SUMMARY:
MR imaging is the preferred technique for the diagnosis, treatment planning, and monitoring of patients with neoplastic CNS lesions. Conventional MR imaging, with gadolinium-based contrast enhancement, is increasingly combined with advanced, functional MR imaging techniques to offer morphologic, metabolic, and physiologic information. This article provides updated recommendations to neuroradiologists, neuro-oncologists, neurosurgeons, and radiation oncologists on the practical applications of MR imaging of neoplastic CNS lesions in adults, with particular focus on gliomas, based on a review of the clinical trial evidence and personal experiences shared at a recent international meeting of experts in neuroradiology, neuro-oncology, neurosurgery, and radio-oncology.
Neoplastic CNS lesions are a heterogeneous group of diseases with a variable outcome that reflects the precision of diagnosis and the delivery of optimal and specific treatment. CNS imaging has a pivotal role in directing management decisions.
The goals and requirements for CNS imaging are multiple and include the establishment of a diagnosis and differential diagnosis, with accurate lesion grading for characterization of tumor biology. Imaging is an essential part of the decision-making process for therapy and later for planning of surgical or radiotherapeutic interventions. In the case of neurosurgery, neuroimaging can precisely define the location and accurately delineate the lesion and its relationship to eloquent gray- and white-matter structures, before intervention. In radiation therapy, imaging can define and demarcate margins for therapy planning. Imaging is mandatory after therapeutic intervention for monitoring disease and possible side effects.
MR imaging is the standard technique for visualizing and characterizing neoplastic CNS lesions, with superior sensitivity compared with alternative modalities.1–4 Diagnosis and treatment planning are routinely based on conventional MR imaging, such as T2-weighted imaging, FLAIR, and T1 unenhanced FSE or GRE. Following contrast-enhanced T1-weighted imaging, sequences including 3D GRE or 2D TSE or FSE are used routinely for assessment of brain tumors. Through enhancing tissue relaxation, gadolinium-based contrast media improve the sensitivity and specificity of conventional and perfusion-weighted MR imaging examinations, with the capability to identify lesions not visible on unenhanced MR imaging and to provide additional information on lesion morphology, delineation, physiology, and biology.2,5
Gadolinium-based contrast media available for use in MR imaging include the following: gadobenate dimeglumine (MultiHance; Bracco, Milan, Italy), gadobutrol (Gadovist/Gadavist; Bayer Pharma, Berlin, Germany), gadodiamide (Omniscan; GE Healthcare, Chalfont St. Giles, United Kingdom), gadopentetate dimeglumine (Bayer Pharma), gadoterate meglumine (Dotarem; Guerbet, Aulay-sous-Bois, France), gadoteridol (ProHance; Bracco), and gadoversetamide (OptiMark; Mallinckrodt, St. Louis, Missouri). These agents are available as 0.5-mol/L formulations, with the exception of gadobutrol, which is a second-generation agent available as a high-concentration 1.0-molar formulation. All currently available gadolinium-based contrast media demonstrate low (<2%) rates of qualitatively similar adverse drug reactions in surveillance studies.6–9 Clinical trial experience supports these observations and additionally indicates no relationship between gadolinium dose and the incidence of adverse reactions.10
In recent years, a number of advanced, nonenhanced, and contrast-enhanced MR imaging techniques have been developed that not only provide better specificity but offer new insights into the pathophysiology of brain tumors, mainly gliomas. These techniques, including MRS, PWI, DCE MR imaging, and DTI, are increasingly incorporated into imaging protocols and complement the morphologic detail of conventional MR imaging studies, with a range of applications including assessment of treatment response (Table 1).11
Table 1:
CNS applications of advanced MR imaging techniques
| Technique | Application |
|---|---|
| DTI and fiber tractography12,13 | Biopsy guidance |
| Determination of functionally eloquent tracts and surgical plan | |
| PWI14–24 | |
| DSC | Differential diagnosis: tumor vs nontumoral lesions, primary vs metastatic lesions, tumor grading, tumor complications, treatment response, pseudoprogression |
| DCE | Differential diagnosis: tumor vs nontumoral tissue, tumor grading, treatment response, pseudoprogression |
| DWI25,26 | Differential diagnosis: tumor vs nontumoral lesions, tumor grading, treatment response |
| ADC27,28 | Glioma grading, differentiation of high cellularity lymphoma |
| MRS29–33 | Differential diagnosis: tumor vs nontumoral lesions, primary vs metastatic lesions, treatment response |
| Blood oxygen level–dependent imaging34 | Neuronal activity, surgical guidance |
| Nuclear medicine, PET35 | Biopsy guidance, treatment response, differential diagnosis: tumor recurrence vs radionecrosis |
| Volumetric imaging36 | Not widely available and implementable |
The evolution of applications and protocols for MR imaging prompts a continued reassessment of the optimal use of this technique in clinical practice. Furthermore, the recognition that contrast enhancement is sometimes nonspecific and may not always be a true surrogate of tumor extension, grading, and treatment response mandates that new criteria be developed and validated to permit accurate assessment of the efficacy of novel therapies. This article reviews current knowledge on the clinical applications of MR imaging of adult neoplastic CNS lesions—in particular, gliomas—and provides recommendations for best practice, derived from a consensus discussion among 9 experts in neuroradiology, neuro-oncology, neurosurgery, and radio-oncology at a recent international meeting, Improving Patient Management by Optimizing MR Imaging of Neoplastic CNS Lesions, held in Zurich, Switzerland (July 13, 2010).
Applications of MR Imaging in Neoplastic CNS Lesions
Differential Diagnosis
The differential diagnosis of tumoral and pseudotumoral (mainly of inflammatory origin) lesions represents a pivotal step in patient assessment that directs subsequent management decisions. Identifying a tumoral lesion at imaging is followed typically by stereotactic biopsy or surgical resection for histologic confirmation. Not infrequently, inflammatory lesions present as single or multiple focal lesions that can be clinically and/or radiologically indistinguishable from a brain tumor. These represent a diagnostic challenge that may require biopsy for definitive diagnosis, which carries significant morbidity and may itself be nondiagnostic.
Conventional MR imaging, including T1-weighted, T2-weighted, and contrast-enhanced T1-weighted imaging, frequently provides imaging features that permit an accurate differential diagnosis between tumoral and pseudotumoral lesions in >50% of cases. Key imaging features include the following:
The Number, Topography, and Morphology of Lesions.
The presence of an additional nonpseudotumoral lesion associated with a tumorlike lesion, involving the periventricular white matter (some with an ovoid shape with the major axis perpendicular to the ventricular wall), the corpus callosum, or the dorsolateral aspect of the spinal cord, is an important feature, which should support the diagnosis of an inflammatory-demyelinating disease of nontumoral origin, particularly in the appropriate clinical setting (young and middle-aged patients without a history of cancer).
The Intrinsic Lesion Architecture.
Not infrequently, inflammatory pseudotumoral lesions show a peculiar pattern of concentric or mosaic bands on T2-weighted images, representing alternating layers of preserved and destroyed myelin (Baló-like pattern), which should be considered pathognomonic for these types of lesions (Fig 1).37
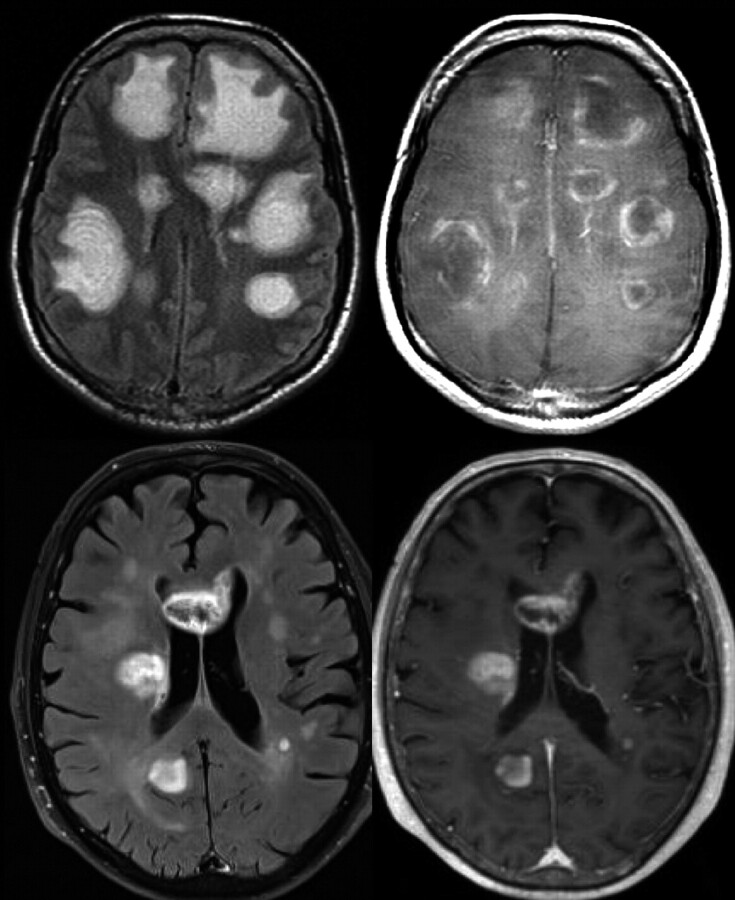
Fig 1.
Intrinsic lesion architecture. A Baló-like pattern (alternating layers of preserved and destroyed myelin) can be identified in a patient with multiple pseudotumoral inflammatory-demyelinating lesions, which shows peripheral contrast uptake (acute disseminated encephalomyelitis) (upper row). This finding is not typically present in high-grade gliomas, as shown in a patient with multiple hemispheric masses that enhanced after contrast administration, which proved to be a multifocal glioblastoma (lower row).
The Pattern of Contrast Medium Uptake.
Acute inflammatory pseudotumoral lesions sometimes show an incomplete ring enhancement on T1-weighted gadolinium-enhanced images, with the open border facing the superficial or deep gray matter. This feature, which may be explained by the reduced degree of inflammatory cell infiltration and blood-brain barrier disruption of these lesions when involving the gray matter, is helpful for distinguishing inflammatory-demyelinating lesions from other focal lesions such as high-grade gliomas or metastases, where the ring enhancement surrounding a central area of necrosis is complete, independent of its relation to the gray matter (Fig 2).
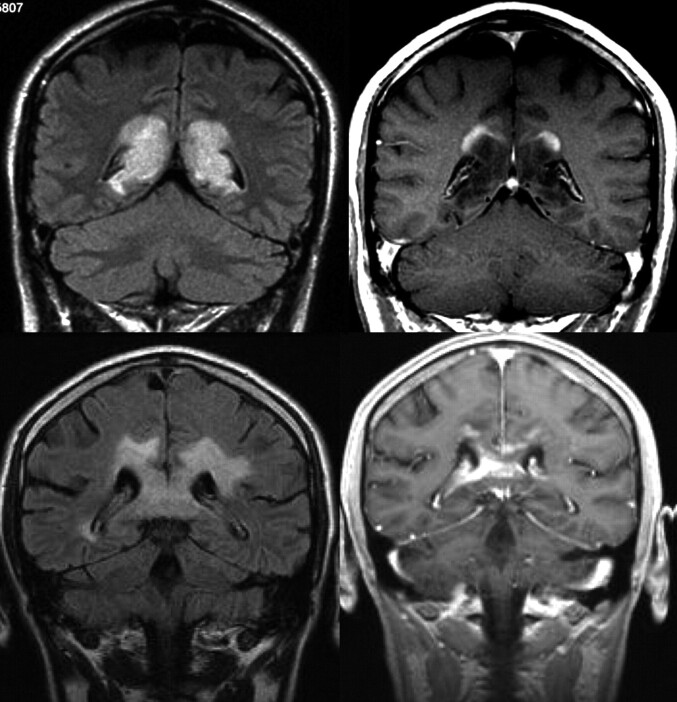
Fig 2.
Patterns of contrast media uptake. A large tumefactive inflammatory lesion involving the corpus callosum shows an open ring enhancement with the open border facing the cortical gray matter (upper row). This feature is not typically seen in high-grade gliomas, where peripheral enhancement is identified even in the margins of the lesion in contact with the gray matter (lower row).
In addition to these features based on conventional MR imaging, advanced but otherwise routine techniques in daily practice, such as DWI sequences, provide useful information for providing an accurate differential diagnosis between pyogenic abscesses and necrotic tumoral lesions (both metastases and high-grade gliomas), because typically the viscous purulent content of abscesses induces a marked diffusion restriction and, as a consequence, strongly reduced ADC.37 However, this technique cannot distinguish necrotic brain tumors from inflammatory pseudotumoral cystic lesions, because in both cases, DWI may show a rim of decreased diffusion (low signal intensity on ADC maps) surrounding the central necrotic or cystic area with increased diffusion (high signal intensity on ADC maps).
The capability of proton MRS to differentiate pseudotumoral lesions from brain tumors is unresolved in the literature. While some authors report that spectral differences are insufficient to provide a precise diagnosis,38,39 others conclude that discrimination is possible by using a pattern-recognition system.40 Metabolites investigated in these studies include N-acetylaspartate (an index of neural tissue viability), choline (a measure of cell membrane attenuation and turnover), glutamate and glutamine (markers of inflammatory processes), and myo-inositol (indicator of glial cell proliferation). Creatinine is a widely used marker of overall brain metabolism. Saindane et al41 reported significant differences in N-acetylaspartate/creatinine ratios between the central regions of pseudotumoral inflammatory lesions and high-grade gliomas. Cianfoni et al42 observed marked elevations of glutamate and glutamine peaks at short TE in pseudotumoral inflammatory lesions that were not typical of high-grade gliomas. Majos et al43 reported discrimination between brain tumors and pseudotumoral lesions by using myo-inositol/N-acetylaspartate at short TEs and choline/N-acetylaspartate at long TEs. Despite these observations, caution is advised before basing a diagnosis on spectroscopic findings alone.
Perfusion MR imaging studies can be helpful in differentiating pseudotumoral inflammatory lesions and high-grade gliomas because only the latter show a significant increase in CBV (Fig 3).44 However, despite the generally decreased cerebral blood flow in inflammatory lesions, a transient increase may be identified in the very early phase of the formation of these lesions.17 A pitfall in perfusion imaging (DSC MR imaging) of lesions is that a very leaky blood-brain barrier can confound CBV measurements and cause lesions with low CBV or perfusion to artifactually demonstrate high perfusion because of the leakiness.
Fig 3.
MR imaging (FLAIR, contrast-enhanced T1-weighted, and cerebral blood flow maps acquired with arterial spin-labeling) obtained in patients with a high-grade necrotic glioma (upper row) and an acute inflammatory-demyelinating lesion (lower row). Observe how, despite similar lesion patterns on both T2- and contrast-enhanced T1-weighted sequences, only the high-grade glioma shows a clear increase in cerebral blood flow.
Conventional MR imaging is capable of distinguishing primary brain tumors from metastatic lesions in most cases, on the basis of the number, location, and architecture of lesions. The use of high-concentration contrast media provides optimal lesion visualization for differential diagnosis. In cases of diagnostic uncertainty, PWI or MRS can increase diagnostic confidence. Elevations of CBV in the peritumoral region (representing tumor infiltration/vascularity) and the choline/creatinine ratio (increased cell membrane turnover) indicate high-grade gliomas rather than metastases.29,45
Primary Tumor Biology and Grading
Histologic Grading and Its Limitations.
Histologic classification remains the criterion standard for grading neoplastic CNS lesions, which reflects the clinical outcome in many instances. The updated, 2007 World Health Organization classification of brain tumors provides each lesion type with a name (based on cell type and histologic criteria), grading (aggressiveness), and a code for whether the mass is benign, malignant, or borderline.46
Critics have noted, however, that problems of classification and grading remain, reflecting both the complexity of neoplastic lesions and the challenges of histologic interpretation, such as sampling error and inter- and intraobserver variability.20 Future revisions of the World Health Organization classification will likely incorporate molecular markers and imaging biomarkers to aid classification of glioma biology. As discussed in this article, MR imaging—particularly by using advanced techniques—can provide additional information on lesion grading relevant to outcome.
Role of MR Imaging and Contrast Enhancement in Tumor Grading.
Low- and high-grade gliomas may be differentiated on conventional MR imaging by characteristic differences in the degree of contrast enhancement and the extent of mass effect and cyst formation, which are elevated in higher lesion grades.47 Low- and high-grade gliomas may, however, present atypically on conventional MR imaging; this outcome has led to investigation of advanced MR imaging for grading.48
PWI, in particular rCBV measurement, has the capability not only of differentiating low- from high-grade gliomas but of predicting progression and survival.14,19,24,49 In 1 study, a threshold value of 1.75 for rCBV derived from DSC had a sensitivity and specificity of 95.0% and 57.5%, respectively, for differentiating low- and high-grade gliomas, with the ability to predict progression-free survival (Fig 4). With further optimization of acquisition and postprocessing methods, this technique has the potential to be an important biomarker of glioma malignancy and patient outcome.20 Quantification of endothelial permeability by using DCE PWI (most commonly by using Ktrans) is an additional technique with the utility for lesion grading that may be combined with DSC.19
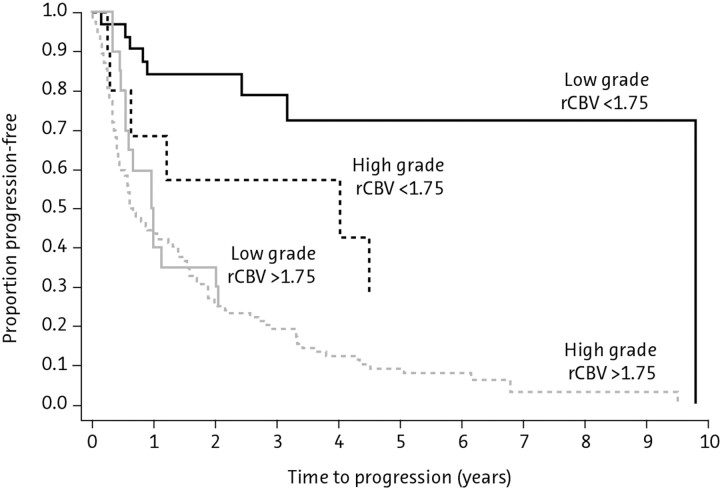
Fig 4.
Kaplan-Meier survival curves for progression-free survival within a low-grade glioma group with low and high rCBV (<1.75 and >1.75, respectively; solid lines) demonstrating a significant difference in time to progression in low-grade gliomas stratified by rCBV alone (P < .0001). Similarly, when comparing high-grade gliomas (broken lines), one sees a significant difference in progression with high-versus-low rCBV (<1.75 versus >1.75) (P < .0001). Among subjects with low rCBV (<1.75), there is a significant difference between low- and high-grade gliomas with respect to progression-free survival (P = .047). However, among subjects with high rCBV (> 1.75), progression-free survival is not significantly different for low-versus-high-grade gliomas (P = .266). Reprinted with permission from Radiology (2008;247:490–98). Copyright 2008, Radiological Society of North America.
The use of MRS for glioma grading is promising, particularly when combined with PWI, but the technique remains largely investigational, with drawbacks of low specificity, long acquisition times, and artifacts dependency, particularly in the postoperative phase.14
Role of Contrast Enhancement in Guiding Biopsy.
The histologic composition of lesions can vary markedly, even within the same lesion mass. Biopsy directed by contrast-enhanced imaging, pre- or perioperatively, increases the likelihood of retrieving the most malignant tissue for histologic diagnosis, which will guide treatment decisions.50–52 The use of high-concentration contrast media and advanced MR imaging techniques provides reliable lesion visualization to direct biopsy.53
PWI in this context has proved its utility to locate regions of high vascularity, and hence malignancy, in lesions.19 Diffusion tensor tractography is a promising advanced MR imaging technique for localizing tumor infiltration in 3D and may have applications for intraoperative surgical planning, avoiding eloquent white matter tracts.54
Identification of Tumor Complications
Neurologic complications associated with the lesion itself or its treatment may require emergency assessment and treatment. Clinically important complications include acute ischemic stroke, intracranial hemorrhage, mass effect, hydrocephalus, infection, and spinal cord compression.55 MR imaging is the recognized technique of choice for identifying and directing the treatment of these complications.55
Treatment Planning (Primary Brain Tumors)
Resection.
Surgical resection, commonly followed by postoperative radiation therapy or chemotherapy or both, represents the treatment of choice for high-grade gliomas. A decision to attempt resection is founded on the MR imaging characteristics of tumor size, location, spatial configuration, and presumed margins, together with assessment of the patient's neurologic and medical condition.50–52
Total resection is superior to partial resection or biopsy for providing histologic material and leads to enhanced patient survival.56,57 Total resection overcomes some, but not all, of the limitations of sampling error. Contrast-enhanced MR imaging postoperatively provides assessment of the success of surgery by locating residual tumor.
Radiation Therapy.
The role of radiation therapy remains controversial for low-grade gliomas.58,59 Several groups have evaluated the impact of radiation therapy, showing that radiation immediately after the primary diagnosis can increase progression-free survival; however, overall survival is comparable with that of patients treated for tumor progression of their low-grade gliomas. For glioblastomas, radiation therapy with temozolomide is demonstrated to prolong survival and delay progression and is currently the standard of care.60–62
For treatment of high-grade gliomas, the radiation oncologist defines gross tumor volumes and clinical target volumes, which are based on pre- and postsurgical imaging. A set of unenhanced and contrast-enhanced CT and MR images is generally necessary for radiation therapy treatment planning. For high-grade gliomas, gross tumor volume is commonly defined as the macroscopic tumor visible on T1 contrast-enhanced MR imaging; however, the clinical target volume comprises T2 or FLAIR hyperintense lesions, adding a necessary safety margin to potential microscopic spread. Additional examinations, such as MRS, can provide important information for intricate target-volume definition. As techniques in radiation oncology have evolved, leading to continuously increasing precision of treatment, more detailed and specific imaging examinations are required.63,64Advanced MR imaging techniques postradiation and -chemotherapy have a pivotal role in distinguishing response from pseudoresponse and true disease progression from pseudoprogression. The timing of these scans is critical for accurate assessment, as discussed below.
Non-MR Imaging Modalities: Pros and Cons
MR imaging represents the technique of choice for visualizing and grading brain tumors. However, CT, with a lower resolution than MR imaging, does have applications in the emergency setting, where an MR imaging protocol may be impracticable on the day of presentation. Perfusion CT can provide noninvasive information on tumor vasculature and angiogenesis within gliomas that correlates with histologic and angiographic markers, with relevance to grading and prognosis.65
PET, most commonly with [18F] fluorodeoxyglucose, has a role for directing biopsy, as a prognostic indicator, and for evaluating disease after therapy.35 Radiolabeled amino acids (eg, 11C-methionine, [18F] fluoroethyl-tyrosine) are valuable PET tracers because of their high uptake in tumor tissue.35 Newer tracers are being investigated for the brain, including [18F] fluorothymidine, which may have even higher specificity than glucose-based tracers.53 The sensitivity of PET is enhanced in combination with MR imaging.35
Posttherapeutic Response Assessment
CNS lesions show great variability in their natural course and response to therapy. MR imaging provides an important reference with which to monitor treatment response.
The Macdonald criteria were developed in 1990 for assessing the posttherapeutic response of high-grade gliomas.66 These criteria define disease progression as an increase in enhancing tumor area ≥25% or the appearance of new enhancing lesions. However, the Macdonald criteria have a number of recognized limitations,67 including the following:
Difficulty of measuring irregularly shaped tumors
Interobserver variability
Lack of assessment of nonenhancing lesion components
Lack of guidance for assessment of multifocal tumors
Difficulty of measuring enhancing lesions in the wall of cystic or surgical cavities
After complete resection, no tissue available for assessment.
In addition, increased gadolinium enhancement is a nonspecific finding that may have a number of causes:
A disrupted blood-brain barrier, which can be influenced by changes in steroid dose or radiologic technique68
Nontumoral processes such as treatment-related inflammation, seizure activity, postsurgical changes, ischemia, subacute radiation effects, pseudoprogression, and radiation necrosis.
As a consequence, contrast enhancement may not correlate with changes observed on T2-weighted or FLAIR imaging.67,69 Advanced MR imaging techniques have utility to overcome the limitations of the Macdonald criteria, as reported in the section below.
Consensus Statement
Conventional MR imaging is the technique of choice for differential diagnosis, tumor grading, and treatment planning of neoplastic CNS lesions.
Alternative imaging modalities (CT, PET) have some applications under specific circumstances, frequently as a complement to MR imaging.
MR imaging is an important reference point for monitoring treatment response and recurrence, but the Macdonald criteria have limitations.
Current Limitations of MR Imaging in Neoplastic CNS Lesions
Response Assessment by the Macdonald Criteria
Advances in therapeutic management highlight the limitations of the Macdonald criteria for assessing tumor response. Approximately 10%–30% of patients with glioblastoma treated with the current standard of care—radiation therapy combined with temozolomide—have been proposed to demonstrate “pseudoprogression,” which is defined as increased gadolinium enhancement that does not reflect true tumor progression. Pseudoprogression is most prevalent within the first 12 weeks after completion of radiation therapy and may, if misinterpreted, lead to premature discontinuation of effective therapy (Fig 5).68–71
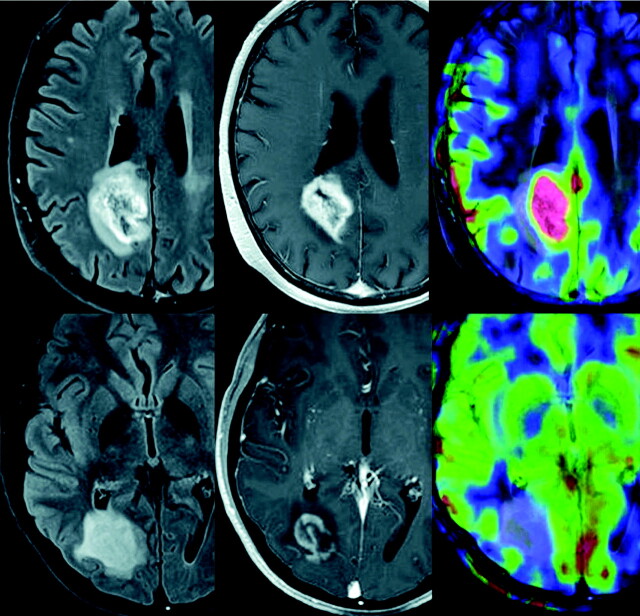
Fig 5.
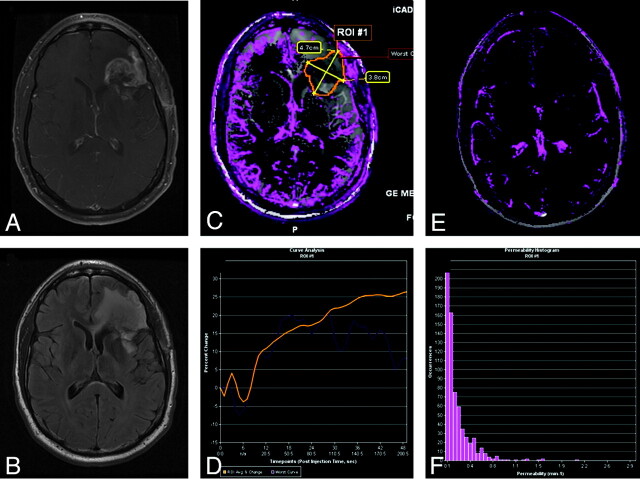
Pseudoprogression in left frontal anaplastic astrocytoma. A, Axial T1-weighted image with contrast shows posttherapeutic brain with nodular contrast enhancement. B, Axial FLAIR image demonstrates increased edema surrounding the enhancing lesion. C, Permeability/Ktrans map with the region of interest. D, DCE MR imaging T1 signal intensity curve demonstrates reduced perfusion and permeability, suggesting pseudoprogression rather than recurrent tumor. Therapy was continued because the findings were thought to be due to pseudoprogression from chemoradiation therapy. E, Permeability/Ktrans color overlay, again confirming decreased vascularity and Ktrans. F, Histogram of each pixel within the region of interest in C, confirming that the permeability is in the lower range, demonstrating pseudoprogression rather than true disease progression. Courtesy of M. Law, Los Angeles, California.
Conversely, antiangiogenic therapies (eg, bevacizumab) can induce a “pseudoresponse” within 1–2 days of initiating treatment, presumably through normalization of the blood-brain barrier.67,69 A subgroup of patients treated with antiangiogenic therapies showed evidence of recurrence, characterized by progressive increases in the nonenhancing component on T2-weighted or FLAIR sequences, which correlate with neurologic deterioration.
New Criteria for Response Assessment
The limitations of the Macdonald criteria, including the recognition that contrast enhancement is nonspecific and may not be a true surrogate of tumor progression or response, have led to the development of new criteria for assessing high-grade gliomas that are summarized in Table 2.67
Table 2:
New criteria for response assessment of high-grade gliomasa
| Standardization of Imaging Definitions |
| Measurable and nonmeasurable disease for contrast-enhancing lesions |
| Measurable disease: 2D contrast-enhancing lesions with clearly defined margins, with 2 perpendicular diameters of at least 10 mm, visible on ≥2 axial sections that are preferably, at most, 5 mm apart |
| Nonmeasurable disease: either unidimensionally measurable lesions, masses with margins not clearly defined, or lesions with maximal perpendicular diameters <10 mm |
| Multiple lesions |
| A minimum of 2 (maximum of 5) largest lesions should be measured on the basis of the sum of products of perpendicular diameters |
| Enhancing lesions are considered target lesions for evaluation of response |
| Definition of progression |
| ≥25% increase in sum of products of perpendicular diameters of enhancing lesions compared with smallest tumor measurement at reference scan (if no decrease) or best response after initiation of therapy |
| Significant increase in T2/FLAIR nonenhancing lesion compared with reference scan or best response |
| Clear progression of nonmeasurable disease |
| Clear clinical deterioration |
| Reference MR imaging |
| Criteria for determining progression are dependent on the time from initial chemotherapy |
| If obtaining the reference MR image immediately postoperative, MR imaging in the first 12 weeks may represent |
| pseudoprogression and pseudoresponse |
| If obtaining the reference scan after that initial 12-week period, then it reduces the likelihood of confusion with pseudoprogression |
| Take note of enhancement outside radiation field; it may indicate progression (Fig 6) |
| A reference MR image should ideally be obtained within 24–48 hours after surgery and no later than 72 hours after surgery, to avoid interpretation of postoperative changes as residual enhancing disease |
Based on Wen et al 2010.67
Fig 6.
Time course of pseudoprogression and change in the reference or baseline MR imaging. Criteria for determining progression are dependent on the time from initial chemotherapy and radiation. If one takes the reference MR image immediately postoperative, the first 12-week MR image may represent pseudoprogression and pseudoresponse. If one takes the reference scan after that initial 12-week period, then it essentially excludes pseudoprogression. Note enhancement outside the radiation field, where any enhancement may indicate disease progression. To avoid interpretation of postoperative changes as residual enhancing disease, one should ideally obtain a reference MR image within 24–48 hours after surgery and no later than 72 hours after surgery.
Advanced MR imaging techniques, particularly PWI, may assist in differentiating posttreatment changes from the true recurrence of high-grade gliomas.72 Pseudoprogression following radiochemotherapy is characterized by a low rCBV in conjunction with “lowish” permeability (blood-brain barrier leakage) and increased choline, whereas true progression is demonstrated by elevated rCBV (>1.75) and permeability (Fig 4).
Consensus Statement
New criteria for response assessment67 have been developed because of limitations with the Macdonald criteria, offering revised definitions of disease progression.
Appropriate timing of MR imaging reference assessments is critical to the interpretation of response and progression.
A reference MR image should be performed before radiation therapy (preferably within 7 days), with repeat scans at 4 and 12 weeks postradiation.
MR imaging to identify the extent of resection should be performed at latest 72 hours postsurgery.
Contrast enhancement beyond the initial radiation field raises the suspicion of recurrent or progressive tumoral disease, whereas enhancement within the radiation portal could represent pseudoprogression.
Advanced MR imaging with PWI offers advantages for the assessment of pseudoprogression.
Protocols and Techniques for MR Imaging of Neoplastic CNS Lesions
Protocol Sequence and Equipment Optimization
Protocol Sequence.
Optimizing the protocol sequence enhances CNS lesion visualization and characterization. Components of a standardized protocol for conventional MR imaging include T1-weighted precontrast, T2-weighted, DWI, and T1-weighted contrast imaging. FLAIR may be substituted for T2 to save time. PWI, MRS, and other advanced MR imaging techniques may be included in the protocol according to the availability of equipment and the clinical scenario (Table 1). Recommendations for an MR imaging protocol that can be adopted in as many institutions as possible are shown in Table 3.
Table 3:
Standard protocol for brain tumor imaging based on expert panel discussion following the framework of the ACRIN 6686 component of the RTOG 0825 protocol73
| Standardized MR imaging protocol |
| 3-Plane localizer/scout (in order of acquisition) |
| T1-weighted precontrast (spin-echo) |
| T2-weighted axial |
| FLAIR (optional to perform after contrast) |
| T1 map (quantitation) for DCE MR imaging—3D gradient-echo T1 or 2D TSE/FSE T1a |
| DWI and/or DTI (can extract DWI data trace/ADC from DTI)a |
| T2* DSC MR imaging (after presaturation DCE MR imaging sequence)a |
| T1-weighted postcontrast (spin-echo) |
| Functional language, auditory, visual, motor testing, and MRSa |
| Can do FLAIR before DSC MR imaging |
| SWI, gradient-echo, additional optional sequencesa |
| General parameter recommendations |
| Section thickness not greater than 5 mm |
| Delay is recommended, which can be built in by performing DWI and/or DTI before acquiring T1 sequences. Another option is to perform FLAIR (or even T2) before T1 sequences, which may give additional sensitivity for leptomeningeal disease74 |
| Target duration ≤30 minutes (maximum, 1.5–2.0 hr) |
Note:—ACRIN indicates American College of Radiology Imaging Network; SWI, susceptibility-weighted imaging; RTOG = Radiation Therapy Oncology Group.
Part of the ACRIN 6686 protocol but can be used as an adjunct in the clinical brain tumor protocol.
Equipment Optimization.
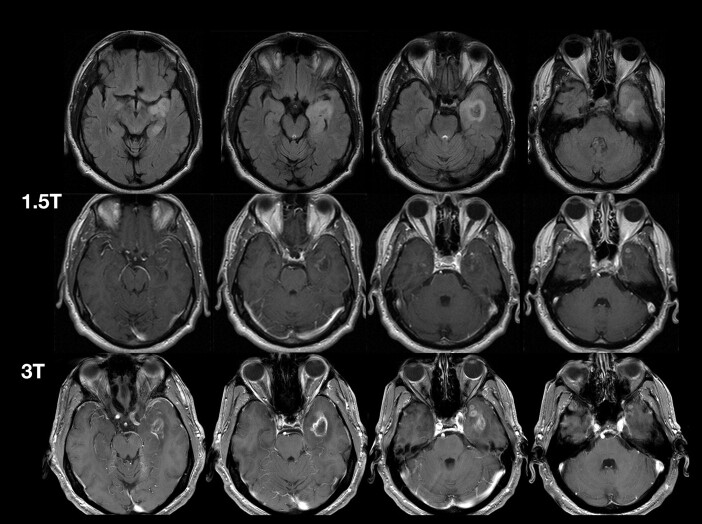
High field strengths provide superior image quality through increased signal intensity. The clinical superiority of 3T over 1.5T has been demonstrated in multiple sclerosis75,76 and, in animal models, in brain tumor imaging.77–79 Case study experience supports superior CNS lesion grading and monitoring with 3T versus 1.5T (Fig 7).
Fig 7.
Comparison of MR images at 1.5 and 3T in a patient with astrocytoma grade III after administration of gadobutrol, 0.1 mmol/kg.
Head coils with ≥32 channels, for use with higher field strengths, offer additional signal intensity–to-noise ratio gains compared with conventional equipment, but these coils are currently uncommon in routine practice.80,81 An industry standard at the time of submission is the 8- or 16-channel head coil.
Timing of Image Acquisition.
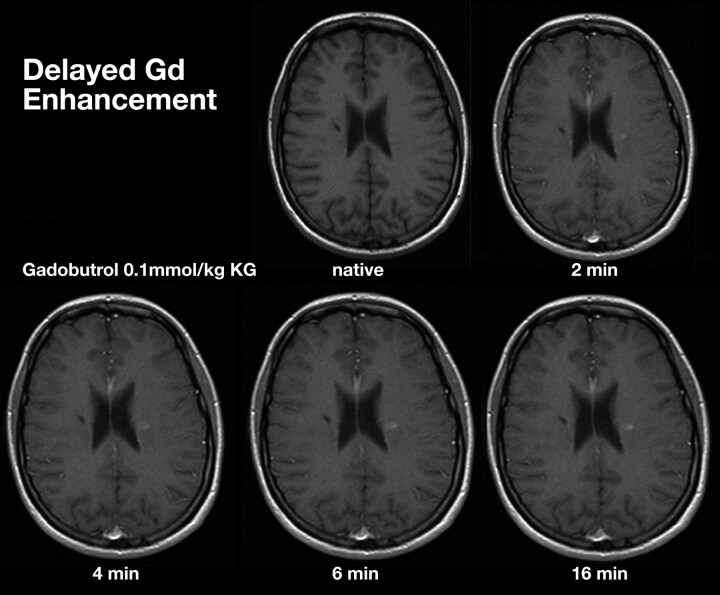
Delays of up to 20 minutes postcontrast injection significantly improve lesion detection rates and the assessment of lesion volume (Fig 8).82,83 Experimental studies by using a rat glioma model suggested that an 8-minute delay postinjection represents a practical compromise between enhanced lesion detection and extended scanning time.84 Delay can be added in by performing DWI and or DTI before acquiring T1 sequences (Table 3).
Fig 8.
Comparison of MR images at increasing time intervals after administration of gadobutrol, 0.1 mmol/kg.
Consensus Statement
Optimization of the protocol sequence enhances CNS lesion characterization.
Components of a standardized protocol for conventional MR imaging include T1-weighted precontrast, T2-weighted (or FLAIR), DWI, and T1-weighted contrast imaging.
Incorporation of additional advanced MR imaging techniques (selected according to the clinical scenario) can provide physiologic data to complement morphologic information.
Higher field strengths (eg, 3T) and head coils with multiple channels, if available, are preferred for superior image quality.
Delays of up to 20 minutes postcontrast injection significantly improve lesion detection rates.
New protocol guidelines have been developed for the assessment of therapeutic response67 because of limitations of the Macdonald criteria relating to pseudoprogression and pseudoresponse, especially after chemoradiotherapy with temozolomide and radiation therapy.
Contrast Medium Choice
Chelate Stability.
Gadolinium-based contrast media can be categorized by their molecular structure into linear and macrocyclic groups. Relative to the agents classified in the linear group, media with a macrocyclic structure (gadobutrol, gadoterate dimeglumine, and gadoteridol) demonstrate an increased stability and a reduced propensity to release gadolinium ions in preclinical experiments that include conditions mimicking renal impairment.85,86
The release of gadolinium ions from certain contrast media has been associated with the rare condition of nephrogenic systemic fibrosis in patients with severe renal impairment.87 In separate recent initiatives, the US Food and Drug Administration and the Committee for Medicinal Products for Human Use of the European Medicines Agency have issued guidance on the risk of nephrogenic systemic fibrosis associated with each gadolinium-based contrast medium, placing the macrocyclic agents into lowest-risk groups.88,89
Contrast Enhancement.
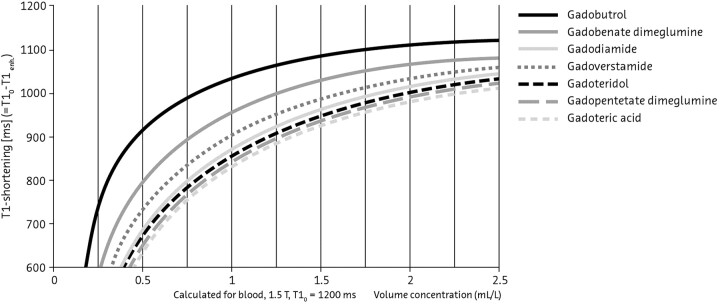
The physicochemical characteristics of contrast media that are associated with the extent of enhancement at MR imaging are their gadolinium concentration and T1 relaxivity. Among current contrast media, gadobutrol possesses the highest gadolinium concentration (1 mol/L) and a high relaxivity, which combine to produce the highest T1 shortening effect per milliliter with the greatest signal intensity (Fig 9).90–92
Fig 9.
Comparison of T1 shortening effect among gadolinium-based contrast media, based on Port et al 200590 and Rohrer et al 2005.92.
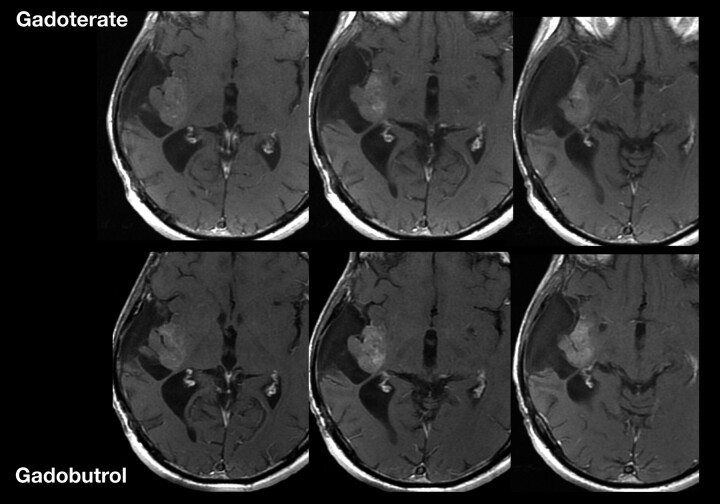
Numerous intraindividual trials have directly compared the imaging characteristics of contrast media in patients with primary CNS lesions or metastases.91,93–104 Among these trials, gadobenate dimeglumine has demonstrated superior lesion enhancement and diagnostic information compared with gadopentetate or gadodiamide,99,100,102 which is attributed to the higher relaxivity of gadobenate. In similarly designed trials, gadobutrol has demonstrated superior performance, including enhanced lesion detection and conspicuity, compared with the 0.5-mol/L agents gadopentetate and gadoterate, administered at the same dose and by using the same field strength (1.5T), reflecting the high T1 shortening effect of gadobutrol (Fig 10).94,98,105
Fig 10.
Recurrent right temporoinsular glioma in a 48-year-old patient. Consecutive axial views of T1-weighted images after a single dose (0.1 mmol/kg body weight) of gadoterate dimeglumine or gadobutrol. On gadobutrol-enhanced images, the tumor presents with significantly stronger contrast enhancement, which allows better delineation of suspected anaplastic tumor from nonenhancing tumor areas and adjacent structures.
Animal glioma models support the clinical observations of superior lesion enhancement with gadobutrol versus 0.5-mol/L agents, both at 1.5T and 3T.77,106,107
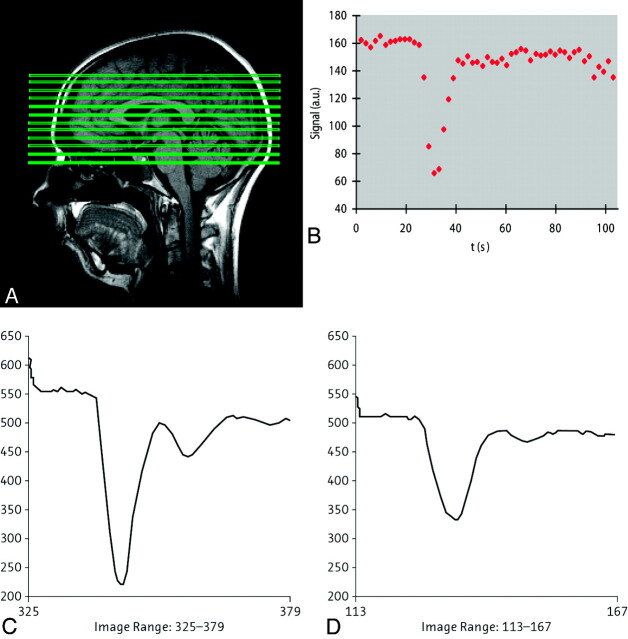
Direct comparison of gadobutrol at 2 concentrations (with the same total dose) in volunteers demonstrated the benefits of the 1 mol/L over a 0.5-mol/L gadolinium concentration for CNS perfusion imaging, which is attributable to the sharper bolus peak and the increased first-pass gadolinium concentration related to a lower injection volume (Figs 11 and 12).108
Fig 11.
A, Sagittal scout MR image with the position of the sections in which perfusion information is acquired. B, Signal intensity–time curve from DSC MR imaging after a bolus injection of a single dose of contrast agent, with substantial signal intensity drop due to the susceptibility effect of the contrast medium. C and D, Signal intensity–time curves from different contrast medium concentrations at a triple dose: 28 mL of the 1.0 mol/L gadobutrol formulation (C) and 56 mL of a 0.5 mol/L gadobutrol formulation (D) in the putamen of the same subject. The susceptibility effect is significantly stronger by using a higher concentration of contrast medium. C and D, reprinted with permission from Radiology (2003;226:880–88). Copyright 2003, Radiological Society of North America.
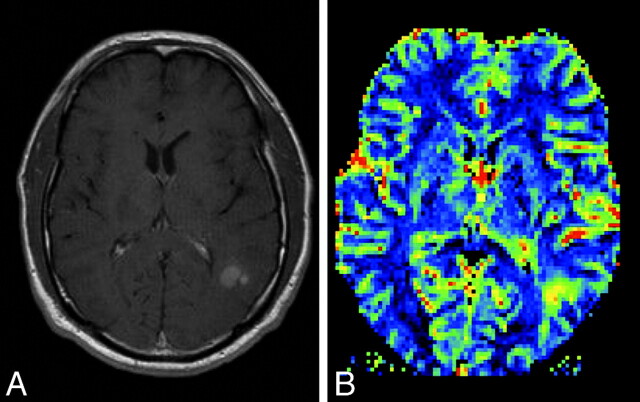
Fig 12.
A, Postcontrast T1-weighted MR image in a patient with a new appearance of a contrast-enhancing lesion in a formerly radiotherapeutically treated fibrillary astrocytoma. From conventional imaging sequences, one cannot differentiate treatment-related blood-brain barrier breakdown and malignization of the tumor. B, rCBF perfusion parameter image shows a highly perfused lesion, which was suspicious and later histologically confirmed as a high-grade tumor nodule within the low-grade astrocytoma.
Dosage Recommendations.
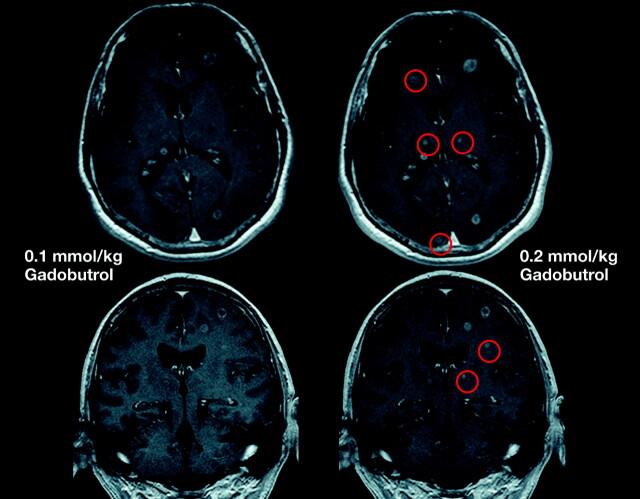
The standard gadolinium dose for contrast media used in MR imaging of the CNS is 0.1 mmol/kg of body weight. Single doses are recommended at many centers for visualization of gliomas, with the option to administer additional doses in cases of diagnostic doubt (Fig 13). For detection of multiple lesions, including metastases, higher concentrations (0.2–0.3 mmol/kg of body weight) may identify additional lesions and are widely used at the initial assessment.4,10,109–119 Double dosing also improves image quality and data quantitation in MR perfusion studies.95
Fig 13.
Comparison of MR images by using gadobutrol at 0.1 and 0.2 mmol/kg. Single-dose (left) and double-dose (right) contrast-enhanced MR images in a patient with cerebral metastases. With the use of double-dose gadobutrol, one can detect substantially more lesions (circles) (see also Kim et al 201098) and lesions already visualized with an improved contrast and a better delineation.
Typically, as for all pharmaceuticals, the lowest dose possible should be used. This is particularly important in the context of the risk of nephrogenic systemic fibrosis and especially in patients with severely impaired renal function (GFR below 30 mL/min/1.73 m2).120
Consensus Statement
Contrast enhancement by using gadolinium-based contrast media is a routine component of the MR imaging protocol for patients with CNS lesions.
Chelate stability is an important consideration in the choice of contrast medium, particularly for patients with renal impairment.
Contrast media with high gadolinium concentration and higher relaxivity are preferred for superior enhancement. Gadobutrol (1 mol/L) offers the highest gadolinium concentration and high relaxivity to provide the highest T1 shortening effect among currently available agents.
A single dose (0.1 mmol/kg of body weight) of gadolinium-based contrast medium is recommended for suspected primary lesions, with a second administration in cases of diagnostic doubt.
In patients with a GFR below 30 mL/min/1.73 m2, a single dose only is recommended (with preference for a macrocyclic). With a GFR of 30–60 mL/min/1.73 m2, a single or double dose may be used (preference for a macrocyclic).
Future Challenges
Standardization of an optimized protocol across centers is an important objective, with benefits for the uniform performance and interpretation of MR imaging studies. However, variability among centers in the equipment and the data-interpretation software that are available and a lack of trial evidence to confirm the clinical benefit of novel MR imaging techniques represent barriers to standardized protocol implementation.
Summary of Expert Meeting Recommendations
Recommendation 1: Applications of MR Imaging in Neoplastic CNS Lesions
MR imaging is the technique of choice for the differential diagnosis, tumor grading, and treatment planning of neoplastic CNS lesions.
Advanced MR imaging techniques provide physiologic data relevant to diagnosis and grading that may assist conventional MR imaging.
Recommendation 2: Limitations of MR Imaging—Posttherapeutic Response Assessment
MR imaging is an important reference point for monitoring treatment response and recurrence, but the Macdonald criteria have limitations.
New criteria for assessing enhancing/nonenhancing lesions67 offer amended guidance for response assessment.
Advanced MR imaging techniques may help assess the posttherapeutic brain when contrast enhancement is nonspecific.
Recommendation 3: Standardized Protocol
Optimization of the protocol sequence enhances CNS lesion characterization.
A standardized protocol sequence for conventional MR imaging includes T1-weighted precontrast, T2-weighted, DWI, and T1-weighted contrast imaging. Additional advanced MR imaging techniques can be selected according to the clinical scenario.
Higher field strengths (eg, 3T versus 1.5T) provide superior image quality, if available.
Delay is recommended. Image acquisition at up to 20 minutes postcontrast injection offers improved lesion detection.
Recommendation 4: Contrast Medium Dose
A single dose (0.1 mmol/kg of body weight) of gadolinium-based contrast medium is recommended for suspected primary lesions, with a second administration in cases of diagnostic doubt.
In patients with a GFR below 30 mL/min/1.73 m2, a single dose only is recommended. With a GFR of 30–60 mL/min/1.73 m2, a single or double dose may be used.
Recommendation 5: Contrast Medium Choice
Contrast media with a macrocyclic structure (eg, gadobutrol, gadoterate dimeglumine, and gadoteridol) have a higher chelate stability than linear agents.
Contrast media with high gadolinium concentration and higher relaxivity are preferred for superior enhancement. Gadobutrol offers the highest gadolinium concentration (1 mol/L) and high relaxivity to provide the highest T1 shortening effect among currently available agents.
ABBREVIATIONS:
- ADC
apparent diffusion coefficient
- CBV
cerebral blood volume
- CNS
central nervous system
- DCE
dynamic contrast-enhanced
- DSC
dynamic susceptibility contrast
- DTI
diffusion tensor imaging
- DWI
diffusion-weighted imaging
- FLAIR
fluid-attenuated inversion recovery
- FSE
fast spin-echo
- GFR
glomerular filtration rate
- GRE
gradient recalled-echo
- Ktrans
volume transfer coefficient
- MRS
MR spectroscopy
- PET
positron-emission tomography
- PWI
perfusion-weighted imaging
- rCBV
relative cerebral blood volume
- TSE
turbo spin-echo
Footnotes
Disclosures: Marco Essig, Consultant: Bayer Healthcare, Details: consultancy agreement. Alex Rovira, Research Support (including provision of equipment or materials): Bayer Schering Pharma; Speaker Bureau: Bayer Schering Pharma, Sanofi-Aventis, Bracco, Merck Serono, Teva Pharmaceutical Industries Ltd, Biogen Idec; Consultant: Novartis. Michael Weller, Speaker Bureau: Bayer Schering, Details: participation in 1 advisory board. Meng Law, Research Support (including provision of equipment or materials): accelerate brain, cancer cure, Details: research grant. Speaker Bureau: Toshiba Medical Systems; Consultant: Bayer Healthcare, Bracco Diagnostics, Fuji Inc, iCAD Inc; Ownership Interest: Prism Clinical Imaging.
The expert meeting and the preparation of the review article were funded by an unrestricted educational grant from Bayer HealthCare. PAREXEL provided editorial support.
References
- 1. Essig M, Weber MA, Tengg-Kobligk H, et al. Contrast-enhanced magnetic resonance imaging of central nervous system tumors: agents, mechanisms, and applications. Top Magn Reson Imaging 2006;17:89–106 [DOI] [PubMed] [Google Scholar]
- 2. Runge VM, Muroff LR, Wells JW. Principles of contrast enhancement in the evaluation of brain diseases: an overview. J Magn Reson Imaging 1997;7:5–13 [DOI] [PubMed] [Google Scholar]
- 3. Schellinger PD, Meinck HM, Thron A. Diagnostic accuracy of MRI compared to CCT in patients with brain metastases. J Neurooncol 1999;44:275–81 [DOI] [PubMed] [Google Scholar]
- 4. Yuh WT, Tali ET, Nguyen HD, et al. The effect of contrast dose, imaging time, and lesion size in the MR detection of intracerebral metastasis. AJNR Am J Neuroradiol 1995;16:373–80 [PMC free article] [PubMed] [Google Scholar]
- 5. Runge VM, Muroff LR, Jinkins JR. Central nervous system: review of clinical use of contrast media. Top Magn Reson Imaging 2001;12:231–63 [DOI] [PubMed] [Google Scholar]
- 6. Forsting M, Palkowitsch P. Prevalence of acute adverse reactions to gadobutrol: a highly concentrated macrocyclic gadolinium chelate—review of 14,299 patients from observational trials. Eur J Radiol 2010;74:e186–e192. Epub 2009 Jul 2 [DOI] [PubMed] [Google Scholar]
- 7. Bleicher AG, Kanal E. Assessment of adverse reaction rates to a newly approved MRI contrast agent: review of 23,553 administrations of gadobenate dimeglumine. AJR Am J Roentgenol 2008;191:W307–11 [DOI] [PubMed] [Google Scholar]
- 8. Herborn CU, Honold E, Wolf M, et al. Clinical safety and diagnostic value of the gadolinium chelate gadoterate meglumine (Gd-DOTA). Invest Radiol 2007;42:58–62 [DOI] [PubMed] [Google Scholar]
- 9. Nelson KL, Gifford LM, Lauber-Huber C, et al. Clinical safety of gadopentetate dimeglumine. Radiology 1995;196:439–43 [DOI] [PubMed] [Google Scholar]
- 10. Engelhorn T, Doerfler A. High-molar contrast agents for CNS application. Imaging Decisions MRI 2008;11:26–32 [Google Scholar]
- 11. Cha S. Update on brain tumor imaging: from anatomy to physiology. AJNR Am J Neuroradiol 2006;27:475–87 [PMC free article] [PubMed] [Google Scholar]
- 12. Field AS, Alexander AL, Wu YC, et al. Diffusion tensor eigenvector directional color imaging patterns in the evaluation of cerebral white matter tracts altered by tumor. J Magn Reson Imaging 2004;20:555–62 [DOI] [PubMed] [Google Scholar]
- 13. Field AS, Alexander AL. Diffusion tensor imaging in cerebral tumor diagnosis and therapy. Top Magn Reson Imaging 2004;15:315–24 [DOI] [PubMed] [Google Scholar]
- 14. Cha S, Yang L, Johnson G, et al. Comparison of microvascular permeability measurements, K(trans), determined with conventional steady-state T1-weighted and first-pass T2*-weighted MR imaging methods in gliomas and meningiomas. AJNR Am J Neuroradiol 2006;27:409–17 [PMC free article] [PubMed] [Google Scholar]
- 15. Cha S. Neuroimaging in neuro-oncology. Neurotherapeutics 2009;6:465–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roberts HC, Pickering RM, Onslow E, et al. The effectiveness of implementing a care pathway for femoral neck fracture in older people: a prospective controlled before and after study. Age Ageing 2004;33:178–84 [DOI] [PubMed] [Google Scholar]
- 17. Cha S. Perfusion MR imaging: basic principles and clinical applications. Magn Reson Imaging Clin N Am 2003;11:403–13 [DOI] [PubMed] [Google Scholar]
- 18. Jackson A, Jayson GC, Li KL, et al. Reproducibility of quantitative dynamic contrast-enhanced MRI in newly presenting glioma. Br J Radiol 2003;76:153–62 [DOI] [PubMed] [Google Scholar]
- 19. Lacerda S, Law M. Magnetic resonance perfusion and permeability imaging in brain tumors. Neuroimaging Clin N Am 2009;19:527–57 [DOI] [PubMed] [Google Scholar]
- 20. Law M, Young RJ, Babb JS, et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 2008;247:490–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li KL, Zhu XP, Checkley DR, et al. Simultaneous mapping of blood volume and endothelial permeability surface area product in gliomas using iterative analysis of first-pass dynamic contrast enhanced MRI data. Br J Radiol 2003;76:39–50 [DOI] [PubMed] [Google Scholar]
- 22. Li KL, Zhu XP, Waterton J, et al. Improved 3D quantitative mapping of blood volume and endothelial permeability in brain tumors. J Magn Reson Imaging 2000;12:347–57 [DOI] [PubMed] [Google Scholar]
- 23. Roberts HC, Roberts TP, Ley S, et al. Quantitative estimation of microvascular permeability in human brain tumors: correlation of dynamic Gd-DTPA-enhanced MR imaging with histopathologic grading. Acad Radiol 2002;9(suppl 1):S151–55 [DOI] [PubMed] [Google Scholar]
- 24. Roberts HC, Roberts TP, Brasch RC, et al. Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: correlation with histologic grade. AJNR Am J Neuroradiol 2000;21:891–99 [PMC free article] [PubMed] [Google Scholar]
- 25. Desprechins B, Stadnik T, Koerts G, et al. Use of diffusion-weighted MR imaging in differential diagnosis between intracerebral necrotic tumors and cerebral abscesses. AJNR Am J Neuroradiol 1999;20:1252–57 [PMC free article] [PubMed] [Google Scholar]
- 26. Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology 2000;217:331–45 [DOI] [PubMed] [Google Scholar]
- 27. Barajas RF, Jr, Rubenstein JL, Chang JS, et al. Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol 2010;31:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murakami R, Hirai T, Sugahara T, et al. Grading astrocytic tumors by using apparent diffusion coefficient parameters: superiority of a one- versus two-parameter pilot method. Radiology 2009;251:838–45 [DOI] [PubMed] [Google Scholar]
- 29. Law M. MR spectroscopy of brain tumors. Top Magn Reson Imaging 2004;15:291–313 [DOI] [PubMed] [Google Scholar]
- 30. Nelson SJ, Cha S. Imaging glioblastoma multiforme. Cancer J 2003;9:134–45 [DOI] [PubMed] [Google Scholar]
- 31. Nelson SJ. Analysis of volume MRI and MR spectroscopic imaging data for the evaluation of patients with brain tumors. Magn Reson Med 2001;46:228–39 [DOI] [PubMed] [Google Scholar]
- 32. Nelson SJ. Multivoxel magnetic resonance spectroscopy of brain tumors. Mol Cancer Ther 2003;2:497–507 [PubMed] [Google Scholar]
- 33. Tedeschi G, Lundbom N, Raman R, et al. Increased choline signal coinciding with malignant degeneration of cerebral gliomas: a serial proton magnetic resonance spectroscopy imaging study. J Neurosurg 1997;87:516–24 [DOI] [PubMed] [Google Scholar]
- 34. Petrella JR, Provenzale JM. MR perfusion imaging of the brain: techniques and applications. AJR Am J Roentgenol 2000;175:207–19 [DOI] [PubMed] [Google Scholar]
- 35. Chen W. Clinical applications of PET in brain tumors. J Nucl Med 2007;48:1468–81 [DOI] [PubMed] [Google Scholar]
- 36. Lin FH, Witzel T, Mandeville JB, et al. Event-related single-shot volumetric functional magnetic resonance inverse imaging of visual processing. Neuroimage 2008;42:230–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iannucci G, Mascalchi M, Salvi F, et al. Vanishing Balò-like lesions in multiple sclerosis. J Neurol Neurosurg Psychiatry 2000;69:399–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Butteriss DJ, Ismail A, Ellison DW, et al. Use of serial proton magnetic resonance spectroscopy to differentiate low grade glioma from tumefactive plaque in a patient with multiple sclerosis. Br J Radiol 2003;76:662–65 [DOI] [PubMed] [Google Scholar]
- 39. Law M, Meltzer DE, Cha S. Spectroscopic magnetic resonance imaging of a tumefactive demyelinating lesion. Neuroradiology 2002;44:986–89 [DOI] [PubMed] [Google Scholar]
- 40. De Stefano N, Caramanos Z, Preul MC, et al. In vivo differentiation of astrocytic brain tumors and isolated demyelinating lesions of the type seen in multiple sclerosis using 1H magnetic resonance spectroscopic imaging. Ann Neurol 1998;44:273–78 [DOI] [PubMed] [Google Scholar]
- 41. Saindane AM, Cha S, Law M, et al. Proton MR spectroscopy of tumefactive demyelinating lesions. AJNR Am J Neuroradiol 2002;23:1378–86 [PMC free article] [PubMed] [Google Scholar]
- 42. Cianfoni A, Niku S, Imbesi SG. Metabolite findings in tumefactive demyelinating lesions utilizing short echo time proton magnetic resonance spectroscopy. AJNR Am J Neuroradiol 2007;28:272–77 [PMC free article] [PubMed] [Google Scholar]
- 43. Majos C, Aguilera C, Alonso J, et al. Proton MR spectroscopy improves discrimination between tumor and pseudotumoral lesion in solid brain masses. AJNR Am J Neuroradiol 2009;30:544–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cha S, Pierce S, Knopp EA, et al. Dynamic contrast-enhanced T2*-weighted MR imaging of tumefactive demyelinating lesions. AJNR Am J Neuroradiol 2001;22:1109–16 [PMC free article] [PubMed] [Google Scholar]
- 45. Law M, Cha S, Knopp EA, et al. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology 2002;222:715–21 [DOI] [PubMed] [Google Scholar]
- 46. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97–109. Epub 2007 Jul 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dean BL, Drayer BP, Bird CR, et al. Gliomas: classification with MR imaging. Radiology 1990;174:411–15 [DOI] [PubMed] [Google Scholar]
- 48. Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 2003;24:1989–98 [PMC free article] [PubMed] [Google Scholar]
- 49. Law M, Wang EY, Oh S, et al. Dynamic susceptibility contrast perfusion MR imaging of low-grade gliomas: a follow-up study of lesions with low vs high relative cerebral blood volume. In: Proceedings of the 42nd Annual Meeting of the American Society of Neuroradiology, Seattle; June 5–11, 2004 [Google Scholar]
- 50. Kelly PJ. Volumetric stereotactic surgical resection of intra-axial brain mass lesions. Mayo Clin Proc 1988;63:1186–98 [DOI] [PubMed] [Google Scholar]
- 51. Kelly PJ. Stereotactic imaging, surgical planning and computer-assisted resection of intracranial lesions: methods and results. Adv Tech Stand Neurosurg 1990;17:77–118 [DOI] [PubMed] [Google Scholar]
- 52. Kelly PJ. Computed tomography and histologic limits in glial neoplasms: tumor types and selection for volumetric resection. Surg Neurol 1993;39:458–65 [DOI] [PubMed] [Google Scholar]
- 53. Weber MA, Henze M, Tüttenberg J, et al. Biopsy targeting gliomas: do functional imaging techniques identify similar target areas? Invest Radiol 2010;45:755–68 [DOI] [PubMed] [Google Scholar]
- 54. Yu CS, Li KC, Xuan Y, et al. Diffusion tensor tractography in patients with cerebral tumors: a helpful technique for neurosurgical planning and postoperative assessment. Eur J Radiol 2005;56:197–204 [DOI] [PubMed] [Google Scholar]
- 55. Law M. Neurological complications. Cancer Imaging 2009;9(spec no A):S71–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006;7:392–401 [DOI] [PubMed] [Google Scholar]
- 57. Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol 2009;27:5874–80. Epub 2009 Nov 9 [DOI] [PubMed] [Google Scholar]
- 58. Shaw EG, Tatter SB, Lesser GJ, et al. Current controversies in the radiotherapeutic management of adult low-grade glioma. Semin Oncol 2004;31:653–58 [DOI] [PubMed] [Google Scholar]
- 59. Shaw EG, Wisoff JH. Prospective clinical trials of intracranial low-grade glioma in adults and children. Neuro Oncol 2003;5:153–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chan MD, Tatter SB, Lesser G, et al. Radiation oncology in brain tumors: current approaches and clinical trials in progress. Neuroimaging Clin N Am 2010;20:401–08 [DOI] [PubMed] [Google Scholar]
- 61. Combs SE. Radiation therapy. Recent Results Cancer Res 2009;171:125–40 [DOI] [PubMed] [Google Scholar]
- 62. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–66. Epub 2009 Mar 9 [DOI] [PubMed] [Google Scholar]
- 63. Pirzkall A, McKnight TR, Graves EE, et al. MR-spectroscopy guided target delineation for high-grade gliomas. Int J Radiat Oncol Biol Phys 2001;50:915–28 [DOI] [PubMed] [Google Scholar]
- 64. Stall B, Zach L, Ning H, et al. Comparison of T2 and FLAIR imaging for target delineation in high grade gliomas. Radiat Oncol 2010;5:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jain R, Gutierrez J, Narang J, et al. In vivo correlation of tumor blood volume and permeability with histologic and molecular angiogenic markers in gliomas. AJNR Am J Neuroradiol 2010;32:388–94. Epub 2010 Nov 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277–80 [DOI] [PubMed] [Google Scholar]
- 67. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010;28:1963–72 [DOI] [PubMed] [Google Scholar]
- 68. van den Bent MJ, Vogelbaum MA, Wen PY, et al. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's criteria. J Clin Oncol 2009;27:2905–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Clarke JL, Chang S. Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr Neurol Neurosci Rep 2009;9:241–46 [DOI] [PubMed] [Google Scholar]
- 70. Brandes AA, Tosoni A, Spagnolli F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol 2008;10:361–67. Epub 2008 Apr 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brandsma D, Stalpers L, Taal W, et al. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 2008;9:453–61 [DOI] [PubMed] [Google Scholar]
- 72. Law M, Lacerda S, Fatterpekar G, et al. Characterization of true disease progression versus pseudoprogression after concomitant radiochemotherapy treatment using perfusion, permeability, and MR spectroscopy in high grade gliomas. In: Proceedings of the 47th Annual Meeting of the Radiological Society of North America, Chicago, Illinois; November 29-December 4, 2009 [Google Scholar]
- 73. RTOG Study Chairs (Coordinating Group). Phase III double-blind placebo-controlled trial of conventional concurrent chemoradiation and adjuvant temozolomide plus bevacizumab versus conventional concurrent chemoradiation and adjuvant temozolomide in patients with newly diagnosed glioblastoma. ACRIN: American College of Radiology Imaging Network. 2009. http://www.acrin.org/PROTOCOLSUMMARYTABLE/PROTOCOL6686.aspx. Accessed January 14, 2011 [Google Scholar]
- 74. Stuckey SL, Goh TD, Heffernan T, et al. Hyperintensity in the subarachnoid space on FLAIR MRI. AJR Am J Roentgenol 2007;189:913–21 [DOI] [PubMed] [Google Scholar]
- 75. Stankiewicz JM, Glanz BI, Healy BC, et al. Brain MRI lesion load at 1.5T and 3T versus clinical status in multiple sclerosis. J Neuroimaging 2011;21:e50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wattjes MP, Lutterbey GG, Harzheim M, et al. Higher sensitivity in the detection of inflammatory brain lesions in patients with clinically isolated syndromes suggestive of multiple sclerosis using high field MRI: an intraindividual comparison of 1.5 T with 3.0 T. Eur Radiol 2006;16:2067–73 [DOI] [PubMed] [Google Scholar]
- 77. Attenberger UI, Runge VM, Morelli JN, et al. Evaluation of gadobutrol, a macrocyclic, nonionic gadolinium chelate in a brain glioma model: comparison with gadoterate meglumine and gadopentetate dimeglumine at 1.5 T, combined with an assessment of field strength dependence, specifically 1.5 versus 3 T. J Magn Reson Imaging 2010;31:549–55 [DOI] [PubMed] [Google Scholar]
- 78. Biswas J, Nelson CB, Runge VM, et al. Brain tumor enhancement in magnetic resonance imaging: comparison of signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) at 1.5 versus 3 Tesla. Invest Radiol 2005;40:792–97 [DOI] [PubMed] [Google Scholar]
- 79. Wintersperger BJ, Runge VM, Biswas J, et al. Brain tumor enhancement in MR imaging at 3 Tesla: comparison of SNR and CNR gain using TSE and GRE techniques. Invest Radiol 2007;42:558–63 [DOI] [PubMed] [Google Scholar]
- 80. Wiggins GC, Triantafyllou C, Potthast A, et al. 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med 2006;56:216–23 [DOI] [PubMed] [Google Scholar]
- 81. Wiggins GC, Polimeni JR, Potthast A, et al. 96-channel receive-only head coil for 3 Tesla: design optimization and evaluation. Magn Reson Med 2009;62:754–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ba-Ssalamah A, Nobauer-Huhmann IM, Pinker K, et al. Effect of contrast dose and field strength in the magnetic resonance detection of brain metastases. Invest Radiol 2003;38:415–22 [DOI] [PubMed] [Google Scholar]
- 83. Schneider G, Kirchin MA, Pirovano G, et al. Gadobenate dimeglumine-enhanced magnetic resonance imaging of intracranial metastases: effect of dose on lesion detection and delineation. J Magn Reson Imaging 2001;14:525–39 [DOI] [PubMed] [Google Scholar]
- 84. Engelhorn T, Schwarz MA, Eyupoglu IY, et al. Dynamic contrast enhancement of experimental glioma an intra-individual comparative study to assess the optimal time delay. Acad Radiol 2010;17:188–93 [DOI] [PubMed] [Google Scholar]
- 85. Frenzel T, Lengsfeld P, Schirmer H, et al. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol 2008;43:817–28 [DOI] [PubMed] [Google Scholar]
- 86. Sieber MA, Lengsfeld P, Frenzel T, et al. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol 2008;18:2164–73 [DOI] [PubMed] [Google Scholar]
- 87. Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006;21:1104–08 [DOI] [PubMed] [Google Scholar]
- 88. European Medicines Agency. European Medicines Agency makes recommendations to minimise risk of nephrogenic systemic fibrosis with gadolinium-containing contrast agents. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/public_health_alerts/2010/09/human_pha_detail_000013.jsp&murl=menus/medicines/medicines.jsp&mid=&jsenabled=true. Accessed January 14, 2011
- 89. US Food and Drug Administration. FDA News Release. New warnings required on use of gadolinium-based contrast agents: enhanced screening recommended to detect kidney dysfunction. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm225286.htm. Accessed January 14, 2011
- 90. Port M, Corot C, Violas X, et al. How to compare the efficiency of albumin-bound and nonalbumin-bound contrast agents in vivo: the concept of dynamic relaxivity. Invest Radiol 2005;40:565–73 [DOI] [PubMed] [Google Scholar]
- 91. Giesel FL, Mehndiratta A, Risse F, et al. Intraindividual comparison between gadopentetate dimeglumine and gadobutrol for magnetic resonance perfusion in normal brain and intracranial tumors at 3 Tesla. Acta Radiol 2009;50:521–30 [DOI] [PubMed] [Google Scholar]
- 92. Rohrer M, Bauer H, Mintorovitch J, et al. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol 2005;40:715–24 [DOI] [PubMed] [Google Scholar]
- 93. Akeson P, Jonsson E, Haugen I, et al. Contrast-enhanced MRI of the central nervous system: comparison between gadodiamide injection and gadolinium-DTPA. Neuroradiology 1995;37:229–33 [DOI] [PubMed] [Google Scholar]
- 94. Anzalone N, Gerevini S, Scotti R, et al. Detection of cerebral metastases on magnetic resonance imaging: intraindividual comparison of gadobutrol with gadopentetate dimeglumine. Acta Radiol 2009;50:933–40 [DOI] [PubMed] [Google Scholar]
- 95. Essig M, Lodemann KP, Le Huu M, et al. Intraindividual comparison of gadobenate dimeglumine and gadobutrol for cerebral magnetic resonance perfusion imaging at 1.5 T. Invest Radiol 2006;41:256–63 [DOI] [PubMed] [Google Scholar]
- 96. Greco A, Parker JR, Ratcliffe CG, et al. Phase III, randomized, double-blind, cross-over comparison of gadoteridol and gadopentetate dimeglumine in magnetic resonance imaging of patients with intracranial lesions. Australas Radiol 2001;45:457–63 [DOI] [PubMed] [Google Scholar]
- 97. Grossman RI, Rubin DL, Hunter G, et al. Magnetic resonance imaging in patients with central nervous system pathology: a comparison of OptiMARK (Gd-DTPA-BMEA) and Magnevist (Gd-DTPA). Invest Radiol 2000;35:412–19 [DOI] [PubMed] [Google Scholar]
- 98. Kim ES, Chang JH, Choi HS, et al. Diagnostic yield of double-dose gadobutrol in the detection of brain metastasis: intraindividual comparison with double-dose gadopentetate dimeglumine. AJNR Am J Neuroradiol 2010;31:1055–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kuhn MJ, Picozzi P, Maldjian JA, et al. Evaluation of intraaxial enhancing brain tumors on magnetic resonance imaging: intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for visualization and assessment, and implications for surgical intervention. J Neurosurg 2007;106:557–66 [DOI] [PubMed] [Google Scholar]
- 100. Maravilla KR, Maldjian JA, Schmalfuss IM, et al. Contrast enhancement of central nervous system lesions: multicenter intraindividual crossover comparative study of two MR contrast agents. Radiology 2006;240:389–400 [DOI] [PubMed] [Google Scholar]
- 101. Oudkerk M, Sijens PE, Van Beek EJ, et al. Safety and efficacy of Dotarem (Gd-DOTA) versus Magnevist (Gd-DTPA) in magnetic resonance imaging of the central nervous system. Invest Radiol 1995;30:75–78 [DOI] [PubMed] [Google Scholar]
- 102. Rowley HA, Scialfa G, Gao PY, et al. Contrast-enhanced MR imaging of brain lesions: a large-scale intraindividual crossover comparison of gadobenate dimeglumine versus gadodiamide. AJNR Am J Neuroradiol 2008;29:1684–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rumboldt Z, Rowley HA, Steinberg F, et al. Multicenter, double-blind, randomized, intra-individual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine in MRI of brain tumors at 3 Tesla. J Magn Reson Imaging 2009;29:760–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Valk J, Algra PR, Hazenberg CJ, et al. A double-blind, comparative study of gadodiamide injection and gadopentetate dimeglumine in MRI of the central nervous system. Neuroradiology 1993;35:173–77 [DOI] [PubMed] [Google Scholar]
- 105. Anzalone N, Colosimo C, Scarabino T, et al. Cerebral neoplastic enhancing lesions: multicenter, randomized, crossover intraindividual comparison between gadobutrol (1.0M) and gadoterate meglumine (0.5M) at 0.1 mmol Gd/kg body weight in a clinical setting. Eur J Radiol In press. [DOI] [PubMed] [Google Scholar]
- 106. Attenberger UI, Runge VM, Jackson CB, et al. Comparative evaluation of lesion enhancement using 1 M gadobutrol vs. 2 conventional gadolinium chelates, all at a dose of 0.1 mmol/kg, in a rat brain tumor model at 3 T. Invest Radiol 2009;44:251–56 [DOI] [PubMed] [Google Scholar]
- 107. Le Duc G, Corde S, Charvet AM, et al. In vivo measurement of gadolinium concentration in a rat glioma model by monochromatic quantitative computed tomography: comparison between gadopentetate dimeglumine and gadobutrol. Invest Radiol 2004;39:385–93 [DOI] [PubMed] [Google Scholar]
- 108. Tombach B, Benner T, Reimer P, et al. Do highly concentrated gadolinium chelates improve MR brain perfusion imaging? Intraindividually controlled randomized crossover concentration comparison study of 0.5 versus 1.0 mol/L gadobutrol. Radiology 2003;226:880–88 [DOI] [PubMed] [Google Scholar]
- 109. Akeson P, Larsson EM, Kristoffersen DT, et al. Brain metastases: comparison of gadodiamide injection-enhanced MR imaging at standard and high dose, contrast-enhanced CT and non-contrast-enhanced MR imaging. Acta Radiol 1995;36:300–06 [PubMed] [Google Scholar]
- 110. Benner T, Reimer P, Erb G, et al. Cerebral MR perfusion imaging: first clinical application of a 1 M gadolinium chelate (Gadovist 1.0) in a double-blinded randomized dose-finding study. J Magn Reson Imaging 2000;12:371–80 [DOI] [PubMed] [Google Scholar]
- 111. Filippi M, Rovaris M, Capra R, et al. A multi-centre longitudinal study comparing the sensitivity of monthly MRI after standard and triple dose gadolinium-DTPA for monitoring disease activity in multiple sclerosis: implications for phase II clinical trials. Brain 1998;121(pt 10):2011–20 [DOI] [PubMed] [Google Scholar]
- 112. Runge VM, Kirsch JE, Burke VJ, et al. High-dose gadoteridol in MR imaging of intracranial neoplasms. J Magn Reson Imaging 1992;2:9–18 [DOI] [PubMed] [Google Scholar]
- 113. Sze G, Johnson C, Kawamura Y, et al. Comparison of single- and triple-dose contrast material in the MR screening of brain metastases. AJNR Am J Neuroradiol 1998;19:821–28 [PMC free article] [PubMed] [Google Scholar]
- 114. Uysal E, Erturk SM, Yildirim H, et al. Sensitivity of immediate and delayed gadolinium-enhanced MRI after injection of 0.5 M and 1.0 M gadolinium chelates for detecting multiple sclerosis lesions. AJR Am J Roentgenol 2007;188:697–702 [DOI] [PubMed] [Google Scholar]
- 115. van der Molen AJ, Bellin MF. Extracellular gadolinium-based contrast media: differences in diagnostic efficacy. Eur J Radiol 2008;66:168–74 [DOI] [PubMed] [Google Scholar]
- 116. van Dijk P, Sijens PE, Schmitz PI, et al. Gd-enhanced MR imaging of brain metastases: contrast as a function of dose and lesion size. Magn Reson Imaging 1997;15:535–41 [DOI] [PubMed] [Google Scholar]
- 117. Vogl TJ, Friebe CE, Balzer T, et al. Diagnosis of cerebral metastasis with standard dose gadobutrol vs. a high dose protocol: intraindividual evaluation of a phase II high dose study[in German]. Radiologe 1995;35:508–16 [PubMed] [Google Scholar]
- 118. Wolansky LJ, Bardini JA, Cook SD, Z, et al. Triple-dose versus single-dose gadoteridol in multiple sclerosis patients. J Neuroimaging 1994;4:141–45 [DOI] [PubMed] [Google Scholar]
- 119. Yuh WT, Halloran JI, Mayr NA, et al. Dose of contrast material in the MR imaging evaluation of central nervous system tumors. J Magn Reson Imaging 1994;4:243–49 [DOI] [PubMed] [Google Scholar]
- 120. Anzalone N. Comparative studies of different gadolinium agents in brain tumors: differences between gadolinium chelates and their possible influence on imaging features. AJNR Am J Neuroradiol 2010;31:981–82 [DOI] [PMC free article] [PubMed] [Google Scholar]