The third coronavirus outbreak in the past 20 years, the SARS-CoV-2 pandemic has caused unprecedented morbidity, mortality, and economic disruption. Safe, effective, and deployable SARS-CoV-2 vaccines are urgently needed to mitigate the consequences of the pandemic and protect from future outbreaks. The accelerated response to COVID-19 includes investments in preclinical and clinical testing and manufacture of multiple vaccine candidates, with efficacy trials in the United States anticipated to start in July.
Controlled human infection models (CHIMs) have been proposed as a strategy for accelerating SARS-CoV-2 vaccine development. Commentaries have focused on the ethical considerations raised by such models, noting their societal benefits and articulating a range of opinions regarding whether the risk is justified.1–3 The controversy they consider hinges on assumptions about the risk posed by purposeful infection (challenge) of humans with SARS-CoV-2 in a world that lacks a reliable treatment for COVID-19. Both the public health imperative that drives the push for a SARS-CoV-2 CHIM and the demand that the risk be justifiable require a model capable of addressing meritorious questions and delivering scientifically sound answers. On behalf of the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) Vaccines Working Group, we have focused on the practical considerations relevant to the development of a SARS-CoV-2 CHIM and the prerequisites for employing such a model.
Traditional vaccine development progresses from preclinical to clinical phases and then to vaccine licensure and production at scale. Current efforts shorten development timelines by compressing and overlapping the stages, accelerating the transition between clinical phases, powering efficacy studies to yield results in a short time frame, and pursuing large-scale manufacture of vaccines before regulatory approval (see diagram). Although the large field trials involved are resource-intensive, they represent the gold standard for evaluation of vaccine efficacy. Participants are exposed to the pathogen in a natural setting, and heterogenous populations at greatest risk for disease acquisition or serious outcomes may be included.
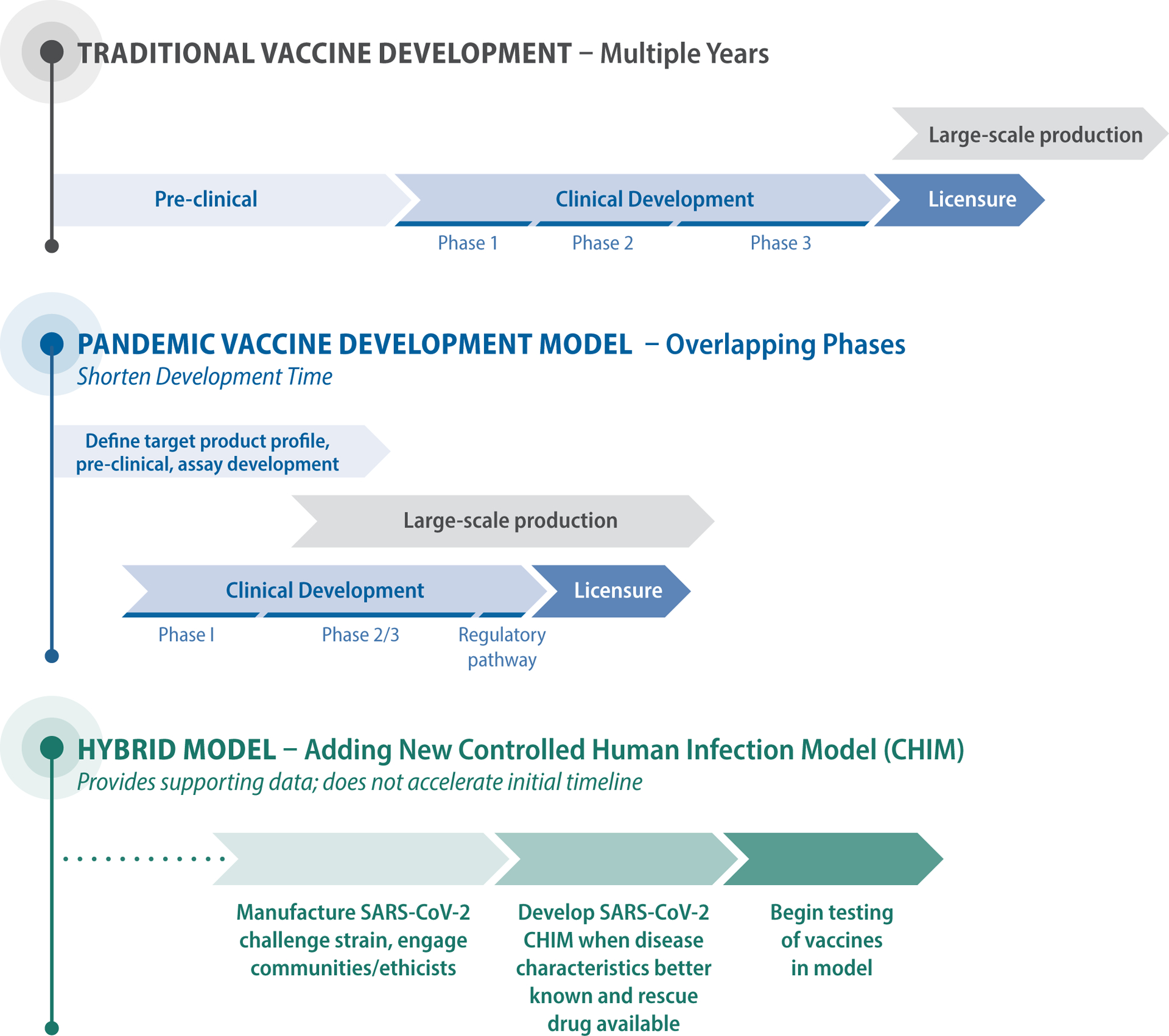
Vaccine Development Models for SARS-CoV-2 Vaccines.
Steps and timelines are shown for traditional and pandemic models of vaccine development. The hybrid approach reflects the addition of a controlled human infection model (CHIM), illustrating the later start date and steps necessary before vaccine testing could begin.
CHIMs, by contrast, require infecting healthy persons with a well-characterized microorganism in order to study pathogenesis, characterize the immune response, and elucidate the efficacy of vaccines or therapeutics. CHIMs can minimize the uncertainty about exposure or disease acquisition inherent in field trials, thereby reducing the number of participants needed to establish the desired end point (e.g., sterilizing immunity, reduced susceptibility to infection, or attenuated disease). Researchers in these studies must ensure that procedures minimizing participant, staff, and community risk are strictly enforced and that CHIMs are rigorously designed to provide scientifically robust data. A key requirement is the preparation of a Good Manufacturing Practice (GMP) challenge “stock” with demonstrated stability and consistent infectivity. This process may take as little as a month for a well-characterized pathogen, or a year if attenuating mutations are introduced to minimize participant risk. Precise preparation and administration of the GMP inoculum; rigorous infection-control procedures; careful participant selection; adherence to regulatory, public health, and ethical guidelines; and close safety monitoring are critical components of the research effort. Even with strict facility engineering controls, stringent discharge criteria, and experienced personnel, there is a potential risk of community spread of the challenge virus. Thus, CHIMs require active community engagement throughout the project.3
By design, the controlled nature of CHIMs limit their generalizability for predicting the effectiveness of a vaccine candidate against natural exposure. Concerns about the generalizability and utility of CHIMs are magnified when disease manifestations vary with patients’ age or coexisting conditions. A model of disease in healthy young volunteers may have questionable scientific validity when extrapolated to older or other at-risk populations that have disproportionate morbidity. Moreover, correlates of protection from SARS-CoV-2 are poorly understood, and may vary with the population or the vaccine construct.
The World Health Organization (WHO) has set forth essential criteria for conducting SARS-CoV-2 challenge studies.4 Central feasibility criteria for a CHIM include: minimizing risk to participants, staff, and the community, and robust scientific and clinical standards. Virtually all recent CHIMs have involved microorganisms that either pose minimal risk for causing severe disease in the enrolled population, have effective oral treatments, or both. Currently, we lack sufficient knowledge of SARS-CoV-2 pathogenesis to inform inclusion and exclusion criteria for a SARS-CoV-2 CHIM. A single death or severe illness in an otherwise healthy volunteer would be unconscionable, and could halt progress. A challenge virus with potentially attenuating mutations may mitigate that risk, though such modification (e.g., by site-directed mutagenesis, codon deoptimization, or serial passage) would increase development time. Critically, a rescue therapy should be available, since even well-established CHIMs have resulted in unexpected severe illness.
Institutions conducting CHIM studies should have Biosafety Level 3 laboratories for handling the virus, appropriate airborne-infection isolation rooms, and access to intensive care facilities. Multiple rounds of challenge are necessary to determine the optimal route of inoculation, the appropriate challenge dose, and clinical and virologic characteristics. Before any vaccine intervention can be tested, a dosing strategy must be found that causes predictable infection with minimal disease severity. Since each challenge round would require an estimated 3 weeks for infection and recovery of participants, with an additional week for facility decontamination and analysis, the dose-escalation period is lengthy. Thus, the development of a robust challenge model for testing SARS-CoV-2 vaccines may be 1 to 2 years. Given that SARS-CoV-2 vaccines will enter Phase 3 trials imminently, these scientific and technical factors alone make CHIMs unlikely to accelerate the establishment of vaccine efficacy.
Though SARS-CoV-2 CHIM development will be laborious, it would mitigate risk if vaccine efficacy studies take longer than expected – for example, because of lower-than-anticipated disease attack rates. The experimental control provided by CHIMs has distinct advantages over field studies for discerning correlates of protection, given the precise timing of infection and the ability to measure immune responses at early and predetermined time points. In addition, SARS-CoV-2 CHIMs could determine the duration of immunity conferred by vaccines undergoing field trials. Development of a SARS-CoV-2 GMP stock, preferably with predicted attenuating mutations, could proceed while appropriate facilities and standard operating procedures are prepared for SARS-CoV-2 CHIMs. Investigators at potential sites should begin soon to engage stakeholders in the scientific, regulatory, public health, and local communities.
In parallel, the development of seasonal coronavirus CHIMs should proceed. Although coronavirus 229E CHIMs were developed beginning in 1967, immunologic characterization was limited to characterizing the presence and kinetics of neutralizing antibodies.5 More comprehensive models could be developed. None of the seasonal coronaviruses cause illness as severe as SARS-CoV, MERS-CoV, and SARS-CoV-2, so challenge studies have favorable risk profiles even if no rescue therapy is available. Proof-of concept development of a seasonal coronavirus CHIM would provide the foundation for characterizing correlates of protection, host mediators of susceptibility, durability of immunity, and any protective or enhancing consequences of sequential heterologous coronavirus infections. These detailed characterizations may provide essential insights into SARS-CoV-2 infections, and seasonal coronavirus CHIM development would optimize administration of challenge virus, timing of sample collection, and analysis strategies during preparation of a SARS-CoV-2 challenge strain.
Large randomized, controlled trials of SARS-CoV-2 vaccines are currently the most efficient, generalizable, and scientifically robust path to establishing vaccine efficacy. SARS-CoV-2 CHIM development might be able to accelerate development of later rounds of vaccine candidates. But a SARS-CoV-2 CHIM could also address essential questions of SARS-CoV-2 immunopathogenesis, duration of vaccine-induced immunity, and correlates of protection in healthy populations. Parallel development of a potentially attenuated SARS-CoV-2 GMP virus, development of a seasonal coronavirus CHIM, and preparation for a SARS-CoV-2 CHIM would represent a broad and sustained research effort toward understanding coronavirus biology and mitigating current and future pandemics.
Acknowledgments
The authors are members of the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) Vaccines Working Group, a public-private partnership sponsored by the National Institutes of Health; its other members are: Paula Annunziato, M.D., Ann Arvin, M.D., Beth Bell, M.D., Susan Buchbinder, M.D., Marco Cavaleri, Ph.D., Lawrence Corey, M.D., Mark Davis, Ph.D., Emilio Emini, Ph.D., Gregory M. Glenn, M.D., Emmanuel Hanon, Ph.D., Barton Haynes, M.D., Peter Hotez, M.D., Ph.D., Kathrin Jansen, Ph.D. (Co-Chair), Antonio Lanzavecchia, M.D., Douglas R. Lowy, M.D. (Co-Chair), Peter Marks, M.D., Ph.D., John Mascola, M.D., Nancy Messonier, M.D., Nelson Michael, M.D., Ph.D., Paul Offit, M.D., Hanneke Schuitemaker, Ph.D., Jonathan Seals, Ph,.D., Jim Tartaglia, Ph.D., and Tal Zaks, M.D., Ph.D.
Footnotes
Disclosure forms provided by the authors are available at NEJM.org.
The views expressed in this article are those of the authors and do not necessarily reflect those of the Department of the Army, the Department of Defense, or the University of Maryland.
References
- 1.Eyal N, Lipsitch M, Smith PG. Human challenge studies to accelerate coronavirus vaccine licensure. J Infect Dis 2020;221(11):1752–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah SK, Miller FG, Darton TC, et al. Ethics of controlled human infection to study COVID-19. Science (80- ) 2020;1076:2016–20. [DOI] [PubMed] [Google Scholar]
- 3.Plotkin SA. Extraordinary diseases require extraordinary solutions. Vaccine 2020;38:3987–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Advisory Group. Feasibility, Potential Value and Limitations of Establishing a Closely Monitored Challenge Model of Experimental COVID-19 Infection and Illness in Healthy Young Adult Volunteers. Geneva: World Health Organization; 2020. [Google Scholar]
- 5.Bradburne AF, Bynoe ML, Tyrrell DAJ. Effects of a “new” human respiratory virus in volunteers. Br Med J 1967;3(5568):767–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


