Abstract
Mosquito specific viruses such as densonucleosis viruses (“densoviruses”) have long been suggested as alternative mosquito control agents in the face of increasing insecticide resistance. Densoviruses are very species-specific and have been found to infect many important mosquito species. While some strains are highly pathogenic, other strains are more benign. Densoviruses have been proposed as a way to reduce mosquito populations through pathogenic interactions, but genetic strategies such as viral paratrangenesis offer new approaches. As small single-stranded DNA viruses, densoviruses can be easily genetically modified for the expression of genes or non-coding RNAs. A growing literature and variety of techniques have shown the potential for the use of densoviruses in the control of mosquitoes or mosquito-borne pathogens as well as the usefulness of densoviruses as molecular tools for understanding mosquito biology.
Introduction
Densoviruses (DNVs), also known as densonucleosis viruses, are 18–22 nm non-enveloped icosahedral viruses in the family Parvoviridae. DNVs replicate in the nuclei of invertebrate hosts forming large cuboidal or circular inclusions [1]. The first described DNV was discovered as a pathogen of wax moth (Galleria mellonella) caterpillars in 1964, although infections previously described in mosquito larvae from California and Louisiana were likely caused by DNVs and not cytoplasmic polyhedrosis viruses as attributed at the time [2–6]. The pathology caused by DNVs was originally described as “virose à noyaux denses” or “viral disease in dense cores” and viral agents capable of causing these morphology changes became known as densonucleosis viruses, or “densoviruses” for short [3].
DNVs have been identified in many invertebrate species including crustaceans and representatives from at least five insect orders [7]. The first mosquito-specific densovirus (MDV), known as Aedes aegypti densovirus (AaeDNV), was found in Ae. aegypti larvae in a Russian lab colony in 1972 [8]. To date, MDVs have been isolated from multiple mosquito species including important disease vectors such as Ae. aegypti, Aedes albopictus, Anopheles gambiae, Anopheles sinensis, Culex pipiens, and Culex pipiens pallans[7–11]. DNVs are common in wild insect populations and more are being discovered through the use of high throughput and shotgun sequencing [12,13]. Many DNVs also persist as chronic infections of insect cell culture lines and therefore sequencing of these lines may reveal additional isolates [14–16]. Most MDVs belong to the genus Brevidensovirus, however, the Cx. pipiens densovirus (CpDNV) has been found to be more serologically similar to Lepidopteran DNVs within the genus Densovirus and genomic data suggests that this MDV may even represent an entirely new genus [17,18].
MDVs infect all developmental stages of the mosquito and can cause larvae to appear sluggish and curved in shape [1,19]. Infected larvae can also have malformed segments and a white colored cuticle with dark shiny areas of melanization [1]. In many cases, infected larvae die before reaching adulthood and MDV strains vary in their ability to produce viable infected adults [9,20]. MDVs can spread horizontally through larval water or between adults during venereal contact (Figure 1) [21,22]. As surface sterilization of mosquito eggs is unable to eliminate MDV infected larvae there appears to be some degree of transovarial transmission from infected female mosquitoes to offspring [23,24]. In fact, vertical transmission rates following surface sterilization of Ae. aegypti eggs have been reported to be as high as 61.7% for AaeDNV [24]. MDVs also vary in their capacity to spread and persist in the environment. In wild populations of Ae. aegypti in Thailand, infections with Thai strain densovirus (AThDNV) as high as 44% have been reported [25]. In one large cage study, AaeDNV was shown to be transmitted to offspring and became established in previously uninfected oviposition sites [26]. The titers in these oviposition sites were too low to cause mortality or influence egg laying, however, the high level of certain MDVs in the wild demonstrates that these viruses have potential as tools for mosquito or pathogen control and it is possible that MDVs could be made more effective using genetic engineering [25].
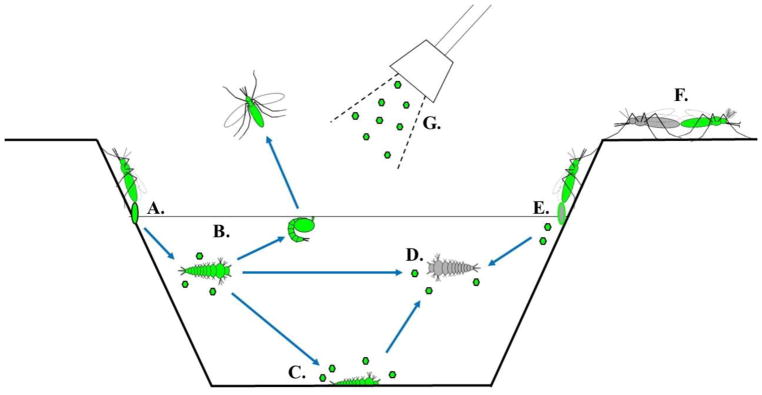
Figure 1. Generalized mosquito densovirus life cycle.
Green indicates DNV virions (hexagons) or infected mosquito lifestages. Gray indicates uninfected mosquito lifestages. A). DNVs can be transovarially transmitted between infected female mosquitoes and their eggs. B). Transstadial transmission of DNV between mosquito stages and throughout the mosquito lifecycle can lead to infected adults. Infected larval stages can shed DNV particles into the environment. C). DNV infection can result in larval death and dead larvae can shed virions into the larval environment. D). DNVs can be transmitted horizontally to uninfected larvae through the larval water. E). Female mosquitoes infected with DNV can deposit virions into the larval environment through egg coatings or secretions during oviposition. F). DNVs can also be transmitted venereally. G). Commercial preparations of DNVs could be applied to larval environments for the purpose of mosquito or pathogen control.
Experimental infections of larvae with MDV in the laboratory have been achieved through several methods. As microinjection is not an ideal option for larval infection due to the fragile nature of larval cuticle, other mechanisms including the use of homogenized infected larvae as food for uninfected larvae have been developed [4]. It has also been reported that larvae can be infected with MDV if placed in culture flasks containing an infected cell line, however, mortality is often high using this method due to overgrowth of bacteria [27]. Recently hatched larvae can also be infected by being placed in a mix of water and culture media or filtered cell lysate from infected cells [9,28]. Divalent cation concentrations have been found to influence MDV infection of larvae and levels of sodium chloride greater than 0.05M have been found to inhibit MDV infection [29]. This information could be critical for predicting the success of MDV application in varied natural habitats.
Mosquito control with wild-type densoviruses
MDVs vary greatly in their pathogenicity across mosquito species. Certain infections can lead to high larval mortality and slowed development times, while others are less pathogenic to larvae but can cause shortened adult lifespans [9,25,30,31]. Furthermore, high cytopathology in mosquito cell culture has not been found to be a true predictor of mortality in infected larvae [31,32]. In one study, Haemagogus equinus densovirus (HeDNV) was found to be highly cytopathic in an Ae. albopictus C6/36 cell line but was not as pathogenic in Ae. aegypti larvae whereas the opposite was true for the closely related AaeDNV [32].
Larval mortality rates induced by MDV infection vary considerably depending on the mosquito species and MDV strain used. The mortality rate of Ae. aegypti larvae infected with AThDNV has been reported to be 51%, yet Ae. albopictus larvae infected with the same viral strain had a mortality rate of 82% [25]. When a high concentration of this same AThDNV strain was used to infect Anopheles minimus, 15.5% mortality was observed [33]. One extensive study documented the pathogenicity of four strains of DNV (AalDNV, AaeDNV, AThDNV, APeDNV) against three strains of Ae. aegypti [34]. The same titer of each DNV strain caused mortality rates of greater than 80% within each Ae. aegypti strain [34]. Such high levels of pathogenicity and mortality are common for MDVs and one DNV isolated from a C6/36 Ae. albopictus cell line (C6/36DNV) has even been reported to cause larval mortalities of up 97.46% in Ae. albopictus [28].
Pathogenicity and prevalence of MDVs can also vary based on environmental factors such as temperature and rainfall, or with larval conditions such as larval density, method of infection, and length of exposure to MDV [30,35]. High concentrations of larvae and MDV has been shown to cause high levels of infection and larval mortality [30]. In field studies of infected An. minimus in Thailand, larval infection was correlated with rainfall two months prior [35]. The environmental factors influencing MDV efficacy have not been extensively studied and more research is needed before control programs employ these viruses.
While larval mortalities are difficult to compare across studies given differences in infection methods, environmental conditions, viral titer, and stage of larvae infected, it is clear that several strains of MDV possess the ability to significantly alter larval survival. The mechanism of MDV based larval mortality is unclear, however it appears that MDVs increase levels of cellular apoptosis [36]. Taking advantage of this natural mortality rate or increasing mortality through genetic engineering could decrease mosquito density. Mosquito population density can also be controlled through reducing rates of reproduction. Some studies have reported decreases in oviposition rate, fecundity, and egg hatch rate from Ae. aegypti females infected with AaeDNV [37]. Others reported no changes in fecundity when studying Ae. albopictus densovirus (AalDNV) in Ae. aegypti and AThDNV in An. minimus [21,33]. Although the ability to alter fecundity differs with MDV strain and mosquito species, a reduction in reproductive success could potentially lead to lower mosquito densities and reduced vectorial capacity.
While a decrease in overall mosquito density is appealing, methods that use this technique are often rapidly made obsolete due the high selection pressures exerted on the population undergoing this suppression. Shortening mosquito lifespan via late-life insecticides would lessening selection pressure by allow mosquitoes to reproduce as usual while hindering the ability of the mosquito to become infectious or to take multiple bloodmeals. A dose-dependent shortening of adult lifespan has been experimentally reported in Ae. aegypti infected with wild-type AaeDNV [37]. Genetically modifying other densoviruses such as the recently discovered An. gambiae densovirus (AgDNV) to act as a biological late-life insecticide targeting older adult An. gambiae has been proposed [38]. This method has heightened potential in An. gambiae, as AgDNV titers peak in 7–10 day old adults and the virus does not cause appreciable mortality in immatures [38].
In addition to altering lifespan, survival, and oviposition rates, MDVs also have the potential to influence mosquito co-infection dynamics and ability to transmit pathogens. Infection of Ae. albopictus mosquitoes with C6/36DNV leads to lower dengue virus strain 2 (DENV-2) titers and may reduce transmission to new hosts [28]. Certain MDVs may even be able to trigger superinfection exclusion or an anti-viral state in which secondary infections with another virus are inhibited. When C6/36 cells chronically infected with AalDNV were challenged with DENV-2 the cells were resistant to superinfection and had much slower DENV-2 virion production [39]. In another study, DENV-3 titers and percentage of infected cells were reduced at various timepoints in the presence of a MDV but through a less clear interaction [40]. MDV-based superinfection exclusion varies depending on the viruses tested and was not seen when C6/36 cells persistently infected with AaeDNV were exposed to the closely related HeDNV [32]. Secondary HeDNV infection still led to severe cell cytopathology and chronic AaeDNV infection did not appear to cause a true anti-viral state. In other cases, persistent and stable in vitro infections with MDVs and other viruses have been observed [41,42]. It is unclear if MDV strain differences account for these varied accounts or if there are specific interactions between MDVs and DENV. More research on DNV-based superinfection suppression is needed to determine the usefulness of MDVs for this type of control.
In relation to field deployment, AaeDNV has been the most extensively studied of the known MDV strains. AaeDNV was discovered in Russia in 1972 and was previously used in a commercial product called Viroden that consisted of homogenized infected Ae. aegypti larvae in a solution of phosphate-buffered saline and glycerol that could be applied to larval habitats [7,43]. While this product was not extensively used commercially, experiments conducted in small artificial ponds resulted in larval and pupal mortalities of 44–86% for mixed populations of native Ukrainian mosquitoes [7]. When applied to natural mosquito larval rearing habitats in a cold region of Russia, 59–76.1% mortality was observed [7]. When Viroden was tested in natural reservoirs in a warmer region of Tajikistan, 43–73% mortality was observed for native species [7]. In all cases, Viroden was applied as a spray to the surface of the water and the preparation was found to be highly stable and resistant to heating, variable pH, UV radiation, and many common environmental stressors [7,43,44]. Additionally, Viroden was found to have a small host range and in laboratory experiments the viral preparation was not infective to white mice, rats, guinea pigs, rabbits, chicken embryos, and cell cultures from warm-blooded animals [7,45]. Infectivity experiments conducted using AalDNV from Ae. albopictus C6/36 cells showed a similar level of target specificity and were unable to establish infections in mice, Drosophila melanogaster, Spodoptera littoralis, vertebrate cell lines (HeLa, BGM, Ma104), or Drosophila cell lines [46]. The Viroden studies demonstrated the effectiveness and target specificity of an applied DNV-based biopesticide in varied field conditions and climates against a variety of mosquito species. While most MDVs have not reached this level of field application, many MDVs have the potential to be used in a similar manner following appropriate testing or genetic modification (Figure 1).
Genetic information
Most MDVs have single stranded negative sense DNA genomes of approximately 4kbs in length, however, CpDNV genomes are ambisense and around 6kb in size [17,18,47]. All DNVs have terminal inverted repeat sequences at both the 5′ and 3′ ends that fold to form hairpins or Y-shaped structures and are essential for capsid packaging [7]. The DNV genome consists of two nonstructural (NS) genes and two viral protein (VP) or capsid genes flanked by inverted terminal repeats (ITRs). Three promoters corresponding to three open reading frames (ORFs) are found within the viral genome positive strand. A left promoter occurs prior to NS1, another mid promoter occurs within the left ORF, and the right promoter is located before the VPs [47]. The small size of the DNV genome has allowed researchers to insert the entire genome of various DNVs into infectious plasmid vectors. Once in a plasmid, the genome may be manipulated using traditional cloning techniques for the purpose of mosquito control or a strategy known as “paratransgenesis”, in which transgenic symbionts are used to alter host biology or ability of the host to transmit pathogens. Transgenic MDVs could be used to express anti-pathogen compounds, insecticidal molecules, or non-coding RNAs within mosquito hosts. Non-coding RNAs (ncRNAs) are RNA molecules that are not translated but instead act through RNA interference (RNAi) or other pathways to alter gene expression. In RNAi, certain ncRNAs of ~22 nucleotides, known as microRNAs (miRNAs), bind to areas of complementary in mRNA transcripts and block translation or lead to the degradation of mRNA transcripts and a reduction in subsequent gene expression. Various genetically modified MDVs have already been tested using some of these techniques.
Mosquito control with genetically modified densoviruses
To date, most genetic modification of densoviruses has been conducted using AaeDNV. In 1994, the first AaeDNV infectious clone known as pUCA was created by adding the entire AaeDNV genome to a bacterial plasmid which was then used to transfect C6/36 mosquito cells [48]. From this plasmid, wild-type infectious AaeDNV was successfully rescued. To further test the potential of AaeDNV as an expression vector, plasmids containing the gene encoding β-galactosidase (β-gal) under the control of each AaeDNV viral promoter were created between the AaeDNV terminal inverted repeats. When these constructs were used to transfect cells, expression levels of β-gal were high for both the mid (p7) and right (p61) ORFs and even higher when cells were co-transfected with a plasmid providing trans-activating NS1. Additionally it was found that recombinant genomic sequences containing β-gal were able to be successfully packaged into infectious viral capsids when a helper construct was provided. [48]. These studies demonstrated that certain AaeDNV promoters were effective at expressing inserted genes and that genetically modified AaeDNV could be packaged into infectious virions.
Later experiments led to the creation of plasmids that express GFP under the control of the AaeDNV p7 or p61 promoter [20]. In these experiments several GFP fusion constructs were tested. Plasmids p7NS1-GFP and p7NS1-GFPp61VP in which GFP expression was under the control of the p7 promoter had the highest levels of observed GFP expression when used to transfect mosquito cell culture [20]. These plasmids were then used to produce transducing virions. Plasmid p7NS1-GFP lacked AaeDNV VP and this was provided using helper plasmids containing either the entire wild-type AaeDNV genome or only the VP gene. The second plasmid, p7NS1-GFPp61VP contained all viral genes and did not require co-transfection with a helper plasmid [20]. Larvae infected with viruses created from these plasmid constructs had observable GFP expression in several organs including the midgut, hindgut, Malpighian tubes, and anal papillae [20]. Unfortunately, this system allowed for recombination between p7NS1-GFP and VP containing helper constructs or within the p7NS1-GFPp61VP construct, resulting in high levels of wild-type AaeDNV. Recombination levels were highest in the p7NS1-GFPp61VP single construct system. Later strategies eliminated this recombination through the use of a recombinant Sindbis virus (single-stranded positive-sense RNA virus) coding for AaeDNV VP under the control of a previously tested Sindbis subgenomic promoter as a helper construct [20,49].
In 2008 the first Anopheles MDV was discovered in An. gambiae Sua5B cells [9]. AgDNV was found to be infectious but not pathogenic to larvae and was able to disseminate and multiply in various adult tissues [9]. Specifically, AgDNV was found to reach high numbers in the fat body and ovaries of An. gambiae [50]. First instar larvae of An. gambiae that were allowed to feed on infected cells for 24 hours or were put into trays containing a mix of filtered infected cell lysate and water for 24 hours were infected with approximately equal efficacy and ~50–60% of surviving larvae remained infected into adulthood as determined by PCR [9]. Vertical transmission was also reported and 28% of larval offspring from infected adults tested positive for the virus [9]. AgDNV was also found to have a minimal influence on adult mosquito survival and transcriptome composition, making it an ideal candidate for paratransgenesis [51].
In order to genetically modify AgDNV and adapt the tool for expression of desired genes, the complete genome including hairpins was purified and added to a pBluescript cloning vector, forming a wild-type AgDNV construct designated pBAgα [9]. A second plasmid construct was created by removing most of the AgDNV genome from between the hairpins and adding in multiple cloning sites. These sites were then used to insert the Drosophila Actin5C promoter, an enhanced GFP (EGFP) gene, and an SV40 termination sequence creating a plasmid subsequently called pAgActinGFP [9]. Moss55 cells co-infected with helper pBAgα and transducing pAgActinGFP quickly expressed fluorescence. Purified viral particles were infective to larvae and fluorescence was observed into mosquito adulthood. GFP expression was observed in 20% of the offspring from infected adults and was later observed in 20% of the F3 generation [9].
Later, a new AgDNV transducing plasmid (pUTRAcGFP) was created that improved upon pAgActinGFP. This plasmid also contained the Actin5C promoter, EGFP sequence and SV40 termination sequence but was made considerably shorter through the removal of a remnant of AgDNV VP that was present in pAgActinGFP [9,50]. Additionally, pUTRAcGFP contained a segment of the 5′ AgDNV untranslated region that was absent from pAgActinGFP [9,50]. Additional constructs were created by switching the promoter used to express EGFP or the termination sequence but all were kept shorter than wild-type AgDNV, as constructs larger than the wild-type DNV genome have reduced and unpredictable transduction due to difficulties in genome packaging [7,48,50]. Of these, the viral construct with a full length Actin5C promotor and EGFP led to the most GFP expression during in vitro and in vivo infections [50]. Another construct developed during this study produced polycystronic expression of multiple genes and demonstrated the potential for use of a two construct AgDNV system in the expression of larger DNA sequences [50]. In DNV vectors that are close to the size of wild-type AgDNV genome, shorter genetic options must be explored and expression of ncRNAs remains an area with potential.
Some experimentation with ncRNA expression has been conducted using a helper-transfection AaeDNV plasmid system to induce RNAi in Ae. albopictus C6/36 cells and larvae [52]. In this study, pUCA, a previously validated plasmid encoding the entire AaeDNV genome, was modified to create a recombinant plasmid lacking the VP gene and containing GFP fused to NS1 [48,52]. An artificial intron containing a small hairpin RNA (shRNA) expression cassette sequence under the control of either an An. gambiae or Ae. aegypti U6 promoter was added to the NS1-GFP fusion area. Following transcription, the intronic shRNA cassette was spliced out and processed into a siRNA sequence and the mature mRNA coding NS1 and GFP was translated. Two different siRNA sequences (siRNA1 and siRNA2) targeting Ae. albopictus V-ATPase mRNA transcripts via RNAi were tested under the control of each U6 promoter. V-ATPase was chosen as a target for this experiment due to its conserved nature, function in cellular invasion of enveloped viruses, and role in essential cellular functions [52]. When Ae. albopictus C6/36 cells were co-infected with helper pUCA and a siRNA-expressing transfecting plasmid, GFP expression was observed in 96% of cells at 60 hours post infection, indicating proper splicing of the shRNA containing intron. RNAi based V-ATPase silencing was most pronounced and sustained when cells or larvae were transfected with plasmids expressing siRNAs driven by the Ae. aegypti U6 promoter. The siRNA2 sequence was the most effective and at 96 hours post infection in vitro V-ATPase expression was reduced by more than 90% using this construct [52]. This construct was also the most effective at knocking down V-ATPase in Ae. albopictus larvae. This construct was also found to be more pathogenic than either wild-type AaeDNV or the other transducing-helper virus combinations in newly emerged Ae. albopictus larvae [52].
Additional work has been done exploring the expression of small ncRNAs via an artificial intron system in non-defective recombinant AaeDNV [53]. To validate intronic splicing, pre-miRNA sequences for aal-let-7 and aal-mir-210 were placed inside the artificial intron within defective AaeDNV plasmids containing the two densovirus non-structural genes fused to DsRed or GFP in the place of the VP gene. These plasmids were used to transfect C6/36 Ae. albopictus cells along with a helper plasmid encoding the full AaeDNV genome. Purified virions produced from these plasmids were also used to infect Ae. albopictus larvae. DsRed or GFP expression indicated proper splicing of the artificial intron was occurring in vitro and in vivo [53]. Splicing was further validated using intron-spanning primers. Following validation of intron splicing, non-defective constructs were created by placing the intron and pre-miRNA sequences, miRNA sponge sequences, or artificial miRNA sequences in the NS1 gene of a plasmid containing the entire AaeDNV genome. These non-defective pre-miRNA and miRNA sponge constructs were shown to alter the levels of aal-let-7 and aal-mir-210 in vitro following plasmid transfection. Similar changes were observed in vivo in infected larvae [53]. Additionally, intronic expression of artificial miRNAs against V-ATPase reduced the level of V-ATPase mRNA in vitro and in vivo [53]. This non-defective AaeDNV intron expression system has recently been slightly modified to increase ease of use and has been used to express a short hairpin sequence against Ae. albopictus V-ATPase [54]. These experiments demonstrate the ability of densoviruses to express small ncRNAs through the use of an artificial intron and show that such vectors can be non-defective.
Disadvantages
There are many gaps in our understanding of MDV basic biology that currently limit the feasibility of using these viruses for mosquito control. Specifically, MDVs have not yet been identified for each medically important mosquito species and for many discovered MDVs, the complete host ranges have yet to be described. Previous research has noted that MDVs tend to have limited host ranges and off-target effects have not been documented, however this must be confirmed for each MDV or genetically modified MDV prior to field application. In addition, the currently known MDVs are often limited to specific tissues. This could be useful if targets are found within the mosquito tissues that each MDV infects, however, this could limit the efficacy of MDV-based control, especially if attempting to inhibit pathogens that do not reside in the same tissues as the virus.
While certain highly pathogenic MDVs have the potential to reduce mosquito numbers, it is unclear if this level of reduction is enough to effectively inhibit mosquito-borne disease transmission. Additionally, highly pathogenic MDVs that kill immatures before reaching adulthood have the potential to exert strong selective pressure. There is always a danger that resistance to MDV tools will develop and that efficacy will decline over time. For the purpose of paratransgenesis as well as reducing the risk of developing resistance, it is more advantageous to infect mosquitoes with a less pathogenic MDV strain that allows mosquitoes to reach adulthood and reproduce but somehow disrupts transmission or shortens the mosquito lifespan.
Another disadvantage is the limited size of the transgenic MDV constructs that can be produced. Unless co-infection techniques with transducing viruses and helper viruses are used, only small amounts of DNA can be added to the MDV construct due to limitations in the capsid capacity [7,48]. Constructs larger or smaller than the wild-type DNV genome have been found to have less efficient packaging. One such construct, 8% larger than wild-type AaeDNV, was found to have a 10% reduction in packaging [48]. There are also problems with deleting parts of the NS1 and NS2 genes in AaeDNV, and potentially in other MDVs, as at least certain areas of these genes seem to play a role in regulating natural VP expression levels [55].
One of the more basic challenges facing large-scale adaptation and deployment of a MDV control strategy is the ability to manufacture the virus in sufficient quantities. The associated costs of producing large amounts of virus are typically high and must be overcome in order for control strategies to have enough viral preparation to be effective. C6/36 cells have been adapted to produce AaeDNV and HeDNV particles in serum-free protein-free Sf-900 II media in spinner flasks [56]. This method is more consistent and easier to handle on a large scale than previous methods using traditional flasks with fetal bovine serum (FBS) supplemented media. While this method represents progress towards easier large-scale production of virus, other obstacles remain for efficient purification of viral particles as well as for the production of MDVs that are not able to grow in C6/36 cells.
Many of the current disadvantages to using MDV as tools to control mosquitoes stem from a lack of basic biological, ecological, and logistical studies of the viruses. While early evidence has been promising, such studies must be continued and expanded to newly discovered MDVs as well as to transgenic strains. Despite some challenges, MDVs have numerous advantages that justify continued research and development as novel methods of control.
Conclusions
MDVs have many characteristics that make them desirable as laboratory tools or as alternatives to traditional pesticides or genetically modified mosquitoes. MDV genomes are small and allow for easy genetic manipulation using standard cloning techniques [9,47,52]. Although the small capsid size of MDVs poses challenges to efficient genome packaging when inserting large genetic segments, research into small ncRNAs is opening up new possibilities for modification and coinfection systems have demonstrated potential for allowing for the expression of longer genetic sequences [9,48,50,52]. MDVs have also been reported to be highly species-specific and no off-target effects have been documented [7,45,46,57]. In field conditions, MDV infections can persist and spread, indicating that modified MDVs may possess the same characteristics and that even minimal application of certain MDVs could lead to stable field infections [21,25,35]. Given these characteristics, MDV-based control efforts have the potential to be cheaper, easier, and less labor intensive than traditional pesticides. While many strains of MDV are highly pathogenic to larvae and could be used to lower overall mosquito density, less pathogenic strains could be engineered for the purpose of paratransgenesis or for use as late-life insecticides [38]. While further development is crucial, MDVs have a clear and demonstrated potential for use in the control of mosquitoes and mosquito-borne pathogens.
Highlights.
Densoviruses are widespread and infect many important mosquito vectors
Certain densovirus strains naturally lead to mosquito mortality
Densoviruses have the potential to be used in lab studies or in field applications
Genetically modified densoviruses can aid in mosquito and pathogen control
Acknowledgments
We thank Dr. Brittany Dodson for her helpful edits and comments on earlier versions of this manuscript. This research was supported by NIH grants R01AI128201, R01AI116636, R21AI128918, U19AI089672, and NSF grant 1645331 to JLR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rebecca M. Johnson, Email: ruj130@psu.edu.
Jason L. Rasgon, Email: jlr54@psu.edu.
References
- 1.Federici BA. Viral pathogens of mosquito larvae. Bull Am Mosq Control Assoc. 1985;6:62–74. [Google Scholar]
- 2.Meynadier G, Vago C, Plantevin G, Atger P. Virose d’un type inhabituel chez le lepidoptere Galleria mellonella. L Rev Zool Agric Appl. 1964;63:207–208. [Google Scholar]
- 3.Vago C, Duthoit JL, Delahaye F. Les lesions nucleaires de la “Virose a noyaux denses” du Lepidoptere Galleria mellonella. Arch Virol. 1965;18:344–349. [Google Scholar]
- 4.Kellen WR, Clark TB, Lindegren JE. A possible polyhedrosis in Culex tarsalis Coquillett (Diptera: Culicidae) J Insect Pathol. 1963;5:98–103. [Google Scholar]
- 5.Clark TB, Chapman HC, Fukuda T. Nuclear-polyhedrosis infections and cytoplasmic-polyhedrosis in Louisiana mosquitoes. J Invertebr Pathol. 1969;14:284–286. doi: 10.1016/0022-2011(69)90120-7. [DOI] [PubMed] [Google Scholar]
- 6.Clark TB, Fukuda T. Field and laboratory observations of two viral diseases in Aedes sollicitans (Walker) in southwestern Louisiana. Mosq News. 1971;31:193–200. [Google Scholar]
- 7.Carlson J, Suchman E, Buchatsky L. Densoviruses for control and genetic manipulation of mosquitoes. Adv Virus Res. 2006;68:361–392. doi: 10.1016/S0065-3527(06)68010-X. [DOI] [PubMed] [Google Scholar]
- 8.Lebedeva OP, Zelenko AP, Kuznetsova MO, Gudz’-Gorban’ OP, Gudz-Gorban OP. The detection of viral infection in larvae of Aedes aegypti. Mikrobiol Zh. 1972;34:70–73. [PubMed] [Google Scholar]
- 9.Ren X, Hoiczyk E, Rasgon JL. Viral paratransgenesis in the malaria vector Anopheles gambiae. PLoS Pathog. 2008;4:e1000135. doi: 10.1371/journal.ppat.1000135. This study details the discovery of the first DNV capable of infecting and disseminating within An. gambiae. This AgDNV was also developed into an expression vector for the purpose of paratrangenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhai Y, Lv X, Sun X, Fu S, Gong Z, Fen Y, Tong S, Wang Z, Tang Q, Attoui H, et al. Isolation and characterization of the full coding sequence of a novel densovirus from the mosquito Culex pipiens pallens. J Gen Virol. 2008;89:195–199. doi: 10.1099/vir.0.83221-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H-L, Zhang Y-Z, Yang W-H, Feng Y, Nasci RS, Yang J, Liu Y-H, Dong C-L, Li S, Zhang B-S, et al. Mosquitoes of western Yunnan province, China: seasonal abundance, diversity, and arbovirus associations. PLoS One. 2013;8:1–13. doi: 10.1371/journal.pone.0077017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma M, Huang Y, Gong Z, Zhuang L, Li C, Yang H, Tong Y, Liu W, Cao W. Discovery of DNA viruses in wild-caught mosquitoes using small RNA high throughput sequencing. PLoS One. 2011;6:e24758. doi: 10.1371/journal.pone.0024758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng TFF, Willner DL, Lim YW, Schmieder R, Chau B, Nilsson C, Anthony S, Ruan Y, Rohwer F, Breitbart M. Broad surveys of DNA viral diversity obtained through viral metagenomics of mosquitoes. PLoS One. 2011;6:e20579. doi: 10.1371/journal.pone.0020579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Neill SL, Kittayapong P, Braig HR, Andreadis TG, Gonzalez JP, Tesh RB. Insect densoviruses may be widespread in mosquito cell lines. J Gen Virol. 1995;76:2067–2074. doi: 10.1099/0022-1317-76-8-2067. [DOI] [PubMed] [Google Scholar]
- 15.Sangdee K, Pattanakitsakul S-N. New genetic variation of Aedes albopictus densovirus isolated from mosquito C6/36 cell line. Southeast Asian J Trop Med Public Health. 2012;43:1122–1133. [PubMed] [Google Scholar]
- 16.Igarashi A. Isolation of a Singh’s Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978;40:531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- 17.Jousset F-X, Baquerizo E, Bergoin M. A new densovirus isolated from the mosquito Culex pipiens (Diptera: culicidae) Virus Res. 2000;67:11–6. doi: 10.1016/s0168-1702(00)00128-3. [DOI] [PubMed] [Google Scholar]
- 18.Baquerizo-Audiot E, Abd-Alla A, Jousset F-X, Cousserans F, Tijssen P, Bergoin M. Structure and expression strategy of the genome of Culex pipiens densovirus, a mosquito densovirus with an ambisense organization. J Virol. 2009;83:6863–6873. doi: 10.1128/JVI.00524-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchatsky LP. Densonucleosis of bloodsucking mosquitoes. Dis Aquat Organ. 1989;6:145–150. [Google Scholar]
- 20.Afanasiev BN, Ward TW, Beaty BJ, Carlson JO. Transduction of Aedes aegypti mosquitoes with vectors derived from Aedes densovirus. Virology. 1999;257:62–72. doi: 10.1006/viro.1999.9621. [DOI] [PubMed] [Google Scholar]
- 21.Barreau C, Jousset F-X, Bergoin M. Venereal and vertical transmission of the Aedes albopictus parvovirus in Aedes aegypti mosquitoes. Am J Trop Med Hyg. 1997;57:126–131. doi: 10.4269/ajtmh.1997.57.126. [DOI] [PubMed] [Google Scholar]
- 22.Barik TK, Suzuki Y, Rasgon JL. Factors influencing infection and transmission of Anopheles gambiae densovirus (AgDNV) in mosquitoes. PeerJ. 2016;4:e2691. doi: 10.7717/peerj.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebedinets NN, Kononko AG. Experimental study of the pathways of densovirus infection transmission in a population of blood-sucking mosquitoes. Meditsinskaya Parazitol. 1989;2:79–83. [PubMed] [Google Scholar]
- 24.Buchatsky LP, Lebedinets NN, Kononko AG. Densonucleosis of bloodsucking mosquitoes: student handbook for biology majors. 1997 [Google Scholar]
- 25.Kittayapong P, Baisley KJ, O’Neill S. A mosquito densovirus infecting Aedes aegypti and Aedes albopictus from Thailand. Am J Trop Med Hyg. 1999;61:612–617. doi: 10.4269/ajtmh.1999.61.612. [DOI] [PubMed] [Google Scholar]
- 26.Wise de Valdez MR, Suchman EL, Carlson JO, Black WC., IV A large scale laboratory cage trial of Aedes densonucleosis virus. J Med Entomol. 2010;47:392–399. doi: 10.1603/me09157. [DOI] [PubMed] [Google Scholar]
- 27.Barreau C, Jousset F-X, Cornet M. An efficient and easy method of infection of mosquito larvae from virus-contaminated cell cultures. J Virol Methods. 1994;49:153–156. doi: 10.1016/0166-0934(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 28.Wei W, Shao D, Huang X, Li J, Chen H, Zhang Q, Zhang J. The pathogenicity of mosquito densovirus (C6/36DNV) and its interaction with Dengue virus type II in Aedes albopictus. Am J Trop Med Hyg. 2006;75:1118–1126. [PubMed] [Google Scholar]
- 29.Becnel JJ. Transmission of viruses to mosquito larvae mediated by divalent cations. J Invertebr Pathol. 2006;92:141–145. doi: 10.1016/j.jip.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Barreau C, Jousset F-X, Bergoin M. Pathogenicity of the Aedes albopictus parvovirus (AaPV), a denso-like virus, for Aedes aegypti mosquitoes. J Invertebr Pathol. 1996;68:299–309. doi: 10.1006/jipa.1996.0100. [DOI] [PubMed] [Google Scholar]
- 31.Ledermann JP, Suchman EL, Black WC, IV, Carlson JO. Infection and pathogenicity of the mosquito densoviruses AeDNV, HeDNV, and APeDNV in Aedes aegypti mosquitoes (Diptera: Culicidae) J Econ Entomol. 2004;97:1828–1835. doi: 10.1093/jee/97.6.1828. [DOI] [PubMed] [Google Scholar]
- 32.Paterson A, Robinson E, Suchman E, Afanasiev B, Carlson J. Mosquito densonucleosis viruses cause dramatically different infection phenotypes in the C6/36 Aedes albopictus cell line. Virology. 2005;337:253–261. doi: 10.1016/j.virol.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 33.Rwegoshora RT, Kittayapong P. Pathogenicity and infectivity of the Thai-strain densovirus (AThDNV) in Anopheles minimus S. L Southeast Asian J Trop Med Public Health. 2004;35:630–634. [PubMed] [Google Scholar]
- 34.Hirunkanokpun S, Carlson JO, Kittayapong P. Evaluation of mosquito densoviruses for controlling Aedes aegypti (Diptera: Culicidae): Variation in efficiency due to virus strain and geographic origin of mosquitoes. Am J Trop Med Hyg. 2008;78:784–790. [PubMed] [Google Scholar]
- 35.Rwegoshora RT, Baisley KJ, Kittayapong P. Seasonal and spatial variation in natural densovirus infection in Anopheles minimus S. L. in Thailand. Southeast Asian J Trop Med Public Health. 2000;31:3–9. [PubMed] [Google Scholar]
- 36.Roekring S, Smith DR. Induction of apoptosis in densovirus infected Aedes aegypti mosquitoes. J Invertebr Pathol. 2010;104:239–241. doi: 10.1016/j.jip.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Suchman EL, Kononko A, Plake E, Doehling M, Kleker B, Black WC, IV, Buchatsky L, Carlson J. Effects of AeDNV infection on Aedes aegypti lifespan and reproduction. Biol Control. 2006;39:465–473. [Google Scholar]
- 38.Ren X, Rasgon JL. Potential for the Anopheles gambiae densonucleosis virus to act as an “evolution-proof” biopesticide. J Virol. 2010;84:7726–7729. doi: 10.1128/JVI.00631-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burivong P, Pattanakitsakul S-N, Thongrungkiat S, Malasit P, Flegel TW. Markedly reduced severity of Dengue virus infection in mosquito cell cultures persistently infected with Aedes albopictus densovirus (AalDNV) Virology. 2004;329:261–269. doi: 10.1016/j.virol.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 40.Mosimann ALP, Bordignon J, Mazzarotto GCA, Motta MCM, Hoffmann F, dos Santos CND. Genetic and biological characterization of a densovirus isolate that affects dengue virus infection. Mem Inst Oswaldo Cruz. 2011;106:285–292. doi: 10.1590/s0074-02762011000300006. [DOI] [PubMed] [Google Scholar]
- 41.Kanthong N, Khemnu N, Sriurairatana S, Pattanakitsakul S-N, Malasit P, Flegel TW. Mosquito cells accommodate balanced, persistent co-infections with a densovirus and Dengue virus. Dev Comp Immunol. 2008;32:1063–1075. doi: 10.1016/j.dci.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Kanthong N, Khemnu N, Pattanakitsakul S-N, Malasit P, Flegel TW. Persistent, triple-virus co-infections in mosquito cells. BMC Microbiol. 2010;10:1–8. doi: 10.1186/1471-2180-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchatskii LP, Kuznetsova MA, Lebedinets NN, Kononko AG. Development and basic properties of the viral preparation viroden. Vopr Virusol. 1987;32:729–733. [PubMed] [Google Scholar]
- 44.Buchatskii LP, Litvinov GS, Lebedinets NN, Filenko OM, Podberezova LM. Effect of temperature on the infectivity of the iridovirus and densonucleosis virus of bloodsucking mosquitoes. Vopr Virusol. 1988;33:603–606. [PubMed] [Google Scholar]
- 45.Vasil’eva VL, Lebedinets NN, Gurval’ AL, Chigir’ TV, Buchatskii LP, Kuznetsova MA. The safety of the prepartation viroden for vertebrate animals. Mikrobiol Zh. 1990;52:73–79. [PubMed] [Google Scholar]
- 46.Jousset F-X, Barreau C, Boublik Y, Cornet M. A parvo-like virus persistently infecting a C6/36 clone of Aedes albopictus mosquito cell line and pathogenic for Aedes aegypti. Virus Res. 1993;29:99–114. doi: 10.1016/0168-1702(93)90052-o. [DOI] [PubMed] [Google Scholar]
- 47.Afanasiev BN, Galyov EE, Buchatsky LP, Kozlov YV. Nucleotide sequence and genomic organization of Aedes densonucleosis virus. Virology. 1991;185:323–336. doi: 10.1016/0042-6822(91)90780-f. [DOI] [PubMed] [Google Scholar]
- 48.Afanasiev BN, Kozlov YV, Carlson JO, Beaty BJ. Densovirus of Aedes aegypti as an expression vector in mosquito cells. Exp Parasitol. 1994;79:322–339. doi: 10.1006/expr.1994.1095. This was the first use of AaeDNV as an expression vector and this study demonstrated that transgenic MDVs could be used to introduce genes of interest. [DOI] [PubMed] [Google Scholar]
- 49.Allen-Miura TM, Afanasiev BN, Olson KE, Beaty BJ, Carlson JO. Packaging of AeDNV-GFP transducing virus by expression of densovirus structural proteins from a Sindbis virus expression system. Virology. 1999;257:54–61. doi: 10.1006/viro.1999.9622. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki Y, Niu G, Hughes GL, Rasgon JL. A viral over-expression system for the major malaria mosquito Anopheles gambiae. Sci Rep. 2014;4:1–9. doi: 10.1038/srep05127. This paper improved upon a previously developed AgDNV expression system and created an easily altered and highly efficient AgDNV over-expression system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren X, Hughes GL, Niu G, Suzuki Y, Rasgon JL. Anopheles gambiae densovirus (AgDNV) has negligible effects on adult survival and transcriptome of its mosquito host. PeerJ. 2014;2:1–11. doi: 10.7717/peerj.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu J, Liu M, Deng Y, Peng H, Chen X. Development of an efficient recombinant mosquito densovirus-mediated RNA interference system and its preliminary application in mosquito control. PLoS One. 2011;6:e21329. doi: 10.1371/journal.pone.0021329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu P, Li X, Gu J, Dong Y, Liu Y, Santhosh P, Chen X. Development of non-defective recombinant densovirus vectors for microRNA delivery in the invasive vector mosquito, Aedes albopictus. Sci Rep. 2016;6:1–13. doi: 10.1038/srep20979. This study details the development of a non-defective DNV vector created by placing an artificial intron in AaeDNV and shows the effectiveness of this strategy in expressing various ncRNAs and altering levels of certain miRNAs and mRNAs in Ae. albopictus cells and larvae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu P-W, Xu J-B, Dong Y-Q, Chen X-G, Gu J-B. Use of a recombinant mosquito densovirus as a gene delivery vector for the functional analysis of genes in mosquito larvae. J Vis Exp. 2017;128:1–8. doi: 10.3791/56121. This paper describes many of the methods useful for artificial-intron-based expression of ncRNAs and DNV larval infection while providing an updated non-defective AaeDNV vector strategy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward TW, Kimmick MW, Afanasiev BN, Carlson JO. Characterization of the structural gene promoter of Aedes aegypti densovirus. J Virol. 2001;75:1325–1331. doi: 10.1128/JVI.75.3.1325-1331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suchman E, Carlson J. Production of mosquito densonucleosis viruses by Aedes albopictus C6/36 cells adapted to suspension culture in serum-free protein-free media. In Vitro Cell Dev Biol Anim. 2004;40:74–75. doi: 10.1290/1543-706x(2004)040<0074:pomdvb>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki Y, Barik TK, Johnson RM, Rasgon JL. In vitro and in vivo host range of Anopheles gambiae densovirus (AgDNV) Sci Rep. 2015;5:1–6. doi: 10.1038/srep12701. This paper enhances our understanding of AgDNV host range and provides data useful for continued development of AgDNV as a paratransgenic and mosquito control agent. [DOI] [PMC free article] [PubMed] [Google Scholar]