Abstract
Background
Antipsychotics improve the positive symptoms of schizophrenia. However, little is known about the extent of antidepressive effects of antipsychotics and their correlation with effects on other symptom domains in schizophrenia. The aim was to investigate whether antidepressive effects of antipsychotics have a significant correlation with the effects on specific symptom domains of schizophrenia.
Methods
Electronic databases were searched to identify eligible studies that reported antidepressive effects of antipsychotics for the treatment of adult patients with schizophrenia in double-blind, randomized placebo-controlled trials (RCTs). Mean change from baseline in depressive symptoms was meta-analyzed, and the correlation with the effects on other symptom domains was examined through meta-regression analysis.
Results
Thirty-five RCTs (13 890 patients) were included in this meta-analysis. Overall, antipsychotics showed greater efficacy than placebo in reducing depressive symptoms, with small to medium effect sizes (standardized mean difference = −0.27, 95% confidence interval −0.32 to −0.22, P < .001). All the antipsychotics, except for chlorpromazine, haloperidol, and ziprasidone, were associated with significantly greater decreases in depressive symptoms compared with placebo (standardized mean difference = −0.19 to −0.40). A higher antidepressive effect was significantly correlated with a higher improvement in Positive and Negative Syndrome Scale/Brief Psychiatric Rating Scale total, positive, and negative, and Positive and Negative Syndrome Scale-general psychopathology symptoms (β = .618, P < .001; β = .476, P < .001; β = .689, P < .001; β = .603, P < .001, respectively).
Conclusions
Second-generation antipsychotics (except for ziprasidone) were associated with small to medium effects sizes on improvement in depressive symptoms among adult patients with schizophrenia. The antidepressive effect of antipsychotics was significantly correlated with improvement in other symptom domains, with the highest correlation observed for improvement in negative symptoms.
PROSPERO registration number
CRD42019133015
Keywords: Antipsychotics, antidepressants, schizophrenia, psychopathology, meta-regression
Significance Statement.
To our knowledge, this study is the first comprehensive meta-analysis that focused on the antidepressive effects of antipsychotics in adult patients with acute schizophrenia. In the present systematic review and meta-analysis of 35 double-blind, placebo-controlled RCTs with a total of 13 890 patients, we found that treatment with second-generation antipsychotics except for ziprasidone was associated with significant improvement of depressive symptoms with small to medium effect size. Furthermore, there was a significant correlation between improvement in depressive symptoms and in psychopathological domain, indicating that some of the reduction in depressive symptoms may be related to the improvement in other symptoms of schizophrenia, in particular negative symptoms.
Despite the extensive use of second-generation antipsychotics in the treatment of schizophrenia, depressive symptoms are still frequently observed and remain a therapeutic challenge in clinical practice. Our findings could renew interest in clinical research on the true antidepressive effect of antipsychotics.
Introduction
A significant proportion of patients with schizophrenia exhibit depressive symptoms during their disease course (Siris, 2000; Siris and Bench, 2003; Conley et al., 2007; Buckley et al., 2009; Majadas et al., 2012). One possible explanation of such depressive symptoms was the early concept of “akinetic depression” that attributed such depression to the extrapyramidal symptoms induced by first-generation antipsychotic (FGA) medications (Van Putten and May, 1978). Another explanation was the notion of “revealed depression” that hypothesized that depression is an inherent part of the illness with the depressive symptoms hidden from clinicians when psychotic symptoms are pronounced, only to be revealed when such psychotic symptoms remit (Hirsch, 1982). A further idea was that of “post-psychotic” depression that hypothesized that reactive depression ensures when individuals with schizophrenia recover and gain insight into their illness and life situation (McGlashan and Carpenter, 1976). Depressive symptoms in schizophrenia have also been thought to be peripheral symptoms caused by comorbid mental disorders (Siris, 2000; Siris and Bench, 2003).
The presence of depressive symptoms in patients with schizophrenia is characterized by an impairment in occupational functioning (Sands and Harrow, 1999), a decreased quality of life (Reine et al., 2003; Sim et al., 2004), and an increased risk of relapse or hospitalization (Mandel et al., 1982). More importantly, depressive symptoms in schizophrenia are closely related to a higher risk of suicide (Bottlender et al., 2000). Thus, depressive symptoms are a major cause of health, social, and financial burden of schizophrenia (Knapp et al., 2004). The frequency of clinically relevant depressive symptoms is greater in the acute phase than in maintenance phase of schizophrenia (Mulholland and Cooper, 2000). Patients with schizophrenia in a stable phase frequently present depressive symptoms despite not being diagnosed with depression (Majadas et al., 2012). Such depressive symptoms in patients with schizophrenia are therefore likely underrecognized and undertreated. Currently available antipsychotics improve the positive symptoms of schizophrenia (Leucht et al., 2017). However, little is known about the extent of antidepressive effect of antipsychotics and about their relationship to the effect on other symptom domains in those with this disorder. Multiple antipsychotics are now approved for use in multiple countries for treatment of treatment-resistant major depressive disorder, and the use of such drugs for treating depression has increased substantially (Gerhard et al., 2014). This further raises the question of how effective these agents are in the treatment of depression in the context of schizophrenia.
We hypothesized that antipsychotics are associated with significant improvement of depressive symptoms in patients with schizophrenia. We aimed to conduct a systematic review and meta-analysis of acute-phase, randomized placebo-controlled trials (RCTs) reporting the efficacy of antipsychotics on depressive symptoms in schizophrenia and to investigate the relationship between antidepressive effect of antipsychotics and effects on the other symptom domains of schizophrenia.
Methods
The meta-analysis followed PRISMA guidelines for reporting meta-analyses of RCTs (Moher et al., 2009). The review protocol was registered at the international prospective register of systematic reviews registration number CRD42019133015.
Study Selection
We selected published and unpublished double-blind RCTs of antipsychotics for adult patients with acute schizophrenia or related disorders; all were placebo controlled. We included all antipsychotics licensed in at least 1 country, except clozapine, as it may be a more efficacious drug (Huhn et al., 2019), and so pooling it with the other compounds would not have been appropriate (only 1 clozapine arm with 16 patients from 1 study [Honigfeld, 1984] had to be excluded on this basis making the impact of this decision negligible). We excluded intramuscular formulations because these are used primarily either for emergency use (short-acting intramuscular drugs) or for relapse prevention (long-acting depot drugs).
Data Sources
We conducted a literature search without language restrictions—using MEDLINE/PubMed, Cochrane library, Scopus, and Embase from database inception (last search: May 18, 2019)—for RCTs of patients with acute schizophrenia. We also searched for unpublished studies, such as conference proceedings and clinical trial registries (http://clinicaltrials.gov/). Search terms included synonyms of (1) schizophrenia AND (2) controlled AND (3) randomized AND (4) clinical trial AND (5) antipsychotic drug. Hand searches of reference lists of relevant publications were also conducted. Because the current investigation was a systematic review and meta-analysis utilizing only secondary sources, informed consent and approval by an ethics board were not required.
Data Extraction
Data were extracted independently by ≥2 reviewers (I.M., T.N., K.H.) experienced in conducting literature searches and data extraction. Disagreements were resolved by consensus. Foreign language papers were translated by bilingual speakers, and data extraction was double checked by 2 investigators (T.N., K.H.) using Google Translate (http://translate.google.com/).
The primary efficacy outcome was set as mean change from baseline to endpoint in depressive symptom scale scores, as represented by the change in the Montgomery Åsberg Depression Rating Scale (MADRS) (Montgomery and Åsberg, 1979), Hamilton Rating Scale for Depression (HAM-D) (Hamilton, 1960), or Calgary Depression Scale for Schizophrenia (CDSS) (Addington et al., 1993). If changes in those scale scores were not available, the change in the score on the Positive and Negative Syndrome Scale (PANSS)-Anxiety/Depression factor (Kay et al., 1987) or on the Brief Psychiatric Rating Scale (BPRS)-Depression cluster (Overall and Gorham, 1962) was used for the calculation of the effect size. Secondary outcomes included symptom severity (positive, negative, general, and total symptoms), Clinical Global Impressions-Severity Illness scale (CGI-S) (Guy, 1976), global functioning, quality of life, all-cause discontinuation at study endpoint, inefficacy-related discontinuation, and intolerability-related discontinuation.
All eligible trials were assessed for methodological quality using the Cochrane Collaboration’s tool for assessing risk of bias (Higgins et al., 2011). We extracted data on study design and patient, illness, and treatment components.
Data Synthesis
All data were double-entered into and meta-analyzed with Comprehensive Meta-Analysis Version 3 (BioStat; Englewood, New Jersey) using a random effects model, as heterogeneity among studies was expected (DerSimonian and Laird, 1986). Continuous outcomes were expressed as the standardized mean difference (SMD) using the inverse variance method, each with their 95% confidence intervals (CIs). For simplicity, we adjusted effect sizes so that SMDs < 0 indicated superiority of antipsychotic drug, independent of whether a lower value (e.g., depressive symptom) or higher value (e.g., functioning, quality of life) was a positive outcome. Dichotomous outcomes were expressed as relative risk (RR) each with their 95% CIs. RR values <1 indicated superiority of antipsychotic treatment for negative outcomes such as discontinuation due to any cause. Number-needed-to-harm (NNH) was calculated when risk differences were significant.
Several articles reported trials with 2 or more experimental groups and only 1 placebo group. To avoid counting the placebo patients twice, we followed the recommendation of the Cochrane Collaboration and divided the placebo group equally into 2 (or more) groups with smaller sample sizes so that the total numbers of participants added up to the original size of the group. We thereby avoided an inflation of sample size, which would lead to an increase of type I errors and thus overoptimistically small SDs. We explored study heterogeneity using the χ2 test of homogeneity and I2 statistics, with P < .05 and I2 > 50%, respectively, indicating significant heterogeneity. All analyses were 2-tailed with an α of .05. In the primary analyses, antipsychotics and placebo were compared at study end point.
We also conducted subgroup and exploratory maximum likelihood random-effects meta-regression analyses of the primary outcome to identify potential moderators or mediators. Subgroup analyses were based on (1) antipsychotic drug type (FGA vs second-generation antipsychotics [SGA]), (2) neuroscience-based nomenclature (NbN) (Zohar et al., 2015), (3) publication year (published 1999 or before, from 2000 to 2009, 2010 or later), (4) country, (5) study sample size (n = ≤400 vs >400), (6) mean age (coded in these ranges: 30–35, 35–40, 40–45, 45–50 years), (7) included patients (schizophrenia, schizophrenia and schizoaffective disorder or schizoaffective disorder), and (8) rating scale (MADRS, HAM-D, or CDSS vs the PANSS anxiety/depression scale or BPRS depression cluster). The NbN categories were defined as follows: M1: receptor antagonists (D2) clopenthixol, fluphenazine, haloperidol, perphenazine, pimozide, pipotiazine, sulpiride, and trifluoperazine; M2: receptor antagonists (D2, 5-HT2) chlorpromazine, iloperidone, loxapine, lurasidone, olanzapine, sertindole, thioridazine, ziprasidone, and zotepine; M3: receptor partial agonists (D2, 5-HT1A) aripiprazole, brexpiprazole, and cariprazine; M4: receptor antagonists (D2, 5-HT2, NE, α2) asenapine, paliperidone, and risperidone; and M5: receptor antagonist (D2, 5-HT2) and reuptake inhibitor (NET) quetiapine.
The secondary outcome measures were regressed on the primary outcome measures to better understand the clinical impact of the primary outcome measures. Finally, we inspected funnel plots. The regression test by (Egger et al., 1997) and the trim-and-fill method by Duval and Tweedie, (2000) were used to examine the presence of publication bias. In this large exploratory set of analyses, no adjustments were made to the P values for the multiple comparisons; therefore, these P values should be interpreted with caution.
Results
Results of the Search
We identified 9572 records and included 35 RCTs with a total of 13 890 participants. The PRISMA flow diagram of the search and screening process is presented in supplementary Figure 1; detailed characteristics of individual included studies are summarized in Table 1.
Table 1.
Characteristics of Included Studies
| Study | Design | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| blinding | |||||||||
| country | |||||||||
| sponsor | Duration | Patients, No. (randomized) treatment arms (n) | Population | Mean age (y) | % Male | % White | Mean dose (mg/d) or range | Depression rating scale | |
| Borison, 1996 | DB | 6 | 109 | Schizophrenia (DSM-III-R) 18–60 y of age | 36.5 | 89.9 | 60.6 | 307 | BPRS depression cluster score |
| NR | QUE (54) | ||||||||
| Pharma | PBO (55) | ||||||||
| Cantillon, 2017 | DB | 7 | 234 | Schizophrenia or schizoaffective disorder (DSM-IV- TR) 18–65 y of age | 35.9 | 76.4 | 5.15 | ARI 15 fixed dose | CDSS |
| International | ARI 15 (20) | ||||||||
| Pharma | [RP5063 15 (58)] | ||||||||
| [RP5063 30 (59)] | |||||||||
| [RP5063 50 (58)] | |||||||||
| PBO (39) | |||||||||
| Canuso, 2010a | DB | 6 | 316 | Schizoaffective disorder (DSM-IV) 18–65 y of age | 37.2 | 64.8 | 46.5 | Lower dose 5.7 Higher dose 11.6 |
HAM-D 21a |
| International | PAL Lower-dose (109) | ||||||||
| Pharma | PAL Higher-dose (100) | ||||||||
| PBO (107) | |||||||||
| Canuso, 2010b | DB | 6 | 311 | Schizoaffective disorder (DSM-IV) 18–65 y of age | 37.6 | 55.9 | 51.3 | 8.6 | HAM-D 21a |
| International | PAL (216) | ||||||||
| Pharma | PBO (95) | ||||||||
| Correll, 2015 | DB | 6 | 636 | Schizophrenia (DSM-IV-TR) 18–65 y of age | 40.1 | 63.1 | 66.5 | BRE 0.25, 2, and 4 fixed dose | PANSS- Anxiety/ Depression |
| International | [BRE 0.25 (90)] | ||||||||
| Pharma | BRE 2 (182) | ||||||||
| BRE 4 (180) | |||||||||
| PBO (184) | |||||||||
| Corrigan, 2004 | DB | 6 | 467 | Schizophrenia (DSM-IV) 18–65 y of age | 37.6 | 65.7 | 31.7 | OLA 15 fixed dose | CDSS |
| International | OLA 15 (93) | ||||||||
| Pharma | [SON 1.5 (96)] | ||||||||
| [SON 10 (99)] | |||||||||
| [SON 60 (91)] | |||||||||
| PBO (87) | |||||||||
| Cutler, 2010 | DB | 6 | 565 | Schizophrenia (DSM-IV) 18–65 y of age | 41.4 | 71.5 | 32.5 | QUE 400, 600, and 800 fixed dose | PANSS- Anxiety/ Depression |
| US | QUE XR 400 (114) | ||||||||
| Pharma | QUE XR 600 (105) | ||||||||
| QUE XR 800 (113) | |||||||||
| QUE IR 800 (116) | |||||||||
| PBO (117) | |||||||||
| Daniel, 1999 | DB | 6 | 302 | Schizophrenia or schizoaffective disorder (DSM- III-R) 18 y or older | 36.6 | 71.2 | 68.2 | ZIP 80 and 160 fixed dose | MADRS |
| International | ZIP 80 (106) | ||||||||
| Pharma | ZIP 160 (104) | ||||||||
| PBO (92) | |||||||||
| Davidson, 2007 | DB | 6 | 618 | Schizophrenia (DSM-IV) 18 y or older | 36.8 | 68 | 49 | OLA 10 PAL 3, 9, and 15 fixed dose |
PANSS- Anxiety/ Depression |
| International | OLA 10 (128) | ||||||||
| Pharma | PAL 3 (127) | ||||||||
| PAL 9 (125) | |||||||||
| [PAL 15 (115)] | |||||||||
| PBO (123) | |||||||||
| Durgam, 2015 | DB | 6 | 617 | Schizophrenia (DSM-IV-TR) 18–60 y of age | 38.5 | 63.2 | 64.0 | ARI 10 CAR 3 and 6 fixed dose |
PANSS-Anxiety/ Depression |
| International | ARI 10 (152) | ||||||||
| Pharma | CAR 3 (155) | ||||||||
| CAR 6 (157) | |||||||||
| PBO (153) | |||||||||
| Egan, 2013 | DB | 4 | 216 | Schizophrenia (DSM-IV-TR) 18–55 y of age | 36.8 | 58.3 | 99.5 | OLA 15 fixed dose |
MADRS |
| International | OLA 15 (47) | ||||||||
| Pharma | [MK-8998 (86)] | ||||||||
| PBO (83) | |||||||||
| Hirayasu, 2010 | DB | 6 | 321 | Schizophrenia (DSM-IV) 20 y or older | 45.3 | 50.6 | 0 | OLA 10 PAL 6 fixed dose |
PANSS-Anxiety/ Depression |
| Japan | OLA 10 (47) | ||||||||
| Pharma | PAL 6 (136) | ||||||||
| PBO (138) | |||||||||
| Higuchi, 2019 | DB | 6 | 460 | Schizophrenia (DSM-IV) 18–75 y of age | 45.6 | 59.1 | 0 | LUR 40 and 80 RIS 4 fixed dose |
PANSS-Anxiety/ Depression |
| International | LUR 40 (131) | ||||||||
| Pharma | LUR 80 (131) | ||||||||
| RIS 4 (65) | |||||||||
| PBO (133) | |||||||||
| Honigfeld, 1984 | DB | 4 | 39 | Schizophrenia (clinical diagnosis) | NR | NR | NR | [CLO 800] CP 1333 |
BPRS depression cluster score |
| US | [CLO (16)] | ||||||||
| NR | CP (15) | ||||||||
| PBO (8) | |||||||||
| Ishigooka, 2018 | DB | 6 | 459 | Schizophrenia (DSM-IV-TR) 18–65 y of age | 44.3 | 47.5 | 0 | BRE 1, 2, and 4 fixed dose | PANSS-Anxiety/ Depression |
| Japan | BRE 1 (115) | ||||||||
| Pharma | BRE 2 (115) | ||||||||
| BRE 4 (113) | |||||||||
| PBO (116) | |||||||||
| Kahn, 2007 | DB | 6 | 588 | Schizophrenia (DSM-IV) 18–65 y of age | 34.2 | 60.2 | 59.2 | QUE 400, 600, and 800 fixed dose |
PANSS-Anxiety/ Depression |
| International | QUE IR 400 (123) | ||||||||
| Pharma | QUE XR 400 (113) | ||||||||
| QUE XR 600 (113) | |||||||||
| QUE XR 800 (121) | |||||||||
| PBO (118) | |||||||||
| Kane, 2010 | DB | 6 | 458 | Schizophrenia (DSM-IV-TR) 18 y or older | 37 to 40 years across the 4 treatment groups | 52% to 68% across the 4 treatment groups | 59% to 64% across the 4 treatment groups | ASE 10 and 20 HAL 8 fixed dose |
CDSS |
| International | ASE 10 (114) | ||||||||
| Pharma | ASE 20 (106) | ||||||||
| HAL 8 (115) | |||||||||
| PBO (123) | |||||||||
| Kane, 2015 | DB | 6 | 674 | Schizophrenia (DSM-IV-TR) 18–65 y of age | 38.4 | 62.8 | 60.4 | BRE 1, 2, and 4 fixed dose | PANSS-Anxiety/ Depression |
| International | BRE 1 (120) | ||||||||
| Pharma | BRE 2 (186) | ||||||||
| BRE4 (184) | |||||||||
| PBO (184) | |||||||||
| Keck, 1998 | DB | 4 | 139 | Schizophrenia or schizoaffective disorder (DSM- III-R) 18–64 y of age | 39.4 | 79.1 | 71.9 | ZIP 40 and 120 fixed dose | BPRS depression cluster scoreb |
| US | ZIP 40 (44) | ||||||||
| Pharma | ZIP 120 (47) | ||||||||
| PBO (48) | |||||||||
| Kinoshita, 2016 | DB | 6 | 532 | Schizophrenia (DSM-IV-TR) 20–64 y of age | 41.4 | 48.1 | 0 | ASE 10 and 20 fixed dose | PANSS-Anxiety/ Depression |
| International | ASE 10 (176) | ||||||||
| Pharma | ASE 20 (182) | ||||||||
| PBO (174) | |||||||||
| Klieser and Lehmann, 1989 | DB | 3 | 75 | Schizophrenia (DSM-III) | 42.6c | 40.8c | NR | HAL 20 fixed dose |
HAM-D |
| Germany | HAL 20 (22) | ||||||||
| NR | [AMIT 150 (20)] | ||||||||
| [TRA 400 (17)] | |||||||||
| PBO (16) | |||||||||
| Landbloom, 2017 | DB | 6 | 360 | Schizophrenia (DSM-IV-TR) 18 y or older | 40.6 | 58.5 | 71.4 | ASE 5 and 10 OLA 15 fixed dose |
PANSS-Anxiety/ Depression |
| International | [ASE 5 (98)] | ||||||||
| Pharma | ASE 10 (113) | ||||||||
| OLA 15 (46) | |||||||||
| PBO (103) | |||||||||
| Lieberman, 2016 | DB | 4 | 335 | Schizophrenia (DSM-IV-TR- Clinical Trials Version) 18–55 y of age | 40.1 | 82.6 | 18.6 | RIS 4 fixed dose |
CDSSd |
| US | RIS 4 (82) | ||||||||
| Pharma | [ITT 007 60 (84)] | ||||||||
| [ITT 007 120 (84)] | |||||||||
| PBO (85) | |||||||||
| Loebel, 2013 | DB | 6 | 488 | Schizophrenia (DSM-IV-TR) 18–75 y of age | 37.2 | 68.3 | 56.6 | LUR 80 and 160 QUE 600 fixed dose |
MADRS |
| International | LUR 80 (125) | ||||||||
| Pharma | LUR 160 (121) | ||||||||
| QUE 600 (120) | |||||||||
| PBO (122) | |||||||||
| Loebel, 2016 | DB | 6 | 412 | Schizophrenia (DSM-IV-TR) 18–75 y of age | 40.8 | 63.7 | 72.7 | LUR 80 or 160 At first 2 wk, ER continued to take LUR 80 mg/d, while ENR were re- randomized in 1:1 ratio either to continue 80 mg/d or receive 160 mg/d for remaining 4 wk |
MADRS |
| International | LUR 80 or 160 (199) | ||||||||
| Pharma | [LUR 20 (101)] | ||||||||
| PBO (112) | |||||||||
| Marder, 2007 | DB | 6 | 444 | Schizophrenia (DSM-IV) 18 y or older | 41.6 | 74 | 43 | OLA 10 PAL 6 and PAL 12 fixed dose |
PANSS- Anxiety/ Depression |
| US | OLA 10 (110) | ||||||||
| Pharma | PAL 6 (112) | ||||||||
| PAL 12 (112) | |||||||||
| PBO (110) | |||||||||
| Marder, 2016 | DB | 6 | 468 | Schizophrenia (DSM-IV-TR) 18–65 y of age | 40.6 | 56.9 | 75.2 | BRE 2–4 QUE 400–800 |
PANSS- Anxiety/ Depression |
| International | BRE (151) | ||||||||
| Pharma | QUE (154) | ||||||||
| PBO (163) | |||||||||
| Meltzer, 2004 | DB | 6 | 481 | Schizophrenia or schizoaffective disorder (DSM-IV) 18–64 y of age | 36.6 | 73.8 | 48.9 | HAL 10 fixed dose |
CDSS |
| US | HAL 10 (98) | ||||||||
| Pharma | [5-HT2a /2c -Ant. (74)] | ||||||||
| [NK3-Ant. (70)] | |||||||||
| [CB1-Ant. (72)] | |||||||||
| [NTS1-Ant. (69)] | |||||||||
| PBO (98) | |||||||||
| Meltzer, 2011 | DB | 6 | 478 | Schizophrenia (DSM-IV-TR) 18–75 y of age | 37.7 | 78.0 | 35.7 | LUR 40 and 120 | MADRS |
| International | LUR 40 (120) | OLA 15 fixed dose |
|||||||
| Pharma | LUR 120 (119) | ||||||||
| OLA 15 (123) | |||||||||
| PBO (116) | |||||||||
| Nakamura, 2009 | DB | 6 | 180 | Schizophrenia (DSM-IV) 18–64 y of age | 40.8 | 76.7 | 33.9 | LUR 80 fixed dose |
MADRS |
| US | LUR 80 (90) | ||||||||
| Pharma | PBO (90) | ||||||||
| Nasrallah, 2013 | DB | 6 | 500 | Schizophrenia (DSM-IV) 18–75 y of age | 38.8 | 69.5 | 49.5 | LUR 40, 80, and 120 fixed dose |
MADRS |
| International | LUR 40 (125) | ||||||||
| Pharma | LUR 80 (123) | ||||||||
| LUR 120 (124) | |||||||||
| PBO (128) | |||||||||
| Potkin, 2015 | DB | 6 | 356 | Schizophrenia (DSM-IV) 18–64 y of age | 41.2 | 74.2 | 49.9 | LUR 20, 40, and 80 HAL 10 fixed dose |
MADRS |
| US | LUR 40 (69) | ||||||||
| Pharma | LUR 80 (71) | ||||||||
| [LUR 20 (71)] | |||||||||
| HAL 10 (73) | |||||||||
| PBO (72) | |||||||||
| NCT01175135 | DB | 4 | 259 | Schizophrenia (DSM-IV) 18–65 y of age | 41.9 | 69.4 | NR | RIS 6 fixed dose | PANSS-Anxiety/ Depression |
| International | RIS 6 (37) | ||||||||
| Pharma | [PF-02545920 10 (74)] | ||||||||
| [PF-02545920 30 (74)] | |||||||||
| PBO (74) | |||||||||
| Included studies = 35, n = 13 890. | Mean age: 39.2 ± 2.9 y | ||||||||
| Country: international (studies = 23, n = 10 387), US (studies =8, n = 2539), Japan (studies = 2, n = 780), Germany (studies = 1, n = 75), NR (studies = 1, n = 109). | % Male: 65.5 ± 10.7 | ||||||||
| Sponsorship: pharmaceuticals (studies = 33, n = 13 776), NR (studies = 2, n = 114). | % White: 46.3 ± 26.2 | ||||||||
| Study duration: mean = 5.7 ± 0.9 weeks (range 3–7) | The number of patients per study: median = 444 (range 39–674) | ||||||||
| Antipsychotics: aripiprazole (studies = 2), asenapine (studies = 3), brexpiprazole (studies = 4), cariprazine (studies = 1), chlorpromazine (study = 1), haloperidol (studies = 4), lurasidone (studies = 7), olanzapine (studies = 7), paliperidone (studies = 6), quetiapine (studies = 5), risperidone (studies = 4), ziprasidone (studies = 2) |
Abbreviations: AMIT, amitriptyline; ARI, aripiprazole; ASE, asenapine; BPRS, brief psychiatry rating scale; BRE, brexpiprazole; CAR, cariprazine; CDSS, Calgary Depression Scale for Schizophrenia; CLO, clozapine; CP, chlorpromazine; DB, double blind; DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision; HAL, haloperidol; HAM-D, Hamilton Rating Scale for Depression; LUR, lurasidone; MADRS, Montgomery Åsberg Depression Rating Scale; NR, not reported; OLA, olanzapine; PANSS, Positive and Negative Syndrome Scale; PBO, placebo; QUE, quetiapine; RIS, risperidone; SON, sonepiprazole; TRA, trazodone; ZIP, ziprasidone. PAL, ER, DSM-III-R ?
aAnti-depressive effect was assessed in patients with baseline HAM-D21 ≥ 16.
bAnti-depressive effect was assessed in patients with baseline BPRS depression cluster score ≥18.
cIncluding 45 patients with major depressive disorder.
dAnti-depressive effect was assessed in patients with baseline CDSS > 6.
Drug groups or numbers in squared brackets were not used in any analysis, neither the primary one nor in a sensitivity analysis.
Description of Included Studies
Studies were published between 1984 and 2019. Twenty-three studies were conducted as multinational clinical trials. Eight studies were conducted in the United States, 2 were in Japan, 1 was in Germany, and 1 was not clear about the study location. Thirty-three studies were sponsored by industry, and sponsors for the remaining 2 studies were not clear or not mentioned. The median number of participants per study was 444 (range: 39–674), and the mean duration was 5.7 weeks (range: 3–7). Thirty-four studies (97.1%) used different versions of DSM and 1 used Research Diagnostic Criteria as the basis for diagnosis. The mean age of participants was 39.2 years (SD = 2.9), 65.5% of participants were men, and 46.3% of patients were White.
The number of studies with each individual antipsychotic were lurasidone (7), olanzapine (7), paliperidone (6), quetiapine (5), brexpiprazole (4), haloperidol (4), risperidone (4), asenapine (3), aripiprazole (2), ziprasidone (2), cariprazine (1), and chlorpromazine (1).
Efficacy of Antipsychotics for Depressive Symptoms
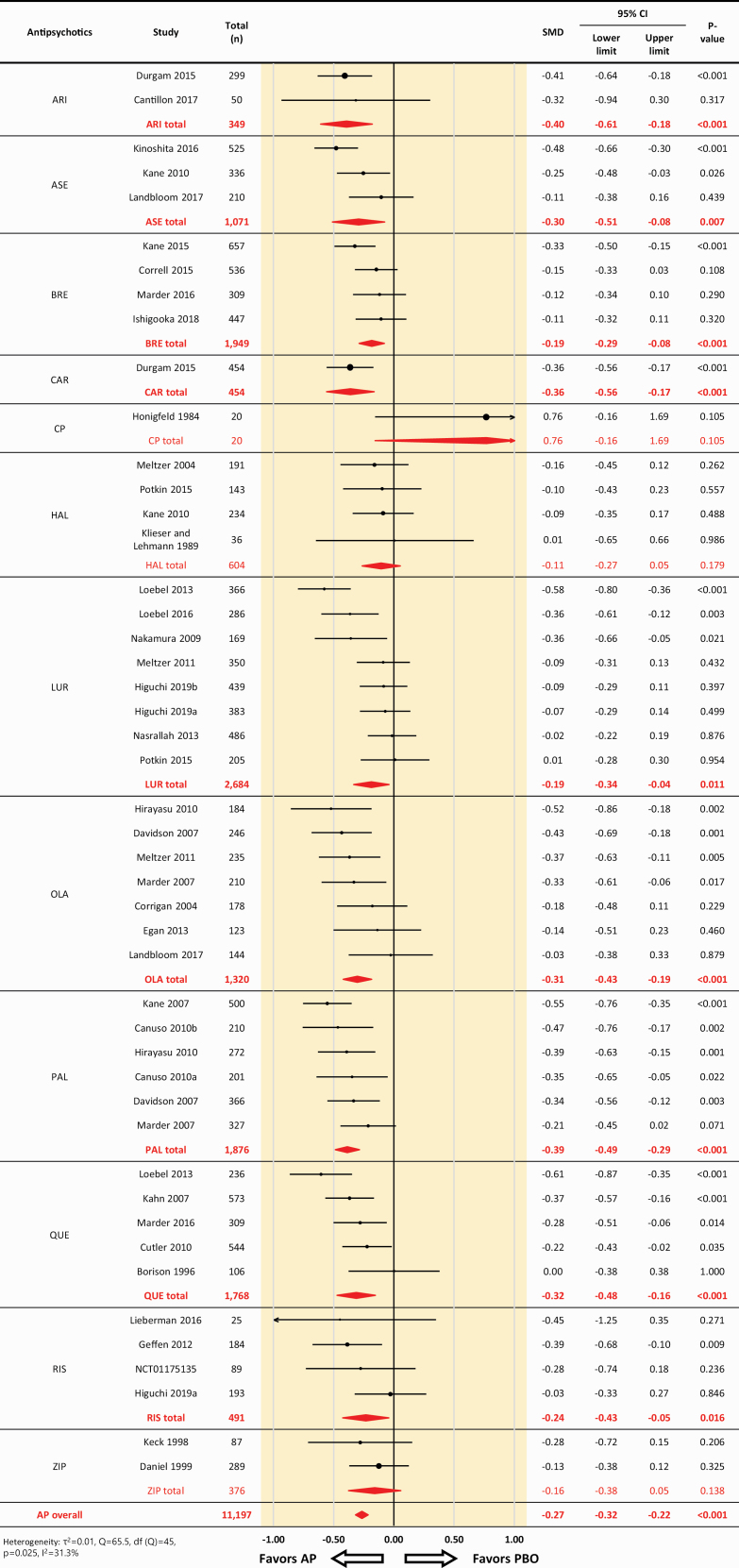
Across 46 comparisons based on 35 RCTs, antipsychotics were more efficacious than placebo for depressive symptoms (46 comparisons, 11 197 participants, SMD: –0.27, 95% CI: –0.32 to –0.22, P < .001; heterogeneity, τ2 = .01, I2 = 31.3%, Q = 65.5, df = 45, P = .025) (Figure 1). All the antipsychotics, except for chlorpromazine, haloperidol, and ziprasidone, were associated with significantly greater decreases in depressive symptoms compared with placebo (SMD = −0.19 to −0.40) (Figure 1; supplementary Table 1).
Figure 1.
Effect sizes of all studies included in the analysis, forest plot. AP, antipsychotic drug; ARI, aripiprazole; ASE, asenapine; BRE, brexpiprazole; CP, chlorpromazine; HAL, haloperidol; CI, confidence interval; LUR, lurasidone; OLA, olanzapine; PAL, paliperidone; PBO, placebo; QUE, quetiapine; RIS, risperidone; SMD, standardized mean difference; ZIP, ziprasidone. SMDs < 0 favor the antipsychotic treatment.
Meta-Regression Analyses
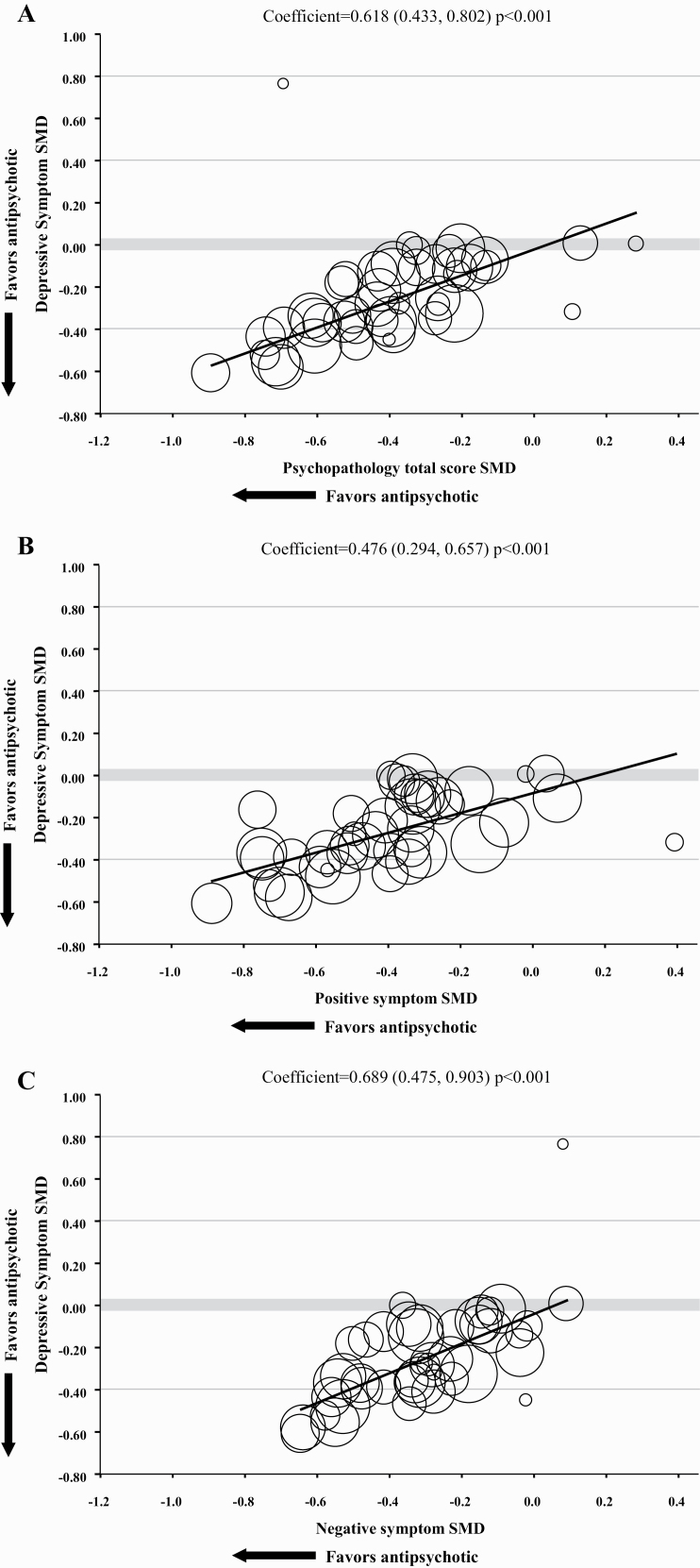
Meta regression analyses showed that the improvement of PANSS/BPRS total score, positive symptom, negative symptom, PANSS-general psychopathology scale score, and CGI-S score significantly predicted the improvement of depressive symptoms with slightly higher correlation with negative symptoms (β = 0.618, 95% CI = 0.433 to 0.802, P < .001; β = 0.476, 95% CI = 0.294 to 0.657, P < .001; β = 0.689, 95% CI = 0.475 to 0.903, P < .001; β = 0.603, 95% CI = 0.302 to 0.904, P < .001; and β = 0.585, 95% CI = 0.343 to 0.826, P < .001; respectively) (Figure 2a–c; supplemental Figure 2a–b).
Figure 2.
Meta-regression analysis of improvement in depressive symptoms on improvement in clinical symptoms. BPRS, Brief Psychiatric Rating Scale; CGI-S, Clinical Global Impressions–Severity illness scale; PANSS, Positive and Negative Syndrome Scale; SMD, standardized mean difference. (a) Correlation between improvement in depressive symptoms and improvement in PANSS/BPRS total score. (b) Correlation between improvement in depressive symptoms and improvement in positive symptoms. (c) Correlation between improvement in depressive symptoms and improvement in negative symptoms.
The improvement of depressive symptoms measured with PANSS/BPRS-depression significantly correlated with the improvement of PANSS/BPRS total score, positive, and negative symptoms, and PANSS-general psychopathology scale score with similar coefficients as found with the primary outcome (β = 0.610, 95% CI = 0.349 to 0.870, P < .001; β = 0.441, 95% CI = 0.219 to 0.663, P < .001; β = 0.688, 95% CI = 0.388 to 0.989, P < .001; and β = 0.667, 95% CI = 0.350 to 0.983, P < .001, respectively) (supplementary Figure 2c–f).
The improvement of depressive symptoms measured with MADRS, HAM-D, or CDSS significantly correlated with the improvement of PANSS/BPRS total score, positive, and negative symptoms (β = 0.622, 95% CI = 0.360 to 0.884, P < .001; β = 0.548, 95% CI = 0.261 to 0.834, P < .001; β = 0.689, 95% CI = 0.380 to 0.998, P < .001, respectively) (supplementary Figure S3a–c); however, it was not significantly correlated with PANSS-general psychopathology scale score (supplementary Figure 3d). The effect size for the improvement of depressive symptoms also had a significant correlation with the improvement of global functioning (β = 0.650, 95% CI = 0.020 to 1.281, P = .043) (supplementary Figure 4). This effect was independent from mean age of participants, percentage of male or white, and publication year (supplementary Figures 5–8). The difference between antipsychotics and placebo decreased with higher baseline PANSS depression score, but baseline MADRS total score was not a statistically significant moderator (supplementary Figures 9 and 10).
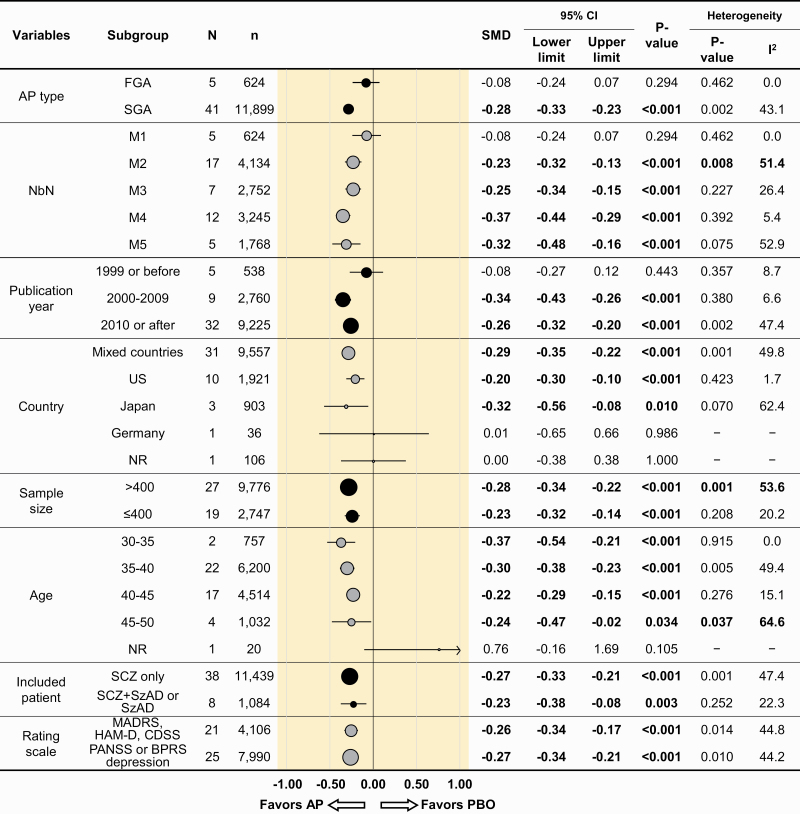
Subgroup Analyses
The summary of subgroup analyses for depressive symptoms is presented in Figure 3. Significant superiority of antipsychotics over the placebo remained in SGA (SMD = −0.28, 95% CI = −0.33 to −0.23, P < .001), but not in FGA (SMD = −0.08, 95% CI = −0.24 to 0.07, P = .294).
Figure 3.
Summary of subgroup analyses for depressive symptoms. AP, antipsychotic drug; BPRS, Brief Psychiatric Rating Scale; CDSS, Calgary Depression Scale for Schizophrenia; FGA, first-generation antipsychotic drug; HAM-D, Hamilton Rating Scale for Depression; MADRS, Montgomery Åsberg Depression Rating Scale; NbN, neuroscience-based nomenclature; PANSS, Positive and Negative Syndrome Scale; PBO, placebo; SCZ, schizophrenia; SGA, second-generation antipsychotic drug; SMD, standardized mean difference; SzAD, schizoaffective disorder. SMDs lower than 0 favor the antipsychotic treatment. Bold case indicates that results of the subgroup were statistically significant. P values <.05 indicate statistical significance. (a) M1–M5 are drug mechanisms of action according to the neuroscience-based nomenclature (NbN). M1: receptor antagonists (D2) clopenthixol, fluphenazine, haloperidol, perphenazine, pimozide, pipotiazine, sulpiride, and trifluoperazine. M2: receptor antagonists (D2, 5-HT2) chlorpromazine, iloperidone, loxapine, lurasidone, olanzapine, sertindole, thioridazine, ziprasidone, and zotepine. M3: receptor partial agonists (D2, 5-HT1A) aripiprazole, brexpiprazole, and cariprazine. M4: receptor antagonists (D2, 5-HT2, NE, α 2) asenapine, paliperidone, and risperidone. M5: receptor antagonist (D2, 5-HT2) and reuptake inhibitor (NET) quetiapine.
Superiority of antipsychotics over the placebo remained in M2-M5 subgroups of NbN (M2, 17 comparisons, 4134 participants, SMD = −0.23, 95% CI = −0.32 to −0.13, P < .001; M3, 7 comparisons, 2752 participants, SMD = −0.25, 95% CI = −0.34 to −0.15, P < .001; M4, 12 comparisons, 3245 participants, SMD = −0.37, 95% CI = −0.44 to −0.29, P < .001, M5, 5 comparisons, 1768 participants, SMD = −0.32, 95% CI = −0.48 to −0.16, P < .001), but not in M1 subgroup of NbN (5 comparisons, 624 participants, SMD = 0.08, 95% CI = −0.24 to 0.07, P = .294). Results for each individual antipsychotic drug separately are appended online (see supplementary Table 1).
Superiority of antipsychotics was moderated by publication year. In RCTs published until 1999 (5 comparisons, 538 participants, SMD = −0.08, 95% CI = −0.27 to 0.12, P = .443) consisting of chlorpromazine (1 RCT), haloperidol (1 RCT), quetiapine (1 RCT), and ziprasidone (2 RCTs), antipsychotics were not significantly superior to the placebo. However, this was not the case in the newer RCTs published from 2000 to 2009 (9 comparisons, 2760 participants, SMD = −0.34, 95% CI = −0.43 to −0.26, P < .001) or after 2010 (32 comparisons, 9225 participants, SMD = −0.26, 95% CI = −0.32 to −0.20, P < .001), which included 3/5 M1 antipsychotics studies (Figure 3). Significant superiority of antipsychotics was also demonstrated in RCTs conducted in mixed countries, the United States, and Japan (31 comparisons, 9557 participants, SMD = −0.29, 95% CI = −0.35 to −0.22, P < .001; 10 comparisons, 1921 participants, SMD = −0.20, 95% CI = −0.30 to −0.10, P < .001; 3 comparisons, 903 participants, SMD = −0.32, 95% CI = −0.56 to −0.08, P = .010, respectively).
Regarding sample size, significant superiority of antipsychotics over the placebo remained in both larger (>400 participants) and smaller RCTs (≤400 participants). Though not statistically significant, the effect sizes tended to be higher in younger patients compared with older patients with the target symptoms for which antipsychotic effects might be most expected (SMD = −0.37 [mean age 30–35 years] and −0.30 [mean age 35–40 years] compared with −0.22 [mean age 40–45 years] and −0.24 [mean age 45–50 years]). Significant superiority of antipsychotics was also confirmed in other examined subpopulations, with similar effect sizes (Figure 3). When analyses were restricted to standard depression scales (HAMD, MADRS, CDSS), effects were similar to that obtained with PANSS and BPRS depression scales (Figure 3, bottom row).
Secondary Outcomes
A summary of the pooled results is presented in supplementary Tables 1b–h and 2. Compared with placebo, antipsychotics were associated with significant improvement of overall symptoms (43 comparisons, 12 048 participants, SMD = −0.41, 95% CI = −0.48 to −0.35, P < .001), positive symptoms (40 comparisons, 12 106 participants, SMD = −0.42, 95% CI = −0.49 to −0.35, P < .001), negative symptoms (41 comparisons, 12 017 participants, SMD = −0.32, 95% CI = −0.37 to −0.26, P < .001), PANSS general psychopathology score (17 comparisons, 5160 participants, SMD = −0.39, 95% CI = −0.49 to −0.30, P < .001), CGI-S (37 comparisons, 10 482 participants, SMD = −0.33, 95% CI = −0.39 to −0.28, P < .001), global functioning (9 comparisons, 2725 participants, SMD = −0.39, 95% CI = −0.48 to −0.31, P < .001), and quality of life (3 comparisons, 1029 participants, SMD = −0.47, 95% CI = −0.60 to −0.34, P < .001) (supplementary Table 1b–h).
There were significant differences between antipsychotics and the placebo in all-cause discontinuation (45 comparisons, 12 882 participants, RR = 0.78, 95% CI = 0.73 to 0.84, P < .001, NNH = −11) and inefficacy-related discontinuation (43 comparisons, 12 931 participants, RR = 0.52, 95% CI = 0.47 to 0.57, P < .001, NNH = −10). However, no significant difference was observed between antipsychotics and the placebo in intolerability related discontinuation (supplementary Table 2).
Publication Bias and Risk of Bias Assessment
No publication bias was detected: the funnel plots were symmetrical (Egger’s regression test for depressive symptoms: intercept = 0.85, 95% CI = −0.52 to 2.22, P = .218, and the trim and fill method yielded almost identical effect size (supplementary Figure 11).
Detailed results of Cochrane risk of bias assessment are presented in supplementary Table 3. Although we included only double-blind RCTs, the reports often did not provide enough details on the sequence generation or allocation concealment. The explanation for the proper blinding methods and the functioning of the blinding were also insufficiently reported.
Discussion
This meta-analysis included 35 double-blind, placebo-controlled RCTs involving 13 890 patients. To our knowledge, this is the first study that focused on the antidepressive effects of antipsychotics and investigated their various aspects in patients with acute schizophrenia.
Our findings suggest that treatment with antipsychotics is associated with significant improvement of depressive symptoms in patients with schizophrenia, but the effect size was small to medium. These results are consistent with a previous meta-analysis (Leucht et al., 2017), though the results are not applicable to all individual antipsychotic drugs. In the current meta-analysis, ziprasidone, haloperidol, and chlorpromazine were not significantly separate from placebo regarding efficacy on depressive symptoms. There was no significant difference between antipsychotics of the M1 group of NbN (i.e., FGA) and placebo in improvement of depressive symptoms. It should be noted, however, that the relatively smaller number of studies (n = 5) of FGAs limits statistical power for detecting effects within that subgroup. Two RCTs of ziprasidone (Keck et al., 1998; Daniel et al., 1999) incorporated into the analysis showed significant improvement of psychiatric symptoms but not antidepressive effects. These results suggest that the mechanism of action on receptors other than dopamine D2 receptors, including serotonin receptors, is related to the antidepressive effects of certain antipsychotic drugs. In other subgroup analysis, there was no statistically significant antidepressive effect by antipsychotics in 5 studies published before 1999, in which ziprasidone, chlorpromazine, and haloperidol are included. Among the antipsychotics found here to show significantly greater improvement in depressive symptoms compared with placebo among patients with schizophrenia, the range in effect sizes across agents was similar to that found in a meta-analysis of the antidepressant effects of SGAs as augmentation agents for use with treatment-resistant depression (SMD range = −0.19 to −0.40 found here, range = −0.27 to −0.43 reported in Zhou et al., 2015).
Except for the difference related to individual antipsychotic drug, the overall favorable efficacy of antipsychotics on depressive symptoms was confirmed by additional sensitivity analyses indicating that effects were independent of influence from a number of moderators and potential confounders. Effects were similar when standard depression scales were separately examined. In the subgroup analysis based on the mean age of participants, the efficacy of antipsychotics was established in all age ranges with numerically higher antidepressive effect in studies of younger participants. Similarly, in the meta-regression analysis of antidepressive effect of antipsychotics and the mean age of participants, there was a tendency for antipsychotics to have greater efficacy in younger participants, although this correlation was not significant. These results may suggest the importance of pharmacological treatment for depressive symptoms beginning at an earlier stage of schizophrenia, though more research is needed to investigate age effects.
In the meta-regression analysis using PANSS/BPRS total, subscale scores, and CGI-S, statistically significant positive correlations with improvement of depressive symptoms were observed with slightly higher correlation for negative symptoms. These results suggest that some of the reduction in depressive symptoms by antipsychotics may be related to the improvement in other symptoms of schizophrenia, in particular negative symptoms. Thus, it is possible that the apparent antidepressive effects of antipsychotics on depression could be in fact be in part an effect on negative symptoms that is being picked up in depression scores. Conversely, it is also possible that negative symptoms may be a manifestation of depression. The overlap between some negative symptoms and depressive symptoms would be consistent with this explanation (Krynicki et al., 2018). To this point, a number of studies have found that negative symptoms cluster into 2 distinct domains: avolition–apathy and expressive deficit (Galderisi et al., 2018). It is possible that the correlation between change in depressive symptoms and change in negative symptoms is a function of 1 of these 2 domains rather than both. One study found that cognitive therapy, a treatment known to impact depressive symptoms, improved avolition–apathy but had no effect on measures of emotional expression in patients with schizophrenia (Grant et al., 2012). Future research is needed to sort out the effects of antipsychotics on the relation between change in depressive symptoms and different dimensions of negative symptoms.
Several other factors may also be responsible for the significant correlations among positive symptoms, negative symptoms, and depressive symptoms. Patients with high levels of treatment adherence may have substantially greater changes on all of these symptom measures compared with patients with relatively lower treatment adherence. Similarly, differences among individuals in the metabolism of the drugs may produce differences in clinical improvement on all measures. In addition, it should be noted that rating scales can be prone to halo effects or correlational error whereby raters tend to let their general impression of a patient’s severity influence their ratings on all items (i.e., a more severely psychotic patient is also more likely to be rated high on items unrelated to psychosis including depression). Such halo effects can increase the correlations among the scales.
Although our results suggest that lower baseline PANSS-depression scale predicted greater difference between antipsychotics and placebo in decrease of depressive symptom scale score, the coefficient was small and such a significant correlation was not observed between baseline MADRS total score and depressive symptom improvement. While previous studies (Conley et al., 2007; Perkins et al., 2008) have identified comorbid depression as a predictor of poor antipsychotic treatment outcome, a participant-level meta-analysis (Furukawa et al., 2015) reported that the difference in symptom reduction between antipsychotics and placebo increased as the baseline severity increased. The association between baseline severity and symptom improvement by antipsychotic treatment may be different between depression and other symptom domains, although the reasons for these controversial results remain unknown. Further studies are needed to investigate the association between baseline severity and depressive symptom improvement by antipsychotics in schizophrenia.
The relation of extrapyramidal symptoms and depression in schizophrenia also needs to be considered. Depression measures cannot always discriminate between depressive symptoms and extrapyramidal (or negative) symptoms. However, a systematic review of depression measures (Lako et al., 2012) found that the CDSS was the most accurate scale for differentiating depression from extrapyramidal and negative symptoms in patients with schizophrenia. Nonetheless, a study in Chinese patients with schizophrenia found that severe levels of akathisia and dyskinesia were significantly associated with greater severity of depressive symptoms (Li et al., 2018). It is possible that severe extrapyramidal symptoms may affect depression symptom severity.
Our results need to be discussed with reference to previous meta-analyses for treatments of depression and negative symptoms in schizophrenia. In 1 meta-analysis (Fusar-Poli et al., 2015), it was reported that treatment with antidepressants was associated with significant improvement of negative symptoms. In another meta-analysis (Helfer et al., 2016), a significant effect of antidepressant augmentation for both depressive and negative symptoms was found. Our results are in line with these previous meta-analyses in the relation of improvement in depressive symptoms and in negative symptoms. In the more recent meta-analysis (Galling et al., 2018), antidepressant augmentation was not superior to placebo for depressive symptom reduction although significantly superior to placebo for negative symptom reduction.
Considering that Galling et al. (Galling et al., 2018) focused on the effects of antidepressants, depressive symptoms in schizophrenia may be qualitatively different from major depressive disorder. As mentioned, there are some important commonalities between depression and negative symptoms in schizophrenia (Andreasen and Olsen, 1982; Carpenter et al., 1985; Siris et al., 1988). Decline in motivation, concentration difficulty, social withdrawal, and anhedonia are common features to both depression and negative symptoms. Other features differentiate these 2 domains of symptoms: feelings of guilt, suicidal ideation, and hopelessness are specifically recognized as depressive symptoms (Lindenmayer et al., 1991; Bermanzohn and Siris, 1992).
Although almost all the SGAs showed significant antidepressive effect in our meta-analysis and the improvement of depressive symptom significantly correlated with the improvement of clinicians’ subjective perception of clinical improvement, as measured with the CGI-S, with similar coefficient as found with the PANSS scores, care should be taken as to whether these effects are clinically meaningful. Given that it is difficult to clinically distinguish depression and negative symptoms in schizophrenia, some of the observed antidepressive effects of SGAs may include the improvement of negative symptoms at least in part. On the other hand, a previous study suggested that psychosocial factors such as loss and social isolation, rather than biological symptoms, were core features for post-psychotic depression (Sandhu et al., 2013). To address these psychosocial factors, nonpharmacological intervention such as psychoeducation and social support may also be needed to treat depression in schizophrenia.
Several limitations should be noted. First, included studies are not primarily aimed to improve depressive symptoms of schizophrenia patients, and this might contribute to the heterogeneity of results. Second, the type of pretreatment and pretrial wash out of the medication might have an influence on the mood of the patient, but no studies reported information on the type and dose of pretrial medications. For this reason, we could not analyze pretreatment drug information as a covariate. Third, there are no clinical trials investigating antidepressive effect of antipsychotics in drug-naïve or in the first-episode schizophrenia patients. This was disappointing considering that depression is prevalent in first-episode schizophrenia (Bustamante et al., 1994; Addington et al., 1998; Jäger et al., 2007), and the effect of antipsychotics on first episode patients is greater than that on multiple episode patients (Jäger et al., 2007). Fourth, no studies reported whether psychotherapy or psychological treatment was applied. If these nonpharmacological treatments were present yet not balanced across treatment groups, these would be confounding factors. Fifth, the causality between improvement of depressive symptoms and that of other psychopathological domains is unknown. Improvement of depressive symptoms may be based on improvement of other psychopathological domains, especially negative symptoms. Alternatively, it may be that improvement of other psychopathological domains was observed due to improvement of depressive symptoms, or reciprocal causation may be occurring between these domains of symptoms. Finally, rating scales for depressive symptoms used in included trials were disparate. The most commonly used rating scale for depressive symptoms was the PANSS-anxiety/depression, which is not designed to assess the depressive symptoms of schizophrenia patients and is not able to distinguish depressive from anxiety and negative symptoms. Though subgroup analyses constrained to studies using standard depression scales yielded similar results as found with the PANSS-anxiety/depression scale, as mentioned, such rating scales can be prone to halo effects. Caution is needed to interpret the results of this meta-analysis because of these halo effects or correlational error.
In the present systematic review and meta-analysis, SGA therapy, except for ziprasidone, demonstrates small to medium treatment effects sizes on depressive symptoms in adult patients with schizophrenia. There was a significant correlation between improvement in depressive symptoms and in psychopathological domain, indicating that some of the reduction in depressive symptoms may be related to the improvement in other symptoms of schizophrenia, in particular negative symptoms. Depressive symptoms are still frequently observed in clinical practice despite the fact that the SGAs have been widely used in the treatment of schizophrenia many years. Taking this into consideration, the results may be overestimating the true antidepressive effect of antipsychotics. While current findings are encouraging in that some antipsychotics are effective for the treatment of depressive symptoms in schizophrenia, further sufficiently large, parallel-group RCTs with the treatment of depressive symptoms as the primary focus would be required to confirm the results of our meta-analysis.
Supplementary Material
Acknowledgments
Authors I.M., T.N., and K.H. had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Editorial and medical writing support was provided Dr Edward Schweizer of Paladin Consulting Group and was funded by Sumitomo Dainippon Pharma Co., Ltd.
There was no funding of this work.
Statement of Interest
I.M. has received honoraria for lectures from Daiichi Sankyo, Sumitomo Dainippon, Janssen, Meiji Seika Pharma, Mochida, MSD, Mylan, Otsuka, Pfizer, Takeda, Tanabe Mitsubishi, and Yoshitomi.
H.Y. has received honoraria for lectures from Eli Lilly, Sumitomo Dainippon, Takeda, MSD, Daiichi Sankyo, Janssen, Esai, UCB Japan, MeijiSeika, Otsuka, Shionogi, and Kyouwa Yakuhin.
K.H. and T.N. are full-time employees of Sumitomo Dainippon Pharma, Japan.
References
- Addington D, Addington J, Maticka-Tyndale E (1993) Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry S22:39–44. [PubMed] [Google Scholar]
- Addington D, Addington J, Patten S (1998) Depression in people with first episode schizophrenia. Br J Psychiatry, Supplement 172:90–92. [PubMed] [Google Scholar]
- Andreasen NC, Olsen S (1982) Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry 39:789–794. [DOI] [PubMed] [Google Scholar]
- Bermanzohn PC, Siris SG (1992) Akinesia: a syndrome common to parkinsonism, retarded depression, and negative symptoms of schizophrenia. Compr Psychiatry 33:221–232. [DOI] [PubMed] [Google Scholar]
- Bottlender R, Strauss A, Möller HJ (2000) Prevalence and background factors of depression in first admitted schizophrenic patients. Acta Psychiatr Scand 101:153–160. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ (2009) Psychiatric comorbidities and schizophrenia. Schizophr Bull 35:383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante S, Maurer K, Löffler W, Häfner H (1994) [Depression in the early course of schizophrenia]. Fortschr Neurol Psychiatr 62:317–329. [DOI] [PubMed] [Google Scholar]
- Carpenter WT Jr, Heinrichs DW, Alphs LD (1985) Treatment of negative symptoms. Schizophr Bull 11:440–452. [DOI] [PubMed] [Google Scholar]
- Conley RR, Ascher-Svanum H, Zhu B, Faries DE, Kinon BJ (2007) The burden of depressive symptoms in the long-term treatment of patients with schizophrenia. Schizophr Res 90:186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel DG, Zimbroff DL, Potkin SG, Reeves KR, Harrigan EP, Lakshminarayanan M (1999) Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology 20:491–505. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa TA, Levine SZ, Tanaka S, Goldberg Y, Samara M, Davis JM, Cipriani A, Leucht S (2015) Initial severity of schizophrenia and efficacy of antipsychotics: participant-level meta-analysis of 6 placebo-controlled studies. JAMA Psychiatry 72:14–21. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, McGuire P (2015) Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull 41:892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi S, Mucci A, Buchanan RW, Arango C (2018) Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry 5:664–677. [DOI] [PubMed] [Google Scholar]
- Galling B, Vernon JA, Pagsberg AK, Wadhwa A, Grudnikoff E, Seidman AJ, Tsoy-Podosenin M, Poyurovsky M, Kane JM, Correll CU (2018) Efficacy and safety of antidepressant augmentation of continued antipsychotic treatment in patients with schizophrenia. Acta Psychiatr Scand 137:187–205. [DOI] [PubMed] [Google Scholar]
- Gerhard T, Akincigil A, Correll CU, Foglio NJ, Crystal S, Olfson M (2014) National trends in second-generation antipsychotic augmentation for nonpsychotic depression. J Clin Psychiatry 75:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PM, Huh GA, Perivoliotis D, Stolar NM, Beck AT (2012) Randomized trial to evaluate the efficacy of cognitive therapy for low-functioning patients with schizophrenia. Arch Gen Psychiatry 69: 121–127. [DOI] [PubMed] [Google Scholar]
- Guy W (1976) ECDEU assessment manual for psychopharmacology, Revised. Washington, DC: US Department of Health, Education and Welfare. [Google Scholar]
- Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer B, Samara MT, Huhn M, Klupp E, Leucht C, Zhu Y, Engel RR, Leucht S (2016) Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry 173:876–886. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch SR (1982) Depression “revealed” in schizophrenia. Br J Psychiatry 140:421–423. [DOI] [PubMed] [Google Scholar]
- Honigfeld G (1984) Clozapine: antipsychotic activity in treatment-resistant schizophrenics. Adv Ther 1:77–97. [Google Scholar]
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, Arndt T, Bäckers L, Rothe P, Cipriani A, Davis J, Salanti G, Leucht S (2019) Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 394:939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger M, Riedel M, Messer T, Laux G, Pfeiffer H, Naber D, Schmidt LG, Gaebel W, Huff W, Heuser I, Kühn KU, Lemke MR, Rüther E, Buchkremer G, Gastpar M, Bottlender R, Strauss A, Möller HJ (2007) Psychopathological characteristics and treatment response of first episode compared with multiple episode schizophrenic disorders. Eur Arch Psychiatry Clin Neurosci 257:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Keck P Jr, Buffenstein A, Ferguson J, Feighner J, Jaffe W, Harrigan EP, Morrissey MR (1998) Ziprasidone 40 and 120 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 4-week placebo-controlled trial. Psychopharmacology 140:173–184. [DOI] [PubMed] [Google Scholar]
- Knapp M, Mangalore R, Simon J (2004) The global costs of schizophrenia. Schizophr Bull 30:279–293. [DOI] [PubMed] [Google Scholar]
- Koreen AR, Siris SG, Chakos M, Alvir J, Mayerhoff D, Lieberman J (1993) Depression in first-episode schizophrenia. Am J Psychiatry 150:1643–1648. [DOI] [PubMed] [Google Scholar]
- Krynicki CR, Upthegrove R, Deakin JFW, Barnes TRE (2018) The relationship between negative symptoms and depression in schizophrenia: a systematic review. Acta Psychiatr Scand 137:380–390. [DOI] [PubMed] [Google Scholar]
- Lako IM, Bruggeman R, Knegtering H, Wiersma D, Schoevers RA, Slooff CJ, Taxis K (2012) A systematic review of instruments to measure depressive symptoms in patients with schizophrenia. J Affect Disord 140:38–47. [DOI] [PubMed] [Google Scholar]
- Leucht S, Leucht C, Huhn M, Chaimani A, Mavridis D, Helfer B, Samara M, Rabaioli M, Bächer S, Cipriani A, Geddes JR, Salanti G, Davis JM (2017) Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry 174:927–942. [DOI] [PubMed] [Google Scholar]
- Li F, Liu XB, Zhong BL (2018) Depressive symptoms in Chinese male inpatients with schizophrenia: prevalence and clinical correlates. Psychiatry Res 264:380–384. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Grochowski S, Kay SR (1991) Schizophrenic patients with depression: psychopathological profiles and relationship with negative symptoms. Compr Psychiatry 32:528–533. [DOI] [PubMed] [Google Scholar]
- Majadas S, Olivares J, Galan J, Diez T (2012) Prevalence of depression and its relationship with other clinical characteristics in a sample of patients with stable schizophrenia. Compr Psychiatry 53:145–151. [DOI] [PubMed] [Google Scholar]
- Mandel MR, Severe JB, Schooler NR, Gelenberg AJ, Mieske M (1982) Development and prediction of postpsychotic depression in neuroleptic-treated schizophrenics. Arch Gen Psychiatry 39:197–203. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Carpenter WT Jr (1976) Postpsychotic depression in schizophrenia. Arch Gen Psychiatry 33:231–239. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Mulholland C, Cooper S (2000) The symptom of depression in schizophrenia and its management. Adv Psychiatr Treat 6:169–177. [Google Scholar]
- Overall JE, Gorham DR (1962) The Brief Psychiatric Rating Scale. Psychol Rep 10:790–812. [Google Scholar]
- Perkins DO, Gu H, Weiden PJ, McEvoy JP, Hamer RM, Lieberman JA; Comparison of Atypicals in First Episode Study Group (2008) Predictors of treatment discontinuation and medication nonadherence in patients recovering from a first episode of schizophrenia, schizophreniform disorder, or schizoaffective disorder: a randomized, double-blind, flexible-dose, multicenter study. J Clin Psychiatry 69:106–113. [DOI] [PubMed] [Google Scholar]
- Reine G, Lançon C, Di Tucci S, Sapin C, Auquier P (2003) Depression and subjective quality of life in chronic phase schizophrenic patients. Acta Psychiatr Scand 108:297–303. [DOI] [PubMed] [Google Scholar]
- Sandhu A, Ives J, Birchwood M, Upthegrove R (2013) The subjective experience and phenomenology of depression following first episode psychosis: a qualitative study using photo-elicitation. J Affect Disord 149:166–174. [DOI] [PubMed] [Google Scholar]
- Sands JR, Harrow M (1999) Depression during the longitudinal course of schizophrenia. Schizophr Bull 25:157–171. [DOI] [PubMed] [Google Scholar]
- Sim K, Mahendran R, Siris SG, Heckers S, Chong SA (2004) Subjective quality of life in first episode schizophrenia spectrum disorders with comorbid depression. Psychiatry Res 129:141–147. [DOI] [PubMed] [Google Scholar]
- Siris SG (2000) Depression in schizophrenia: perspective in the era of “atypical” antipsychotic agents. Am J Psychiatry 157:1379–1389. [DOI] [PubMed] [Google Scholar]
- Siris SG, Adan F, Cohen M, Mandeli J, Aronson A, Casey E (1988) Postpsychotic depression and negative symptoms: an investigation of syndromal overlap. Am J Psychiatry 145:1532–1537. [DOI] [PubMed] [Google Scholar]
- Siris SG, Bench C (2003) Depression and schizophrenia. In: Schizophrenia. 2nd ed. (Hirsch SR, Weinberger D, eds.), pp 142–167. Oxford, UK: Blackwell Publishing Ltd. [Google Scholar]
- Van Putten T, May RP (1978) “Akinetic depression” in schizophrenia. Arch Gen Psychiatry 35:1101–1107. [DOI] [PubMed] [Google Scholar]
- Zhou X, Keitner GI, Qin B, Ravindran AV, Bauer M, Del Giovane C, Zhao J, Liu Y, Fang Y, Zhang Y, Xie P (2015) Atypical antipsychotic augmentation for treatment-resistant depression: a systematic review and network meta-analysis. Int J Neuropsychopharmacol 25:pyv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar J, Stahl S, Moller HJ, Blier P, Kupfer D, Yamawaki S, Uchida H, Spedding M, Goodwin GM, Nutt D (2015) A review of the current nomenclature for psychotropic agents and an introduction to the neuroscience-based nomenclature. Eur Neuropsychopharmacol 25:2318–2325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.