Abstract
BACKGROUND AND PURPOSE:
Endovascular treatment of bifurcation middle cerebral artery aneurysms with a wide neck could be challenging, and many lesions are still treated by a surgical approach. The pCONus is a newly emerging device for wide-neck bifurcation intracranial aneurysms. To date, a single report on the treatment of intracranial aneurysms including all locations has been published. We report our experience with pCONus in the treatment of wide-neck MCA aneurysms.
MATERIALS AND METHODS:
MCA aneurysms treated with pCONus in 4 European centers were retrospectively reviewed.
RESULTS:
Forty MCA aneurysms (mean dome size, 7.7 mm; mean neck size, 5.6 mm) were treated in 40 patients (mean age, 62 years). Aneurysm coiling was performed after deployment of 1 pCONus in 95% (38/40) of cases and after deployment of 2 pCONus devices in 5% (2/40). No procedural angiographic complications were observed. Reversible neurologic complications were noted in 5% (2/40), and permanent neurologic complication, in 2.5% (1/40) at 1 month. There was no mortality. No aneurysms bled or rebled after treatment. Immediate angiographic results were complete aneurysm occlusion in 25% (10/40), neck remnant in 47.5% (19/40), and aneurysm remnant in 27.5% (11/40). Follow-up (mean, 6.8 months) was available for 33 aneurysms (82.5%). Stable or improved results were observed in all except 3 cases, including 48.5% complete occlusions (16/33), 30.3% neck remnants (10/33), and 21.2% aneurysm remnants (7/33). There was no in-stent stenosis or jailed branch occlusion. There was no angiographic recurrence of initially totally occluded aneurysms.
CONCLUSIONS:
MCA aneurysms with a wide neck are amenable to treatment with pCONus.
Although the superiority of endovascular treatment compared with surgery appears unaffected by aneurysm location in the randomized International Subarachnoid Aneurysm Trial,1 management of middle cerebral artery aneurysms remains a matter of debate. Nevertheless many institutions still use surgical clipping as the first treatment for MCA aneurysms.2,3 Balloon and stent-assisted techniques have widened the indications for endovascular treatment of MCA aneurysms with a wide neck and/or unfavorable anatomy that were otherwise unsuitable for coiling.4,5 However, the risk of procedure-related morbidity and mortality is not negligible, especially with double-stent placement in Y and X configurations.4,6,7 The widespread adoption of endovascular treatment for MCA aneurysms with unfavorable anatomy requires an improvement of the safety of the endovascular approach. A new device, the pCONus aneurysm implant (phenox, Bochum, Germany), has recently been developed to improve the safety of endovascular treatment of these challenging aneurysms. To date, a single published article on intracranial aneurysms treated with pCONus reported a series including some cases of MCA aneurysms.8 The aim of this study was to evaluate the results in the treatment of wide-neck MCA aneurysms with the pCONus device.
Materials and Methods
From June 2012 to July 2014, the clinical and angiographic outcomes of 40 consecutive patients treated at 4 institutions with the pCONus device and coils for MCA aneurysms were retrospectively analyzed. “Wide neck” was defined as a neck of >4 mm. The decision to assist the aneurysm coiling with a pCONus device was made at the discretion of the operator. All patients were informed of the procedure. In each center, the procedures were performed according to the local institutional policy.
The pCONus is a new stentlike self-expanding nitinol implant with 4 distal petals, which is fully retrievable and electrolytically detachable. The distal intra-aneurysmal inner diameter of the pCONus is additionally crossed by 6 polyamide fibers, creating a mechanical barrier between the aneurysm and the parent vessel for preventing coil protrusion into the lumen. The proximal extra-aneurysmal end of the implant and the 4 distal loops carry segmental radiopaque markers made of platinum-iridium wire. The pCONus is compatible with standard microcatheters with an inner diameter of 0.021 inches. In a suitable working projection, the distal end of the pCONus was deployed in the middle of the aneurysm sac, and the device with the microcatheter was gently pulled back; this step brings the petals more proximal to the neck of the aneurysm. Thereafter, a second microcatheter was inserted through the shaft into the aneurysm sac, and coiling was then performed.
All patients were treated under general anesthesia and full anticoagulation. In addition, double antiplatelet therapy was administered preoperatively according to the operator's protocol. A Multiplate test (Verum Diagnostica, Munich, Germany), VASP test (Lancet Laboratories, Durban, South Africa), or VerifyNow P2Y12 test (Accumetrics, San Diego, California) was systematically performed before the procedure to confirm sufficient inhibition of the platelet function. In unruptured aneurysms, a single dose of 500 mg acetylsalicylic acid and 600 mg clopidogrel was given the day before or 75 mg acetylsalicylic acid and 75 mg clopidogrel/per day were administered 5 days before the procedure. If the platelet-function inhibition was not sufficient, the patient was directly premedicated with 180 mg ticagrelor or 60 mg prasugrel. In case of SAH, patients received 600 mg clopidogrel via a gastric tube and 500 mg acetylsalicylic acid intravenously during the procedure. Postprocedural medication included 75 mg clopidogrel for 3 months and acetylsalicylic acid (75–160 mg) orally during 12 months or for life according to each center's protocol. Postprocedural medication was the same in both the ruptured and unruptured aneurysm group.
Clinical Events
Any clinical event appearing in the postoperative course was noted. A neurologic assessment was performed before and after the treatment, at discharge, and at follow-up.
Angiographic Follow-Up
Angiographic images were acquired in anteroposterior, lateral, and working projections before and immediately after treatment. Angiographic images obtained immediately after endovascular treatment were compared with those obtained at each angiographic follow-up.
Immediate aneurysm occlusion after the procedure and at follow-up was classified by using the simplified 3-point scale (complete occlusion, neck remnant, aneurysm remnant).9 In addition, the aneurysm changes were also evaluated (ie, increase or decrease in size of aneurysm neck remnants or aneurysm remnants). Two readers (B.G., A.B.) independently evaluated all the angiograms. Disagreements were solved by consensus.
The angiographic follow-up protocol consisted of a first angiographic follow-up performed from 3 to 6 months after the procedure and a second one performed at 1 year.
Results
Forty MCA bifurcation aneurysms were treated with the pCONus (23 on the right side and 17 on the left). Aneurysms dome sizes ranged from 2.7 to 17.4 mm (mean, 7.7 mm), and neck sizes ranged from 2.8 to 13.8 mm (mean, 5.6 mm; ≥4 mm in 35 aneurysms). Forty patients (27 women and 13 men) ranged from 36 to 77 years of age (mean, 62 years). Ninety percent (36/40) of aneurysms were unruptured, 5% (2/40) were recanalized (all were previously treated endovascularly), and 5% (2/40) were ruptured. Thirty-eight aneurysms were treated with 1 pCONus device, and in 2 patients, the aneurysm was treated by using 2 pCONus devices. In 4 patients, the pCONus treatment was performed after failure of aneurysm endovascular treatment with the WEB aneurysm embolization system (Sequent Medical, Aliso Viejo, California). Coiling was performed with bare platinum coils in 97.5% of cases (39/40).
Immediate Angiographic Results
In all cases, the pCONus devices were successfully deployed. Anatomic outcome is detailed in the On-line Table. Immediate angiograms showed complete occlusion in 25% (10/40), neck remnant in 47.5% (19/40), and aneurysm remnant in 27.5% (11/40).
Angiographic Follow-Up
Angiographic follow-up from 3 to 12 months (mean, 6.8 months) was available in 33 aneurysms (82.5%), and 14 aneurysms (35%) had a 12-monthfollow-up. Angiographic follow-up showed complete occlusion in 48.5% (16/33), neck remnants in 30.3% (10/33), and aneurysm remnants in 21.2% (7/33). Adequate occlusion (total occlusion and neck remnant) was obtained in 78.8% (26/33) (On-line Table and Figs 1–3).
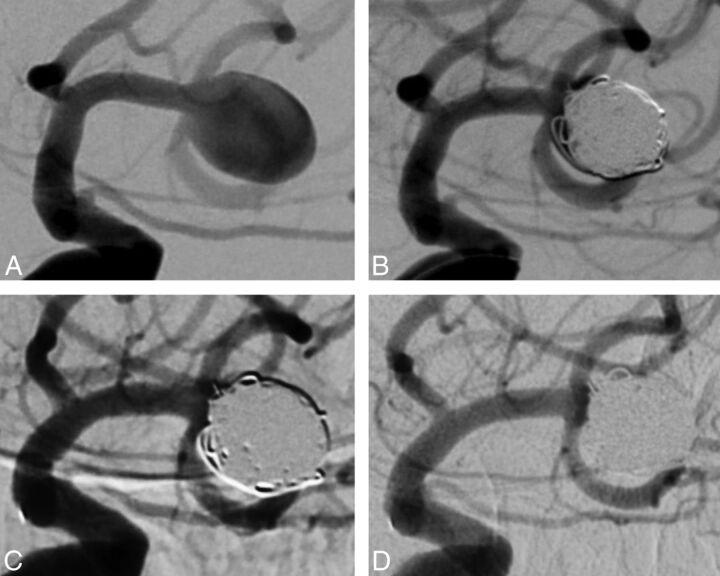
Fig 1.
Case 1, patient 1. Left unruptured MCA aneurysm in a 76-year-old woman. A, Subtracted angiography of the internal carotid artery shows a wide-neck MCA aneurysm. B, Subtracted angiography of the internal carotid artery at the end of the procedure shows a neck remnant. C, Subtracted angiography of the internal carotid artery at 3 months shows complete aneurysm occlusion. D, Subtracted angiography of the internal carotid artery at 12 months shows persistent aneurysm occlusion.
Fig 2.
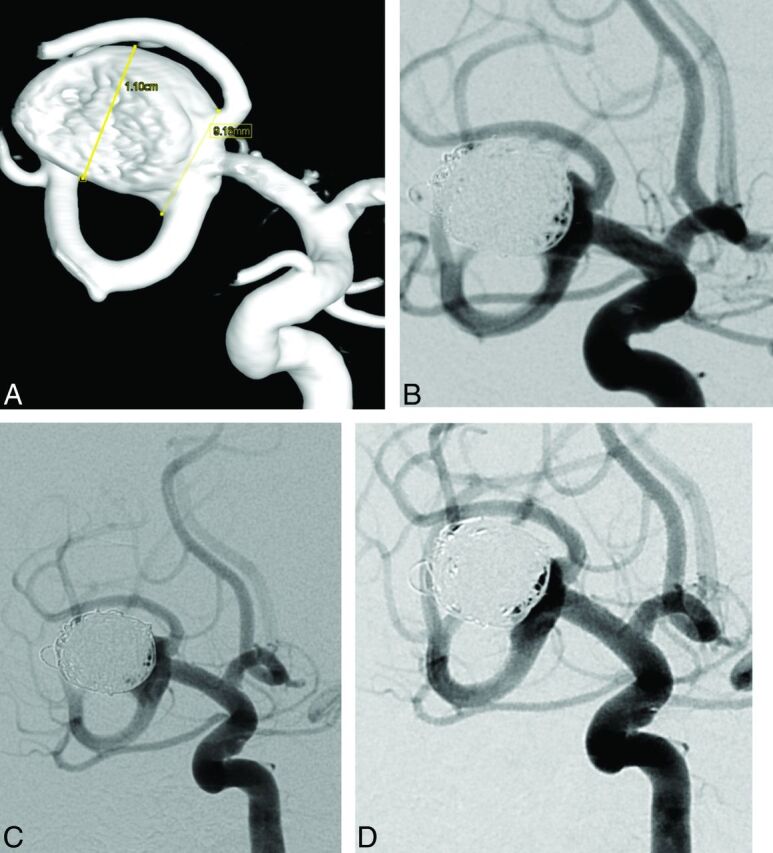
Case 2, patient 33. Right unruptured MCA aneurysm in a 50-year-old woman. A, 3D reconstruction after rotational angiography shows a large MCA aneurysm with a 9-mm neck. The superior branch is emerging from the neck of the aneurysm. B, Subtracted angiography of the internal carotid artery at the end of the procedure shows a neck remnant. Subtracted angiographies of the internal carotid artery at 6 months (C) and at 12 months (D) show a stable neck remnant.
Fig 3.
Case 3, patient 36. Left unruptured MCA aneurysm in a 41-year-old man. A, 3D reconstruction after rotational angiography and subtracted angiography (B) of the internal carotid artery shows a 4.2-mm MCA aneurysm with a 5.1-mm neck. C, Subtracted angiography of the internal carotid artery at the end of the procedure shows a neck remnant. D, Subtracted angiography of the internal carotid artery at 6 months shows a neck remnant.
At follow-up, among these 33 aneurysms, aneurysm occlusion improved in 42.4% (14/33), was stable in 48.4% (16/33), and worsened in 9.2% (3/33). In 5 cases, the aneurysm remnants increased in size, requiring further endovascular procedures in 4 of them. No in-stent stenosis or jailed branch occlusion was observed. During the first year of follow-up, retreatment was performed in 17.5% of cases (7/40) due to persistent or increase in size of aneurysm remnants (6/7) or an important increase in size of the neck remnant (1/7). The size of the retreated aneurysms was >10 mm in 57.1% (4/7). Retreated aneurysms had a neck size of ≥6 mm in 71.4% (5/7). Postoperative angiographic outcome included 1 complete occlusion, 4 neck remnants, and 2 aneurysm remnants.
Procedural and Clinical Complications
No angiographic thromboembolic event or intraoperative rupture was observed. Reversible neurologic complications were noted in 5% of the patients (2/40). In 1 case (patient 3), the patient sustained a reversible right hemiparesis immediately after the procedure. A thromboembolic incident with a new ischemic lesion on MR imaging was observed. The patient was asymptomatic at discharge (mRS 0). In the other case (patient 35), the patient had a transient hypostenia of her left hand immediately after the procedure. The mRS scale was 1 at discharge. A patient (patient 1) presented with a permanent neurologic complication with right hemiparesis and aphasia, due to an ischemic lesion. No specific treatment was started. The patient improved within 2 days and was discharged with NIHSS 3 and mRS 1. Consequently, permanent morbidity was 2.5% and mortality was 0% at 1 month. There were no delayed neurologic deficits or deaths at follow-up.
pCONus in Subarachnoid Hemorrhage
Two aneurysms (5%) were treated with pCONus in the setting of subarachnoid hemorrhage. The first was an 11.1-mm right MCA aneurysm with a 10.6-mm neck in a man 46 years of age who presented with a Fisher grade 4 SAH (patient 5). The final angiogram showed a residual aneurysm. Follow-up angiography performed 3 months and 1 year later demonstrated that the residual aneurysm was unchanged. The second case was an 11.1-mm aneurysm of the right MCA bifurcation in a 53-year-old woman with a Fisher grade 3 SAH (patient 14). The postprocedural angiogram showed a neck remnant. The follow-up angiogram at 3 months demonstrated an aneurysm remnant with worsening angiographic findings.
Discussion
The use of pCONus in MCA bifurcation aneurysms with a wide neck allows a relatively efficient and safe endovascular treatment of the aneurysm despite the very specific population. In fact, we reported 1 permanent neurologic complication (2.5%) and no death. These findings compare favorably with the morbidity and mortality of endovascular treatment of MCA aneurysms reported by Brinjikji et al5 (3.9% and 1.2%, respectively). In the first published series, the safety of the pCONus was also highlighted because no clinically evident complication associated with its use was observed in 28 wide-neck aneurysms, including 9 recently ruptured ones.8
An endovascular approach to MCA aneurysms often requires the use of a balloon- or stent-assisted coiling technique. In a recent prospective cohort of 131 consecutive nonselected MCA aneurysms (34.2% ruptured aneurysms), balloon assistance was required in 60.3%.4 As previously reported, the balloon-assisted coiling technique does not seem to increase the complication rate compared with simple coiling in unruptured and ruptured aneurysms.4,10 Similar results concerning safety have also been reported by using stent-assisted coiling of wide-neck aneurysms.11–14 In a multicenter study reporting the use of the Neuroform stent (Stryker Neurovascular, Kalamazoo, Michigan),12 permanent morbidity and mortality rates were 1%, respectively, at 12- to 18-month follow-up. In another article, treatment-related permanent morbidity was 1.6% and mortality was 0% by using the Solitaire AB stent (Covidien, Irvine, California).13 On the contrary, other authors reported that intracranial stent placement, especially with double stents in Y and X configurations, seems to highly increase the risk of clinical complications.6,7 The rate of procedure-related permanent neurologic deficits in one study was 10% in 97 patients with complex and wide-neck bifurcation aneurysms.6 The odds of developing complications were 4.8× greater when stents were used in the treatment of nonselected MCA aneurysms.4 In addition, the MCA location is more likely to be associated with procedural complications in stent-assisted coiling, as recently outlined.15 We encountered no case of in-stent stenosis at follow-up, a rate lower than that reported (4.2%) in a series of stent-assisted coiling of 52 unruptured MCA aneurysms with longer follow-up (mean, 14 months).16 However, angiography was performed in only 58% of patients.
The University of California, San Francisco, surgery group reported a 4.6% permanent morbidity rate and a 5.3% mortality rate after surgery in 631 MCA aneurysms in 543 patients,2 which seems less safe than the endovascular outcome. However, for the morbidity-mortality rate, one must consider that ruptured aneurysms were present in 51.9% in the University of California, San Francisco, series, whereas our series included only 5% ruptured lesions. In the International Subarachnoid Aneurysm Trial, patients with a ruptured MCA aneurysm treated with endovascular coiling had a significantly better outcome at 1, 7, and 18 years compared with those treated by surgery.1,17,18 Nevertheless, to date, no randomized clinical trials have addressed the question of the first treatment for wide-neck MCA aneurysms, to our knowledge. In addition, although the rate of aneurysm recurrence appears low after surgical clipping, it is difficult to compare the rate of aneurysm recurrence after endovascular treatment versus surgical clipping because only a few surgical series reported long-term angiographic follow-up.7,8 In the University of California, San Francisco, surgical series, long-term follow-up with angiography was performed in only 106 of 480 patients (22%).2
Although stent placement is generally avoided in acutely ruptured aneurysms because of the requirement of dual antiplatelet medication,19 2 aneurysms of our series were ruptured. More complications were also reported in cases of ruptured MCA aneurysms managed by a surgical approach (8.5% versus 4.9%).2 In the setting of acute SAH, an intrasaccular flow disrupter, such as the WEB device, could be a therapeutic solution due to the lack of antiplatelet agents. However, it may not provide the immediate protection from rebleeding offered by coiling or surgical clipping. In a recent small series of 6 patients with acute ruptured aneurysms (3 MCA and 3 anterior communicating artery aneurysms) treated with the WEB device, no rebleeding was reported.20 The use of flow diverters is relatively limited in cases of bifurcation aneurysms because preservation of the bifurcation branches is unpredictable.21 However, no clinical complication was recently reported after treatment of 5 large and 3 giant MCA aneurysms,22 whereas other authors reported a 16% (4/25) rate of morbidity after treatment of 25 MCA aneurysms with the Pipeline Embolization Device (Covidien).23
In the past, the TriSpan (Boston Scientific, Natick, Massachusetts) had been specifically developed for the treatment of wide-neck aneurysms. However, the TriSpan is no longer available, and few data were published. In 1 series, the authors evaluated the TriSpan neck-bridge device to assist coiling in a series of patients with wide-neck bifurcation aneurysms of the anterior circulation (6 MCA aneurysms, 6 anterior communicating artery aneurysms, 1 carotid siphon aneurysm, and 1 carotid bifurcation aneurysm).24 A complication and severe procedure-related complication occurred in 6 cases (37.5%) and 1 case (6.25%), respectively. Late aneurysmal outcome, assessed by MRA or angiography at 1–39 months (mean, 12.9 months) posttreatment, was available in 8 patients and showed complete occlusion in 2 (14.3%), neck remnant in 2 (14.3%), and aneurysm remnant in 4 (28.6%) cases. A novel assisted coiling device, the PulseRider aneurysm neck reconstruction device (Pulsar Vascular, San Jose, California), intended for use in the treatment of wide-neck aneurysms arising at bifurcations, was used in 3 patients; however, the article is in press and no follow-up is available with this new device.25,26
In the present series, immediate adequate aneurysm occlusion (complete occlusion or neck remnant) was achieved in most cases (72.5%). Moreover, no case of angiographic in-stent stenosis or jailed branch occlusion was observed. Although long-term follow-up is available in only 35% of cases in our series, pCONus seems to achieve a durable treatment with a low rate of angiographic recurrence. No angiographic recurrence of initially totally occluded aneurysms was observed. In addition, the pCONus could promote progressive aneurysm thrombosis because 42.4% of aneurysms demonstrated a better angiographic result at short- or midterm follow-up. Previously published series have also reported a progressive thrombosis phenomenon after stent-assisted coiling.7,13
In a series of 33 wide-neck MCA aneurysms treated by using the WEB device, a similar adequate occlusion rate (83.3%) has been reported at follow-up.27 However, in our series, the rate of neck remnants with the pCONus (30.3%) was inferior to that reported by using the WEB device (56.7%). In addition, the pCONus could be an effective treatment after WEB-placement failure. Actually, in 4 aneurysms in our series in which the WEB deployment had failed, a successful treatment with pCONus was performed in all 4 aneurysms with complete occlusion in 3. However, longer follow-up is mandatory to evaluate the efficacy of this treatment in terms of aneurysm recanalization. In our series, all patients were followed by using angiography, which remains the criterion standard. At the present, artifacts introduced by the pCONus limit the value of MR imaging as a follow-up technique, especially for the evaluation of the parent artery where the extra-aneurysmal part of the pCONus is deployed. Contrast administration could improve vessel lumen visualization.28
The present study has some limitations including the number of patients being relatively small and the long-term follow-up lacking in some cases. However, our preliminary evaluation of this new endovascular approach for the treatment of MCA aneurysms with unfavorable anatomy shows that the pCONus device can be an interesting and safe option for such complex lesions.
Conclusions
In our selected population, pCONus stent-assisted coiling safely allows endovascular treatment of wide-neck MCA aneurysms that are usually considered surgical due to defavorable anatomy. Longer follow-up is mandatory to evaluate the efficacy of this treatment in terms of aneurysm recanalization.
Supplementary Material
Footnotes
Disclosures: Marta Aguilar Pérez—UNRELATED: Consultancy: phenox; Other: phenox (proctoring). Francis Turjman—UNRELATED: Consultancy: Codman,* Covidien,* Stryker,* Sequent Medical*; Grants/Grants Pending: Covidien*; Payment for Lectures (including service on Speakers Bureaus): Codman,* Covidien*; Payment for Development of Educational Presentations: Codman,* Covidien*; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Covidien,* Codman,* Stryker.* Werner Weber—UNRELATED: Consultancy: Proctor for the pCONus and p64 Flow Modulation Device; Payment for Lectures (including service on Speakers Bureaus): phenox (products: pCONus and flow-diverter p64 Flow Modulation Device), Comments: German Society Meeting in Cologne 2013 and 2014. Hans Henkes—RELATED: Consulting Fee or Honorarium: phenox, Comments: I have a consulting contract and a proctoring contract with phenox, which includes compensation on a fee-for-service basis; Support for Travel to Meetings for the Study or Other Purposes: phenox, Comments: Compensation for expenses related to the participation in meetings with presentation of p64 Flow Modulation Device data; Other: phenox, Comments: I am cofounder and shareholder of phenox; UNRELATED: Board Membership: phenox, Comments: Expenses related to board meetings are compensated; Grants/Grants Pending: Siemens (cooperation in relation to DynaCT),* Covidien (stroke research support)*; Payment for Lectures (including service on Speakers Bureaus): Covidien; Patents (planned, pending or issued): Dendron GmbH Bochum, phenox,* Comments: participation in numerous patents filed by Dendron GmbH and phenox; Royalties: phenox; Stock/Stock Options: phenox, Comments: I am cofounder and shareholder of phenox; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: ab medica,* Comments: compensation for physician training courses. Alessandra Biondi—RELATED: Consulting Fee or Honorarium: phenox; UNRELATED: Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Stryker Neurovascular, Covidien. *Money paid to the institution.
References
- 1. Molyneux A, Kerr R, Stratton I, et al. ; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–74 [DOI] [PubMed] [Google Scholar]
- 2. Rodríguez-Hernández A, Sughrue ME, Akhavan S, et al. Current management of middle cerebral artery aneurysms: surgical results with a “clip first” policy. Neurosurgery 2013;72:415–27 [DOI] [PubMed] [Google Scholar]
- 3. Pasqualin A, Meneghelli P, Cozzi F, et al. Surgical exclusion of unruptured middle cerebral artery aneurysms: experience of 126 cases. Acta Neurochir Suppl 2014;119:25–31 [DOI] [PubMed] [Google Scholar]
- 4. Gory B, Rouchaud A, Saleme S, et al. Endovascular treatment of middle cerebral artery aneurysms for 120 nonselected patients: a prospective cohort study. AJNR Am J Neuroradiol 2014;35:715–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brinjikji W, Lanzino G, Cloft HJ, et al. Endovascular treatment of middle cerebral artery aneurysms: a systematic review and single-center series. Neurosurgery 2011;68:397–402; discussion 402 [DOI] [PubMed] [Google Scholar]
- 6. Bartolini B, Blanc R, Pistocchi S, et al. “Y” and “X” stent-assisted coiling of complex and wide-neck intracranial bifurcation aneurysms. AJNR Am J Neuroradiol 2014;35:2153–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Piotin M, Blanc R, Spelle L, et al. Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke 2010;41:110–15 [DOI] [PubMed] [Google Scholar]
- 8. Aguilar-Pérez M, Kurre W, Fischer S, et al. Coil occlusion of wide-neck bifurcation aneurysms assisted by a novel intra- to extra-aneurysmatic neck-bridging device (pCONus): initial experience. AJNR Am J Neuroradiol 2014;35:965–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003;34:1398–403 [DOI] [PubMed] [Google Scholar]
- 10. Pierot L, Spelle L, Leclerc X, et al. Endovascular treatment of unruptured intracranial aneurysms: comparison of safety of remodeling technique and standard treatment with coils. Radiology 2009;251:846–55 [DOI] [PubMed] [Google Scholar]
- 11. Biondi A, Janardhan V, Katz JM, et al. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: strategies in stent deployment and midterm follow-up. Neurosurgery 2007;61:460–68; discussion 468–69 [DOI] [PubMed] [Google Scholar]
- 12. Gentric JC, Biondi A, Piotin M, et al. ; French SENAT Investigators. Safety and efficacy of Neuroform for treatment of intracranial aneurysms: a prospective, consecutive, French multicentric study. AJNR Am J Neuroradiol 2013;34:1203–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gory B, Klisch J, Bonafé A, et al. Solitaire AB stent-assisted coiling of wide-necked intracranial aneurysms: short-term results from a prospective, consecutive, European multicentric study. Neuroradiology 2013;55:1373–78 [DOI] [PubMed] [Google Scholar]
- 14. Gory B, Klisch J, Bonafé A, et al. Solitaire AB stent-assisted coiling of wide-necked intracranial aneurysms: mid-term results from the SOLARE study. Neurosurgery 2014;75:215–19; discussion 219 [DOI] [PubMed] [Google Scholar]
- 15. Chalouhi N, Jabbour P, Singhal S, et al. Stent-assisted coiling of intracranial aneurysms: predictors of complications, recanalization, and outcome in 508 cases. Stroke 2013;44:1348–53 [DOI] [PubMed] [Google Scholar]
- 16. Vendrell JF, Costalat V, Brunel H, et al. Stent-assisted coiling of complex middle cerebral artery aneurysms: initial and midterm results. AJNR Am J Neuroradiol 2011;32:259–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molyneux AJ, Kerr RS, Yu LM, et al. ; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005;366:809–17 [DOI] [PubMed] [Google Scholar]
- 18. Molyneux AJ, Birks J, Clarke A, et al. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 2015;385:691–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bodily KD, Cloft HJ, Lanzino G, et al. Stent-assisted coiling in acutely ruptured intracranial aneurysms: a qualitative, systematic review of the literature. AJNR Am J Neuroradiol 2011;32:1232–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caroff J, Mihalea C, Dargento F, et al. Woven Endobridge (WEB) device for endovascular treatment of ruptured intracranial wide-neck aneurysms: a single-center experience. Neuroradiology 2014;56:755–61 [DOI] [PubMed] [Google Scholar]
- 21. Gory B, Bonafé A, Pierot L, et al. Safety and efficacy of flow-diverter stents in endovascular treatment of intracranial aneurysm: interest of the prospective DIVERSION observational study. J Neuroradiol 2014;41:93–96 [DOI] [PubMed] [Google Scholar]
- 22. Zanaty M, Chalouhi N, Tjoumakaris SI, et al. Flow diversion for complex middle cerebral artery aneurysms. Neuroradiology 2014;56:381–87 [DOI] [PubMed] [Google Scholar]
- 23. Yavuz K, Geyik S, Saatci I, et al. Endovascular treatment of middle cerebral artery aneurysms with flow modification with the use of the Pipeline embolization device. AJNR Am J Neuroradiol 2014;35:529–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Keukeleire K, Vanlangenhove P, Defreyne L. Evaluation of a neck-bridge device to assist endovascular treatment of wide-neck aneurysms of the anterior circulation. AJNR Am J Neuroradiol 2008;29:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spiotta AM, Chaudry MI, Turk AS, et al. Initial experience with the PulseRider for the treatment of bifurcation aneurysms: report of first three cases in the USA. J Neurointerv Surg 2015. January 5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26. Gory B, Spiotta AM, Mangiafico S, et al. PulseRider stent-assisted coiling of wide-neck bifurcation aneurysms: periprocedural results in an international series. AJNR Am J Neuroradiol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pierot L, Klisch J, Cognard C, et al. Endovascular WEB flow disruption in middle cerebral artery aneurysms: preliminary feasibility, clinical, and anatomical results in a multicenter study. Neurosurgery 2013;73:27–34; discussion 34–35 [DOI] [PubMed] [Google Scholar]
- 28. Lövblad KO, Yilmaz H, Chouiter A, et al. Intracranial aneurysm stenting: follow-up with MR angiography. J Magn Reson Imaging 2006;24:418–22 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.