Abstract
The characterization of pulmonary arterial hypertension (PAH) relies mainly on right heart catheterization (RHC). Electrical impedance tomography (EIT) provides a non-invasive estimation of lung perfusion that could complement the hemodynamic information from RHC. To assess the association between impedance variation of lung perfusion (ΔZQ) and hemodynamic profile, severity, and prognosis, suspected of PAH or worsening PAH patients were submitted simultaneously to RHC and EIT. Measurements of ΔZQ were obtained. Based on the results of the RHC, 35 patients composed the PAH group, and eight patients, the normopressoric (NP) group. PAH patients showed a significantly reduced ΔZQ compared to the NP group. There was a significant correlation between ΔZQ and hemodynamic parameters, particularly with stroke volume (SV) (r = 0.76; P < 0.001). At 60 months, 15 patients died (43%) and 1 received lung transplantation; at baseline they had worse hemodynamics, and reduced ΔZQ when compared to survivors. Patients with low ΔZQ (≤154.6%.Kg) presented significantly worse survival (P = 0.033). ΔZQ is associated with hemodynamic status of PAH patients, with disease severity and survival, demonstrating EIT as a promising tool for monitoring patients with pulmonary vascular disease.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease of the pulmonary circulation encompassing an intense vascular remodeling process, leading to severe disruption of vascular mechanics, right ventricle dysfunction and, ultimately, premature death [1, 2]. Right heart catheterization (RHC) remains the most appropriate method for PAH diagnosis with significant prognostic information [2]. Although different imaging modalities provide significant noninvasive information about pulmonary vascular physiology, PAH severity as well as prognosis [3–6], methods for estimation of lung perfusion remain scarce.
Electrical impedance tomography (EIT) is a non-invasive imaging tool that identifies both lung ventilation and perfusion simultaneously based on measurements of thoracic impedance changes [7]. While the entry of air in the lungs causes impedance to increase, because of its low resistivity, the flow of blood into the pulmonary circulation during systole leads to a decrease in the thoracic impedance signal. Since these two phenomena occur at different frequencies, it is possible to separate the signal of perfusion from that of ventilation [8].
Despite the potential for clinical application, there is limited information about the use of EIT on PAH patients. In one study of eight patients with idiopathic PAH (IPAH), a single patient responded to the vasodilatation test; in this patient, there was correlation between impedance change related to lung perfusion (ΔZQ) and the change on pulmonary vascular resistance (PVR) and mean pulmonary artery pressure (mPAP). The authors suggested that EIT reliably measured pulmonary intra-vascular blood volume changes [9]. In another study [10], there was a significantly reduced ΔZQ in IPAH compared to healthy volunteers, probably indicating an impairment of pulmonary vascular mechanics. Our hypothesis is that EIT carries pathophysiological information, reflecting PAH severity.
The main objective of this study was to assess EIT as a noninvasive prognostic imaging modality in PAH through its ability to reflect PAH severity according to RHC findings. Thus, the association between ΔZQ and the hemodynamic profile, disease severity, and survival of PAH patients was evaluated.
Materials and methods
The study received the approval of the Research Ethics Committee of the Heart Institute, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, approval number: 1392/06. The form of consent obtained was written.
Study population and design
We prospectively studied adult patients with suspected PAH or diagnosed PAH referred for invasive hemodynamic evaluation. For those patients under diagnostic evaluation, pulmonary hypertension was suspected by the combination of suggested symptoms/signs plus the finding of systolic PAP ≥40 mmHg in the transthoracic echocardiography. All other patients included in the study already had the diagnosis of pulmonary hypertension (Table 1).
Table 1. Baseline characteristics of the study population.
| NP | PAH | p | |
|---|---|---|---|
| (n = 8) | (n = 35) | ||
| Demographics | |||
| Sex, Female:Male | 6 (3): 2 (1) | 26 (2.9): 9 (1) | |
| Age, years | 40.1 ± 15 | 42.8 ± 14.5 | 0.37 |
| Weight, Kg.m-2 | 62.4 ± 15 | 65.9 ± 16.2 | 0.29 |
| Height, m | 1.56 ± 0.06 | 1.60 ± 0.1 | 0.11 |
| Functional Class | |||
| CF I/II | 8 (100%) | 24 (68,6%) | 0.90 |
| CF III/IV | - | 11 (31,4%) | |
| Biomarkers | |||
| BNP (ng/dL) | 65.6 ± 105.4 | 247 ± 304.3 | 0.006 |
| Hemodynamics | |||
| mPAP, mm Hg | 19.1 ± 4 | 55.5 ± 16.2 | <0.001 |
| PWP, mm Hg | 9.1 ± 3.4 | 10.4 ± 3.1 | 0.15 |
| SV, mL | 75.6 ± 22.6 | 53.7 ± 18.7 | 0.013 |
| CO, L.min-1 | 6.4 ± 2.2 | 4.1 ± 1.1 | <0.001 |
| PVR, Woods | 2.3 ± 1.6 | 11.7 ± 6.4 | <0.001 |
| Compl, mL.mm Hg-1 | 4.6 ± 2.2 | 1.3 ± 0.9 | <0.001 |
| Etiologies | |||
| IPAH | - | 15 (42.9%) | |
| CTD | 3 (37.5%) | 9 (25.7%) | |
| Schistosomiasis | - | 4 (11.4%) | |
| Portopulmonary | 3 (37.5%) | 3 (8.6%) | |
| HIV | - | 2 (5.7%) | |
| Congenital cardiac shunts | - | 2 (5.7%) | |
| Sickle cell disease | 1 (12.5%) | - | |
| Other | 1 (12.5%) | - | |
| Treatment | |||
| Sildenafil | - | 9 (25.7%) | |
| Bosentan | - | 3 (8.6%) | |
| Combined therapy | - | 5 (14.3%) | |
| Naïve | - | 18 (51.4%) | |
Definitions of abbreviations: NP = normopressoric; PAH = pulmonary arterial hypertension; BNP = brain natriuretic peptide; mPAP = mean pulmonary arterial pressure; PWP = pulmonary wedge pressure; SV = stroke volume; CO = cardiac output; NYAH = New York Heart Association; PVR = pulmonary vascular resistance; PVC = pulmonary vascular compliance; IPAH = idiopathic pulmonary arterial hypertension; CTD = collagenous tissue diseases; HIV = human immunodeficiency virus; PDE5i = phosphodiesterase type 5 inhibitors; ERA = endothelin-1 receptor antagonists.
The continuous variables are presented as mean ± standard deviation (SD), when normally distributed, and otherwise as median and interquartile [25–75%] ranges.
Pulmonary arterial hypertension was defined by a resting mPAP ≥25 mmHg during RHC, with a pulmonary wedge pressure (PWP) ≤15 mmHg, in the absence of significant lung parenchyma disease, left heart dysfunction and chronic thromboembolic disease [2, 11]. The EIT acquisition was performed simultaneously to RHC. Based on RHC findings, patients were discriminated into 2 groups: PAH group and those with normal hemodynamics, named here as “normopressoric” (NP) group.
The study was conducted at the Hemodynamic Laboratory of a tertiary pulmonary hypertension reference center. After the diagnosis of PAH, all the patients were followed periodically on an outpatient basis at the same institution. There were no losses to follow-up.
The protocol was approved by the local ethics committee and all participants gave written informed consent.
Right heart catheterization
A complete hemodynamic invasive evaluation was performed in all patients using standard techniques for RHC [7]. Hemodynamic measurements included mean right atrial pressure, mPAP, PWP, and cardiac output (CO) determined by the thermodilution technique. Cardiac index (CI) was calculated as CO divided by body surface area. Pulmonary vascular resistance (PVR) was calculated as (mPAP–PWP) divided by CO. Pulmonary vascular compliance (PVC) was calculated as systolic volume (SV) divided by pulse pressure (systolic PAP–diastolic PAP).
EIT protocol
The EIT measurements and image acquisition were performed with the platform Enlight 1800, a 32-electrode device (Timpel, Sao Paulo, Brazil).
Image acquisition protocol
The 32-electrodes were attached circumferentially to the surface of the patients’ thorax at about the level of the fourth intercostal space. The EKG-gated technique was used in order to eliminate the ventilatory impedance oscillations [12]. Patients remained under resting condition, in supine position. One hundred cardiac cycles were automatically averaged to obtain one complete data set. Image files were recorded for 3 minutes for posterior off-line analysis. Since the obtained impedance images are relative images, the measurement of ΔZQ are expressed as percent values (%).
Off-line image analysis
The recorded file was analyzed using a dedicated software developed in Labview 7.1 (National Instruments, USA). This software was validated in another study from our group [13]. A temporal series of 50 images per second was acquired, each image comprising a 32x32 matrix, in which each value represented a pixel.
To quantify the impedance change within the lungs from the images, we analyzed the images using regions of interest (ROI). ROI analysis was performed using individual masks for each file for both ventilation and perfusion [14]. We built perfusion masks following a stepwise approach, as follows: 1. Cardiac pixels–which include the potential anatomical areas corresponding to both the heart and the great vessels–tend to be out of phase with lung pixels, because the lungs receive the blood ejected during systole. Based on the phase lag of each pixel in the EKG-gated image and a typical lung pixel, cardiac pixels were automatically excluded; 2. Typical lung pixels were determined by the identification of a decrease in ΔZQ value in the ΔZQ vs. time curves immediately after the point corresponding to EKG-gating (Fig 2); 3. The lung mask comprised of all pixels after the exclusion of cardiac pixels; 4. The sum of the mask-derived pixel amplitudes was multiplied by body weight [14] to yield the ΔZQ measure.
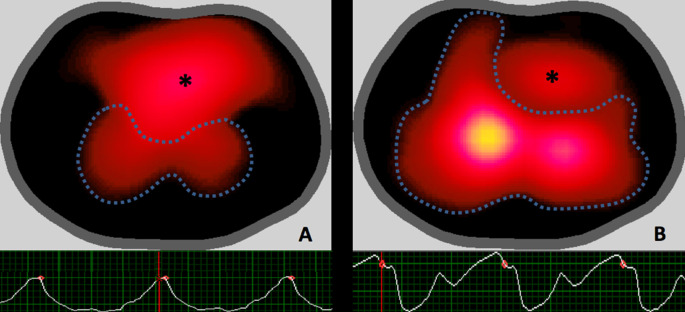
Fig 2.
EIT images and ΔZQ vs. time curves of the pulmonary arterial hypertension group (A) and of the normopressoric group (B). The dashed lines represent the pixels corresponding to the pulmonary area; the central yellow area corresponds to the pixels with higher ΔZQ values, while the dark-red regions to the pixels with lower ΔZQ values; * refers to the cardiac pixels. Below, the ΔZQ vs. time curves: the red points indicate gating to the QRS complex of the ECG–it correlates with ventricular systole and filling of the pulmonary circulation, which is responsible for the descent of the ΔZQ value; note the difference in the amplitude and waveforms.
Statistical analysis
The continuous variables are presented as mean ± standard deviation (SD), when normally distributed, otherwise as median and interquartile [25–75%] ranges. The Student t-test and the Mann-Whitney test were used for group comparison, as appropriate. The Fisher exact test was used to compare categorical variables. For the estimation of the correlation between the ΔZQ and hemodynamic parameters, we used the Pearson correlation analysis. ΔZQ was dichotomized in low vs high ΔZQ based on its median value. Survival curves were described using the Kaplan-Meier Product Time Limit method, and the low vs high ΔZQ groups were compared with the Log-rank test. Hazard ratios and 95% confidence intervals were estimated with Cox proportional hazards analysis in a multivariable model in which both ΔZQ and BNP were included. P values <0.05 were considered significant. The analysis was performed using SPSS software, version 17.0) and R (version 3.0.2).
Results
A total of 55 patients were submitted to RHC. The hemodynamic measurements were normal in eight patients, who represented the normopressoric group. Forty-seven patients received the diagnosis of pulmonary hypertension, of whom 12 were excluded (6 due to EIT signal/file problems; 4 due to PWP >15 mm Hg; and 2 patients due to lack of reliable measure of CO). Thirty-five patients composed the PAH group.
Baseline characteristics of both groups are presented in Table 1. All NP patients were symptomatic at the time of the RHC. Only 30% of the PAH patients had NYHA III/IV, despite a severe hemodynamic profile. The brain natriuretic peptide (BNP) levels were normal in NP group and were consistently elevated in the PAH group. Six etiologies of PAH were identified, of which 70% were composed by idiopathic PAH (IPAH) and connective tissue disease associated PAH (CTD_PAH). More than half of the PAH group was composed of newly diagnosed, treatment-naïve patients.
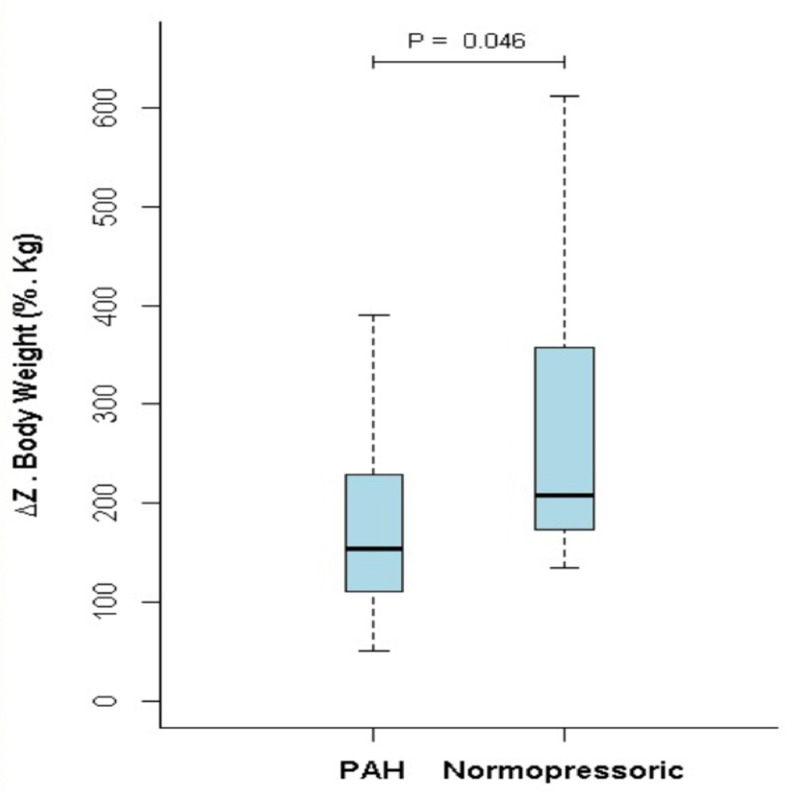
ΔZQ was significantly lower in the PAH group compared to the NP group (177.8±90.3 vs. 277.8±160.5%.Kg; p = 0.046) (Fig 1). Fig 2 illustrates the typical images of ΔZQ and its respective variation over time for both groups.
Fig 1. Comparison between the ΔZQ value of the pulmonary arterial hypertension group and the normopressoric group.
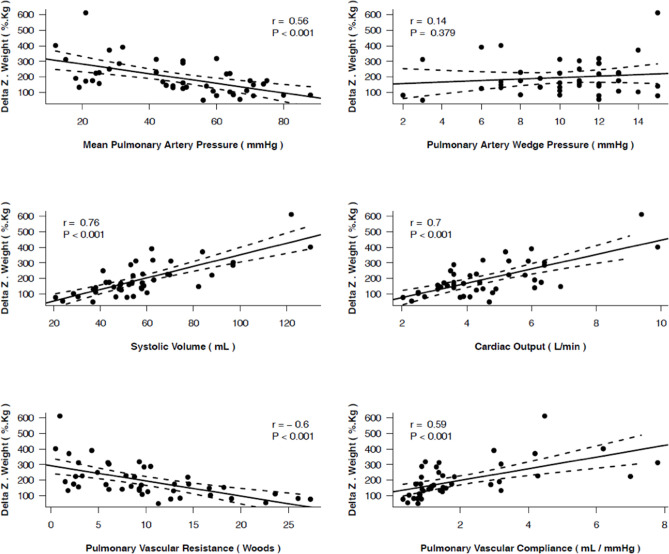
ΔZQ correlated with mPAP, PVC, PVR, CO, and especially with SV (Fig 3).
Fig 3. Correlations between ΔZQ and invasive hemodynamic parameters.
During follow-up, 15 patients died and one received bilateral lung transplantation successfully. Fourteen of them were female (87%). There was no difference in mean age between survivors and non-survivors. Non-survivors had a worse hemodynamic profile: CO (3.6±1.0 vs. 4.7±1.2 L/min; p = 0.004), SV (44.2±12.1 vs. 63.9±19 mL; p < 0.001), mPAP (60.4±13.7 vs. 47.8±16.8 mmHg; p = 0.01), PVR (14.6±6.6 vs. 8.6±4.6 Woods Unit (WU); p = 0.02), PVC (0.9±0.3 vs. 1.8±1.2 mL.mmHg-1; p = 0.002); there was no difference in PWP (10.3±3.1 vs. 10.4±3.2 mL.mmHg-1; p = NS).
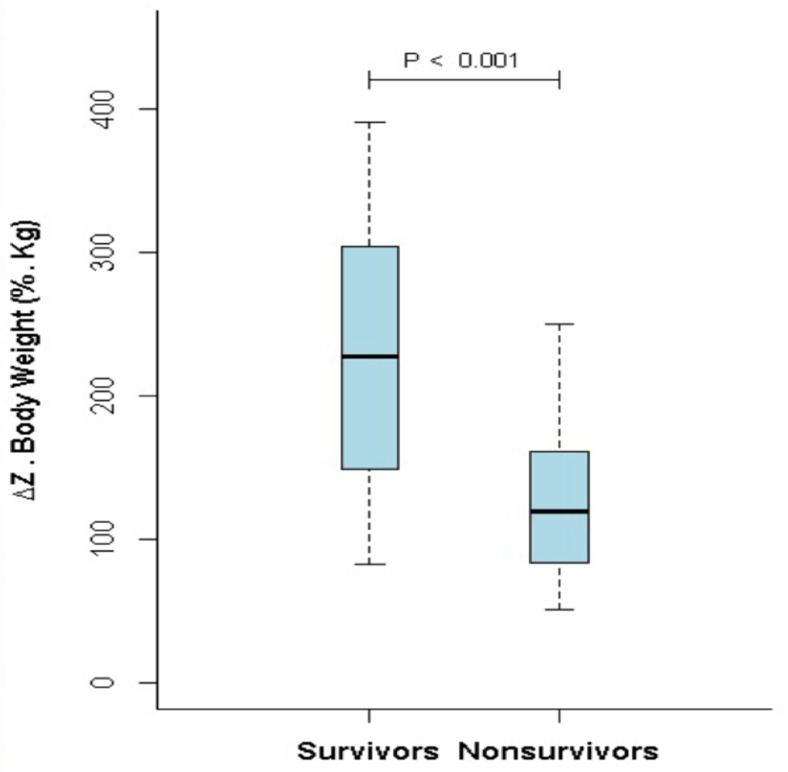
ΔZQ was significantly reduced in the non-survivors in comparison to the survivors (127.2±54.3 vs. 231.6±91%.Kg; p < 0.001) (Fig 4).
Fig 4. Comparison between the ΔZQ value of the survivors and the nonsurvivors groups.
Patients with low ΔZQ (≤154.6%.Kg) also had a worse hemodynamic profile when compared to the patients with high ΔZQ: CO (3.8±1.3 vs. 4.5±1.1 L/min; p = 0.037), SV (45.3±15.4 vs. 62.7±18 mL; p = 0.002), mPAP (62.3±12 vs. 45.8±16.3 mmHg; p < 0.001), PVR (15.1±6.5 vs. 8±3.9 WU; p < 0.001), PVC (0.9±0.4 vs. 1.7±1.2 mL.mmHg-1; p = 0.004); there was no difference in PWP (9.9±3.8 vs. 11±2.2 mL.mmHg-1; p = NS).
BNP measurements were available in 28 patients. In a multivariable analysis, ΔZQ independently predicted survival (HR 0.98, 95%CI 0.97–0.99; p = 0.011) whereas BNP did not (HR 1.0, 95%CI 0.99–1.0; p = 0.928).
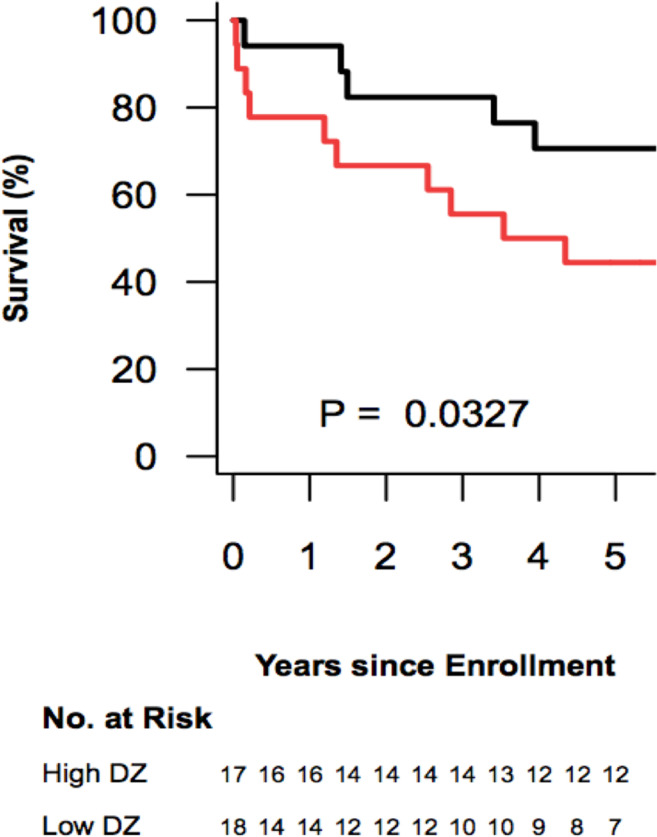
The median survival in the low-ΔZQ group was 3.94 years, while median survival was not reached in the high-ΔZQ group. Patients with low ΔZQ (≤154.6%.Kg) had presented a worse survival when compared to the patients with high ΔZQ (p = 0.033) (Fig 5).
Fig 5. Kaplan-Meier transplant-free survival estimates in the high-ΔZQ group (black) and in the low-ΔZQ group (red) according to the median of the ΔZQ.
Discussion
The present study analyzed the application of EIT, an emergent imaging modality, to patients with suspected or confirmed PAH. Our results demonstrated that ΔZQ shows a significant association with the hemodynamic profile, the disease severity, and the prognosis of PAH patients.
Seventy-five percent of our PAH group were composed by women, with mean age in the fifth decade of life, and nutritional status in the range of overweight. These findings are in line with the data from recent registries [15–17]. Furthermore, our patients, the majority of whom had IPAH or CTD_PAH, had severe and advanced forms of PAH evidenced by their poor hemodynamic profile.
Patients with PAH presented with a significantly reduced ΔZQ in comparison to the NP individuals. A similar result was presented by a Dutch study, in 2006, which identified an important reduction in ΔZQ in 21 patients with IPAH compared to 30 healthy volunteers [10]. Nevertheless, three different aspects between the two studies are relevant: first, we used a newer generation of EIT technology which comprises a 32x32 pixel matrix, a noncircular mesh and a better image temporal resolution; second, our control group was composed of patients with conditions related to PAH, all symptomatic, who had clinical indication for RHC, but did not confirm the presence of pulmonary hypertension. These patients where named here as normopressoric (NP) and represented more than 14% (8/55) of the patients referred to RHC, and more than 30% (8/26) of those who performed the RHC in search for PH diagnosis. In a study from our group, about 20% of the individuals that underwent RHC as part of the diagnostic evaluation for the presence of pulmonary hypertension presented normal pressure levels [18]. Distinctly of health volunteers, it is not possible to assert that the NP patients do not have incipient disease. Even so, there was a significant difference between the PAH and the NP groups [19]. Including patients with indication for RHC rather than healthy volunteers in the control group was important to avoid spectrum bias. Finally, our PAH group was composed of six different forms of PAH whereas only IPAH patients were included in the Dutch study. Our patient mix reflects more closely patients with suspected PAH in clinical practice.
The second relevant finding of our study refers to the correlation of the ΔZQ to the hemodynamic profile. The ΔZQ correlated both to PVC and PVR, mechanical constituents of pulmonary circulation, as well as to SV, the surrogate of right ventricular function. Only one clinical study observed correlation of ΔZQ to hemodynamic parameters in a single patient who presented a progressive increase in ΔZQ parallel to a decrease in mPAP and PVR during the vasoreactivity test [9]. Correlation between ΔZQ and SV was not evaluated in that study. On the other hand, our study demonstrated significant correlations between ΔZQ and all hemodynamic parameters except for PWP. Such a difference can be explained by two factors: (i) our population was composed of 43 patients with hemodynamic data–to the best of our knowledge, this is the largest clinical investigation of EIT in PAH in the presence of simultaneous hemodynamic data; and (ii) our systematic approach to exclude non-pulmonary pixels [20]. Thus, our measurement of ΔZQ probably reflected more accurately the actual magnitude of the pulmonary intra-vascular blood volume variation, illustrating the so sought non-invasive and functional information about the microvascular pulmonary bed. This finding can place EIT as a promising form of assessing the pulmonary circulation physiology and PAH pathophysiology.
Another noteworthy result of our study is the correlation between ΔZQ and SV. An MRI study demonstrated that indexed SV <25 mL.(m2)-1 and indexed right ventricular diastolic volume >84 mL.(m2)-1 were significantly associated with worse prognosis in IPAH [5]. Stroke volume has a pivotal role on the severity and survival of PAH patients, especially because it represents the impairment of the right ventricle (RV) due to the pathological changes in pulmonary vascular mechanics. A recent study [21] suggested that SV provides significant prognostic information even in patients already stratified as presenting low-risk of disease progression. Despite some difficulties reported in estimating SV by means of EIT [22], one can also understand EIT as a non-invasive tool for assessment of disease severity given the good correlation of SV and ΔZQ (r = 0.76; p < 0.001) in our study as well as in an experimental study of our group [14]. This assumption is strengthened by the finding of an even more pronounced reduction of ΔZQ in the 16 patients who died or received lung transplantation during follow-up, in accordance with a significant worse hemodynamic profile in this group, including a significantly lower SV.
In the current state of PAH management, even after great achievements in the understanding and management, overall mortality in PAH is still elevated [17, 23–25]. In our study, the median survival in the low ΔZQ group was almost four years, and EIT was capable of discriminating those patients with higher risk of death or lung transplantation. Risk assessment is paramount in estimating the prognosis of PAH patients during follow-up [25–27]. Multidimensional scores combining clinical, echocardiographic, biological, hemodynamic, and exercise variables have been used to stratify patients in low or high-risk of death or lung transplantation [25–27]. These scores have used noninvasive parameters in an attempt to improve the prognostic ability of invasive hemodynamic parameters. We showed that electrical impedance tomography is also a non-invasive method that carries prognostic information in PAH patients. Thus, it is reasonable to suggest the incorporation of EIT in future multimodal scores as well as its use for the serial follow-up assessments of PAH patients.
Two aspects related to the EIT assessment are noteworthy. The first was our choice to only include lung pixels on the pulsatility analysis. This approach is better than measuring the heart pulsatility because the heart position in relation to the electrode belt can introduce measurement error [11, 22]. Additionally, lung pulsatility can further decrease in more severe PAH because of lower vascular compliance. The second aspect was the correction to body weight. As well as in a recent experimental study of our group [14], that also observed a strong association between ΔZQ and SV, the correlation between ΔZQ and SV was improved by the incorporation of weight to the ΔZQ value. Since EIT measurements are relative images based on changes in impedance in relation to a reference, the ΔZQ is expressed as a percentage change in impedance in relation to the reference condition. The advantage of this approach is the improvement in the robustness of the image reconstruction. In contrast, it produces outputs that are insensible to the absolute values of impedance. It means that an equal absolute perturbation inside thoraces of different sizes and different muscle-to-fat proportion will produce distinct impedance changes, reinforcing the importance of anthropometric correction.
Additionally, information about reproducibility and feasibility deserves consideration. The reproducibility of EIT has been studied in both ventilation and perfusion studies [22, 28–31]. In a model for estimating SV by means of EIT, compared to cardiac MRI, a mean error of -10±12.8 mL was found [30]. Two of these studies [22, 31] showed that the core difficulty in EIT-based SV monitoring is that purely amplitude-based features (that is the case of our study) are strongly influenced by other factors: posture, electrode contact impedance and lung or heart conductivity. The EIT technology consists in installing two auto-adhesive belts over the thorax of a patient by a trained technician. Each belt is connected to a cable that transfers information between the belts and the (trans-)portable EIT device, whose digital/touchable interface also allows a simple operation by a trained technician. These characteristics demonstrate the feasibility of EIT for bedside evaluation in hospitalized patients [32], and probably for the outpatient ones.
Our study has some limitations. First, we did not explore the potential of EIT as a diagnostic tool for pulmonary hypertension, and we only monitored each patient once. Second, there are some limitations related to the EIT technology itself. EKG-gating requires a regular heart rhythm. In patients with arrhythmias such as atrial fibrillation, ΔZQ can be significantly influenced by the ventilation signal. As a result, six patients were excluded because of inadequate image reconstruction or filtering. Additionally, it is known that the pulsatile signal at the cardiac frequency is not purely due to lung perfusion: the impedance changes related to vascular lung pulses can be related to other determinants such as PAP and surrounding lung tissue compliance [33]. The indicator dilution technique could help in the understanding if our results would express strictly lung perfusion [34].
Finally, there are still many challenges ahead before EIT can be incorporated in the field of pulmonary hypertension. Many gaps of knowledge should be explored in future studies. For example, the usefulness of EIT for the diagnosis of PAH or for monitoring response to PAH specific treatment is yet unknown.
In conclusion, given its close association with the hemodynamic profile, disease severity, and survival of PAH patients, EIT can be seen as a promising non-invasive tool for monitoring patients with pulmonary vascular disease.
Supporting information
(CSV)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo). http://www.fapesp.br/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016;4(4):306–22. 10.1016/S2213-2600(15)00543-3 [DOI] [PubMed] [Google Scholar]
- 2.ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2015; 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. 10.1016/j.jacc.2004.02.029 [DOI] [PubMed] [Google Scholar]
- 4.Forfia PR, Fisher MR, Mathai FC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–41. 10.1164/rccm.200604-547OC [DOI] [PubMed] [Google Scholar]
- 5.van Wolferen SA, Marcus JT, Boonstra A, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–7. 10.1093/eurheartj/ehl477 [DOI] [PubMed] [Google Scholar]
- 6.Vonk-Noordegraaf A, Souza R. Cardiac magnetic resonance imaging: what can it add to our knowledge of the right ventricle in pulmonary arterial hypertension? Am J Cardiol. 2012;110(6 Suppl):25S–31S. 10.1016/j.amjcard.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 7.Costa ELV, Amato MBP. Electrical impedance tomography in critical ill patients. Clin Pulm Med. 2013;20(4):178–86. [Google Scholar]
- 8.Smit HJ, Vonk Noordegraaf A, Marcus JT, Boonstra A, de Vries PM, Postmus PV. Determinants of pulmonary perfusion measured by electrical impedance tomography. Eur J Appl Physiol. 2004;92:45–9. 10.1007/s00421-004-1043-3 [DOI] [PubMed] [Google Scholar]
- 9.Smit HJ, Vonk Noordegraaf A, Roeleveld RJ, et al. Epoprostenol-induced vasodilatation in patients with pulmonary hypertension measured by electrical impedance tomography. Physiol Meas. 2002;23:237–43. 10.1088/0967-3334/23/1/324 [DOI] [PubMed] [Google Scholar]
- 10.Smit HJ, Vonk Noordegraaf A, Boonstra A, Vries PM, Postmus PE. Assessment of the pulmonary volume pulse in idiopathic pulmonary arterial hypertension by means of electrical impedance tomography. Respiration. 2006;73:597–602 10.1159/000088694 [DOI] [PubMed] [Google Scholar]
- 11.Costa ELV, Jardim C, Bogossian HGB, Amato MBP, Carvalho CRR, Souza R. Acute vasodilator test in pulmonary arterial hypertension: Evaluation of two response criteria. Vascular Pharmacology. 2005,43:143–7. 10.1016/j.vph.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 12.Eyüboglu BM, Brown BH, Barber DC, Seagar AD. Localization of cardiac related impedance changes in the thorax. Clin Phys Physiol Meas. 1987,8:167–73. 10.1088/0143-0815/8/4a/021 [DOI] [PubMed] [Google Scholar]
- 13.da Silva Ramos FJ, Hovnanian A, Souza R, Azevedo LCP, Amato MBP, Costa ELV. Estimation of stroke volume and stroke volume changes by electrical impedance tomography. Anesth Analg. 2018;126(1):102–10. 10.1213/ANE.0000000000002271 [DOI] [PubMed] [Google Scholar]
- 14.Proença M, Braun F, Solà J, et al. Non-invasive monitoring of pulmonary artery pressure from timing information by EIT: experimental evaluation during induced hypoxia. Physiol Meas. 2016;3:713–36. 10.1088/0967-3334/37/6/713 [DOI] [PubMed] [Google Scholar]
- 15.Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States. How REVEAL differs from historic and non-US contemporary registries. Chest. 2011;139(1):128–37. 10.1378/chest.10-0075 [DOI] [PubMed] [Google Scholar]
- 16.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary hypertension results: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186(8):790–6. 10.1164/rccm.201203-0383OC [DOI] [PubMed] [Google Scholar]
- 17.Alves JL Jr, Gavilanes F, Jardim C, et al. Pulmonary arterial hypertension in the southern hemisphere. Results from a registry of incident brazilian cases. Chest. 2015;147(2):495–501. 10.1378/chest.14-1036 [DOI] [PubMed] [Google Scholar]
- 18.Gavilanes F, Alves JL Jr, Fernandes C, et al. Left ventricular disfunction in patients with suspected pulmonary arterial hypertension. J Bras Pneumol. 2014; 40(6):609–16. 10.1590/S1806-37132014000600004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whyte K, Hoette S, Hervé P, et al. The association between rest and mild-to-moderate exercise pulmonar artery pressure. Eur Respir J. 2012;39(2):313–8. 10.1183/09031936.00019911 [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Ortega NRS, Galizia MS, Borges JB, Amato MBP. Fuzzy modeling of electrical impedance tomography image of the lungs. Clinics. 2008;63(3):363–70. 10.1590/s1807-59322008000300013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weatherald J, Boucly A, Chemla D, et al. Prognostic value of follow-up hemodynamic variables after initial management in pulmonary arterial hypertension. Circulation. 2018;137:693–704. 10.1161/CIRCULATIONAHA.117.029254 [DOI] [PubMed] [Google Scholar]
- 22.Braun F, Proença M, Adler A, Riedel T, Thiran J-P, Solà J. Accuracy and reliability of noninvasive stroke volume monitoring via ECG-gated 3D electrical impedance tomography in healthy volunteers. PLoS One. 2018;13(1):e0191870. 10.1371/journal.pone.0191870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humbert M, Montani D, Souza R. Predicting survival in pulmonary arterial hypertension. Time to combine markers. Chest. 2011;139(6):1263–4. 10.1378/chest.10-2868 [DOI] [PubMed] [Google Scholar]
- 24.Delcroix M, Staehler G, Gall H, et al. Risk assessment in medically treated chronic thromboembolic pulmonary hypertension patients. Eur Respir J. 2018;52(5):180024 10.1183/13993003.00248-2018 [DOI] [PubMed] [Google Scholar]
- 25.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Heart J. 2016;37: 67–119. 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 26.Boucly Athénaïs, Weatherald Jason, Savale Laurent, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50(2):1700889. 10.1183/13993003.00889-2017 [DOI] [PubMed] [Google Scholar]
- 27.Boucly A, Weatherald J, Humbert M, Sitbon O. Risk assessment in pulmonary arterial hypertension. Eur Respir J. 2018;51(3):1800279. 10.1183/13993003.00279-2018 [DOI] [PubMed] [Google Scholar]
- 28.Frerichs I, Schmitz G, Pulletz S, et al. Reproducibility of regional lung ventilation distribution measured by electrical impedance tomography during mechanical ventilation. Physiol Meas. 2007;28(7):S261–7. 10.1088/0967-3334/28/7/S19 [DOI] [PubMed] [Google Scholar]
- 29.Reifferscheid F, Elke G, Pulletz S, et al. Regional ventilation distribution determined by electrical impedance tomography: reproducibility and effects of posture and chest plane. Respirology. 2011;16(3):523–31. 10.1111/j.1440-1843.2011.01929.x [DOI] [PubMed] [Google Scholar]
- 30.Proença M. IEEE Biomedical Circuits and Systems Conference (BioCAS) Proceedings. IEEE Trans Biomed Circuits Syst. 2014;8(5):605–750. [PubMed] [Google Scholar]
- 31.Braun F, Proença M, Wendler A, et al. Noninvasive measurement of stroke volume changes in critically ill patients by means of electrical impedance tomography. J Clin Monit Comput. 2020;34(5):903–11. 10.1007/s10877-019-00402-z [DOI] [PubMed] [Google Scholar]
- 32.Bachmann MC, Morais C, Bugedo G, et al. Electrical impedance tomography in acute respiratory distress syndrome. Crit Care. 2018;22(1):263. 10.1186/s13054-018-2195-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellige G, Hahn G. Cardiac-related impedance changes obtained by electrical impedance tomography: an acceptable parameter for assessment of pulmonary perfusion? Critical Care. 2011;15:430. 10.1186/cc10231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borges JB, Suarez-Sipmann F, Bohm SH, et al. Regional lung perfusion estimated by electrical impedance tomography in a piglet model of lung collapse. J Appl Physiol. 2012;112:225–36. 10.1152/japplphysiol.01090.2010 [DOI] [PubMed] [Google Scholar]