Abstract
BACKGROUND AND PURPOSE:
MTI has the potential to detect abnormalities in normal-appearing white and gray matter on conventional MR imaging. Early detection methods and disease progression markers are needed in HD research. Therefore, we investigated MTI parameters and their clinical correlates in premanifest and manifest HD.
MATERIALS AND METHODS:
From the Leiden TRACK-HD study, 78 participants (28 controls, 25 PMGC, 25 MHD) were included. Brain segmentation of cortical gray matter, white matter, caudate nucleus, putamen, pallidum, thalamus, amygdala, and hippocampus was performed using FSL's automated tools FAST and FIRST. Individual MTR values were calculated from these regions and MTR histograms constructed. Regression analysis of MTR measures from all gene carriers with clinical measures was performed.
RESULTS:
MTR peak height was reduced in both cortical gray (P = .01) and white matter (P = .006) in manifest HD compared with controls. Mean MTR was also reduced in cortical gray matter (P = .01) and showed a trend in white matter (P = .052). Deep gray matter structures showed a uniform pattern of reduced MTR values (P < .05). No differences between premanifest gene carriers and controls were found. MTR values correlated with disease burden and motor and cognitive impairment.
CONCLUSIONS:
Throughout the brain, disturbances in MTI parameters are apparent in early HD and are homogeneous across white and gray matter. The correlation of MTI with clinical measures indicates the potential to act as a disease monitor in clinical trials. However, our study does not provide evidence for MTI as a marker in premanifest HD.
HD is a progressive neurodegenerative genetic brain disorder with clinical features consisting of motor signs, cognitive impairment, and psychiatric disturbances. Disease onset is typically during midlife.1 Since genetic testing became available for this autosomal dominant inheritable disease, it has become possible to identify premanifest gene carriers and, in this way, ascertain with certainty that they will eventually develop the disease. The disease is caused by a genetic defect on chromosome 4 that results in an expanded polyglutamine in the gene coding for the huntingtin protein.2 This mutant huntingtin predominantly affects the brain, resulting in malfunction and loss of neurons. Histopathologically, the disease is characterized by cellular loss of gray matter structures, most profoundly that of the medium spiny neurons within the striatum, and also significant white matter volume loss.3
Sensitive and reliable biomarkers are needed for evaluating clinical trials in HD. The challenges in this field relate to the fact that a biomarker should be able to monitor pathophysiological changes not only in the manifest phase but also in the preceding premanifest stage, when no overt symptoms exist. MR imaging characterization of brain changes is regarded as a potential source of biomarkers, as previous studies have shown that atrophy of the striatum is already apparent a decade or more before symptom onset.4–6 In addition, abnormalities in white matter7,8 and cortical gray matter5 have been reported. It is likely that the underlying pathologic processes resulting in brain atrophy occur before or in concurrence with the volumetric changes.
MTI has the potential to quantify the pathologic changes in central nervous system disorders in the normal-appearing white and gray matter on conventional MR imaging sequences.9,10 MTI offers a way of examining tissue structure and structural components that are normally not resolvable with conventional MR imaging.11 This allows for examination of structural integrity in a different and possibly more sensitive manner than volumetric changes alone. The technique of MTI relies on interaction between protons in free fluid and protons bound to macromolecules. The magnetization saturation and relaxation within macromolecules affect the observable signal intensity. The MTR, representing the percentage of variation in the MR signal intensity between the saturated and unsaturated acquisitions, is an effective and simple MTI measure to use as a clinical application. MTI has been used to characterize many different disorders, including multiple sclerosis, Alzheimer disease, and Parkinson disease.9
This study aims to examine MTR measures in a well-defined premanifest and manifest HD population, and to determine associations between MTR and clinical features of HD. By examining MTR in this sample, we aim to advance understanding of the timing of pathophysiological changes in HD and also evaluate the suitability of MTI/MTR as a potential biomarker for HD.
Materials and Methods
Subjects
Of the 90 participants from the Leiden TRACK-HD study, 12 did not receive MTI scanning due to either unexpected claustrophobia or time constraints of the full TRACK-HD protocol, resulting in 78 participants. The cohort consisted of 3 groups: 28 healthy controls, 25 PMGC, and 25 MHD. Inclusion criteria for the PMGC consisted of genetically confirmed expanded CAG repeat ≥40, a disease burden score (calculated as ([CAG repeat length–35.5] × Age) of >25012 and absence of motor abnormalities on the UHDRS, defined as a total motor score of ≤5. Inclusion criteria for MHD consisted of genetically confirmed CAG repeat ≥40, presence of motor abnormalities on the UHDRS-total motor score of >5. In addition, a total functional capacity score of 7 or higher was required to ensure that the HD group was in the earliest disease stages. Healthy gene-negative family members, spouses, or partners were recruited as control subjects. Exclusion criteria consisted of significant (neurologic) comorbidity, active major psychiatric disturbance, and MR imaging incompatibility. Full details on recruitment are available from the TRACK-HD baseline paper.5 Local institutional review board approval and written informed consent were obtained from all participants.
Imaging Sequences
All 78 participants underwent scanning on a 3T whole-body scanner (Philips Healthcare, Best, the Netherlands) with an 8-channel receive and transmit coil. T1-weighted image volumes were acquired using an ultrafast gradient-echo 3D acquisition sequence with the following imaging parameters: TR = 7.7 ms, TE = 3.5 ms, flip angle = 8°, FOV = 24 cm, matrix size 224 × 224 × 164, with sagittal sections to cover the entire brain with a section thickness of 1.0 mm.
A 3D gradient MTI sequence was subsequently performed with the following parameters: TR = 100 ms, TE = 11 ms, flip angle = 9°, matrix 224 × 180 × 144 mm, and voxel size 1.0 × 1.0 × 7.2 mm. Two consecutive imaging sets were acquired, one with and one without a saturation pulse. The imaging parameters are identical to those described by Jurgens et al.13 Total scanning time for T1-weighted and MTI sequences was 12 minutes maximum.
Postprocessing
T1-weighted images were segmented using FAST14 and FIRST15,16 from FSL.17 This provided individual brain masks for the total white matter, cortical gray matter, caudate nucleus, putamen, pallidum, thalamus, amygdala, and hippocampus. To correct for possible partial volume effects, an eroded mask of these segmentations was created by removing 1 voxel in-plane for all named VOIs. All brain masks were then registered to the MTI volumes using the transform obtained from linear registration of the T1-weighted volume with 7 degrees of freedom (FSL FLIRT). MTR is calculated per voxel as M0-Ms/M0, whereby Ms is the saturated image and M0 is the unsaturated image. The mean MTR per VOI was calculated. Additionally, to represent voxel-based MTR variations/variability within each VOI, we constructed MTR histograms and calculated MTR peak height using FSL-STATs. Mean MTR and MTR peak height normalized for the size of the volume of interest were the primary outcome variables.
Clinical Measures
A total measure of motor dysfunction was obtained with the UHDRS-total motor score (range 0–124). Quantification of subtle motor dysfunction by measuring variability of dominant-hand finger tapping and tongue protrusion force was achieved with force transducer-based quantitative motor assessments.18,19 The tapping and tongue measures are expressed as a logarithmic number; higher numbers represent more motor disturbances. Total functional capacity score (range 0–13) and MMSE for global assessment of cognitive functioning (range 0–30) were obtained. Cognitive scores included the total scores from the Symbol Digit Modality Test, Stroop word reading card, Trail-Making Test A and B, and verbal fluency. For the Trail-Making Test, a subtraction of Trail-Making Test B minus Trail-Making Test A was used to minimize the potential effects of motor speed on performance. The University of Pennsylvania Smell Identification Test (Sensonics, Haddon Heights, New Jersey) quantifies smell ability with a 20-item smell test and is known to correlate to clinical features of neurodegenerative diseases.20 An IQ estimate was obtained with the Dutch Adult Reading Test (a validated translation of the National Adult Reading Test). For assessment of psychiatric disturbances the Beck Depression Inventory II, the Problem Behaviour Assessment, short version, and the Frontal Systems Behavior21 were used. Predicted years until disease onset was calculated for PMGC as described in the TRACK-HD baseline paper. For a more detailed description of these clinical assessments, see Tabrizi et al (2009).5
Statistics
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS, Version 17.0.2; SPSS, Chicago, Illinois). An analysis of variance was conducted for all demographic variables. For group comparisons, all MTR values were analyzed in a 3-group analysis of variance, with post hoc analysis to determine differences between groups.
Hierarchical multiple regression analysis was performed to ascertain the relationship of MTR values with clinical measures. For this analysis only gene carriers (premanifest + manifest) were included, as the aim was to examine the relationship to disease progression. MTR values and 14 different clinical assessments were assessed for all regions of interest. In the hierarchical regression, age and sex were entered at step 1, thus correcting for the influence of these variables. This was applied for all motor and general assessments; for the specific cognitive tasks (Symbol Digit Modality Test, Stroop word reading, verbal fluency, and Trail-Making Test), IQ was also entered at step 1, as IQ can have a significant impact on cognitive scores.
Results
Demographic variables (Table 1) show that there were no differences between the groups in terms of age or CAG repeat length but that there was a significant difference (P < .05) between groups for all clinical tests, except for IQ and the Frontal Systems Behavior scores.
Table 1:
Group characteristics
| Control Mean (SD) | PMGC Mean (SD) | MHD Mean (SD) | PBetween Groups | |
|---|---|---|---|---|
| N | 28 | 25 | 25 | |
| Age | 48.3 (8.0) | 43.8 (8.5) | 48.4 (10.9) | .131 |
| CAG larger allele | n.a. | 42.72 (2.6) | 43.73 (2.8) | .182 |
| UHDRS TMS | 2.3 (2.3) | 2.5 (1.5) | 22.9 (11.4) | .000* |
| TFC | 12.96 (0.2) | 12.56 (0.8) | 10.2 (2.1) | .000* |
| YTO | n.a. | 7.06 (1.99) | n.a. | n.a. |
| IQ | 104 (9) | 100 (11) | 99 (12) | .260 |
| MMSE | 29.1 (1.2) | 28.7 (1.5) | 27.2 (2.6) | .001* |
| Tongue force | 3.56 (0.40) | 3.97 (0.51) | 4.88 (0.65) | .000* |
| Tapping | 11.6 (5.8) | 16.7 (8.5) | 30.9 (18.2) | .000* |
| SDMT | 50.6 (9.3) | 50.7 (10.2) | 35.7 (11.10) | .000* |
| Stroop | 98.1 (14.2) | 93.0 (13.7) | 76.7 (20.6) | .000* |
| TMT | 33.5 (23.5) | 43.2 (26.9) | 90.3 (73.6) | .000* |
| Verbal fluency | 26.8 (8.7) | 33.3 (14.1) | 20.8 (14.5) | .016* |
| UPSIT | 15.89 (2.9) | 14.6 (2.5) | 12.23 (3.8) | .000* |
| BDI | 4.8 (5.9) | 7.0 (7.7) | 11.1 (10.2) | .020* |
| PBA | 6.4 (8.1) | 7.6 (8.5) | 14.6 (14.7) | .017* |
| FrSBe | 78.1 (19.6) | 85.9 (23.8) | 87.3 (21.6) | .259 |
Note:—Group characteristics.
n.a. indicates not applicable; TMS, total motor score; TFC, total functional capacity; YTO, predicted years to disease onset; SDMT, Symbol Digit Modality Test; TMT, Trail-Making Test; UPSIT, University of Pennsylvania Smell Identification Test; BDI-II, Beck-Depression Inventory, 2nd version; PBA, Problem Behaviour Assessment, short version; FrSBe, Frontal Systems Behavior.
indicates a significant finding (P < 0.05).
MTR peak height was significantly reduced in the MHD group compared with either controls or PMGC in the following regions: white matter, gray matter, putamen, pallidum, amygdala, left thalamus (with a trend for the right thalamus), and right hippocampus. No significant results were found between the controls and PMGC. The mean MTR value was significantly lower between MHD and controls in the following regions: gray matter, both caudate nuclei, both thalami, and right putamen. All MTR values are shown for all regions, as examined for each group (On-line Table 1) .
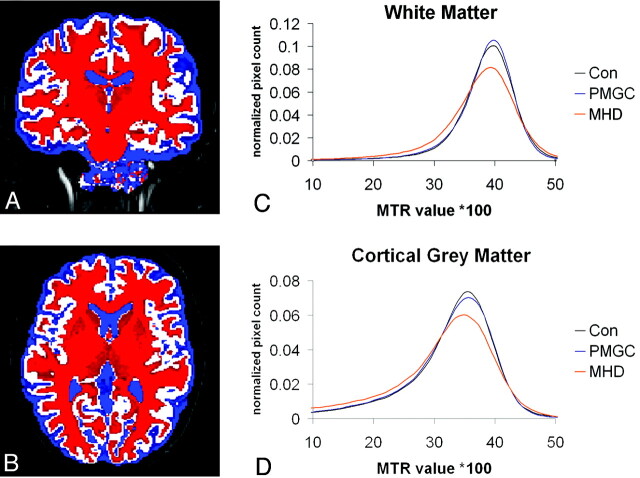
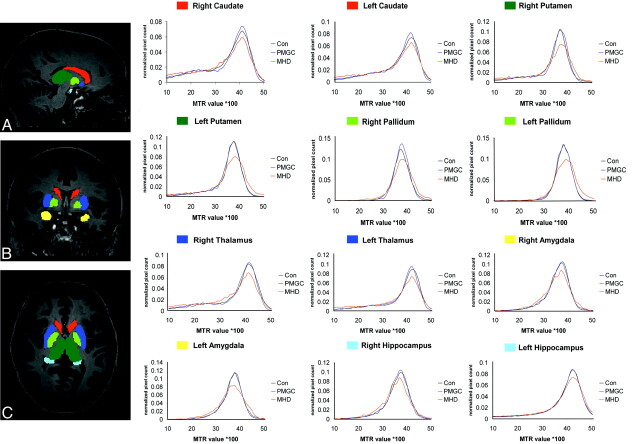
Overall, the MTR histograms showed similar patterns for all study groups in white and cortical gray matter (Fig 1) as well as all subcortical gray matter regions separately (Fig 2); in all histograms, the MHD group displayed a lower and broader histogram compared with controls and PMGC.
Fig 1.
MTR histogram for the white matter (C) and cortical gray matter (D), corrected for volume size of the region for 3 groups. An example segmentation acquired with the FAST software showing white matter (red), gray matter (white), and CSF (blue). The subcortical gray matter structures were subtracted from these masks.
Fig 2.
MTR histogram for 6 deep gray matter structures bilaterally, corrected for volume size of the region for 3 groups. Red = caudate nucleus; dark blue = putamen; light green = pallidum; dark green = thalamus; yellow = amygdala; light blue = hippocampus; Con = controls.
The regression analysis revealed several highly significant correlations between the MTR values and clinical measures (On-line Table 2). The disease burden score was significantly correlated with both cortical gray and white matter MTR peak height and mean MTR. The deep gray matter structures mainly showed a correlation of MTR peak height to the disease burden, except the right caudate nucleus and right putamen. The motor tests also correlated significantly with the MTR values in most regions of interest, predominantly with the UHDRS-total motor score and the tapping measure, and only minimally with the tongue measure. The cognitive measures showed correlations in the following regions: cortical gray matter, white matter, thalamus, left putamen, right pallidum, and left amygdala. The total functional capacity showed a correlation with white matter and the left putamen. The smell identification test was correlated to MTR values in both cortical gray and white matter, as in the caudate nucleus, amygdala, and thalamus. The MMSE and the measures of behavioral/psychiatric functioning revealed no correlation to any structures and are therefore not displayed in On-line Table 2.
Discussion
MTI applied in HD reveals disturbances throughout the brain in early HD compared with controls and PMGC. Disease burden, and quantitative motor and cognitive measures, have a strong correlation with MTR values, leading to the conclusion that MTI can possibly be used to track disease progression. No abnormalities are quantifiable in the premanifest stages of the disease compared with controls, which suggests that MTI, though perhaps a good disease monitor, is not an early marker of the disease.
Currently, conventional structural MR imaging and DTI are the 2 most widely applied methods in HD research with respect to the search for a MR imaging biomarker covering all disease stages of HD. Only 3 reports on MTI in HD are available.13,22,23 The value derived from MTI is the MTR value per brain voxel and is thought to represent structural integrity. The value quantifies the exchange of magnetization from the nonwater components in the region at hand. The most frequently reported outcome measures of MTR are mean MTR and MTR peak height. Mean MTR represents the average MTR value of all voxels in a region of interest, with lower mean MTR corresponding to poorer integrity. MTR peak height reflects the most frequently occurring MTR value in a region of interest when all the MTR values are set out in a MTR histogram. When each MTR value occurs less frequently, the histogram becomes broader and the maximum peak height decreases. This reduction represents reduced capacity to optimally exchange magnetization over the region of interest, hence representing reduced structural integrity.13,24 For example, in white matter, myelin is the main component and therefore MTR is thought to relate to myelinization or myelin integrity. To which cellular structure, whether neurons or glia cells, MTR in gray matter specifically refers is unknown. In our study, it does not solely reflect atrophy, as the differences found in peak height were corrected for size of the volume examined, thereby accounting for atrophy. From histopathological studies we know that medium spiny neurons in HD are most affected, making these the most likely source of the differences.
In the current study, we found that both MTR measures were significantly reduced in the manifest stages of HD in cortical gray matter, deep gray matter structures, and white matter. This finding conflicts with the findings of Mascalchi et al, who reported no differences between a group of 21 gene carriers (of which 19 manifest HD) and controls.22 The differences could be explained by the fact that Mascalchi et al applied a different (manual) segmentation technique, used a lower field strength, included a slightly smaller group, and did not examine MTR peak height. In our study, mean MTR does show significant results but not in the white matter. In general we found that the peak height tended to be the more sensitive MTR measure rather than the mean MTR. The study by Jurgens et al13 is comparable to our study, with the same type of scanner and analysis. We replicate their findings, as they also demonstrated a lack of group difference between PMGC and controls. Furthermore, the clinical correlation of the MTI peak height with clinical measures in gene carriers is confirmed in our study, and this knowledge is extended from a premanifest study group to both PMGC and MHD.13 Ginestroni et al23 applied a similar methodology to our study, as both used the FSL tools for segmentation. The main difference between these 2 studies is that we examined explicitly premanifest and manifest groups separately instead of a “gene carrier group with a range of clinical severity.” The outcomes of the studies are highly comparable with reduced MTR in subcortical and cortical gray matter. The absence of white matter differences in mean MTR is similar in both reports. However, we did find white matter differences with an outcome measure not examined by Ginestroni et al23, namely, MTR peak height. Finally, the correlation of MTR values with clinical measures was similarly reported by both studies.23
The finding of reduced MTR measures throughout the brain is remarkably homogeneous. This seems in contrast to the volumetric data available in HD research. Striatal degeneration is the key feature of brain pathology in HD, yet evermore evidence is building up that, though the damage starts in the striatum, HD is truly a whole brain disease, as numerous volumetric studies demonstrate widespread volumetric loss in both gray and white matter.25 So the seemingly paradoxic homogeneity is really not that surprising.
The application of MTI and its relationship to clinical severity has been demonstrated in other neurologic diseases such as multiple sclerosis and Alzheimer disease.10,26–30 Our findings indicate that MTI measures in HD correlate with disease burden, specific motor tasks, and cognitive measures in this study population. The finding of correlations of MTI with specific clinical parameters indicates that MTI is a good reflection of the disease status, as shown by motor and cognitive measures. Furthermore, the disease burden, which encompasses the CAG-repeat length, has been demonstrated to correlate with striatal degeneration and predicted time to disease onset,12 therefore indirectly linking MTI outcomes to such measures.
The lack of significant group differences between PMGC and controls was unexpected. We anticipated differences on the basis of previous reports on white matter integrity loss in premanifest HD using DTI.7,8,31 DTI can be used to examine protons in free water and their diffusive properties in more than 1 way, namely, the strength of the directional of diffusivity (fractional anisotropy), the average amount of diffusivity (mean diffusivity), and also the amount of diffusivity in either the radial and axial direction. DTI has the potential for examining and quantifying many features of brain tissue, all captured by the terms structural integrity and/or organization. In white matter, DTI is heavily influenced by axonal membranes and myelin sheaths.32 In contrast, MTI can be used to examine tissue structure according to the protons bound to macromolecules.33 As myelin is the main component of white matter, MTI is thought to mainly represent myelin integrity. Therefore, these techniques characterize fundamentally different aspects of brain tissue, possibly explaining (part of) the differences found between DTI studies and MTI studies. The question remains whether DTI or MTI is more sensitive in detecting the pathologic neuronal integrity breakdown. However, answering this question was not the aim of this study.
The potential role for MTI as a biomarker in HD is apparent, as there are both significant differences between groups and a clear relationship to clinical measures. However, MTI may be sensitive to a particular (early) disease state and not to all disease stages in HD. Longitudinal follow-up is needed to confirm this. The biomarker role for MTI has already been suggested in Alzheimer disease by Ridha et al,30 and the reports of using MTI as a biomarker in MS are building,34 strengthening the possibility for MTI as a biomarker in neurodegenerative disorders such as HD. TRACK-HD is specifically designed for longitudinal assessment of potential biomarkers and is therefore the ideal platform to confirm the findings longitudinally.
Limitations of our study relate to the fact that the automated segmentation technique has not specifically been validated for HD. However, we used these only for obtaining the brain regions of interest. Furthermore, we accounted for some possible incorrect segmentation and/or partial volume effects using an eroded version of the brain masks. A limitation could also be that we have chosen a region of interest–based analysis as opposed to voxelwise analysis. However, as the morphology of the structures at hand changes due to the disease, registration issues could be a severe problem, not to mention that the mean MTR can remain constant while intensities do change. Therefore, region of interest–based analysis is potentially more sensitive. Futhermore, we examined voxel-based variations within structures by representing this in histograms of each VOI. Another limitation could be that the exploratory nature of this study accounted for a high number of correlations included, which could lead to false-positive results. It seems, however, that MTI measures are fairly stable in every region we examined and provide a rather uniform picture of group differences and clinical correlation outcomes. Finally, a limitation of MTI in general is the limited reproducibility across centers, as the magnetization transfer phenomenon is dependent on many technical parameters and lack of a standardized protocol.9
Conclusions
MTI demonstrates that whole-brain disturbances are apparent in early HD and, furthermore, that these structural integrity differences seem to be relatively homogeneous throughout the brain in early HD. The strong correlations to clinical features, especially motor and cognitive measures, suggest that there is potential for this analysis to serve as a disease monitor in future clinical trials. However, MTI does not seem to be an early marker of HD, as no disturbances in MTI measures can be detected in the premanifest stages of the disease.
Supplementary Material
Acknowledgments
We wish to thank the TRACK-HD participants, the “CHDI/High Q Foundation,” a not-for-profit organization dedicated to finding treatments for HD, for providing financial support, and all TRACK-HD investigators for their efforts in conducting this study.
ABBREVIATIONS:
- CAG
cytosine-adenosine-guanine
- HD
Huntington disease
- MHD
early manifest HD patients
- MMSE
Mini-Mental State Examination
- MTI
magnetization transfer imaging
- MTR
magnetization transfer ratio
- PMGC
premanifest HD gene carriers
- UHDRS
United Huntington's Disease Rating Scale
Footnotes
Disclosures: Simon van den Bogaard—RELATED: Grant: CHDI.* Eve Dumas—RELATED: CHDI.* Ralf Reilmann—UNRELATED: Consultancy: CHDI,* Siena Biotech,* Novartis Pharma,* Meda Pharma;* Payment for Lectures (including service on speakers bureaus): Temmler Pharma. Julie Stout—RELATED: Grant: The work was supported by the CHDI Foundation; my institution, Monash University, received grant funds for this work;* Support for Travel to Meetings for the Study or Other Purposes: The central Track-HD study funds at University College London paid for my travel to Steering Committee meetings for this study. David Craufurd—RELATED: Grant: CHDI;* Support for Travel to Meetings for the Study or Other Purposes: CHDI, Comments: Travel expenses actually incurred for meetings directly related to the study. Raymund Roos—RELATED: CHDI.* (* Money paid to institution)
Previously presented in the form of a poster at: 2010 European Huntington's Disease Network (EHDN) Annual Meeting, Prague, Czech Republic, 3–5 September 2010.
CHDI/High Q Foundation (http://www.chdifoundation.org) is a not-for-profit organization dedicated to finding treatments for Huntington disease. They provided financial support for this study.
References
- 1. Novak MJ, Tabrizi SJ. Huntington's disease. BMJ 2010;340:c3109. [DOI] [PubMed] [Google Scholar]
- 2. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell 1993;72:971–83 [DOI] [PubMed] [Google Scholar]
- 3. de la Monte SM, Vonsattel JP, Richardson EP, Jr.. Morphometric demonstration of atrophic changes in the cerebral cortex, white matter, and neostriatum in Huntington's disease. J Neuropathol Exp Neurol 1988;47:516–25 [DOI] [PubMed] [Google Scholar]
- 4. Paulsen JS, Langbehn DR, Stout JC, et al. Detection of Huntington's disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry 2008;79:874–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tabrizi SJ, Langbehn DR, Leavitt BR, et al. Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol 2009;9:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van den Bogaard SJ, Dumas EM, Acharya TP, et al. Early atrophy of pallidum and accumbens nucleus in Huntington's disease. J Neurol 2010;258:412–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosas HD, Tuch DS, Hevelone ND, et al. Diffusion tensor imaging in presymptomatic and early Huntington's disease: selective white matter pathology and its relationship to clinical measures. Mov Disord 2006;21:1317–25 [DOI] [PubMed] [Google Scholar]
- 8. Dumas EM, van den Bogaard SJ, Ruber ME, et al. Early changes in white matter pathways of the sensorimotor cortex in premanifest Huntington's disease. Hum Brain Mapp 2012;33:203–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Filippi M, Rocca MA. Magnetization transfer magnetic resonance imaging of the brain, spinal cord, and optic nerve. Neurotherapeutics 2007;4:401–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dehmeshki J, Chard DT, Leary SM, et al. The normal appearing grey matter in primary progressive multiple sclerosis: a magnetisation transfer imaging study. J Neurol 2003;250:67–74 [DOI] [PubMed] [Google Scholar]
- 11. McGowan JC. The physical basis of magnetization transfer imaging. Neurology 1999;53:S3–7 [PubMed] [Google Scholar]
- 12. Penney JB, Jr, Vonsattel JP, MacDonald ME, et al. CAG repeat number governs the development rate of pathology in Huntington's disease. Ann Neurol 1997;41:689–92 [DOI] [PubMed] [Google Scholar]
- 13. Jurgens CK, Bos R, Luyendijk J, et al. Magnetization transfer imaging in “premanifest” Huntington's disease. J Neurol 2010;257:426–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 2001;20:45–57 [DOI] [PubMed] [Google Scholar]
- 15. Patenaude B, Smith SM, Kennedy DN, et al. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011;56:907–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patenaude B. Bayesian statistical models of shape and appearance for subcortical brain segmentation [Thesis]. 2007; available at http://users.fmrib.ox.ac.uk/∼brian/brianp_thesis.pdf [DOI] [PMC free article] [PubMed]
- 17. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:S208–19 [DOI] [PubMed] [Google Scholar]
- 18. Bechtel N, Scahill RI, Rosas HD, et al. Tapping linked to function and structure in premanifest and symptomatic Huntington disease. Neurology 2010;75:2150–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reilmann R, Bohlen S, Klopstock T, et al. Tongue force analysis assesses motor phenotype in premanifest and symptomatic Huntington's disease. Mov Disord 2010;25:2195–202 [DOI] [PubMed] [Google Scholar]
- 20. McKinnon J, Evidente V, Driver-Dunckley E, et al. Olfaction in the elderly: a cross-sectional analysis comparing Parkinson's disease with controls and other disorders. Int J Neurosci 2010;120:36–39 [DOI] [PubMed] [Google Scholar]
- 21. Grace J, Stout JC, Malloy PF. Assessing frontal lobe behavioral syndromes with the frontal lobe personality scale. Assessment 1999;6:269–84 [DOI] [PubMed] [Google Scholar]
- 22. Mascalchi M, Lolli F, Della NR, et al. Huntington disease: volumetric, diffusion-weighted, and magnetization transfer MR imaging of brain. Radiology 2004;232:867–73 [DOI] [PubMed] [Google Scholar]
- 23. Ginestroni A, Battaglini M, Diciotti S, et al. Magnetization transfer MR imaging demonstrates degeneration of the subcortical and cortical gray matter in Huntington disease. AJNR Am J Neuroradiol 2010;31:1807–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Filippi M, Rocca MA, Comi G. The use of quantitative magnetic-resonance-based techniques to monitor the evolution of multiple sclerosis. Lancet Neurol 2003;2:337–46 [DOI] [PubMed] [Google Scholar]
- 25. Rosas HD, Koroshetz WJ, Chen YI, et al. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology 2003;60:1615–20 [DOI] [PubMed] [Google Scholar]
- 26. van Buchem MA, Grossman RI, Armstrong C, et al. Correlation of volumetric magnetization transfer imaging with clinical data in MS. Neurology 1998;50:1609–17 [DOI] [PubMed] [Google Scholar]
- 27. Khaleeli Z, Sastre-Garriga J, Ciccarelli O, et al. Magnetisation transfer ratio in the normal appearing white matter predicts progression of disability over 1 year in early primary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 2007;78:1076–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ge Y, Grossman RI, Udupa JK, et al. Magnetization transfer ratio histogram analysis of gray matter in relapsing-remitting multiple sclerosis. AJNR Am J Neuroradiol 2001;22:470–75 [PMC free article] [PubMed] [Google Scholar]
- 29. Hayton T, Furby J, Smith KJ, et al. Grey matter magnetization transfer ratio independently correlates with neurological deficit in secondary progressive multiple sclerosis. J Neurol 2009;256:427–35 [DOI] [PubMed] [Google Scholar]
- 30. Ridha BH, Tozer DJ, Symms MR, et al. Quantitative magnetization transfer imaging in Alzheimer disease. Radiology 2007;244:832–37 [DOI] [PubMed] [Google Scholar]
- 31. Magnotta VA, Kim J, Koscik T, et al. Diffusion tensor imaging in preclinical Huntington's disease. Brain Imaging and Behavior 2009;3:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 2002;15:435–55 [DOI] [PubMed] [Google Scholar]
- 33. Laule C, Vavasour IM, Kolind SH, et al. Magnetic resonance imaging of myelin. Neurotherapeutics 2007;4:460–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Filippi M, Agosta F. Magnetic resonance techniques to quantify tissue damage, tissue repair, and functional cortical reorganization in multiple sclerosis. Prog Brain Res 2009;175:465–82 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.