Abstract
Introduction:
20.8% of the United States population and 67% of the European population speak two or more languages. Intraoperative different languages, mapping, and localization are crucial. The aim of this investigation is to address three questions between BL and ML patients: 1) Are there differences in complications (i.e. seizures) and DECS techniques during intra-operative brain mapping? 2) Is EOR different? and 3) Are there differences in the recovery pattern post-surgery?
Methods:
Data from 56 patients that underwent left-sided awake craniotomy for tumors infiltrating possible dominant hemisphere language areas from September 2016 to June 2019 were identified and analyzed in this study; 14 BL and 42 ML control patients. Patient demographics, education level, and the age of language acquisition were documented and evaluated. fMRI was performed on all participants.
Results:
0 (0%) BL and 3 (7%) ML experienced intraoperative seizures (P = 0.73). BL patients received a higher direct DECS current in comparison to the ML patients (average = 4.7, 3.8, respectively, P=0.03). The extent of resection was higher in ML patients in comparison to the BL patients (80.9 vs 64.8, respectively, P =0.04). The post-operative KPS scores were higher in BL patients in comparison to ML patients (84.3, 77.4, respectively, P = 0.03). BL showed lower drop in post-operative KPS in comparison to ML patients (−4.3, −8.7, respectively, P = 0.03).
Conclusion:
We show that BL patients have a lower incidence of intra-operative seizures, lower EOR, higher post-operative KPS and tolerate higher DECS current, in comparison to ML patients.
Keywords: Bilingual patients, Intraoperative speech mapping, Language cortex, Electrocorticography, Extent of resection, Direct cortical stimulation
Introduction
Approximately 20.8% of the population in the United States and 67% of the European population speak two or more languages.[1,2] This number continues to rise with the increase in global connectivity.[2,1] Related to second language proficiency, an important feature referred to as the age of acquisition (AoA) has been defined as the age at which monolinguals acquire their second language.[3–6] These linguistic features of bilingualism have been shown to impact structural organization, cortical representation of language, and neuroplasticity.[3,7–15] For example, several studies suggest that language proficiency is an important determinant of spatial language localization, where proficiency in the second language is hypothesized to be inferior to the first language and leads to specific localization patterns.[8,5,9,16] In contrast, recent fMRI studies demonstrate that AoA may also independently influence second-language cortical representation. [17,10,18–20] In early bilinguals (acquisition before the age of 6–9 years), the first language (L1) and the second language (L2) showed cortical regions overlap, while late bilinguals (acquisition after the age of 6–9 years) L1 and L2 have spatially separate cortical regions.[17,10,18–20,16,21,14,22–26] Late bilinguals are hypothesized to have decreased neuroplasticity and require enhanced recruitment of neural circuits, thereby producing more diffuse cortical language representation.[17,10,18–20,16,21,14,22–26] The mechanistic underpinnings of bilingual language representation and contributory linguistic features remains poorly understood and controversial. In recent years, there has been increasing interest in understanding the functional and behavioral sciences in bilingual individuals in order to uncover fundamental concepts in language processing and cognition.[22,27,28,24,29]
In both bilingual and monolingual individuals diagnosed with brain lesions (epileptogenic foci or tumors) located in the dominant hemisphere, careful direct cortical brain mapping must be conducted to identify the eloquent areas in order to maximize resection with preservation of function.[24,30,31] Prior studies demonstrated that speech and language areas in monolingual individuals are localized to certain cortical regions during direct cortical brain mapping.[32,33,24,34–37] However, in bilingual individuals, additional cortical regions may exist and must be identified (Figure 1).[30,23,24] Intra-operative speech mapping in conjunction with functional radiographic studies have observed that language co-localization is more common with early bilingualism.[24] The degree of spatial overlap between functional language areas for each language remains debated. This study presents an analysis of brain lesion resection for bilingual and monolingual speakers in order to discuss the clinical and management implications of brain surgery for bilingual patients.
Figure 1:
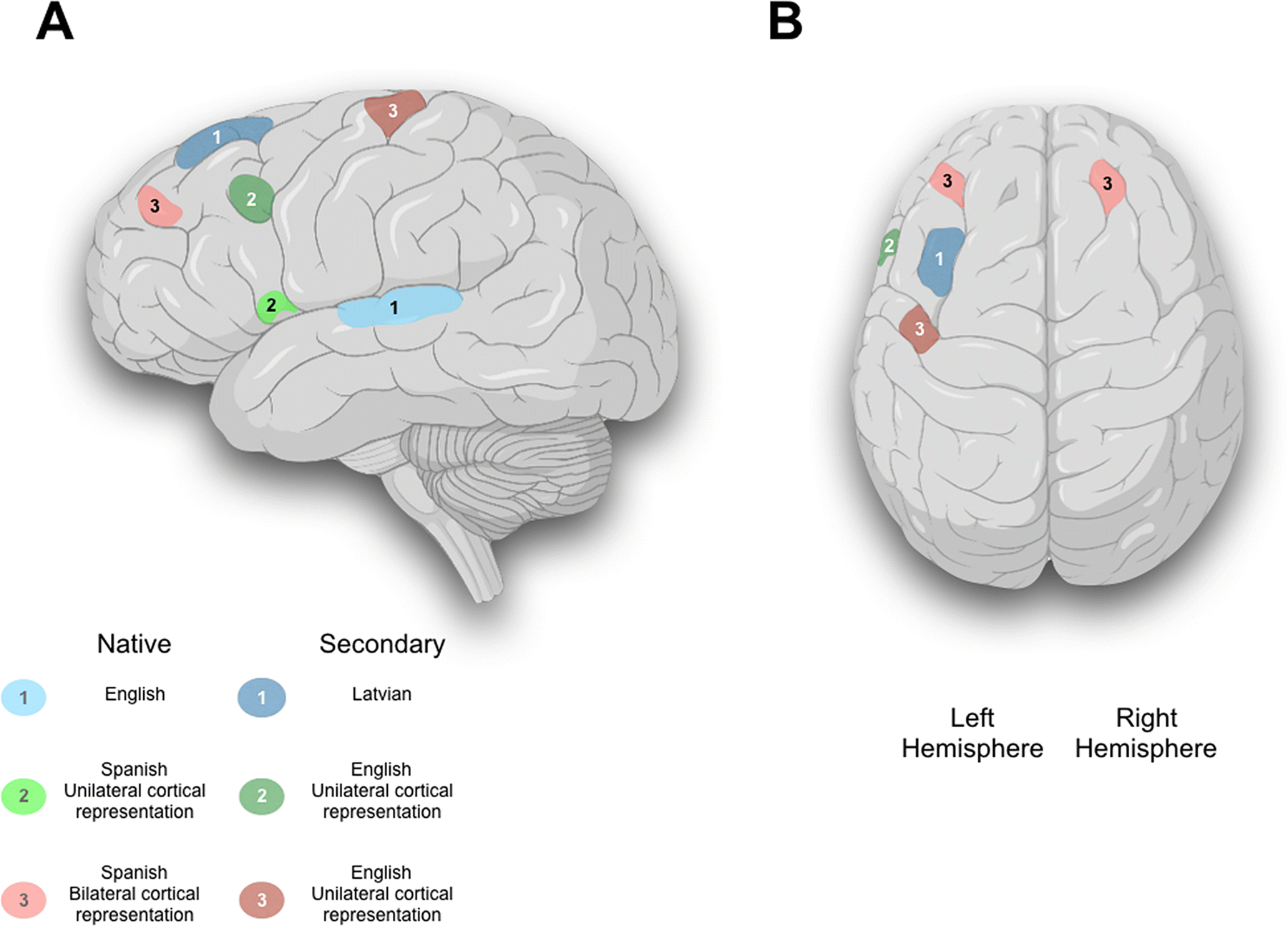
A 3D reconstruction of a brain image highlighting variation in language localization and lateralization derived from fMRI and intraoperative direct cortical stimulation in three patients. A) Sagittal brain illustration showing the native and the secondary language for three patients. Patient#1 showed a unilateral cortical representation of both English (Native), and Latvian (Secondary) languages, and was represented by light blue and blue color, respectively. Patient#2 showed a unilateral cortical representation of both Spanish (Native), and English (Secondary) languages, and was represented by light green and green color, respectively. Patient#3 showed a bilateral cortical representation of Spanish (Native), while unilateral cortical representation of English (Secondary) language, and was represented by light red and red color, respectively. B) Axial brain illustration showing the unilateral and bilateral cortical language representation.
Therefore, the aim of this study was to investigate whether there is a difference in clinical outcomes between bilingual and monolingual patients who underwent awake craniotomy for glioma resection.
Materials and Methods
Patient Selection
After approval by our institutional review board, data from 56 patients who underwent an awake craniotomy from September 2016 to June 2019 (Dates were restricted to within the past 4 years to reflect the most recent advances and techniques in awake brain surgery with brain mapping) performed by two neurosurgeons (A.Q.H. and K.L.C.) for lesions located in potential eloquent brain regions (eloquent language areas) were collected; 14 bilingual (BL) and 42 monolingual (ML) patients were identified and analyzed. All patients underwent left-sided procedures for intra-axial lesions located in one of the following regions: primary language cortex, Broca’s, Wernicke’s, or other language areas; posterior inferior frontal gyrus, posterior superior temporal gyrus; however, some lesions infiltrated adjacent motor or sensory areas. Pre-operative functional MRI (fMRI) was done for cortical language localization and was projected intraoperatively through merging with the navigation (Figure 2). Pathology was grouped into high-grade or low-grade using World Health Organization 2016 classification system with astrocytoma, oligodendroglioma, and oligoastrocytoma considered low-grade.[38] All the tumor pathology samples were reviewed by a senior neuropathologist (M.J.). All patients had received an education equivalent to or higher than a college degree. The proficiency of the second language in our study was determined by the patient report (speaker’s point of view), as well as the evaluation by our certified translators (listener’s point of view).[4,39–42] All patients in our study acquired the second language after six years of age and considered as late bilinguals.[17,10,18–20,16,21,14] An interpreter was used intraoperatively to aid with the language tasks. Object naming, non-word repetition, word comprehension neuropsychology paradigms were tested intraoperatively.
Figure 2:
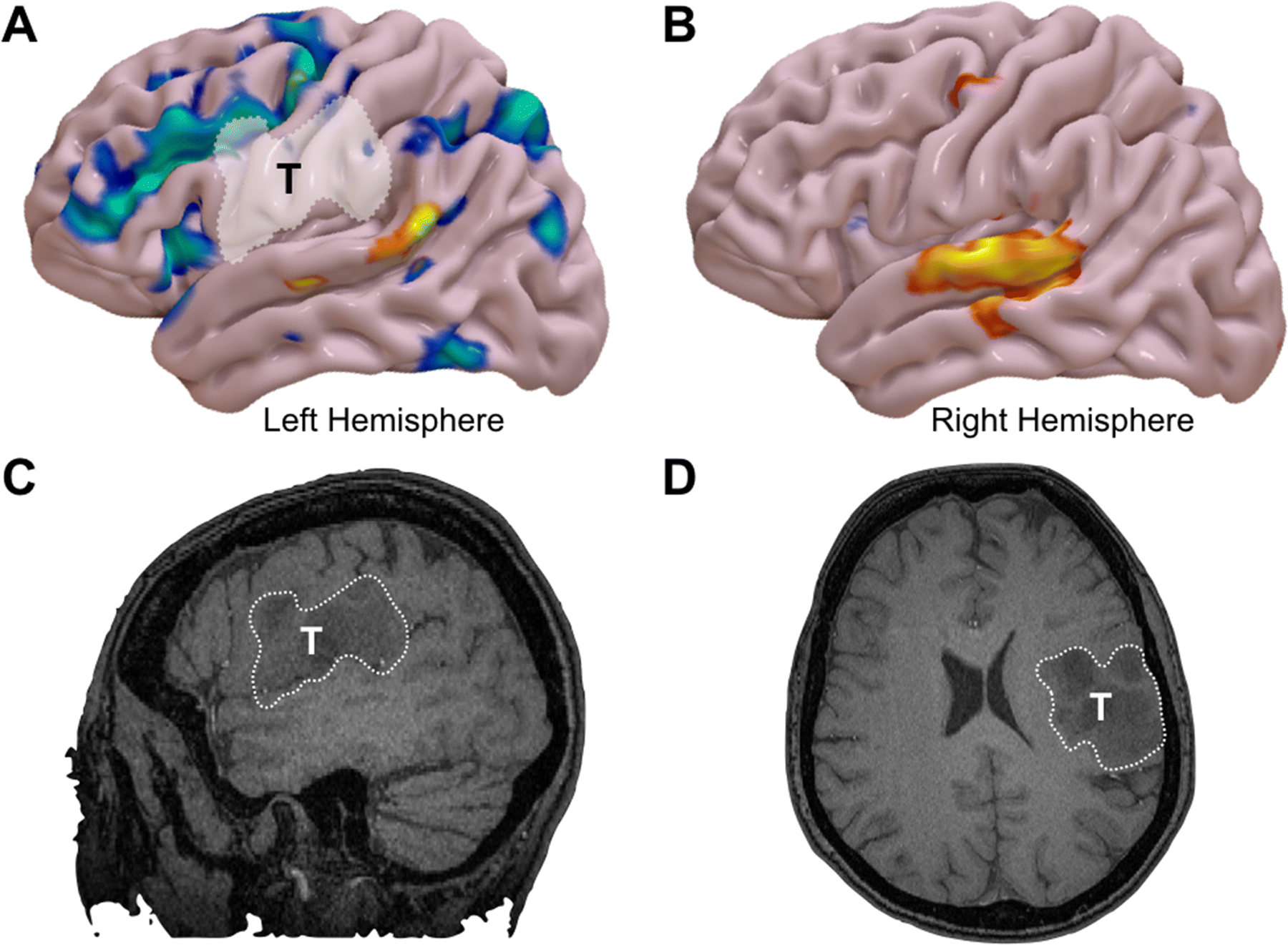
Reconstructive functional MRI (fMRI) results from a word-generation task in a bilingual patient with a WHO Grade III astrocytoma of the left frontoparietal operculum. A) The results for the patient’s native language is shown in blue and secondary language in orange. The native language shows an expected pattern of language activation in the left hemisphere, including both frontal and posterior language areas. B) fMRI representation of the secondary language in the right hemisphere. Non-contrast enhancing T1-weighted imaging showing the tumor in C) Left-sided sagittal cut and D) Axial cut on the left hemisphere.
Pre-operative Evaluation
All patients underwent pre-operative and post-operative MRI and pre-operative fMRI. Pre-operative and post-operative Karnofsky Performance Scale (KPS) scores were documented and presence of seizures pre-operatively or intra-operatively was noted. Non-language related deficits were also documented as noted in EMR (Electronic Medical Records).
Tumor Volumetric Evaluation
The pre-operative low-grade gliomas tumor volume was obtained using the T2-weighted MRI with gadolinium contrast as well as the fluid-attenuated inversion recovery (FLAIR) axial cuts. For high grade gliomas, the volume of an enhancing tumor was measured using a T1-weighted MRI with contrast. The Horos software Version 3 (LGPL-3.0) was used to calculate the tumor volume. The post-operative residual tumor volume was calculated using the MRI images obtained within 48 hours of the surgery, as previously reported.[43–47] The extent of resection (EOR) was . [46,47,43,48,49,44]
Electrocorticography/Stimulation Parameters
Customized high-density circular grid (patent application no. PCT/US2018/039956) consisted of a 22-channel hollow silastic® grid array with 0.3 mm platinum sensors separated by 0.5 cm for ECoG recording and identification of after-discharges during functional brain mapping (Figure 3A).[45,50] ECoG was recorded with a 128-channel digital video-EEG system (XLTEK, Natus Biomedical, San Carlos, CA) for analysis post-processing. The referential recording used band-pass filters from 0.1 to 100 Hz, a sampling rate of 512 Hz, and 16-bit analog-digital conversion. Monopolar recording and bipolar montage reformatting clarified epileptiform activity. Electrode impedance was checked, and a 60 Hz notch filter used in real-time to eliminate artifact during ECoG interpretation. Stimulation parameters for functional brain mapping including frequency of 50 hertz, pulse width of 500 microseconds, and flexible current setting titrated from 1 up to 6 milliamperes in trains up to 4 seconds until functional deficit or after-discharge was elicited. Stimulation intensity was less than that imposing after-discharges.
Figure 3:
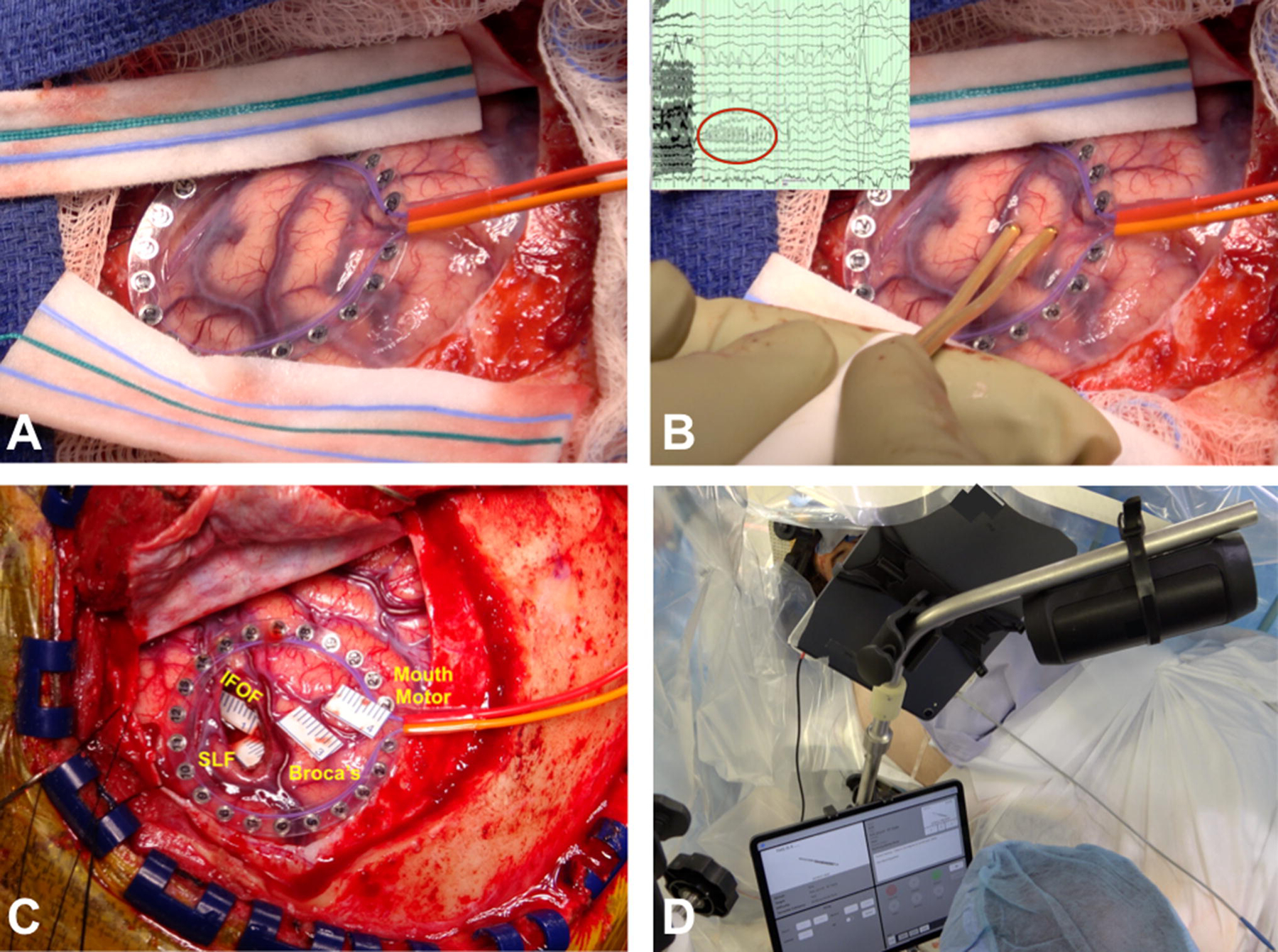
A) Intra-operative surgical field photograph showing the circular grid over the brain surface. B) Direct cortical stimulation using Ojemann stimulator with showing after-discharge on the electrocorticography (ECoG) monitoring using the circular grid. C) Intra-operative photograph showing the stickers identifying the eloquent cortical and subcortical areas: mouth motor, Broca’s, IFOF (inferior fronto-occipital fasciculus), and SLF (superior longitudinal fasciculus). D) Showing intra-operative neuropsychology language testing in both languages for bilingual patients.
Intra-operative Identification of Bilingual areas
The operative techniques of our awake craniotomies were described in the previous literature.[51,52,43,53,34,24] Cortical and subcortical language areas in the surgical field were identified using a handheld bipolar stimulator with two ball-tip electrodes known as the Ojemann Stimulator (Integra Lifesciences) for speech coherence and fluency assessment (Figure 3B). Endpoints were considered as either maximal stimulation without the interference of function, appearance of after-discharges on intra-operative electrocorticography (ECoG) (Figure 3B), and/or speech symptoms such as speech arrest (Figure 3C). Additional direct electrical cortical stimulation (DECS) was applied to the surrounding anatomical structures to ensure thorough identification of the speech and language areas. Intra-operative language testing was conducted by neuropsychologist (D.S.) in both languages for bilingual patients (Figure 3D) (Video 1). Areas of negative mapping were excluded from the analysis. Mapping of the surgical field was done systematically, starting with the areas of high suspicion for eloquence based on the pre-operative fMRI. The surgeons (A.Q.H. and K.L.C.) in our study start DCES at 2 mA for all patients.
Statistical Analysis
R Studio (Version 1.0.143, R. RStudio, Inc., Boston, MA) was utilized to analyze the patient data. Significance was considered at α ≤ 0.05. Patient outcome measures between monolingual and bilingual patients were analyzed using Wilcox, McNemar, and ANOVA testing. Additionally, a subgroup analysis of high-grade gliomas only was conducted (Supplementary Table 1). Continuous variables were reported as mean (SD) and categorical variables as counts (percentage).
Results
Patient Characteristics
From September 2016 to June 2019, a total of 56 patients (14 BL and 42 ML) underwent craniotomy for intra-axial lesions located near eloquent cortex. In our study, our statistical analysis with or without the inclusion of low-grade gliomas didn’t show any significant difference in pre-operative, intra-operative and post-operative patients characteristics. (Table 1, and Supplementary Table 1) Therefore, we present the data from high-grade and low-grade gliomas in Table 1, and the subset analysis of high-grade gliomas only in Supplementary Table 1. Baseline patient characteristics were similar between the groups and included patient demographics, pre-operative seizures, seizures medications, pre-operative tumor volumes, and Karnofsky Performance Score (KPS) are summarized in Table 1. In our cohort, 10 (71%) BL were male and 20 (47%) ML were male (P= 0.22). The average age was 45.2 years for BL and 50.5 years for ML patients (P= 0.18). A total of 3 (21.4%) BL and 14 (33%) ML presented with pre-operative seizures (P = 0.40). 2 (66%) BL and 8 (57.1%) ML patients were treated with monotherapy anti-seizure medications regimen (P = 0.76). However, 1 (33%) BL and 6 (42.8%) ML patients were treated with dual-therapy anti-seizure medications regimen (P = 0.76). Levetiracetam (Keppra®) was the anti-seizure medication of choice for 14 (100%) BL and 12 (85.7%) ML patients, and Phenytoin (Dilantin®) was the drug of choice for 0 (0%) BL and 4 (28.6%) ML patients (P = 0.48, and P = 0.29, respectively). Overall, no statistically significant difference was found in anti-seizure medication treatment between BL and ML. (Table 1) Pre-operative KPS scores for BL and ML patients were similar between groups (BL = 88.6 vs ML = 86.1, P = 0.50) (Table 1). Pre-operative tumor volume for BL and ML patients were comparable between the two groups (BL = 38.1 cm3 vs ML = 36.7 cm3, P = 0.83). The percentage of high-grade tumors in BL compared to ML and the tumor pathologies were not statistically significant different (Table 1). Tumor location was categorized based on lobe and functional cortex involvement. No differences were seen in tumor locations between BL and ML patients (frontal lobe, parietal lobe, temporal lobe, P = 0.09, P = 0.08, and P = 0.16, respectively) (Table 1).
Table 1.
Univariate analysis of patient characteristics, intra-operative findings, tumor characteristics, and outcomes of bilingual versus monolingual patients. Categorical and binary data is represented by counts; continuous data is represented by means.
| Bilingual (n=14) | Monolingual (n=42) | P-Value | |
|---|---|---|---|
| Preoperative patient characteristics | |||
| Age | 45.2 | 50.5 | 0.18 |
| Male | 10 (71%) | 20 (47%) | 0.22 |
| Pre-KPS | 88.6 | 86.1 | 0.67 |
| Pre-Op Vol (cm3) | 38.1 | 36.7 | 0.83 |
| Pre-op Seizures | 3 (21.4%) | 14 (33%) | 0.40 |
| Monotherapy | 2 (66%) | 8 (57.1%) | 0.76 |
| Dual Therapy | 1 (33%) | 6 (42.8%) | 0.76 |
| Levetiracetam (Keppra®) | 3(100%) | 12 (85.7%) | 0.48 |
| Phenytoin (Dilantin®) | 0 (0%) | 4 (28.6%) | 0.29 |
| Other | 1 (33%) | 4 (28.6%) | 0.87 |
| Intra-operative analysis of BL vs ML Patients | |||
| Intra-Op Seizures | 0 (0%) | 3 (7%) | 0.73 |
| # of Stimulations (Range) | 98.8 (86–123) | 94.2(75 −124) | 0.12 |
| mAMPS* (Range) | 4.7 (3– 6) | 3.8(2.5 −6.5) | 0.03 |
| Post-operative outcome measures | |||
| Seizure | 1 (7%) | 0 (0%) | 0.56 |
| KPS | 84.3 | 77.4 | 0.03 |
| ΔKPS | −4.3 | −8.7 | 0.03 |
| Post-Op Vol (cm3) | 13.5 | 8.1 | 0.03 |
| EOR (%) | 64.8 | 80.9 | 0.04 |
| Trans. Lang. Def. | 5 (36%) | 9 (21%) | 0.48 |
| Hospital Stay (days) | 4 | 5.3 | 0.64 |
| Pathology† | |||
| High Grade | 11 (79%) | 34 (81%) | 0.85 |
| Low Grade | 3 (21%) | 8 (19%) | 0.85 |
| Glioblastoma | 8(57%) | 21(50%) | 0.87 |
| Oligodendroglioma | 2(14%) | 6(14%) | 1 |
| Anaplastic Astrocytoma | 3(21%) | 13(26%) | 1 |
| Astrocytoma | 1(7%) | 2(5%) | 0.73 |
| Tumor location‡ | |||
| Frontal Lobe | 9 (64%) | 16 (38%) | 0.09 |
| Parietal Lobe | 8 (57%) | 13 (31%) | 0.08 |
| Temporal Lobe | 5 (36%) | 24 (57%) | 0.16 |
KPS = Karnofsky Performance Score; Preop = Preoperative; BL = Bilingual; ML = Monolingual.
Largest current that did not evoke after discharges. AMPS = Amperes. EOR = Extent of Resection; Δ = Delta; Trans. Lang. Def. = Transient Language Deficits. Vol = Volume.
Tumors were classified using the World Health Organization 2016 classification of diffuse gliomas.
Tumor may be located in overlapping eloquent regions.
Intra-operative Mapping
Intra-operatively, 0 (0%) BL and 3 (7%) ML experienced seizures (P = 0.73) (Table 1). Intra-operative stimulation-induced seizures were the most common events during awake procedures with brain mapping and were terminated by using our intraoperative treatment protocol: 1) Repeat electrical stimulus, 2) Cold saline irrigation and, 3) Administration of medication (either benzodiazepine or Propofol).[52,43,54–56] The frequency and the applied current (mAMPS) of the intra-operative DECS was recorded. Our data showed that both groups (BL and ML) received the same average number of stimulations during intra-operative mapping (BL = 98.8 vs ML = 94.2, P = 0.12) (Table 1). However, BL patients required a higher applied current in order to locate eloquent areas compared to ML patients (BL = 4.7 mAMPS vs ML = 3.8 mAMPS, P = 0.03) (Table 1).
Patient Outcome
Post-operatively, 1(7%) BL and 0 (0%) ML suffered a seizure (P = 0.56) (Table 1). The post-operative KPS scores were higher in BL in comparison to ML patients (BL = 84.3 vs ML = 77.4, P = 0.03) (Table 1). Additionally, the average drop in KPS was smaller for BL patients compared to ML (BL = −4.3 vs ML = −8.7, P = 0.03) (Table 1). The average EOR was significantly lower for BL patients than ML patients (BL = 64.8% vs ML = 80.9%, P = 0.04) (Table 1). Finally, length of hospital stay did not show any significant difference between BL and ML groups (BL = 4 days vs ML = 5.3 days, P = 0.64) (Table 1).
Discussion
In this study, we examined the role of bilingualism as a prognostic indicator for intra-operative course and post-operative outcome in patients undergoing awake craniotomy for intra-axial brain tumors. To our knowledge, this is the first study of its kind to address these questions and the largest series comparing the electrical current and number of stimulations of DECS during intra-operative mapping between monolingual and bilingual patients with intra-axial brain tumors.
In our study, intra-operative DECS showed that bilingual patients received similar numbers of stimulation trials, with a higher electrical current per stimulation in order to produce a response in the eloquent cortex when compared to monolingual patients (P = 0.12 and P = 0.03, respectively) (Table 1). This observation needs to be interpreted carefully due to the possibility of false positive mapping. We observed that despite the electrical current per stimulation difference, the bilingual patients had no intra-operative seizures, while 3 monolingual patients had intra-operative seizures (P = 0.73) (Table 1). It is important to note that these findings should be interpreted with caution given the small sample size and the lack of statistical differences; however, monolingual patients may be at a greater risk of seizures if similar or lower electrical stimulation current is applied. We hypothesize that the ability of bilingual patients to tolerate higher electrical stimulation current is due to the anatomical distribution of language networks.[57,58] This observation in the present study will need to be further elucidated in larger cohorts of patients. It might seem contradictory that in bilingual patients, who might have two different speech areas, we do not see a greater number of stimulations. However, we attribute this to limited cortical exposure thus the second language might not be exposed during surgery and the only entire exposed surface was mapped in both bilingual and monolingual patients.
A significantly lower extent of resection was achieved in bilingual patients in comparison to monolingual patients (64.8% vs 80.9%, respectively) (P = 0.04), with a significantly higher post-operative residual tumor volume in bilingual patients in comparison to the monolingual patients (13.5 vs 8.1 cm3, respectively) (P = 0.03) (Table 1). Given into consideration that all of the surgeries in our cohort were done by two neurosurgeons and the EOR did not show any significant differences between the surgeons (P = 0.52). The reason for lower EOR in BL is unclear. Therefore, we should be cautious in our interpretation of these observations. A potential explanation for these observations includes 1) the operative techniques and surgeons’ experience of performing surgery on bilingual patients, 2) the differences in the functional organization between BL and ML, and the age of secondary language acquisition. From an operative standpoint and as evidenced by the observed higher EOR in the ML group, a more aggressive resection was possible in this group despite using a similar mapping approach and methods and a high degree of expertise in mapping BL by one of the neurosurgeons. In addition, a higher post-operative KPS scores and lower post-operative KPS drops were observed for BL patients in comparison to the ML patients (Table 1). This would necessarily include resection of more normal, functional brain as well in ML patients which could then contribute to a lower KPS in these patients when compared to BL patients who underwent a lesser EOR. Similar findings have been reported by our group previously, showing that patients who underwent awake craniotomy tend to experience a higher drop in KPS immediately post-operatively in comparison to the patients who underwent surgery under general anesthesia (−5.9 vs −4.5, respectively).[43]
Alternatively, or more likely in tandem, it is possible that language organization also plays a contributory role. From a linguistics perspective, prior work has established that BL individuals harbor a more diffuse, robust language organization and connectivity in comparison to the ML individuals.[59–61,16,62,63,24,64–66,7,17] Furthermore, studies have noted that surgery itself can promote functional reorganization, and this is also an important consideration particularly in bilingual patients where the strategy for resection may be spatially distributed given a wider distribution of language function.[57,58,67–69,35,23,70,71] The findings of the present study (lower EOR in BL, and higher post-operative KPS scores and lower post-operative KPS drops) are in line with these potential explanations; however, future studies will be required that account for possible confounders such as location of functional organization, extent of resection, and location of second language representation. Additionally, presence and extent of functional reorganization pre-operatively and post-operatively would be required to elucidate the mechanisms behind the findings of the present study.[72,73,12,74,57] The age of secondary language acquisition plays an important role in cortical language distribution, as individuals who acquired the secondary language after the age of seven years old tends to show more diffuse cortical language representations.[59–61,16,62,63,24,64–66,7,17] On the contrary, bilingual individuals who acquired the second language before the age of seven years old, tend to have more localized cortical language representations. Since all of our bilingual patient population acquired the second language after the age of seven years old, we hypothesize that the lower EOR and the lower drop in post-operative KPS in the bilingual patients is a result of the diffuse cortical distribution of secondary language. Taken together, these findings suggest that bilingual patients may experience better surgical outcomes following resection of intra-axial brain tumors. In the present study, there may be evidence that is suggestive of the presence of a differential functional organization in monolingual and bilingual patients that contributes to improved recovery. Prior studies have noted that the surgery itself can promote functional reorganization, and this is an important consideration particularly in bilingual patients where the strategy for resection may be spatially distributed given a wider distribution of language function.[75,76,15,66,77–79]
Interestingly, 1 (7%) bilingual patient experienced a post-operative seizure, while none of the monolingual patients experienced seizures (P = 0.56) (Table 1). Despite the statistical insignificance for this finding, we believe this warrant deeper investigation in subsequent studies. Important considerations and potential explanations include the magnitude of electrical stimulation applied and the underlying differences in brain network organization in bilingual patients. Bilingual patients received a higher applied current during intra-operative stimulation, which could potentially promote increased cortical excitability since one known unwanted effect of cortical stimulation is seizures. Secondly, due to the robustness of cortical and subcortical networks in bilingual patients, their brains may be more excitable at baseline and have a lower threshold for seizures as a consequence of increased network interconnectivity.[57,58]
Limitations
Several limitations are noteworthy. One is the selection bias inherent to the retrospective nature of the study and additionally, the selection associated with only including patients undergoing awake craniotomy. Although the language of the patients was limited to only Arabic, English, Spanish, German, Estonian, Lithuanian, and French, we believe that the findings reported here have implications for bilingual patients in general since they are consistent with previous studies of different languages.[24,75] Additionally, all the patients in this study are late bilinguals (language acquisition after the age of 6–9 years), and therefore these results may not extend to early bilinguals.[17] It is also important to note that intra-operative electrical stimulation-based studies, showed heterogeneous results, varying from a total cortical overlap between L1 and L2, to partially separated cortical regions between L1 & L2, to spatially separate cortical regions between the two languages.[19–26,15,70,80,81,75,59,64]. Given the prevailing limitations in understanding both fMRI and intraoperative electrical stimulation in language, even in monolingual patients, this retrospective study design does not allow inferences to be made about language localization in bilingual patients, but rather is intended to shed light on the potential outcomes differences and serve as a basis for further exploration. Although, functional MRI was performed on the patients preoperatively, however, the discrepancies between the functional MRI and the intraoperative language mapping were not included in our cohort. In addition, it is possible that recruitment of additional cognitive brain regions, such as attention networks, produce more unpredictable activation on fMRI during mapping of a non-native language, which could potentially confound interpretation. To date, it is unknown how these variations in activation, as seen in Figure 1C, Patient#3, relate to the incidence of stimulation-induced language effects and the tolerance for resection. Given the small sample size of BL patients, instances of low EOR in BL patients might skew these results. Although it is known that the type of brain tumor can also have an impact on EOR, the impact of tumor type on language networks in bilinguals has not been investigated and would likely offer important insights into language connectivity and intra-operative course in patients undergoing surgery for eloquently-located brain tumors.[82] Ultimately, a prospective study evaluating the intra-operative (seizures, number of DECS, current) and post-operative (EOR, KPS, ∆ KPS) outcomes between ML and BL patients would be needed to further elucidate differences between these cohorts.
Conclusion
In summary, the primary goal of this study was to investigate (1) any differences in complications (i.e., seizures) and DECS techniques during intra-operative brain mapping, (2) differences in the extent of resection (EOR) and (3) any differences in the recovery pattern post-surgery, between ML and BL patient population. We have shown that BL patient has a lower incidence of intra-operative seizures, tolerates higher DECS current, lower EOR, and higher post-operative KPS compared to ML patients. Furthermore, we describe important clinical perioperative differences between monolingual and bilingual patients, including seizure incidence and cortical stimulation parameters such as current. Taken together, these results suggest a difference in the outcome of these two patient populations; however, the underlying mechanism remains unclear.
Supplementary Material
Video 1: This 32-year-old right-handed, Hispanic male presented with a history of seizures and was diagnosed with a left frontal lobe lesion, for which he underwent a near-total resection back in 2013. The pathology was consistent with WHO grade II Oligodendroglioma, IDH-mutant, and 1p19q co-deleted. The recommendations were for him to continue to monitor the lesion with a serial of MRI scans, which remained stable. His most recent scan in February 2020 revealed left frontal expected post-surgical changes, with an increase in the surrounding T2/FLAIR hyperintensity signals with slight fullness of the parenchyma at the superior and inferior aspects. These findings were consistent with the evidence of disease progression vs. recurrence. The patient was taken to the operating room for a left-sided awake craniotomy in a supine position; a curvilinear skin incision was done, and the musculocutaneous flap was elevated after removal of the previous titanium plates and miniscrews. Two burr holes were placed and connected using a footplate, and the bone flap was removed. The dura was opened in a curvilinear fashion. Ojemann stimulator and the circular grid was used for cortical brain mapping; at the same time, neuropsychological testing in both English and Spanish was conducted. A prolonged and after-discharge and focal electrographic seizure was detected and was aborted using cold water irrigation. Tumor resection proceeded in tandem with neuropsychological testing. The patient tolerated the surgery well with no issues or complications and was discharged on a postoperative day 3 after an unremarkable hospital course. The pathology was consistent with Oligodendroglioma, IDH-mutant, and 1p19q co-deleted (WHO grade II). The postoperative imaging showed gross total resection with expected postoperative changes.
Funding:
AQH was supported by the Mayo Clinic Professorship and a Clinician Investigator award, and Florida State Department of Health Research Grant, and the Mayo Clinic Graduate School, as well as the NIH (R43-CA221490, R01-CA200399, R01-CA195503, and R01-CA216855). PB was supported by NIH/NIBIB (P41-EB018783, R01-EB026439), the NIH/NINDS (U01-NS108916 and U24-NS109103). PB, AR, and KR were supported by NIH/NINDS (U01-NS108916).
Abbreviations:
- ML
monolingual
- BL
Bilingual
- EOR
extent of resection
- DECS
direct electrical cortical stimulation
- KPS
Karnofsky Performance Scale
- fMRI
functional MRI
- AoA
Age of Acquisition
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Declarations
Conflict of Interest /Competing interests: Drs. Tatum, Quiñones-Hinojosa, and ReFaey filed a patent disclosing the circular grid device and technology (patent application no. PCT/ US2018/039956). The rest of the authors have no conflict of interest. The abstract was presented as an Oral Presentation for the Sunrise Science and Late Breaking Session 7: Stereotactic and Functional during the 2019 Congress of Neurological Surgeons Annual Meeting, October 19–23, in San Francisco, CA.
Ethics approval: The institutional review board approved this study. IRB# 16-009946
References
- 1.Bureau USC (2011) Language Use in the United States. https://www2.census.gov/library/publications/2013/acs/acs-22/acs-22.pdf. Accessed November 2019
- 2.TNS Opinion & Social at the request of Directorate-General Education and Culture D-GfTaD-GfI (2012) Europeans and their Languages. Special Eurobarometer 386, vol 2019. [Google Scholar]
- 3.Kovelman I, Baker SA, Petitto L-AJBl, cognition (2008) Age of first bilingual language exposure as a new window into bilingual reading development. 11 (2):203–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennon P (1990) Investigating Fluency in EFL: A Quantitative Approach*. 40 (3):387–417. doi: 10.1111/j.1467-1770.1990.tb00669.x [DOI] [Google Scholar]
- 5.De Jong NHJLAQ (2018) Fluency in second language testing: Insights from different disciplines. 15 (3):237–254 [Google Scholar]
- 6.Fillmore CJ, Kempler D, Wang WS (2014) Individual differences in language ability and language behavior. Academic Press, [Google Scholar]
- 7.Kovelman I, Baker SA, Petitto LA (2008) Bilingual and monolingual brains compared: a functional magnetic resonance imaging investigation of syntactic processing and a possible “neural signature” of bilingualism. J Cogn Neurosci 20 (1):153–169. doi: 10.1162/jocn.2008.20011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wattendorf E, Festman JJARoAL (2008) Images of the multilingual brain: the effect of age of second language acquisition. 28:3–24 [Google Scholar]
- 9.Leonard MK, Torres C, Travis KE, Brown TT, Hagler DJ Jr, Dale AM, Elman JL, Halgren EJPo (2011) Language proficiency modulates the recruitment of non-classical language areas in bilinguals. 6 (3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KH, Relkin NR, Lee KM, Hirsch J (1997) Distinct cortical areas associated with native and second languages. Nature 388 (6638):171–174. doi: 10.1038/40623 [DOI] [PubMed] [Google Scholar]
- 11.Tu L, Wang J, Abutalebi J, Jiang B, Pan X, Li M, Gao W, Yang Y, Liang B, Lu Z, Huang R (2015) Language exposure induced neuroplasticity in the bilingual brain: a follow-up fMRI study. Cortex 64:8–19. doi: 10.1016/j.cortex.2014.09.019 [DOI] [PubMed] [Google Scholar]
- 12.Herbet G, Moritz-Gasser S, Lemaitre AL, Almairac F, Duffau H (2019) Functional compensation of the left inferior longitudinal fasciculus for picture naming. Cogn Neuropsychol 36 (3–4):140–157. doi: 10.1080/02643294.2018.1477749 [DOI] [PubMed] [Google Scholar]
- 13.Duffau H (2015) Stimulation mapping of white matter tracts to study brain functional connectivity. Nat Rev Neurol 11 (5):255–265. doi: 10.1038/nrneurol.2015.51 [DOI] [PubMed] [Google Scholar]
- 14.Yetkin O, Yetkin FZ, Haughton VM, Cox RWJAJoN (1996) Use of functional MR to map language in multilingual volunteers. 17 (3):473–477 [PMC free article] [PubMed] [Google Scholar]
- 15.Roux F-E, Trémoulet MJJoN (2002) Organization of language areas in bilingual patients: a cortical stimulation study. 97 (4):857–864 [DOI] [PubMed] [Google Scholar]
- 16.Perani D, Paulesu E, Galles NS, Dupoux E, Dehaene S, Bettinardi V, Cappa SF, Fazio F, Mehler J (1998) The bilingual brain. Proficiency and age of acquisition of the second language. Brain 121 ( Pt 10):1841–1852. doi: 10.1093/brain/121.10.1841 [DOI] [PubMed] [Google Scholar]
- 17.Bloch C, Kaiser A, Kuenzli E, Zappatore D, Haller S, Franceschini R, Luedi G, Radue EW, Nitsch C (2009) The age of second language acquisition determines the variability in activation elicited by narration in three languages in Broca’s and Wernicke’s area. Neuropsychologia 47 (3):625–633. doi: 10.1016/j.neuropsychologia.2008.11.009 [DOI] [PubMed] [Google Scholar]
- 18.Abutalebi J, Annoni J-M, Zimine I, Pegna AJ, Seghier ML, Lee-Jahnke H, Lazeyras F, Cappa SF, Khateb AJCC (2008) Language control and lexical competition in bilinguals: an event-related fMRI study. 18 (7):1496–1505 [DOI] [PubMed] [Google Scholar]
- 19.Briellmann RS, Saling MM, Connell AB, Waites AB, Abbott DF, Jackson GDJB, Language (2004) A high-field functional MRI study of quadri-lingual subjects. 89 (3):531–542 [DOI] [PubMed] [Google Scholar]
- 20.Perani D, Abutalebi J, Paulesu E, Brambati S, Scifo P, Cappa SF, Fazio FJHbm (2003) The role of age of acquisition and language usage in early, high-proficient bilinguals: An fMRI study during verbal fluency. 19 (3):170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vingerhoets G, Van Borsel J, Tesink C, Van den Noort M, Deblaere K, Seurinck R, Vandemaele P, Achten EJN (2003) Multilingualism: an fMRI study. 20 (4):2181–2196 [DOI] [PubMed] [Google Scholar]
- 22.Paradis M (2003) Differential use of cerebral mechanisms in bilinguals. In: Mack MTBM (ed) Mind, brain, and language: Multidisciplinary perspectives. Lawrence Erlbaum, London, pp 351–370 [Google Scholar]
- 23.Sierpowska J, Fernandez-Coello A, Gomez-Andres A, Camins A, Castaner S, Juncadella M, Gabarros A, Rodriguez-Fornells A (2018) Involvement of the middle frontal gyrus in language switching as revealed by electrical stimulation mapping and functional magnetic resonance imaging in bilingual brain tumor patients. Cortex 99:78–92. doi: 10.1016/j.cortex.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 24.Walker JA, Quinones-Hinojosa A, Berger MS (2004) Intraoperative speech mapping in 17 bilingual patients undergoing resection of a mass lesion. Neurosurgery 54 (1):113–117; discussion 118 [DOI] [PubMed] [Google Scholar]
- 25.Yokoyama S, Okamoto H, Miyamoto T, Yoshimoto K, Kim J, Iwata K, Jeong H, Uchida S, Ikuta N, Sassa Y, Nakamura W, Horie K, Sato S, Kawashima R (2006) Cortical activation in the processing of passive sentences in L1 and L2: an fMRI study. Neuroimage 30 (2):570–579. doi: 10.1016/j.neuroimage.2005.09.066 [DOI] [PubMed] [Google Scholar]
- 26.Halsband U (2006) Bilingual and multilingual language processing. J Physiol Paris 99 (4–6):355–369. doi: 10.1016/j.jphysparis.2006.03.016 [DOI] [PubMed] [Google Scholar]
- 27.Kousta ST, Vinson DP, Vigliocco G (2008) Investigating linguistic relativity through bilingualism: the case of grammatical gender. J Exp Psychol Learn Mem Cogn 34 (4):843–858. doi: 10.1037/0278-7393.34.4.843 [DOI] [PubMed] [Google Scholar]
- 28.McComsey M (2015) Bilingual Spaces: Approaches to Linguistic Relativity in Bilingual Mexico.
- 29.Hernandez AE, Martinez A, Kohnert K (2000) In search of the language switch: An fMRI study of picture naming in Spanish-English bilinguals. Brain Lang 73 (3):421–431. doi: 10.1006/brln.1999.2278 [DOI] [PubMed] [Google Scholar]
- 30.Ojemann G, Ojemann J, Lettich E, Berger M (1989) Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg 71 (3):316–326. doi: 10.3171/jns.1989.71.3.0316 [DOI] [PubMed] [Google Scholar]
- 31.Van Buren JM, Fedio P, Frederick GC (1978) Mechanism and localization of speech in the parietotemporal cortex. Neurosurgery 2 (3):233–239. doi: 10.1227/00006123-197805000-00009 [DOI] [PubMed] [Google Scholar]
- 32.Bilotta F, Stazi E, Delfini R, Rosa G (2011) Language testing during awake “anesthesia” in a bilingual patient with brain lesion adjacent to Wernicke’s area. Anesth Analg 112 (4):938–939. doi: 10.1213/ANE.0b013e31820bd1a4 [DOI] [PubMed] [Google Scholar]
- 33.Sanai N, Mirzadeh Z, Berger MS (2008) Functional outcome after language mapping for glioma resection. N Engl J Med 358 (1):18–27. doi: 10.1056/NEJMoa067819 [DOI] [PubMed] [Google Scholar]
- 34.Quinones-Hinojosa A, Ojemann SG, Sanai N, Dillon WP, Berger MS (2003) Preoperative correlation of intraoperative cortical mapping with magnetic resonance imaging landmarks to predict localization of the Broca area. J Neurosurg 99 (2):311–318. doi: 10.3171/jns.2003.99.2.0311 [DOI] [PubMed] [Google Scholar]
- 35.Ojemann SG, Berger MS, Lettich E, Ojemann GA (2003) Localization of language function in children: results of electrical stimulation mapping. J Neurosurg 98 (3):465–470. doi: 10.3171/jns.2003.98.3.0465 [DOI] [PubMed] [Google Scholar]
- 36.Berger MS (1996) Minimalism through intraoperative functional mapping. Clin Neurosurg 43:324–337 [PubMed] [Google Scholar]
- 37.Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA (1994) Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery 34 (4):567–576; discussion 576 [DOI] [PubMed] [Google Scholar]
- 38.Wesseling P, Capper D (2017) WHO 2016 Classification of Gliomas. Neuropathol Appl Neurobiol. doi: 10.1111/nan.12432 [DOI] [PubMed] [Google Scholar]
- 39.Riggenbach HJDp (1991) Toward an understanding of fluency: A microanalysis of nonnative speaker conversations. 14 (4):423–441 [Google Scholar]
- 40.Bosker HR, Pinget A-F, Quené H, Sanders T, De Jong NHJLT (2013) What makes speech sound fluent? The contributions of pauses, speed and repairs. 30 (2):159–175 [Google Scholar]
- 41.Kahng JJLL (2014) Exploring utterance and cognitive fluency of L1 and L2 English speakers: Temporal measures and stimulated recall. 64 (4):809–854 [Google Scholar]
- 42.Révész A, Ekiert M, Torgersen ENJAL (2016) The effects of complexity, accuracy, and fluency on communicative adequacy in oral task performance. 37 (6):828–848 [Google Scholar]
- 43.Eseonu CI, Rincon-Torroella J, ReFaey K, Lee YM, Nangiana J, Vivas-Buitrago T, Quinones-Hinojosa A (2017) Awake Craniotomy vs Craniotomy Under General Anesthesia for Perirolandic Gliomas: Evaluating Perioperative Complications and Extent of Resection. Neurosurgery. doi: 10.1093/neuros/nyx023 [DOI] [PubMed] [Google Scholar]
- 44.Eseonu CI, ReFaey K, Garcia O, Raghuraman G, Quinones-Hinojosa A (2017) Volumetric Analysis of Extent of Resection, Survival, and Surgical Outcomes for Insular Gliomas. World Neurosurg 103:265–274. doi: 10.1016/j.wneu.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 45.ReFaey K, Chaichana KL, Feyissa AM, Vivas-Buitrago T, Brinkmann BH, Middlebrooks EH, McKay JH, Lankford DJ, Tripathi S, Bojaxhi E, Roth GE, Tatum WO, Quiñones-Hinojosa A (2019) A 360° electronic device for recording high-resolution intraoperative electrocorticography of the brain during awake craniotomy. J Neurosurg:1–8. doi: 10.3171/2019.4.JNS19261 [DOI] [PubMed] [Google Scholar]
- 46.Chaichana KL, Jusue-Torres I, Lemos AM, Gokaslan A, Cabrera-Aldana EE, Ashary A, Olivi A, Quinones-Hinojosa A (2014) The butterfly effect on glioblastoma: is volumetric extent of resection more effective than biopsy for these tumors? J Neurooncol 120 (3):625–634. doi: 10.1007/s11060-014-1597-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, Raza SM, Pascual-Gallego M, Ibrahim A, Hernandez-Hermann M, Gomez L, Ye X, Weingart JD, Olivi A, Blakeley J, Gallia GL, Lim M, Brem H, Quinones-Hinojosa A (2014) Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol 16 (1):113–122. doi: 10.1093/neuonc/not137 not137 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eseonu CI, Eguia F, ReFaey K, Garcia O, Rodriguez FJ, Chaichana K, Quinones-Hinojosa A (2017) Comparative volumetric analysis of the extent of resection of molecularly and histologically distinct low grade gliomas and its role on survival. J Neurooncol 134 (1):65–74. doi: 10.1007/s11060-017-2486-9 [DOI] [PubMed] [Google Scholar]
- 49.Eseonu CI, Rincon-Torroella J, ReFaey K, Quinones-Hinojosa A (2017) The Cost of Brain Surgery: Awake vs Asleep Craniotomy for Perirolandic Region Tumors. Neurosurgery 81 (2):307–314. doi: 10.1093/neuros/nyx022 [DOI] [PubMed] [Google Scholar]
- 50.Tatum WO, McKay JH, ReFaey K, Feyissa AM, Ryan D, Ritaccio A, Middlebrooks E, Yelvington K, Roth G, Acton E, Grewal S, Chaichana K, Quinones-Hinojosa A (2020) Detection of after-discharges during intraoperative functional brain mapping in awake brain tumor surgery using a novel high-density circular grid. Clin Neurophysiol 131 (4):828–835. doi: 10.1016/j.clinph.2019.12.416 [DOI] [PubMed] [Google Scholar]
- 51.Norma Arechiga KR, Rincon-Torroella Jordina, Chaichana Kaisorn L., Quiñones-Hinojosa (2016) Video Atlas of Neurosurgery: Contemporary Tumor and Skull Base Surgery, vol 1. Cortical/Subcortical Motor Mapping for Gliomas. Elsevier Philadelphia, [Google Scholar]
- 52.Eseonu CI, Rincon-Torroella J, Lee YM, ReFaey K, Tripathi P, Quinones-Hinojosa A (2018) Intraoperative Seizures in Awake Craniotomy for Perirolandic Glioma Resections That Undergo Cortical Mapping. J Neurol Surg A Cent Eur Neurosurg. doi: 10.1055/s-0037-1617759 [DOI] [PubMed] [Google Scholar]
- 53.Eseonu CI, ReFaey K, Garcia O, John A, Quinones-Hinojosa A, Tripathi P (2017) Awake Craniotomy Anesthesia: A Comparison of the Monitored Anesthesia Care and Asleep-Awake-Asleep Techniques. World Neurosurg 104:679–686. doi: 10.1016/j.wneu.2017.05.053 [DOI] [PubMed] [Google Scholar]
- 54.Nossek E, Matot I, Shahar T, Barzilai O, Rapoport Y, Gonen T, Sela G, Grossman R, Korn A, Hayat D, Ram Z (2013) Intraoperative seizures during awake craniotomy: incidence and consequences: analysis of 477 patients. Neurosurgery 73 (1):135–140; discussion 140. doi: 10.1227/01.neu.0000429847.91707.97 [DOI] [PubMed] [Google Scholar]
- 55.Lima GLO, Dezamis E, Corns R, Rigaux-Viode O, Moritz-Gasser S, Roux A, Duffau H, Pallud J (2017) Surgical resection of incidental diffuse gliomas involving eloquent brain areas. Rationale, functional, epileptological and oncological outcomes. Neurochirurgie 63 (3):250–258. doi: 10.1016/j.neuchi.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 56.Yuan Y, Peizhi Z, Xiang W, Yanhui L, Ruofei L, Shu J, Qing M (2016) Intraoperative seizures and seizures outcome in patients underwent awake craniotomy. J Neurosurg Sci [DOI] [PubMed] [Google Scholar]
- 57.Middlebrooks EH, Yagmurlu K, Szaflarski JP, Rahman M, Bozkurt B (2017) A contemporary framework of language processing in the human brain in the context of preoperative and intraoperative language mapping. Neuroradiology 59 (1):69–87. doi: 10.1007/s00234-016-1772-0 [DOI] [PubMed] [Google Scholar]
- 58.Yagmurlu K, Middlebrooks EH, Tanriover N, Rhoton AL Jr. (2016) Fiber tracts of the dorsal language stream in the human brain. J Neurosurg 124 (5):1396–1405. doi: 10.3171/2015.5.JNS15455 [DOI] [PubMed] [Google Scholar]
- 59.Ojemann GA, Whitaker HA (1978) The bilingual brain. Arch Neurol 35 (7):409–412. doi: 10.1001/archneur.1978.00500310011002 [DOI] [PubMed] [Google Scholar]
- 60.Ojemann GA, Whitaker HA (1978) Language localization and variability. Brain Lang 6 (2):239–260. doi: 10.1016/0093-934x(78)90061-5 [DOI] [PubMed] [Google Scholar]
- 61.Rapport RL, Tan CT, Whitaker HA (1983) Language function and dysfunction among Chinese- and English-speaking polyglots: cortical stimulation, Wada testing, and clinical studies. Brain Lang 18 (2):342–366. doi: 10.1016/0093-934x(83)90024-x [DOI] [PubMed] [Google Scholar]
- 62.Calabrese P, Neufeld H, Falk A, Markowitsch HJ, Muller C, Heuser L, Gehlen W, Durwen HF (2001) [Word generation in bilinguals--fMRI study with implications for language and memory processes]. Fortschr Neurol Psychiatr 69 (1):42–49. doi: 10.1055/s-2001-10467 [DOI] [PubMed] [Google Scholar]
- 63.Hernandez AE, Dapretto M, Mazziotta J, Bookheimer S (2001) Language switching and language representation in Spanish-English bilinguals: an fMRI study. Neuroimage 14 (2):510–520. doi: 10.1006/nimg.2001.0810 [DOI] [PubMed] [Google Scholar]
- 64.Roux FE, Lubrano V, Lauwers-Cances V, Tremoulet M, Mascott CR, Demonet JF (2004) Intra-operative mapping of cortical areas involved in reading in mono- and bilingual patients. Brain 127 (Pt 8):1796–1810. doi: 10.1093/brain/awh204 [DOI] [PubMed] [Google Scholar]
- 65.Tham WW, Rickard Liow SJ, Rajapakse JC, Choong Leong T, Ng SE, Lim WE, Ho LG (2005) Phonological processing in Chinese-English bilingual biscriptals: an fMRI study. Neuroimage 28 (3):579–587. doi: 10.1016/j.neuroimage.2005.06.057 [DOI] [PubMed] [Google Scholar]
- 66.Giussani C, Roux FE, Lubrano V, Gaini SM, Bello L (2007) Review of language organisation in bilingual patients: what can we learn from direct brain mapping? Acta Neurochir (Wien) 149 (11):1109–1116; discussion 1116. doi: 10.1007/s00701-007-1266-2 [DOI] [PubMed] [Google Scholar]
- 67.Yamao Y, Matsumoto R, Kunieda T, Arakawa Y, Kobayashi K, Usami K, Shibata S, Kikuchi T, Sawamoto N, Mikuni N, Ikeda A, Fukuyama H, Miyamoto S (2014) Intraoperative dorsal language network mapping by using single-pulse electrical stimulation. Human Brain Mapping 35 (9):4345–4361. doi: 10.1002/hbm.22479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Enatsu R, Kubota Y, Kakisaka Y, Bulacio J, Piao Z, O’Connor T, Horning K, Mosher J, Burgess RC, Bingaman W, Nair DR (2013) Reorganization of posterior language area in temporal lobe epilepsy: A cortico-cortical evoked potential study. Epilepsy Research 103 (1):73–82. doi: 10.1016/j.eplepsyres.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 69.Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK (2007) Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci U S A 104 (43):17163–17168. doi: 10.1073/pnas.0702116104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernández-Coello A, Havas V, Juncadella M, Sierpowska J, Rodríguez-Fornells A, Gabarrós A (2017) Age of language acquisition and cortical language organization in multilingual patients undergoing awake brain mapping. J Neurosurg 126 (6):1912–1923. doi: 10.3171/2016.5.JNS152791 [DOI] [PubMed] [Google Scholar]
- 71.Sierpowska J, Gabarros A, Ripolles P, Juncadella M, Castaner S, Camins A, Plans G, Rodriguez-Fornells A (2013) Intraoperative electrical stimulation of language switching in two bilingual patients. Neuropsychologia 51 (13):2882–2892. doi: 10.1016/j.neuropsychologia.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 72.Rivera-Rivera PA, Rios-Lago M, Sanchez-Casarrubios S, Salazar O, Yus M, Gonzalez-Hidalgo M, Sanz A, Avecillas-Chasin J, Alvarez-Linera J, Pascual-Leone A, Oliviero A, Barcia JA (2017) Cortical plasticity catalyzed by prehabilitation enables extensive resection of brain tumors in eloquent areas. J Neurosurg 126 (4):1323–1333. doi: 10.3171/2016.2.JNS152485 [DOI] [PubMed] [Google Scholar]
- 73.Deverdun J, van Dokkum LEH, Le Bars E, Herbet G, Mura T, D’Agata B, Picot MC, Menjot N, Molino F, Duffau H, Moritz Gasser S (2019) Language reorganization after resection of low-grade gliomas: an fMRI task based connectivity study. Brain Imaging Behav. doi: 10.1007/s11682-019-00114-7 [DOI] [PubMed] [Google Scholar]
- 74.Gatignol P, Duffau H, Capelle L, Plaza M (2009) Naming performance in two bilinguals with frontal vs. temporal glioma. Neurocase 15 (6):466–477. doi: 10.1080/13554790902950434 [DOI] [PubMed] [Google Scholar]
- 75.Lucas TH, McKhann GM, Ojemann GAJJon (2004) Functional separation of languages in the bilingual brain: a comparison of electrical stimulation language mapping in 25 bilingual patients and 117 monolingual control patients. 101 (3):449–457 [DOI] [PubMed] [Google Scholar]
- 76.Li P, Legault J, Litcofsky KA (2014) Neuroplasticity as a function of second language learning: anatomical changes in the human brain. Cortex 58:301–324. doi: 10.1016/j.cortex.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 77.Schafer RJ, Constable RT (2009) Variation in language networks in monolingual and bilingual English speakers: consequences for language mapping for surgical preplanning. J Clin Exp Neuropsychol 31 (8):945–954. doi: 10.1080/13803390902766879 [DOI] [PubMed] [Google Scholar]
- 78.Duffau H (2005) Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol 4 (8):476–486. doi: 10.1016/S1474-4422(05)70140-X [DOI] [PubMed] [Google Scholar]
- 79.Costello TG (2014) Awake craniotomy and multilingualism: language testing during anaesthesia for awake craniotomy in a bilingual patient. J Clin Neurosci 21 (8):1469–1470. doi: 10.1016/j.jocn.2013.11.053 [DOI] [PubMed] [Google Scholar]
- 80.Bello L, Acerbi F, Giussani C, Baratta P, Taccone P, Songa VJN (2006) Intraoperative language localizationin multilingual patients with gliomas. 59 (1):115–125 [DOI] [PubMed] [Google Scholar]
- 81.Cervenka MC, Boatman-Reich D, Ward J, Franaszczuk PJ, Crone NJFihn (2011) Language mapping in multilingual patients: electrocorticography and cortical stimulation during naming. 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erturk Cetin O, Isler C, Uzan M, Ozkara C (2017) Epilepsy-related brain tumors. Seizure 44:93–97. doi: 10.1016/j.seizure.2016.12.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: This 32-year-old right-handed, Hispanic male presented with a history of seizures and was diagnosed with a left frontal lobe lesion, for which he underwent a near-total resection back in 2013. The pathology was consistent with WHO grade II Oligodendroglioma, IDH-mutant, and 1p19q co-deleted. The recommendations were for him to continue to monitor the lesion with a serial of MRI scans, which remained stable. His most recent scan in February 2020 revealed left frontal expected post-surgical changes, with an increase in the surrounding T2/FLAIR hyperintensity signals with slight fullness of the parenchyma at the superior and inferior aspects. These findings were consistent with the evidence of disease progression vs. recurrence. The patient was taken to the operating room for a left-sided awake craniotomy in a supine position; a curvilinear skin incision was done, and the musculocutaneous flap was elevated after removal of the previous titanium plates and miniscrews. Two burr holes were placed and connected using a footplate, and the bone flap was removed. The dura was opened in a curvilinear fashion. Ojemann stimulator and the circular grid was used for cortical brain mapping; at the same time, neuropsychological testing in both English and Spanish was conducted. A prolonged and after-discharge and focal electrographic seizure was detected and was aborted using cold water irrigation. Tumor resection proceeded in tandem with neuropsychological testing. The patient tolerated the surgery well with no issues or complications and was discharged on a postoperative day 3 after an unremarkable hospital course. The pathology was consistent with Oligodendroglioma, IDH-mutant, and 1p19q co-deleted (WHO grade II). The postoperative imaging showed gross total resection with expected postoperative changes.


