Abstract
Heterotopic pregnancy (HP) is the coexistence of extrauterine and intrauterine pregnancies. This case is rare, difficult to diagnose, and threatening if left untreated. Incidental rate is estimated 1 in 30,000 spontaneous pregnancies and higher in assisted reproductive techniques. HP is often missed because of the detection of intrauterine sacs; therefore, comprehensive and systematic ultrasonography (USG) is needed, especially when there is ectopic pregnancy suspicion or when there is free fluid in the pelvis. A 46-year nulligravida with 13-year primary infertility history underwent frozen embryo transfer process with positive beta-human chorionic gonadotropin 2 weeks after the procedure. Clinical pregnancy is expressed by gestational sac findings at 6-week gestation. Two weeks later, she complained of lower right abdominal pain accompanied by spots from the birth canal. USG showed intrauterine pregnancy and sac appropriate to 8-week gestation and adnexal mass accompanied by a ring of fire image. The patient underwent right salpingectomy, recovered well, and continued her pregnancy. In vitro fertilization is the main risk factor for multiple and ectopic pregnancies. Clinical manifestations are similar to pregnancy loss or ectopic pregnancy. Specific risk factor must be acknowledged by the physician prior initial examination to rule out HP. Transvaginal ultrasound is useful in making the diagnosis of HP, especially in early pregnancy.
Keywords: Heterotopic, laparoscopy, ultrasound
INTRODUCTION
Heterotopic pregnancy (HP) is a condition where intrauterine gestational sac coexists with extrauterine gestational sac. This condition is believed to happen as a result of dizygotic implantation in different locations. In the modern assisted reproductive technology (ART) era, the incidence of HP increased from 1 of 30,000 pregnancy cases to 1 of 3900 pregnancy cases. HP can cause life-threatening condition; therefore, doctors should be able to quickly and appropriately make diagnosis and give treatments.[1,2]
CASE REPORT
A 46-year-old nulligravida with 13 years of primary infertility history underwent in vitro fertilization (IVF) process in Yasmin Fertility Clinic, RSCM Kencana. From all examinations, there was no significant problem from both the woman and her spouse. Ovum pickup was done, resulted in 11 oocytes and six embryos through intracytoplasmic sperm injection process. The first IVF trial was done toward three transfer embryos. Biochemical pregnancy happened but then failed because beta-human chorionic gonadotropin (β-HCG) level decreased. The second trial was done through frozen embryo trial (FET), resulted in positive β-HCG and progesterone level (1023.20 mIU/mL and 9.550 ng/mL each) 2 weeks after FET. Clinical pregnancy was announced at 6-week pregnancy, with one intrauterine gestational sac without free fluid or other adnexal mass at the moment [Figure 1].
Figure 1.
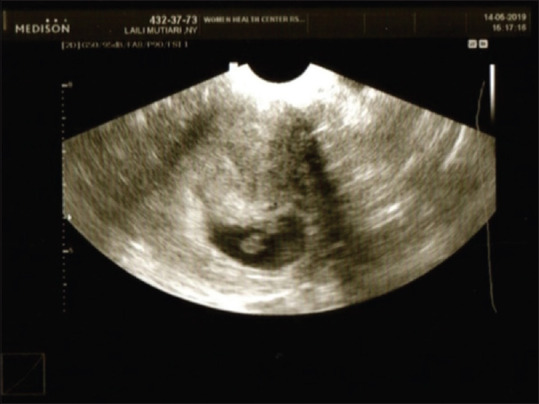
Gestational sac at 6-week pregnancy result of transvaginal ultrasound picture shows gestational sac appropriate to 6-week pregnancy, without free fluid or other mass
The patient came back with abdominal pain in the right lower quadrant 2 weeks later. Through transvaginal ultrasound (TVUS), one intrauterine gestational sac appropriate to 8-week pregnancy with positive heartbeat was found, along with a mass in the right adnexa with the impression of gestational sac and free fluid in pelvic cavity to Douglas pouch [Figure 2].
Figure 2.
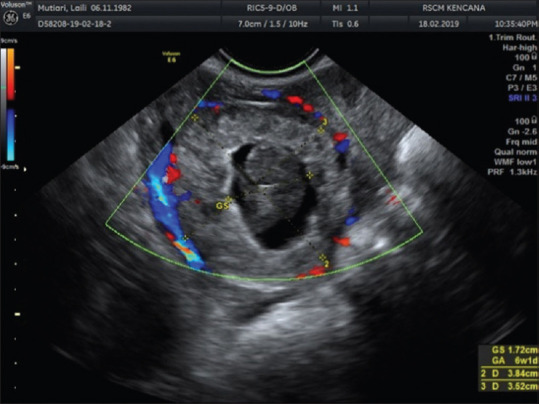
Gestational sac in the right adnexa gestational sac appropriate to 8-week pregnancy along with hypoechoic image around it which shows free fluid existence in adnexa
HP was diagnosed, and salpingectomy-laparoscopy was decided to be done. Intraoperative findings found a smooth-surfaced mass, 3 cm in diameter, coming from the right tube ampulla with active bleeding, corresponding to an ectopic pregnancy [Figure 3]. The base of the right tube, mesovarium, to the fimbriae was clamped, coagulated, and cut, and then confirmation of no more active bleeding was done [Figure 4]. The patient is hospitalized for 2 days without any complication. The intrauterine pregnancy was maintained, and the patient is continuing her pregnancy in a good condition.
Figure 3.
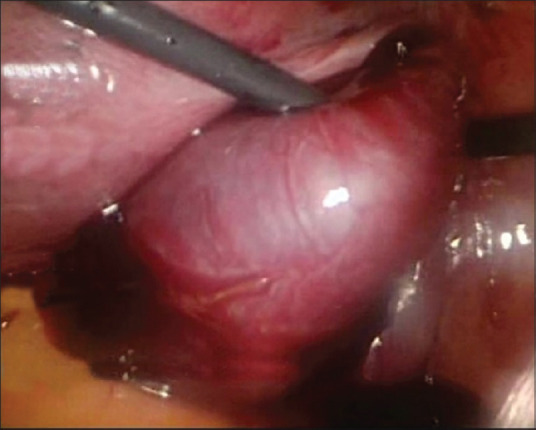
Smooth-surfaced mass in the ampulla of the right tube; a mass, 3 cm in diameter, with smooth surface in pars ampulla of the right tube, along with active bleeding which gives the impression of an ectopic pregnancy sac mass
Figure 4.
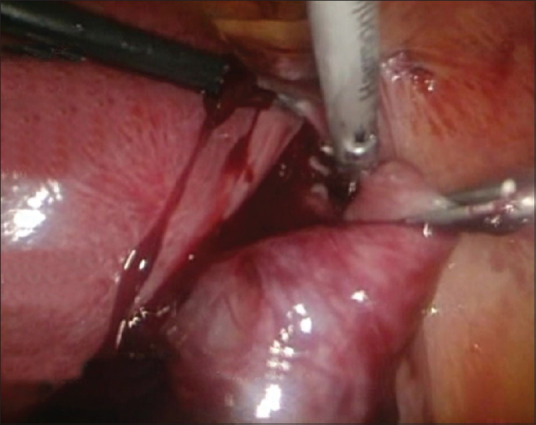
Right salpingectomy was done; proximal of the right fallopian tube, mesovarium, to the fimbriae was clamped, coagulated, and cut
DISCUSSION
HP is estimated in 1 of 30,000 pregnancies. With the rise of ART including superovulation, intrauterine insemination, and IVF, the incidence increased to approximately 1 of 3900 pregnancies. Data analysis in the United States of America in 1999–2002 reported the incidence in 1.5/1000 pregnancies with ART.[1,2,3]
The most significant factor is the high incidence of twin pregnancy after fertility treatment, with an average of 5%–10%, 10%–30%, and 35% following citrate clomiphene, gonadotropin, and IVF. Glassner reported two cases of HP in patients who used citrate clomiphene and concluded that the incidence of HP was 1 of 900 pregnancies. Berger and Taymor explained two cases; one followed citrate clomiphene therapy and the other followed gonadotropin therapy; both went through laparotomy-salpingectomy due to ruptured ectopic pregnancy while both intrauterine pregnancies were maintained until term. IVF is the main risk factor for multiple and ectopic pregnancies, especially when it is for mechanical infertility problem, where a tubal abnormality is an independent risk factor for ectopic pregnancy.[1,2,3]
Pelvic or abdominal pain almost always exists, unilaterally or bilaterally, localized or spread; they can also coexist with subdiaphragm or shoulder pain depends on the existence of intra-abdominal bleeding. Abnormal uterine bleeding usually comes in the form of spotting, and in 75% of cases, caused by the escape of some decidual parts. Secondary amenorrhea does not always happen, and 50% of ectopic pregnancy patients had complained about spotting in their expected menstruation date, so suspicion of being pregnant is almost nonsexist. Nearly 5%–10% of ectopic pregnancy cases expel “decidual cast,” which is very similar to conception result. Overall or localized tense feeling happens in 80% of cases, along with cervical pain and adnexal tensing in 75% of ectopic pregnancy cases. Unilateral mass in adnexa can be palpated in 50% of cases. Two weeks before hospital admittance, the patient complained of spotting significant (one time per day); TVUS was only to confirm intrauterine pregnancy. There is no hypovolemic shock in our patient; therefore, laparoscopy is more preferable.[4]
HP sign is the existence of complex adnexal mass or fluid in the pelvic area. Clinician usually ignores the existence of mass in adnexa after visualizing intrauterine pregnancy. Ectopic pregnancy can be similar to lutein corpus, but in further stage, fetal pole and yolk sac exist with heart activities, which make diagnosis easier. Free fluid in abdomen may be a sign of ruptured tube, but ascites may still be associated with ovarian hyperstimulation syndrome. In our case, findings of adnexal mass similar to gestational sac with fetal echo inside were found when the patient came back with right abdominal pain, so the diagnosis of HP is made.[5]
TVUS role is useful in making the diagnosis of HP, especially in early pregnancy. In 5–6-week pregnancy, the sensitivity of the tool is only 56%. If the pregnancy is <6 weeks, diagnosis can be seen from the existence of activity signs from fetal heart pulse. The confound in TVUS examination is that adnexal sac can be seen like bleeding in luteum corpus or an ovarian cyst and the limited amount of free fluid in pelvic made detection by ultrasonography (USG) more difficult in the first trimester of a patient going through IVF.[6,7] The presence of adnexal mass along with intrauterine pregnancy can cause an error in diagnosis, mistaking it for corpus luteum cyst, and visualization of adnexal mass is not possible until the mass size is around 2 cm, around 7 weeks of gestation, or detected through pulsation from embryonic pole.[7] Doppler USG may be useful in scanning adnexal mass suspicion. With high velocity, the low resistance of Doppler signal, which is associated with developing trophoblast, the increase of trophoblast flow, shows “ring of fire” image. Identification of this type is that the pattern of flow in adnexal mass increases the sensitivity of ectopic pregnancy diagnosis from 53% to 73% with transabdominal USG. Colored Doppler from TVUS increases the sensitivity and specificity to 96% and 93%.[7,8]
Unlike in ectopic pregnancy, where failure to visualize intrauterine pregnancy when β-hCG level reached 1700 mIU/mL is a strong indicator, in HP, β-hCG level reflects the presence of normal intrauterine pregnancy and associated with gestational age.
Case from Yu et al. shows that 44% of ectopic pregnancies in HP were not detected at the initial ultrasound examination and were diagnosed on repeated examination. This study also suggests an initial examination is well performed in 6-7 weeks of gestational age.[9] Lv et al. also identified specific risk factor, for example, previous tubal surgery must be acknowledged by physicians in fertility setting.[10]
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This study was approved by the institutional review board of Faculty of Medicine Universitas Indonesia no. ND-1238/UN2.F1/ETIK/PPM.00.02/2019 on 7th October 2019 that this case report can be done without any Ethical Approval as long as the author can disclose patient anonymity and other patient's data.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tal J, Haddad S, Gordon N, Timor-Tritsch I. Heterotopic pregnancy after ovulation induction and assisted reproductive technologies: A literature review from 1971 to 1993. Fertil Steril. 1996;66:1–2. doi: 10.1016/s0015-0282(16)58378-2. [DOI] [PubMed] [Google Scholar]
- 2.Flisser E, Licciardi F. Subchorionic hematoma associated with heterotopic pregnancy following in vitro fertilization: A case report. J Reprod Med. 2006;51:503–6. [PubMed] [Google Scholar]
- 3.Clayton HB, Schieve LA, Peterson HB, Jamieson DJ, Reynolds MA, Wright VC. A comparison of heterotopic and intrauterine-only pregnancy outcomes after assisted reproductive technologies in the United States from 1999 to 2002. Fertil Steril. 2007;87:303–9. doi: 10.1016/j.fertnstert.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 4.Cirillo D, Di Spiezio Sardo A, Cirillo P, Pellicano M, Guida M, Nappi C. Conservative laparoscopic treatment of heterotopic ovarian and intrauterine pregnancy following ovulation induction with gonadotropins. Acta Obstet Gynecol Scand. 2006;85:1269–71. doi: 10.1080/00016340600613550. [DOI] [PubMed] [Google Scholar]
- 5.Brunette DD, Roline C. Heterotopic pregnancy resulting from in vitro fertilization. Am J Emerg Med. 2011;29:960, e1–2. doi: 10.1016/j.ajem.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Wen H, Xu D, Chen LQ, He J. Management and pregnancy outcomes of heterotopic pregnancy. Zhonghua Fu Chan Ke Za Zhi. 2018;53:768–75. doi: 10.3760/cma.j.issn.0529-567x.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Singhal M, Ahuja CK, Saxena AK, Dhaliwal L, Khandelwal N. Sonographic appearance of heterotopic pregnancy with ruptured ectopic tubal pregnancy. J Clin Ultrasound. 2010;38:509–11. doi: 10.1002/jcu.20715. [DOI] [PubMed] [Google Scholar]
- 8.Melendez J, Paraskevopolou SM, Foo X, Yoong W. Heterotopic pregnancy: Tubal ectopic pregnancy with a viable IVF intrauterine pregnancy. J Obstet Gynaecol. 2010;30:742–3. doi: 10.3109/01443615.2010.501414. [DOI] [PubMed] [Google Scholar]
- 9.Yu Y, Xu W, Xie Z, Huang Q, Li S. Management and outcome of 25 heterotopic pregnancies in Zhejiang, China. Eur J Obstet Gynecol Reprod Biol. 2014;180:157–61. doi: 10.1016/j.ejogrb.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 10.Lv S, Wang Z, Liu H, Peng J, Song J, Liu W, et al. Management strategies of heterotopic pregnancy following in vitro fertilization-embryo transfer. Taiwan J Obstet Gynecol. 2020;59:67–72. doi: 10.1016/j.tjog.2019.11.010. [DOI] [PubMed] [Google Scholar]


