Abstract
BACKGROUND AND PURPOSE:
Noninvasive imaging of cerebral aneurysms is still considered inferior to conventional angiography. The purpose of this study was to evaluate the diagnostic accuracy of ivACT in the assessment of intracranial aneurysms compared with 3D-DSA.
MATERIALS AND METHODS:
We included 13 patients with 15 incidental unruptured saccular aneurysms scheduled for diagnostic angiographic work-up in our study. In each patient, we performed an ivACT and a conventional angiography including a 3D rotational run. During postprocessing, MPR images were generated for each technique. Maximal aneurysm diameter, neck diameter, aneurysm height, maximum width, bulge height, parent artery diameter, and angle between the parent artery and aneurysm apex were measured for each aneurysm.
RESULTS:
3D-DSA and ivACT both provided images of high quality without artificial disturbances (ie, motion artifacts). Measurements of all parameters resulted in comparable values for both modalities with a strong correlation (P ≤ .001).
CONCLUSIONS:
ivACT is feasible for the noninvasive visualization of saccular cerebral aneurysms and may provide reliable diagnostic information for the assessment of aneurysm size and geometry comparable with conventional intra-arterial 3D rotational angiography. These preliminary results might be a first promising step to replacing conventional angiography in preinterventional aneurysm imaging.
The prevalence of cerebral aneurysms is considered approximately 4%–7% of the population.1–3 Because of the ever-widening use of neuroimaging techniques, the number of incidentally found and subsequently treated aneurysms is rising. Before interventional therapy, the acquisition of accurate data on aneurysm geometry is of crucial importance. Precise knowledge of the aneurysm neck and originating arteries is a key factor for deciding whether to perform microsurgical clipping or endovascular coiling. The criterion standard for the assessment of size and geometry of intracranial aneurysms is still conventional angiography,4 especially 3D-DSA.5–7 However, disadvantages of conventional diagnostic angiography are the cost and its invasiveness, even if the complication rate can be considered low at experienced centers.8 Noninvasive methods for aneurysm visualization such as CTA or MRA9—14 have indeed benefited from more and more improved techniques, but they have not yet reached the accuracy of 3D-DSA.15–17
FPCT in combination with intravenous contrast medium injection is a recently described method capable of visualization of intracranial vessels.18 The higher spatial resolution of FPCT in comparison with MSCT should also be an advantage in the visualization of cerebral aneurysms. The aim of our study was to evaluate the accuracy of ivACT in assessing aneurysms in humans compared with 3D-DSA.
Materials and Methods
Ethics committee approval was obtained before this study. Thirteen patients with 15 cerebral aneurysms were enrolled in our study. According to institutional guidelines, most patients with incidental findings of aneurysms at MR imaging or CT undergo further evaluation by DSA including 3D rotational angiography. If further therapy is considered, the day after conventional angiography we perform, in every patient, an ivACT after the serum creatinine level is checked. Only patients with an unruptured saccular aneurysm were included in this study. Fusiform, dissecting, or ruptured aneurysms were not included.
DSA
Both DSA and ivACT were performed on a biplane flat-panel detector angiographic system (Axiom Artis dBA; Siemens, Erlangen, Germany). With standard angiographic methods (transfemoral route), we used a diagnostic catheter to obtain standard posteroanterior, lateral, and oblique projections (2D). After this, 3D rotational angiography was performed with a 5-second run (a 5-second DSA program as provided by the manufacturer) with selective 4-mL/s injection of contrast material into the ICA or vertebral artery by using a power injector (Accutron HP-D, Medtron, Saarbrücken, Germany). The rotational angiographic data were transferred to a workstation (Leonardo, Siemens) to generate MPR images. The whole angiographic investigation required about 60 mL of contrast material (iomepol, Imeron 300; Bracco Imaging, Konstanz, Germany).
ivACT
ACT in combination with intravenous contrast medium injection was performed the day after DSA. For image acquisition, we used a dedicated FPCT program (a 10-second DSA program) with a native and a contrast-enhanced run. We applied the “bolus-watching” technique,19 which allows the beginning of data acquisition exactly at the point when the arteries have the best contrast material opacification. Sixty milliliters of contrast agent (Imeron 300) was injected into an antecubital vein at a rate of 5 mL/s by using a power injector (Accutron HP-D, Medtron) followed by a saline chaser (60 mL, injection rate 5 mL/s). Data acquisition per run was performed by using the following parameters: acquisition time, 10 seconds per run; 70 kV; 512 × 512 matrix; projection on 30 × 40 cm flat panel size; 200° total angle; 0.8°/frame; 250 frames total; dose, 1.2 μGy/frame. The dataset was transferred to a commercially available workstation (Leonardo) for postprocessing.
Postprocessing and Image Analysis
For each technique, image reconstructions were performed by using commercially available software (DynaCT, Siemens). The software allows the reconstruction of different image impressions. The modes “native fill,” kernel type “HU,” and image impression “normal” were used. All 3D-DSA and ivACT data were anonymized and stored in random order. For image analysis, the volumetric data were loaded into the 3D application to perform MPRs. MPR reconstructions of each aneurysm were generated in transverse, coronal, and sagittal orientations with a section thickness of 0.5 mm. All images were independently evaluated by 2 experienced neuroradiologists in consensus reading. Measurements were performed on the workstation with an electronic caliper. We recorded the following, previously described20 dimensions of aneurysm geometry: maximal diameter, neck diameter, aneurysm height, maximum width, and bulge height (the distance of the neck plane to the plane of maximal diameter). In addition, the parent artery diameter and the angle between parent artery and aneurysm apex were measured.
Statistical analysis was performed by using the Statistical Package for the Social Sciences (SPSS, Chicago, Illinois). The mean values and the SDs were calculated for each dimension. For analysis of the correlation between the variables, a linear 2-sided correlation (Pearson r) test was performed. Significance level was set as P ≤ .001.
Results
The patient population consisted of 10 women and 3 men for a total of 13 patients with 15 cerebral aneurysms. The average age of the patients was 61.3 ± 14.8 years (range, 23–76 years). Twelve (80%) aneurysms were in the anterior and 3 aneurysms (20%) were in the posterior circulation. The mean values and SDs of each aneurysm parameter measured for both modalities are shown in the Table. The highest correlation (r = 1.000) was found for the maximal aneurysm width; the lowest, for the parent artery diameter (r = 0.950). The Pearson correlation coefficient reached a significant level in all measured variables (P ≤ .001).
Correlation of the mean values of aneurysm dimensions measured by 3D-DSA and ivACT
| 3D-DSA (mean) | ivACT (mean) | Pearson Correlation Coefficent | |
|---|---|---|---|
| Maximum diameter | 1.414 ± 1.035 cm | 1.413 ± 1.027 cm | 0.999 |
| Neck diameter | 0.651 ± 0.607 cm | 0.655 ± 0.585 cm | 0.999 |
| Aneurysm height | 1.077 ± 0.780 cm | 1.087 ± 0.776 cm | 0.998 |
| Maximum width | 1.055 ± 0.846 cm | 1.064 ± 0.830 cm | 1.000 |
| Bulge height | 0.405 ± 0.251 cm | 0.404 ± 0.246 cm | 0.990 |
| Parent artery diameter | 0.336 ± 0.110 cm | 0.339 ± 0.108 cm | 0.950 |
| Aneurysm/artery angle (SD) | 103.333° (43.322°) | 103.667° (43.956°) | 0.997 |
Representative Cases
Case 1.
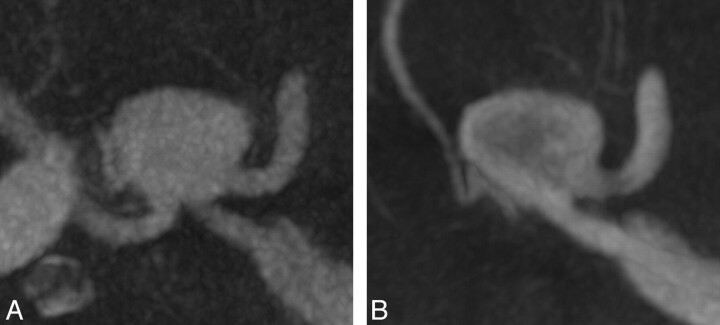
A 59-year-old patient presented with nausea and diplopia. MR imaging revealed bilateral giant aneurysms of the distal part of internal carotid artery with an ophthalmoplegic effect on the left side. Angiographic images before successful endovascular treatment by flow-diverter stent deployment are shown in Fig 1. The preinterventional assessment of the appropriate stent length was accurately determined on ivACT.
Fig 1.
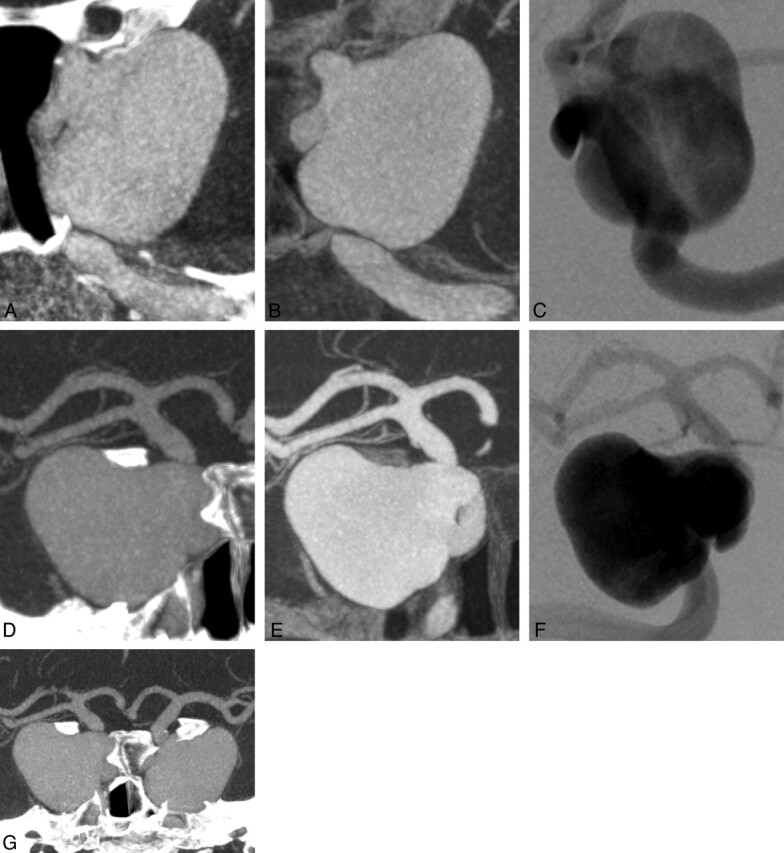
Bilateral giant siphon aneurysms illustrated by maximum-intensity-projection (MIP) reconstructions of ivACT (A and D) and 3D-DSA (B and E) with the correlating 2D-DSA images (C and F). G, MIP reconstruction of ivACT of both aneurysms. ivACT allows the depiction of both aneurysms in only 1 examination step. In a comparison of both modalities, 3D-DSA provides a more distinct image impression, but the required information (ie, for device usage) can also be obtained by MIP reconstructions of ivACT.
Case 2.
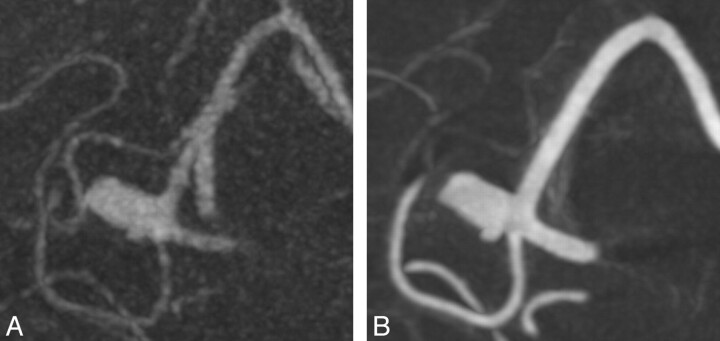
A 70-year-old patient underwent MR imaging because of trigeminal affection. As an incidental finding, an aneurysm of the ACA was revealed (Fig 2), which was successfully treated by endovascular coiling. In ivACT, both A1 segments of the ACA were visible in 1 acquisition step in contrast to 3D-DSA, where a second contralateral contrast agent injection was needed.
Fig 2.
An aneurysm of the ACA is presented by maximum-intensity-projection reconstructions of ivACT (A) and 3D-DSA (B). With ivACT, also the right-sided A1 and A2 segments of the ACA are highlighted in contrast to 3D-DSA.
Case 3.
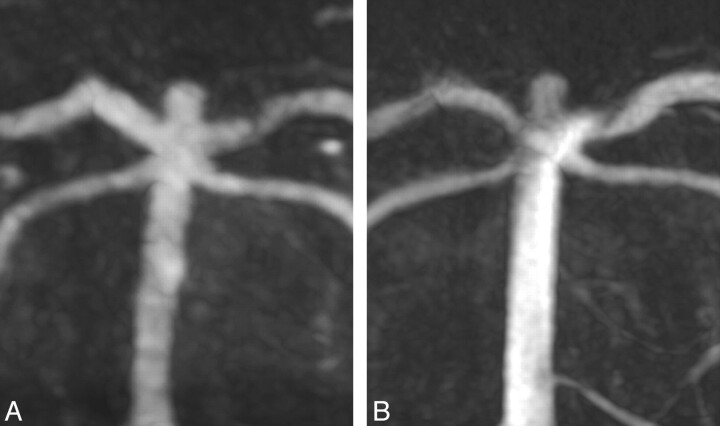
A broad-based aneurysm of the right-sided pericallosal artery was demonstrated, which was incidentally found by MR imaging, actually performed because of headache in a 61-year-old patient (Fig 3). Further treatment was surgical clipping.
Fig 3.
Correlating maximum-intensity-projection reconstructions of ivACT (A) and 3D-DSA (B) both show a broad-based aneurysm of the right-sided pericallosal artery. 3D-DSA offers some more clarity for treatment, but the image information gained by ivACT seems sufficient for treatment planning.
Case 4.
MR imaging of a 58-year-old patient revealed a basilar tip aneurysm. The further angiographic aneurysm evaluation is shown in Fig 4. This case demonstrates that aneurysm evaluation by ivACT is also viable in the posterior circulation.
Fig 4.
A small basilar tip aneurysm is demonstrated by maximum-intensity-projection reconstructions of ivACT (A) and 3D-DSA (B). Both techniques seem to provide nearly identical images of the aneurysm.
Discussion
Conventional angiography with 3D-DSA is considered the criterion standard for aneurysm evaluation.5–7 Although noninvasive aneurysm visualization with CTA or MRA has improved by technical advances recently, the accuracy of conventional angiography, especially in the visualization of aneurysm geometry, has not yet been achieved.15–17,21 CTA is frequently used as a noninvasive, broadly available, fast, and feasible tool, especially in acute settings for aneurysm detection,22 but there are still limitations through bone artifacts near the skull base and its confined spatial resolution. MRA can provide thin source images of intracranial vessels, but the disadvantages are a long acquisition time as well as artifacts by patient movement or flow phenomena.15
With the newly introduced combination of FPCT with intravenous contrast medium injection, a new tool for cerebral aneurysm visualization might be available. Its feasibility concerning vessel visualization has been shown in animal models so far.18 The aim of our study was to evaluate the accuracy of ivACT in assessing aneurysms in humans compared with 3D-DSA.
For both modalities, we compared the following, previously published20 indices of aneurysm dimensions: maximal diameter, neck diameter, aneurysm height, maximum width, bulge height, parent artery diameter, and the angle between the parent artery and the aneurysm apex. The results showed a strong significant correlation between both techniques.
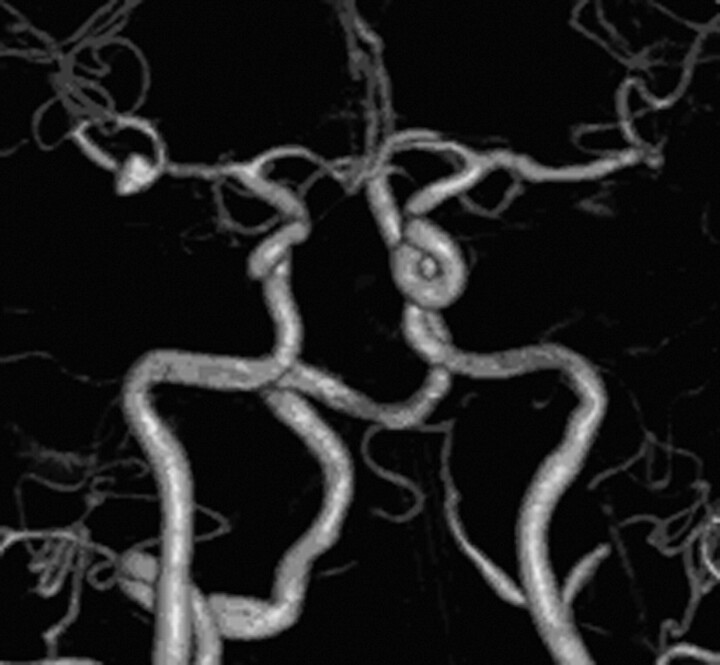
As a major advantage, ivACT is a noninvasive procedure in contrast to conventional angiography. Complications of conventional angiography (ie, cerebrovascular ischemic events and so forth) can be avoided, and hospitalization of the patients is not necessary. Thus the examinations can be performed on an outpatient basis. The acquisition of ivACT is less time-consuming than DSA, and in addition, the whole cerebral circulation can be evaluated in 1 step (Fig 5), which might be especially helpful in the visualization of ≥2 cerebral aneurysms at the same time.
Fig 5.
Volume rendering technique reconstruction of ivACT shows opacification of all intracranial vessels in the anteroposterior view by only 1 contrast agent injection. An aneurysm in the bifurcation of the right middle cerebral artery is revealed.
However, the accuracy for detecting additional aneurysms by using ivACT was not the aim of this study and would probably need a larger number of patients. The ivACT nicely illustrates aneurysms located within the anterior as well as posterior circulation without limitations in separating vessels from bony structures due to beam-hardening artifacts (ie, near the skull base). In comparison with CTA, the promising issue of this novel technique seems to be the option to generate images with a superior spatial resolution and a reduced interference of bony structures so that these reported technical limitations23 of CTA might be avoided. Comparative studies between CTA and ivACT are planned, now that the feasibility of ivACT in comparison with 3D rotational angiography as the criterion standard has been shown.
In some cases, for example in giant aneurysms, it may be possible to observe flow disturbances in conventional angiography. Such flow-related problems have not been experienced in our cases with the ivACT; instead, a more homogeneous contrasted vessel view was obtained. Due to the contrast opacification of all intracranial vessels at the same time, contralateral vessel segments and their relation to an aneurysm can also be estimated; this estimation can be helpful in the preinterventional decision-making, for example, in ACA aneurysms. Moreover, ivACT leads to a uniform contrast mixing within all cerebral vessels. By intravenous injection, a dilution of the contrast medium bolus can be gained, which results in a less intense vessel opacification using ivACT. Hereby, high contrast artifacts, which might occur in 3D-DSA through direct intra-arterial contrast medium injection, can be avoided, and the performance of thresholding can be improved, respectively. Overall, this might result in more precise measurements of intracranial vessels.
On the other hand, no dynamic information about the flow direction and velocity can be gained from ivACT because data acquisition starts when all intracranial vessels have full contrast opacification. Compared with 3D-DSA, a higher amount of contrast agent is needed per ivACT acquisition run (60 versus 18–24 mL), and the acquisition time is somewhat longer (10 versus 5 seconds). However, a second contrast application for visualization of other aneurysms is not necessary as opposed to with DSA. Through additional image reconstruction processes, thrombosed parts of an aneurysm can also be illustrated, which are not visible on DSA.
In this context, radiation dose should be mentioned. Preliminary internal, not yet published, data (T. Struffert 2011) concerning the radiation dose showed a slightly increased effective patient dose of 3D-DSA compared with the biplane DSA series (1.2 versus 1 mSV) (T. Struffert). The measured CT dose indexw of 3D-DSA amounts to approximately 9 mGy, and the dose-length product, to 180 mGy · cm.24
Preliminary results from internal unpublished investigations with a calibrated ionization chamber and thermoluminescence dosimetry as well as a Monte Carlo tool and virtual phantom models considered the effective dose of ivACT at a level of approximately 1.5 mSv (T. Struffert).
Because of intravenous contrast administration with ivACT, all intracranial vessels could be imaged in 1 step; thus, the reduced total number of required 2D and 3D series warrants the slightly increased radiation dose of the ivACT compared with the 3D-DSA. Here collimation can function as an effective tool for dose reduction of 3D-DSA and ivACT, respectively. In general, a constant dose optimization is of crucial importance, and further radiation dose measurements are mandatory.
Our preliminary results indicate that ivACT could function as a noninvasive tool for the evaluation of unruptured saccular cerebral aneurysms with quality comparable with 3D rotational angiography. Potentially this might be a first step toward new standards in the preinterventional visualization of incidentally found aneurysms.
Certainly our study is limited by the small number of patients, but the number seems sufficient to show the feasibility and accuracy of ivACT and to provide a good impression of this new technique.
As another shortcoming, the mean aneurysm diameter in our study was relatively high, and only a few of the small and smallest aneurysms were included so that further validation with an increased number of cases with such small aneurysms (<3 mm) should be added to our results. Because we included only incidental aneurysms, an objective for further investigations could be cases with acute SAH for proving whether ivACT can demonstrate similar results in cases with ruptured aneurysms. Additionally, the ability to perform ivACT is restricted by the patient population. Patient cooperation is of crucial importance to obtain usable images. Restless und noncompliant patients, such as those who are elderly or infirm or have SAH, might not be suitable for ivACT. Further objectives could be a more optimized injection of contrast agent and better postprocessing programs.
Conclusions
Our preliminary data indicate that noninvasive ivACT may provide reliable information for the assessment of intracranial aneurysms. The measured aneurysm dimensions are comparable with the results of 3D conventional angiography and can be considered sufficient for clinical decision-making of the appropriate therapeutic strategy. Further evaluation with a larger number of patients, including those with smaller nonsaccular aneurysms, is mandatory to validate this new technique in the clinical setting.
ABBREVIATIONS:
- ACA A1
anterior cerebral artery proximal to the origin of the anterior communicating artery
- ACT
angiographic CT
- FPCT
flat-detector CT
- ivACT
ACT with intravenous contrast medium injection
- MPR
multiplanar reformation
- MSCT
multisection CT
- 3D-DSA
3D rotational digital subtraction angiography
References
- 1. Campi A, Ramzi N, Molyneux AJ, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT). Stroke 2007;38:1538–44 [DOI] [PubMed] [Google Scholar]
- 2. Molyneux AJ, Kerr RS, Birks J, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol 2009;8:427–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schievink WI, Torres VE, Piepgras DG, et al. Saccular intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1992;3:88–95 [DOI] [PubMed] [Google Scholar]
- 4. Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med 2006;355:928–39 [DOI] [PubMed] [Google Scholar]
- 5. Anxionnat R, Bracard S, Ducrocq X, et al. Intracranial aneurysms: clinical value of 3D digital subtraction angiography in the therapeutic decision and endovascular treatment. Radiology 2001;218:799–808 [DOI] [PubMed] [Google Scholar]
- 6. Sugahara T, Korogi Y, Nakashima K, et al. Comparison of 2D and 3D digital subtraction angiography in evaluation of intracranial aneurysms. AJNR Am J Neuroradiol 2002;23:1545–52 [PMC free article] [PubMed] [Google Scholar]
- 7. Tanoue S, Kiyosue H, Kenai H, et al. Three-dimensional reconstructed images after rotational angiography in the evaluation of intracranial aneurysms: surgical correlation. Neurosurgery 2000;47:866–71 [DOI] [PubMed] [Google Scholar]
- 8. Fifi JT, Meyers PM, Lavine SD, et al. Complications of modern diagnostic cerebral angiography in an academic medical center. J Vasc Interv Radiol 2009;20:442–47 [DOI] [PubMed] [Google Scholar]
- 9. Okahara M, Kiyosue H, Yamashita M, et al. Diagnostic accuracy of magnetic resonance angiography for cerebral aneurysms in correlation with 3D-digital subtraction angiographic images: a study of 133 aneurysms. Stroke 2002;33:1803–08 [DOI] [PubMed] [Google Scholar]
- 10. Adams WM, Laitt RD, Jackson A. The role of MR angiography in the pretreatment assessment of intracranial aneurysms: a comparative study. AJNR Am J Neuroradiol 2000;21:1618–28 [PMC free article] [PubMed] [Google Scholar]
- 11. Numminen J, Tarkiainen A, Niemelä M, et al. Detection of unruptured cerebral artery aneurysms by MRA at 3.0 Tesla: comparison with multislice helical computed tomographic angiography. Acta Radiol 2011;52:670–74. Epub 2011 Apr 27 [DOI] [PubMed] [Google Scholar]
- 12. Luo Z, Wang D, Sun X, et al. Comparison of the accuracy of subtraction CT angiography performed on 320-detector row volume CT with conventional CT angiography for diagnosis of intracranial aneurysms. Eur J Radiol 2012;81:118–22 [DOI] [PubMed] [Google Scholar]
- 13. Li MH, Li YD, Tan HQ, et al. Contrast-free MRA at 3.0 T for the detection of intracranial aneurysms. Neurology 2011;77:667–76. Epub 2011 Jul 20 [DOI] [PubMed] [Google Scholar]
- 14. Ramasundara S, Mitchell PJ, Dowling RJ. Bone subtraction CT angiography for the detection of intracranial aneurysms. J Med Imaging Radiat Oncol 2010;54:526–33 [DOI] [PubMed] [Google Scholar]
- 15. Kouskouras C, Charitanti A, Giavroglou C, et al. Intracranial aneurysms: evaluation using CTA and MRA—correlation with DSA and intraoperative findings. Neuroradiology 2004;46:842–50 [DOI] [PubMed] [Google Scholar]
- 16. Yoon DY, Lim KJ, Choi CS, et al. Detection and characterization of intracranial aneurysms with 16-channel multi-detector row CT angiography: a prospective comparison of volume-rendered images and digital subtraction angiography. AJNR Am J Neuroradiol 2007;28:60–67 [PMC free article] [PubMed] [Google Scholar]
- 17. Jayaraman MV, Mayo-Smith WW, Tung GA, et al. Detection of intracranial aneurysms: multi-detector row CT angiography compared with DSA. Radiology 2004;230:510–18 [DOI] [PubMed] [Google Scholar]
- 18. Struffert T, Doelken M, Adamek E, et al. Flat-detector computed tomography with intravenous contrast material application in experimental aneurysms: comparison with multislice CT and conventional angiography. Acta Radiol 2010;51:431–37 [DOI] [PubMed] [Google Scholar]
- 19. Struffert T, Kloska S, Engelhorn T, et al. Optimized intravenous flat detector CT for non-invasive visualization of intracranial stents: first results. Eur Radiol 2011;21:411–18 [DOI] [PubMed] [Google Scholar]
- 20. Hoh B, Sistrom C, Firment C, et al. Bottleneck factor and height-width ratio: association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery 2007;61:714–22 [DOI] [PubMed] [Google Scholar]
- 21. Romijn M, Gratama van Andel HA, van Walderveen MA, et al. Diagnostic accuracy of CT angiography with matched mask bone elimination for detection of intracranial aneurysms: comparison with digital subtraction angiography and 3D rotational angiography. AJNR Am J Neuroradiol 2008;29:134–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsumoto M, Sato M, Nakano M, et al. Three-dimensional computerized tomography angiography-guided surgery of acutely ruptured cerebral aneurysms. J Neurosurg 2001;94:718–27 [DOI] [PubMed] [Google Scholar]
- 23. Chappell ET, Moure FC, Good MC. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: a meta-analysis. Neurosurgery 2003;52:624–31 [DOI] [PubMed] [Google Scholar]
- 24. Kyriaku Y, Richter G, Doerfler A, et al. Neuroradiologic applications with routine C-arm flat panel detector CT: evaluation of patient dose measurements. AJNR Am J Neuroradiol 2008;29:1930–36 [DOI] [PMC free article] [PubMed] [Google Scholar]