Abstract
Background
Silexan is a lavender essential oil with established anxiolytic and calming efficacy. Here we asked whether there is a potential for abuse in human patients.
Methods
We carried out a phase I abuse liability single-center, double-blind, 5-way crossover study in healthy users of recreational central nervous system depressants. They received single oral doses of 80 mg (therapeutic dose) and 640 mg Silexan, 2 mg and 4 mg lorazepam (active control) and placebo in randomized order, with 4- to 14-day washout periods between treatments. Pharmacodynamic measures included validated visual analogue scales assessing positive, negative, and sedative drug effects and balance of effects; a short form of the Addiction Research Center Inventory; and a drug similarity assessment. The primary outcome measure was the individual maximum value on the drug liking visual analogue scale during 24 hours post-dose.
Results
Forty participants were randomized and 34 were evaluable for pharmacodynamic outcomes. In intraindividual head-to-head comparisons of the drug liking visual analogue scale maximum value, both doses of Silexan were rated similar to placebo whereas differences were observed between Silexan and lorazepam and between placebo and lorazepam (P < .001). These data were supported by all secondary measures of positive drug effects and of balance of effects. Differences between placebo and both doses of Silexan were always negligible in magnitude. Moreover, Silexan showed no sedative effects and was not perceived to be similar to commonly used drugs that participants had used in the past.
Conclusions
Silexan did not exhibit any abuse potential in a standard abuse potential detection screen study and is unlikely to be recreationally abused.
Keywords: Silexan, lavender oil, anxiolytic drug, abuse potential assessment, recreational drug users
Significance Statement.
Drugs for the treatment of anxiety disorders affect the central nervous system (CNS) and are therefore generally suspected to have a potential for abuse. This double-blind study compared the abuse liability of Silexan, an anxiolytic agent from lavender oil, to those of placebo and lorazepam, a tranquilizer with known abuse and addictive potential. The participants were non-dependent, recreational drug users with known CNS depressant abuse, who received 1 dose of each study treatment in randomized order. After each administration, participants completed an inventory of rating scales to describe the subjective effects of the drugs and their similarity to other drugs they had personally used before. Overall, the effects of Silexan were similar to those of placebo while important differences were observed between Silexan and lorazepam on the one hand and lorazepam and placebo on the other. The results indicate that Silexan does not exhibit abuse potential and is very unlikely to be recreationally abused.
Introduction
Silexan is a proprietary essential oil from Lavandula angustifolia complying with the monograph Lavender oil of the Ph. Eur. (Ph. Eur.; Council of Europe, 2015). It exceeds the quality definition of the Ph. Eur. Silexan has been approved in Germany at a daily dose of 1 × 80 mg as a medicinal product for the treatment of restlessness related to anxious moods.
A comprehensive characterization of the pharmacological profile of Silexan has been published (Müller et al., 2015; Kasper et al., 2018). In summary, Silexan causes a potent inhibition of voltage dependent calcium channels in synaptosomes, primary hippocampal neurons, and stably overexpressing cell lines (Schuwald et al., 2013), which leads to an attenuation of the overreaching, situationally inadequate stress response of the central nervous system (CNS) associated with anxiety and mood disorders (Satpute et al., 2012). Silexan also significantly reduces the 5-hydroxy-tryptamine (5-HT)1A binding potential in several brain clusters, which may lead to an increase of extracellular serotonin levels (Baldinger et al., 2014). Moreover, a recently completed preclinical assay shows that lavender essential oil has a significant, dose-dependent effect on the serotonin transporter and a dose-dependent affinity to the glutamate n-methyl-D-aspartate receptor, which may contribute to the anxiolytic and calming effects of lavender, whereas no affinity for the gamma-aminobutyric acid (GABA)A-benzodiazepine receptor was observed. Randomized, double-blind–controlled clinical trials have demonstrated that Silexan has a significant anxiolytic effect in conditions such as generalized anxiety disorder (GAD), subthreshold anxiety disorder, mixed anxiety and depressive disorder, and anxiety-related restlessness and agitation (Kasper et al., 2016, 2017; Generoso et al., 2017; Möller et al., 2019).
In controlled clinical trials, adverse events (AEs) observed more frequently during exposure to Silexan compared with patients treated with placebo were mainly limited to gastrointestinal disturbances and allergic skin reactions, while the drug has not been associated with increased rates of unwanted CNS effects compared with placebo (Kasper et al., 2017, 2018). Moreover, despite its efficacy as an anxiolytic drug, no sedative effects of Silexan were observed (Kumar, 2013; Silenieks et al., 2013; Müller et al., 2015; Kasper et al., 2018), and even when abruptly discontinued, Silexan did not cause any withdrawal syndromes (Gastpar et al., 2017).
Following speculations based on the clinically observed anti-agitation properties of L. angustifolia essential oils, Huang and colleagues (2008) performed an in vitro investigation to test whether these effects might be attributable to a benzodiazepine-like action on GABAA receptors. The authors concluded, however, that observed anti-agitation and CNS depressant effects were unlikely to reflect a sedative interaction with any of the ionotropic receptors examined in their study. Moreover, results from a pre-clinical abuse liability study also demonstrate that Silexan is not recognized as benzodiazepine-like by Sprague-Dawley rats trained to discriminate a diazepam cue (Silenieks et al., 2013).
Even though the study performed by Silenieks et al. (2013) did not show any signal indicative of an interoceptive property or abuse potential in an animal model, the pharmacological profile of Silexan as well as clinical data suggest that the drug has effects on the CNS. Therefore, a human abuse liability study was performed to investigate whether there is potential for abuse in humans in accordance with the FDA Draft Guidance for Industry: Assessment of Abuse Potential of Drugs (Food and Drug Administration, 2010) that applied when the trial was performed.
Methods
Objective and Design
This study was a randomized, double-blind, double-dummy, placebo and positive controlled, single center, phase I, 5-way crossover study performed to evaluate whether there is an abuse potential of single oral doses of Silexan compared with the benzodiazepine lorazepam and placebo in healthy, non-dependent, recreational CNS depressant users.
The positive control in an abuse liability study is typically a controlled substance from the same pharmacological class with a comparable indication as the investigational product. Lorazepam was chosen because Silexan was shown to have similar therapeutic effects as lorazepam in a double-blind, randomized trial in patients with GAD (Woelk and Schläfke, 2010), also indicating that the appropriate pharmacological class for a positive control in this study was the sedative (benzodiazepine) class.
The trial was performed at the Phase I unit of Syneos Health (formerly INC Research Early Phase), in Toronto, Canada.
Investigational Products
Lavender oil is a complex, multi-ingredient mixture of more than 160 different substances (Kasper, 2013) whose anxiolytic and calming properties have been attributed primarily to linalool and linalyl acetate that are among the main constituents (Setzer, 2009). Silexan is produced from L. angustifolia flowers by steam distillation using a well-defined and standardized manufacturing process to assure high batch-to-batch consistency. The product was available as gelatin capsules containing 80 or 160 mg of Silexan.
Placebo capsules were produced to be indistinguishable from Silexan capsules in all outward aspects. They were aromatized with 0.08 mg lavender oil for masking the smell of the product.
Lorazepam lactose tablets were obtained as merchandise and were encapsulated to maintain the blind.
Participants
Participants were male or female, healthy, non-dependent recreational users of CNS depressant drugs between 18 and 55 years of age with a body mass index ranging between 18.0 and 29.9 kg/m2 and a minimum weight of 50 kg. Recreational drug users were considered to be the most face valid population to study because they represent individuals at an increased risk for abuse of compounds with potential sedative effects, and they could provide meaningful ratings of drug experiences with a lower risk of false negative results (Balster and Bigelow, 2003; Griffiths et al., 2003; McColl and Sellers, 2006). Eligible participants had to have at least 10 non-therapeutic lifetime experiences with CNS depressant drugs and at least 1 experience within the last 12 weeks before the screening visit.
Main specific exclusion criteria were substance or alcohol dependence (excluding nicotine and caffeine) within the last 12 months as well as participation in a substance or alcohol rehabilitation program at any time. Participants with a history or presence of clinically significant physical or mental illness as well as those presenting with a positive drug screening or breath alcohol test were also excluded.
In addition to the requirement that participants were qualified based on self-reported historical account and medical assessments, the study used a pharmacological qualification phase to ensure that those who met the drug use history criteria were also able to distinguish lorazepam from placebo and liked the subjective effects of the drug.
Ethical Conduct
The protocol was reviewed and approved by the Ontario Institutional Review Board, ON IRB registration #IRB00000776, approval no. CR00046829. All patients provided written informed consent. The principles of Good Clinical Practice and the Declaration of Helsinki were adhered to.
Study Design, Treatments, and Schedule
The study consisted of 8 visits and included 4 phases: screening, qualification, treatment (five 3-day clinic visits), and follow-up. A sufficient number of participants were screened and qualified to randomize approximately 40 participants into the treatment phase and complete at least 30 participants.
The study procedures started with an outpatient screening visit during which informed consent was obtained and screening assessments were completed to determine patient eligibility for the study. Within 28 days of the screening visit, eligible patients returned to the clinical research unit as in-patients for a 4-day (3-night) qualification phase. To ensure that patients could discriminate between the active control and placebo, they received single oral doses of 3 mg lorazepam and a matching placebo separated by approximately 24 hours in a randomized, double-blind, 2-way crossover drug discrimination test. Pharmacodynamic (PD) and safety assessments were conducted pre-dose and up to 23 hours post-dose, as applicable. The qualification phase was followed by a 28-day washout period.
During the treatment phase, participants returned for five 3-day in-patient visits during each of which they received a single dose of 1 of the following drugs in randomized order: placebo, 2 mg lorazepam, 4 mg lorazepam, 80 mg Silexan, or 640 mg Silexan. Ten treatment sequences were generated according to a Williams square design (Williams, 1949), and each participant was randomly assigned to 1 of these sequences in ascending order of their participant number determined at inclusion into screening. The randomization code was generated by a designated, unblinded statistician who was otherwise not involved into the trial using SAS statistical software (SAS Institute, Inc., Cary, NC, USA). The smell of the investigational product was matched by flavoring the capsules containing Silexan placebo with 1/1000 of the amount of lavender oil contained in the Silexan 80-mg capsules.
Participants were admitted to the clinical research unit on the day before dosing. Single-dose drug administration occurred on the next day of each treatment period, followed by PD, pharmacokinetic (PK), and safety assessments conducted up to 24 hours post-dose. Participants were discharged approximately 24 hours after each drug administration following completion of the 24-hour post-dose procedures. Between any 2 treatment visits, a 4- to 14-day washout period was observed.
A follow-up visit was conducted approximately 5 to 10 days after the last drug administration in the treatment phase or at the time of early withdrawal from the study and included standard safety assessments.
Assessments
PD assessments were administered during a training session on the admission day of each treatment visit and were then collected over 24 hours post-dosing. Participants rated their perceived subjective state as well as the effects of the investigational drugs using the paper-based, validated, 101-point, unipolar or bipolar visual analogue scales (VASs) shown in Table 1 and presented as a horizontal, 100-mm-long line, with the question text presented above it. The Drug Similarity VAS was used to compare the investigational drug received during a particular visit with those out of a compilation of 15 drug classes with which the participant reported enough personal experience (according to the individual subject screening assessment) so that it could be used as a standard for comparison.
Table 1.
Visual Analogue Scales Used During PD Assessments
| Group | Scale | Question text | Response anchors | Schedule |
|---|---|---|---|---|
| Balance of effects | Drug liking | At this moment, my liking for this drug is … | 0: Strong disliking 50: Neither like nor dislike 100: Strong liking |
A |
| Overall drug liking | Overall, my liking for this drug is … | 0: Strong disliking 50: Neither like nor dislike 100: Strong liking |
C | |
| Take drug Again | I would take this drug again … | 0: Definitely not 100: Definitely so | C | |
| Positive effects | Good effects | At this moment, I feel good drug effects … | 0: Not at all 100: Extremely |
A |
| High | At this moment, I feel high … | 0: Not at all 100: Extremely |
B | |
| Negative effects | Bad effects | At this moment, I feel bad drug effects … | 0: Not at all 100: Extremely |
A |
| Sedation | Alertness/ drowsiness | At this moment, my mental state is … | 0: Very drowsy 50: Neither drowsy nor alert 100: Very alert |
B |
| Other effects | Any effects | At this moment, I feel any drug effect … | 0: Not at all 100: Extremely |
A |
| Dizziness | I am feeling dizzy … | 0: Not at all 100: Extremely |
B | |
| Drug similarity | How similar is the drug you most recently received to … | 0: Not at all similar 100: Very similar | D |
Abbreviations: A, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, and 24 hours post-dose; B, pre-dose + schedule A; C, 12 and 24 hours post-dose; D, 12 hours post-dose; PD, pharmacodynamic.
In addition to the VASs, the test battery also included the Euphoria (morphine-benzedrine group), Dysphoria (lysergic acid diethylamide), and Sedation (pentobarbital-chlorpromazine-alcohol group) scales from the 49-item version of the Addiction Research Center Inventory (ARCI; Martin et al., 1971), which were administered according to schedule B shown in Table 1.
PK assessments were performed for determining the plasma concentrations of linalool and lorazepam. During each treatment visit, samples were taken at pre-dose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 12, 14, and 24 hours post-dose.
Safety assessments included AEs reported by the participant spontaneously or upon questioning or observed by the investigator, vital signs, clinical laboratory assessments (hematology, clinical chemistry, urinalysis), as well as physical and ECG examinations.
Data Analysis
For each PD scale assessed more than once within each treatment, the intraindividual maximum and/or minimum values (depending on the relevance), the time between drug administration, and the intraindividual maximum and/or minimum values as well as the time-averaged area under the effect curve were determined from the time profiles. Comparisons were made between Silexan (80 mg and 640 mg) and lorazepam (2 mg and 4 mg), between Silexan (80 mg and 640 mg) and placebo, and between lorazepam (2 mg and 4 mg) and placebo. The pre-defined primary endpoint of the study was the individual maximum value achieved on the Drug Liking VAS.
PD endpoints for the treatment phase were analyzed using linear mixed-effect analyses of covariance, which included treatment, period, sequence, and first-order carryover effect as fixed effects, baseline (pre-dose) measurement as covariate where applicable, and participant nested within treatment sequence as random effect. From each model, adjusted means, 95% confidence intervals (CIs), and P values for treatments and treatment differences were computed. If the distribution of the mixed-model residuals differed obviously from normality (P value of the Shapiro-Wilk-W-test <.05), pairwise treatment group comparisons were assessed using Wilcoxon’s signed rank test on the within-participant differences. All P values are 2-sided; P ≤ .05 was considered to be statistically important. It should be noted that, in the context of this study, P values were obtained and should be interpreted as standardized estimates of population effects rather than as confirmatory measures. Adjustments for multiple testing were therefore not required.
PK analyses of the concentration vs time profiles for linalool and lorazepam were carried out via non-compartmental methods to estimate PK parameters in plasma according to the model independent approach.
The primary population for the PD analyses included all randomized participants who participated in all treatment visits and completed at least 1 post-dose assessment of the Drug Liking VAS for each treatment. PK assessments were based on all randomized participants who received at least 1 dose of an investigational product from whom at least 1 PK sample was obtained after dosing and who had no protocol deviations that excluded them from the analysis. Safety analyses included all randomized participants who received any investigational product (Silexan, lorazepam, or placebo) starting from the treatment phase.
Sample size calculations were based on the reduction of abuse potential by Silexan compared with lorazepam measured by the Drug Liking VAS intraindividual maximum value. Based on unpublished data collected at the investigational site with a comparable positive control with lorazepam, an average intraindividual difference of 15 points and a SD of 24.5 points were assumed. Using a paired t test with a 2-sided type I error level of α = .05, 30 participants were required to achieve a target power ≥90%. Assuming a 25% dropout rate, 40 participants were to be randomized with the intention to complete approximately 30 participants.
Results
Baseline Characteristics and Disposition of Participants
The study was performed between April and August 2015 (first participant in to last subject participant out) in a Phase I unit in Toronto, Ontario, Canada. A total of 95 participants were screened for eligibility, 40 were randomized, and 34 (85% of 40) completed the study as scheduled (Figure 1). Treatment period 1 was completed by all randomized participants, period 2 by 38, and periods 3 through 5 by 34.
Figure 1.
Disposition of participants.
All 40 randomized participants received at least 1 dose of study drug and comprised the safety population as well as the PK population. The 34 participants who completed all treatment periods were included in the PD population.
At inclusion, the study participants were between 24 and 54 years of age. The majority of participants were white men (Table 2). Medical history findings ongoing at screening were mostly eye disorders (ametropia) and allergies. All randomized participants reported using CNS depressants during the past year, followed by opioids and morphine derivatives, cannabinoids, and stimulants, all of which had been used by more than 70% of participants.
Table 2.
Demographic and Baseline Characteristics
| Safety/PK population (n = 40) | PD population (n = 34) | |
|---|---|---|
| Age (y) | 40.1 ± 8.7, 24 – 54 | 39.3 ± 8.0, 24 – 54 |
| Sex | ||
| Female | 10 (25.0%) | 8 (23.5%) |
| Male | 30 (75.0%) | 26 (76.5%) |
| Race | ||
| White | 35 (87.5%) | 30 (88.2%) |
| Black/African American | 3 (7.5%) | 2 (5.9%) |
| Asian | 2 (5.0%) | 2 (5.9%) |
| BMI (kg/m2) | 25.3 ± 2.7, 19.5 – 29.7 | 25.5 ± 2.7, 19.5 – 29.7 |
| Recreational drug use, last year | ||
| CNS depressants | 40 (100.0%) | 34 (100.0%) |
| Opioids/morphine derivatives | 36 (90.0%) | 30 (88.2%) |
| Cannabinoids | 30 (75.0%) | 24 (70.6%) |
| Stimulants | 29 (72.5%) | 24 (70.6%) |
| Hallucinogens | 6 (15.0%) | 5 (14.7%) |
| Dissociative anesthetics | 1 (2.5%) | 1 (2.9%) |
Abbreviations: BMI, body mass index; PD, pharmacodynamic; PK, pharmacokinetic.
Values are mean ± SD and range, or count and %.
PD Measures
Drug-Liking at this Moment (Primary Outcome Measure)
For placebo as well as for Silexan 80 mg and 640 mg, median individual maximum drug liking scores of 51 points were observed that were thus close to the neutral position of the scale (at 50 points), compared with medians of 76 and 80.5 points for lorazepam 2 mg and 4 mg, respectively. For Silexan and placebo, the time profiles show a slight increase of the mean drug-liking scores above the neutral position at 50 points between 1 and 2.5 hours post-dose, to mean (± SD) values peaking at 52.9 ± 12.4 points for Silexan 80 mg, 58.6 ± 17.7 points for the 640-mg dose, and 53.5 ± 5.5 points for placebo. For lorazepam, mean drug-liking scores reached their individual maximum at 2.9 and 4.3 hours after dosing for 2 mg and 4 mg, respectively, with time profile mean values peaking at 66.9 ± 20.4 and 69.4 ± 19.7 points for 2 and 4 mg, respectively, and were still above neutral at 12 hours after dosing.
Table 3 and Figure 2 present intraindividual comparisons between the different doses of Silexan and lorazepam on the one hand and placebo on the other. Statistically important, dose-dependent differences between lorazepam and placebo demonstrate the assay sensitivity of the trial to distinguish between a CNS depressant effect and no pharmacological effect and thus underline the validity of the design and the between-treatment comparisons made. In contrast to lorazepam, the median difference between both doses of Silexan and placebo was determined to be 0, indicating that the drug-liking effect of the herbal active substance was not systematically distinguishable from placebo by experienced recreational drug users. Consequently, head-to-head comparisons between lorazepam and Silexan (Table 3) also showed statistically important intraindividual differences in maximum drug-liking that were similar to those between lorazepam and placebo.
Table 3.
Drug-Liking at This Moment: Individual Maximum Values, Differences Between Drug and Placebo (PD Population, n = 34)
| Comparison | Median differencea | IQR | P b |
|---|---|---|---|
| Silexan 80 mg vs placebo | 0.0 | (−1) – 10 | .2600 |
| Silexan 640 mg vs placebo | 0.0 | (−2) – 14 | .2809 |
| Lorazepam 2 mg vs placebo | 19.5 | 11 – 31 | <.0001 |
| Lorazepam 4 mg vs placebo | 24.5 | 15 – 36 | <.0001 |
| Silexan 80 mg vs lorazepam 2 mg | −18.0 | (−29) – (−7) | <.0001 |
| Silexan 640 mg vs lorazepam 2 mg | −12.0 | (−24) – 0 | .0005 |
| Silexan 80 mg vs lorazepam 4 mg | −23.0 | (−34) – (−15) | <.0001 |
| Silexan 640 mg vs lorazepam 4 mg | −20.5 | (−27) – (−1) | <.0001 |
Abbreviations: IQR, interquartile range (25th–75th percentile); PD, pharmacodynamic.
aPositive values denote greater liking for the drug named first.
bWilcoxon signed rank test in intraindividual differences.
Figure 2.
Assessment of balance of drug effects: difference in maximum effect between drug and placebo (medians and interquartile ranges for lorazepam 2 mg [LOR 2 mg], 4 mg [LOR 4 mg], and for Silexan 80 mg [SIL 80 mg] and 640 mg [SIL 640 mg]; pharmacodynamic population, n = 34).
Secondary Measures of Balance of Effects
Results for the overall drug-liking and take drug again scales (Table 4; Figure 2) were consistent with those for the primary outcome measure. While the scores observed after administration of both doses of Silexan were on or close to the placebo level, statistically important higher scores were observed for lorazepam 2 mg and 4 mg compared with both placebo and Silexan.
Table 4.
Secondary PD Outcome Measures: Individual Maximum or Minimum
| Comparison | Overall drug-likinga | Take drug againb | Good effectsb | Higha | ARCI MBGa | Bad effectsa | ARCI LSDGa | Alertness/ drowsinessb,c | ARCI PCAGb | Any effectsa | Dizzinessa |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Silexan 80 mg vs placebo | 0.0 | 2.5 | 1.7 | 0.0 | 0.0 | 0.0 | 0.0 | −3.4 | 0.4 | 1.5 | 0.0 |
| Silexan 640 mg vs placebo | 0.0 | 11.5 | 13.9* | 1.0* | 0.0 | 0.0 | 0.0 | −1.4 | −0.7 | 0.5* | 0.0 |
| Lorazepam 2 mg vs placebo | 25.0*** | 52.3*** | 45.5*** | 44.0*** | 2.0*** | 1.0 | 0.5*** | −19.2*** | 4.0*** | 55.0*** | 4.5*** |
| Lorazepam 4 mg vs placebo | 31.0*** | 60.0*** | 52.9*** | 51.5*** | 5.0*** | 6.0*** | 2.0*** | −23.7*** | 5.6*** | 60.0*** | 22.5*** |
| Silexan 80 mg vs lorazepam 2 mg | −24.5*** | −49.9*** | −43.8*** | −30.0*** | −1.0*** | 0.0 | 0.0*** | 15.8*** | −3.5*** | −43.5*** | −3.5*** |
| Silexan 640 mg vs lorazepam 2 mg | −14.0*** | −40.8*** | −31.6*** | −27.0*** | −1.0* | 0.0 | 0.0** | 17.8*** | −4.7*** | −30.5*** | −3.0*** |
| Silexan 80 mg vs lorazepam 4 mg | −29.0*** | −57.5*** | −51.2*** | −45.5*** | −4.0*** | −2.5** | −2.0*** | 20.3*** | −5.2*** | −46.0*** | −23.5*** |
| Silexan 640 mg vs lorazepam 4 mg | −23.5*** | −48.5*** | −39.0*** | −45.5*** | −2.0*** | −4.0** | −2.0*** | 22.3*** | −6.4*** | −32.5*** | −17.0*** |
Abbreviations: ARCI, Addiction Research Center Inventory; LSDG, LSD Group; MBG, Morphine-Benzedrine Group; PCAG, Pentobarbital-Chlorpromazine-Alcohol Group; PD, pharmacodynamic.
Adjusted mean or median of intraindividual differences and P value; PD population, n = 34. Positive values denote higher values on applicable scale for the drug named first.
* P ≤ .05; ** P ≤ .01; *** P ≤ .001.
aMedian differences, Wilcoxon signed rank test P values; bAdjusted mean differences, analysis of covariance P values.
cMinimum effect is analyzed.
Positive Effects
Positive drug effects were assessed using the good effects and high VASs as well as the ARCI morphine-benzedrine group (“euphoria”) subscale (Table 4). Maximum values for Silexan 80 mg did not differ statistically important from those observed for placebo for any of the positive effects measures. For the 640-mg dose, a slight elevation over the placebo level was observed for the good effects (scale medians: Silexan 640 mg 4 points, placebo 2 points) and high VASs (medians: Silexan 640 mg 1.5 points, placebo 0 points, which represents the expected placebo response on this unipolar scale) but not for the ARCI Morphine-benzedrine group subscale. Intraindividual differences between lorazepam and placebo were, however, more than threefold larger for the good effects scale and more than fortyfold larger for the high VAS than those between Silexan and placebo. Moreover, in head-to-head comparisons, values of all positive effects measures for both Silexan doses were always statistically importantly lower than for any dose of lorazepam.
Negative Effects
The bad effects VAS as well as the ARCI LSD group subscale were available for assessing negative drug effects perceived subjectively. All treatments were generally associated with low scores for negative effects: mean and median individual maximum scores for the bad effects VAS were highest for lorazepam 4 mg (median 6.5) and much lower and similar for all other treatments (mean ≤1.0). Consequently, statistically important intra-individual differences were observed between lorazepam 4 mg and placebo as well as both doses of Silexan, but not between other treatments (Table 4). For the ARCI LSD group subscale, both doses of lorazepam showed statistically important, more pronounced negative effects scores than Silexan or placebo, between which only marginal differences were observed.
Sedative Effects
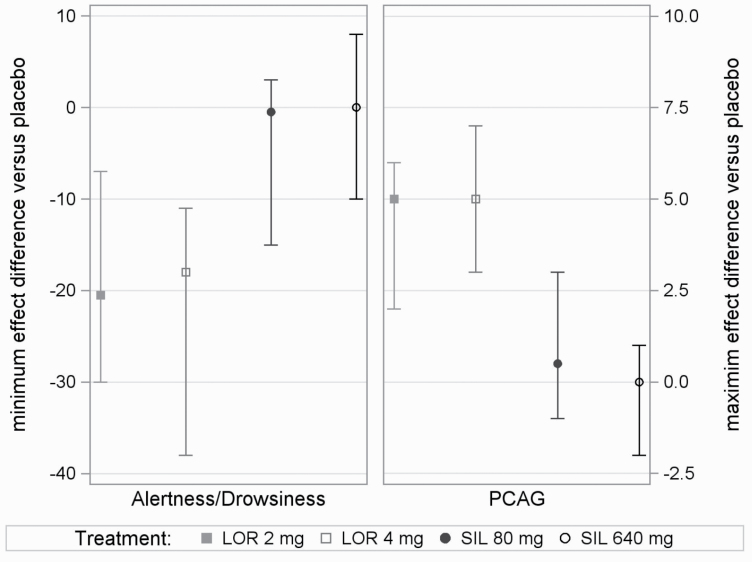
All treatments, including placebo, exhibited a post-dose decrease in the alertness/drowsiness mean scores, indicating increased drowsiness. Following pre-dose mean values between 52 and 55 points, the lowest mean values observed for placebo, Silexan 80 mg, and Silexan 640 mg at post-dose were 46.2 ± 17.5 (at 2.5 hours), 43.4 ± 19.0 (at 2 hours), and 46.0 ± 18.4 points (at 2.5 hours), respectively. Lorazepam, however, showed the greatest decrease over the longest time interval, from approximately 1 hour to 8 hours post-dose for lorazepam 2 mg to 12 hours post-dose for lorazepam 4 mg, with mean values as low as 26.4 ± 15.7 points (at 3 hours) for the 2-mg dose and 30.3 ± 19.0 points (at 2.5 hours) for the 4-mg dose. For the ARCI phenobarbital-chlorpromazine-alcohol group subscale, Silexan 640 mg exhibited the lowest mean scores (i.e., least sedation), followed by placebo and then Silexan 80 mg, although mean scores for these treatments overlapped for most of the time course of assessment while the scores for lorazepam always indicated higher sedation. In head-to-head comparisons for the 2 sedation scores, within-participant differences between lorazepam and Silexan or placebo were always statistically important while differences between Silexan and placebo were statistically negligible (Table 4; Figure 3).
Figure 3.
Sedative effects: difference in minimum or maximum effect between drug and placebo (medians and interquartile ranges for lorazepam 2 mg [LOR 2 mg], 4 mg [LOR 4 mg], and for Silexan 80 mg [SIL 80 mg] and 640 mg [SIL 640 mg]). PCAG, Addiction Research Center Inventory Pentobarbital-Chlorpromazine-Alcohol Group scale; pharmacodynamic population, n = 34.
Other PD Measures
On the 101-point any effects VAS, mean and median maximum scores were highest for lorazepam 4 mg and lorazepam 2 mg, with scores above 65. Silexan 640 mg, Silexan 80 mg, and placebo all had mean and median scores below 35. For the dizziness VAS, average maximum scores were again highest for lorazepam, followed by placebo and slightly lower mean scores for both doses of Silexan. Treatment differences between Silexan and placebo on one hand and lorazepam on the other were always statistically important, whereas those between Silexan 80 mg and placebo were not (Table 4). For the comparison between the Silexan 640 mg dose and placebo, a P value of .04 was determined for the any effects scale, with a median difference of 0.5 for Silexan 640 mg compared with placebo. The median differences for the comparison of lorazepam (2 mg and 4 mg) and placebo were 55.0 and 60.0 points, respectively. No appreciable difference between Silexan 640 mg and placebo was seen for the dizziness VAS (P = .91).
Drug similarity assessment median scores for lorazepam indicated that participants felt it was similar to CNS depressants such as other benzodiazepines. Silexan 80 mg was not similar to any drug previously experienced by the participants. Interestingly, of all the active treatments, Silexan 640 mg was the only 1 scored as similar to placebo.
PK Measures
Peak and overall exposures for linalool increased with increasing dose of Silexan. Median time to reach peak exposure was slightly longer for Silexan 640 mg than for the 80-mg dose. Mean terminal elimination half-lives were 2.5 hours and 8.8 hours for Silexan 80 mg and 640 mg, respectively. Overall, the PK profiles of linalool and lorazepam were as expected and supported the treatment compliance in the study.
Safety
During randomized treatment, the highest incidence of AEs within the first 24 hours of drug administration was observed for lorazepam 4 mg (88.9%, 32 of 36 participants exposed), followed by lorazepam 2 mg (86.5%, 32 of 37 participants), Silexan 640 mg (63.9%, 23 of 36 participants), Silexan 80 mg (60.0%, 21 of 35 participants), and placebo (50.0%, 18 of 36 participants).
Somnolence was the most common AE overall and appeared most frequently after lorazepam administration. Dizziness too occurred with the highest incidence in the lorazepam groups. Eructation was observed following administration of Silexan but not lorazepam. The incidence of euphoric mood was highest after administration of lorazepam.
No clear dose-related increase in the percentage of patients with AEs was reported for lorazepam or for Silexan. However, a dose-related increase in the number of events was observed for lorazepam (2 mg: 53 events; 4 mg: 74 events) but not for Silexan (80 mg: 44 events; 640 mg: 42 events).
There were no serious or severe AEs during randomized treatment. Moderate intensity AEs occurred only after exposure to lorazepam. No participant exhibited any suicidal ideation or behavior at any time during the study.
Discussion
Anxiety disorders are among the most prevalent psychiatric conditions (Wittchen et al., 2011; Haller et al., 2014). They may cause profound suffering and functional impairment and adversely affect quality of life even in their subthreshold form (Karsten et al., 2013; Möller et al., 2019). According to currently applicable clinical practice guidelines (e.g., National Collaborating Centre for Mental Health, 2011; Bandelow et al., 2012; Baldwin et al., 2014), recommended pharmacological treatments for anxiety disorders include antidepressant drugs such as selective serotonin reuptake inhibitors and selective norepinephrine reuptake inhibitors, sedative-hypnotic drugs such as benzodiazepines, and pregabalin. Benzodiazepines and pregabalin in particular have been associated with abuse potential and dependence based on clinical observations and through their mechanisms of action (e.g., Nothdurfter et al., 2012; Baldwin et al., 2013; Engin et al., 2014; Schjerning et al., 2016; Schwienteck et al., 2017). Silexan may thus be an interesting therapeutic option for the pharmacotherapy of anxiety-related disorders. While the herbal active substance was demonstrated to have similar effects as lorazepam and paroxetine in the treatment of patients with GAD (Woelk and Schläfke, 2010; Kasper et al., 2014), no sedative, addictive, or withdrawal effects have been reported to date in controlled clinical trials (Kumar, 2013; Silenieks et al., 2013; Müller et al., 2015; Gastpar et al., 2017; Kasper et al., 2018) even prior to a human abuse potential study. This study was designed to close this gap by investigating whether there is an abuse potential of Silexan in a sample of recreational drug users with a history of CNS depressant abuse.
The results did not reveal any signal for an abuse potential of Silexan, neither at the registered therapeutic dose of 80 mg nor at the supratherapeutic, eightfold dose of 640 mg. In contrast to lorazepam, measures of drug effect for Silexan were consistently in the neutral range of bipolar scales between 40 and 60 points, within which responses to placebo are typically observed (Setnik et al., 2017), or in the lower range of unipolar scales. In intraindividual head-to-head comparisons of Silexan (both doses) with placebo, median or mean differences were at or close to zero for all PD outcomes investigated, including the primary outcome measure. By contrast, differences between Silexan and both doses of lorazepam were found for all PD outcomes except for the bad effects scale (where differences to lorazepam 2 mg were negligible because this dose was also not found to be associated with appreciable negative effects). Moreover, even though the dose of Silexan in the 640-mg condition exceeded the therapeutic dose of 80 mg eightfold, no consistent relationship between dose and negative effect was observed.
For the good effects scale, the high scale, and the any effects scale included as secondary PD outcomes, comparisons between Silexan 640 mg and placebo showed intraindividual differences that were associated with descriptive P values in the range between .03 and .02. For the same scales, differences between both doses of lorazepam and placebo were, however, much more pronounced (see Table 4), and even though the P values determined for these comparisons between Silexan 640 mg and placebo were formally in the range <.05, it appears to be very unlikely that the differences may be of any appreciable clinical significance.
We would like to emphasize that, instead of focusing on isolated P values below a more or less arbitrary threshold of .05, which resulted from mean value differences of questionable clinical importance, assessments of abuse potential need to take into account all the evidence collected and should thus be based on the overall clinical picture. In this regard, we consider the risk that Silexan, even at a dose of 640 mg, may be attractive for recreational use to be extremely remote. This interpretation is supported by the results of the drug similarity rating where Silexan 640 mg was identified as being similar to placebo but not to any drug used non-therapeutically by the participants.
The PD results of this trial in healthy recreational drug users thus confirm the results already observed in a rat model (Silenieks et al., 2013) according to which Silexan, even at the eightfold amount of the currently registered therapeutic daily dose, is not recognized as benzodiazepine-like and is therefore unlikely to share the potential of benzodiazepines to induce the development of tolerance, dependence, and addiction.
Assay sensitivity of the study was demonstrated by showing that the study procedures were successful in selecting participants who were able to discriminate reliably between the active control lorazepam and placebo. Participants rated both doses of lorazepam clearly higher than placebo on all positive drug effects and balance of effects measures, and there were also negative and sedating effects of lorazepam, notably for the 4-mg dose. Moreover, all PD outcomes consistently showed a dose-dependent increase of the effects of lorazepam. These observations were supported by the fact that lorazepam was clearly identified as a CNS depressant drug but not as placebo in the drug similarity test.
Regarding the similarities between Silexan and pregabalin concerning the inhibition of voltage dependent calcium channels observed by Schuwald et al. (2013), it should be noted that the 2 substances were also shown to interact with different therapeutic targets. This explains the major differences between their pharmacological profiles, notably the absence of a sedating effect of Silexan compared with pregabalin (Müller et al., 2015).
Single doses of 80 mg and 640 mg Silexan were well tolerated, with no dose-related increase in the number of AEs or in the percentage of participants with AEs. Events observed after the administration of Silexan with a higher rate than for the remaining treatments were limited to mild eructation (and mild dysgeusia along with it), which is known as an adverse reaction to the oral administration of herbal essential oils, including lavender oil (e.g., Kasper, 2013).
Limitations of this study include that only responses to single doses of the investigational drugs were investigated, and, moreover, only subjective measures of drug effects were used rather than self-administration models as direct assessments of abuse. It is worth mentioning, however, that the design of the study, including its endpoints, was consistent with the guidance on the investigation of abuse potential that applied when the study was conducted (Health Canada, 2007; Food and Drug Administration, 2010) and that the assay sensitivity of the trial was demonstrated through a well-recognized active control.
In conclusion, for all measures of abuse potential investigated, the therapeutic dose of Silexan did not differ by any statistically important margin from placebo on all primary and secondary endpoints; the supratherapeutic dose was not different from placebo on the primary endpoint as well as on most secondary endpoints and was assessed to be similar to placebo rather than to drugs used recreationally by the participating participants. Where differences from placebo were observed, their magnitude was very small. Therefore, Silexan did not exhibit abuse potential and is unlikely to be recreationally abused. Silexan was devoid of sedative effects and well tolerated.
Acknowledgments
The study presented in this paper was sponsored by Dr. Willmar Schwabe GmbH & Co. KG, manufacturer of Silexan. A first draft of the manuscript was prepared by Andreas Völp, PhD, an independent medical writer who was reimbursed by Dr. Willmar Schwabe GmbH & Co. KG.
Statement of Interest
Erich Seifritz has received honoraria from Schwabe GmbH for educational lectures. He has further received educational grants and consulting fees from Janssen Cilag, Lundbeck, Angelini, Otsuka, Servier, Ricordati, Vifor, Sunovion, and Mepha. Hans-Jürgen Möller has received grant/research support, consulting fees, and honoraria within the last years from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen Cilag, Lundbeck, MSD, Novartis, Organon, Otsuka, Pfizer, Schwabe, Sepracor, Servier, and Wyeth. Hans-Peter Volz has served as a consultant or on advisory boards for Astra/Zeneca, Eli Lilly, Lundbeck, Pfizer, Schwabe, Janssen, Otsuka, Merz, Wyeth, and Neuraxpharm and has served on speakers’ bureaus for Astra/Zeneca, Eli Lilly, Lundbeck, Schwabe, Janssen, Merz, Wyeth, Lichtwer, Steigerwald, Hormosan, Neuraxpharm, and Bristol-Myers Squibb.
Walter E. Müller has received grant support from Schwabe and UCB as well as speaker’s honoraria from Lundbeck, Neuraxpharm, and Schwabe. Talar Hopyan is an employee of Syneos Health (previously INC Research/Inventiv Health) and consults with various pharmaceutical and biotech companies. Anna Wacker is an employee of Dr. Willmar Schwabe GmbH & Co. KG, manufacturer of Silexan.
Sandra Schläfke is an employee of Dr. Willmar Schwabe GmbH & Co. KG, manufacturer of Silexan. Siegfried Kasper received grants/research support, consulting fees, and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, Celegne GmbH, Eli Lilly, Janssen-Cilag Pharma GmbH, KRKA-Pharma, Lundbeck A/S, Mundipharma, Neuraxpharm, Pfizer, Sage, Sanofi, Schwabe, Servier, Shire, Sumitomo Dainippon Pharma Co. Ltd., and Takeda.
References
- Baldinger P, Höflich AS, Mitterhauser M, Hahn A, Rami-Mark C, Spies M, Wadsak W, Lanzenberger R, Kasper S (2014) Effects of Silexan on the serotonin-1A receptor and microstructure of the human brain: a randomized, placebo-controlled, double-blind, cross-over study with molecular and structural neuroimaging. Int J Neuropsychopharmacol 18:pyu063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DS, Ajel K, Masdrakis VG, Nowak M, Rafiq R (2013) Pregabalin for the treatment of generalized anxiety disorder: an update. Neuropsychiatr Dis Treat 9:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DS, Anderson IM, Nutt DJ, Allgulander C, Bandelow B, den Boer JA, Christmas DM, Davies S, Fineberg N, Lidbetter N, Malizia A, McCrone P, Nabarro D, O’Neill C, Scott J, van der Wee N, Wittchen HU (2014) Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol 28:403–439. [DOI] [PubMed] [Google Scholar]
- Balster RL, Bigelow GE (2003) Guidelines and methodological reviews concerning drug abuse liability assessment. Drug Alcohol Depend 70:S13–S40. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Sher L, Bunevicius R, Hollander E, Kasper S, Zohar J, Möller HJ; WFSBP Task Force on Mental Disorders in Primary Care; WFSBP Task Force on Anxiety Disorders, OCD and PTSD (2012) Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract 16:77–84. [DOI] [PubMed] [Google Scholar]
- Council of Europe (2015) European Pharmacopoeia. 8th ed. Strasbourg, France: Council of Europe. [Google Scholar]
- Engin E, Bakhurin KI, Smith KS, Hines RM, Reynolds LM, Tang W, Sprengel R, Moss SJ, Rudolph U (2014) Neural basis of benzodiazepine reward: requirement for α2 containing GABAA receptors in the nucleus accumbens. Neuropsychopharmacology 39:1805–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (2010) Guidance for industry-assessment of abuse potential of drugs. Draft guidance. Silver Spring, MD: U.S. Department of Health and Human Services. [Google Scholar]
- Gastpar M, Müller WE, Volz HP, Möller HJ, Schläfke S, Dienel A, Kasper S (2017) Silexan does not cause withdrawal symptoms even when abruptly discontinued. Int J Psychiatry Clin Pract 21:1–4. [DOI] [PubMed] [Google Scholar]
- Generoso MB, Soares A, Taiar IT, Cordeiro Q, Shiozawa P (2017) Lavender oil preparation (Silexan) for treating anxiety: an updated meta-analysis. J Clin Psychopharmacol 37:115–117. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Ator NA (2003) Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend 70:S41–S54. [DOI] [PubMed] [Google Scholar]
- Haller H, Cramer H, Lauche R, Gass F, Dobos GJ (2014) The prevalence and burden of subthreshold generalized anxiety disorder: a systematic review. BMC Psychiatry 14:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada (2007) Clinical assessment of abuse liability for drugs with central nervous system activity. Ottawa: Minister of Public Works and Government Services Canada. [Google Scholar]
- Huang L, Abuhamdah S, Howes MJ, Dixon CL, Elliot MS, Ballard C, Holmes C, Burns A, Perry EK, Francis PT, Lees G, Chazot PL (2008) Pharmacological profile of essential oils derived from Lavandula angustifolia and Melissa officinalis with anti-agitation properties: focus on ligand-gated channels. J Pharm Pharmacol 60:1515–1522. [DOI] [PubMed] [Google Scholar]
- Karsten J, Penninx BW, Verboom CE, Nolen WA, Hartman CA (2013) Course and risk factors of functional impairment in subthreshold depression and anxiety. Depress Anxiety 30:386–394. [DOI] [PubMed] [Google Scholar]
- Kasper S (2013) An orally administered lavandula oil preparation (Silexan) for anxiety disorder and related conditions: an evidence based review. Int J Psychiatry Clin Pract 17Suppl 1:15–22. [DOI] [PubMed] [Google Scholar]
- Kasper S, Gastpar M, Müller WE, Volz HP, Möller HJ, Schläfke S, Dienel A (2014) Lavender oil preparation Silexan is effective in generalized anxiety disorder–a randomized, double-blind comparison to placebo and paroxetine. Int J Neuropsychopharmacol 17:859–869. [DOI] [PubMed] [Google Scholar]
- Kasper S, Volz HP, Dienel A, Schläfke S (2016) Efficacy of Silexan in mixed anxiety-depression–a randomized, placebo-controlled trial. Eur Neuropsychopharmacol 26:331–340. [DOI] [PubMed] [Google Scholar]
- Kasper S, Möller HJ, Volz HP, Schläfke S, Dienel A (2017) Silexan in generalized anxiety disorder: investigation of the therapeutic dosage range in a pooled data set. Int Clin Psychopharmacol 32:195–204. [DOI] [PubMed] [Google Scholar]
- Kasper S, Müller WE, Volz HP, Möller HJ, Koch E, Dienel A (2018) Silexan in anxiety disorders: clinical data and pharmacological background. World J Biol Psychiatry 19:412–420. [DOI] [PubMed] [Google Scholar]
- Kumar V (2013) Characterization of anxiolytic and neuropharmacological activities of Silexan. Wien Med Wochenschr 163:89–94. [DOI] [PubMed] [Google Scholar]
- López V, Nielsen B, Solas M, Ramírez MJ, Jäger AK (2017) Exploring pharmacological mechanisms of lavender (Lavandula angustifolia) essential oil on central nervous system targets. Front Pharmacol 8:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR (1971) Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther 12:245–258. [DOI] [PubMed] [Google Scholar]
- McColl S, Sellers EM (2006) Research design strategies to evaluate the impact of formulations on abuse liability. Drug Alcohol Depend 83Suppl 1:S52–S62. [DOI] [PubMed] [Google Scholar]
- Möller HJ, Volz HP, Dienel A, Schläfke S, Kasper S (2019) Efficacy of Silexan in subthreshold anxiety: meta-analysis of randomised, placebo-controlled trials. Eur Arch Psychiatry Clin Neurosci 269:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller WE, Schuwald AM, Nöldner M, Kasper S, Friedland K (2015) Pharmakologische Grundlagen der therapeutischen Anwendung von Silexan (Lasea®). Psychopharmakotherapie 22:3–14. [Google Scholar]
- National Collaborating Centre for Mental Health (NCCMH) (2011) Generalised anxiety disorder in adults. The NICE guideline on management in primary, secondary and community care. London: RCPsych Publications. [Google Scholar]
- Nothdurfter C, Rupprecht R, Rammes G (2012) Recent developments in potential anxiolytic agents targeting GABAA/BzR complex or the translocator protein (18kDa) (TSPO). Curr Top Med Chem 12:360–370. [DOI] [PubMed] [Google Scholar]
- Satpute AB, Mumford JA, Naliboff BD, Poldrack RA (2012) Human anterior and posterior hippocampus respond distinctly to state and trait anxiety. Emotion 12:58–68. [DOI] [PubMed] [Google Scholar]
- Schjerning O, Rosenzweig M, Pottegård A, Damkier P, Nielsen J (2016) Abuse potential of pregabalin: a systematic review. CNS Drugs 30:9–25. [DOI] [PubMed] [Google Scholar]
- Schuwald AM, Nöldner M, Wilmes T, Klugbauer N, Leuner K, Müller WE (2013) Lavender oil-potent anxiolytic properties via modulating voltage dependent calcium channels. PloS One 8:e59998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwienteck KL, Li G, Poe MM, Cook JM, Banks ML, Stevens Negus S (2017) Abuse-related effects of subtype-selective GABAA receptor positive allosteric modulators in an assay of intracranial self-stimulation in rats. Psychopharmacology (Berl) 234:2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setnik B, Roland CL, Pixton G, Webster L (2017) Measurement of drug liking in abuse potential studies: a comparison of unipolar and bipolar visual analog scales. J Clin Pharmacol 57:266–274. [DOI] [PubMed] [Google Scholar]
- Setzer WN (2009) Essential oils and anxiolytic aromatherapy. Nat Prod Commun 4:1305–1316. [PubMed] [Google Scholar]
- Silenieks LB, Koch E, Higgins GA (2013) Silexan, an essential oil from flowers of Lavandula angustifolia, is not recognized as benzodiazepine-like in rats trained to discriminate a diazepam cue. Phytomedicine 20:172–177. [DOI] [PubMed] [Google Scholar]
- Williams EJ (1949) Experimental designs balanced for the estimation of residual effects of treatments. Austr J Scientific Res 2:149–168. [Google Scholar]
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, Olesen J, Allgulander C, Alonso J, Faravelli C, Fratiglioni L, Jennum P, Lieb R, Maercker A, van Os J, Preisig M, Salvador-Carulla L, Simon R, Steinhausen HC (2011) The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21:655–679. [DOI] [PubMed] [Google Scholar]
- Woelk H, Schläfke S (2010) A multi-center, double-blind, randomised study of the lavender oil preparation Silexan in comparison to Lorazepam for generalized anxiety disorder. Phytomedicine 17:94–99. [DOI] [PubMed] [Google Scholar]