Abstract
Background
Oral ivermectin is a safe broad spectrum anthelminthic used for treating several neglected tropical diseases (NTDs). Currently, ivermectin use is contraindicated in children weighing less than 15 kg, restricting access to this drug for the treatment of NTDs. Here we provide an updated systematic review of the literature and we conducted an individual-level patient data (IPD) meta-analysis describing the safety of ivermectin in children weighing less than 15 kg.
Methodology/Principal findings
A systematic review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for IPD guidelines by searching MEDLINE via PubMed, Web of Science, Ovid Embase, LILACS, Cochrane Database of Systematic Reviews, TOXLINE for all clinical trials, case series, case reports, and database entries for reports on the use of ivermectin in children weighing less than 15 kg that were published between 1 January 1980 to 25 October 2019. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO): CRD42017056515. A total of 3,730 publications were identified, 97 were selected for potential inclusion, but only 17 sources describing 15 studies met the minimum criteria which consisted of known weights of children less than 15 kg linked to possible adverse events, and provided comprehensive IPD. A total of 1,088 children weighing less than 15 kg were administered oral ivermectin for one of the following indications: scabies, mass drug administration for scabies control, crusted scabies, cutaneous larva migrans, myiasis, pthiriasis, strongyloidiasis, trichuriasis, and parasitic disease of unknown origin. Overall a total of 1.4% (15/1,088) of children experienced 18 adverse events all of which were mild and self-limiting. No serious adverse events were reported.
Conclusions/Significance
Existing limited data suggest that oral ivermectin in children weighing less than 15 kilograms is safe. Data from well-designed clinical trials are needed to provide further assurance.
Author summary
Oral ivermectin is a safe and efficacious drug for the treatment of neglected tropical diseases. To date, ivermectin is not indicated in children weighing less than 15 kg because there have been insufficient safety data to support a change of recommendation. A PRISMA-level systematic review was conducted, and 97 potential sources were identified. All lead investigators were contacted to share individual patient data if they could provide the minimum criteria. These were the known weights of the children less than 15 kg in whom there were possible adverse events. A total of 17 investigators replied, sharing individual-level patient data (IPD) from 15 studies, which represent a database of 1,088 children weighing less than 15 kg treated with oral ivermectin. Overall 18 adverse events were reported in 1.4% (15/1,088) of children, all of which were mild and self-limiting. No serious adverse events were recorded. These data suggest that ivermectin is safe for use in children weighing less than 15 kilograms. Further data from well-designed clinical trials are needed to assess the safety of oral ivermectin at escalating doses in children weighing less than 15 kg.
Introduction
Ivermectin is a safe broad-spectrum anthelminthic drug registered for the treatment of several neglected tropical diseases (NTDs) including onchocerciasis, lymphatic filariasis, scabies, and strongyloidiasis. These NTDs frequently afflict small children but ivermectin is not currently indicated or licensed for use in children weighing less than 15 kg because of a lack of safety evidence. Because of this contraindication, alternatives to oral ivermectin are used in children weighing less than 15 kg that may be less effective (e.g. topical creams for scabies) or potentially toxic (e.g. lindane for scabies, thiabendazole for strongyloidiasis).
Ivermectin was developed initially for human use in the control of Onchocerca volvulus transmission because it kills microfilariae rapidly and it inhibits release of microfilariae from the gravid female worms. It is unlikely that infants and young children contribute substantially to the transmission of O. volvulus because of the biology of the parasite; it takes more than one year from entry of L3 larvae into the human body for these larvae to mature, find a mate, become fertile, and start releasing microfilariae [1,2]. Field evaluations indicate that it is very uncommon for children aged less than 5 years to be microfilaridermic [1,3]. Therefore, during drug development, it was not considered necessary to evaluate the safety and efficacy of ivermectin in children weighing less than 15 kg for inclusion in mass drug administrations (MDAs) for onchocerciasis control.
While there is a contraindication on the ivermectin package insert for treating children weighing less than 15 kg, it is likely that there have been millions of children weighing less than 15 kg who have been given ivermectin during MDA campaigns for onchocerciasis and lymphatic filariasis in Africa. Over 400 million people were treated with ivermectin during MDAs in 2019 alone, primarily in Africa, with over four billion treatments administered via MDA to date [4]. To expedite ivermectin delivery during MDAs, ivermectin dosing is usually based on height and not weight [5] with a cut-off for administration of 90 cm [4]. Based on WHO Child Growth Standards, the median weight for a 90 cm child is 12.7 kg for boys and 12.5 kg for girls, thus a 90 cm child weighing 15kg would be on the 97th weight percentile for boys and the 98th percentile for girls [6]. Therefore, it is likely that children with a weight between 11 and 15 kg in practice are routinely administered ivermectin during MDAs. As NTDs may cause growth stunting in children, for example infection with soil-transmitted helminths (STHs) or onchocerciasis-associated Nakalanga syndrome [7] some children do not reach 90 cm until they are older, and thus are denied ivermectin during MDA campaigns which could eliminate the very parasites causing their stunted growth. Infants one to three months of age have been treated with oral ivermectin (200 μg/kg) for scabies, with one dose [8,9], or two doses spaced one to two weeks apart [9,10], with no neurological events reported.
In mammals, ivermectin is prevented from crossing the blood brain barrier (BBB) by active efflux via P-glycoprotein (P-gp) at the luminal membrane of capillary endothelial cells [11]. However, neurotoxic complications have been observed in certain dog breeds (e.g. Collies, Sheepdogs) following ivermectin treatment. It was discovered that these dog breeds have a four base pair deletion in the ABCB1 gene [12] that causes complete loss of transport function of P-gp allowing entry of ivermectin into the central nervous system [13]. A recent case report illustrates that nonsense mutations in the ABCB1 gene leading to a loss of function of P-gp associated with a neurological adverse event in a 13-year-old boy following a single dose of ivermectin (200 μg/kg) [14]. Concerns have been raised regarding the use of ivermectin in infants due to a partially formed BBB.
During early drug development, preclinical toxicity studies of ivermectin were performed in neonatal rats and macaques. Neurotoxicity was observed in 1- to 2- day old rats (CRCD strain), which manifested as ataxia, ptosis, and decreased activity. The rat neonatal LD50 was 2.3 mg/kg, approximately twenty-fold lower than the adult LD50 of 42.8–52.8 mg/kg [15]. However, neonatal rats may not be an ideal model for ivermectin toxicity for human infants. In general, human and primate BBB development begins earlier in gestation and proceeds more rapidly than it does in rodents [16]. By birth, the ratio of human P-gp expression of newborns to adults is higher in humans [17] compared to rats [18]. Neonatal macaques are a better model for humans. Macaques aged 6–13 days old given oral ivermectin (100 μg/kg) for 14 days experienced no AEs [15]. In humans, P-gp reaches adult levels of expression three to six months after birth [16].
A systematic review of the safety of oral ivermectin in small children published previously [19], concluded that the limited available literature suggests that ivermectin is well tolerated by small children with no serious or long-term adverse effects. Here we provide an updated and more detailed review of the published literature on the safety of ivermectin in children weighing less than 15 kg using individual-level patient data (IPD).
Methods
Ethics statement
Data included in this analysis were obtained in accordance with ethical approvals from the location of origin. Data were requested to be shared anonymized. A non-human subjects use protocol was established for the acquisition of IPD, reviewed by the Walter Reed Army Institute of Research (WRAIR#2458). Consent was not obtained because data were analyzed anonymously.
Literature review
Reports on the use of ivermectin in children weighing less than 15 kg were searched for on the following databases: MEDLINE via PubMed, Web of Science, Ovid Embase, LILACS, Cochrane Database of Systematic Reviews, TOXLINE, and ClinicalTrials.gov. Databases were searched in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of individual participant data (PRISMA) statement (S1 Checklist) [20]. Clinical trials, case series, case reports, and database entries published between 1 January 1980 to 25 October 2019 in any language describing ivermectin use in small children were identified. The US Food and Drug Administration, the Australian Adverse Drug Reactions Advisory Committee, the EudraVigilance system, and Vigibase (maintained by the WHO Collaborating Centre for International Drug Monitoring) were contacted for case reports of ivermectin adverse reactions in children weighing less than 15 kg however, these databases do not collect weights of individuals so no data could be contributed to this study. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO): CRD42017056515. Three independent investigators undertook the review process and extracted the data (PJ, WM, KCK), resolving discrepancy through discussion. Search terms included but were not restricted to: (soolantra OR sklice OR oramec OR mk933 OR "mk 933" OR ivomec OR eqvalan OR eqvalen OR epimer OR diapec OR cardomec OR stromectol OR mectizan OR ivermectin) AND (child or children OR newborn* OR infan* or new-born* or perinat* or neonat* or baby* or babies or toddler* or boy or boys or girl or girls or kid or kids or pediatric* or paediatric* or peadiatric* or prematur* or preterm* OR "low birth weight" or vlbw or lbw or preschool*). Screening was based on the title and abstract, and if treatment based on age (≤5 years old) or weight (<15 kg) was not clearly defined, then a review of the manuscript was performed. To confirm if ivermectin was used in children weighing less than 15 kg, authors of possible reports of ivermectin use in small children were contacted via email on at least 3 attempts in English, and when warranted in French, Portuguese, Spanish, or Swedish. In some cases, attempts were made to call and/or message authors via ResearchGate.
Lead investigators of identified studies were contacted to share individual-level patient data IPD. Only studies including weight of ivermectin-treated children less than 15 kg and adverse events data were considered for the final IPD meta-analysis. Adverse events were defined as the appearance or worsening of any undesirable sign, symptom, or medical condition after starting the study drug, even if the event was not considered related to the study drug. Serious Adverse Events (SAEs) were defined as any untoward medical occurrence that at any dose: resulted in death; was life-threatening; required inpatient hospitalization or resulted in prolongation of existing hospitalization; resulted in persistent or significant disability/incapacity; was a congenital anomaly/birth defect; or was a medically important event or reaction [21].
Additional patient-level data including disease indication for ivermectin use, manufacturer, brand name, dose, regimen, and delivery method were requested. IPD shared with the WorldWide Antimalarial Resistance Network (WWARN) were curated and standardized as described in a data management plan [22]. Any data inconsistencies or missing information were resolved directly with the investigators. Descriptive summaries were made to describe the risk of selection bias and reporting bias from the IPD contributing studies.
Outcome
The primary outcome was the incidence of adverse events in children weighing less than 15 kg that ingested oral ivermectin.
Statistical analysis
Since data were limited and heterogenous, mostly descriptive analyses were conducted. Incidences of AEs, their grading and causality classification, as determined by the on-site principal investigator or physician in each study were pooled and analyzed. Incidence of any AEs in children weighing less than 15 kg who were treated for scabies was estimated by pooling data from cohort, MDA or randomized studies (excluding case reports/series) using fixed logistic regression model (stata command metapreg). Heterogeneity between studies was quantified using I2 measure. Exact confidence intervals are presented for individual studies and for rate of SAE. Risk of bias was assessed based on study design and AE elicitation method.
Results
Systematic review summary
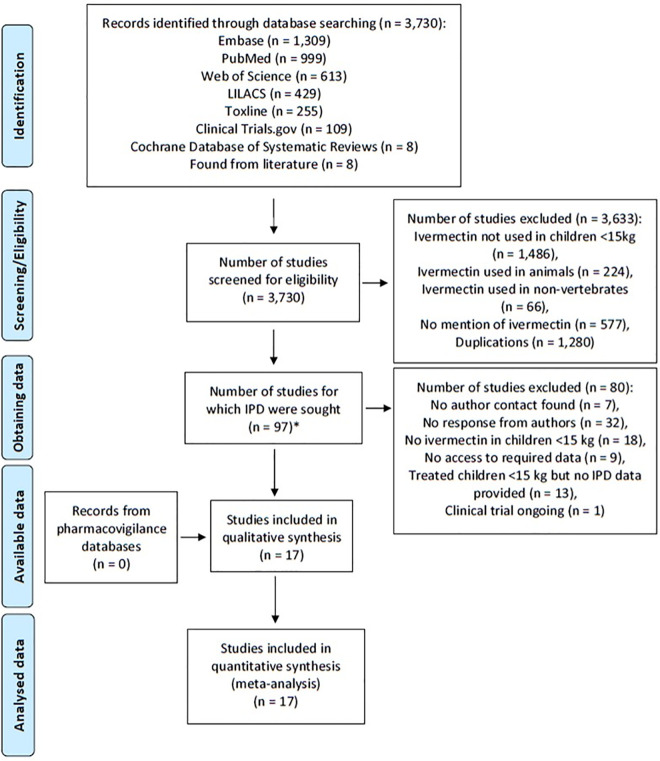
A total of 3,730 potential publications were identified and assessed for eligibility from literature database searches, of which 97 were selected for potential inclusion in the review (Fig 1). We attempted to contact all authors of the publications, and received responses for only 59.8% (58/97) of publications. Of responding authors, 29.3% (17/58) successfully provided IPD on ivermectin use in children weighing less than 15 kg, while 31.0% (18/58) confirmed that oral ivermectin was not provided to children weighing less than 15 kg, 8.6% (5/58) reported they no longer had access to the data, 6.9% (4/58) reported that ivermectin was used in children weighing less than 15 kg but weight or adverse event data were not recorded or were not available, 22.4% (13/58) reported they used ivermectin in children weighing less than 15 kg but failed to provide the IPD data, and 1.7% (1/58) reported that children weighing less than 15 kg will be treated with oral ivermectin but the study has not started yet (S1 Table). Overall, 17 reports describing 15 studies provided IPD. Characteristics of the 15 included studies are summarized in Table 1. Study designs utilized include: case reports 46.7% (7/15; 10 subjects), case series 26.7% (4/15; 175 subjects), cohort studies 6.7% (1/15; 4 subjects), MDA 6.7% (1/15; 838 subjects), a prospective observational study 6.7% (1/15; 17 subjects), and a randomized controlled trial 6.7% (1/15; 44 subjects). No issues were identified in IPD integrity.
Fig 1. PRISMA IPD flow diagram.
Fig 1 depicts the total number of studies identified from systematic review, rationale for exclusion, and number of studies included in the final analyses. * See S1 Table for list of studies and rationale for inclusion or exclusion from the review.
Table 1. Study characteristics.
| Study ID | Pubmed ID | Country | Study Type | Year(s) | Indication | Brand Name | Manufacturer | Number Doses | Days b/w doses | Dose μg/kg | Number of Subjects | AE Method | Cohort Description |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 29576324 | France | Prospective observational study | 2015–2017 | Scabies | Stromectol | MSD France | Two | 7–10 | 150–400 | 17 | Passive reporting | Sarcoptes scabei positive children seen at one of seven French departments of dermatology |
| 2 | 28063594 29576324 |
France | Case report | 2017 | Scabies | Stromectol | MSD France | Two | 10 | 100 | 1* | Passive reporting | Single child seen at clinic |
| 3 | 31073620 | France | Case series | 2017 | Cutaneous Larva Migrans | Stromectol | Unknown | One | n/a | 200 | 1 | Observed in clinic | Autochthonous cases in France |
| 4 | 30468533 | France | Case report | 2017 | Cutaneous Larva Migrans | Stromectol | MSD France | One | n/a | 272 | 1 | Observed in clinic | Single child seen at clinic |
| 5 | 23094746 | Venezuela | Case report | 2012 | Strongyloidiasis | Kilox | Laboratorios Bussie | Two | 1 | 200 | 1 | Observed in clinic | Single child seen at clinic |
| 6 | 30857778 | France | Case report | 2019 | Pthiriasis | Stromectol | MSD France | Two | 8 | 400 | 1 | Passive reporting | Single child seen at clinic |
| 7 | 16045712 | Mexico | Case report | 2005 | Scabies | Unknown | Unknown | One | n/a | 200 | 1 | Passive reporting | Single child seen at clinic |
| 8 | no PMID** | Brazil | Case report | 2002 | Crusted scabies | Unknown | Unknown | Two | 7 | 200 | 1 | Observed in clinic | Single child seen at clinic |
| 9 | 26962825 | Spain | Case report | 2013 | Strongyloidiasis | Stromectol | MSD France | Two | 1 | 200 | 4 | Observed in the clinic | Strongyloides stercoralis positive children; one published and three unpublished case reports |
| 10 | 29165211 | Peru | Case series | 2012–2015 | Myiasis | Quanox oral | Siegfried | One | n/a | 300 | 1 | Observed in the clinic | Single child seen at clinic |
| 11 | 12139665 | Mexico | Case series | 2002 | Crusted scabies / Scabies/ Cutaneous Larva Migrans | Unknown | Unknown | One or two | 14 | 150–200 | 4 | Passive reporting | Sarcoptes scabei or cutaneous larva migrans positive children at least 14 mo that were not able to be treated with topical creams |
| 12 | 31344258 | France | Case series | 2012–2015 | Scabies | Stromectol | MSD France | One or two | 7–15 | 94–556 | 169 | Reported at follow up visit at 14 days | Sarcoptes scabei positive children weighing less than 15 kg; standardized anonymous form was sent to members of the French Society of Paediatric Dermatology |
| 13 | 30223985 | Solomon Islands | MDA trial | 2015 | Scabies MDA | Stromectol | MSD Australia | One or two | 3–19 | 200–400 | 838 | Reported at second visit | All residents eligible to participate, excludes pregnant and breastfeeding women or children weighing less than 12.5kg |
| 14 | 29617737 | Côte d’Ivoire | Randomized Controlled Trial | 2017 | Trichuriasis | milled 0.5 mg tablet | Elea Laboratorios | Single dose | n/a | 81–500 | 44 | Reported at follow up visits at 3,24,72 hours | Trichuris trichiura positive children 2–12 yo |
| 15 | 27548286 | Cambodia | Cohort study | 2012–2014 | Strongyloidiasis | Stromectol | MSD Netherlands | Single dose | n/a | 200 | 4 | Passive reporting | Strongyloides stercoralis positive residents over 2 yo |
| 16 | 29059195 27548286 |
Cambodia | Cohort study | 2012–2014 | Strongyloidiasis | Stromectol | MSD Netherlands | Single dose | n/a | 200 | 4*** | Passive reporting | Strongyloides stercoralis positive residents over 2 yo |
| 17 | 28070007 | Guadeloupe | Case report | 2013 | Parasitic disease of unknown origin before the diagnosis of angiostrongyliasis | Stromectol | MSD France | Single dose | n/a | 353 | 1 | Observed in the clinic | Single child seen at hospital |
* Single case included in Study ID 1
** No Pubmed ID; Dermatol. Argent; 8(3):136–140, jul.-aug. 2002. Available at: https://www.dermatolarg.org.ar/index.php/dermatolarg/article/view/357/168
*** These four cases included in Study ID 15
IPD summary
From the 17 reports, there were 1,088 confirmed instances in which oral ivermectin was given to children weighing less than 15 kg. The majority of the treated children (80.2%, 867/1,081) were younger than five years of age with an overall median age of 36 months (range 1–132 months). Median weight was 13.0 kg (range 4.0–14.9 kg). Height was only available in 5 out of 15 studies, for 10.9% (119/1,088) of children, with a median of 84 cm (range 54–103 cm) and 28.6% (34/119) were 90 cm or taller.
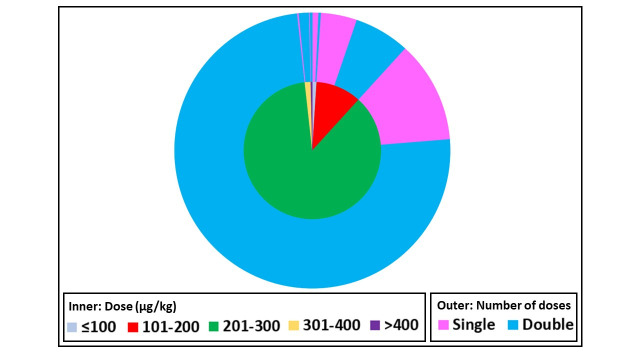
Of ivermectin-treated children, 82.8% (901/1,088) received two doses of ivermectin anywhere from 1 to 19 days apart. Although 200 μg/kg of ivermectin is the standard oral dose for most NTDs, 86.5% (941/1,088) children received a dose between 201–300 μg/kg and 1.7% (19/1,088) received doses above 300 μg/kg (Fig 2). Overall, the median dose administered was 221 μg/kg (range 81–556 μg/kg). The doses administered were significantly higher in the two-dose regimen (median 231 μg/kg, range 94–556, n = 901) compared to the single-dose regimen (median 214 μg/kg, range 81–500 μg/kg, n = 187), (p<0.001, Mann Whitney test).
Fig 2. Ivermectin dose and number administered to children weighing less than 15 kg.
Fig 2 depicts the ivermectin dose administered in μg/kg (inner ring) and the number of doses administered (outer ring) that were collected for the IPD database (n = 1,088).
Oral ivermectin was administered to children weighing less than 15 kg for a total of nine indications including: scabies MDA 77.0% (838/1,088), scabies individual treatment 17.3% (188/1,088), trichuriasis 4.0% (44/1,088), strongyloidiasis 0.8% (9/1,088), cutaneous larva migrans 0.4% (4/1,088), crusted scabies 0.2% (2/1,088), myiasis 0.1% (1/1,088), pthiriasis 0.1% (1/1,088), and parasitic disease of unknown origin 0.1% (1/1,088). Instances of ivermectin administration to children weighing less than 15 kg were reported from ten countries including: Solomon Islands 77.0% (838/1,088), France 17.4% (189/1,088), Côte d’Ivoire 4.0% (44/1,088), Mexico 0.5% (5/1,088), Cambodia 0.4% (4/1,088), Spain 0.4% (4/1,088), Brazil 0.1% (1/1,088), Guadeloupe 0.1% (1/1,088), Peru 0.1% (1/1,088), and Venezuela 0.1% (1/1,088).
In total, 18 AEs were reported from 1.4% (15/1,088) of ivermectin-treated children and none of the AEs (0/18, 95%CI 0–0.33%) were deemed SAEs (Table 2). The most common AEs reported were diarrhea 0.4% (4/1,088) and eczema 0.5% (5/1,088). Headache, itching and vomiting were each reported twice 0.2% (2/1,088) and joint pain, abdominal pain, and symmetrical edema of the feet were each reported once 0.1% (1/1,088) (Tables 2 and S2). Twelve patients reported only one AE, while three patients reported 2 AEs: headache and diarrhea (n = 1), abdominal pain and vomiting (n = 1), diarrhea and itching (n = 1). All AEs were deemed possibly related to ivermectin administration, except for the joint pain which was classified as unlikely related to ivermectin administration. Adverse events were related to scabies treatment or MDA for scabies in 50% (9/18), trichuriasis in 44.4% (8/18), and strongyloidiasis in 5.6% (1/18) of patients. A pooled prevalence of AEs in the scabies, crusted scabies, or scabies MDA data derived from 3 studies, was estimated as 0.88% (95%CI 0.48–1.68) (Table 3). Seven of the AEs occurred in children that were co-administered either topical creams for scabies (4/7), oral azithromycin (2/7), or intravenous iron plus vitamin supplements (1/7) (Table 2). No adverse events were reported in the 1.3% (14/1,081) of children three months or younger. Of 128 children who received ≤200 μg/kg ivermectin, 7.0% (9/128) experienced an AE, of 960 children who received >200 μg/kg ivermectin 0.6% (6/960) reported an AE, and of 19 children who received >300 μg/kg ivermectin none reported an AE. All AEs were considered mild, self-limiting, and resolved without further intervention.
Table 2. Listing of individual adverse events (n = 18) following ivermectin ingestion.
None of the AEs were classified as serious adverse events.
| AE ID | Patient ID | Study ID | AE term | Relation to IVM | Time Reported (Days) | Weight (kg) | Age (mo) | Number of doses | Dose (μg/kg) | Indication | Concomitant Medication |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 11 | Diarrhea | Possible | 10 | 10 | 10 | 2 | 300 | Scabies | Topical Cream |
| 2 | 2 | 13 | Diarrhea | Possible | 1 | 10 | 24 | 1 | 100 | Trichuriasis | |
| 3 | 3 | 13 | Diarrhea | Possible | 3 | 11 | 24 | 1 | 182 | Trichuriasis | |
| 4 | 4 | 12 | Diarrhea | Possible | 8 | 13 | 24 | 2 | 231 | Scabies MDA | Azithromycin |
| 5 | 5 | 11 | Eczema | Possible | 10 | 10 | 12 | 2 | 150 | Scabies | |
| 6 | 6 | 11 | Eczema | Possible | 7 | 10 | 16 | 2 | 200 | Scabies | Topical Cream |
| 7 | 7 | 11 | Eczema | Possible | 10 | 10 | 18 | 2 | 150 | Scabies | Topical Cream |
| 8 | 8 | 11 | Eczema | Possible | 8 | 10.1 | 14 | 2 | 297 | Scabies | Topical Cream |
| 9 | 9 | 11 | Eczema | Possible | 8 | 11 | 31 | 2 | 205 | Scabies | |
| 10 | 2 | 13 | Headache | Possible | 1 | 10 | 24 | 1 | 100 | Trichuriasis | |
| 11 | 10 | 13 | Headache | Possible | 1 | 13 | 60 | 1 | 192 | Trichuriasis | |
| 12 | 3 | 13 | Itching | Possible | 3 | 11 | 24 | 1 | 182 | Trichuriasis | |
| 13 | 11 | 13 | Itching | Possible | 1 | 13.2 | 24 | 1 | 114 | Trichuriasis | |
| 14 | 12 | 12 | Joint Pain | Unlikely | 8 | 13 | 36 | 2 | 231 | Scabies MDA | Azithromycin |
| 15 | 13 | 13 | Abdominal Pain | Possible | 1 | 14 | 60 | 1 | 107 | Trichuriasis | |
| 16 | 14 | 11 | Vomiting | Possible | 10 | 11 | 32 | 2 | 273 | Scabies | |
| 17 | 13 | 13 | Vomiting | Possible | 3 | 14 | 60 | 1 | 107 | Trichuriasis | |
| 18 | 15 | 4 | Symmetrical Edema of the Feet | Possible | 1 | 4.7 | 18 | 2 | 200 | Strongyloidiasis | IV Iron and Vitamin Supplement |
AE, adverse event; IVM, ivermectin; kg, kilograms; mo, months; IV, intravenous
Table 3. Pooled AE incidence in children with Scabies, Crusted scabies, or participating in MDAs for scabies (excluding case studies) from a fixed effects logistic regression model.
I2 statistics (variation in log-odds attributable to heterogeneity) = 21.4%.
| Study ID | Number of patients | Number of AE | AE incidence (%) | 95% CI |
|---|---|---|---|---|
| 1 | 17 | 0 | 0.00% | 0.00% - 19.51% |
| 12 | 169 | 7 | 4.14% | 1.68% - 8.35% |
| 13 | 838 | 2 | 0.24% | 0.03% - 0.86% |
| Overall—Fixed Effects | 0.88% | 0.46% - 1.68% | ||
| Bécourt et al. 2013 | 15 | 2 | 13.33 | 1.66–40.46 |
| Overall—Fixed Effects | 1.06% | 0.59% - 1.90% |
Risk of bias
A single patient case report or small case series does not allow for estimation of AE frequency, but provides descriptive or narrative results. Therefore, case reports or case series in our review (study IDs 2–11,17) [8,23–32] with patients (n = 16) seen in the clinic were categorized as high risk of selection and reporting bias.
Among the remaining studies, low risk of selection bias is expected in the randomized control study (study ID 14) [33] (n = 44) and the MDA trial (study ID 13) [34] in which all eligible persons in the study area were treated (n = 838) and participants from randomly selected villages were followed up for AEs, and moderate risk in the prospective studies (study IDs 1,15,16) [35–37] (n = 21). Only two studies actively asked for AEs (study ID 12,14) [9,33] (n = 213), while MDA (study ID 13) [34] (n = 838) and cohort studies (study ID 15,16) [35,36] (n = 4) used passive reporting of AEs, and were categorized a high risk for reporting bias.
One study identified by the review [10] could not be included in the analysis because AEs were not linked to individual patients. The article reported that among 15 children weighing<15kg treated for scabies, two experienced AEs (one was nervous and irritable and the other scratched intensely). Including this study would slightly increase the pooled AE frequency to 1.06% (95% CI 0.59–1.90%) (Table 3). In any other studies (n = 79) for which IPD could not be obtained, data published could not be used in the synthesis as the AEs and number of patients studied were not presented explicitly in the weight group of interest. Consequently, we cannot assess representativeness of the IPD collated in relation to all ivermectin use data available in this weight group.
Discussion
This systematic review identified 97 reports where ivermectin was potentially used in children weighing less than 15 kg for numerous indications including: scabies MDA, scabies, crusted scabies, strongyloidiasis, onchocerciasis, lymphatic filariasis, baylisascariasis, head lice, pubic lice, cutaneous larva migrans, gnathostomiasis, trichuriasis, tungiasis, trombidiasis, rosacea, capillariasis, myiasis, intestinal acariasis, and parasitic disease of unknown origin. However, IPD reporting both the weight and adverse events could only be acquired from 17 reports, accounting for 1,088 children, treating the following indications: scabies MDA, scabies, crusted scabies, strongyloidiasis, pthiriasis, cutaneous larva migrans, trichuriasis, myiasis, and parasitic disease of unknown origin (Table 1). The evidence summarized here indicates that oral ivermectin caused AEs in 1.4% (15/1,088) children weighing less than 15 kg and there were no SAEs reported. With a sample size of 1000, the probability of observing at least one AE with prevalence of 1 in 1000 is 0.632, and >0.999 for adverse events with incidence 1 in 100. Thus, the upper 95% confidence interval for the true incidence of AEs following ivermectin administration to children <15kg is 1 in 362.
All data contributors rated the AEs as being possibly caused by ivermectin treatment with the exception of joint pain which was rated as unlikely to be caused by ivermectin. Of the reported AEs, headache 2 (0.18%), vomiting 2 (0.18%), abdominal pain 1 (0.09%), diarrhea 4 (0.37%), and itching 2 (0.18%) have all previously been described for ivermectin and in the current study occurred at rates less than stated on ivermectin package inserts [38,39]. Our study results therefore suggest that ivermectin is as safe and well-tolerated in children weighing less than 15 kg as indeed it is for persons weighing more than 15 kg. One patient given oral ivermectin for strongyloidiasis had symmetrical edema of the feet, but this could not be directly attributed to ivermectin because the child was extremely malnourished and was also given intravenous fluids, iron, and supplements [26]. A limitation of this study is the lack of responses from several authors 32.0% (31/97) and inability to contribute IPD-level data after confirmation of oral ivermectin use in children weighing less than 15 kg 13.4% (13/97) from published reports.
This evidence has been compiled into an IPD database, held at WWARN, and available upon request to by contacting the study authors. Our hope is that this database can be used for regulatory review regarding the safety of ivermectin in children weighing less than 15 kg. These data can be difficult to compile as they are generated from a wide range of treatment indications from around the world. The following sections discuss key issues surrounding use of ivermectin in children weighing less than 15 kg.
Ivermectin treatment and mass drug administration for scabies
Several trials of ivermectin MDA for the control of scabies have been performed in the South Pacific [34,40,41]. Adequate population coverage is critical for the success of scabies MDA. The use of oral ivermectin has logistical and compliance advantages over topical creams. Furthermore, MDA of oral ivermectin was found to be more effective than topical permethrin [40]. Children not treated by ivermectin during MDA serve as a reservoir for future community re-infection unless adequately treated with topical treatments. One MDA trial for scabies in the Solomon Islands co-administered ivermectin and azithromycin for the control of scabies and impetigo. Azithromycin is indicated for use in children weighing 12.5 kg or more, and in the trial in the Solomon Islands the 12.5 kg limit was also used for ivermectin for pragmatic reasons. A total of 838 children weighing 12.5 to less than 15 kg received ivermectin as part of MDA, and only two adverse events were reported in this group [34].
In 2018, Ethiopia performed the largest scabies ivermectin MDA to date, treating 1,634,271 persons with oral ivermectin. Drug administration was not based on height or weight but by age. Children two to six years old were administered a single 3 mg ivermectin tablet, with 147,380 children in this age range treated [42]. Based on WHO Child Growth Standards, the median weight for a 24-month-old child is 12.2 kg for boys and 11.5 kg for girls, with children achieving a median 15.0 kg weight at 40 months for boys and at 42 months for girls [6]. Enbiale et al. report that roughly 30% of children aged two to six years old were given more costly and logistically difficult to administer topical permethrin or sulfur creams instead of oral ivermectin, which may have occurred because of confusion with onchocerciasis guidelines indicating that children under five years of age should not be treated with ivermectin [42]. In this review, of the children weighing less than 15 kg treated with oral ivermectin, 19.8% (214/1,081) were five years of age or older. While this data set is limited, it suggests that treating by age with five years old as a cutoff would still frequently provide ivermectin to children weighing less than 15 kg. Of the children weighing less than 15 kg treated with oral ivermectin, 28.6% (34/119) were 90 cm or taller. This illustrates that the 90 cm cutoff for ivermectin administration during MDAs means that children weighing less than 15 kg are routinely treated with ivermectin. Establishing the safety of ivermectin in children weighing less than 15 kg would remove the imprecise weight, height, and age restriction barriers and facilitate more streamlined ivermectin MDAs.
Scabies is recognized in France as a common clinical problem, including in small children. In 2014, the French Medicines Regulatory Agency “Agence Nationale de Sécurité du Médicament”, published a decision algorithm for treatment of scabies in children aged less than one year. This algorithm recommends treatment with two doses of oral ivermectin (200 μg/kg) one week apart in infants who fail scabies treatment with permethrin or benzyl benzoate topical creams, or have a contraindication for topical esdepallethrin spray (e.g. asthma) [43]. A survey of French dermatologists found that oral ivermectin was unlikely to be used in infants with common scabies weighing less than 10 kg (15%). However, willingness to use oral ivermectin in children weighing less than 10 kg increased: if the child had asthma (25%), after topical cream failure (40%), complications with impetigo (60%), profuse scabies (70%), or if the child was unlikely to be seen again follow-up (65%). Furthermore, survey respondents were willing to treat scabies in children less than three months with oral ivermectin [44]. Indeed, in this current review, of the 14 children that were three months or younger that were administered oral ivermectin, all of them were treated for scabies and 92.9% (13/14) were treated in France. France appears to be the first country with clear guidance to use oral ivermectin off-label for treatment of scabies in children weighing less than 15 kg.
Ivermectin treatment for soil-transmitted helminthiasis and strongyloidiasis
Ivermectin has been used for the treatment of young children weighing less than 15 kg with infections due to STHs or Strongyloides stercoralis. The combination of ivermectin and albendazole is superior to either drug alone for the treatment of Trichuris trichiura [45] and albendazole is superior to ivermectin for the treatment of hookworm. The Global Program for the Elimination of Lymphatic Filariasis (GPELF) co-administered ivermectin and albendazole to 52 million school-aged children via MDA in 2015 [46] and this combination has been added to the WHO List of Essential Medicines for Children [47]. However, the combination is still not used in children weighing less than 15 kg because of the ivermectin contraindication. Part of the GPELF rationale for albendazole and ivermectin co-administration was increased secondary effects against STHs [48]. The triple-drug therapy of ivermectin, diethylcarbamazine, and albendazole (IDA) was recently shown to be superior for the treatment of lymphatic filariasis [49] and Merck has pledged an additional 100 million ivermectin treatments annually to use in triple-drug therapy IDA MDAs outside of Africa [50]. However, with the success of the GPELF against lymphatic filariasis, MDA is now scaling back in many areas. If community-wide MDA programs with ivermectin and albendazole are to continue in the future, then STH programs will have to shift strategies from school-based administration of albendazole to community-wide MDAs with combinations of ivermectin and albendazole, and inclusion of children weighing less than 15 kilograms could be considered.
A recent clinical trial demonstrated that ivermectin for T. trichiura infection is safe in preschool-aged children, some of whom weighed less than 15 kg (n = 44) [33], but safety studies of the combination of ivermectin and albendazole in children weighing less than 15 kg are lacking. STH infection at a young age is associated with growth stunting, impairment of cognitive development, and reduced school attendance. Co-administration of ivermectin and albendazole during MDAs to small children infected with STHs could positively impact childhood development in many settings.
Expanding ivermectin use for children weighing less than 15 kg during MDAs could have marked health benefits for this group through impact on S. stercoralis burden. Repeated annual ivermectin MDA for onchocerciasis control in Colombia [51] and Ecuador [52] demonstrated marked and sustained reductions in S. stercoralis prevalence. Three rounds of annual ivermectin and albendazole MDA in Argentina [53] and two rounds of annual ivermectin MDA in Australia [54] led to marked reduction on S. stercoralis prevalence. Strongyloidiasis can be life threatening in small children and ivermectin is a safe and effective cure for infected children weighing less than 15 kg [26,29,35,36,55].
Ivermectin for myiasis
The administration of oral ivermectin for myiasis has proven to be effective as it prevents complications in surgery and reduces the difficulty of mechanical removal of the larvae [56]. Children are vulnerable to nasal [57], oral [57,58], ocular [59–61], and auricular myiasis [57,62]. Treatment of children with oral ivermectin (200–350 μg/kg) for myiasis is effective [30,59–61,63] but use in children weighing less than 15 kg has been extremely limited due to the current contraindication [38,39].
Onchocerciasis, onchocerciasis-associated epilepsy and Nodding Syndrome
While the prevalence of onchocerciasis microfilaridermia in children aged less than five years is expected to be low, these children can become infected in areas of high O. volvulus transmission and present disease manifestations, especially pruritus [64]. The presence of troublesome itching may produce general fatigue, insomnia, and distraction at school [65,66]. It is possible that itching could be exacerbated in O. volvulus infected children weighing less than 15 kg that were treated with ivermectin, however, no studies were identified that assessed ivermectin in this weight class and disease combination. Trials in O. volvulus infected children weighing less than 15 kg are recommended before large scale ivermectin MDA rollout in onchocerciasis endemic regions in this weight class. In addition, the relative risk of excess mortality associated with onchocerciasis is higher, for a given microfilarial load, for younger age groups [67]. Furthermore, there is a consistent association between onchocerciasis and the incidence of onchocerciasis-associated epilepsy and Nodding Syndrome when the infection is intense and occurs at a very young age [68–73]. Nodding Syndrome, a form of epilepsy, may be caused by an autoimmune reaction to O. volvulus infection [74] and afflicts children between three and 18 years old [75]. Onchocerciasis-endemic regions where ivermectin MDAs are routinely performed have reduced [76–79]. All of these observations suggest that inclusion of children weighing less than 15 kg in ivermectin MDA will benefit child health in onchocerciasis endemic areas.
Ivermectin use for malaria control
Ivermectin has been proposed as a novel malaria control tool due to its ability to kill Anopheles mosquitoes and ivermectin MDA can suppress Plasmodium transmission [80–82]. When using ivermectin MDA as a malaria control agent, coverage is the most critical component of efficacy [83]. The contraindication of ivermectin for children weighing less than 15 kg excludes roughly 20% of the population in malaria endemic areas, potentially inhibiting MDA efficacy. In Africa, children under five years old are frequently bitten by Anopheles as evidenced by high rates of new malaria infections in this population [82]. Untreated small children serve an important reservoir of Plasmodium and can facilitate onwards transmission to mosquitoes.
Clinical trials have evaluated the safety, and efficacy of ivermectin along with the antimalarial drugs artemether-lumefantrine [84] and dihydroartemisinin-piperaquine [85,86] in adults. However, safety data in children and infants are needed in order to expand administration of these combinations to younger age groups. Currently in the African Sahel, seasonal malaria chemoprophylaxis (SMC) with sulfadoxine-pyrimethamine plus amodiaquine (SP-AQ) is given annually to 10–15 million children less than five years old, as an effective tool to reduce clinical incidence of severe malaria. Combining ivermectin MDAs with SMC would simultaneously target the parasite and vector, likely further reducing malaria in young children [87]. Before ivermectin and SP-AQ can be co-administered, safety and efficacy studies should be performed. Finally, ivermectin has been shown to inhibit liver-stage development of Plasmodium berghei [88], but its prophylactic efficacy in humans is debatable. Field evidence of repeated ivermectin (150 μg/kg) MDAs demonstrated that ivermectin treatment reduced the likelihood of infection and number of P. falciparum clones per infection beyond predicted community-wide transmission benefit, suggestive of ivermectin liver-stage prophylactic effect [82]. However, a recent controlled human malaria infection trial with single dose ivermectin (400 μg/kg) in adults failed to prevent P. falciparum infection [89]. If ivermectin can indeed inhibit Plasmodium development in treated humans, then this makes the inclusion of children weighing less than 15 kg in ivermectin MDAs for malaria control even more critical as they suffer a higher burden of morbidity and mortality from malaria.
Pediatric ivermectin formulations and dosage
Oral Ivermectin is typically delivered as 3 mg or 6 mg tablets, and while the 3 mg tablet size is relatively small, 5.5 mm diameter for Stromectol (Merck & Co., Whitehouse Station, NJ, USA), it can be difficult for young children to swallow oral tablets. Since there is no pediatric ivermectin formulation available in France, the French Medicines Regulatory Agency recommends to crush a whole 3 mg tablet for children weighing 10–15 kg or half of a three mg tablet for children weighing less than 10 kg, mix thoroughly in 10 mL of water, and administer [43]. In the clinical setting, there was no evidence of children weighing less than 15 kg choking when swallowing crushed ivermectin (3 mg) mixed in liquid (n = 169) [9]. A recent study milled three mg tablets into smaller 0.5 mg minitablets to provide to children aged two to 12 years old, many of whom weighed less than 15 kg (n = 43) [33]. In Latin America, a liquid oral formulation has been manufactured and used in several trials for treatment of young children with head lice in Colombia [90–92], and specifically in infants weighing less than 15kg for strongyloidiasis in Venezuela [26] and myiasis in Peru [30]. A liquid oral, minitablet, oro-dispersible minitablet, or multiparticulate formulation for small children would be advantageous for use in clinical treatment and MDA settings. The considerations outlined in this manuscript underline the need for the development of an adapted pediatric formulation of ivermectin.
The ideal dose of ivermectin for different indications in small children is not known. Available data suggest that adult based dosing is not appropriate for children and that dose escalation efficacy trials in children weighing less than 15 kg are needed. Recent pharmacokinetic analyses indicate that ivermectin treated children aged less than 12 years reach half the peak concentration and total exposure of adults [93] and a dose increase for young children was suggested [94]. A multi-center analysis found that ivermectin was significantly more effective at treating scabies in children weighing less than 15 kg when the dose was more than 200 μg/kg compared to less than 200 μg/kg [9]. This suggests that the standard 200 μg/kg dose of ivermectin may not reach effective levels in small children for all NTDs. This review identified 939 children treated with 201–300 μg/kg, 17 children treated with 301–400 μg/kg, and four children treated with >400 μg/kg. This limited evidence suggests that ivermectin doses greater >200 μg/kg are potentially safe and well tolerated in children weighing less than 15 kg. One limitation of this review is that most of the evidence 92.6% (1,007/1,088) is derived from two studies, one MDA study in the Solomon Islands [34] and a case series from France [9]. A multi-country, randomized, double-blind, placebo-controlled, dose escalation (200, 400, 800 μg/kg) study, dubbed the Ivermectin Safety in Small Children (ISSC) trial (NCT04332068) is currently underway, which is designed to assess the safety, pharmacokinetics, and efficacy of oral ivermectin in scabies-infected children weighing less than 15 kg [95]. An additional concern to ivermectin dosing in children weighing less than 15 kg are potential drug-drug interactions with other drugs frequently used in MDA populations (e.g. antiretrovirals) that may alter safety or pharmacokinetics.
A call for clarity in data reporting
Age is not a limiting criterion listed on most ivermectin package inserts but weight <15 kg and/or height <90 cm when used in MDAs are directly contraindicated [38]. However, there is a common misconception reported in the literature that children aged less than five years should not be administered ivermectin. Not treating children under five years of age would further restrict ivermectin use, as these children frequently weigh 15 kg or more or are 90 cm or taller [6]. Reports of ivermectin use in case studies, series, or trials frequently group subjects by age rather than by weight, providing no conclusive evidence for ivermectin safety in children weighing less than 15 kg. Of the 97 possible reports identified in this review, only four stated the number of children weighing less than 15 kg that were administered oral ivermectin [9,10,34,95]. Here we call for greater clarity when reporting ivermectin use in children to include the weight of any child below 15 kg and reporting of any associated AEs to the individual-patient level. An IPD call for ivermectin safety in children weighing less than 15 kg has been organized by WWARN and data from future publications or clarifications to publications cited in S1 Table can be contributed by contacting the study authors.
Conclusion
Millions of children weighing less than 15 kg are currently denied access to ivermectin treatment due to the current label indication. In order to remove this barrier and improve treatment equity, further evidence in children weighing less than 15 kg must be gathered and clearly compiled for review. The theoretical concern regarding the potential for neurotoxicity of ivermectin in infancy has not been confirmed. This IPD meta-analysis provide new evidence to be considered by medicines regulatory authorities and policy makers regarding the safety of ivermectin in children weighing less than 15 kg. The data provides limited but encouraging evidence that ivermectin is safe and well-tolerated in small children weighing less than 15 kg. The ivermectin tolerability and safety profile in children weighing less than 15 kg is similar to that in heavier individuals. Further investigation is warranted through well-designed clinical trials in children weighing less than 15 kg with the objective of optimizing dosing and characterizing the safety profile the prescribing restriction in young children can be lifted.
Supporting information
Preferred Reporting Items for Systematic Reviews and Meta-Analyses–Individual Patient Data (PRISMA-IPD).
(DOCX)
Response results and inclusion decisions of selected studies contacted by study team.
(DOCX)
Number and classification of adverse events reported for each study included in the systematic analysis.
(DOCX)
Acknowledgments
We would like to thank Saowalak Booncharoenraksa, Librarian at the Armed Forces Research Institute of Medical Sciences, for her support searching the literature and obtaining articles. We would like to thank Caitlin Richmond and Rebekah Burrow at WWARN for their guidance on the IPD process and development.
Disclaimer
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25.
Data Availability
Requests for access will be reviewed by a Data Access Committee to ensure that use of data protects the interests of the participants and researchers according to the terms of ethics approval and principles of equitable data sharing. Requests can be submitted by email to malariaDAC@iddo.org via the Data Access Form available at WWARN.org/accessing-data. The WWARN is registered with the Registry of Research Data Repositories (re3data.org).
Funding Statement
KCK was supported in part by a Fellowship from the US National Research Council. WM is supported in part by a Fellowship from the National Council for Scientific and Technological Development (CNPq) and by Fapem (Pró-Estado). BP and SS are supported by the Drugs for Neglected Diseases initiative (DNDi). A full list of DNDi’s donors can be found at http://www.dndi.org/donors/donors/. The Infectious Disease Data Observatory and WorldWide Antimalarial Research Network are supported by the Bill & Melinda Gates Foundation and the Exxon Mobil Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nelson G. Human onchocerciasis: notes on the history, the parasite and the life cycle. Annals Tropical Medicine Parasitology. 1991;85(1):83–95. [DOI] [PubMed] [Google Scholar]
- 2.Duke B. The population dynamics of Onchocerca volvulus in the human host. Tropical Medicine Parasitology. 1993;44(2):61–8. [PubMed] [Google Scholar]
- 3.Filipe J, Boussinesq M, Renz A, Collins R, Vivas-Martinez S, Grillet M, et al. Human infection patterns and heterogeneous exposure in river blindness. Proceedings National Academy Sciences USA. 2005;102(42):15265–70. 10.1073/pnas.0502659102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mectizan Donation Program. 2019 Annual Highlights Celebrating Milestones & Looking to the Future: Merck & Co., Inc; 2020 [Available from: https://mectizan.org/wp-content/uploads/2020/06/MDP_AH19_051920.pdf.
- 5.Alexander N, Cousens S, Yahaya H, Abiose A, Jones B. Ivermectin dose assessment without weighing scales. Bulletin of the World Health Organization. 1993;71(3–4):361–6. [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization. 2006. [Google Scholar]
- 7.Föger K, Gora-Stahlberg G, Sejvar J, Ovuga E, Jilek-Aall L, Schmutzhard E, et al. Nakalanga Syndrome: Clinical Characteristics, Potential Causes, and Its Relationship with Recently Described Nodding Syndrome. PLoS Neglected Tropical Diseases. 2017;11(2):e0005201. 10.1371/journal.pntd.0005201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mauleón-Fernández C, del Mar Sáez-de-Ocariz M, Rodríguez-Jurado R, Durán-McKinster C, Orozco-Covarrubias L, Ruiz-Maldonado R. Nodular scabies mimicking urticaria pigmentosa in an infant. Clinical and Experimental Dermatology. 2005;30:595–6. 10.1111/j.1365-2230.2005.01832.x [DOI] [PubMed] [Google Scholar]
- 9.Levy M, Martin L, Bursztejn A-C, Chiaverini C, Miquel J, Mahé E, et al. Ivermectin safety in infants and children under 15 kg treated for scabies: a multicentric observational study. British Journal of Dermatology. 2020;182(4):1003–6. 10.1111/bjd.18369 [DOI] [PubMed] [Google Scholar]
- 10.Bécourt C, Marguet C, Balguerie X, Joly P. Treatment of scabies with oral ivermectin in 15 infants: a retrospective study on tolerance and efficacy. British Journal of Dermatology. 2013;169(4):931–3. 10.1111/bjd.12454 [DOI] [PubMed] [Google Scholar]
- 11.Edwards G. Ivermectin: does P-glycoprotein play a role in neurotoxicity? Filaria Journal. 2003;2(Suppl 1):S8. 10.1186/1475-2883-2-S1-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mealey K, Bentjen S, Gay J, Cantor G. Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics. 2001;11(8):727–33. 10.1097/00008571-200111000-00012 [DOI] [PubMed] [Google Scholar]
- 13.Geyer J, Gavrilova O, Petzinger E. Brain penetration of ivermectin and selamectin in mdr1a,b P-glycoprotein- and bcrp- deficient knockout mice. Journal of Veterinary Pharmacology and Therapeutics. 2009;32(1):87–96. 10.1111/j.1365-2885.2008.01007.x [DOI] [PubMed] [Google Scholar]
- 14.Baudou E, Lespine A, Durrieu G, André F, Gandia P, Durand C, et al. Serious Ivermectin Toxicity and Human ABCB1 Nonsense Mutations. New England Journal of Medicine. 2020;383(8):787–9. 10.1056/NEJMc1917344 [DOI] [PubMed] [Google Scholar]
- 15.Lankas G, Gordon L. Toxicology. In: Campbell W, editor. Ivermectin and Abamectin: Springer; 1989. p. 89–112. 10.1016/0300-483x(89)90081-4 [DOI] [Google Scholar]
- 16.Virgintino D, Errede M, Robertson D, Capobianco C, Girolamo F, Vimercati A, et al. Immunolocalization of tight junction proteins in the adult and developing human brain. Histochemistry and cell biology. 2004;122(1):51–9. 10.1007/s00418-004-0665-1 [DOI] [PubMed] [Google Scholar]
- 17.Lam J, Baello S, Iqbal M, Kelly L, Shannon P, Chitayat D, et al. The ontogeny of P-glycoprotein in the developing human blood-brain barrier: implication for opioid toxicity in neonates. Pediatric Research. 2015;78(4):417–21. 10.1038/pr.2015.119 [DOI] [PubMed] [Google Scholar]
- 18.Ek C, Wong A, Liddelow S, Johansson P, Dziegielewska K, Saunders N. Efflux mechanisms at the developing brain barriers: ABC-transporters in the fetal and postnatal rat. Toxicology Letters. 2010;197(1):51–9. 10.1016/j.toxlet.2010.04.025 [DOI] [PubMed] [Google Scholar]
- 19.Wilkins A, Steer A, Cranswick N, Gwee A. Question 1: Is it safe to use ivermectin in children less than five years of age and weighing less than 15 kg? Archives of disease in childhood. 2018;103(5):514–9. 10.1136/archdischild-2017-314505 [DOI] [PubMed] [Google Scholar]
- 20.Stewart L, Clarke M, Rovers M, Riley R, Simmonds M, Stewart G, et al. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA. 2015;313(16):1657–65. 10.1001/jama.2015.3656 [DOI] [PubMed] [Google Scholar]
- 21.Food US and Administration Drug. Part 312 Investigational New Drug Application In: Services DoHaH, editor.: Code of Federal Regulations; 2019. [Google Scholar]
- 22.WorldWide Antimalarial Resistance Network. Clinical Module: Data Management and Statistical Analysis Plan, Version 1.2 2012 [Available from: www.wwarn.org/sites/default/files/ClinicalDMSAP.pdf.
- 23.Finon A, Desoubeaux G, Nadal M, Georgescou G, Baran R, Maruani A. Scabies of the nail unit in an infant. Annales de Dermatologie et de Venereologie. 2017;144(5):356–61. 10.1016/j.annder.2016.09.043 [DOI] [PubMed] [Google Scholar]
- 24.Del Giudice P, Hakimi S, Vandenbos F, Magana C, Hubiche T. Autochthonous Cutaneous Larva Migrans in France and Europe. Acta Dermato-Venereologica. 2019;99(9):805–8. 10.2340/00015555-3217 [DOI] [PubMed] [Google Scholar]
- 25.Robert M, Faisant A, Cognet O, Rabodonirina M, Peyron F, Piquemal M, et al. Autochthonous and persistent cutaneous larva migrans in an infant successfully treated by topic albendazole ointment. Journal of the European Academy of Dermatology Venereology. 2019;33(4):e163–e4. 10.1111/jdv.15356 [DOI] [PubMed] [Google Scholar]
- 26.Chaccour C, Del Pozo J. Case 23–2012: A man with abdominal pain and weight loss. New England Journal of Medicine. 2012;367(17):1670–1. 10.1056/NEJMc1210168 [DOI] [PubMed] [Google Scholar]
- 27.Ouedraogo M, Ventejou S, Leducq S, Desoubeaux G, Maruani A. Crusts on the Eyelashes. The Journal of Pediatrics. 2019;209:254–.e1. 10.1016/j.jpeds.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 28.Piquero-Casals J, Piquero-Casals V, La Rotta E, Menta Simonsen Nico M. Crusted scabies in cushingoid child treated with oral ivermectin. Dermatologia Argentina. 2002;8(3):136–9. [Google Scholar]
- 29.Soriano-Arandes A, Sulleiro E, Zarzuela F, Ruiz E, Clavería I, Espasa M. Discordances Between Serology and Culture for Strongyloides in an Ethiopian Adopted Child With Multiple Parasitic Infections: A Case Report. Medicine (Baltimore). 2016;95(10):e3040. 10.1097/MD.0000000000003040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Failoc-Rojas V, Molina-Ayasta C, Salazar-Zuloeta J, Samamé A, Silva-Díaz H. Case Report: Myiasis due to Cochliomyia hominivorax and Dermatobia hominis: Clinical and Pathological Differences between Two Species in Northern Peru. The American journal of tropical medicine and hygiene. 2018;98(1):150–3. 10.4269/ajtmh.16-0437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sáez-de-Ocariz M, McKinster C, Orozco-Covarrubias L, Tamayo-Sánchez L, Ruiz-Maldonado R. Treatment of 18 children with scabies or cutaneous larva migrans using ivermectin. Clinical and Experimental Dermatology. 2002;27(4):264–7. 10.1046/j.1365-2230.2002.01050.x [DOI] [PubMed] [Google Scholar]
- 32.Dard C, Piloquet J, Qvarnstrom Y, Fox L, M’Kada H, Hebert J, et al. First Evidence of Angiostrongyliasis Caused by Angiostrongylus cantonensis in Guadeloupe, Lesser Antilles. The American journal of tropical medicine and hygiene. 2017;96(3):692–7. 10.4269/ajtmh.16-0792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wimmersberger D, Coulibaly J, Schulz J, Puchkow M, Huwyler J, N’Gbesso Y, et al. Efficacy and Safety of Ivermectin Against Trichuris trichiura in Preschool-aged and School-aged Children: A Randomized Controlled Dose-finding Trial. Clinical Infectious Diseases. 2018;67(8):1247–55. 10.1093/cid/ciy246 [DOI] [PubMed] [Google Scholar]
- 34.Romani L, Marks M, Sokana O, Nasi T, Kamoriki B, Wand H, et al. Feasibility and safety of mass drug coadministration with azithromycin and ivermectin for the control of neglected tropical diseases: a single-arm intervention trial. Lancet Global Health. 2018;6(10):e1132–e8. 10.1016/S2214-109X(18)30397-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forrer A, Khieu V, Schindler C, Schär F, Marti H, Char M, et al. Ivermectin Treatment and Sanitation Effectively Reduce Strongyloides stercoralis Infection Risk in Rural Communities in Cambodia. PLoS Neglected Tropical Diseases. 2016;10(8):e0004909. 10.1371/journal.pntd.0004909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forrer A, Khieu V, Schär F, Hattendorf J, Marti H, Neumayr A, et al. Strongyloides stercoralis is associated with significant morbidity in rural Cambodia, including stunting in children. PLoS Neglected Tropical Diseases. 2017;11(10):e0005685. 10.1371/journal.pntd.0005685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chinazzo M, Desoubeaux G, Leducq S, Bessis D, Droitcourt C, Mahe E, et al. Prevalence of Nail Scabies: A French Prospective Multicenter Study. The Journal of Pediatrics. 2018;197:154–7. 10.1016/j.jpeds.2018.01.038 [DOI] [PubMed] [Google Scholar]
- 38.Merck Sharp & Dohme (France). Mectizan Package Insert. 2014.
- 39.Merck Sharp & Dohme (Australia). Stromectol Package Insert. 2014.
- 40.Romani L, Whitfeld M, Koroivueta J, Kama M, Wand H, Tikoduadua L, et al. Mass Drug Administration for Scabies Control in a Population with Endemic Disease. The New England journal of medicine. 2015;373(24):2305–13. 10.1056/NEJMoa1500987 [DOI] [PubMed] [Google Scholar]
- 41.Marks M, Taotao-Wini B, Satorara L, Engelman D, Nasi T, Mabey D, et al. Long Term Control of Scabies Fifteen Years after an Intensive Treatment Programme. PLoS Neglected Tropical Diseases. 2015;9(12):e0004246. 10.1371/journal.pntd.0004246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enbiale W, Baynie T, Ayalew A, Gebrehiwot T, Getanew T, Ayal A, et al. "Stopping the itch": mass drug administration for scabies outbreak control covered for over nine million people in Ethiopia. The Journal of Infection in Developing Countries. 2020;14(6.1):28s–35s. 10.3855/jidc.11701 [DOI] [PubMed] [Google Scholar]
- 43.Berthe-Aucejo A, Prot-Labarthe S, Pull L, Lorrot M, Touratier S, Trout H, et al. Treatment of scabies and Ascabiol supply disruption: what about the pediatric population? Archives de Pediatrie. 2014;21(6):670–5. 10.1016/j.arcped.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 44.Lê M, Richard M, Baumstarck K, Hesse S, Gaudy-Marqueste C, Grob J, et al. Evaluation of practices in the management of scabies in children. Annales de Dermatologie et de Venereologie 2017;144(5):341–8. 10.1016/j.annder.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 45.Palmeirim M, Hurlimann E, Knopp S, Speich B, Belizario V, Joseph S, et al. Efficacy and safety of co-administered ivermectin plus albendazole for treating soil-transmitted helminths: A systematic review, meta-analysis and individual patient data analysis. PLoS Neglected Tropical Diseases. 2018;12(4):e0006458. 10.1371/journal.pntd.0006458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization. Global programme to eliminate lymphatic filariasis: progress report, 2015. Weekly Epidemiology Record. 2016;39(91):441–60.
- 47.World Health Organization. Model List of Essential Medicines for Children, 7th List. Geneva: World Health Organization; 2019. [Google Scholar]
- 48.Ottesen E, Ismail M, Horton J. The role of albendazole in programmes to eliminate lymphatic filariasis. Parasitology Today. 1999;15(9):382–6. 10.1016/s0169-4758(99)01486-6 [DOI] [PubMed] [Google Scholar]
- 49.Weil G, Bogus J, Christian M, Dubray C, Djuardi Y, Fischer PU, et al. The safety of double- and triple-drug community mass drug administration for lymphatic filariasis: A multicenter, open-label, cluster-randomized study. PLoS medicine. 2019;16(6):e1002839. 10.1371/journal.pmed.1002839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merck. Program expanded to reach additional 100 million people annually for lymphatic filariasis in support of new, evidence-based WHO guidelines Merck Newsroom Home2017 [Available from: https://www.mrknewsroom.com/news-release/corporate-news/merck-commemorates-30-years-mectizan-donation-program-progress.
- 51.Knudson A, Ariza Y, López M, Fajardo O, Reyes P, Moncada L, et al. The effect of ivermectin on geohelminth frequency (i.e. as used in the onchocerciasis control program in Colombia). Revista de Salud Publica (Bogota, Colombia). 2012;14(4):681–94. [PubMed] [Google Scholar]
- 52.Anselmi M, Buonfrate D, Espinoza A, Prandi R, Marquez M, Gobbo M, et al. Mass administration of ivermectin for the elimination of Onchocerciasis significantly reduced and maintained low the prevalence of Strongyloides stercoralis in Esmeraldas, Ecuador. PLoS Neglected Tropical Diseases. 2015;9(11):e0004150. 10.1371/journal.pntd.0004150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Echazu A, Juarez M, Vargas P, Cajal S, Cimino R, Heredia V, et al. Albendazole and ivermectin for the control of soil-transmitted helminths in an area with high prevalence of Strongyloides stercoralis and hookworm in northwestern Argentina: A community-based pragmatic study. PLoS Neglected Tropical Diseases. 2017;11(10):e0006003. 10.1371/journal.pntd.0006003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kearns T, Currie B, Cheng A, McCarthy J, Carapetis J, Holt D, et al. Strongyloides seroprevalence before and after an ivermectin mass drug administration in a remote Australian Aboriginal community. PLoS Neglected Tropical Diseases. 2017;11(5):e0005607. 10.1371/journal.pntd.0005607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ordóñez L, Angulo E. Efficacy of ivermectin in the treatment of children parasitized by Strongyloides stercoralis. Biomedica. 2004;24(1):33–41. [PubMed] [Google Scholar]
- 56.Osorio J, Moncada L, Molano A, Valderrama S, Gualtero S, Franco-Paredes C. Role of ivermectin in the treatment of severe orbital myiasis due to Cochliomyia hominivorax. Clinical Infectious Diseases. 2006;43(6):e57–9. 10.1086/507038 [DOI] [PubMed] [Google Scholar]
- 57.Singh I, Gathwala G, Yadav S, Wig U, Jakhar K. Myiasis in children: the Indian perspective. International Journal of Pediatric Otorhinolaryngology. 1993;25(1–3):127–31. 10.1016/0165-5876(93)90045-5 [DOI] [PubMed] [Google Scholar]
- 58.Reddy M, Das N, Vivekananda M. Oral myiasis in children. Contemporary Clinical Dentistry. 2012;3(Suppl 1):S19–22. 10.4103/0976-237X.95097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taba K, Vanchiere J, Kavanaugh A, Lusk J, Smith M. Successful treatment of ophthalmomyiasis interna posterior with ivermectin. Retinal Cases & Brief Reports. 2012;6(1):91–4. 10.1097/ICB.0b013e318208859c [DOI] [PubMed] [Google Scholar]
- 60.Kan B, Otranto D, Fossen K, Åsbakk K. Dermal swellings and ocular injury after exposure to reindeer. N Engl J Med. 2012;367(25):2456–7. 10.1056/NEJMc1201434 [DOI] [PubMed] [Google Scholar]
- 61.Landehag J, Skogen A, Åsbakk K, Kan B. Human myiasis caused by the reindeer warble fly, Hypoderma tarandi, case series from Norway, 2011 to 2016. Euro Surveillance. 2017;22(29). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuca K, Caksen H, Sakin Y, Yuca S, Kiriş M, Yilmaz H, et al. Aural myiasis in children and literature review. Tohoku Journal of Experimental Medicine. 2005;206(2):125–30. 10.1620/tjem.206.125 [DOI] [PubMed] [Google Scholar]
- 63.Kan B, Asen C, Åsbakk K, Jaenson T. Suspected lice eggs in the hair of a boy revealed dangerous parasite. Lakartidningen. 2010;107(26–28):1694–7. [PubMed] [Google Scholar]
- 64.Remme J. The global burden of onchocerciasis in 1990. WHO: Geneva. 2004. [Google Scholar]
- 65.World Health Organization. Importance of oncho skin disease. Report of a multicountry study. Geneva: World Health Organization; 1995. [Google Scholar]
- 66.Brieger W, Oshiname F, Ososanya O. Stigma associated with onchocercal skin disease among those affected near the Ofiki and Oyan Rivers in western Nigeria. Social Science and Medicine. 1998;47(7):841–52. 10.1016/s0277-9536(98)00007-0 [DOI] [PubMed] [Google Scholar]
- 67.Walker M, Little MP, Wagner KS, Soumbey-Alley EW, Boatin BA, Basanez MG. Density-dependent mortality of the human host in onchocerciasis: relationships between microfilarial load and excess mortality. PLoS Neglected Tropical Diseases. 2012;6(3):e1578. 10.1371/journal.pntd.0001578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winkler A, Friedrich K, Konig R, Meindl M, Helbok R, Unterberger I, et al. The head nodding syndrome—clinical classification and possible causes. Epilepsia. 2008;49(12):2008–15. 10.1111/j.1528-1167.2008.01671.x [DOI] [PubMed] [Google Scholar]
- 69.Kaiser C, Pion S, Boussinesq M. Head nodding syndrome and river blindness: a parasitologic perspective. Epilepsia. 2009;50(10):2325–6. 10.1111/j.1528-1167.2009.02280.x [DOI] [PubMed] [Google Scholar]
- 70.Kaiser C, Pion SD, Boussinesq M. Case-control studies on the relationship between onchocerciasis and epilepsy: systematic review and meta-analysis. PLoS Neglected Tropical Diseases. 2013;7(3):e2147. 10.1371/journal.pntd.0002147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pion SD, Kaiser C, Boutros-Toni F, Cournil A, Taylor MM, Meredith SE, et al. Epilepsy in onchocerciasis endemic areas: systematic review and meta-analysis of population-based surveys. PLoS Neglected Tropical Diseases. 2009;3(6):e461. 10.1371/journal.pntd.0000461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chesnais C, Nana-Djeunga H, Njamnshi A, Lenou-Nanga C, Boullé C, Bissek A, et al. The temporal relationship between onchocerciasis and epilepsy: a population-based cohort study. Lancet Infectious Diseases. 2018;18(11):1278–86. 10.1016/S1473-3099(18)30425-0 [DOI] [PubMed] [Google Scholar]
- 73.Chesnais C, Bizet C, Campillo J, Njamnshi W, Bopda J, Nwane P, et al. A Second Population-Based Cohort Study in Cameroon Confirms the Temporal Relationship Between Onchocerciasis and Epilepsy. Open Forum Infect Dis. 2020;7(6):ofaa206. 10.1093/ofid/ofaa206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson T, Tyagi R, Lee P, Lee M, Johnson K, Kowalak J, et al. Nodding syndrome may be an autoimmune reaction to the parasitic worm Onchocerca volvulus. Science Translational Medicine. 2017;9(377):e6953. 10.1126/scitranslmed.aaf6953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colebunders R, Njamnshi A, van Oijen M, Mukendi D, Kashama J, Mandro M, et al. Onchocerciasis-associated epilepsy: From recent epidemiological and clinical findings to policy implications. Epilepsia Open. 2017;2(2):145–52. 10.1002/epi4.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levick B, Laudisoit A, Tepage F, Ensoy-Musoro C, Mandro M, Bonareri Osoro C, et al. High prevalence of epilepsy in onchocerciasis endemic regions in the Democratic Republic of the Congo. PLoS Neglected Tropical Diseases. 2017;11(7):e0005732. 10.1371/journal.pntd.0005732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colebunders R, Mandro M, Mokili J, Mucinya G, Mambandu G, Pfarr K, et al. Risk factors for epilepsy in Bas-Uele Province, Democratic Republic of the Congo: a case-control study. International Journal of Infectious Diseases. 2016;49:1–8. 10.1016/j.ijid.2016.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gumisiriza N, Kaiser C, Asaba G, Onen H, Mubiru F, Kisembo D, et al. Changes in epilepsy burden after onchocerciasis elimination in a hyperendemic focus of western Uganda: a comparison of two population-based, cross-sectional studies. Lancet Infect Diseases. 2020;20(11):1315–23. [DOI] [PubMed] [Google Scholar]
- 79.Gumisiriza N, Mubiru F, Siewe Fodjo J, Mbonye Kayitale M, Hotterbeekx A, Idro R, et al. Prevalence and incidence of nodding syndrome and other forms of epilepsy in onchocerciasis-endemic areas in northern Uganda after the implementation of onchocerciasis control measures. Infectious Diseases of Poverty. 2020;9(1):12. 10.1186/s40249-020-0628-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kobylinski K, Sylla M, Chapman P, Sarr M, Foy B. Short Report: Ivermectin Mass Drug Administration to Humans Disrupts Malaria Parasite Transmission in Senegalese Villages. The American journal of tropical medicine and hygiene. 2011;85(1):3–5. 10.4269/ajtmh.2011.11-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alout H, Krajacich B, Meyers J, Grubaugh N, Brackney D, Kobylinski K, et al. Evaluation of ivermectin mass drug administration for malaria transmission control across different West African environments. Malaria Journal. 2014;13:e417. 10.1186/1475-2875-13-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Foy B, Alout H, Seaman J, Rao S, Magalhaes T, Wade M, et al. Efficacy and risk of harms of repeat ivermectin mass drug administrations for control of malaria (RIMDAMAL): a cluster-randomised trial. Lancet. 2019;393(10180):1517–26. 10.1016/S0140-6736(18)32321-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Slater H, Walker P, Bousema T, Okell L, Ghani A. The potential impact of adding ivermectin to a mass treatment intervention to reduce malaria transmission: a modelling study. Journal of Infectious Diseases. 2014;210(12):1972–80. 10.1093/infdis/jiu351 [DOI] [PubMed] [Google Scholar]
- 84.Ouédraogo A, Bastiaens G, Tiono A, Guelbéogo W, Kobylinski K, Ouédraogo A, et al. Efficacy and safety of the mosquitocidal drug ivermectin to prevent malaria transmission after treatment: A double-blind, randomized, clinical trial. Clinical Infectious Diseases. 2015;60(3):357–65. 10.1093/cid/ciu797 [DOI] [PubMed] [Google Scholar]
- 85.Smit M, Ochomo E, Aljayyoussi G, Kwambai T, Abong’o B, Chen T, et al. Safety and mosquitocidal efficacy of high-dose ivermectin when co-administered with dihydroartemisinin-piperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): a randomised, double-blind, placebo-controlled trial. Lancet Infectious Diseases. 2018;18(6):615–26. 10.1016/S1473-3099(18)30163-4 [DOI] [PubMed] [Google Scholar]
- 86.Kobylinski K, Jittamala P, Hanboonkunupakarn B, Pukrittayakamee S, Pantuwattana K, Phasomkulsolsil S, et al. Safety, pharmacokinetics, and mosquito-lethal effects of ivermectin in combination with dihydroartemisinin-piperaquine and primaquine in healthy adult Thai subjects Clinical Pharmacology and Therapeutics. 2020;107(5):1221–30. 10.1002/cpt.1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Slater H, Foy B, Kobylinski K, Chaccour C, Watson O, Hellewell J, et al. Ivermectin as a novel complementary malaria control tool to reduce incidence and prevalence: a modelling study. Lancet Infect Dis. 2020;20(4):498–508. 10.1016/S1473-3099(19)30633-4 [DOI] [PubMed] [Google Scholar]
- 88.Mendes AM, Albuquerque IS, Machado M, Pissarra J, Meireles P, Prudencio M. Inhibition of Plasmodium Liver Infection by Ivermectin. Antimicrobial Agents and Chemotherapy. 2017;61(2):e02005–16. 10.1128/AAC.02005-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Metzger W, Theurer A, Pfleiderer A, Molnar Z, Maihofer-Braatting D, Bissinger A, et al. Ivermectin for causal malaria prophylaxis: a randomised controlled human infection trial. Tropical Medicine and International Health. 2020;25(3):380–6. 10.1111/tmi.13357 [DOI] [PubMed] [Google Scholar]
- 90.Jairo V, Ahumada N, González F. Pediculosis capitis: Tratamiento de 100 niños con Ivermectina. Act Terap Dermatol. 1997;20:99–103. [Google Scholar]
- 91.Jairo V. Ivermectina en pediculosis capitis. Act Terap Dermatol. 1998;21:448–51. [Google Scholar]
- 92.Jairo V. Uso de Ivermectina en ninos. Dermatologia Pediátrica Latinoamericana 2003;1(1):61–5. [Google Scholar]
- 93.Schulz J, Coulibaly J, Schindler C, Wimmersberger D, Keiser J. Pharmacokinetics of ascending doses of ivermectin in Trichuris trichiura-infected children aged 2–12 years. The Journal of Antimicrobial Chemotherapy. 2019;74(6):1642–7. 10.1093/jac/dkz083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brussee J, Schulz J, Coulibaly J, Keiser J, Pfister M. Ivermectin Dosing Strategy to Achieve Equivalent Exposure Coverage in Children and Adults. Clinical Pharmacology and Therapeutics. 2019;106(3):661–7. 10.1002/cpt.1456 [DOI] [PubMed] [Google Scholar]
- 95.Kobylinski K, von Seidlein L. Ivermectin Safety in Small Children (NCT04332068). University of Oxford; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preferred Reporting Items for Systematic Reviews and Meta-Analyses–Individual Patient Data (PRISMA-IPD).
(DOCX)
Response results and inclusion decisions of selected studies contacted by study team.
(DOCX)
Number and classification of adverse events reported for each study included in the systematic analysis.
(DOCX)
Data Availability Statement
Requests for access will be reviewed by a Data Access Committee to ensure that use of data protects the interests of the participants and researchers according to the terms of ethics approval and principles of equitable data sharing. Requests can be submitted by email to malariaDAC@iddo.org via the Data Access Form available at WWARN.org/accessing-data. The WWARN is registered with the Registry of Research Data Repositories (re3data.org).