scholar]
- Elik A, Yanık DK, Göğüş F. Microwave-assisted extraction of carotenoids from carrot juice processing waste using flaxseed oil as a solvent. Lwt. 2020 doi: 10.1016/j.lwt.2020.109100. [DOI] [Google Scholar]
- El Kantar S, Boussetta N, Lebovka N, et al. Pulsed electric field treatment of citrus fruits: Improvement of juice and polyphenols extraction. Innov Food Sci Emerg Technol. 2018;46:153–161. doi: 10.1016/j.ifset.2017.09.024. [DOI] [Google Scholar]
- El Kantar S, Boussetta N, Rajha HN, et al. High voltage electrical discharges combined with enzymatic hydrolysis for extraction of polyphenols and fermentable sugars from orange peels. Food Res Int. 2018;107:755–762. doi: 10.1016/j.foodres.2018.01.070. [DOI] [PubMed] [Google Scholar]
- El Kantar S, Rajha HN, Boussetta N, et al. Green extraction of polyphenols from grapefruit peels using high voltage electrical discharges, deep eutectic solvents and aqueous glycerol. Food Chem. 2019;295:165–171. doi: 10.1016/j.foodchem.2019.05.111. [DOI] [PubMed] [Google Scholar]
- El-Messery TM, El-Said MM, Demircan E, Ozçelik B. Microencapsulation of natural polyphenolic compounds. Acta Sci Pol Technol Aliment. 2019;18:25–34. doi: 10.17306/J.AFS.0597. [DOI] [PubMed] [Google Scholar]
- Estevez-Areco S, Guz L, Candal R, Goyanes S. Release kinetics of rosemary (Rosmarinus officinalis) polyphenols from polyvinyl alcohol (PVA) electrospun nanofibers in several food simulants. Food Packag Shelf Life. 2018;18:42–50. doi: 10.1016/j.fpsl.2018.08.006. [DOI] [Google Scholar]
- Ezhilarasi PN, Karthik P, Chhanwal N, Anandharamakrishnan C. Nanoencapsulation techniques for food bioactive components: a review. Food Bioprocess Technol. 2013;6:628–647. doi: 10.1007/s11947-012-0944-0. [DOI] [Google Scholar]
- Fante L, Noreña CPZ. Enzyme inactivation kinetics and colour changes in Garlic (Allium sativum L.) blanched under different conditions. J Food Eng. 2012;108:436–443. doi: 10.1016/j.jfoodeng.2011.08.024. [DOI] [Google Scholar]
- Fayaz SM, Abdoli MA, Baghdadi M, Karbasi A. Ag removal from e-waste using supercritical fluid: improving efficiency and selectivity. Int J Environ Stud. 2020;00:1–15. doi: 10.1080/00207233.2020.1834305. [DOI] [Google Scholar]
- Félix G, Ancheyta J, Trejo F. Sensitivity analysis of kinetic parameters for heavy oil hydrocracking. Fuel. 2019;241:836–844. doi: 10.1016/j.fuel.2018.12.058. [DOI] [Google Scholar]
- Fernández-López JA, Angosto JM, Giménez PJ, León G. Thermal stability of selected natural red extracts used as food colorants. Plant Foods Hum Nutr. 2013;68:11–17. doi: 10.1007/s11130-013-0337-1. [DOI] [PubMed] [Google Scholar]
- Ferrentino G, Giampiccolo S, Morozova K, et al. Supercritical fluid extraction of oils from apple seeds: process optimization, chemical characterization and comparison with a conventional solvent extraction. Innov Food Sci Emerg Technol. 2020;64:102428. doi: 10.1016/j.ifset.2020.102428. [DOI] [Google Scholar]
- Fonteles TV, Costa MGM, de Jesus ALT, et al. Power ultrasound processing of cantaloupe melon juice: effects on quality parameters. Food Res Int. 2012;48:41–48. doi: 10.1016/j.foodres.2012.02.013. [DOI] [Google Scholar]
- Frontuto D, Carullo D, Harrison SM, et al. Optimization of pulsed electric fields-assisted extraction of polyphenols from potato peels using response surface methodology. Food Bioprocess Technol. 2019;12:1708–1720. doi: 10.1007/s11947-019-02320-z. [DOI] [Google Scholar]
- Fuleky G, Czinkota I. Hot water percolation ( HWP ): a new rapid soil extraction method Fiileky and Czinkota. Plant Soil Chem. 1993;157(1):131–135. doi: 10.1007/BF02390235. [DOI] [Google Scholar]
- Fu Y, Shao L, Liu H, et al. Unexpected decrease in yield and antioxidants in vegetable at very high CO2 levels. Environ Chem Lett. 2015;13:473–479. doi: 10.1007/s10311-015-0522-6. [DOI] [Google Scholar]
- Galiano F, Figoli A, Conidi C, et al. Functional properties of punica granatum L. juice clarified by hollow fiber membranes. Processes. 2016;4:1–16. doi: 10.3390/pr4030021. [DOI] [Google Scholar]
- Gallo M, Formato A, Giacco R, et al. Mathematical optimization of the green extraction of polyphenols from grape peels through a cyclic pressurization process. Heliyon. 2019;5:1–21. doi: 10.1016/j.heliyon.2019.e01526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan D’Alessandro L, Kriaa K, Nikov I, Dimitrov K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep Purif Technol. 2012;93:42–47. doi: 10.1016/j.seppur.2012.03.024. [DOI] [Google Scholar]
- Gao M, Liu CZ. Comparison of techniques for the extraction of flavonoids from cultured cells of Saussurea medusa Maxim. World J Microbiol Biotechnol. 2005;21:1461–1463. doi: 10.1007/s11274-005-6809-1. [DOI] [Google Scholar]
- Giacobbo A, Bernardes AM, de Pinho MN. Sequential pressure-driven membrane operations to recover and fractionate polyphenols and polysaccharides from second racking wine lees. Sep Purif Technol. 2017;173:49–54. doi: 10.1016/j.seppur.2016.09.007. [DOI] [Google Scholar]
- Gómez-Estaca J, Gavara R, Hernández-Muñoz P. Encapsulation of curcumin in electrosprayed gelatin microspheres enhances its bioaccessibility and widens its uses in food applications. Innov Food Sci Emerg Technol. 2015;29:302–307. doi: 10.1016/j.ifset.2015.03.004. [DOI] [Google Scholar]
- Gómez-Mejía E, Rosales-Conrado N, León-González ME, Madrid Y. Citrus peels waste as a source of value-added compounds: extraction and quantification of bioactive polyphenols. Food Chem. 2019;295:289–299. doi: 10.1016/j.foodchem.2019.05.136. [DOI] [PubMed] [Google Scholar]
- Goñi SM, Salvadori VO. Kinetic modelling of colour changes during beef roasting. Procedia Food Sci. 2011;1:1039–1044. doi: 10.1016/j.profoo.2011.09.155. [DOI] [Google Scholar]
- González E, Gómez-Caravaca AM, Giménez B, et al. Evolution of the phenolic compounds profile of olive leaf extract encapsulated by spray-drying during in vitro gastrointestinal digestion. Food Chem. 2019;279:40–48. doi: 10.1016/j.foodchem.2018.11.127. [DOI] [PubMed] [Google Scholar]
- Govindarajalu AK, Ponnuchamy M, Sivasamy B, et al. A cellulosic paper-based sensor for detection of starch contamination in milk. Bull Mater Sci. 2019;42:1–6. doi: 10.1007/s12034-019-1958-2. [DOI] [Google Scholar]
- Hameed A, Hussain SA, Ijaz MU, et al. Antioxidant activity of polyphenolic extracts of filamentous fungus Mucor circinelloides (WJ11): Extraction, characterization and storage stability of food emulsions. Food Biosci. 2020;34:100525. doi: 10.1016/j.fbio.2019.100525. [DOI] [Google Scholar]
- Hansen J, Møller I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal Biochem. 1975;68:87–94. doi: 10.1016/0003-2697(75)90682-X. [DOI] [PubMed] [Google Scholar]
- Harouna-Oumarou HA, Fauduet H, Porte C, Ho YS. Comparison of kinetic models for the aqueous solid-liquid extraction of Tilia sapwood a continuous stirred tank reactor. Chem Eng Commun. 2007;194:537–552. doi: 10.1080/00986440600992511. [DOI] [Google Scholar]
- Hashemi Z, Ebrahimzadeh MA, Khalili M. Sun protection factor, total phenol, flavonoid contents and antioxidant activity of medicinal plants from iran. Trop J Pharm Res. 2019;18:1443–1448. doi: 10.4314/tjpr.v18i7.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim N, Daniel O, Rahaman E. A preliminary study: kinetic model of drying process of pumpkins (Cucurbita moschata) in a Convective Hot Air Dryer. Agric Agric Sci Procedia. 2014;2:345–352. doi: 10.1016/j.aaspro.2014.11.048. [DOI] [Google Scholar]
- Hauser B. Membrane-assisted solvent extrac-tion of triazines and other semivolatile contaminants directly coupled to large-volume injection / GC-MS. Glob Anal Solut. 2002;I:1–8. [Google Scholar]
- Hawthorne SB, Grabanski CB, Martin E, Miller DJ. Comparisons of Soxhlet extraction, pressurized liquid extraction, supercritical fluid extraction and subcritical water extraction for environmental solids: Recovery, selectivity and effects on sample matrix. J Chromatogr A. 2000;892:421–433. doi: 10.1016/S0021-9673(00)00091-1. [DOI] [PubMed] [Google Scholar]
- Herodež ŠS, Hadolin M, Škerget M, Knez Ž. Solvent extraction study of antioxidants from Balm (Melissa officinalis L.) leaves. Food Chem. 2003;80:275–282. doi: 10.1016/S0308-8146(02)00382-5. [DOI] [Google Scholar]
- He X, Wang L. Experimental determination of moisture diffusivity of birch. IOP Conf Ser Earth Environ Sci. 2020 doi: 10.1088/1755-1315/546/4/042012. [DOI] [Google Scholar]
- Hoe BC, Chan ES, Nagasundara Ramanan R, Ooi CW. Recent development and challenges in extraction of phytonutrients from palm oil. Compr Rev Food Sci Food Saf. 2020;19:4031–4061. doi: 10.1111/1541-4337.12648. [DOI] [PubMed] [Google Scholar]
- Holman BWB, Ponnampalam EN, Kilgannon AK, et al. Moisture content, fatty acid profile and oxidative traits of aged beef subjected to different temperature-time combinations. Meat Sci. 2019;157:107876. doi: 10.1016/j.meatsci.2019.107876. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Bala BK. Drying of hot chilli using solar tunnel drier. Sol Energy. 2007;81:85–92. doi: 10.1016/j.solener.2006.06.008. [DOI] [Google Scholar]
- Huynh NT, Smagghe G, Gonzales GB, et al. Enzyme-assisted extraction enhancing the phenolic release from cauliflower (Brassica oleracea L. var. botrytis) outer leaves. J Agric Food Chem. 2014;62:7468–7476. doi: 10.1021/jf502543c. [DOI] [PubMed] [Google Scholar]
- Iriondo-Dehond M, Miguel E, Del Castillo MD. Food byproducts as sustainable ingredients for innovative and healthy dairy foods. Nutrients. 2018;10:1–24. doi: 10.3390/nu10101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isik BS, Altay F, Capanoglu E. The uniaxial and coaxial encapsulations of sour cherry (Prunus cerasus L.) concentrate by electrospinning and their in vitro bioaccessibility. Food Chem. 2018;265:260–273. doi: 10.1016/j.foodchem.2018.05.064. [DOI] [PubMed] [Google Scholar]
- JARDe O, Komesu A, Rogez H. Enzyme-assisted extraction of phenolic compounds from murucizeiro leaves (Byrsonima crassifolia) Sci Plena. 2020;16:1–9. doi: 10.14808/sci.plena.2020.051501. [DOI] [Google Scholar]
- Jazini MH, Hatamipour MS. A new physical pretreatment of plum for drying. Food Bioprod Process. 2010;88:133–137. doi: 10.1016/j.fbp.2009.06.002. [DOI] [Google Scholar]
- Kadam DM, Goyal RK, Gupta MK. Mathematical modeling of convective thin layer drying of basil leaves. J Med Plant Res. 2011;5:4721–4730. [Google Scholar]
- Karacabey E, Bayindirli L, Artik N, Mazza G. Modeling solid-liquid extraction kinetics of trans-resveratrol and trans-ε-viniferin from grape cane. J Food Process Eng. 2013;36:103–112. doi: 10.1111/j.1745-4530.2011.00660.x. [DOI] [Google Scholar]
- Karageorgou I, Grigorakis S, Lalas S, Makris DP. Enhanced extraction of antioxidant polyphenols from Moringa oleifera Lam. leaves using a biomolecule-based low-transition temperature mixture. Eur Food Res Technol. 2017;243:1839–1848. doi: 10.1007/s00217-017-2887-1. [DOI] [Google Scholar]
- Kiai H, Raiti J, El-Abbassi A, Hafidi A. Recovery of phenolic compounds from table olive processing wastewaters using cloud point extraction method. J Environ Chem Eng. 2018;6:1569–1575. doi: 10.1016/j.jece.2018.05.007. [DOI] [Google Scholar]
- Kiew PL, Mat Don M. Screening and empirical kinetic models of collagen extraction from selected Malaysian freshwater fish. J Food Process Eng. 2013;36:428–438. doi: 10.1111/j.1745-4530.2012.00683.x. [DOI] [Google Scholar]
- Kitrytė V, Kraujalienė V, Šulniūtė V, et al. Chokeberry pomace valorization into food ingredients by enzyme-assisted extraction: Process optimization and product characterization. Food Bioprod Process. 2017;105:36–50. doi: 10.1016/j.fbp.2017.06.001. [DOI] [Google Scholar]
- Kothari V, Gupta A, Naraniwal M. Comparative study of various methods for extraction of antioxidant and antibacterial compounds from plant seeds. J Nat Remedies. 2012;12:162–173. doi: 10.18311/jnr/2012/271. [DOI] [Google Scholar]
- Kratky L, Zamazal P. Economic feasibility and sensitivity analysis of fish waste processing biorefinery. J Clean Prod. 2020;243:118677. doi: 10.1016/j.jclepro.2019.118677. [DOI] [Google Scholar]
- Kuai L, Liu F, Ma Y, et al. Regulation of nano-encapsulated tea polyphenol release from gelatin films with different Bloom values. Food Hydrocoll. 2020;108:106045. doi: 10.1016/j.foodhyd.2020.106045. [DOI] [Google Scholar]
- Lanças FM, Queiroz MEC, de Silva ICE. Seed oil extraction with supercritical carbon dioxide modified with pentane. Chromatographia. 1994;39:687–692. doi: 10.1007/BF02274584. [DOI] [Google Scholar]
- Lee KY, Rahman MS, Kim AN, et al. Quality characteristics and storage stability of low-fat tofu prepared with defatted soy flours treated by supercritical-CO2 and hexane. Lwt. 2019;100:237–243. doi: 10.1016/j.lwt.2018.10.073. [DOI] [Google Scholar]
- Liu D, Vorobiev E, Savoire R, Lanoisellé JL. Intensification of polyphenols extraction from grape seeds by high voltage electrical discharges and extract concentration by dead-end ultrafiltration. Sep Purif Technol. 2011;81:134–140. doi: 10.1016/j.seppur.2011.07.012. [DOI] [Google Scholar]
- Liu ZW, Zeng XA, Ngadi M. Enhanced extraction of phenolic compounds from onion by pulsed electric field (PEF) J Food Process Preserv. 2018;42:4–11. doi: 10.1111/jfpp.13755. [DOI] [Google Scholar]
- Liu Z, Esveld E, Vincken JP, Bruins ME. Pulsed electric field as an alternative pre-treatment for drying to enhance polyphenol extraction from fresh tea leaves. Food Bioprocess Technol. 2019;12:183–192. doi: 10.1007/s11947-018-2199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Fan Y, Xi J. Recent advances in high voltage electric discharge extraction of bioactive ingredients from plant materials. Food Chem. 2019;277:246–260. doi: 10.1016/j.foodchem.2018.10.119. [DOI] [PubMed] [Google Scholar]
- Li R, Fan H, Shen L, et al. Inkjet printing assisted fabrication of polyphenol-based coating membranes for oil/water separation. Chemosphere. 2020;250:126236. doi: 10.1016/j.chemosphere.2020.126236. [DOI] [PubMed] [Google Scholar]
- Llompart M, Celeiro M, Dagnac T. Microwave-assisted extraction of pharmaceuticals, personal care products and industrial contaminants in the environment. TrAC–Trends Anal Chem. 2019;116:136–150. doi: 10.1016/j.trac.2019.04.029. [DOI] [Google Scholar]
- Loginova KV, Lebovka NI, Vorobiev E. Pulsed electric field assisted aqueous extraction of colorants from red beet. J Food Eng. 2011;106:127–133. doi: 10.1016/j.jfoodeng.2011.04.019. [DOI] [Google Scholar]
- Lohith Kumar DH, Sarkar P. Encapsulation of bioactive compounds using nanoemulsions. Environ Chem Lett. 2018;16:59–70. doi: 10.1007/s10311-017-0663-x. [DOI] [Google Scholar]
- Lončarić A, Celeiro M, Jozinović A, et al. Green extraction methods for extraction of polyphenolic compounds from blueberry pomace. Foods. 2020;9:1–22. doi: 10.3390/foods9111521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luengo E, Álvarez I, Raso J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov Food Sci Emerg Technol. 2013;17:79–84. doi: 10.1016/j.ifset.2012.10.005. [DOI] [Google Scholar]
- Luo X, Cui J, Zhang H, et al. Ultrasound assisted extraction of polyphenolic compounds from red sorghum (Sorghum bicolor L.) bran and their biological activities and polyphenolic compositions. Ind Crops Prod. 2018;112:296–304. doi: 10.1016/j.indcrop.2017.12.019. [DOI] [Google Scholar]
- Luque de Castro MD, Priego-Capote F. Soxhlet extraction: past and present panacea. J Chromatogr A. 2010;1217:2383–2389. doi: 10.1016/j.chroma.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Mahindrakar KV, Rathod VK. Ultrasonic assisted aqueous extraction of catechin and gallic acid from Syzygium cumini seed kernel and evaluation of total phenolic, flavonoid contents and antioxidant activity. Chem Eng Process–Process Intensif. 2020;149:107–841. doi: 10.1016/j.cep.2020.107841. [DOI] [Google Scholar]
- Makris DP, Passalidi V, Kallithraka S, Mourtzinos I. Optimization of polyphenol extraction from red grape pomace using aqueous glycerol/tartaric acid mixtures and response surface methodology. Prep Biochem Biotechnol. 2016;46:176–182. doi: 10.1080/10826068.2015.1015562. [DOI] [PubMed] [Google Scholar]
- Maleki M, Shahidi F, Varidi MJ, Azarpazhooh E. Hot air drying kinetics of novel functional carrot snack: Impregnated using polyphenolic rich osmotic solution with ultrasound pretreatment. J Food Process Eng. 2020;43:1–11. doi: 10.1111/jfpe.13331. [DOI] [Google Scholar]
- Marić M, Grassino AN, Zhu Z, et al. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci Technol. 2018;76:28–37. doi: 10.1016/j.tifs.2018.03.022. [DOI] [Google Scholar]
- Maroun RG, Chacar S (2018) Antioxidant molecules of byproduct: from extraction using innovative technologies to bioactivre properties
- Maza M, Álvarez I, Raso J. Thermal and Non-Thermal Physical Methods for Improving Polyphenol Extraction in Red Winemaking. Beverages. 2019;5:47. doi: 10.3390/beverages5030047. [DOI] [Google Scholar]
- Meini MR, Cabezudo I, Boschetti CE, Romanini D. Recovery of phenolic antioxidants from Syrah grape pomace through the optimization of an enzymatic extraction process. Food Chem. 2019;283:257–264. doi: 10.1016/j.foodchem.2019.01.037. [DOI] [PubMed] [Google Scholar]
- Milea Ștefania A, Aprodu I, Vasile AM, et al. Widen the functionality of flavonoids from yellow onion skins through extraction and microencapsulation in whey proteins hydrolysates and different polymers. J Food Eng. 2019;251:29–35. doi: 10.1016/j.jfoodeng.2019.02.003. [DOI] [Google Scholar]
- Mouratoglou E, Malliou V, Makris DP. Novel glycerol-based natural eutectic mixtures and their efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from agri-food waste biomass. Waste Biomass Valorization. 2016;7:1377–1387. doi: 10.1007/s12649-016-9539-8. [DOI] [Google Scholar]
- Munekata PES, Alcántara C, Žugčić T, et al. Impact of ultrasound-assisted extraction and solvent composition on bioactive compounds and in vitro biological activities of thyme and rosemary. Food Res Int. 2020;134:109242. doi: 10.1016/j.foodres.2020.109242. [DOI] [PubMed] [Google Scholar]
- Mustapa AN, Martin A, Gallego JR, et al. Microwave-assisted extraction of polyphenols from Clinacanthus nutans Lindau medicinal plant: energy perspective and kinetics modeling. Chem Eng Process Process Intensif. 2015;97:66–74. doi: 10.1016/j.cep.2015.08.013. [DOI] [Google Scholar]
- Nadar SS, Rao P, Rathod VK. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res Int. 2018;108:309–330. doi: 10.1016/j.foodres.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Natolino A, Da Porto C. Kinetic models for conventional and ultrasound assistant extraction of polyphenols from defatted fresh and distilled grape marc and its main components skins and seeds. Chem Eng Res Des. 2020;156:1–12. doi: 10.1016/j.cherd.2020.01.009. [DOI] [Google Scholar]
- Nishad J, Saha S, Kaur C. Enzyme- and ultrasound-assisted extractions of polyphenols from Citrus sinensis (cv. Malta) peel: A comparative study. J Food Process Preserv. 2019;43:1–13. doi: 10.1111/jfpp.14046. [DOI] [Google Scholar]
- Olszewska MA, Gędas A, Simões M. Antimicrobial polyphenol-rich extracts: applications and limitations in the food industry. Food Res Int. 2020;134:109214. doi: 10.1016/j.foodres.2020.109214. [DOI] [PubMed] [Google Scholar]
- Olszowy M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol Biochem. 2019;144:135–143. doi: 10.1016/j.plaphy.2019.09.039. [DOI] [PubMed] [Google Scholar]
- Oroian M, Dranca F, Ursachi F. Comparative evaluation of maceration, microwave and ultrasonic- assisted extraction of phenolic compounds from propolis. J Food Sci Technol. 2019 doi: 10.1007/s13197-019-04031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroian M, Ursachi F, Dranca F. Ultrasound-assisted extraction of polyphenols from crude pollen. Antioxidants. 2020;9:1–15. doi: 10.3390/antiox9040322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio-Tobón JF. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J Food Sci Technol. 2020;57:4299–4315. doi: 10.1007/s13197-020-04433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman SNS, Mustapa AN, Ku Hamid KH. Extraction of polyphenols from Clinacanthus nutans Lindau (C. nutans) by vacuum solvent-free microwave extraction (V-SFME) Chem Eng Commun. 2020 doi: 10.1080/00986445.2020.1727452. [DOI] [Google Scholar]
- Ozel MZ, Kaymaz H. Superheated water extraction, steam distillation and Soxhlet extraction of essential oils of Origanum onites. Anal Bioanal Chem. 2004;379:1127–1133. doi: 10.1007/s00216-004-2671-5. [DOI] [PubMed] [Google Scholar]
- Pandey A, Tripathi S. Extraction of pharmaceutical drugs. J Pharmacogn Phytochem. 2014;2:115–119. [Google Scholar]
- Paraiso IL, Revel JS, Stevens JF. Potential use of polyphenols in the battle against COVID-19. Curr Opin Food Sci. 2020;32:149–155. doi: 10.1016/j.cofs.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasrija D, Anandharamakrishnan C. Techniques for Extraction of Green Tea Polyphenols: A Review. Food Bioprocess Technol. 2015;8:935–950. doi: 10.1007/s11947-015-1479-y. [DOI] [Google Scholar]
- Pătrăuţanu OA, Lazăr L, Popa VI, Volf I. Influence of particle size and size distribution on kinetic mechanism of spruce bark polyphenols extraction. Cellul Chem Technol. 2019;53:71–78. doi: 10.35812/cellulosechemtechnol.2019.53.08. [DOI] [Google Scholar]
- Peiró S, Luengo E, Segovia F, et al. Improving polyphenol extraction from lemon residues by pulsed electric fields. Waste Biomass Valorization. 2019;10:889–897. doi: 10.1007/s12649-017-0116-6. [DOI] [Google Scholar]
- Périno-Issartier S, Zill-e-Huma A-V, Chemat F. Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products. Food Bioprocess Technol. 2011;4:1020–1028. doi: 10.1007/s11947-010-0438-x. [DOI] [Google Scholar]
- Piccolella S, Crescente G, Candela L, Pacifico S. Nutraceutical polyphenols: new analytical challenges and opportunities. J Pharm Biomed Anal. 2019;175:112774. doi: 10.1016/j.jpba.2019.07.022. [DOI] [PubMed] [Google Scholar]
- Pripakhaylo AV, Magomedov RN, Maryutina TA. Separation of heavy oil into narrow fractions by supercritical fluid extraction using a CO2–toluene mixture. J Anal Chem. 2019;74:401–409. doi: 10.1134/S1061934819040105. [DOI] [Google Scholar]
- Puértolas E, Saldaña G, Condón S, et al. Evolution of polyphenolic compounds in red wine from Cabernet Sauvignon grapes processed by pulsed electric fields during aging in bottle. Food Chem. 2010;119:1063–1070. doi: 10.1016/j.foodchem.2009.08.018. [DOI] [Google Scholar]
- Quiles-Carrillo L, Mellinas C, Garrigos MC, et al. Optimization of Microwave-Assisted Extraction of Phenolic Compounds with Antioxidant Activity from Carob Pods. Food Anal Methods. 2019;12:2480–2490. doi: 10.1007/s12161-019-01596-3. [DOI] [Google Scholar]
- Qu W, Pan Z, Ma H. Extraction modeling and activities of antioxidants from pomegranate marc. J Food Eng. 2010;99:16–23. doi: 10.1016/j.jfoodeng.2010.01.020. [DOI] [Google Scholar]
- Radha Krishnan K, Sivarajan M, Babuskin S, et al. Kinetic modeling of spice extraction from S. aromaticum and C. cassia. J Food Eng. 2013;117:326–332. doi: 10.1016/j.jfoodeng.2013.03.011. [DOI] [Google Scholar]
- Rajha HN, Boussetta N, Louka N, et al. A comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Res Int. 2014;65:462–468. doi: 10.1016/j.foodres.2014.04.024. [DOI] [Google Scholar]
- Rajha HN, Abi-Khattar AM, El Kantar S, et al. Comparison of aqueous extraction efficiency and biological activities of polyphenols from pomegranate peels assisted by infrared, ultrasound, pulsed electric fields and high-voltage electrical discharges. Innov Food Sci Emerg Technol. 2019;58:102212. doi: 10.1016/j.ifset.2019.102212. [DOI] [Google Scholar]
- Rakshit M, Srivastav PP, Bhunia K. Kinetic modeling of ultrasonic-assisted extraction of punicalagin from pomegranate peel. J Food Process Eng. 2020 doi: 10.1111/jfpe.13533. [DOI] [Google Scholar]
- Rathore SK, Bhatt S, Dhyani S, Jain A. Preliminary phytochemical screening of medicinal plant Ziziphus mauritiana Lam. Fruits Int J Curr Pharm Res. 2012;4:160–162. [Google Scholar]
- Rayguru M, Routray A. Mathematical modeling of thin layer drying kinetics of stone apple slices. Int Food Res J. 2012;19:1503–1510. [Google Scholar]
- Ribeiro AM, Estevinho BN, Rocha F. Edible films prepared with different biopolymers, containing polyphenols extracted from elderberry (Sambucus nigra L.), to protect food products and to improve food functionality. Food Bioprocess Technol. 2020;13:1742–1754. doi: 10.1007/s11947-020-02516-8. [DOI] [Google Scholar]
- Rijo P, Falé PL, Serralheiro ML, et al. Optimization of medicinal plant extraction methods and their encapsulation through extrusion technology. Meas J Int Meas Confed. 2014;58:249–255. doi: 10.1016/j.measurement.2014.08.045. [DOI] [Google Scholar]
- Roberts JS, Kidd DR, Padilla-Zakour O. Drying kinetics of grape seeds. J Food Eng. 2008;89:460–465. doi: 10.1016/j.jfoodeng.2008.05.030. [DOI] [Google Scholar]
- Rodil R, Schellin M, Popp P. Analysis of polycyclic aromatic hydrocarbons in water and beverages using membrane-assisted solvent extraction in combination with large volume injection-gas chromatography-mass spectrometric detection. J Chromatogr A. 2007;1163:288–297. doi: 10.1016/j.chroma.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Rodsamran P, Sothornvit R. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Biosci. 2019;28:66–73. doi: 10.1016/j.fbio.2019.01.017. [DOI] [Google Scholar]
- Romani A, Campo M, Urciuoli S, et al. An industrial and sustainable platform for the production of bioactive micronized powders and extracts enriched in polyphenols from Olea europaea L. and Vitis vinifera L. Wastes Front Nutr. 2020;7:1–18. doi: 10.3389/fnut.2020.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román GC, Jackson RE, Gadhia R, et al. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev Neurol (Paris) 2019;175:724–741. doi: 10.1016/j.neurol.2019.08.005. [DOI] [PubMed] [Google Scholar]
- Roselló-Soto E, Barba FJ, Parniakov O, et al. High voltage electrical discharges, pulsed electric field, and ultrasound assisted extraction of protein and phenolic compounds from Olive Kernel. Food Bioprocess Technol. 2015;8:885–894. doi: 10.1007/s11947-014-1456-x. [DOI] [Google Scholar]
- Rowland I. Optimal nutrition: Fibre and phytochemicals. Proc Nutr Soc. 1999;58:415–419. doi: 10.1017/S0029665199000543. [DOI] [PubMed] [Google Scholar]
- J S, V D, B M, et al. Modelling of the process of solid-liquid extraction of total polyphenols from soybeans. Czech J Food Sci. 2010;28:206–212. doi: 10.17221/200/2009-CJFS. [DOI] [Google Scholar]
- Safdar MN, Kausar T, Nadeem M. Comparison of ultrasound and maceration techniques for the extraction of polyphenols from the mango peel. J Food Process Preserv. 2017;41:1–13. doi: 10.1111/jfpp.13028. [DOI] [Google Scholar]
- Saini A, Panesar PS, Bera M. Comparative study on the extraction and quantification of polyphenols from citrus peels using maceration and ultrasonic technique. Curr Res Nutr Food Sci. 2019;7:678–685. doi: 10.12944/CRNFSJ.7.3.08. [DOI] [Google Scholar]
- Saini A, Panwar D, Panesar PS, Bera MB. Encapsulation of functional ingredients in lipidic nanocarriers and antimicrobial applications: a review. Environ Chem Lett. 2020 doi: 10.1007/s10311-020-01109-3. [DOI] [Google Scholar]
- Sakaki K, Yokochi T, Suzuki O, Hakuta T. Supercritical fluid extraction of fungal oil using CO2, N2O, CHF3 and SF6. J Am Oil Chem Soc. 1990;67:553–557. doi: 10.1007/BF02540765. [DOI] [Google Scholar]
- Sanches Silva A, Reboredo-Rodríguez P, Sanchez-Machado DI, et al. Evaluation of the status quo of polyphenols analysis: Part II—Analysis methods and food processing effects. Compr Rev Food Sci Food Saf. 2020 doi: 10.1111/1541-4337.12626. [DOI] [PubMed] [Google Scholar]
- Sánchez-Vicente Y, Cabañas A, Renuncio JAR, Pando C. Supercritical fluid extraction of peach (Prunus persica) seed oil using carbon dioxide and ethanol. J Supercrit Fluids. 2009;49:167–173. doi: 10.1016/j.supflu.2009.01.001. [DOI] [Google Scholar]
- Sanchez V, Baeza R, Galmarini MV, et al. Freeze-Drying Encapsulation of Red Wine Polyphenols in an Amorphous Matrix of Maltodextrin. Food Bioprocess Technol. 2013;6:1350–1354. doi: 10.1007/s11947-011-0654-z. [DOI] [Google Scholar]
- Sanna V, Roggio AM, Pala N, et al. Effect of chitosan concentration on PLGA microcapsules for controlled release and stability of resveratrol. Int J Biol Macromol. 2015;72:531–536. doi: 10.1016/j.ijbiomac.2014.08.053. [DOI] [PubMed] [Google Scholar]
- Santos DT, Veggi PC, Meireles MAA. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J Food Eng. 2012;108:444–452. doi: 10.1016/j.jfoodeng.2011.08.022. [DOI] [Google Scholar]
- Santos NC, da Silva WP, Barros SL, et al. Red rice (Oryza sativa L.) use in flour production: Convective drying and bioactive quality. J Food Process Eng. 2020;43:1–10. doi: 10.1111/jfpe.13490. [DOI] [Google Scholar]
- Saravanan A, Kumar PS, Hemavathy RV, et al. Methods of detection of food-borne pathogens: a review. Environ Chem Lett. 2020 doi: 10.1007/s10311-020-01072-z. [DOI] [Google Scholar]
- Sarkis JR, Boussetta N, Blouet C, et al. Effect of pulsed electric fields and high voltage electrical discharges on polyphenol and protein extraction from sesame cake. Innov Food Sci Emerg Technol. 2015;29:170–177. doi: 10.1016/j.ifset.2015.02.011. [DOI] [Google Scholar]
- Sato T, Ikeya Y, Adachi S, ichi, , et al. Extraction of strawberry leaves with supercritical carbon dioxide and entrainers: Antioxidant capacity, total phenolic content, and inhibitory effect on uric acid production of the extract. Food Bioprod Process. 2019;117:160–169. doi: 10.1016/j.fbp.2019.07.003. [DOI] [Google Scholar]
- Savic IM, Savic Gajic IM. Optimization of ultrasound-assisted extraction of polyphenols from wheatgrass (Triticum aestivum L.) J Food Sci Technol. 2020;57:2809–2818. doi: 10.1007/s13197-020-04312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellin M, Popp P. Membrane-assisted solvent extraction of seven phenols combined with large volume injection-gas chromatography-mass spectrometric detection. J Chromatogr A. 2005;1072:37–43. doi: 10.1016/j.chroma.2004.10.066. [DOI] [PubMed] [Google Scholar]
- Seifzadeh N, Ali Sahari M, Barzegar M, et al. Evaluation of polyphenolic compounds in membrane concentrated pistachio hull extract. Food Chem. 2019;277:398–406. doi: 10.1016/j.foodchem.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Sen KK, Chouhan KBS, Tandey R, et al. Impact of microwaves on the extraction yield of phenolics, flavonoids, and triterpenoids fom centella leaves: an approach toward digitzed robust botanical extraction. Pharmacogn Mag. 2017;13:179–188. doi: 10.4103/pm.pm. [DOI] [Google Scholar]
- Shafaei SM, Masoumi AA, Roshan H. Analysis of water absorption of bean and chickpea during soaking using Peleg model. J Saudi Soc Agric Sci. 2016;15:135–144. doi: 10.1016/j.jssas.2014.08.003. [DOI] [Google Scholar]
- Shewale S, Rathod VK. Extraction of total phenolic content from Azadirachta indica or (neem) leaves: Kinetics study. Prep Biochem Biotechnol. 2018;48:312–320. doi: 10.1080/10826068.2018.1431784. [DOI] [PubMed] [Google Scholar]
- Shirsath SR, Sable SS, Gaikwad SG, et al. Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach: Effect of different operating parameters. Ultrason Sonochem. 2017;38:437–445. doi: 10.1016/j.ultsonch.2017.03.040. [DOI] [PubMed] [Google Scholar]
- Shukla A, Tyagi R, Shukla RK. The effect of two different extraction methods for antioxidant activity on petroleum ether extract of Casearia tomentosa leaves. Int J Adv Technol Eng Sci. 2016;04:377–385. [Google Scholar]
- Skrypnik L, Novikova A. Response surface modeling and optimization of polyphenols extraction from apple pomace based on nonionic emulsifiers. Agronomy. 2020 doi: 10.3390/agronomy10010092. [DOI] [Google Scholar]
- Sridhar A, Ponnuchamy M, Kumar PS, Kapoor A. Food preservation techniques and nanotechnology for increased shelf life of fruits, vegetables, beverages and spices: a review. Environ Chem Lett. 2020;2:1–21. doi: 10.1007/s10311-020-01126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stübler AS, Lesmes U, Heinz V, et al. Digestibility, antioxidative activity and stability of plant protein-rich products after processing and formulation with polyphenol-rich juices: kale and kale–strawberry as a model. Eur Food Res Technol. 2019;245:2499–2514. doi: 10.1007/s00217-019-03362-5. [DOI] [Google Scholar]
- Sturzoiu A, Stroescu M. Empirical Models Applied for Kinetics Extraction of β-carotene from Rosa canina. REV CHIM. 2011;62:3–7. [Google Scholar]
- Tao Y, Zhang Z, Sun DW. Kinetic modeling of ultrasound-assisted extraction of phenolic compounds from grape marc: Influence of acoustic energy density and temperature. Ultrason Sonochem. 2014;21:1461–1469. doi: 10.1016/j.ultsonch.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Taşkın B, Aksoylu Özbek Z. Optimisation of microwave effect on bioactives contents and colour attributes of aqueous green tea extracts by central composite design. J Food Meas Charact. 2020;14:2240–2252. doi: 10.1007/s11694-020-00471-8. [DOI] [Google Scholar]
- Tavasi V, Bettens RPA, Leong LP. Temperature and solvent effects on radical scavenging ability of phenols velmurugan thavasi. J Phys Chem A. 2009;113:3068–3077. doi: 10.1021/jp806679v. [DOI] [PubMed] [Google Scholar]
- Terefe NS, Matthies K, Simons L, Versteeg C. Combined high pressure-mild temperature processing for optimal retention of physical and nutritional quality of strawberries (Fragaria × ananassa) Innov Food Sci Emerg Technol. 2009;10:297–307. doi: 10.1016/j.ifset.2008.12.003. [DOI] [Google Scholar]
- Theodorou MK, Kingston-Smith AH, Winters AL, et al. Polyphenols and their influence on gut function and health in ruminants: A review. Environ Chem Lett. 2006;4:121–126. doi: 10.1007/s10311-006-0061-2. [DOI] [Google Scholar]
- Tian B, Qiao Y, yun, Tian yu Y, et al. Effect of heat reflux extraction on the structure and composition of a high-volatile bituminous coal. Appl Therm Eng. 2016;109:560–568. doi: 10.1016/j.applthermaleng.2016.08.104. [DOI] [Google Scholar]
- Toǧrul IT, Pehlivan D. Modelling of drying kinetics of single apricot. J Food Eng. 2003;58:23–32. doi: 10.1016/S0260-8774(02)00329-1. [DOI] [Google Scholar]
- Towler G, Sinnott RAY (2013) Principles, practice and economics of plant and process design
- Tresserra-Rimbau A, Lamuela-Raventos RM, Moreno JJ. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem Pharmacol. 2018;156:186–195. doi: 10.1016/j.bcp.2018.07.050. [DOI] [PubMed] [Google Scholar]
- Treutter D. Significance of flavonoids in plant resistance: a review. Environ Chem Lett. 2006;4:147–157. doi: 10.1007/s10311-006-0068-8. [DOI] [Google Scholar]
- Vella FM, Cautela D, Laratta B. Characterization of polyphenolic compounds in cantaloupe melon by-products. Foods. 2019;8:2–11. doi: 10.3390/foods8060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma ML, Rani V. Biosensors for toxic metals, polychlorinated biphenyls, biological oxygen demand, endocrine disruptors, hormones, dioxin, phenolic and organophosphorus compounds: a review. Environ Chem Lett. 2020 doi: 10.1007/s10311-020-01116-4. [DOI] [Google Scholar]
- Vetal MD, Lade VG, Rathod VK. Extraction of ursolic acid from Ocimum sanctum by ultrasound: Process intensification and kinetic studies. Chem Eng Process Process Intensif. 2013;69:24–30. doi: 10.1016/j.cep.2013.01.011. [DOI] [Google Scholar]
- Vincelet C, Roussel JM, Benanou D. Experimental designs dedicated to the evaluation of a membrane extraction method: Membrane-assisted solvent extraction for compounds having different polarities by means of gas chromatography-mass detection. Anal Bioanal Chem. 2010;396:2285–2292. doi: 10.1007/s00216-009-3449-6. [DOI] [PubMed] [Google Scholar]
- Vishwasrao C, Chakraborty S, Ananthanarayan L. Partial purification, characterisation and thermal inactivation kinetics of peroxidase and polyphenol oxidase isolated from Kalipatti sapota (Manilkara zapota) J Sci Food Agric. 2017;97:3568–3575. doi: 10.1002/jsfa.8215. [DOI] [PubMed] [Google Scholar]
- Wang L, Wu Y, Liu Y, Wu Z. Complex enzyme-assisted extraction releases antioxidative phenolic compositions from guava leaves. Molecules. 2017;22:1–15. doi: 10.3390/molecules22101648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Martínez-Hernández A, de Lamo-Castellví S, et al. Low-energy membrane-based processes to concentrate and encapsulate polyphenols from carob pulp. J Food Eng. 2020 doi: 10.1016/j.jfoodeng.2020.109996. [DOI] [Google Scholar]
- Wen L, Zhang Z, Sun DW, et al. Combination of emerging technologies for the extraction of bioactive compounds. Crit Rev Food Sci Nutr. 2020;60:1826–1841. doi: 10.1080/10408398.2019.1602823. [DOI] [PubMed] [Google Scholar]
- Wikiera A, Mika M, Starzyńska-Janiszewska A, Stodolak B. Endo-xylanase and endo-cellulase-assisted extraction of pectin from apple pomace. Carbohydr Polym. 2016;142:199–205. doi: 10.1016/j.carbpol.2016.01.063. [DOI] [PubMed] [Google Scholar]
- Wilińska A, de Figueiredo Rodrigues AS, Bryjak J, Polakovič M. Thermal inactivation of exogenous pectin methylesterase in apple and cloudberry juices. J Food Eng. 2008;85:459–465. doi: 10.1016/j.jfoodeng.2007.08.009. [DOI] [Google Scholar]
- Xiao W, Han L, Shi B. Microwave-assisted extraction of flavonoids from Radix Astragali. Sep Purif Technol. 2008;62:614–618. doi: 10.1016/j.seppur.2008.03.025. [DOI] [Google Scholar]
- Xiao H, Lin Q, Liu GQ, et al. Inhibitory Effects of Green Tea Polyphenols on the Retrogradation of Starches from Different Botanical Sources. Food Bioprocess Technol. 2013;6:2177–2181. doi: 10.1007/s11947-011-0739-8. [DOI] [Google Scholar]
- Xi J, He L, Yan L. Kinetic modeling of pressure-assisted solvent extraction of polyphenols from green tea in comparison with the conventional extraction. Food Chem. 2015;166:287–291. doi: 10.1016/j.foodchem.2014.06.026. [DOI] [PubMed] [Google Scholar]
- Xi J, He L, Yan L, gong, Continuous extraction of phenolic compounds from pomegranate peel using high voltage electrical discharge. Food Chem. 2017;230:354–361. doi: 10.1016/j.foodchem.2017.03.072. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhang Y, He C. Ultrasonically assisted extraction of isoflavones from stem of Pueraria lobata (Willd.) Ohwi and Its Mathematical Model. Chinese J Chem Eng. 2007;15:861–867. doi: 10.1016/s1004-9541(08)60015-4. [DOI] [Google Scholar]
- Xu S, Lu D, Wang P, et al. Polyphenol engineered membranes with dually charged sandwich structure for low-pressure molecular separation. J Memb Sci. 2020;601:117885. doi: 10.1016/j.memsci.2020.117885. [DOI] [Google Scholar]
- Yan LG, Deng Y, Ju T, et al. Continuous high voltage electrical discharge extraction of flavonoids from peanut shells based on “annular gap type” treatment chamber. Food Chem. 2018;256:350–357. doi: 10.1016/j.foodchem.2018.02.129. [DOI] [PubMed] [Google Scholar]
- Yuliarti O, Goh KKT, Matia-Merino L, et al. Extraction and characterisation of pomace pectin from gold kiwifruit (I) Food Chem. 2015;187:290–296. doi: 10.1016/j.foodchem.2015.03.148. [DOI] [PubMed] [Google Scholar]
- Yu K, Wu Y, Hu Z, et al. Modeling thermal degradation of litchi texture: Comparison of WeLL model and conventional methods. Food Res Int. 2011;44:1970–1976. doi: 10.1016/j.foodres.2010.03.027. [DOI] [Google Scholar]
- Zhang H, Wang Z. Phase transition and release kinetics of polyphenols encapsulated lyotropic liquid crystals. Int J Pharm. 2019;565:283–293. doi: 10.1016/j.ijpharm.2019.05.021. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zeng G, Pan Y, Qi N. Difference research of pectins extracted from tobacco waste by heat reflux extraction and microwave-assisted extraction. Biocatal Agric Biotechnol. 2018;15:359–363. doi: 10.1016/j.bcab.2018.06.022. [DOI] [Google Scholar]
- Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: A comprehensive review. Chinese Med (United Kingdom) 2018;13:1–26. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wang X, Ma Z, et al. Removal of phenolic substances from wastewater by algae. A review Environ Chem Lett. 2020;18:377–392. doi: 10.1007/s10311-019-00953-2. [DOI] [Google Scholar]
- Zhou Y, Xu XY, Gan RY, et al. Optimization of ultrasound-assisted extraction of antioxidant polyphenols from the seed coats of red sword bean (Canavalia gladiate (Jacq.) DC.) Antioxidants. 2019;8:1–13. doi: 10.3390/antiox8070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Živković J, Šavikin K, Janković T, et al. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep Purif Technol. 2018;194:40–47. doi: 10.1016/j.seppur.2017.11.032. [DOI] [Google Scholar]
- Zlatanović S, Ostojić S, Micić D, et al. Thermal behaviour and degradation kinetics of apple pomace flours. Thermochim Acta. 2019;673:17–25. doi: 10.1016/j.tca.2019.01.009. [DOI] [Google Scholar]
- Zuo YB, Zeng AW, Yuan XG, Yu KT. Extraction of soybean isoflavones from soybean meal with aqueous methanol modified supercritical carbon dioxide. J Food Eng. 2008;89:384–389. doi: 10.1016/j.jfoodeng.2008.05.004. [DOI] [Google Scholar]
- Zwingelstein M, Draye M, Besombes JL, et al. Viticultural wood waste as a source of polyphenols of interest: Opportunities and perspectives through conventional and emerging extraction methods. Waste Manag. 2020;102:782–794. doi: 10.1016/j.wasman.2019.11.034. [DOI] [PubMed] [Google Scholar]