Abstract
Background
Little is known regarding the association of cannabis use with brain structure in adolescents with bipolar disorder (BD). This subject is timely, given expanded availability of cannabis contemporaneously with increased social acceptance and diminished societal constraints to access. Therefore, we set out to examine this topic in a sample of adolescents with BD and healthy control (HC) adolescents.
Methods
Participants included 144 adolescents (47 BD with cannabis use [BDCB+; including 13 with cannabis use disorder], 34 BD without cannabis use [BDCB−], 63 HC without cannabis use) ages 13–20 years. FreeSurfer-processed 3T MRI with T1-weighted contrast yielded measures of cortical thickness, surface area (SA), and volume. Region of interest (amygdala, hippocampus, ventrolateral prefrontal cortex, ventromedial prefrontal cortex, and anterior cingulate cortex) analyses and exploratory vertex-wise analysis were undertaken. A general linear model tested for between-group differences, accounting for age, sex, and intracranial volume.
Results
Vertex-wise analysis revealed significant group effects in frontal and parietal regions. In post-hoc analyses, BDCB+ exhibited larger volume and SA in parietal regions, and smaller thickness in frontal regions, relative to HC and BDCB−. BDCB− had smaller volume, SA, and thickness in parietal and frontal regions relative to HC. There were no significant region of interest findings after correcting for multiple comparisons.
Conclusion
This study found that cannabis use is associated with differences in regional brain structure among adolescents with BD. Future prospective studies are necessary to determine the direction of the observed association and to assess for dose effects.
Keywords: cannabis, bipolar disorder, adolescent, neuroimaging
Significance Statement.
Approximately 1 in 3 adolescents with bipolar disorder (BD) have comorbid substance use disorder, with cannabis being the most commonly used drug. In adults with BD, cannabis use and cannabis use disorders are associated with decreased treatment adherence and more severe illness course, including increased symptom severity and delayed recovery. However, adolescence is a critical period of dynamic neurobiological and behavioral changes. Due to this critical developmental epoch, adolescents are more susceptible to developmental disturbances induced by exogenous substances. However, there is a lack of research regarding the brain-based implications of cannabis use in adolescents with BD. Therefore, this study examined the neuro-structural associations of cannabis use in adolescents with BD. This study will inform future studies regarding the mechanism and directionality of cannabis use in adolescents with BD, leading to further understanding of the causes and effects of cannabis use.
Bipolar disorder (BD) is a highly complex and impairing condition characterized by recurrent mood episodes (Birmaher et al., 2006). BD affects 2%–5% of adolescents and is the fourth leading cause of adolescent disability worldwide (Kozloff et al., 2010). In addition to mood symptoms, approximately 1 in 3 adolescents with BD have comorbid substance use disorders (Wilens et al., 2004; Goldstein et al., 2008). Similar to adults, cannabis is the most commonly used drug among adolescents with BD (Goldstein et al., 2008). Furthermore, adolescents with BD have higher rates of cannabis use and greater likelihood of progression to cannabis use disorders (CUD) relative to the general population (Wittchen et al., 2007; Tyler et al., 2015). Cannabis use has been associated with increased symptom severity and decreased treatment response in individuals with BD (Van Rossum et al., 2009; Agrawal et al., 2011). In addition to the association of cannabis use with concurrent and future psychiatric symptoms and disorders, cannabis use has been associated with brain structure alterations in individuals with and without mood disorders (Lim et al., 2013; Jacobus and Tapert, 2014).
Compared with adulthood, adolescence is a critical period of neurodevelopment and maturation. During this period, the brain is particularly sensitive to the deleterious effects of environmental factors; substance use, including cannabis, is thought to interfere with the natural process of brain maturation and potentially lead to psychosocial and/or biological consequences (Squeglia et al., 2009). While a number of studies have reported cannabis-related differences in grey matter structure in adolescents, the directionality of these results vary (Squeglia et al., 2009; Ashtari et al., 2011; Lopez-Larson et al., 2011; Jacobus and Tapert, 2014). For example, some studies have shown that heavy cannabis users display smaller hippocampal volumes and smaller thickness in prefrontal cortical regions compared with controls (Ashtari et al., 2011; Lopez-Larson et al., 2011), whereas other studies have found no differences in hippocampal volume as well as larger parietal thickness (Medina et al., 2009; Lopez-Larson et al., 2011).
The inconsistency regarding neuroanatomical correlates of cannabis use may be due to a number of factors such as duration, frequency, and potency of cannabis use as well as age of cannabis use onset (Nader and Sanchez, 2018). Although there are numerous studies regarding the association of brain structure and cannabis use and regarding the association of brain structure and BD, only 1 prior study, to our knowledge, has examined neuro-structural correlates of cannabis use in adolescents with BD). The study included 14 adolescents with BD (n = 7 with CUD) and found increased frontal grey matter regional volumes and decreased temporal grey matter regional volumes in those with CUD in areas also implicated in BD (Jarvis et al., 2008). Thus far, there are no studies to our knowledge focused on cannabis use among adolescents with BD that have included a control group or that have evaluated structural neuroimaging phenotypes other than volume.
Given the high prevalence of cannabis use and CUD in adolescents with BD, together with the paucity of literature on this topic, it is important to further examine the neuro-structural correlates of cannabis use within this population. With an increase in global legalization, including Canada, and the related increase in access to cannabis and decrease in perceptions of cannabis risks, understanding the neuro-structural correlates of cannabis use among adolescents with BD is especially timely (Fischer and Rehm, 2017; Picard, 2017; Rotermann, 2020). We therefore set out to investigate this topic in a sample of adolescents with BD and healthy control (HC) adolescents. Based on prior neuroimaging studies regarding BD and cannabis use independently, we evaluated cortical volume, surface area (SA), and thickness in the following regions of interest (ROI): amygdala, hippocampus, prefrontal cortex (PFC), and anterior cingulate cortex, as well as in an exploratory vertex-wise analysis. Although volume is a product of thickness and SA, we examined each of these 3 MRI phenotypes as they are driven by different environmental, genetic, and developmental processes (Lochhead et al., 2004; Frazier et al., 2005; Wierenga et al., 2014). While cognizant that the cross-sectional design of this study precludes causal inferences, hypotheses were based on the premise of enhanced susceptibility to putative neurotoxic effects of cannabis. We hypothesized that brain structure measures would decline linearly across groups: BD cannabis users (BDCB+) < BD non-users (BDCB−) < HC.
MATERIALS AND METHODS
This study was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre. Written informed consent was obtained from all participants and their parent/guardian(s).
Participants
A total of 144 adolescent participants, ages 13 to 20, were recruited for this study, including 81 with BD (type I, type II, or not otherwise specified) and 63 HC. There were 47 adolescents in the BDCB+ group and 34 adolescents in the BDCB− group. BD participants were primarily recruited from the Centre for Youth Bipolar Disorder, a subspecialty clinical research program located at Sunnybrook Health Sciences Centre in Toronto, Canada. HC participants were recruited primarily from the community via advertisements. HC participants had no lifetime history of major psychiatric disorders, including mood and psychotic disorders, no anxiety disorders or alcohol/drug dependence in the past 3 months, and no first- or second-degree family history of BD or psychosis.
For all participants, current and lifetime psychiatric diagnoses were assessed using the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Present and Lifetime Version (KSADS-PL) (Kaufman et al., 1997), a semi-structured diagnostic interview incorporating information from the adolescent and their parent/guardian. To assess mood symptoms, we used the expanded KSADS Mania Rating Scale (Axelson et al., 2003) and KSADS Depression Rating Scale (Chambers et al., 1985). Cannabis use was evaluated during the KSADS-PL interview. Participants were asked whether they have ever used cannabis before, even if they have only tried it once. If participants answered yes, a substance use supplement was administered to determine whether they met diagnostic criteria for a CUD. Lifetime history of physical and/or sexual abuse was obtained from the KSADS-PL post-traumatic stress disorder screening questions. The Hollingshead Four-Factor Index was used to assess the socioeconomic status of parents and the Children’s Global Assessment Scale was used as a measure of current and lifetime functioning (Hollingshead, 1975; Shaffer et al., 1983). Participants were enrolled from 2012 to 2019. DSM-V was published in 2013, and the DSM-V version of K-SADS-PL was only available in 2016. Diagnoses in the current study are therefore based on DSM-IV. All interviewers had bachelors and/or masters degrees and completed rigorous training under the supervision of the principal investigator. Diagnoses and symptom ratings were reviewed and confirmed by a licensed child-adolescent psychiatrist.
The current study was a secondary analysis based on 2 other studies that employed the following exclusion criteria: (1) unable to give informed consent; and/or (2) had any infectious illness within the past 14 days; and/or (3) had an existing cardiac condition, auto-immune illness, or inflammatory illness; and/or (4) were currently taking any anti-inflammatory, anti-platelet, anti-lipidemic, anti-hypertensive, or hypoglycemic agents; and/or (5) had any MRI contraindications (i.e., cardiac pacemaker, or any metal in the body); and/or (6) had a history of severe neurological or cognitive impairments; and/or (7) substance dependence in the last 3 months. Lifetime cannabis use was not an exclusion criteria for those studies, and there were 7 HC participants with lifetime cannabis use. Due to the small cell size and lack of HC participants with CUD, these 7 HC were excluded from the present analyses.
MRI Methods
MRI data was collected on a 3 Tesla Philips Achieva medical scanner using an 8-channel head receiver coil and body coil transmission. Structural images were collected using T1 weighted high-resolution fast-field echo images, which were used to achieve grey matter and white matter image contrast. The following parameters were used for the T1 weighted image: repetition time = 9.5 milliseconds, echo time = 2.3 milliseconds, inversion time = 1400 milliseconds, spatial resolution = 0.94 × 1.17 × 1.2 mm, 256 × 164 × 140 matrix, flip angle = 8°, field of view = 240 × 191 mm2, scan duration = 8 minutes 56 seconds, 140 slices.
Prior to pre-processing, T1-weighted images were visually inspected by 2 independent raters to assess for motion or other image artifacts. For each image, a score between 0 and 3 was given based on overall image quality (i.e., number of artifacts due to excessive movement while in the scanner, contrast between white matter and grey matter, or otherwise poor image quality). If the scores between raters were incongruent, images were inspected a second time to ensure that a consensus was achieved following discussion. T1-weighted images found to be of poor quality (those images with a score of 3) were excluded from the data set before processing. Quality control and parcellation accuracy were also completed after pre-processing. T1 quality and parcellation accuracy were assessed using a scoring system by raters to exclude poor-quality images from the data set. If possible, parcellations with poor accuracy were edited.
Estimates of cortical volume, SA, and thickness were performed using FreeSurfer v6.0 (Fischl, 2012). Pre-processing included resampling the original 3D coronal images to 1-mm isotropic voxel resolution, intensity normalization (Sled et al., 1998), registration to Montreal Neurological Institute space, and automated skull stripping (Ségonne et al., 2004). Automated parcellation (Fischl et al., 2002, 2004) then proceeded with cortical surface reconstruction. This included generation of binary white matter masks in 2 hemispheres, which were used to produce a mesh of white matter surface smoothed to remove voxel-based effects and also corrected for topological defects (Fischl et al., 2001). White matter and pial surfaces were then extracted and spherically inflated to be registered to a canonical template (Fischl et al., 1999). Lastly, a parcellation algorithm was used to map the individual brains to the Destrieux cortical atlas (Destrieux et al., 2010) and based on spatial landmarks, curvatures, and sulcal depth to assign gyral labels of interest (Fischl et al., 2004). For subcortical volumes, automated segmentation occurred independently and involved nonlinear registration to the MNI305 probabilistic atlas to label various subcortical structures (Fischl et al., 2002).
To create cortical and subcortical ROI measurements, multiple individual regions were summed using the Desikan-Killiany atlas (Desikan et al., 2006). The anterior cingulate cortex included the caudal anterior cingulate, and rostral anterior cingulate, the ventromedial prefrontal cortex included the lateral and medial orbitofrontal cortex, and the ventrolateral PFC included the pars orbitalis and pars triangularis. Bilateral amygdala was considered as a single estimate and similarly for the hippocampus. Cortical measures of volume and SA were calculated by summing the values of each region. Thickness was calculated proportional to the SA of each component region.
Statistical Analysis
BD and HC participants were age and sex matched. For demographic and clinical characteristics, normality was evaluated using the Shapiro-Wilks test for all continuous variables. ANOVA or Kruskal-Wallis was used to assess between-group differences for all continuous variables, while independent samples t tests or Mann-Whitney U tests were used to assess within-BD differences for continuous variables. Categorical variables were examined using chi-squared tests. Two-tailed statistical significance was set as P < .05.
For ROI analysis, all assumptions were tested prior to analysis, which was conducted in MATLAB R2018b using a GLM. ROI measures were the outcome variables, group membership was the fixed factor (i.e., BDCB+, BDCB−, HC), and age and sex were covariates. For volume and SA analyses only, intracranial volume was added as an additional covariate. Statistical significance was defined as P < .05; the family-wise error approach was used to correct for multiple comparisons (i.e., correcting for the number of ROIs examined, P < .01).
For whole-brain vertex-wise analysis, a GLM FreeSurfer-based shell script was used (Fischl, 2012). Sex, age, and intracranial volume were used as covariates for volume and SA, whereas only sex and age were used as covariates for thickness. In this analysis, we corrected for multiple comparisons using Monte Carlo simulations thresholded at 1.3 (P < .05). Surface-based smoothing with a full width at half-maximum of 10 mm was selected based on previous literature (Fischl, 2012). Post-hoc analyses were completed similarly to ROI analysis in MATLAB using a GLM.
RESULTS
Demographic characteristics are summarized in Table 1. There were significant between-group differences in age, which was therefore included as a covariate in subsequent analyses. Clinical characteristics of the BD group are summarized in Table 2. In addition, within the HC group, 1 had smoked, 8 had ADHD, 2 had physical abuse, 1 had police contact, 3 had suicidal ideation, 5 had any anxiety, 1 had SSRI use, and 4 had stimulant use at some point in their lifetime.
Table 1.
Demographic Characteristics
| BDCB+ | BDCB− | HC | ||||
|---|---|---|---|---|---|---|
| (n = 47) | (n = 34) | (n = 63) | Test statistic | P value | Effect size | |
| Age | 17.45 ± 1.18 | 17.07 ± 1.70 | 16.99 ± 1.68 | F = 1.30 | .27 | η 2 = 0.02 |
| Sex (% female) | 31 (66.0) | 20 (58.82) | 34 (53.97) | χ 2 = 1.60 | .45 | V = 0.11 |
| SES | 4.15 ± 0.98 | 4.38 ± 0.78 | 4.27 ± 0.95 | H = 1.10 | .58 | η 2 = 0.01 |
| Race (% Caucasian) | 35 (74.47) | 26 (76.47) | 32 (50.79) | χ 2 = 9.35 | .009a,b | V = 0.26 |
| Intact family | 26 (55.32) | 23 (67.65) | 43 (68.25) | χ 2 = 2.23 | .33 | V = 0.12 |
| Tanner Stage (1–5) | 4.49 ± 0.62 | 4.32 ± 0.68 | 4.25 ± 0.65 | H = 3.82 | .15 | η 2 = 0.01 |
| BMI (adjusted) | 24.40 ± 4.30 | 23.19 ± 4.57 | 21.94 ± 3.60 | H = 11.38 | .003a | η 2 = 0.07 |
| CGAS: most severe past episode | 43.66 ± 8.96 | 43.73 ± 8.60 | — | U = 770.0 | .96 | d = 0.008 |
| CGAS: highest past year | 68.04±11.69 | 68.67 ± 10.38 | 88.56 ± 13.69 | F = 83.36 | <.00a,b | η 2 = 0.54 |
| CGAS: current episode (past month) | 64.23 ± 12.56 | 64.58 ± 11.98 | 88.29 ± 6.68 | F = 96.53 | <.001a,b | η 2 = 0.58 |
Abbreviations: BDCB−, BD non-users; BDCB+, BD cannabis users; BMI = body mass index; CGAS, Children’s Global Assessment Scale; HC, healthy controls; SES, socio-economic status.
Values are reported in mean ± SD unless otherwise indicated.
aSignificant (BDCB+ vs HC).
bSignificant (BDCB− vs HC).
Table 2.
Clinical Characteristics of BDCB+ and BDCB−
| BDCB+ (n = 47) |
BDCB− (n = 34) |
Test statistic | P value | Effect size | |
|---|---|---|---|---|---|
| BD-I | 20 (42.6) | 10 (29.4) | χ 2 = 1.68 | ||
| BD-II | 13 (27.7) | 10 (29.4) | .43 | V = 0.14 | |
| BD-NOS | 14 (29.8) | 14 (41.2) | |||
| Age of onset | 15.25 ± 2.5 | 14.30 ± 2.9 | U = 583.0 | .06* | d = 0.36 |
| Clinical characteristics | |||||
| Lifetime psychosis | 7 (14.9) | 3 (8.8) | χ 2 = 0.67 | .41 | V = 0.09 |
| Lifetime suicide attempts | 12 (25.5) | 1 (2.9) | χ 2 = 7.47 | .006* | V = 0.30 |
| Lifetime self-injurious behavior | 23 (48.9) | 17 (50.0) | χ 2 = 0.01 | .93 | V = 0.01 |
| Lifetime suicidal ideation | 29 (61.7) | 22 (64.7) | χ 2 = 0.08 | .78 | V = 0.03 |
| Police contact/arrest | 12 (25.6) | 7 (20.6) | χ 2 = 0.27 | .60 | V = 0.06 |
| Lifetime physical and/or sexual abuse | 3 (6.4) | 2 (5.9) | χ 2 = 0.009 | .93 | V = 0.01 |
| Lifetime psychiatric hospitalization | 24 (51.1) | 14 (41.2) | χ 2 = 0.77 | .38 | V = 0.10 |
| Current depression score | 16.28 ± 12.85 | 14.14 ± 9.40 | U = 722.0 | .46 | d = 0.19 |
| Lifetime depression score | 30.60 ± 11.98 | 28.68 ± 12.67 | t = 0.70 | .49 | d = 0.16 |
| Current mania score | 9.83 ± 11.10 | 8.94 ± 9.28 | U = 783.0 | .88 | d = 0.09 |
| Lifetime mania score | 30.98 ± 11.31 | 31.03 ± 10.34 | t = −0.21 | .98 | d = 0.005 |
| Lifetime comorbid diagnoses | |||||
| ADHD | 21 (44.7) | 16 (47.1) | χ 2 = 0.05 | .83 | V = 0.02 |
| Any anxiety | 37 (78.7) | 26 (76.5) | χ 2 = 0.06 | .81 | V = 0.03 |
| SUD | 16 (34.0) | 2 (5.9) | χ 2 = 9.05 | .003* | V = 0.33 |
| ODD | 13 (27.7) | 7 (20.6) | χ 2 = 0.53 | .47 | V = 0.08 |
| CD | 2 (4.3) | 1 (2.9) | χ 2 = 0.10 | .76 | V = 0.03 |
| Nicotine use (yes/no) | 10 (21.3) | 2 (5.9) | χ 2 = 3.71 | .05 | V = 0.21 |
| Alcohol dependence (yes/no) | 7 (14.89) | 0 | χ 2 = 5.54 | .02* | V = 0.26 |
| Alcohol abuse (yes/no) | 6 (12.77) | 1 (2.94) | χ 2 = 2.41 | .12 | V = 0.17 |
| Family psychiatric history | |||||
| Mania/hypomania | 8 (17.0) | 9 (26.5) | χ 2 = 1.22 | .27 | V = 0.12 |
| Depression | 25 (53.2) | 19 (55.9) | χ 2 = 0.15 | .70 | V = 0.04 |
| Anxiety | 26 (55.3) | 14 (41.2) | χ 2 = 1.29 | .26 | V = 0.13 |
| ADHD | 12 (25.5) | 8 (23.6) | χ 2 = 0.02 | .90 | V = 0.02 |
| Lifetime medications | |||||
| SGA | 34 (72.3) | 26 (76.5) | χ 2 = 0.18 | .68 | V = 0.05 |
| Lithium | 11 (23.4) | 9 (26.5) | χ 2 = 0.10 | .75 | V = 0.04 |
| SSRI antidepressants | 16 (34.0) | 9 (26.5) | χ 2 = 0.53 | .47 | V = 0.08 |
| Non-SSRI antidepressants | 11(23.4) | 5 (14.7) | χ 2 = 0.94 | .33 | V = 0.11 |
| Stimulants | 7 (14.9) | 9 (26.5) | χ 2 = 1.67 | .20 | V = 0.14 |
| Current medications | |||||
| SGA | 29 (61.7) | 20 (58.8) | χ 2 = 0.07 | 0.80 | V = 0.03 |
| Lithium | 8 (17.0) | 7 (20.6) | χ 2 = 0.17 | 0.68 | V = 0.05 |
| SSRI antidepressants | 4 (8.5) | 3 (8.8) | χ 2 = 0.002 | 0.96 | V = 0.005 |
| Non-SSRI antidepressants | 2 (4.3) | 2 (5.9) | χ 2 = 0.11 | 0.74 | V = 0.04 |
| Stimulants | 1 (2.1) | 4 (11.8) | χ 2 = 3.16 | 0.08 | V = 0.20 |
Abbreviations: ADHD, attention deficit-hyperactivity disorder; BDCB−, BD non-users; BDCB+, BD cannabis users; CD, conduct disorder; HC, healthy controls; NOS, not otherwise specified; ODD, oppositional defiant disorder; SGA, second-generation antipsychotic; SSRI, selective serotonin reuptake inhibitor; SUD, substance use disorder.
Values are reported in mean ± SD unless otherwise indicated. Depression score based on depression rating scale; mania score based on mania rating scale.
*Significant.
ROI Analysis
ROI analysis revealed a group effect in left ventromedial PFC (P = .04), which did not survive correction for multiple comparisons (P = .01). There were no other significant ROI findings.
Vertex-Wise Analysis
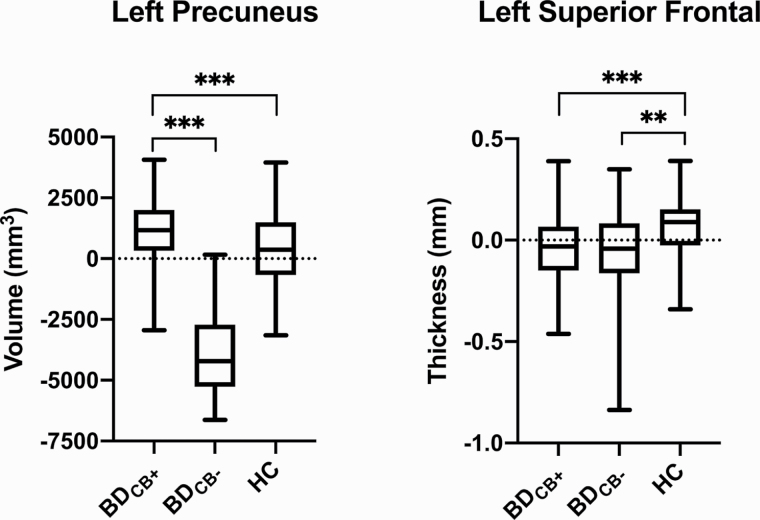
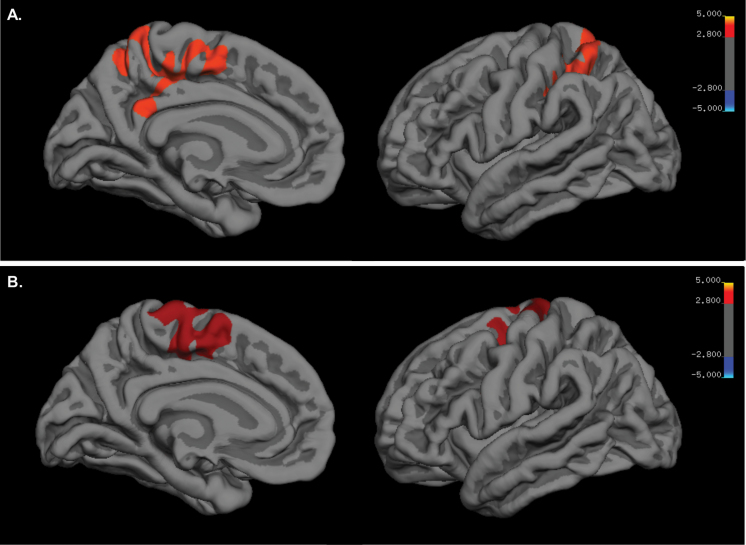
Vertex-wise analysis revealed significant group differences in 8 clusters: left and right precuneus volume (P < .001; P < .001), left inferior parietal lobe volume (P < .001), left superior parietal lobe SA (P < .001), left and right superior frontal gyri thickness (P < .001; P < .001), left precentral gyrus thickness (P = .001), and left medial orbitofrontal cortical thickness (P < .001). Post-hoc analysis revealed that, relative to HC, BDCB+ had larger left inferior parietal lobe volume (P = .003), left and right precuneus volume (P < .001; P < .001), and left superior parietal lobe SA (P = .009) as well as smaller precentral gyrus thickness (P = .001), medial orbitofrontal cortical thickness (P < .001), and left and right superior frontal gyri thickness (P < .001; P < .001). Relative to BDCB−, BDCB+ had larger left precuneus volume (P < .001), left inferior parietal lobe volume (P < .001), and left superior parietal lobe SA (P < .001) as well as smaller left medial orbitofrontal cortical thickness (P = .003). Finally, relative to HC adolescents, BDCB− had smaller left inferior parietal lobe volume (P = .01), left superior parietal lobe SA (P = .002), left precentral gyrus thickness (P = .003), and left and right superior frontal gyri thickness (P = .001; P = .002). All post-hoc results remained significant after correcting for multiple comparisons (P < .017). Tables 3 and 4 display the vertex-wise results for group analyses as well as post-hoc analyses. Figures 1 and 2 depict the findings in the left precuneus and left superior frontal gyrus. All findings remained unchanged in sensitivity analysis further controlling for nicotine use.
Table 3.
Vertex-Wise Analyses
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Peak cluster region | Cortical measure | Cluster Size (mm2) | cwp | x | y | z | Additional region/s |
| Left superior parietal | Surface Area | 2528.33 | 0.0105 | −29.7 | −47.5 | 37.6 | Inferior parietal |
| Left precuneus | Volume | 3514.5 | 0.0001 | −11.4 | −57.8 | 54.1 | Supramarginal, superior parietal, inferior parietal, postcentral, paracentral, isthmus cingulate, posterior cingulate, superior frontal |
| Left inferior parietal | Volume | 1948.11 | 0.0009 | −29.1 | −67 | 36.8 | Superior parietal |
| Right precuneus | Volume | 1854.35 | 0.0009 | 9.7 | −70 | 38.9 | Superior parietal, inferior parietal |
| Left superior frontal | Thickness | 1912.88 | 0.0012 | −6.3 | −2.2 | 57.9 | Precentral gyrus, paracentral |
| Left precentral gyrus | Thickness | 1638.33 | 0.0045 | −47.2 | 1.1 | 46.6 | Caudle middle frontal |
| Left medial orbitofrontal | Thickness | 1218.21 | 0.0302 | −6.9 | 24.6 | −12.2 | Rostral middle frontal, frontal pole, lateral orbitofrontal, rostral middle frontal |
| Right superior frontal | Thickness | 1030.91 | 0.0444 | 8.1 | 22.9 | 41.1 | No other regions present |
Abbreviations: cwp, cluster wide P value; MNI, Montreal Neurological Institute and Hospital.
Monte Carlo Z simulation threshold was set at 1.3 for all analyses.
Table 4.
Vertex-Wise Post-Hoc Analyses
| Post-hoc groups | Cortical measure | Peak cluster region | Test statistic (F) | P value | Effect size (η 2) |
|---|---|---|---|---|---|
| BDCB+ > BDCB− | Left superior parietal | Surface Area | 23.346 | <.001* | 0.303 |
| Left precuneus | Volume | 25.913 | <.001* | 0.330 | |
| Left inferior parietal | Volume | 22.986 | <.001* | 0.299 | |
| Right precuneus | Volume | 12.541 | <.001* | 0.175 | |
| BDCB+ < BDCB− | Left superior frontal | Thickness | 0.260 | .611 | 0.004 |
| Left precentral gyrus | Thickness | 0.152 | .698 | 0.002 | |
| Left medial orbitofrontal | Thickness | 8.750 | .004* | 0.119 | |
| Right superior frontal | Thickness | 0.573 | .450 | 0.008 | |
| BDCB+ > HC | Left superior parietal | Surface Area | 6.935 | .009* | 0.101 |
| Left precuneus | Volume | 14.508 | <.001* | 0.199 | |
| Left inferior parietal | Volume | 9.172 | .003* | 0.131 | |
| Right precuneus | Volume | 19.477 | <.001* | 0.259 | |
| BDCB+ < HC | Left superior frontal | Thickness | 12.642 | <.001* | 0.168 |
| Left precentral gyrus | Thickness | 10.497 | .001* | 0.141 | |
| Left medial orbitofrontal | Thickness | 19.415 | <.001* | 0.247 | |
| Right superior frontal | Thickness | 13.606 | <.001* | 0.180 | |
| BDCB− < HC | Left superior parietal | Surface Area | 10.042 | .002* | 0.142 |
| Left precuneus | Volume | 3.989 | .048* | 0.059 | |
| Left inferior parietal | Volume | 6.790 | .010* | 0.099 | |
| Left superior frontal | Thickness | 10.758 | .001* | 0.145 | |
| Left precentral gyrus | Thickness | 9.460 | .003* | 0.128 | |
| Left medial orbitofrontal | Thickness | 1.895 | .171 | 0.027 | |
| Right superior frontal | Thickness | 9.853 | .002* | 0.133 | |
| BDCB− > HC | Right precuneus | Volume | 0.806 | .371 | 0.012 |
Abbreviations: BD, bipolar disorder; BDCB+, BD cannabis users; BDCB−, BD non-users.
*Significant.
Figure 1.
Graphs for vertex-wise analyses. There was a significant group effect in the left precuneus (P < .001) and left superior frontal gryus (P < .001). Post-hoc analyses revealed volume in the left precuneus was larger in BDCB+ relative to BDCB− and HC, whereas thickness in the left superior frontal gyrus was smaller in BDCB+ and BDCB− relative to HC.
Figure 2.
Brain maps for vertex-wise analyses. (A) Brain map of the left hemisphere of a cluster with the peak vertex located in the left precuneus (P < .001). (B) Brain map of the left hemisphere of a cluster with the peak vertex located in the left superior frontal gyrus (P = .001).
Discussion
This study examined brain volume, SA, and thickness among adolescents in 3 groups: BDCB+, BDCB−, and HC. Between-group differences were observed in 8 frontal and parietal regions. Overall, there was a pattern of findings whereby BDCB+ had larger volume and/or SA in parietal regions and smaller thickness in frontal regions compared with both HC and BDCB−. Furthermore, BDCB− displayed smaller volume, SA, and thickness in these regions compared with HC. This study advances prior research on this subject by virtue of a larger sample size, integrating a control group, and evaluating multiple structural neuroimaging phenotypes.
The direction of the between-group differences in this study differs according to brain region and imaging phenotype. Regional differences in genetic vs environmental effects may be contributory. A study examining regional differences in genetic and environmental influences on brain structure in a healthy adolescent population found that regions with primarily environmental influences (>80%) were predominantly in the parietal lobe and regions with primarily genetic contributions (>80%) were predominantly in the frontal lobe (Yang et al., 2012). Different neurostructural phenotypes (i.e., volume, SA, thickness) are also differentially affected by environmental and genetic factors (Lenroot et al., 2009; Winkler et al., 2010). Moreover, SA shows much more variation both within and between subjects compared with thickness, and genetic factors that control SA appear to differ from those that influence thickness (Lenroot et al., 2009; Winkler et al., 2010). Furthermore, while volume measures are based on the product of SA and thickness, volume appears to be driven by SA more so than by thickness (Winkler et al., 2010). Taken together, these studies suggest that current findings regarding SA in the parietal lobe are more susceptible to environmental influences, whereas thickness in the frontal lobe is more related to genetic influences.
Cannabis is known to have pleiotropic neurodevelopmental effects, with both increases and decreases in regional brain structures (Lopez-Larson et al., 2011; Jacobus and Tapert, 2014). In studies of adolescents and adults with BD who have identified differences in parietal lobe structure, these have primarily been in the direction of smaller structure (Frazier et al., 2005; Li et al., 2011; Altamura et al., 2018; Hibar et al., 2018). In contrast, studies of adolescents and adults using cannabis have found both larger and smaller parietal regions relative to controls (Mata et al., 2010; Lopez-Larson et al., 2011; Jacobus and Tapert, 2014; Price et al., 2015). Prior studies in adolescents have found that cannabis use and BD are each associated with differences in PFC structure, primarily reduction (Dickstein et al., 2005; Price et al., 2015; Nader and Sanchez, 2018). While most research on this topic has been cross-sectional, there is prior evidence that smaller volumes in frontal regions predate initiation of cannabis and other substance use in adolescents (Cheetham et al., 2012; Lippard et al., 2017).
In this cross-sectional study, it cannot be determined whether observed differences comprise causes or effects of cannabis use. We speculate that our findings regarding larger parietal lobe volume and SA may reflect effects of cannabis on the brain, whereas our findings regarding reduced frontal cortical thickness may reflect genetic predisposition to cannabis use. Previous literature has shown us that smaller PFC structure and reduced activity have been linked to impaired impulse and decision-making in adults and adolescents with BD (Dickstein et al., 2005; Ha et al., 2009; Mazzola-Pomietto et al., 2009; Chen et al., 2011). Studies have also shown poorer white matter integrity in fronto-parietal circuitry among adolescent cannabis users (Bava et al., 2019; Jacobus and Tapert, 2014). White matter integrity in these regions has been linked to neurocognitive performance on measures of attention, processing speed, and working memory as well as to emotional functioning and prospective risk taking in substance users (Medina et al., 2007; Jacobus et al., 2013). Finally, the findings of smaller volume, SA, and thickness in frontal and parietal regions of the in BDCB− relative to HC converge with prior studies (Frazier et al., 2005; Li et al., 2011; Lim et al., 2013).
Several limitations of this study warrant consideration. First, the cross-sectional methodology precludes causal or directional inferences. As previously mentioned, the current study cannot directly examine whether present findings reflect predisposition to and/or consequences of cannabis use. Second, the sample size was not powered to detect small effect sizes such that there may be additional regions also associated with cannabis use that were not detected in this study. This study was also not powered for the complicated multivariable models that would be needed to parse the independent association of cannabis use with brain structure after controlling for various clinical characteristics such as comorbidity, treatment, and family psychiatric history. Such studies are needed and will require substantially larger sample sizes. Third, this study did not have an HC cannabis use group, hindering our ability to examine whether neuro-structural correlates of cannabis use differ between BD and HC groups. Fourth, urine toxicology was not used in this study; this could have led to underreporting of cannabis use and biased the results towards negative findings. Finally, this study did not collect information regarding cannabis potency, quantity or duration of use, and could not evaluate for related associations with brain structure.
Despite these limitations, the current study advances knowledge regarding the association between cannabis use and brain structure in relation to adolescent BD. Future studies based on larger samples, using prospective methodology, and examining dose effects (e.g., potency, frequency, duration) are warranted. Future research should also include neurocognitive measures and other measures that would signal whether observed neuro-structural differences are beneficial or detrimental. In Canada, cannabis has been legalized for adults at least 19 years of age, and increased availability and acceptance of cannabis use is anticipated (Fischer and Rehm, 2017; Picard, 2017). While preliminary and cross-sectional, our findings suggest that adolescents with BD may comprise a group in whom cannabis-related neuro-structural effects are pronounced. In conclusion, in this case control study regarding the association of cannabis use with brain structure in adolescent BD, we found significant findings driven by larger volume and/or SA, and smaller cortical thickness among BDCB+ vs HC. Given the context of increasing global legalization, increased potency, and increased belief regarding salutary health effects of cannabis, addition research on this topic is greatly needed.
Acknowledgments
We acknowledge the contribution of the staff at the Centre for Youth Bipolar Disorder at Sunnybrook Health Sciences Centre and thank the adolescents and their families for their participation.
This study was supported by the Ontario Mental Health Foundation (1010589 to B. I. Goldstein) and the Canadian Institutes of Health Research (MOP-136947 to B. I. Goldstein).
Statement of Interest
Dr B. I. Goldstein receives grant or research support from the Brain and Behavior Research Foundation (NARSAD), Brain Canada, the Canadian Institutes of Health Research, the Heart and Stroke Foundation, National Institute of Mental Health, the Ontario Ministry of Research and Innovation, and the departments of psychiatry of Sunnybrook Health Sciences Centre and the University of Toronto. Dr Bradley MacIntosh receives grant or research support from the Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council, and the NARSAD. The remaining authors have no financial disclosures to report.
References
- Agrawal A, Nurnberger JI Jr, Lynskey MT; Study TBG (2011) Cannabis involvement in individuals with bipolar disorder. Psychiatry Res 185:459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura AC, Maggioni E, Dhanoa T, Ciappolino V, Paoli RA, Cremaschi L, Prunas C, Orsenigo G, Caletti E, Cinnante CM, Triulzi FM, Dell’osso B, Yatham L, Brambilla P (2018) The impact of psychosis on brain anatomy in bipolar disorder: a structural MRI study. J Affect Disord 233:100–109. Available at: 10.1016/j.jad.2017.11.092. Accessed January 28, 2020. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, Gee J, Sevy S, Kumra S (2011) Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res 45:1055–1066. Available at: https://www.ncbi.nlm.nih.gov/pubmed/21296361. Accessed June 11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, Ryan N (2003) A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol 13:463–470. Available at: https://www.ncbi.nlm.nih.gov/pubmed/14977459. Accessed March 14, 2019. [DOI] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF (2019) Altered white matter microstructure in adolescent substance users. Psychiatry Res - Neuroimaging 173:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Keller M (2006) Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry 63:175–183. Available at: https://www.ncbi.nlm.nih.gov/pubmed/16461861. Accessed April 3, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M (1985) The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch Gen Psychiatry 42:696–702. Available at: https://www.ncbi.nlm.nih.gov/pubmed/4015311. Accessed March 14, 2019. [DOI] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons JG, Yücel M, Lubman DI (2012) Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol Psychiatry 71:684–692. [DOI] [PubMed] [Google Scholar]
- Chen C-H, Suckling J, Lennox BR, Ooi C, Bullmore ET (2011) A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord 13:1–15. Available at: http://doi.wiley.com/10.1111/j.1399-5618.2011.00893.x. Accessed May 7, 2020. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. Available at: https://www.sciencedirect.com/science/article/pii/S1053811906000437. Accessed July 19, 2019. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E (2010) Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53:1–15. Available at: https://www.sciencedirect.com/science/article/pii/S1053811910008542. Accessed July 19, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney DS, Pine DS, Leibenluft E (2005) Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry 62:734–741. Available at: https://www.ncbi.nlm.nih.gov/pubmed/15997014. Accessed May 7, 2020. [DOI] [PubMed] [Google Scholar]
- Fischer B, Rehm J (2017) Cannabis use, legalization and youth health. CMAJ 189:E971–E972. Available at: https://www.ncbi.nlm.nih.gov/pubmed/28739851. Accessed January 22, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B (2012) FreeSurfer. Neuroimage 62:774–781. Available at: https://www.ncbi.nlm.nih.gov/pubmed/22248573. Accessed July 19, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RBH, Dale AM (1999) High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8:272–284. Available at: http://doi.wiley.com/10.1002/%28SICI%291097-0193%281999%298%3A4%3C272%3A%3AAID-HBM10%3E3.0.CO%3B2-4. Accessed July 19, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM (2001) Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 20:70–80. Available at: http://ieeexplore.ieee.org/document/906426/. Accessed July 19, 2019. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. Available at: https://www.sciencedirect.com/science/article/pii/S089662730200569X. Accessed July 19, 2019. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004) Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. Available at: https://academic.oup.com/cercor/article-lookup/doi/10.1093/cercor/bhg087. Accessed July 19, 2019. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Breeze JL, Makris N, Giuliano AS, Herbert MR, Seidman L, Biederman J, Hodge SM, Dieterich ME, Gerstein ED, Kennedy DN, Rauch SL, Cohen BM, Caviness VS (2005) Cortical gray matter differences identified by structural magnetic resonance imaging in pediatric bipolar disorder. Bipolar Disord 7:555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Strober MA, Birmaher B, Axelson DA, Esposito-Smythers C, Goldstein TR, Leonard H, Hunt J, Gill MK, Iyengar S, Grimm C, Yang M, Ryan ND, Keller MB (2008) Substance use disorders among adolescents with bipolar spectrum disorders. Bipolar Disord 10:469–478. Available at: https://www.ncbi.nlm.nih.gov/pubmed/18452443. Accessed June 17, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TH, Ha K, Kim JH, Choi JE (2009) Regional brain gray matter abnormalities in patients with bipolar II disorder: a comparison study with bipolar I patients and healthy controls. Neurosci Lett 456:44–48. Available at: https://www.ncbi.nlm.nih.gov/pubmed/19429131. Accessed May 19, 2020. [DOI] [PubMed] [Google Scholar]
- Hibar DP, et al. (2018) Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. EJ Canales-Rodriguez 20:932–942. Available at: http://enigma.ini.usc.edu/proto. Accessed January 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A (1975) Four factor index of social status. Available at: https://sociology.yale.edu/sites/default/files/files/yjs_fall_2011.pdf#page=21. Accessed March 18, 2020.
- Jacobus J, Tapert SF (2014) Effects of cannabis on the adolescent brain. Curr Pharm Des 20:2186–2193. Available at: https://www.ncbi.nlm.nih.gov/pubmed/23829363. Accessed June 11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Thayer RE, Trim RS, Bava S, Frank LR, Tapert SF (2013) White matter integrity, substance use, and risk taking in adolescence. Psychol Addict Behav 27:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis K, DelBello MP, Mills N, Elman I, Strakowski SM, Adler CM (2008) Neuroanatomic comparison of bipolar adolescents with and without cannabis use disorders. J Child Adolesc Psychopharmacol 18:557–563. Available at: https://www.ncbi.nlm.nih.gov/pubmed/19108660. Accessed January 7, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1997) Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988. Available at: https://www.ncbi.nlm.nih.gov/pubmed/9204677. Accessed March 6, 2020. [DOI] [PubMed] [Google Scholar]
- Kozloff N, Cheung AH, Schaffer A, Cairney J, Dewa CS, Veldhuizen S, Kurdyak P, Levitt AJ (2010) Bipolar disorder among adolescents and young adults: results from an epidemiological sample. J Affect Disord 125:350–354. Available at: https://www.ncbi.nlm.nih.gov/pubmed/20226535. Accessed April 22, 2020. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Kendler KS, Evans AC, Giedd JN (2009) Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp 30:163–174. Available at: http://doi.wiley.com/10.1002/hbm.20494. Accessed March 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Cui L, Deng W, Ma X, Huang C, Jiang L, Wang Y, Collier DA, Gong Q, Li T (2011) Voxel-based morphometric analysis on the volume of gray matter in bipolar I disorder. Psychiatry Res Neuroimaging 191:92–97. [DOI] [PubMed] [Google Scholar]
- Lim CS, Baldessarini RJ, Vieta E, Yucel M, Bora E, Sim K (2013) Longitudinal neuroimaging and neuropsychological changes in bipolar disorder patients: review of the evidence. Neurosci Biobehav Rev 37:418–435. [DOI] [PubMed] [Google Scholar]
- Lippard ETC, Mazure CM, Johnston JAY, Spencer L, Weathers J, Pittman B, Wang F, Blumberg HP (2017) Brain circuitry associated with the development of substance use in bipolar disorder and preliminary evidence for sexual dimorphism in adolescents. J Neurosci Res 95:777–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead RA, Parsey RV, Oquendo MA, Mann JJ (2004) Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Available at: www.elsevier.com/locate/biopsych. Accessed January 28, 2020. [DOI] [PubMed]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D (2011) Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav Brain Res 220:164–172. Available at: https://www.ncbi.nlm.nih.gov/pubmed/21310189. Accessed June 11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata I, Perez-Iglesias R, Roiz-Santiañez R, Tordesillas-Gutierrez D, Pazos A, Gutierrez A, Vazquez-Barquero JL, Crespo-Facorro B (2010) Gyrification brain abnormalities associated with adolescence and early-adulthood cannabis use. Brain Res 1317:297–304. [DOI] [PubMed] [Google Scholar]
- Mazzola-Pomietto P, Kaladjian A, Azorin JM, Anton JL, Jeanningros R (2009) Bilateral decrease in ventrolateral prefrontal cortex activation during motor response inhibition in mania. J Psychiatr Res 43:432–441. [DOI] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF (2007) Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry 48:592–600. Available at: http://doi.wiley.com/10.1111/j.1469-7610.2007.01728.x. Accessed January 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF (2009) Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol 14:457–468. Available at: https://www.ncbi.nlm.nih.gov/pubmed/19650817. Accessed January 28, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader DA, Sanchez ZM (2018) Effects of regular cannabis use on neurocognition, brain structure, and function: a systematic review of findings in adults. Am J Drug Alcohol Abus 44:4–18. Available at: https://www.ncbi.nlm.nih.gov/pubmed/28498718. Accessed May 7, 2019. [DOI] [PubMed] [Google Scholar]
- Picard A (2017) Panel provides guidelines for safe cannabis use - The Globe and Mail. Available at: https://www.theglobeandmail.com/news/national/panel-provides-guidelines-for-safe-cannabis-use/article35441871/. Accessed January 22, 2019.
- Price JS, McQueeny T, Shollenbarger S, Browning EL, Wieser J, Lisdahl KM (2015) Effects of marijuana use on prefrontal and parietal volumes and cognition in emerging adults. Psychopharmacology (Berl) 232:2939–2950. Available at: http://link.springer.com/10.1007/s00213-015-3931-0. Accessed May 7, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotermann M (2020) Health reports what has changed since cannabis was legalized?Health Rep 31:11–20. Available at: 10.25318/82-003-x202000200002-eng. Accessed April 20, 2020. [DOI] [PubMed] [Google Scholar]
- Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B (2004) A hybrid approach to the skull stripping problem in MRI. Neuroimage 22:1060–1075. Available at: https://www.sciencedirect.com/science/article/pii/S1053811904001880. Accessed August 8, 2019. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S (1983) A children’s global assessment scale (CGAS). Arch Gen Psychiatry 40:1228–1231. jamanetwork.com. Available at: https://jamanetwork.com/journals/jamapsychiatry/article-abstract/493197 Accessed March 18, 2020. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97. Available at: http://ieeexplore.ieee.org/document/668698/. Accessed July 19, 2019. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF (2009) The influence of substance use on adolescent brain development. Clin EEG Neurosci 40:31–38. Available at: https://www.ncbi.nlm.nih.gov/pubmed/19278130. Accessed June 11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler E, Jones S, Black N, Carter LA, Barrowclough C (2015) The relationship between bipolar disorder and cannabis use in daily life: an experience sampling study. PLoS One 10:e0118916. Available at: https://www.ncbi.nlm.nih.gov/pubmed/25738578. Accessed June 13, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rossum I, Boomsma M, Tenback D, Reed C, van Os J, Board EA (2009) Does cannabis use affect treatment outcome in bipolar disorder? A longitudinal analysis. J Nerv Ment Dis 197:35–40. Available at: https://www.ncbi.nlm.nih.gov/pubmed/19155808. Accessed June 13, 2019. [DOI] [PubMed] [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S (2014) Unique developmental trajectories of cortical thickness and surface area. Neuroimage 87:120–126. Available at: https://www.ncbi.nlm.nih.gov/pubmed/24246495. Accessed January 30, 2020. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Kwon A, Ditterline J, Forkner P, Moore H, Swezey A, Snyder L, Henin A, Wozniak J, Faraone SV (2004) Risk of substance use disorders in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 43:1380–1386. Available at: https://www.ncbi.nlm.nih.gov/pubmed/15502597. Accessed July 28, 2019. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC (2010) Cortical thickness or grey matter volume? The Importance of selecting the phenotype for imaging genetics studies. Neuroimage 53:1135–1146. Accessed March 6, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU, Frohlich C, Behrendt S, Gunther A, Rehm J, Zimmermann P, Lieb R, Perkonigg A (2007) Cannabis use and cannabis use disorders and their relationship to mental disorders: a 10-year prospective-longitudinal community study in adolescents. Drug Alcohol Depend 88Suppl 1:S60–S70. Available at: https://www.ncbi.nlm.nih.gov/pubmed/17257779. Accessed June 13, 2019. [DOI] [PubMed] [Google Scholar]
- Yang Y, Joshi AA, Joshi SH, Baker LA, Narr KL, Raine A, Thompson PM, Damasio H (2012) Genetic and environmental influences on cortical thickness among 14-year-old twins. Neuroreport 23:702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]