Abstract
Objective:
We leveraged decomposition analysis, commonly used in labor economics, to understand determinants of cost differences related to location of admission in children undergoing neonatal congenital heart surgery.
Design:
A retrospective cohort study
Setting:
Pediatric Health Information Systems database
Patients:
Neonates (<30 days old) undergoing their index congenital heart surgery between 2004-2013
Measurements:
A decomposition analysis with bootstrapping determined characteristic (explainable by differing covariate levels) and structural effects (if covariates are held constant) related to cost differences. Covariates included center volume, age at admission, prematurity, sex, race, genetic or major non-cardiac abnormality, RACHS-1 score, payor, admission year, cardiac arrest, infection, and delayed sternal closure.
Main Results:
Of 19,984 infants included [10,491 (52%) to CICU/PICU and 9,493 (48%) to NICU], admission to the NICU had overall higher average costs ($24,959±3,260, p<0.001) vs. CICU/PICU admission. Characteristic effects accounted for higher costs in the NICU ($28,958±2,044, p<0.001). Differing levels of prematurity, genetic syndromes, hospital volume, age at admission and infection contributed to higher NICU costs, with infection rate providing the most significant contribution ($13,581, p<0.001). Aggregate structural effects were not associated with cost differences for those admitted to the NICU vs. CICU/PICU (p=0.1). Individually, prematurity and age at admission were associated with higher costs due to structural effects for infants admitted to the NICU vs. CICU/PICU.
Conclusions:
The difference in cost between NICU and CICU/PICU admissions is largely driven by differing prevalence of risk factors between these units. Infection rate was a modifiable factor that accounted for the largest difference in costs between admitting units.
Keywords: Intensive care unit, neonate, congenital heart surgery, cost, admission
Introduction
Caring for children with congenital heart disease (CHD) is costly with congenital heart defects accounting for the highest resource use of all birth defects. (1) Annual admissions in the United States for CHD cost an estimated $5.6 billion, which comprises 15% of the costs for all pediatric care even though it accounts for only 3.7% of pediatric admissions. (2) Neonates undergoing congenital heart surgery require highly specialized, resource-intensive perioperative care. In the current economic climate, investigators and policy-makers are exploring strategies targeted at providing the highest value by directing care delivery to areas that improve outcomes and minimize cost. (3-6)
Specialization of care is associated with improved outcomes, reduced resource use, and cost reduction.(7-11) We have shown that neonates requiring congenital heart surgery admitted to a dedicated cardiac intensive care unit (CICU) have lower resource use and lower total hospital costs compared to those admitted to a neonatal intensive care unit (NICU).(12) However, the factors that contribute to these differences in cost remain unclear.
Decomposition analysis, a classic economics approach, recently has been widely adopted in the health disparities literature (13-16), including understanding inequities by socioeconomic status. (17) This statistical approach quantifies the extent whereby differences in an outcome between two groups are explained by differences in covariate levels (characteristic effects) or differences in covariate effects (structural effects). (18, 19) The classic example of a decomposition analysis identified determinants of wage gaps between men and women in the 1950’s. (18) In this analysis, characteristic effects described the difference in wages explained by differing education levels (or other relevant covariates) between men and women. Structural effects described the difference in wages if education levels (or other relevant covariates) between men and women were the same. In other words, the structural effects quantify the effect of “discrimination”, the differences unexplained by differing covariate levels. (18, 19) While typically used in labor market and discrimination literature, the technique can be used to study group differences for any continuous outcome variable. (20) In this case, it allows us to identify factors contributing to costs in each type of intensive care setting. The aim of this study is to identify potential determinants of the difference in cost by location of admission in neonates undergoing congenital heart surgery using decomposition analysis.
Materials and Methods
The Pediatric Health Information Systems (PHIS) database is an administrative database that includes inpatient admissions from 52 pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, KS). Data quality and reliability are assured through a joint effort between the Children’s Hospital Association and participating hospitals. The dataset includes demographics, dates of admission and discharge, vital status at discharge and billing data for medications, laboratory tests, surgical procedures, imaging procedures, clinical services and supplies. Data are de-identified at the time of collection and reviewed for reliability and validity prior to inclusion in the database.(21) For this study, data from all 44 hospitals with data submitted during our study time frame were included. Given the de-identified nature of the data in the PHIS database, the need for Institutional Review Board approval was waived as has been described in prior publications. (12, 22)
Study Population:
Infants undergoing congenital heart surgery at <30 days of age with a RACHS-1 (23) score of 2-6 and discharged from a PHIS hospital between January 2004 and December 2013 were included. Cardiac surgeries in infants <30 days by definition must be classified with a RACHS-1 score of ≥2. Infants initially admitted to any unit other than an ICU were excluded (5%).
Data Collection:
Study variables were gathered from PHIS using available demographic information and International Classification of Diseases, Ninth-Revision (ICD-9) and billing codes. Demographic data included gender, race and ethnicity. Patient characteristics included admission age, admission year, prematurity, and the presence of genetic or major non-cardiac abnormality. Complications (delayed sternal closure, cardiac arrest, medical complications, surgical complications and infections) were collected using a combination of flags defined in the PHIS system and ICD-9 codes. A complete list of the findings flagged as medical complications, surgical complications or infections are in Appendix 1.
Center data included hospital census region and the volume of cardiac cases per year. Volume of cardiac cases was calculated using the total number of neonatal cases reported per hospital divided by the number of years reporting to PHIS. Hospital volume was divided into tertiles for the analyses.
The primary exposure of interest was the initial admission location. As described in our prior publication (12), admission location was classified as CICU, pediatric intensive care unit (PICU) or NICU based on the charges documented on the first day of admission to the pediatric hospital. Given that some specialization of cardiac care occurs in some PICUs, the PICU group was analyzed with the CICU group. As the exposure of initial admission location and resulting care decisions will influence downstream outcomes and resource use, the location of initial admission was treated similar to an “intention to treat” variable in a randomized control trial by assigning patients to their initial admission ICU exposure cohort, as described in our prior publication (12).
The primary outcomes for this study were the characteristic and structural effects, both overall and by individual covariate of interest, attributing to differences in total hospital cost between infants initially admitted to a CICU/PICU versus those admitted to a NICU. Costs were only collected for the index hospitalization. Total and category specific charge data were collected. Data from hospitals missing charge-to-cost ratios (3/44, 7%) were excluded. Total cost was calculated by multiplying total hospital charges per patient by department-specific charge-to-cost ratios. Costs were then adjusted for geographic area and for inflation to 2013 dollars using the Health Care Finance Administration wage/price index and the All Urban Consumers Consumer Price Index. (6, 24)
Covariates were chosen a priori based on hypothesized clinical significance and potential for contributing to a cost difference between the CICU/PICU and NICU. Covariates considered included center volume, age at admission, prematurity, sex, race, genetic or major non-cardiac abnormality, RACHS-1 score, payor, admission year, cardiac arrest, infection, and delayed sternal closure. These covariates were decomposed to explain the contributions of each of these covariates to the difference in cost between the CICU/PICU and NICU. Infection and genetic or major non-cardiac abnormality were defined using flags defined in the PHIS database as described previously (12).
Statistical Analysis:
Descriptive statistics were reported as mean (standard deviation) for continuous variables and as frequency (percentage) for categorical variables. We compared variables between NICU and CICU/PICU by using t-tests and Chi-square tests or Fisher exact test, as appropriate. Decomposition analyses are usually conducted using linear models. (18, 19) However, an alternative approach is necessary when the data of interest are not normally distributed, as is typically the case with healthcare costs. Therefore, we used a recently developed approach for detailed decomposition with non-linear models (25) that explicitly takes into account non-linearity by using a generalized linear model (GLM). Specifically, we applied a GLM with a log link and gamma distribution, similar to previous studies modeling healthcare costs. (26) Standard errors were estimated by bootstrapping with 200 iterations.
Results
Of 19,984 infants who met study criteria, 10,491 (52%) were admitted to the CICU/PICU and 9,493 (48%) were admitted to the NICU. Patients admitted to the NICU (Table 1) were more likely younger in age, admitted at a lower volume hospital, and have a genetic syndrome. They were less likely to have a RACHS-1 score of 6.
Table 1:
The distribution of relevant covariates between the CICU/PICU and NICU admissions
| Covariates | CICU/PICU n=10,491 (52%) |
NICU n=9,493 (48%) |
p-value |
|---|---|---|---|
| Male | 6,268 (60%) | 5,669 (60%) | 0.90 |
| Hospital neonatal cardiac surgery volume per year, mean (SDa) | 83.9 (37.8) | 66.2 (29.2) | <0.001 |
| Age at admission (median, IQRb) | 1 (0,5) | 0 (0,2) | <0.001 |
| RACHS-1 score | <0.001 | ||
| 2 | 2,302 (22%) | 1,979 (21%) | |
| 3 | 3,800 (36%) | 3,505 (37%) | |
| 4 | 2,654 (25%) | 2,620 (28%) | |
| 5 | 65 (1%) | 55 (1%) | |
| 6 | 1,670 (16%) | 1,334 (14%) | |
| Payor | <0.001 | ||
| Government | 4,955 (47.2%) | 4,595 (48.4%) | |
| Private | 3,791 (36.1%) | 3,734 (39.3%) | |
| Other | 1,745 (16.6%) | 1,164 (12.3%) | |
| Race | <0.001 | ||
| White | 5,351 (51.0%) | 4,772 (50.3%) | |
| Black | 1,071 (10.2%) | 859 (9.0%) | |
| Asian | 89 (0.8%) | 134 (1.4%) | |
| American-Indian | 1,594 (15.2%) | 1,451 (15.3%) | |
| Other | 1,847 (17.6%) | 1,852 (19.5%) | |
| Missing | 539 (5.1%) | 425 (4.5%) | |
| Admission year | <0.001 | ||
| 2003 | 55 (0.5%) | 85 (0.9%) | |
| 2004 | 899 (8.6%) | 858 (9.0%) | |
| 2005 | 1,031 (9.8%) | 934 (9.8%) | |
| 2006 | 1,196 (11.4%) | 1,022 (10.8%) | |
| 2007 | 1,113 (10.6%) | 1,097 (11.6%) | |
| 2008 | 1,006 (9.6%) | 1,176 (12.4%) | |
| 2009 | 871 (8.3%) | 1,037 (10.9%) | |
| 2010 | 869 (8.3%) | 967 (10.2%) | |
| 2011 | 1,133 (10.8%) | 875 (9.2%) | |
| 2012 | 1,162 (11.1%) | 756 (8.0%) | |
| 2013 | 1,156 (11.0%) | 686 (7.2%) | |
| Prematurity | 915 (8.7%) | 1,428 (15.0%) | <0.001 |
| Genetic syndrome | 1,150 (11.0%) | 1,366 (14.4%) | <0.001 |
| Infection | 2,973 (28.3%) | 3,678 (38.7%) | <0.001 |
| Delayed sternal closure | 3 (0.03%) | 9 (0.1%) | 0.06 |
| Cardiac arrest | 776 (7%) | 695 (7%) | 0.84 |
SD= standard deviation,
IQR = Interquartile Range
The overall differential in average total hospital cost of those admitted to the CICU/PICU was $24,551 less than those admitted to the NICU (p<0.001). When this difference was decomposed (Figure 1), the aggregate characteristic effect was responsible for a difference of −$30,306 in infants who were admitted to the CICU/PICU compared to those admitted to the NICU (p<0.001). The covariates with differing levels between the CICU/PICU and NICU that contributed to the characteristic difference included prematurity, genetic syndromes, hospital volume, age at admission and rate of infection (Table 2). Differing rates of infection in those admitted to the NICU vs. the CICU/PICU were the largest contributor to differences in characteristic effects.
Figure 1:
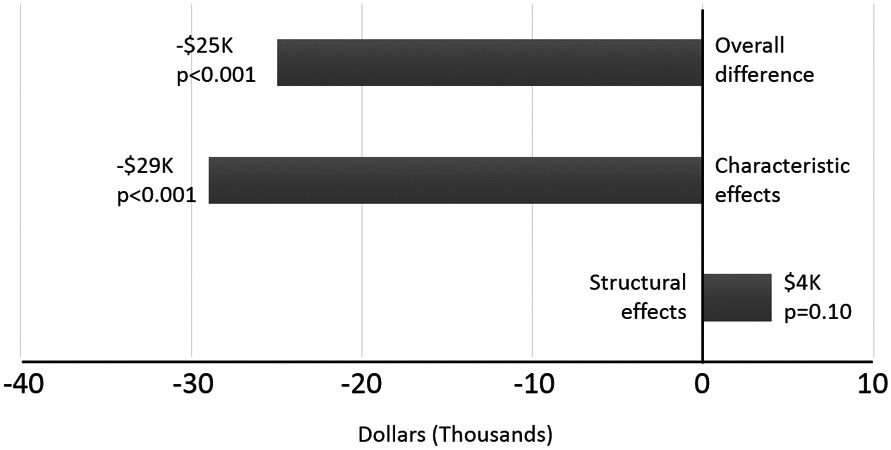
Figure displaying overall, characteristic and structural differences between admissions to the NICU and CICU/PICU. (NICU admission referent)
Table 2:
Characteristic Effects.
| Covariate | Estimated Difference |
SEMa | p-value |
|---|---|---|---|
| Aggregate characteristic effect | −$28,958 | $2,044 | <0.001 |
| Hospital volume per year | −$10,332 | $967 | <0.001 |
| Age at admission (days) | −$4,222 | $662 | <0.001 |
| Prematurity | −$2,326 | $353 | <0.001 |
| Genetic syndrome | −$1,423 | $297 | <0.001 |
| RACHS Score (2 referent) | |||
| 3 | −$220 | $281 | 0.44 |
| 4 | −$1,417 | $487 | 0.004 |
| 5 | $49 | $151 | 0.74 |
| 6 | $1,787 | $509 | <0.001 |
| Infection | −$13,581 | $1,120 | <0.001 |
| Delayed sternal closure | −$26 | $24 | 0.28 |
| Cardiac Arrest | $145 | $456 | 0.75 |
Model adjusted for race, gender, payor, and year of admission.
SEM=standard error of the mean
When the structural effects were examined (covariate levels held constant), there was no difference for those admitted to the CICU/PICU compared those admitted to the NICU (Table 3). However, when the individual covariates were examined, age at admission, prematurity, and RACHS-1 severity categories 3, 4 and 6 were associated with lower costs in the CICU/PICU versus the NICU.
Table 3:
Structural effects.
| Covariate | Estimated difference | SEMa | p-value |
|---|---|---|---|
| Aggregate structural effect | $3,999 | $2,682 | 0.14 |
| Hospital volume per year | $420 | $5,116 | 0.94 |
| Age at admission (days) | −$4,834 | $1,268 | <0.001 |
| Prematurity | −$1,322 | $643 | 0.04 |
| Genetic syndrome | $1,048 | $963 | 0.28 |
| RACHS score (2 referent) | |||
| 3 | $9,915 | $2,097 | <0.001 |
| 4 | $5,610 | $1,753 | 0.001 |
| 5 | −$71 | $247 | 0.77 |
| 6 | $4,693 | $1,399 | 0.001 |
| Infection | −$2,112 | $2,111 | 0.32 |
| Delayed sternal closure | $27 | $45 | 0.55 |
| Cardiac arrest | $276 | $1136 | 0.81 |
Model adjusted for race, gender, payor, and year of admission.
SEM=standard error of the mean
Discussion
This study is the first to identify factors associated with higher costs with admission to the NICU vs. CICU/PICU for infants undergoing congenital heart surgery. Through our novel application of economic analyses, we found that differing levels of covariates (e.g. prematurity, age at admission, and hospital volume, rate of infection) between the CICU/PICU and NICU (characteristic effect) accounted for the majority of the higher costs in the NICU. Overall, when these factors were held constant (structural effect), costs were similar between CICU/PICU and NICU admissions. However, when individual factors were further examined, prematurity and age at admission were associated with higher costs due to structural effects in the NICU. Importantly, differing rates of infection between the units, a modifiable target, was the largest contributor to cost differences.
Our findings are supported by prior studies of costs in children undergoing surgery for CHD. These investigations found that complications and a prolonged length of stay were associated with the highest hospital costs across multiple types of surgery. (27) Similar findings were reported in a study where 28% of the difference in cost between hospitals was explained by differences in LOS and complication rates, suggesting these two factors drive resource use. (27) Our study is the first to identify the difference in infection rates between CICU/PICU versus NICU admissions as the largest contributor to the cost differences seen for neonatal CHD admissions between these units. Since infection rates in patients undergoing congenital heart surgery are high (an estimated 28-38% in our study), targeting strategies that lead to a reduction in the rate of infections in other neonatal populations seems warranted to reduce costs in infants undergoing congenital heart surgery. (28-30)
We identified other covariates responsible for cost differences between the CICU/PICU and NICU that were not easily modifiable. Specifically, prematurity and genetic syndromes were more common in the NICU and contributed to their higher costs. This is not surprising as the association of prematurity and genetic syndromes with higher cost and increased risk of complications in children with CHD has been reported previously. (31-33) Age at admission, center volume and RACHS-1 score may be driven by factors that are also difficult to modify or they may serve as surrogates for problems within the health care system. Interestingly, infants with higher RACHS-1 scores were more likely to be admitted to the CICU/PICU where their care cost less than similar admissions to the NICU.
For characteristic effects, we found higher volume neonatal cardiac surgical centers were associated with lower total hospital costs for those admitted to the CICU/PICU. This supports our prior findings where hospital cardiac surgical volume, location of admission, and total hospital cost were associated with a higher likelihood of being admitted to a CICU and lower total hospital costs. (12) Other studies also reported lower costs in higher volume cardiac centers, particularly for the most complex operations. (3, 6, 27) These investigators suggested that the volume-cost relationship may be mediated by quality of care, with higher volume and lower cost hospitals demonstrating a shorter length of stay, fewer complications, a lower failure to rescue rate, and improved survival. (3, 6, 27, 34) Our findings demonstrated an association of higher volume centers with lower cost, independent of complications such as cardiac arrest and infection but we could not test for unmeasured confounders that were not collected in the PHIS database.
Overall, there was no difference in cost between being admitted to the CICU/PICU vs. the NICU when the specified risk factors were held constant. However, when age at admission and prematurity were held constant, costs were higher in the NICU. It is possible cost structures differ between the CICU/PICU vs. NICU or that the comprehensiveness of their protocols for testing or treatment varied enough to account for the differences in costs. As the ICD-9 code for prematurity does not account for the degree of prematurity, it is also possible that more premature infants were admitted to the NICU contributing, at least in part, to their higher costs.
Limitations:
As with any retrospective study, there is a possibility of uncontrolled confounding. Specifically, we did not have information regarding the physician decision-making process with respect to admission unit, or other measures of quality such as accreditation by professional centers. As birth weight in grams and gestational age in weeks are frequently missing or inaccurate in PHIS, ICD-9-DM codes were used to allow comparisons with previous rigorous studies using administrative data. (35) By using billing codes, we cannot know the severity of the covariates for which we adjusted. For example, there could be a systematic difference in the severity of infections by unit of admission that was unrepresented in this dataset. The level of cardiac specialization within the group billed as PICU is not completely known, and likely represents a mix of general PICUs and dedicated CICUs. (12) We did not have information on care prior to admission or transfer to the PHIS hospital. Since our information was limited to the hospital admission, we could not evaluate the effects of this hospital stay on subsequent outcomes. While PHIS data quality are rigorously confirmed through the Children’s Hospital Association and submitting hospitals, misclassification may exist and cannot be quantified.
Conclusions:
The difference in cost between CICU/PICU and NICU admissions is largely driven by differing prevalence of risk factors between these units. However, the rate of infections, a modifiable factor, accounted for the largest difference in costs between admitting units. Lowering the rate of infection in infants undergoing congenital heart surgery may thus have the added benefit of decreasing total hospital cost in addition to decreasing their comorbidity burden. Further study identifying the specific risk factors for infection in this cohort is warranted.
Supplementary Material
Acknowledgments
Copyright form disclosure: Dr. Nelson disclosed government work. Dr. Sheng’s institution received funding from the National Institutes of Health. Dr. Greene’s institution received funding from CSL, AstraZeneca, and Boehringer-Ingleheim, and he received funding from Durect Corporation and Jannsen Pharmaceuticals. The remaining authors have disclosed that they do not have any potential conflicts of interest
Footnotes
The work was performed at Ann & Robert H. Children’s Hospital of Chicago, and the University of Utah School of Medicine
Article tweet: Differing infection rates contribute most to the cost difference between NICU and CICU/PICU admissions in neonates undergoing heart surgery.
Contributor Information
Joyce T. Johnson, Division of Pediatric Cardiology, Ann & Robert H. Lurie Children’s Hospital of Chicago, Northwestern University Feinberg School of Medicine.
Kirsen L. Sullivan, Department of Statistics, Purdue University.
Richard E. Nelson, Division of Epidemiology, University of Utah School of Medicine.
Xiaoming Sheng, Division of Biostatistics, University of Utah School of Medicine.
Guo Wei, Division of Biostatistics, University of Utah School of Medicine.
Tom H. Greene, Division of Biostatistics, University of Utah School of Medicine.
David K. Bailly, Division of Pediatric Critical Care Medicine, Primary Children’s Hospital, University of Utah School of Medicine.
Aaron W. Eckhauser, Division of Pediatric Cardiovascular Surgery, Primary Children’s Hospital, University of Utah School of Medicine.
Bradley S. Marino, Division of Pediatric Cardiology, Ann & Robert H. Lurie Children’s Hospital of Chicago, Northwestern University Feinberg School of Medicine.
L. LuAnn Minich, Division of Pediatric Cardiology, Primary Children’s Hospital, University of Utah School of Medicine.
Nelangi M. Pinto, Division of Pediatric Cardiology, Primary Children’s Hospital, University of Utah School of Medicine.
References:
- 1.Russo CA and Elixhauser A. Hospitalizations for birth defects, 2004 Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]: Agency for Healthcare Research and Quality (US); 2007. [PubMed] [Google Scholar]
- 2.Simeone RM, Oster ME, Cassell CH, et al. Pediatric inpatient hospital resource use for congenital heart defects. Birth Defects Res A Clin Mol Teratol. 2014;100:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasquali SK, Jacobs JP, Bove EL, et al. Quality-cost relationship in congenital heart surgery. Ann Thorac Surg. 2015;100:1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connor JA, Gauvreau K and Jenkins KJ. Factors associated with increased resource utilization for congenital heart disease. Pediatrics. 2005;116:689–695. [DOI] [PubMed] [Google Scholar]
- 5.Smith AH, Gay JC and Patel NR. Trends in resource utilization associated with the inpatient treatment of neonatal congenital heart disease. Congenit Heart Dis. 2014;9:96–105. [DOI] [PubMed] [Google Scholar]
- 6.Chan T, Kim J, Minich LL, et al. Surgical volume, hospital quality, and hospitalization cost in congenital heart surgery in the United States. Pediatr Cardiol. 2015;36:205–213. [DOI] [PubMed] [Google Scholar]
- 7.Kulaylat AS, Pappou E, Philp MM, et al. Emergent Colon Resections: Does Surgeon Specialization Influence Outcomes? Dis Colon Rectum. 2019;62:79–87. [DOI] [PubMed] [Google Scholar]
- 8.Lee DS, Stukel TA, Austin PC, et al. Improved outcomes with early collaborative care of ambulatory heart failure patients discharged from the emergency department. Circulation. 2010;122:1806–1814. [DOI] [PubMed] [Google Scholar]
- 9.Watkins AC, Ghoreishi M, Maassel NL, et al. Programmatic and surgeon specialization improves mortality in isolated coronary bypass grafting. Ann Thorac Surg. 2018;106:1150–1158. [DOI] [PubMed] [Google Scholar]
- 10.Slattery E and Harewood GC. Specialty-specific admission: a cost-effective intervention? Ir J Med Sci. 2012;181:87–91. [DOI] [PubMed] [Google Scholar]
- 11.Eastaugh SR. Hospital specialization and cost efficiency: benefits of trimming product lines. Hosp Health Serv Adm. 1992;37:223–35. [PubMed] [Google Scholar]
- 12.Johnson JT, Wilkes JF, Menon SC, et al. Admission to dedicated pediatric cardiac intensive care units is associated with decreased resource use in neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2018;155:2606–2614. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson JW and VanderWeele TJ. Decomposition analysis to identify intervention targets for reducing disparities. Epidemiology. 2018;29:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isong IA, Rao SR, Bind M-A, et al. Racial and Ethnic Disparities in Early Childhood Obesity. Pediatrics. 2018;141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taber DR, Robinson WR, Bleich SN, et al. Deconstructing race and gender differences in adolescent obesity: Oaxaca-blinder decomposition. Obesity. 2016;24:719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo B-K, Hasebe T and Szilagyi PG. Decomposing racial/ethnic disparities in influenza vaccination among the elderly. Vaccine. 2015;33:2997–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'donnell O, Van Doorslaer E, Wagstaff , et al. Analyzing health equity using household survey data: a guide to techniques and their implementation: The World Bank; 2007. [Google Scholar]
- 18.Oaxaca R Male-Female Wage Differentials in Urban Labor Markets. Int'l Econ Rev. 1973;693:2525981693. [Google Scholar]
- 19.Blinder AS. Wage discrimination: reduced form and structural estimates. J Hum Resour. 1973:436–455. [Google Scholar]
- 20.Jann B A Stata implementation of the Blinder-Oaxaca decomposition. Stata J. 2008;8:453–479. [Google Scholar]
- 21.Children’s Hospital Association. Pediatric Health Information Systems (PHIS), 2014. Available at: https://www.childrenshospitals.org/programs-and-services/data-analytics-and-research/pediatric-analytic-solutions/pediatric-health-information-system. Accessed May 30, 2014.
- 22.Johnson JT, Marino BS, Klugman D, et al. National variation in the use of tracheostomy in patients with congenital heart disease. Pediatr Crit Care Med. 2017;18:958–964. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins KJ, Gauvreau K, Newburger JW, et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–8. [DOI] [PubMed] [Google Scholar]
- 24.Keren R, Luan X, Localio R, et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166:1155–1164. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser B Detailed decompositions in nonlinear models. Appl Econ Lett. 2015;22:25–29. [Google Scholar]
- 26.Mihaylova B, Briggs A, O'Hagan A, et al. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2011;20:897–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasquali SK, He X, Jacobs ML, et al. Excess costs associated with complications and prolonged length of stay after congenital heart surgery. Ann Thorac Surg. 2014;98:1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher D, Cochran KM, Provost LP, et al. Reducing central line–associated bloodstream infections in North Carolina NICUs. Pediatrics. 2013;132:e1664–e1671. [DOI] [PubMed] [Google Scholar]
- 29.Wilder KA, Wall B, Haggard D, et al. CLABSI Reduction Strategy. Adv Neonatal Care. 2016;16:170–177. [DOI] [PubMed] [Google Scholar]
- 30.Turcotte RF, Brozovich A, Corda R, et al. Health care-associated infections in children after cardiac surgery. Pediatr Cardiol. 2014;35:1448–1455. [DOI] [PubMed] [Google Scholar]
- 31.Alsoufi B, Gillespie S, Mahle WT, et al. The effect of noncardiac and genetic abnormalities on outcomes following neonatal congenital heart surgery. Semin Thorac Cardiovasc Surg. 2016;28:105–114. [DOI] [PubMed] [Google Scholar]
- 32.Furlong-Dillard J, Bailly D, Amula V, et al. Resource use and morbidities in pediatric cardiac surgery patients with genetic conditions. J Pediatr. 2018;193:139–146. e1. [DOI] [PubMed] [Google Scholar]
- 33.Costello JM, Pasquali SK, Jacobs JP, et al. Gestational age at birth and outcomes after neonatal cardiac surgery: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Circulation. 2014;129:2511–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasquali SK, He X, Jacobs JP, et al. Evaluation of failure to rescue as a quality metric in pediatric heart surgery: an analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg. 2012;94:573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lillehei CW, Gauvreau K and Jenkins KJ. Risk adjustment for neonatal surgery: a method for comparison of in-hospital mortality. Pediatrics. 2012;130:e568–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


