Lightscapes affect life and death of elephant seals during oceanic migrations.
Abstract
Like landscapes of fear, animals are hypothesized to strategically use lightscapes based on intrinsic motivations. However, longitudinal evidence of state-dependent risk aversion has been difficult to obtain in wild animals. Using high-resolution biologgers, we continuously measured body condition, time partitioning, three-dimensional movement, and risk exposure of 71 elephant seals throughout their 7-month foraging migrations (N = 16,000 seal days). As body condition improved from 21 to 32% fat and daylength declined from 16 to 10 hours, seals rested progressively earlier with respect to sunrise, sacrificing valuable nocturnal foraging hours to rest in the safety of darkness. Seals in superior body condition prioritized safety over energy conservation by resting >100 meters deeper where it was 300× darker. Together, these results provide empirical evidence that marine mammals actively use the three-dimensional lightscape to optimize risk-reward trade-offs based on ecological and physiological factors.
INTRODUCTION
Conflicting demands to forage, rest, and avoid predators are the cornerstone of eco-evolutionary dynamics (1, 2). Although many animals are obligately nocturnal or diurnal, physiological and ecological factors such as starvation and predation can lead to nuanced shifts in temporal niches (3, 4). Yet, these state-dependent shifts have only been measured using a cross-sectional approach; rest timing and fat stores have never been measured through time in individual wild animals. Here, we show that migrating wild animals prioritize predator avoidance over resource acquisition through strategic use of the lightscape as they gain body condition.
These findings support predictions about state-dependent niche partitioning between predator and prey that have received strong empirical support with regard to spatial partitioning (5–7) but have proved difficult to test for temporal partitioning (4, 8, 9). As nutritional state and environmental cues change markedly throughout migration, failing to adjust activities to local sunrise or sunset cues should cause substantial mismatches in predator-prey interactions (10), especially for mesopredators that rely on darkness for both prey acquisition and predator avoidance (11). The potential for temporal mismatches is considerable in the ocean where daily light-dark cycles prompt diel vertical movements of numerous prey species (12). Here, marine mesopredators can optimize energy intake by foraging at night on prey closer to the ocean surface and ensure safety by resting at night when their predators’ visual acuity is compromised. As a result, nighttime is optimal for both feeding and resting, and individuals must choose between competing priorities. It is unknown whether mesopredators resolve this trade-off by adjusting their daily schedules to those of their prey or their predators or whether priorities shift as a function of intrinsic or extrinsic states (13).
Our objective was to determine whether wildlife adjust their use of the lightscape based on nutritional state and photoperiod. Northern elephant seals, Mirounga angustirostris, are a model system for addressing this question because they are easily accessible for both deploying and recovering archival biologgers, which allows for large sample sizes of high-resolution data. Adult female elephant seals travel 10,000 km across the North Pacific Ocean, diving continuously for ~23 min followed by only ~2 min at the ocean surface for 7 consecutive months (fig. S1).
Predators are thought to play an important role in driving elephant seal behavior at sea. The core habitats of elephant seal predators including white sharks (14), salmon sharks (15), sleeper sharks (16), and killer whales (17) overlap with elephant seal habitat near coastal regions (18). Overlaps between offshore elephant seal foraging locations and predator distributions have not been documented, although elephant seals appear to show antipredator behaviors across their entire foraging range [e.g., resting at depth rather than at the ocean surface (19) and exhibiting deeper diving in response to predator vocalizations (20)]. During foraging migrations, elephant seals have no access to habitat refugia or groups (21), so time allocation and spatial utilization are the only plausible predator avoidance strategies.
In 6% of dives each day, seals passively drift through the water column and are entirely inactive while they presumably process food and/or sleep (19, 22). Their rate of vertical drift through the water column provides a metric of buoyancy (23) and can be used to infer rest timing and body condition simultaneously (24). Because seals have not evolved unihemispheric slow wave sleep (25), resting at sea should increase predation risk by their predators, especially in high ambient light conditions during the day and near the ocean surface (26). Although elephant seals could adjust their use of the lightscape by resting during nighttime or deeper in the water column, state-dependent behavioral plasticity has not yet been investigated.
We obtained time-light-depth data at 4-s intervals throughout the entire oceanic foraging migrations of 71 elephant seals using advanced biologgers (N = 459,930 dives). We identified drift dives (N = 37,254) as those with bottom phases characterized by a constant rate of drift (depth/time) through the water column (fig. S2) (24, 27). The ambient light level at the start of the dive (light level at surface) and the ambient light level and depth at the start and end of each drift segment were calculated. We also estimated body condition for each seal on each day as described by Pirotta et al. (23). Data are presented relative to the daily timing of sunrise and sunset measured by each seal, which accounts for the wide range of daylengths that seals experience across seasons and locations (fig. S3). The circular variable “drift timing” was coded such that 0° represents time of sunrise and 180° represents time of sunset independent of the date. The daylength, lipid, latitude, and longitude variables were centered such that a value of 0 represents the average. To estimate the contribution of body condition, photoperiod, and location to the timing of rest, we performed circular mixed-effects regression models (28) and compared model fits using deviance information criterion (DIC) and Watanabe-Akaike information criterion (WAIC).
To describe how risk and reward vary in relation to ambient light, we instrumented an additional 12 seals with acceleration loggers affixed to the lower jaw. The loggers measured variation in feeding success (the rate of prey capture attempts) and predation risk (the ocean surface light level) (27). We also evaluated the risk associated with daylight by extracting Argos satellite transmissions for the 42 seals that stopped transmitting between 2004 and 2018 and were presumed to have died at sea because they were never again observed at the colony (29). We calculated time of day (daytime or nighttime) of all received Argos transmissions based on the date, latitude, and longitude and compared the time of day of the last transmission (presumably when the animal died) to all other transmissions (when the animal was alive).
RESULTS
After sunrise each day, the light level at the ocean surface increased by 100,000-fold, foraging success was reduced by 50%, and dive efficiency was lowered (Fig. 1). Seals were slightly more likely to die during the daytime (69% of last Argos transmissions, indicating mortality) than expected by random chance (56% of all Argos transmissions), although this trend was not significant (χ2 = 2.9182, P = 0.087) (fig. S4). Given higher feeding efficiency and safety from predators at night, each seal must balance foraging in darkness to maximize prey acquisition with exhaustion from continuous foraging.
Fig. 1. Diel differences in risk, reward, and efficiency for elephant seals.
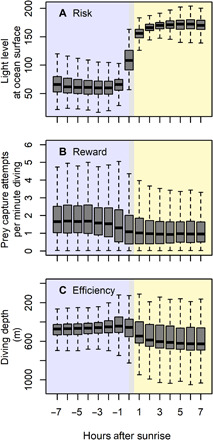
Each day, the transition from nighttime (blue) to daytime (yellow) is associated with (A) higher light levels, a proxy for predation risk, (B) lower feeding success, and (C) deeper diving (note the inverted y axis). Therefore, foraging efficiency is highest at night and coincides with the ideal time to rest in the safety of darkness.
Across the migration, elephant seals scheduled their drift dives near sunrise, following a night of shallower, high-efficiency foraging dives, and preceding a day of deeper, lower-efficiency foraging dives. These drift dives occurred progressively earlier throughout the migration, both in GMT time and relative to sunrise (Fig. 2C and figs. S5 and S6). As a result, the proportion of behavioral rest that occurred during the daytime decreased from 80 to 30% throughout the migration. Similarly, foraging dives occurred progressively earlier throughout the migration, but this change (range, 4 hours) was notably less than the change in the timing of drift dives (range, 6 hours) (Fig. 2D). Thus, as daylength declined and nighttime opportunities increased, this new temporal space was allocated to optimize the timing of resting more so than foraging. The model with all main effects (lipid, daylength, latitude, longitude, and date), including date*daylength and date*lipid interactions, showed the best fit (table S1). In other words, the model estimates suggest that rest timing is related to the explanatory variables lipid and daylength, which both vary with time, along with latitude and longitude.
Fig. 2. Body condition and activity timing throughout migration in elephant seals.
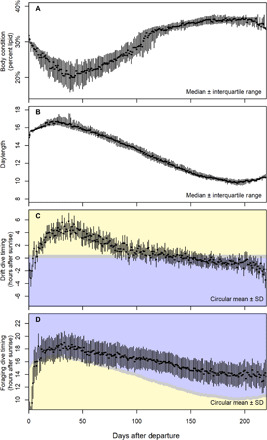
Throughout the foraging migration, elephant seals gain body condition and perform drift dives progressively earlier in the day relative to sunrise. (A) After departing the beach, animals lose body condition for approximately 1 month as they transition to offshore foraging grounds and then gain fat throughout their migration. (B) Because of horizontal movement and seasonal changes, daylength gradually decreases throughout the migration. (C) Concomitant with increasing body condition and shortening daylength is a change in rest phenology, such that animals drift progressively earlier in the day relative to sunrise. (D) Animals also forage progressively earlier throughout the trip, although this rate of change is less than the change in drift dive timing. Blue represents nighttime, gray represents twilight, and yellow represents daytime such that the transition from blue to yellow in (C) represents sunrise, whereas the transition from yellow to blue in (D) represents sunset.
The shift in nocturnality was tightly linked to body condition gains throughout the migration (Fig. 3). Seals in poor body condition started their drift dives just after sunrise, whereas seals in good body condition started their drift dives just before sunrise (fig. S7). Seals departed the beach at 31 ± 2% lipid, lost lipid mass while transiting to foraging areas, and then gained lipid before transiting back to the beach and arriving with 32 ± 3% lipid (Fig. 2A). At the beginning of the migration, a seal with average body condition experiencing average photoperiod drifted at 7.75% of total daylight time after sunrise on average. As seals gained body condition, they rested progressively earlier (3.14% of photoperiod per SD increase in percent lipid on average). This markedly increased the duration of rest occurring during the daytime, such that seals at 15% body fat rested 70% during the daytime and seals with 35% body fat rested only 20% during the daytime (fig. S8C). The timing of foraging dives did not have the same relationship with body condition (fig. S8D).
Fig. 3. Links between body condition and activity timing in migrating elephant seals.
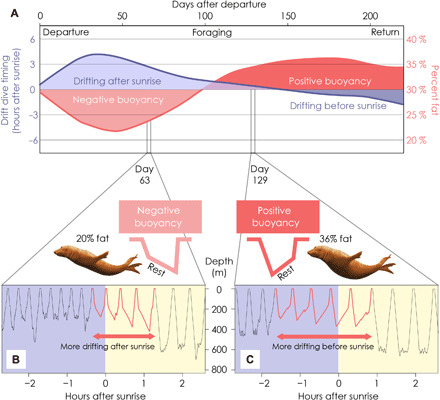
Combining body condition and rest timing shows (A) that the switch to drifting before sunrise (in safer, darker conditions) occurs just after seals switch to positive buoyancy during the middle of the foraging migration. Example diving records of a single elephant seal across 5 hours reveal a state-dependent predator avoidance strategy of (B) shallower, daytime drifts during early migration when the seal is in inferior body condition and (C) deeper, nighttime drifts during late migration while in superior body condition. Colors represent nighttime (blue) and daytime (yellow). Arrows extend from the first drift dive each day to the end of the consecutive drift dives. Note the inverted y axis that represents depth.
These data refute the hypothesis that seals always postpone rest until higher priority feeding occurs. Instead, it appears that as seals gain body condition throughout the migration, they are more likely to fill the nighttime opportunities with low-risk rest rather than high-reward feeding. In other words, seals are more motivated to feed throughout the night during the outbound trip than in the inbound trip when they are in better body condition and can afford to prioritize resting in safer nighttime conditions.
The shift in time allocation was also strongly associated with the change in daylength across the migration. As daylength declined, seals spent more time resting at night, while the timing of foraging remained mostly unchanged (Fig. 2). Specifically, for each hour increase in daylength, the rest timing moved further away from sunrise by 10.99% of photoperiod on average. This suggests a behavioral response to predation risk after sunrise predominantly drives the timing of rest rather than circadian rhythm or a lagged effect of foraging timing. The timing of rest was different in the first and last few weeks of the migration (Fig. 2), likely because adult female elephant seals rarely feed along the coast, where the predation risk is high (30). Seals also rested earlier (more often during nighttime) at lower latitudes (table S2), possibly because their white shark predators are more abundant there (31).
In addition to strategic changes in their activity patterns, seals actively modified their use of three-dimensional space to mediate their exposure to risk. After actively swimming down in the water column, seals began to drift at different depths based on their body condition. Specifically, seals in poor body condition began to drift at shallower depths with higher ambient light levels. In contrast, seals in good body condition began to drift at deeper depths with darker ambient light levels (Fig. 4). Across consecutive drift dives, seals drifted progressively deeper as the sun rose, which minimized ambient light levels (fig. S9). Because of differences in buoyancy state (i.e., seals <30% fat were negatively buoyant and sank while drifting, whereas seals >30% fat were positively buoyant and floated while drifting), all seals finished drifting at similar depths with comparable light levels (fig. S10). Thus, seals with more lipid stores appeared to minimize the risk of detection by predators when they drift up into shallower waters with higher ambient light. This suggests that seals can perceive their buoyancy and actively choose their starting drift depth as a predator avoidance strategy; however, the mechanism that triggers the termination of the drift phase (e.g., depth or duration) remains a mystery.
Fig. 4. Resting depth varies with body condition and time of day in elephant seals.
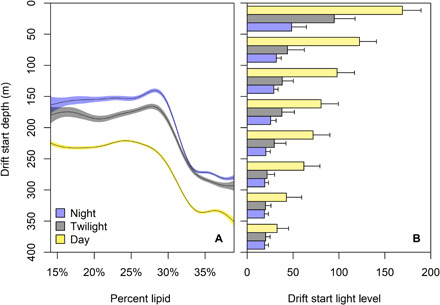
During daytime hours (yellow) when surface light levels and resulting predation risk are higher, seals strategically drift deeper and at lower light levels than during twilight (gray) and nighttime (blue). Seals in superior body condition begin their drifts (A) deeper (note the inverted y axis) and (B) at lower light levels as compared to seals in inferior body condition, presumably due to their positive buoyancy.
DISCUSSION
Given the difficulty of measuring predation events, including the predator species and the three-dimensional location of death, our interpretation of the observed patterns is limited to inference in the context of existing data. Previous research has demonstrated that elephant seal diet items (32, 33) and prey capture rates (34) remain relatively consistent across the foraging migration, suggesting that potential alternative hypotheses regarding prey distribution or diet switches are unlikely to be driving the observed patterns in rest timing throughout elephant seal foraging migrations. Observational data support predator avoidance rather than prey consumption as the driving factor; fresh wounds and scars from white shark attacks are frequently observed on adult elephant seals at the haul-out sites (35), whereas it is exceedingly rare to observe seals nearing starvation at the haul-out site. Starvation is also unlikely given that seals appear to have little competition for their specialized dietary niche (34) and do not routinely dive near their physiological limits (36). Thus, predation is the most likely source of mortality for adult elephant seals.
Together, these data revealed that changes in nutritional state and photoperiod motivate migrating elephant seals to adaptively modify their behavior throughout long-distance foraging migrations. In addition, these data establish photoperiod as a fundamental ecological constraint (37) and, moreover, suggest that seasonal changes in photoperiod confer different advantages across trophic levels. Whereas a longer daylength may benefit apex predators that rely on light to feed, it may be detrimental for species in which foraging and resting are optimal at night. Last, the relative safety of the twilight zone has implications for the evolution of diving physiology and sensory biology in seals and other air-breathing marine vertebrates (38).
Inactivity during drift dives is hypothesized to be associated with behavioral sleep, when reduced sensory responsiveness may increase the risk of predation. Regardless of the function of drift dives, our data suggest that inactivity may be an essential yet overlooked mortality risk for animals under continuous pressure to forage and remain vigilant during migration. Advancements in biotelemetry devices will allow a more comprehensive understanding of the correlation between behavioral and electrophysiological sleep—an essential physiological state for almost all animals (39)—in the ecological context in which it evolved (40).
The balance between predation risk and feeding efficiency is often responsible for the emergence of obligate diurnal or nocturnal strategies, especially in ectotherms, where day-night temperature fluctuations are more consequential than in endotherms (41). We have shown that a wild mesopredator uses an adaptive and state-dependent strategy for maximizing reward while mitigating risk. Given that sleep is highly conserved across animal taxa (42, 43), active risk aversion via adaptive plasticity in diel behavior is likely to be commonplace in other wildlife, especially for mesopredators in which synergistic effects of predators and prey are most apparent (38). As humans continue to threaten the ability of wild animals to forage efficiently and sleep safely (44, 45), we must work to understand how physiology and ecology interact to shape the timing of fundamental behaviors.
MATERIALS AND METHODS
Instrumentation
We instrumented elephant seals between 2004 and 2012 after the molting season (May–June) and recovered instruments 219 ± 18 days later (December–January). Each seal was instrumented with an Argos satellite tag (Wildlife Computers, USA; N = 113), an MK9 time-depth recorder (Wildlife Computers, USA; N = 71), and/or a Kami Kami jaw accelerometer (Little Leonardo, Japan; N = 12). Procedures for tag attachment and removal along with dive identification and dive type categorization using the IKNOS Toolbox are detailed by Robinson et al. (21). Across seals, departure date varied by 42 days (June 06 ± 10 days; range, 15 May to 26 June), photoperiod ranged from 5.7 to 21.7 hours per day, sunrise ranged from 12:00 to 18:00 GMT, and sunset ranged from 01:00 to 8:00 GMT (fig. S3). Within each seal, there was marked variation in daylength (7.8 ± 1.6 hours), sunrise hour (5.7 ± 2.3 hours GMT), and sunset hour (9.8 ± 4.6 hours GMT) due to seasonal and spatial variation (fig. S3).
Dive behavior and feeding efficiency
We used data from the time-depth recorders to infer body condition and lightscape during the drifting phase of each drift dive. To do this, we wrote a custom MATLAB algorithm to identify individual drift segments based on fine-scale changes in depth over time (drift rate, measured in meters per second). For each drift dive with a maximum depth exceeding 200 m, we calculated the dominant drift rate using kernel density estimation (24). We identified the consecutive data points for which the first derivative of the drift rate fell within ±0.38 m/s of the dominant drift rate (fig. S2). This was considered the drifting segment for each drift dive. We then used a cubic spline to calculate the dominant drift rate each day and excluded drift segments that fell outside ±0.03 m/s of the daily dominant drift rate (26% of total drift dives) (fig. S2). To quantify how feeding efficiency (rate of prey capture attempts) and predation risk (ocean surface light level as a proxy) varied by hour, 12 seals were instrumented with Kami Kami acceleration loggers attached to the jaw. Jaw motion events, indicative of prey capture attempts, were identified from the raw acceleration data using a 0.3-g amplitude surge acceleration threshold as described in Naito et al. (34). Foraging dives considered were those where the number of jaw motion events exceeded zero (N = 95,682 dives made by 12 seals).
As compared to their predators, elephant seals are highly proficient at nighttime foraging due to their highly innervated vibrissal sensory systems that can be used to track prey (46–48). In addition, the visual systems of elephant seals are well adapted to dim light conditions (49), and elephant seals have been shown to capture bioluminescent prey (32, 50–52), which helps explain why feeding success is not compromised at night. Thus, we did not expect elephant seal foraging success to vary widely by time of day.
Body condition
We estimated daily lipid mass for each seal using the hierarchical state-space model from Pirotta et al. (23) and input data including the daily median drift rate, daily median dive metrics (ascent rate, descent rate, time spent at the bottom during a dive, total number of dives, and dive duration), Argos location estimates, and morphometric measurements at the beginning and end of the migration (lipid mass, nonlipid mass, and pup mass). The model assumes a functional form for the variation of nonlipid mass throughout the trip. Here, we used a linear increase in maternal nonlipid mass, accounting for pup mass in the final third of the trip (23, 53). We fitted the model in a Bayesian framework using JAGS run from the R package runjags (54), setting the same priors described for the original model. Markov chain Monte Carlo (MCMC) algorithms were optimized during 5000 adaptation iterations and then iterated until convergence of the lipid mass estimates. We ran three parallel chains, starting at different initial values, and convergence was assessed as described by Pirotta et al. (23). Because of computational limitations, the model was run separately on three equally sized subsets of individuals. Before analyzing maternal body composition, we subtracted pup mass from the nonlipid mass of pregnant females, because we were interested in quantifying maternal body condition irrespective of the mass of the pup.
Light and time of day
We quantified sunrise, sunset, daylength, and time of day based on the time-light data measured by the MK9 loggers. The instruments measure relative light intensity with a logarithmic response (a change of 20 readings corresponds to a 10-fold change of light intensity within the range of 25 to 225). A light measurement of 25 corresponds to ~5 × 10−12 W*cm−2 (dark), and a light measurement of 225 corresponds to ~5 × 10−2 W*cm−2 (light).
Using these relative light measurements when seals were at the ocean surface, we quantified the daylength experienced by seals using the TwGeos package with light threshold 110 and a Lowess smoother with span 0.08 using the lowess package in R (21). For each seal, we calculated the difference between the date of each dive and the date of the nearest sunrise time in hours such that 0 coincides with sunrise, −12 represents 12 hours before sunrise, and +12 represents 12 hours after sunrise. We then categorized time of day based on time since sunrise and sunset: twilight (0.5 hour after sunrise and 0.5 hour before sunset), daytime (when hours after sunset fell below 24 minus daylength), and nighttime (when hours after sunrise did not exceed daylength). Differences in the date of migration start and, thus, body composition trajectories were adjusted by using Days After Departure instead of Julian Days. For visualization purposes, hour metrics were analyzed using circular median and mean deviation in the package psych in R. We present descriptive statistics as mean ± SD, and figures show median ± interquartile range or circular mean ± SD. We used a generalized additive mixed model to characterize the relationships between drift depth and body composition with the individual as a random effect: depth ~ s(body condition) + (1|individual). Including model uncertainty for lipid (by resampling 100 times and fitting the GAMM (generalized additive mixed model) to these new data) did not affect the conclusions (fig. S11).
Daily activity timing as a function of intrinsic and extrinsic factors
To evaluate the contributions of body condition, daylength, latitude, and longitude to the daily timing of activity, we performed circular mixed-effects model analyses using the R package bpnreg (55). This package implements circular regression and circular mixed-effects regression models as described by Cremers et al. (28), Cremers and Klugkist (56), Cremers et al. (57), and Cremers et al. (58). First, the circular outcome “Drift” was coded such that 0° represents time of sunrise and 180° represents time of sunset independent of the date. This means that an increase or decrease of 1° represents a 1/1.8 = 0.56% change in the total daytime or nighttime. For example, a drift segment at 10° takes place at 5.6% of total daylight time after sunrise, and a decrease of 2° means that a drift takes place 1.12% total daylight time closer to sunrise than the average drift. The variable daylength, lipid, latitude, and longitude were centered such that a value of 0 represents the average. To check whether the MCMC sampler converged, we evaluated the traceplots for the linear regression coefficients of both bivariate components of the circular regression model. Four indicators of model fit were used to choose between the competing models: two versions of the DIC (DIC and DICalt) and two versions of the WAIC (WAIC and WAIC2) [for details, see Gelman et al. (59)]. Statistics closer to zero indicate better model fit. Lipid mass estimates resampled from the posterior distribution N = 100 times did not significantly affect results. When including model uncertainty for lipid (by resampling 100 values and fitting the best-fitting model to these new datasets), average model fits (SD) for the best-fitting model are as follows: DIC = 102,069 (34), DICalt = 102,219 (32), WAIC = 102,129 (11), WAIC2 = 102,130 (11).
Supplementary Material
Acknowledgments
We are grateful to the past and present members of the Costa lab, especially field leaders C. Kuhn, S. Simmons, J. Hassrick, M. Fowler, B. McDonald, S. Peterson, R. Holser, and T. Keates, as well as A. Marm Kilpatrick, J. Estes, and T. Williams for the helpful discussions and the Año Nuevo State Park staff and docents for facilitating and sharing our research. Fieldwork was completed at the University of California Natural Reserve System’s Año Nuevo Reserve. Research was approved by the University of California Santa Cruz Institutional Animal Care and Use Committee no. Costd1709 and the National Marine Fisheries Service marine mammal research permit no. 19108. Funding: Financial support for this research was provided by an NSF Postdoctoral Research Fellowship in Biology and UC Santa Cruz Chancellor’s Postdoctoral Fellowship (R.S.B.), as well as the Office of Naval Research, the E&P Sound and Marine Life Joint Industry Project of the International Association of Oil and Gas Producers (D.P.C.), and the Novo Nordisk Foundation (“Harnessing The Power of Big Data to Address the Societal Challenge of Aging”, NNF17OC0027812) (J.C.). Author contributions: Conceptualization: R.S.B., J.M.K.-B., and D.P.C. Data collection: D.P.C., D.E.C., P.W.R., T.A., A.T., and Y.N. Visualization: R.S.B. and J.M.K.-B. Analysis: R.S.B., J.M.K.-B., E.P., and J.C. All authors read and edited the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: Data and code used in this analysis are available at https://github.com/roxannebeltran/LightscapesOfFear. All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/12/eabd9818/DC1
REFERENCES AND NOTES
- 1.J. S. Brown, Annales Zoologici Fennici (JSTOR, Helsinki, 1992), vol. 29, pp. 301–309.
- 2.McNamara J. M., Houston A. I., Starvation and predation as factors limiting population size. Ecology 68, 1515–1519 (1987). [Google Scholar]
- 3.Metcalfe N. B., Fraser N. H., Burns M. D., State-dependent shifts between nocturnal and diurnal activity in salmon. Proc. R. Soc. B Biol. Sci. 265, 1503–1507 (1998). [Google Scholar]
- 4.Bennie J. J., Duffy J. P., Inger R., Gaston K. J., Biogeography of time partitioning in mammals. Proc. Natl. Acad. Sci. 111, 13727–13732 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frid A., Baker G. G., Dill L. M., Do resource declines increase predation rates on North Pacific harbor seals? A behavior-based plausibility model. Mar. Ecol. Prog. Ser. 312, 265–275 (2006). [Google Scholar]
- 6.Sinclair A., Arcese P., Population consequences of predation-sensitive foraging: The Serengeti wildebeest. Ecology 76, 882–891 (1995). [Google Scholar]
- 7.Heithaus M. R., Frid A., Wirsing A. J., Dill L. M., Fourqurean J. W., Burkholder D., Thomson J., Bejder L., State-dependent risk-taking by green sea turtles mediates top-down effects of tiger shark intimidation in a marine ecosystem. J. Anim. Ecol. 76, 837–844 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Kronfeld-Schor N., Dayan T., Elvert R., Haim A., Zisapel N., Heldmaier G., On the use of the time axis for ecological separation: Diel rhythms as an evolutionary constraint. Am. Nat. 158, 451–457 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Lima S. L., Bednekoff P. A., Temporal variation in danger drives antipredator behavior: The predation risk allocation hypothesis. Am. Nat. 153, 649–659 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Furey N. B., Armstrong J. B., Beauchamp D. A., Hinch S. G., Migratory coupling between predators and prey. Nat. Ecol. Evol. 2, 1846–1853 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Lima S. L., Rattenborg N. C., Lesku J. A., Amlaner C. J., Sleeping under the risk of predation. Anim. Behav. 70, 723–736 (2005). [Google Scholar]
- 12.Behrenfeld M. J., Gaube P., Della Penna A., O’Malley R. T., Burt W. J., Hu Y., Bontempi P. S., Steinberg D. K., Boss E. S., Siegel D. A., Hostetler C. A., Tortell P. D., Doney S. C., Global satellite-observed daily vertical migrations of ocean animals. Nature 576, 257–261 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Houston A. I., McNamara J. M., Hutchinson J. M., General results concerning the trade-off between gaining energy and avoiding predation. Philos. Trans. Biol. Sci. 341, 375–397 (1993). [Google Scholar]
- 14.Carlisle A. B., Kim S. L., Semmens B. X., Madigan D. J., Jorgensen S. J., Perle C. R., Anderson S. D., Chapple T. K., Kanive P. E., Block B. A., Using stable isotope analysis to understand the migration and trophic ecology of northeastern Pacific white sharks (Carcharodon carcharias). PLOS ONE 7, e30492 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Queiroz N., Humphries N. E., Couto A., Vedor M., da Costa I., Sequeira A. M. M., Mucientes G., Santos A. M., Abascal F. J., Abercrombie D. L., Abrantes K., Acuña-Marrero D., Afonso A. S., Afonso P., Anders D., Araujo G., Arauz R., Bach P., Barnett A., Bernal D., Berumen M. L., Bessudo Lion S., Bezerra N. P. A., Blaison A. V., Block B. A., Bond M. E., Bonfil R., Bradford R. W., Braun C. D., Brooks E. J., Brooks A., Brown J., Bruce B. D., Byrne M. E., Campana S. E., Carlisle A. B., Chapman D. D., Chapple T. K., Chisholm J., Clarke C. R., Clua E. G., Cochran J. E. M., Crochelet E. C., Dagorn L., Daly R., Cortés D. D., Doyle T. K., Drew M., Duffy C. A. J., Erikson T., Espinoza E., Ferreira L. C., Ferretti F., Filmalter J. D., Fischer G. C., Fitzpatrick R., Fontes J., Forget F., Fowler M., Francis M. P., Gallagher A. J., Gennari E., Goldsworthy S. D., Gollock M. J., Green J. R., Gustafson J. A., Guttridge T. L., Guzman H. M., Hammerschlag N., Harman L., Hazin F. H. V., Heard M., Hearn A. R., Holdsworth J. C., Holmes B. J., Howey L. A., Hoyos M., Hueter R. E., Hussey N. E., Huveneers C., Irion D. T., Jacoby D. M. P., Jewell O. J. D., Johnson R., Jordan L. K. B., Jorgensen S. J., Joyce W., Keating Daly C. A., Ketchum J. T., Klimley A. P., Kock A. A., Koen P., Ladino F., Lana F. O., Lea J. S. E., Llewellyn F., Lyon W. S., MacDonnell A., Macena B. C. L., Marshall H., McAllister J. D., McAuley R., Meÿer M. A., Morris J. J., Nelson E. R., Papastamatiou Y. P., Patterson T. A., Peñaherrera-Palma C., Pepperell J. G., Pierce S. J., Poisson F., Quintero L. M., Richardson A. J., Rogers P. J., Rohner C. A., Rowat D. R. L., Samoilys M., Semmens J. M., Sheaves M., Shillinger G., Shivji M., Singh S., Skomal G. B., Smale M. J., Snyders L. B., Soler G., Soria M., Stehfest K. M., Stevens J. D., Thorrold S. R., Tolotti M. T., Towner A., Travassos P., Tyminski J. P., Vandeperre F., Vaudo J. J., Watanabe Y. Y., Weber S. B., Wetherbee B. M., White T. D., Williams S., Zárate P. M., Harcourt R., Hays G. C., Meekan M. G., Thums M., Irigoien X., Eguiluz V. M., Duarte C. M., Sousa L. L., Simpson S. J., Southall E. J., Sims D. W., Global spatial risk assessment of sharks under the footprint of fisheries. Nature 572, 461–466 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Van Den Hoff J., Morrice M. G., Sleeper shark (Somniosus antarcticus) and other bite wounds observed on southern elephant seals (Mirounga leonina) at Macquarie Island. Mar. Mamm. Sci. 24, 239–247 (2008). [Google Scholar]
- 17.Ford J. K., Ellis G. M., Barrett-Lennard L. G., Morton A. B., Palm R. S., Balcomb III K. C., Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters. Can. J. Zool. 76, 1456–1471 (1998). [Google Scholar]
- 18.White T. D., Ferretti F., Kroodsma D. A., Hazen E. L., Carlisle A. B., Scales K. L., Bograd S. J., Block B. A., Predicted hotspots of overlap between highly migratory fishes and industrial fishing fleets in the northeast Pacific. Sci. Adv. 5, eaau3761 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crocker D. E., Boeuf B. J. L., Costa D. P., Drift diving in female northern elephant seals: Implications for food processing. Can. J. Zool. 75, 27–39 (1997). [Google Scholar]
- 20.Fregosi S., Klinck H., Horning M., Costa D. P., Mann D., Sexton K., Hückstädt L. A., Mellinger D. K., Southall B. L., An animal-borne active acoustic tag for minimally invasive behavioral response studies on marine mammals. Anim. Biotelemetry 4, 9 (2016). [Google Scholar]
- 21.Robinson P. W., Costa D. P., Crocker D. E., Gallo-Reynoso J. P., Champagne C. D., Fowler M. A., Goetsch C., Goetz K. T., Hassrick J. L., Hückstädt L. A., Kuhn C. E., Maresh J. L., Maxwell S. M., McDonald B. I., Peterson S. H., Simmons S. E., Teutschel N. M., Villegas-Amtmann S., Yoda K., Foraging behavior and success of a mesopelagic predator in the northeast Pacific Ocean: Insights from a data-rich species, the northern elephant seal. PLOS ONE 7, e36728 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitani Y., Andrews R. D., Sato K., Kato A., Naito Y., Costa D. P., Three-dimensional resting behaviour of northern elephant seals: Drifting like a falling leaf. Biol. Lett. 6, 163–166 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirotta E., Schwarz L. K., Costa D. P., Robinson P. W., New L., Modeling the functional link between movement, feeding activity, and condition in a marine predator. Behav. Ecol. 30, 434–445 (2019). [Google Scholar]
- 24.Robinson P. W., Simmons S. E., Crocker D. E., Costa D. P., Measurements of foraging success in a highly pelagic marine predator, the northern elephant seal. J. Anim. Ecol. 79, 1146–1156 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Kendall-Bar J. M., Vyssotski A. L., Mukhametov L. M., Siegel J. M., Lyamin O. I., Eye state asymmetry during aquatic unihemispheric slow wave sleep in northern fur seals (Callorhinus ursinus). PLOS ONE 14, e0217025 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heithaus M. R., Frid A., Optimal diving under the risk of predation. J. Theor. Biol. 223, 79–92 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Materials and methods are available as supplementary materials at the Science website.
- 28.Cremers J., Mainhard T., Klugkist I., Assessing a Bayesian embedding approach to circular regression models. Methodology 14, 69–81 (2018). [Google Scholar]
- 29.Klaassen R. H., Hake M., Strandberg R., Koks B. J., Trierweiler C., Exo K.-M., Bairlein F., Alerstam T., When and where does mortality occur in migratory birds? Direct evidence from long-term satellite tracking of raptors. J. Anim. Ecol. 83, 176–184 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Jorgensen S. J., Reeb C. A., Chapple T. K., Anderson S., Perle C., Van Sommeran S. R., Fritz-Cope C., Brown A. C., Klimley A. P., Block B. A., Philopatry and migration of Pacific white sharks. Proc. Biol. Sci. 277, 679–688 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Block B. A., Jonsen I. D., Jorgensen S. J., Winship A. J., Shaffer S. A., Bograd S. J., Hazen E. L., Foley D. G., Breed G. A., Harrison A. L., Ganong J. E., Swithenbank A., Castleton M., Dewar H., Mate B. R., Shillinger G. L., Schaefer K. M., Benson S. R., Weise M. J., Henry R. W., Costa D. P., Tracking apex marine predator movements in a dynamic ocean. Nature 475, 86–90 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Yoshino K., Takahashi A., Adachi T., Costa D. P., Robinson P. W., Peterson S. H., Hückstädt L. A., Holser R. R., Naito Y., Acceleration-triggered animal-borne videos show a dominance of fish in the diet of female northern elephant seals. J. Exp. Biol. 223, jeb212936 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Goetsch C., Conners M. G., Budge S. M., Mitani Y., Walker W. A., Bromaghin J. F., Simmons S. E., Reichmuth C., Costa D. P., Energy-rich mesopelagic fishes revealed as a critical prey resource for a deep-diving predator using quantitative fatty acid signature analysis. Front. Mar. Sci. 5, 430 (2018). [Google Scholar]
- 34.Naito Y., Costa D. P., Adachi T., Robinson P. W., Fowler M., Takahashi A., Unravelling the mysteries of a mesopelagic diet: A large apex predator specializes on small prey. Func. Ecol. 27, 710–717 (2013). [Google Scholar]
- 35.B. J. LeBoeuf, D. E. Crocker, Great White Sharks (Elsevier, 1996), pp. 193–205. [Google Scholar]
- 36.Hassrick J., Crocker D. E., Teutschel N. M., McDonald B. I., Robinson P. W., Simmons S. E., Costa D. P., Condition and mass impact oxygen stores and dive duration in adult female northern elephant seals. J. Exp. Biol. 213, 585–592 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Prugh L. R., Golden C. D., Does moonlight increase predation risk? Meta-analysis reveals divergent responses of nocturnal mammals to lunar cycles. J. Anim. Ecol. 83, 504–514 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Frid A., Burns J., Baker G. G., Thorne R. E., Predicting synergistic effects of resources and predators on foraging decisions by juvenile Steller sea lions. Oecologia 158, 775–786 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Ball N. J., Amlaner C. J., A synthesis of sleep in wild birds. Behaviour 87, 85–119 (1983). [Google Scholar]
- 40.Randler C., Sleep, sleep timing and chronotype in animal behaviour. Anim. Behav. 94, 161–166 (2014). [Google Scholar]
- 41.Fenn M. G., Macdonald D. W., Use of middens by red foxes: Risk reverses rhythms of rats. J. Mammal. 76, 130–136 (1995). [Google Scholar]
- 42.Siegel J. M., Clues to the functions of mammalian sleep. Nature 437, 1264–1271 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warkentin K. M., Adaptive plasticity in hatching age: A response to predation risk trade-offs. Proc. Natl. Acad. Sci. U.S.A. 92, 3507–3510 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaynor K. M., Hojnowski C. E., Carter N. H., Brashares J. S., The influence of human disturbance on wildlife nocturnality. Science 360, 1232–1235 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Kronfeld-Schor N., Visser M. E., Salis L., van Gils J. A., Chronobiology of interspecific interactions in a changing world. Philos. Trans. R. Soc. B Biol. Sci. 372, 20160248 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanke W., Wieskotten S., Marshall C., Dehnhardt G., Hydrodynamic perception in true seals (Phocidae) and eared seals (Otariidae). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 199, 421–440 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Eberhardt W. C., Wakefield B. F., Murphy C. T., Casey C., Shakhsheer Y., Calhoun B. H., Reichmuth C., Development of an artificial sensor for hydrodynamic detection inspired by a seal’s whisker array. Bioinspir. Biomim. 11, 056011 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Murphy C. T., Reichmuth C., Mann D., Vibrissal sensitivity in a harbor seal (Phoca vitulina). J. Exp. Biol. 218, 2463–2471 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Levenson D. H., Schusterman R. J., Pupillometry in seals and sea lions: Ecological implications. Can. J. Zool. 75, 2050–2057 (1997). [Google Scholar]
- 50.Vacquié-Garcia J., Royer F., Dragon A. C., Viviant M., Bailleul F., Guinet C., Foraging in the darkness of the Southern Ocean: Influence of bioluminescence on a deep diving predator. PLOS ONE 7, e43565 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campagna C., Dignani J. P., Blackwell S. B., Marin M. R., Detecting bioluminescence with an irradiance time-depth recorder deployed on southern elephant seals. Mar. Mamm. Sci. 17, 402–414 (2001). [Google Scholar]
- 52.Goulet P., Guinet C., Campagna C., Campagna J., Tyack P. L., Johnson M., Flash and grab: Deep-diving southern elephant seals trigger anti-predator flashes in bioluminescent prey. J. Exp. Biol. 223, jeb222810 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.New L. F., Clark J. S., Costa D. P., Fleishman E., Hindell M. A., Klanjšček T., Lusseau D., Kraus S., McMahon C., Robinson P. W., Schick R. S., Schwarz L. K., Simmons S. E., Thomas L., Tyack P., Harwood J., Using short-term measures of behaviour to estimate long-term fitness of southern elephant seals. Mar. Ecol. Prog. Ser. 496, 99–108 (2014). [Google Scholar]
- 54.Denwood M. J., runjags: An R package providing interface utilities, model templates, parallel computing methods and additional distributions for MCMC models in JAGS. J. Stat. Softw. 71, 9 (2016). [Google Scholar]
- 55.J. Cremers, bpnreg: Bayesian Projected Normal Regression Models for Circular Data (R package version 1.0.2, 2019); https://CRAN.R-project.org/package=bpnreg.
- 56.Cremers J., Klugkist I., One direction? A tutorial for circular data analysis using R with examples in cognitive psychology. Front. Psychol. 9, 2040 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cremers J., Mulder K. T., Klugkist I., Circular interpretation of regression coefficients. Brit. J. Math. Stat. Psychol. 71, 75–95 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cremers J., Pennings H. J., Mainhard T., Klugkist I., Circular modelling of circumplex measurements for interpersonal behavior. Assessment 1073191119858407, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.A. Gelman, J. Carlin, A. Vehtari, D. Rubin, H. S. Stern, D. Dunson, Bayesian Data Analysis (Chapman&Hall/CRC, Boca Raton, FL, ed. 3, 2014). [Google Scholar]
- 60.De Vos A., Justin O’Riain M., Meyer M. A., Kotze P. G. H., Kock A. A., Behavior of Cape fur seals (Arctocephalus pusillus pusillus) in relation to temporal variation in predation risk by white sharks (Carcharodon carcharias) around a seal rookery in False Bay, South Africa. Mar. Mamm. Sci. 31, 1118–1131 (2015). [Google Scholar]
- 61.Miller P. J. O. M., Shapiro A. D., Deecke V. B., The diving behaviour of mammal-eating killer whales (Orcinus orca): Variations with ecological not physiological factors. Can. J. Zool. 88, 1103–1112 (2010). [Google Scholar]
- 62.Boustany A. M., Davis S. F., Pyle P., Anderson S. D., le Boeuf B. J., Block B. A., Expanded niche for white sharks. Nature 415, 35–36 (2002). [DOI] [PubMed] [Google Scholar]
- 63.Moxley J. H., Skomal G., Chisholm J., Halpin P., Johnston D. W., Daily and seasonal movements of Cape Cod gray seals vary with predation risk. Mar. Ecol. Prog. Ser. 644, 215–228 (2020). [Google Scholar]
- 64.Williams T. M., Blackwell S. B., Richter B., Sinding M.-H. S., Heide-Jørgensen M. P., Paradoxical escape responses by narwhals (Monodon monoceros). Science 358, 1328–1331 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Laroche R. K., Kock A. A., Dill L. M., Oosthuizen W. H., Running the gauntlet: A predator–prey game between sharks and two age classes of seals. Anim. Behav. 76, 1901–1917 (2008). [Google Scholar]
- 66.Hammerschlag N., Martin R. A., Fallows C., Effects of environmental conditions on predator–prey interactions between white sharks (Carcharodon carcharias) and Cape fur seals (Arctocephalus pusillus pusillus) at Seal Island, South Africa. Environ. Biol. Fishes 76, 341–350 (2006). [Google Scholar]
- 67.Deecke V. B., Ford J. K., Slater P. J., The vocal behaviour of mammal-eating killer whales: Communicating with costly calls. Anim. Behav. 69, 395–405 (2005). [Google Scholar]
- 68.Martin R. A., Hammerschlag N., Marine predator–prey contests: Ambush and speed versus vigilance and agility. Marine Biol. Res. 8, 90–94 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/12/eabd9818/DC1


