Abstract
“Candidatus Liberibacter asiaticus” (CLas) and Spiroplasma citri are phloem-limited bacteria that infect citrus and are transmitted by insect vectors. S. citri causes citrus stubborn disease (CSD) and is vectored by the beet leafhopper in California. CLas is associated with the devastating citrus disease, Huanglongbing (HLB), and is vectored by the Asian citrus psyllid. CLas is a regulatory pathogen spreading in citrus on residential properties in southern California and is an imminent threat to spread to commercial citrus plantings. CSD is endemic in California and has symptoms in citrus that can be easily confused with HLB. Therefore, the objective of this study was to develop a multiplex qPCR and duplex droplet digital PCR (ddPCR) assay for simultaneous detection of CLas and S. citri to be used where both pathogens can co-exist. The multiplex qPCR assay was designed to detect multicopy genes of CLas—RNR (5 copies) and S. citri–SPV1 ORF1 (13 copies), respectively, and citrus cytochrome oxidase (COX) as internal positive control. Absolute quantitation of these pathogens was achieved by duplex ddPCR as a supplement for marginal qPCR results. Duplex ddPCR allowed higher sensitivity than qPCR for detection of CLas and S. citri. ddPCR showed higher tolerance to inhibitors and yielded highly reproducible results. The multiplex qPCR assay has the benefit of testing both pathogens at reduced cost and can serve to augment the official regulatory protocol for CLas detection in California. Moreover, the ddPCR provided unambiguous absolute detection of CLas and S. citri at very low concentrations without any standards for pathogen titer.
Introduction
Citrus is severely affected by fastidious vascular colonizing bacteria such as “Candidatus Liberibacter asiaticus” (CLas) and Spiroplasma citri (S. citri). The fastidious bacteria are introduced directly into phloem sieve tubes by phloem feeding insect vectors. CLas is a Gram-negative, α –proteobacterium [1] associated with the devastating citrus disease Huanglongbing (HLB), known as citrus greening. CLas is transmitted by the Asian citrus psyllid (ACP), Diaphorina citri Kuwayama (Hemiptera: Psyllidae). HLB is widely being distributed in Asia, South Africa, Central America, South America and some parts of United States viz., Florida, Texas and California [2]. S. citri is a wall-less Gram-positive bacteria that causes citrus stubborn disease (CSD) [3]. CSD is an endemic disease and widely distributed in semi-arid regions of California, where citrus is grown mostly as an irrigated crop [4]. S. citri is transmitted by the beet leafhopper, Neoaliturus (Circulifer) tenellus (Baker) (Hemiptera: Cicadellidae) [5] in the United States and Circulifer haematoceps (Mulsant & Rey) (Hemiptera: Cicadellidae) [6] in the Mediterranean region.
HLB and CSD have latent periods of several months to a year or more. Symptoms of HLB and CSD can be easily confused with each other and nutritional disorders [7] (Fig 1). In general, both the diseases are difficult to diagnose and differentiate at the early stages of infection. Fruit symptoms include irregular shape or small lopsided fruits with varying size and maturity on the same tree. During later stages of infection, the plant shows twig decline, stunted growth, low yield and in case of HLB, eventually leads to death of tree (Fig 1).
Fig 1.
Comparison of qPCR-confirmed Washington navel tree infected with Huanglongbing (HLB) (Left) and Citrus stubborn disease (CSD) (Right). (A) CLas infected tree in suburban southern California; (B) Blotchy mottle leaves and distorted fruits symptoms of HLB. (C) S. citri infected tree (left) next to a healthy tree (right) in a field in central California; (D) Chlorotic leaves with shortened internodes and (E) smaller misshapen fruit, with seasonal stylar-end greening typical of CSD. HLB pictures provided by Magally Luque-Williams, CDFA.
Detection of these diseases are challenging due to seasonal fluctuation and sporadic distribution of bacterial titer within the tree [8–10]. The spread of HLB into commercial citrus trees and presumptive co-infection with S. citri is eminent with the widespread of distribution of ACP and establishment of HLB in residential properties of southern California. Although CSD reduces tree vigor and contributes to loss of production, dual infection may cause a more rapid and deadly tree demise. In a greenhouse study, coinfection of these bacterial pathogens led to severe yellowing, dieback symptoms and later the plant died within 18 months post inoculation (dpi) in sweet orange, while the plants infected only with CLas or S. ctiri survived during the period observed [11].
A grower-funded citrus pest detection program (CPDP) surveys the commercial citrus in central California. HLB survey is of high priority to CPDP. Field inspectors visually inspect every tree on the perimeter of a grove for HLB symptoms and the ACP. Since HLB symptoms are similar to CSD symptoms and CSD is endemic in survey areas of CPDP, accurate diagnosis of HLB and CSD is very critical for implementation of timely control measures. Misdiagnosis of these diseases can lead to false corrective measures and unnecessary regulatory actions. Therefore, a multiplex detection of two bacterial pathogens is needed to distinguish these pathogens in a timely and cost-effective manner. Nucleic acid-based techniques such as real time quantitative PCR (qPCR) and droplet digital PCR (ddPCR) are currently available tools for early detection of CLas and S. citri in singleplex reactions. Detection of CLas utilizes 16S and RNR genes either by qPCR or ddPCR [12–15], whereas, S. citri was detected using spiralin SP1 and prophage ORF1 genes by qPCR or ddPCR [4, 10, 16, 17]. In this study, a multiplex qPCR assay was developed and validated for simultaneous detection of CLas and S. citri and included the citrus COX gene as an internal control. In addition, a duplex ddPCR was developed for absolute quantification of CLas and S. citri at very low copy numbers without the use of a standard curve.
Material and methods
Pathogens and DNA isolation
Citrus tissues infected with CLas and S. citri were obtained from the Contained Research Facility, University of California, Davis; ARS-USDA, Parlier and citrus fields in the San Joaquin Valley. Total DNA was extracted from citrus tissues by the Cetrimonium bromide (CTAB) method [18]. Nucleic acid quality and quantity was measured using Qubit 3.0 (Thermo Fisher Scientific, USA).
Primers and probes
Primers and probes used in qPCR and ddPCR are listed in Table 1. TaqMan probes were synthesized by labeling the 5’ terminal nucleotide with 6-carboxy-fluorescein (FAM), VIC and Texas Red for RNR, ORF1 and COX genes, respectively, and the 3’ terminal nucleotide with Minor groove binder/nonfluorescent quencher (Thermo Fisher Scientific, USA).
Table 1. Primer and probe sequences used for detection of “Candidatus Liberibacter asiaticus” and Spiroplasma citri.
| Organism | Target Gene | Primer/Probe name | Sequence (5’-3’) | Amplicon length | Reference |
|---|---|---|---|---|---|
| “Candidatus Liberibacter asiaticus” | nrdB, β-subunit of ribonucleotide reductase (RNR) | RNR F | CATGCTCCATGAAGCTACCC | 80 bp | [13] |
| RNR R | GGAGCATTTAACCCCACGAA | ||||
| RNR P | 6FAM/CCTCGAAATCGCCTATGCAC/ | ||||
| MGB/NFQ | |||||
| Spiroplasma citri | SPV1 ORF1 | ORF1 F | TGGCAGTTTTGTTTAGTCATCC | 190 bp | [10] |
| Prophage (ORF 1) | ORF1 R | GGGTCTAAACGCCGTTAAAGT | |||
| ORF1 P | VIC/TTGGGTTTGGTTATTCCATT/ MGB/NFQ | ||||
| Citrus | Cytochrome oxidase subunit 1 (COX)a | COX F | GTATGCCACGTCGCATTCCAGA | 68 bp | [12] |
| COX R | GCCAAAACTGCTAAGGGCATTC | ||||
| COX-P | TexasRed/ATCCAGATGCTTACGCTG G/MGB/NFQ |
aInternal control gene for multiplex qPCR assay.
Multiplex quantitative PCR
The multiplex qPCR-based detection of CLas and S. citri was carried out in duplex and triplex assay. Duplex qPCR assays are currently used for routine detection of CLas and S. citri with RNR/COX and ORF1/COX primers and probes, respectively. The triplex qPCR assay was developed in this study to detect RNR/ORF1/COX genes using tenfold serial dilutions of dually infected citrus DNA. The efficiency of triplex qPCR assay was later compared with duplex assay. The primer and probe concentration in triplex reaction mixture for RNR F/R was 0.15 μM and 0.08 μM, respectively; ORF1 F/R and probe was 0.30 μM and 0.15 μM, respectively; COX F/R was 0.30 μM and 0.15 μM, respectively. The reaction mixture contained 10 μl of 2x Perfecta qPCR ToughMix with low ROX and 1 μl of infected citrus DNA template at ~400 ng/μl. The qPCR was performed in CFX96 Real-Time System (Bio-Rad, USA). The thermal cycling consisted of initial denaturation of 3 min at 95°C, followed by 40 cycles of 95°C for 15 sec and 63°C for 40 sec. Two sets of duplex qPCR reactions were performed for detection of RNR/COX and ORF1/COX using tenfold serial dilutions of CLas and S. citri infected DNA, respectively.
Plasmid construction
The RNR (80 bp) gene was amplified from DNA extracted from HLB-infected citrus leaves using RNR F/R primers. The prophage gene ORF1 (533 bp) was amplified from S. citri DNA Prophage ORF1 F/R primers. The amplicons were ligated in pGEM-T Easy vector (Promega) and transformed in JM-109 (Promega). The positive plasmids were linearized using SpeI restriction enzyme (New England Biolabs, UK) and the concentrations were measured using dsDNA high sensitivity assay kit in Qubit 3.0 fluorometer (Thermo Fisher Scientific, USA). Ten-fold serial dilutions of the linearized plasmids were made to assess analytical sensitivity, linearity, and dynamic range of ddPCR in singleplex and duplex ddPCR assays.
Thermal gradient optimization of duplex ddPCR
The optimal annealing temperature for duplex ddPCR assay with RNR and ORF1 primers was determined by a thermal gradient in the S1000TM Thermal cycler (Bio-Rad, USA). The temperature range were 53°C, 53.7°C, 55.1°C, 57°C, 59.2°C, 61.1°C, 62.4°C and 63°C. The reaction mixture contained the same amount of CLas and S. citri DNA with primers/probes concentrations of 0.9 μM/ 0.25 μM. Optimization of duplex ddPCR assay with RNR and ORF1 primers was achieved using linearized plasmids. The duplex ddPCR reaction mixture (20 μl) contained 2x ddPCR Supermix for probes (no dUTP) (BioRad, USA), 0.9 μM of ORF1 and RNR primers, 0.25 μM of ORF1 and RNR probes, and 1 μl of plasmid DNA. The singleplex ddPCR reaction contained the same amount of primers and probes as in duplex assay for CLas or S. citri. The 20 μl reaction mixture and 70 μl of droplet generation oil was used for generation of emulsion droplets in QX 200 droplet generator (BioRad, USA) using DG8 cartridge. The droplet emulsions were loaded in a 96-well PCR plate and sealed with pierceable foil using a PX1 PCR plate sealer (Bio-Rad, USA). PCR amplification was carried out in a C1000TM thermal cycler. The thermal cycling conditions consisted of 10 min initial denaturation at 95°C, followed by 40 cycles of denaturation at 94°C for 30 sec and annealing/extension at 57°C for 1 min, with a ramp of 2°C/sec. and a final 10 min incubation at 98°C for enzyme deactivation. After thermal cycling, the plate was placed in a QX 200 droplet reader (Bio-Rad, USA) for analyzing each individual droplet by a detector.
Assessing inter-assay and intra-assay variability of duplex ddPCR
The inter-assay and intra-assay variation of duplex ddPCR assay was carried out using CLas and S. citri, dual infected leaf DNA samples. The inter-assay variation was determined by measuring the copy number of CLas and S. citri between three different assays in triplicate. The intra-assay variation was determined by measuring the copy number of CLas and S. citri within the assay in three replications. Coefficient of Variation (CV) was calculated by standard deviation/mean.
Estimation of tolerance to residual matrices on duplex ddPCR
The tolerance of duplex ddPCR assay for inhibitors in citrus leaf samples was evaluated with reactions containing different concentration of inhibitors in citrus. The reaction was spiked with same amount of S. citri and CLas plasmids DNA (ORF1 and RNR) and different quantities of citrus healthy leaf extract (1 to 5μl). The effect of inhibitors in leaf extract were assessed relative to the mean measured signals in each sample with no added inhibitors. Healthy citrus leaf extracts were used to estimate the tolerance of duplex ddPCR assays.
Data analysis
The qPCR data were analyzed by the BioRad CFX Manager 3.1 software. The ddPCR data were analyzed with QuantaSoft analysis software version 1.7 (Bio-Rad, USA). The positive droplets with amplified products were discriminated from negative droplets by applying a threshold above the negative droplets. Reactions with more than 10,000 accepted droplets per well were used for analysis. The linear regression and P-value of the ddPCR assay were determined by plotting the measured copies of ddPCR and comparing them with expected values of serial dilution of plasmid DNA, CLas/S. citri infected citrus DNA in Excel. The Poisson error and total error was obtained using QuantaSoft software.
Results
Real time quantitative PCR
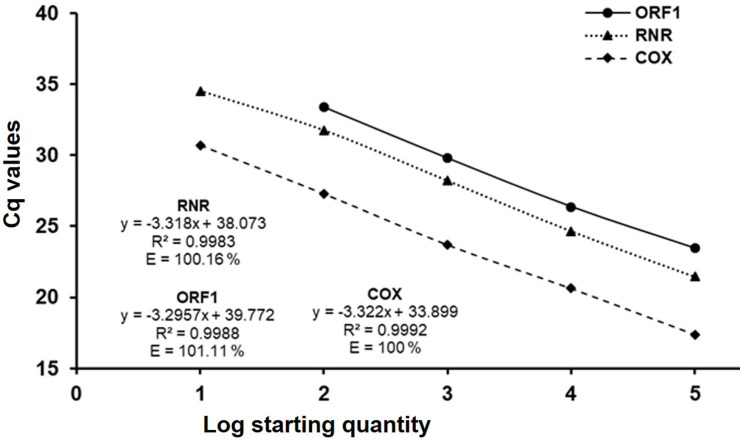
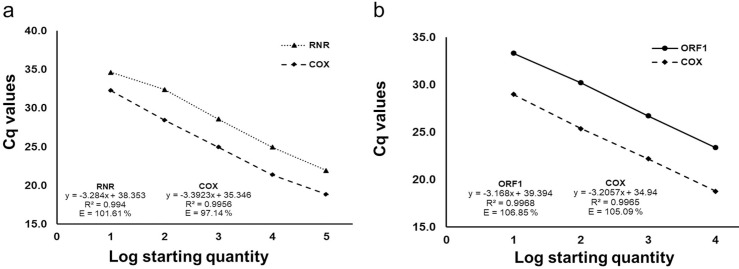
The triplex qPCR assay regression curve showed good linearity with ten-fold dilutions of CLas and S. citri infected leaf DNA for RNR (R2 = 0.9983), ORF1 (R2 = 0.9988) and COX (R2 = 0.9992). The PCR efficiency was 100.16, 101.11 and 100 for RNR, ORF1 and COX, respectively (Fig 2). Duplex assay for detection of CLas showed a linearity of 99.4% and 99.5%, with an efficiency of 101.6% and 97.14% for RNR and COX, respectively (Fig 3A). S. citri duplex detection assay showed linearity of 106.85% and 105.9% for ORF1 and COX, respectively (Fig 3B). Comparison of triplex and duplex assay Ct values for RNR, ORF1 and COX values did not show significant changes for detection of CLas and S. citri. Detection limit of both triplex and duplex assays was 0.04 ng of total DNA from dually infected with CLas and S. citri.
Fig 2. Calibration curve of triplex qPCR assays with tenfold serial dilution of “Candidatus Liberibacter asiaticus” and Spiroplasma citri infected DNA (400 ng to 0.004 ng).
Gene specific targets were RNR (dotted line) and ORF1 (unbroken line) for detection of CLas and S. citri, respectively, and COX (dash line) for citrus DNA.
Fig 3. Calibration curve of duplex qPCR assays with tenfold serial dilution of “Candidatus Liberibacter asiaticus” and Spiroplasma citri DNA (400 ng to 0.004 ng) performed using RNR (dotted line), ORF1 (unbroken line) and COX (dash line) gene specific primers.
(a) Detection of CLas by duplex qPCR assay using RNR and COX gene; (b) Detection of S. citri by duplex qPCR assay using ORF1, and COX gene for citrus DNA.
Optimization of duplex ddPCR
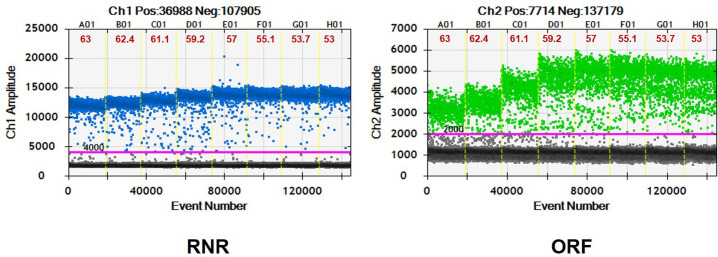
The optimum annealing temperature for CLas and S. citri was selected based on the fluorescence amplitude that differs from the positive and negative droplets in duplex ddPCR assay. An annealing temperature of 57°C was chosen for RNR and ORF1 gene primers and probes for the subsequent duplex ddPCR experiments (Fig 4).
Fig 4. Thermal gradient droplet digital PCR for optimizing annealing temperature.
(A) RNR of “Candidatus Liberibacter asiaticus”; (B) ORF1 of Spiroplasma citri. Eight ddPCR reactions with an annealing temperature gradient ranging from 53°C to 63°C are divided by vertical dotted yellow lines. The pink line is the threshold, above which are positive droplets (blue/Green) and below are negative droplets (gray) without any target DNA.
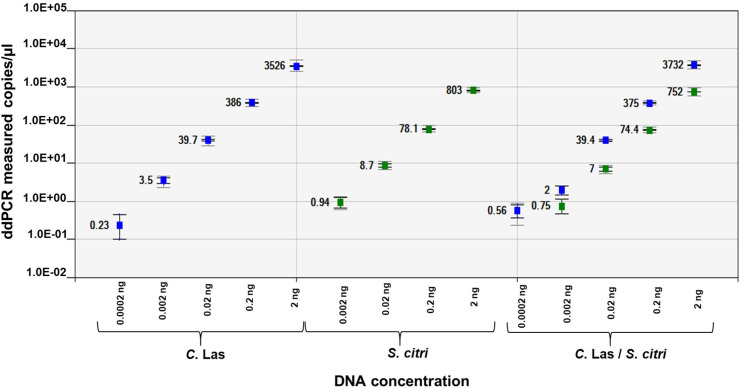
The linear regression curve was obtained by plotting the Log10 transformed values between the serially diluted plasmid DNA and expected values in singleplex and duplex ddPCR assays. The RNR and ORF1 plasmid DNA showed R2 = 1 in singleplex assay and R2 = 1 and R2 = 0.999 respectively, in duplex assay. The sensitivity of RNR and ORF1 was 3 copies and 2 copies in singleplex assay. The sensitivity of RNR and ORF1 was 2 copies in duplex ddPCR assay (Fig 5; S1 Table).
Fig 5. Linear regression of the singleplex and duplex ddPCR assays for RNR plasmid DNA and ORF1 plasmid DNA for detection of “Candidatus Liberibacter asiaticus” (CLas) and Spiroplasma citri (S. citri) respectively.
The Pearson correlation coefficient of singleplex RNR plasmid DNA regression curve (y = 0.8521x-11.405) is 1 and ORF1 plasmid DNA (0.7658x + 6.2768) is 1, respectively. Pearson correlation coefficient of duplex RNR plasmid DNA regression curve (0.871x - 4.3816) is 1 and ORF1 plasmid DNA (0.7414x + 28.637) is 0.999, respectively. The inner error bars indicate the Poisson 95% confidence interval (CI) and the outer error bars show the total 95% CI of replicates. (P<0.0001).
Singleplex and duplex ddPCR assay of CLas and S. citri dual infected DNA showed five and four orders of magnitude respectively, between the target input amounts and ddPCR measured values. The RNR and ORF1 primers showed good linearity of R2 = 1 and R2 = 0.999 respectively, in singleplex assay and R2 = 1 in duplex assay. The sensitivity of CLas in dual infected citrus leaf DNA was 5 copies and 11 copies in singleplex and duplex ddPCR assay, respectively. The sensitivity of S. citri in dual infected citrus leaf DNA was 19 copies and 15 copies in singleplex and duplex ddPCR assay, respectively (S2 Table). There was an excellent correlation (P<0.0001) in copy number of CLas and S. citri between singleplex and duplex ddPCR assays with linearized plasmids and dual infected citrus leaf DNA (Fig 6).
Fig 6. Linear regression of the singleplex and duplex ddPCR assays for detection of “Candidatus Liberibacter asiaticus” (RNR gene) and Spiroplasma citri (ORF1 gene) in dually infected citrus leaf DNA.
The Pearson correlation coefficient of singleplex RNR and ORF 1 DNA regression curve is 0.999 and 1, respectively. Pearson correlation coefficient of duplex RNR and ORF1 DNA regression curve is 1. The inner error bars indicate the Poisson 95% confidence interval (CI) and the outer error bars show the total 95% CI of replicates. (P<0.0001).
Repeatability and reproducibility of duplex ddPCR assay
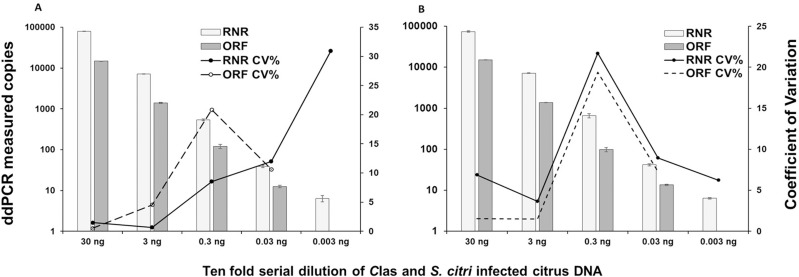
The absolute quantification of CLas and S. citri in ten-fold dilution of dual infected citrus leaf DNA produced good repeatability (intra-assay) and reproducibility (inter-assay). The co-efficient of variation of RNR in inter-assay was better compared to intra-assay especially in low titer samples. The co-efficient of variation of ORF1 was better in inter and intra assay (Fig 7).
Fig 7.
Intra-assay (A) and Inter-assay (B) variation of duplex ddPCR assay for detection of RNR gene of “Candidatus Liberibacter asiaticus” and ORF1 gene of Spiroplasma citri in ten-fold dilutions of dual infected citrus leaf DNA. Bar represents the average of triplicate ddPCR values of each dilutions. CV means coefficient of variation.
Influence of residual matrices on duplex ddPCR assay
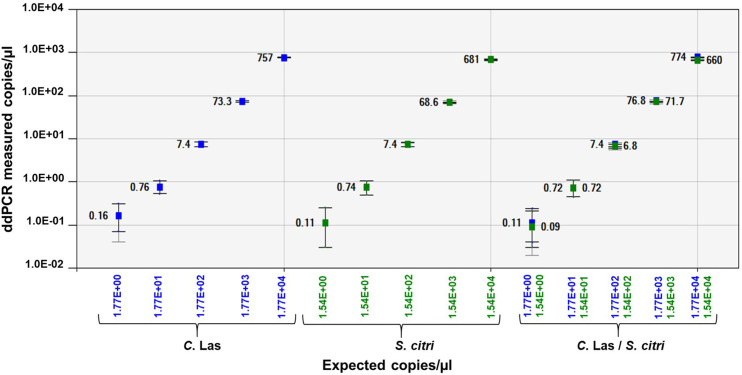
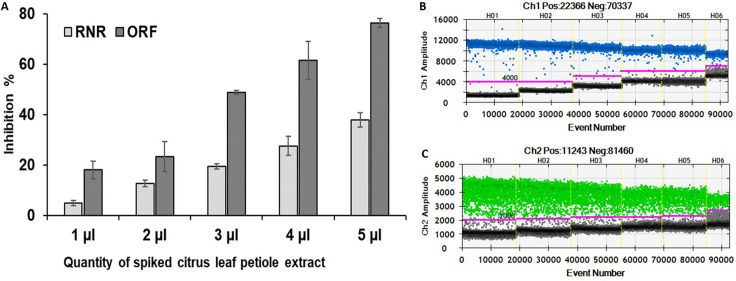
The tolerance of duplex ddPCR assay to citrus leaf extract was estimated using RNR and ORF1 primers (Fig 8). The fluorescent signals of positive droplets and negative droplets gradually decreased and increased respectively, with the increasing amount of citrus extract for both CLas and S. citri. In contrast, the other parameter affected by the presence of the residual matrices for the ddPCR were S. citri and CLas titer, which decreased with the increasing amount of citrus extract.
Fig 8. Influence of citrus leaf petiole extract on quantification of “Candidatus Liberibacter asiaticus” and Spiroplasma citri by duplex ddPCR assays for RNR gene and ORF1 gene respectively.
(A) ddPCR reaction mixture was spiked with different quantity of citrus leaf petiole extract and equal amount of citrus leaf DNA. Error bars denote standard error of inhibition between three replicates of each reaction. (B, C) one dimensional plot showing only one of three replications for RNR gene and ORF1 gene with citrus leaf petiole extracts.
Discussion
CLas and S. citri are pathogens that colonize the phloem tissues and induce similar symptoms in citrus. Both pathogens are unevenly distributed and the seasonal fluctuation in titer makes the early detection of pathogens challenging. Misdiagnosis of CLas would lead to costly regulatory actions and impact the grower. RNA and DNA targets multiplexing by qPCR analysis has been shown for citrus pathogens [19–24]. Duplex ddPCR has been reported for simultaneous detection of two gene targets for unambiguous detection of CLas [14] and S. citri [10]. However, concurrent detection of CLas and S. citri by qPCR and ddPCR is lacking. In this study, sensitive multiplex qPCR technique was developed for simultaneous detection of these two pathogens using RNR (CLas), ORF1 (S. citri) and COX (citrus internal control) gene primers. A duplex ddPCR was also developed for absolute quantification and detection of these bacterium in dually infected samples.
In areas where both HLB and CSD occur, the multiplex assay developed in this study tests for both pathogens simultaneously without sacrificing efficacy and loss of sensitivity. Inconclusive results from qPCR Ct values at the upper limits is always a critical concern. In such cases, duplex ddPCR assay can provide unambiguous detection and absolute quantification of CLas, S. citri or both without need of standards [10, 14]. ddPCR was more sensitive compared to qPCR with citrus tissue dually infected with CLas and S. citri. qPCR detected CLas and S. citri up to 0.004 ng of total DNA, whereas ddPCR detected up to a dilution of 0.002 ng for S. citri and 0.0002 ng for CLas.
While ddPCR is robust, reliable, and tolerant to PCR inhibitors, the cost for ddPCR is ~2.3x greater than qPCR due to expendables like gaskets, cartridges and droplet oil needed to test samples each 96-well plate. Additionally, the time required for a ddPCR run is ~2.3x longer than qPCR to assay the same samples in a 96-well plate. Notably, qPCR is a real time assay; whereas ddPCR is an end point assay. Therefore, the best application for ddPCR is as a secondary test to confirm results of questionable qPCR results.
Previous qPCR and ddPCR methods have been essentially for CLas [12, 14] and S. citri [10, 17]. This report combines these protocols to test for both pathogens in a duplex reaction from the same sample and well. Multiplex detection of CLas and S. citri will be useful in California as HLB spreads from established citrus trees in residential properties to commercial citrus groves. High incidence of CSD has been reported to range from 4 to 60% in certain San Joaquin Valley orchards based on qPCR results [16]. Additional S. citri infected trees will likely be found with additional sampling to account for seasonality and erratic pathogen titer. Moreover, S. citri is not a regulated pathogen in California and CSD-affected citrus trees are rarely removed. Therefore, presence of CSD must be accounted for during testing of CLas in HLB-suspect trees where the outcome is eradication or rouging of infected trees.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
We thank Robert DeBorde (United States Department of Agriculture-Agricultural Research Service, San Joaquin Valley Agricultural Sciences Center, Parlier, CA) for technical assistance. Mention of trade names or commercial products in this publication is solely for providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
RY 5300-191 Citrus Research Board RY Yok-18 California citrus Nursery Board RY 2034-22000-013-10D United States Department of Agriculture, Agricultural Research Service, Base Project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jagoueix S, Bové JM, Garnier M. The phloem-limited bacterium of greening disease of citrus is a member of the alpha subdivision of the Proteobacteria. Int. J. Syst. Bacteriol. 1994; 44:379–386. 10.1099/00207713-44-3-379 . [DOI] [PubMed] [Google Scholar]
- 2.Wang N, Trivedi P. Citrus huanglongbing: a newly relevant disease presents unprecedented challenges. Phytopathology 2013; 103:652–65 10.1094/PHYTO-12-12-0331-RVW [DOI] [PubMed] [Google Scholar]
- 3.Saglio P, L’Hospital M, Laflèche D, Dupont G, Bové JM, Tully JG, et al. Spiroplasma citri gen. and sp. nov.: a mycoplasmalike organism associated with ‘‘stubborn” disease of citrus. Int. J. Syst. Bacteriol. 1973; 23:191–204 [Google Scholar]
- 4.Yokomi RK, Mello AFS, Saponari M, Fletcher J. Polymerase chain reaction-based detection of Spiroplasma citri associated with citrus stubborn disease. Plant Dis. 2008; 92:253–260 10.1094/PDIS-92-2-0253 [DOI] [PubMed] [Google Scholar]
- 5.Liu HY, Gumpf DJ, Oldfield GN, Calavan EC. The relationship of Spiroplasma citri and Circulifer tenellus. Phytopathology 1983; 73:585–590 [Google Scholar]
- 6.Bové JM, Fos A, Lallemand J, Raie A, Ali Y, Ahmed N, et al. Epidemiology of Spiroplasma citri in the Old World, p 295–299. In. Proc. 10th Conf. Int. Organ. Citrus Virol. IOCV, Riverside, CA: 1988; https://escholarship.org/uc/item/3j51z50h [Google Scholar]
- 7.Pagliaccia D, Shi J, Pang Z, Hawara E, Clark K, Thapa SP, et al. A pathogen secreted protein as a detection marker for citrus huanglongbing. Frontiers in microbiology. 2017; 26:8:2041 10.3389/fmicb.2017.02041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauer AV, Zanutto CA, Nocchi PT, Machado MA, Bock CH, Nunes WM. Seasonal variation in populations of ‘Candidatus Liberibacter asiaticus’ in citrus trees in Paraná state, Brazil. Plant Disease. 2015; 5:99:1125–32 10.1094/PDIS-09-14-0926-RE [DOI] [PubMed] [Google Scholar]
- 9.Hajeri S, Yokomi R. K. Reliable Sampling tissue and seasonality for consistent detection of ‘Candidatus Liberibacter asiaticus’ by qPCR. Curr Agri Res 2019; 8. 10.12944/CARJ.8.1.01 [DOI] [Google Scholar]
- 10.Maheshwari Y, Selvaraj V, Hajeri S, Yokomi R. Application of droplet digital PCR for quantitative detection of Spiroplasma citri in comparison with real time PCR. PLoS ONE 2017; 12(9): e0184751. 10.1371/journal.pone.0184751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osman F, Pagliaccia D, Vidalakis G. Real-time PCR co-detection of ‘Candidatus Liberibacter’ species and S. citri. Citrograph 2017; 8:64–68 [Google Scholar]
- 12.Li WB, Hartung JS, Levy L. Quantitative real-time PCR for detection and identification of ‘Candidatus Liberibacter’ species associated with citrus Huanglongbing. J. Microbiol. Meth. 2006; 66:104–115 [DOI] [PubMed] [Google Scholar]
- 13.Zheng Z, Xu M, Bao M, Wu F, Chen J, and Deng X. Unusual Five Copies and Dual Forms of nrdB in ‘Candidatus Liberibacter asiaticus’: Biological Implications and PCR Detection Application. Scientific Reports 2016; 6:39020 10.1038/srep39020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selvaraj V, Maheshwari Y, Hajeri S, Chen J, McCollum TG, Yokomi R. Development of a duplex droplet digital PCR assay for absolute quantitative detection of “Candidatus Liberibacter asiaticus”. PLoS ONE 2018; 13:e0197184 10.1371/journal.pone.0197184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong X, Liu X, Lou B, Zhou C, Wang X. Development of a sensitive and reliable droplet digital PCR assay for the detection of ‘Candidatus Liberibacter asiaticus’. J. Integrative Agric. 2018; 17:483–487. [Google Scholar]
- 16.Yokomi RK, Sisterson M. Validation and comparison of a hierarchal sampling plan for estimating incidence of citrus stubborn disease. In: Proc. 18th Conf. Int. Organ. Citrus Virol. IOCV, Riverside, CA.2011; http://iocv.org/proceedings/eighteen/Yokomi_and_Sisterson.pdf. [Google Scholar]
- 17.Wang X, Doddapaneni H, Chen J, Yokomi RK. Improved real-time PCR diagnosis of citrus stubborn disease by targeting prophage genes of Spiroplasma citri. Plant Disease. 2015; 99:149–154 10.1094/PDIS-06-14-0572-RE [DOI] [PubMed] [Google Scholar]
- 18.Doyle JJ. DNA protocols for plants. In: Molecular Techniques in Taxonomy. Hewitt G, Johnson AWB, Young JPW. (eds.). NATO ASI Ser. H, Cell Biol. 57. Springer-Verlag, Berlin. 1991; 283±293. [Google Scholar]
- 19.Roy A, Fayad A, Barthe G, and Brlansky RH. 2005. A multiplex polymerase chain reaction method for reliable, sensitive and simultaneous detection of multiple viruses in citrus trees. J. Virol Meth. 129:47–55 10.1016/j.jviromet.2005.05.008 [DOI] [PubMed] [Google Scholar]
- 20.Adkar-Purushothama C, Quaglino F, Casati P, Bianco PA. Reverse transcription-duplex-polymerase chain reaction for simultaneous detection of Citrus tristeza virus and ‘Candidatus Liberibacter’ from citrus plants. J Plant Dis. and Prot. 2010; 117:241–24 [Google Scholar]
- 21.Loconsole G, Önelge N, Yokomi RK, Abou Kubaa R, Savino V, Saponari M. Rapid differentiation of citrus Hop stunt viroid variants by real-time RT-PCR and high resolution melting analysis. Mol. Cell. Probes 2013; 27:221–229 10.1016/j.mcp.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 22.Osman F, Dang T, Bodaghi S. Vidalakis G. One-step multiplex RT-qPCR detects three citrus viroids from different genera in a wide range of hosts. J. Virol. Meth. 2017; 245:40–52 [DOI] [PubMed] [Google Scholar]
- 23.Saponari M, Loconsole G, Liao H-H, Jiang B, Savino V, Yokomi RK. Validation of high-throughput real time polymerase chain reaction assays for simultaneous detection of invasive citrus pathogens. J. Virol. Methods 2013; 193:478–486 10.1016/j.jviromet.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 24.Saponari M, Zicca S, Loconsole G, Navarro Beatriz, Di Serio F. 2019. Detection of Citrus tristeza virus and Coinfecting Viroids, 67–78. In Catara AF, Bar-Joseph M, and Licciardello. (eds.). Citrus Tristeza Virus: Methods and Protocols, Methods in Molecular Biology vol. 2015 10.1007/978-1-4939-9558-5_6 Springer Nature [DOI] [PubMed] [Google Scholar]