Abstract
Background:
Women on the liver transplant waitlist are at greater risk of hospitalization compared with men, but whether this impacts length of stay (LOS) post-transplant is unknown. We aimed to evaluate gender disparities in post-transplant LOS, an important surrogate of health resource utilization post-transplant.
Methods:
Using the UNOS/OPTN registry, we analyzed all non-Status 1 adult deceased donor liver transplant recipients without exception points from 2008–2017. Poisson regression associated female gender with post-transplant LOS.
Results:
Of 27,294 transplant recipients, 36% were female. Women were more likely to be hospitalized pre-transplant than men (44% vs 39%, p<0.01). Post-transplant, women were more likely to have prolonged (≥20d) LOS (25% vs 22%, p<0.01). In univariable analysis, female gender was associated with longer post-transplant LOS (IRR 1.09, 95%CI 1.06–1.12, p<0.01). Prolonged pre-transplant admission was also associated with post-transplant LOS (IRR 1.83, 95%CI 1.77–1.89, p<0.01). In multivariable analysis, female gender remained independently associated with post-transplant LOS (aIRR 1.05, 95%CI 1.02–1.08, p<0.01), after adjustment for age, UNOS region, insurance type, MELDNa, cirrhosis complications, and donor risk index. Pre-transplant hospitalization mediated this relationship, explaining 14.1% (95%CI 9.7–25.4%) of the total effect.
Conclusions:
Women who undergo deceased donor liver transplant have increased health care utilization in the peri-transplant period compared with men. Reducing gender disparities in liver transplantation, including the disproportionate burden of health care utilization by women pre- and post-transplant, will require interventions targeted at preventing hospitalization among women on the transplant waitlist and developing tools aimed at better characterizing the severity of end-stage liver disease in women.
Keywords: Female, Liver Transplantation, Length of Stay, Transplant Recipients, Health Resources, Hospitalization
LAY SUMMARY
Women stay in the hospital longer after liver transplant than men, in large part because they are hospitalized more before they undergo transplant. This likely results in higher healthcare costs and more time away from home for women who undergo transplant. Additional support for women early on, while they are waiting for a liver transplant, may help decrease the amount of time they spend in the hospital after transplant as well.
INTRODUCTION
Women have been shown to have worse transplant-related outcomes than men.1 Rates of liver transplant are lower for women on the waitlist,2–6 and women are more likely to die or become too sick for transplant than men.2,7,8 We have also shown that women on the liver transplant waitlist are at increased risk of hospitalization compared with men, independent of severity of illness.9 Thus, women with cirrhosis on the liver transplant waitlist have higher health care resource utilization than men, and they experience worse outcomes. Despite this well-documented gender disparity among patients on the liver transplant waitlist, the data are less clear on whether there are gender disparities in outcomes and resource utilization after liver transplantation.10,11
Previous studies have shown that both resource utilization and clinical characteristics in the peri-transplant period determine outcomes after liver transplantation. Recipient factors such as pre-transplant MELDNa, sarcopenia, and life support, donor factors such as age and cause of death, as well as operative factors such as cold ischemia time and duration of surgery are all known to be associated with increased post-transplant mortality.12–21 Both pre- and post-transplant health care utilization factors – including length of stay and intensive care unit (ICU) needs – independently predict post-transplant outcomes as well.19,20,22 In addition to predicting survival, post-transplant length of stay has been shown to be a key driver of liver transplantation cost and is relatively consistent across institutions, making it a reliable surrogate for resource utilization in the peri-transplant period.13,23
Given increased rates of hospitalization among women on the liver transplant waitlist, we hypothesized that there would be gender disparities in peri-transplant health resource utilization as well. In particular, we sought to determine whether women have longer post-transplant length of stay, and whether this affects short-term outcomes post-liver transplant.
MATERIALS AND METHODS
All patients who underwent deceased donor liver transplantation in the United Network for Organ Sharing/Organ Procurement and Transplantation Network (UNOS/OPTN) registry from January 1, 2008 through December 31, 2017 were evaluated for inclusion in this study. Patients who were <18 years old at time of listing, those listed as Status 1, and those with fulminant hepatic failure were excluded. Due to differences in severity of illness at time of transplant, those who had hepatocellular carcinoma (HCC), received MELD exception points or underwent a living donor liver transplantation were also excluded. As death during hospitalization could result in shorter length of stay than expected, patients who died during hospitalization (3.4% of cohort, statistically similar rates between women and men) were excluded from primary analyses.
Covariates
Data were obtained from the UNOS/OPTN registry as of January 31, 2018. Demographic data included gender, age at transplant, race, height, weight, ABO group, insurance type, and UNOS region. Given regional variation in MELD scores at time of transplant which may be associated with peri-transplant health resource utilization and outcomes, regions were categorized as low (3, 8, 10, 11), medium (1, 2, 6) or high (4, 5, 7, 9) based on median MELD score at time of transplant.3 Disease-specific data collected at time of transplant included disease etiology, history of prior transplant, MELDNa, sodium, portal vein thrombus, hepatic encephalopathy, albumin, ascites, and Karnofsky Performance Status score. Health care utilization data collected included date of admission, date of transplant, date of discharge, and ICU admission immediately prior to transplant. Donor risk index (DRI) was calculated as described previously by Feng et al. based on donor-specific characteristics.12
Outcomes
The primary outcome in this study was length of stay (LOS) post-transplant, defined as the difference between date of discharge and date of transplant. This outcome was further categorized into non-prolonged versus prolonged LOS, defined as ≥20 days, which was the 75th percentile LOS cut-off in our cohort. We hypothesized that hospitalization pre-transplant was a mediator in the effect of gender on post-transplant length of stay. To explore and quantify this effect, we developed mediation models for prolonged length of stay using hospitalization pre-transplant as a mediating variable. In addition, we performed a subgroup analysis excluding all patients with hospitalization >24 hours prior to transplant. Although patients who died during hospitalization were excluded from primary analysis, a secondary analysis including these patients was also performed. Secondary outcomes included mortality at 3 months, 6 months and one year. Subgroup analysis was performed to evaluate whether gender disparities were affected by the Share 35 policy, which was implemented June 18, 2013 and mandates broader regional organ sharing for patients with a MELDNa score ≥35.
Statistical Analysis
Continuous variables were compared between groups by the Wilcoxon rank sum test. Categorical variables were compared between groups by the chi square test. Unadjusted models were used to assess the association of all listed covariates with primary and secondary outcomes. All covariates with a P < 0.2 in univariate analysis were considered for inclusion in multivariate models. Those not reaching significance of P < .05 were sequentially eliminated. Length of stay as a count outcome was modeled using a zero-truncated Poisson regression with robust standard errors, and logistic regression was used to associate covariates with prolonged (≥20 days) post-transplant length of stay. To explore this effect of hospitalization pre-transplant as a mediating variable, we utilized the MedEff module in Stata 15.0 using our regression models for the binary outcome of LOS >20 days.24 Cox proportional hazard regression was used to associate covariates with 3-month, 6-month, 1-year and overall post-transplant mortality, as short-term mortality has been shown to be associated with peri-transplant factors.14,20 Follow-up time was defined as the interval between the date of transplant and the date of death or last follow-up. Patients remaining alive at last follow-up were censored at time of last follow-up. Two-sided P< .05 were considered statistically significant. In primary and secondary analyses, we tested for interactions between gender and health resource utilization variables (e.g. length of stay, ICU stay) given known gender differences in pre-transplant health resource utilization.9 Analyses were performed using Stata 15.0 statistical software (College Station, TX). This study was approved by the institutional review board at the University of California, San Francisco.
RESULTS
There were 27,294 deceased donor liver transplant recipients during the study period. Of these, 9,700 (36%) were female and 20,080 (74%) were non-Hispanic white. Median pre-transplant laboratory MELDNa was 29 [interquartile range (IQR) 22–35]. Compared with men, women in the cohort were less likely to be White (71% vs 75%, p <0.001) and less likely to have cirrhosis due to Hepatitis C (23% vs 35%, p<0.001) or alcohol (23% vs 33%, p < 0.001) (Table 1). Comorbidities including diabetes and obesity, as well as listing MELDNa, were similar between women and men. At the time of transplant, women had been on the waitlist for longer than men (51d vs 47d, p=0.001) and MELDNa at time of transplant was higher (29 vs 28, p<0.001). Women and men had clinically similar rates of hepatic encephalopathy, ascites, and portal vein thrombosis though women were more likely to have had dialysis in the week prior to transplant (22% vs 19%, p<0.001) and to be on life support at the time of transplant (11% vs 8%, p<0.001). Functional status, as measured by Karnofsky Performance Score (KPS), was slightly worse in women compared with men at time of transplant, with a larger proportion of women having KPS of 40 or less (54% vs 50%, p<0.001). Donor-risk index was 1.5 (IQR 1.2–1.8) in women and 1.4 (IQR 1.2–1.7) in men (p<0.001).
Table 1.
Characteristics of patients who underwent liver transplantation in the United States 2008–2017 †
| Women (n = 9,700) | Men (n = 17,594) | p-value | ||
|---|---|---|---|---|
| Demographics, comorbidities, and liver disease history | ||||
| Age at listing, years | 55 (48–61) | 55 (48–60) | <0.001 | |
| Ethnicity | White | 70.5 | 75.3 | <0.001 |
| Black | 11.2 | 8.4 | ||
| Hispanic | 14.2 | 12.9 | ||
| Asian | 2.4 | 2.3 | ||
| Other | 1.7 | 1.2 | ||
| Liver diagnosis | Alcohol | 16.2 | 26.2 | <0.001 |
| Hepatitis C | 23.1 | 33.2 | ||
| NASH/Cryptogenic | 28.5 | 20.2 | ||
| Hepatitis B | 1.1 | 2.2 | ||
| Autoimmune/cholestatic | 23.1 | 9.4 | ||
| Other | 8.0 | 8.9 | ||
| History of prior transplant | 2.5 | 2.7 | 0.17 | |
| Height, cm | 163 (158–168) | 178 (173–183) | <0.001 | |
| Obesity | 42.1 | 41.6 | 0.42 | |
| Diabetes | 25.0 | 24.9 | 0.80 | |
| Insurance | Private | 52.1 | 56.0 | <0.001 |
| Medicare | 17.4 | 14.5 | ||
| Medicaid | 28.1 | 24.4 | ||
| Blood type | A | 35.1 | 36.5 | 0.14 |
| B | 13.7 | 13.7 | ||
| AB | 5.7 | 5.4 | ||
| O | 45.4 | 44.4 | ||
| Region risk | Low | 49.0 | 48.9 | <0.001 |
| Medium | 15.6 | 17.9 | ||
| High | 35.4 | 33.3 | ||
| Time on waitlist, days | 51 (11–209) | 47 (11–186) | 0.001 | |
| Clinical characteristics at time of transplant | ||||
| Age, years | 56 (49–62) | 55 (49–61) | <0.001 | |
| MELDNa | 29 (23–35) | 28 (22–35) | <0.001 | |
| Hepatic encephalopathy | 73.5 | 74.1 | 0.32 | |
| Ascites | 86.2 | 87.2 | 0.02 | |
| Portal vein thrombus | 11.3 | 11.7 | 0.34 | |
| Dialysis | 23.9 | 20.7 | <0.001 | |
| Life support | 10.6 | 8.4 | <0.001 | |
| Karnofsky Performance Status | 40 (20–60) | 50 (20–70) | <0.001 | |
| KPS Category | Unable to care for self | 52.9 | 49.2 | <0.001 |
| Unable to work | 37.0 | 38.4 | ||
| Normal activity | 10.1 | 12.4 | ||
| Donor risk index | 1.5 (1.2–1.8) | 1.4 (1.2–1.7) | <0.001 | |
| Health resource utilization pre-transplant | ||||
| Hospitalized pre-transplant | 44.0 | 39.0 | <0.001 | |
| Prolonged hospitalization (> 10d) pre-transplant | 24.1 | 19.8 | <0.001 | |
| Transplanted from ICU | 18.0 | 14.1 | <0.001 | |
Data presented as percent or median (interquartile range)
Nonalcoholic steatohepatitis (NASH); Model for End-Stage Liver Disease with Sodium (MELDNa); Karnofsky Performance Score (KPS); intensive care unit (ICU).
Pre-transplant hospitalization
Of the 41% of patients who were hospitalized for >1 day prior to transplant, 52% had a prolonged pre-transplant hospitalization (>10 days), and 36% were transplanted from the intensive care unit (ICU). Women were significantly more likely to be hospitalized in the immediate pre-transplant period than men (44% vs 39%, p<0.001), and were more likely to have a prolonged pre-transplant hospitalization (24% vs 20%, p<0.001). Women were also more likely to be transplanted from the ICU than men (18% vs 15%, p<0.001).
Post-transplant length of stay
Regarding post-transplant outcomes, the median post-transplant length of stay was 11 days for both women (IQR 8–20) and men (IQR 7–18), though the length of stay distribution differed significantly between genders on Wilcoxon rank-sum analysis (p<0.001), as shown in Figure 1. Women were also significantly more likely to have a prolonged post-transplant hospitalization (≥ 20 days) than men (25% vs 22%, p<0.001). Compared to women without prolonged hospitalization, women with prolonged hospitalization were older (56 vs 55, p<0.001), more likely to be non-White (32% vs 29%, p=0.01), and to have non-private insurance (52% vs 47%, p<0.001) (Table 2). Women with prolonged hospitalization were also more likely to have diabetes (27% vs 24%, p=0.02), to have higher MELDNa scores (33 vs 27, p<0.001), more complications of cirrhosis at transplant (including hepatic encephalopathy, dialysis, and ascites), and to be unable to care for themselves at time of transplant (73% vs 46%, p<0.001).
Figure 1. Post-Liver Transplant Length of Stay by Gender.
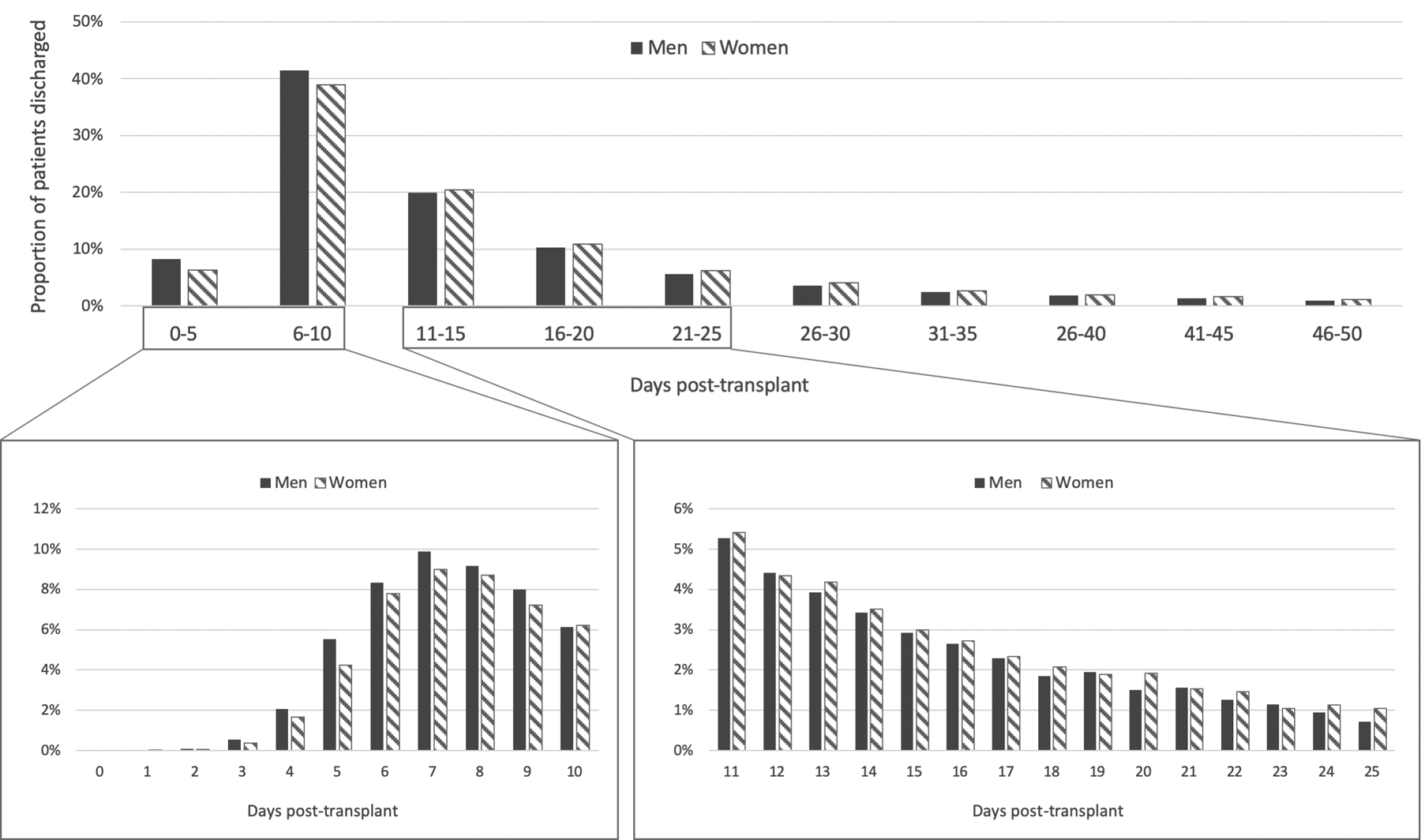
Excludes patients with post-transplant length of stay over 50 days (n=1,268). Solid bars represent men and shaded bars represent women. Median length of stay was 11 days for both women and men, but distribution of length of stay differed significantly (Wilcoxon Rank-Sum p <0.001).
Table 2.
Characteristics of women who underwent liver transplantation in the United States 2008–2017, by length of stay †
| Length of stay <20 days (n = 7,246) |
Length of stay ≥20 days (n = 2,454) |
p-value | ||
|---|---|---|---|---|
| Demographics, comorbidities, and liver disease history | ||||
| Age at listing, years | 55 (48–61) | 56 (49–62) | <0.001 | |
| Ethnicity | White | 71.2 | 68.5 | <0.001 |
| Black | 11.6 | 9.8 | ||
| Hispanic | 12.9 | 17.8 | ||
| Asian | 2.4 | 2.6 | ||
| Other | 1.9 | 1.4 | ||
| Liver diagnosis | Alcohol | 15.8 | 17.2 | 0.05 |
| Hepatitis C | 23.2 | 22.7 | ||
| NASH/Cryptogenic | 19.2 | 20.7 | ||
| Hepatitis B | 9.0 | 8.8 | ||
| Autoimmune/cholestatic | 23.7 | 21.2 | ||
| Other | 7.9 | 8.6 | ||
| History of prior transplant | 2.3 | 2.9 | 1.1 | |
| Height, cm | 163 (158–168) | 163 (158–168) | <0.001 | |
| Obesity | 41.8 | 43.0 | 0.31 | |
| Diabetes | 24.4 | 26.8 | 0.02 | |
| Insurance | Private | 53.5 | 48.0 | <0.001 |
| Medicaid | 16.5 | 20.1 | ||
| Medicare | 27.6 | 29.7 | ||
| Region risk | Low | 53.6 | 35.4 | 0.003 |
| Medium | 14.9 | 17.9 | ||
| High | 31.5 | 46.7 | ||
| Clinical characteristics at time of transplant | ||||
| Age, years | 55 (48–61) | 57 (50–63) | <0.001 | |
| MELDNa | 27 (22–34) | 33 (27–39) | <0.001 | |
| Hepatic encephalopathy | 71.8 | 78.5 | <0.001 | |
| Ascites | 85.6 | 87.8 | 0.008 | |
| Portal vein thrombus | 10.9 | 12.3 | 0.07 | |
| Dialysis | 16.5 | 38.7 | <0.001 | |
| Life support | 10.6 | 8.4 | <0.001 | |
| Karnofsky Performance Status | 50 (30–70) | 30 (20–50) | <0.001 | |
| KPS Category | Unable to care for self | 46.0 | 73.1 | <0.001 |
| Unable to work | 42.0 | 22.4 | ||
| Normal activity | 12.0 | 4.5 | ||
| Donor risk index | 1.5 (1.2–1.8) | 1.5 (1.2–1.8) | 0.05 | |
| Health resource utilization pre-transplant | ||||
| Hospitalized pre-transplant | 36.3 | 66.7 | <0.001 | |
| Prolonged hospitalization (> 10d) pre-transplant | 17.2 | 44.5 | <0.001 | |
| Transplanted from ICU | 11.9 | 36.1 | <0.001 | |
Data presented as percent or median (interquartile range)
Nonalcoholic steatohepatitis (NASH); Model for End-Stage Liver Disease with Sodium (MELDNa); Karnofsky Performance Score (KPS); intensive care unit (ICU).
On univariable Poisson regression, female gender was independently associated with post-transplant length of stay (IRR 1.09, 95% CI 1.06–1.12, p<0.001). Other variables associated with post-transplant length of stay on univariable analysis were: age, Hispanic ethnicity, BMI, autoimmune liver disease, UNOS region, history of previous transplant, transplant laboratory MELDNa, DRI, as well as pre-transplant albumin, hepatic encephalopathy, ascites, portal vein thrombosis, life support, and Karnofsky Performance Status (Table 3). In addition, hospitalization for >24 hours pre-transplant, as well as ICU admission pre-transplant were associated with significantly increased post-transplant length of stay (IRR 1.72, 95% 1.67–1.77, p<0.001 and IRR 1.93, 95% CI 1.86–2.00, p<0.001, respectively).
Table 3.
Predictors of post-transplant length of stay among patients who underwent liver transplant in the United States 2008–17
| Univariable | Multivariable † | |||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | p-value | aIRR | 95% CI | p-value | |
| Female gender | 1.09 | 1.06–1.12 | <0.001 | 1.04 | 1.01–1.07 | 0.007 |
| Age (at transplant) per year | 1.01 | 1.00–1.01 | <0.001 | 1.01 | 1.00–1.01 | <0.001 |
| Race | ||||||
| White | Ref | 0.91–0.99 | 0.013 | |||
| Black | 0.95 | 1.12–1.23 | <0.001 | |||
| Hispanic | 1.17 | 0.99–1.18 | 0.08 | |||
| Asian | 1.08 | |||||
| Nonwhite race | 1.07 | 1.04–1.11 | <0.001 | |||
| Body mass index | 1.00 | 0.99–1.00 | 0.001 | 0.99 | 0.99–1.00 | <0.001 |
| Diabetes | 1.04 | 1.01–1.08 | 0.02 | |||
| Liver diagnosis | ||||||
| Alcohol | Ref | 0.96–1.03 | 0.76 | |||
| Hepatitis C | 0.99 | 0.97–1.07 | 0.49 | |||
| NASH | 1.02 | 0.92–1.03 | 0.28 | |||
| Cryptogenic | 0.97 | 0.94–1.23 | 0.31 | |||
| Hepatitis B | 1.07 | 0.87–0.96 | <0.001 | |||
| Autoimmune/cholestatic | 0.91 | |||||
| Prior transplant | 1.22 | 1.11–1.34 | <0.001 | 1.21 | 1.10–1.33 | <0.001 |
| Days on waitlist | 1.00 | 1.00–1.00 | 0.28 | |||
| Insurance | ||||||
| Private | Ref | 1.09–1.18 | <0.001 | Ref | 1.03–1.11 | 0.001 |
| Medicaid | 1.14 | 1.10–1.18 | <0.001 | 1.07 | 1.05–1.12 | <0.001 |
| Medicare | 1.14 | 1.09 | ||||
| MELDNa at transplant per point | 1.03 | 1.03–1.03 | <0.001 | 1.01 | 1.01–1.01 | <0.001 |
| Albumin at transplant per point | 1.13 | 1.10–1.16 | <0.001 | |||
| Hepatic encephalopathy | 1.25 | 1.22–1.28 | <0.001 | 1.09 | 1.07–1.12 | <0.001 |
| Ascites | 1.08 | 1.03–1.13 | 0.002 | 0.90 | 0.85–0.94 | <0.001 |
| Dialysis at transplant | 1.64 | 1.59–1.70 | <0.001 | |||
| Life support at transplant | 2.08 | 1.99–2.18 | <0.001 | 1.57 | 1.50–165 | <0.001 |
| Portal vein thrombus at transplant | 1.08 | 1.03–1.13 | 0.001 | 1.07 | 1.02–1.11 | 0.002 |
| KPS category prior to transplant | ||||||
| 80 or more | ||||||
| 50–70 | Ref | Ref | ||||
| 40 or less | 1.10 | 1.71–1.87 | <0.001 | 1.04 | 1.00–1.09 | 0.07 |
| 1.79 | 1.71–1.87 | <0.001 | 1.37 | 1.31–1.44 | <0.001 | |
| Hospitalization pre-transplant | 1.72 | 1.67–1.77 | <0.001 | 1.21 | 1.16–1.26 | <0.001 |
| Transplant from ICU | 1.93 | 1.86–2.00 | <0.001 | 1.24 | 1.18–1.29 | <0.001 |
| Donor risk index per point | 0.95 | 0.92–0.99 | 0.006 | 1.04 | 1.01–1.08 | 0.05 |
Adjusted for year and region
Incidence rate ratio (IRR); confidence interval (CI); Nonalcoholic steatohepatitis (NASH); Model for End-Stage Liver Disease with Sodium (MELDNa); Karnofsky Performance Score (KPS); intensive care unit (ICU).
On multivariable analysis, after adjustment for age, ethnicity, UNOS region, insurance type, BMI, DRI, MELDNa, hepatic encephalopathy, ascites, portal vein thrombosis, life support, and KPS, female gender remained independently associated with our primary outcome of post-transplant length of stay (aIRR 1.04, 95% CI 1.01–1.07, p=0.007). On sensitivity analysis including all patients, including those who died in the hospital, the same covariates were associated with post-transplant length of stay. Female gender was also associated with our secondary outcome of prolonged (≥20-day) post-transplant length of stay on univariable (OR 1.22, 95% CI 1.15–1.29, p<0.001) and multivariable (adjusted OR 1.17, 95% CI 1.10–1.25, p<0.001) analysis.
We hypothesized that pre-transplant health care utilization factors, specifically pre-transplant hospitalization and prolonged pre-transplant hospitalization, were mediating variables on the effect of gender on prolonged post-transplant length of stay. On mediation analysis using multivariable logistic regression for prolonged post-transplant length of stay, we found that 14.1% (95% CI 9.7–25.4%) of the total effect of gender on prolonged post-transplant length of stay was mediated by pre-transplant hospitalization >24 hours. Similarly, prolonged pre-transplant hospitalization (>10 days) explained 18.7% (95% CI 12.8–24.6%) of the total effect of gender on prolonged post-transplant length of stay. These findings are further supported on subgroup analysis excluding all patients with pre-transplant hospitalization (n=11,131, 40.8%). Even among this subgroup, female gender is independently associated with post-transplant length of stay (aIRR 1.04, 95% CI 1.01–1.08, p = 0.02) and 18% increased odds of prolonged post-transplant length of stay (aOR 1.18, 95% CI 1.07–1.30, p=0.001) on multivariable analyses.
In order to determine whether there were any changes in gender disparities in resource utilization as a result of policy changes—specifically the Share 35 policy, we performed a subgroup analysis looking at gender differences before and after June 18, 2013. Women who were transplanted in the post-Share 35 era had significantly higher rates of prolonged length of stay compared to women transplanted in the in the pre-Share 35 era (26% vs 24%, p = 0.008). Men, in contrast, had similar rates of prolonged length of stay before and after the change in policy (22% vs 22%, p = 0.94). This increasing disparity is also demonstrated in Figure 2, which plots post-transplant length of stay by year for women and men.
Figure 2. Gender Disparity in Post-Transplant Length of Stay Over Time, 2008–2017.
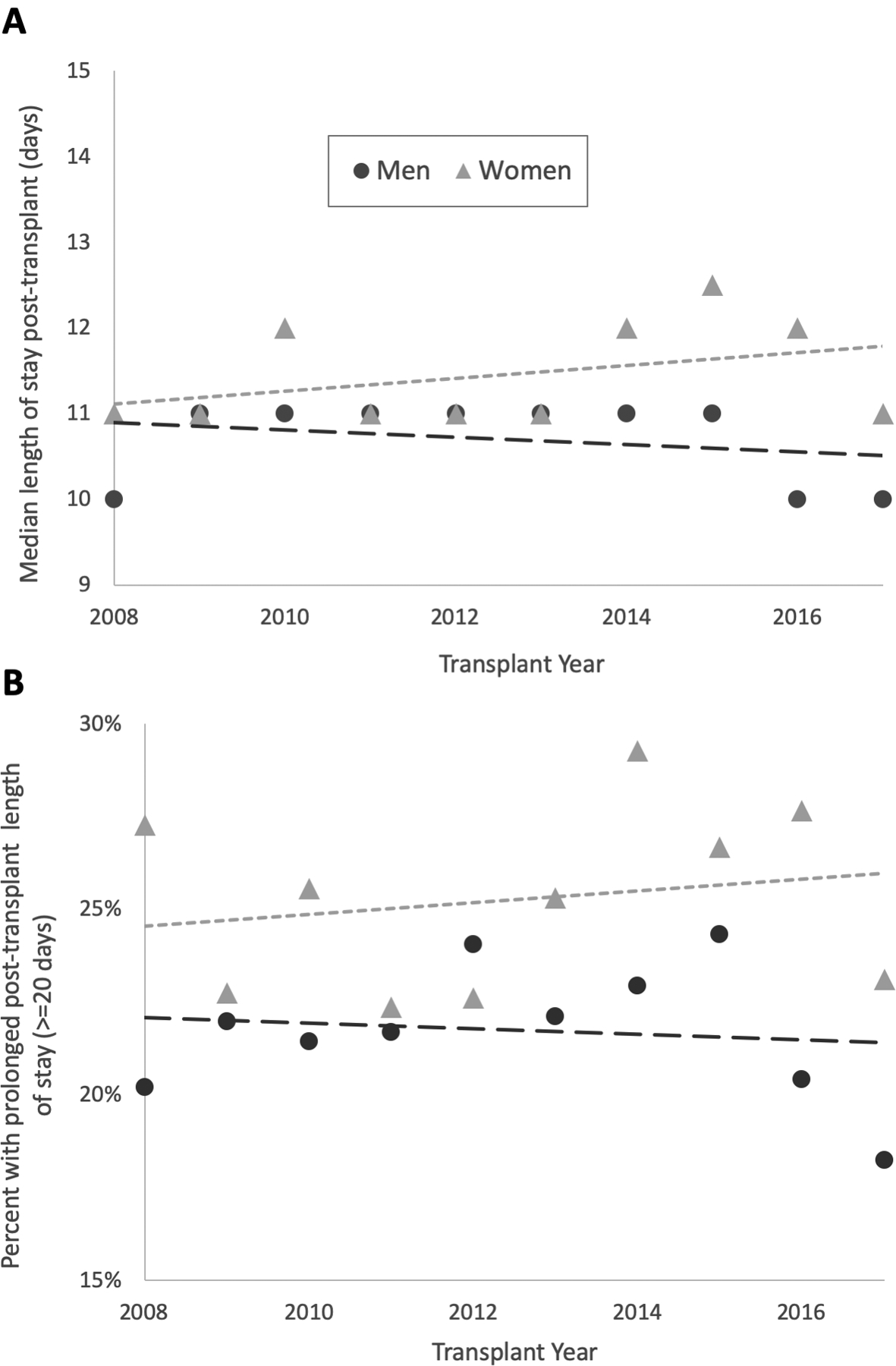
Panel A shows median length of stay post-liver transplant for women (triangles) and men (circles) by year. Panel B shows the percent of women (triangles) and men (circles) with prolonged length of stay (≥ 20 days) post-transplant by year. Short dashed line is linear best fit line for women; long dashed line is linear best fit for men in both panels.
Post-transplant mortality
380 (3.8%) women and 608 (3.3%) men died in the hospital (p = 0.06) following liver transplant, with no significant difference in median post-transplant length of stay prior to death (16 days for women and 19 days for men, p = 0.19). Among patients who did not die during initial transplant hospitalization, three- and six-month post-transplant mortality were 1.3% and 2.9%, respectively; one-year mortality was 5.2%. Female gender was not independently associated with post-transplant mortality at any time point, nor was it associated with overall post-transplant mortality on univariable Cox regression (HR 0.98, 95% CI 0.92–1.05, p = 0.55). Patients with prolonged post-transplant hospitalizations, however, had nearly twice the risk of death compared with those without prolonged hospitalizations on univariable (HR 1.98, 95% CI 1.86–2.11, p<0.001) and multivariable (aHR 1.95, 95% CI 1.83–2.09, p<0.001) Cox regression. Interestingly, as shown in Figure 3, among the subgroup of patients with prolonged hospitalization, female gender was associated with a 13% decreased risk of post-transplant mortality (HR 0.87, 95% CI 0.78–0.97, p = 0.016). In contrast, among those without prolonged hospitalization, there was no difference in mortality between women and men (HR 1.00, 95% CI 0.92–1.08, p = 0.99). There was a trend toward an interaction between gender and prolonged hospitalization on post-transplant mortality (p = 0.053).
Figure 3. Post-Transplant Waitlist Mortality by Gender and Prolonged Length of Stay Post-Transplant.
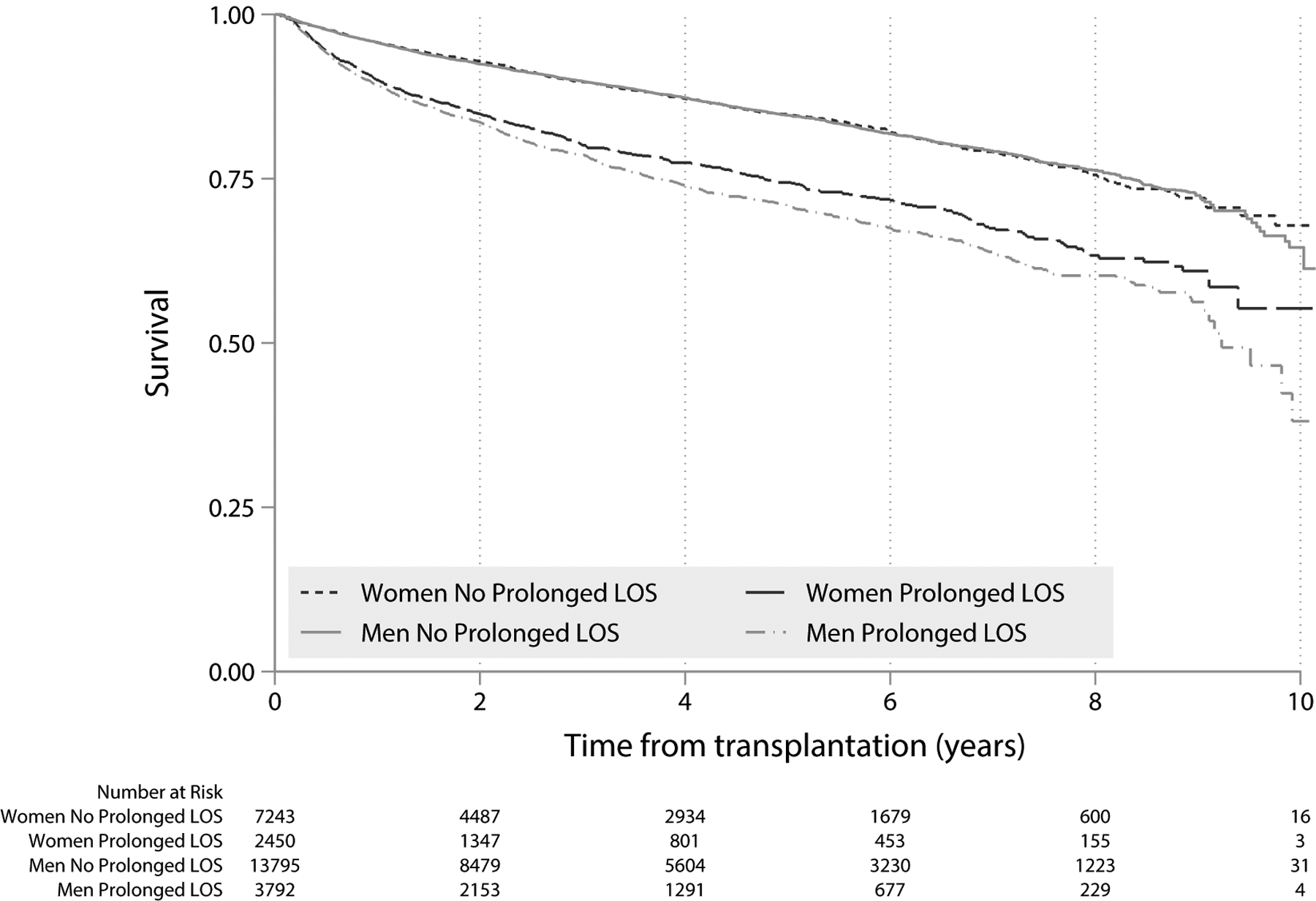
Kaplan-Meier survival curve for patients who underwent liver transplantation in the US (2008–2017), and survived initial post-transplant hospitalization. Black lines represent women and grey lines represent men. Prolonged post-transplant length of stay (LOS) defined as ≥20 days. For patients without prolonged LOS, mortality between women and men did not differ (Kaplan Log-Rank p = 0.99). For patient with prolonged LOS, mortality in women was significantly lower than in men (Kaplan Log-Rank p = 0.016).
DISCUSSION
While women on the liver transplant waitlist have higher rates of mortality, dropout and hospitalization than men, gender disparities in outcomes and resource utilization in the peri-transplant period are less well-established. In this study, we aimed to determine whether there were gender differences in post-transplant hospital length of stay and short-term mortality among a contemporary cohort of patients that underwent liver transplant in the United States (US). We found that women had both significantly longer post-transplant length of stay than men, and were significantly more likely to have prolonged post-transplant hospitalizations, but surprisingly, these differences did not result in decreased short-term survival among women.
Several previously published studies have sought to identify predictors of length of stay post-liver transplant, yet only one identified female gender as a significant predictor of prolonged length of stay.16 Of note, most of these previous studies utilized transplant data from prior to 2010. Over time, spurred by the introduction of MELD-based organ allocation, the US has witnessed trends of increasing recipient disease severity and decreasing graft quality, which has resulted in changing characteristics and outcomes of the waitlist and post-transplant populations, including an increasing proportion of female transplant recipients.25 In addition, in 2013, the Share 35 allocation policy was introduced to prioritize liver transplant for patients with high MELD, further increasing the proportion of female transplant recipients among patients with MELD ≥35.26 This change may have intensified the post-transplant length of stay disparity between women and men. On subgroup analysis in the present study, we found this to be true – the proportion of women with prolonged post-transplant length of stay significantly increased since June 2013, after Share 35 was implemented, but the proportion of men with prolonged length of stay remained similar. Additionally, the differences in median length of stay between women and men seems to be increasing over time. These findings highlight the need for studies that better quantify gender disparities in the setting of changing demographics and practice patterns in the post-Share 35 era.
What explains our observed disparity in post-transplant length of stay between women and men? Similar to prior studies,16,17,21 we found that several demographic, pre-transplant clinical, and donor-related factors were associated with longer post-transplant length of stay. However, the gender disparity persisted even after adjustment for all these factors. Interestingly, several measures of pre-transplant health resource utilization, including pre-transplant hospitalization, prolonged length of stay, and ICU admission – all of which were higher in women – mediated a portion, but not all, of women’s longer length of stay post-transplant. Thus, we believe that the gender disparities we observed in post-transplant length of stay are likely in part explained by the same factors that explain women’s poorer waitlist outcomes, factors such as physical deconditioning, non-liver related comorbidities (e.g. depression, chronic pain) and sociodemographic factors (e.g. lack of caregiver support). Conservative clinical management in the presence of such factors may exacerbate the observed disparity. This hypothesis is supported by previous studies of transplant patients showing that these specific, non-hepatic factors are all independent predictors of post-transplant length of stay and post-discharge rehabilitation needs.18,27–29 In the present study, low Karnofsky Performance Status at the time of transplant – perhaps a surrogate of physical deconditioning – explained some of the gender disparity we observed, though the other aforementioned factors could not be explored using the UNOS/OPTN data.
Similar to previously published studies,19,22 we found that prolonged post-transplant hospitalization was associated with significantly increased short-term post-transplant mortality. Interestingly, this effect was significantly stronger among men than women - women in our cohort did not have increased mortality rates despite longer post-transplant length of stay. These findings suggest either that the gender disparities we observed are limited to the peri-transplant period, or that women have increased resilience following liver transplantation than men. This may relate to women being sicker than MELDNa reflects prior to transplant as measured by factors that resolve in the short-term post-liver transplant, such as deconditioning, frailty or renal dysfunction. There may also be biologic differences in liver regeneration between women and men, or improved management of other comorbidities post-transplant. Although our observed gender disparities in health resource utilization did not translate to disparities in post-transplant mortality, they undoubtedly have significant implications in post-transplant quality of life and peri-transplant costs. Moreover, the fact that gender disparities are limited to the pre- and peri-transplant periods suggests that they may be more easily addressed through interventions targeted at improving care for women on the liver transplant waitlist pre-transplant, and would not require ongoing interventions post-transplant.
We acknowledge the following limitations to this study. First, while we adjusted for several demographic and pre-transplant variables that could explain post-transplant length of stay, other variables that have been shown to be important predictors of length of stay and gender disparities in other studies – such as operative and post-transplant hospitalization details, comorbidities, and length of ICU stay – were not available in the UNOS/OPTN database. The dataset is also missing post-discharge details – such as need for rehab services, subsequent functional assessments, and quality of life metrics – which may help explain both the disparities in length of stay (i.e. wait time for skilled nursing facility beds) and the unexpectedly similar mortality rates between women and men. Second, several of the health resource utilization metrics used in this analysis – such as need for hospital admission, indication for ICU admission pre-transplant and length of stay post-transplant – may be subject to variability by centers or individual providers. Third, this study excludes patients with MELD exception points; previous studies have shown that women are less likely to receive exception points than men,30 which may exacerbate our observed disparities. We performed a similar analysis on only patients with MELD exception points (data not shown) and obtained similar results. Finally, this study represents a large cohort with the statistical power to make clinically insignificant differences statistically significant. However, even seemingly small gender disparities in length of stay may result in significant increases in the cost of care, as the number of women undergoing transplant – particularly those with high MELD – is rising, and the disparity seems to be worsening. During our study period, the disparities we identified translated into increased length of stay for nearly 1000 women, which has significant implications for both costs and quality of life.
The present study advances our knowledge of gender disparities in liver transplantation by exploring disparities in outcomes and health resource utilization in the peri-transplant period. Our findings that the well-known disadvantages for women on the liver transplant waitlist are not limited to pre-transplant, but in fact carry forward to the peri-transplant period as well, highlight that gender disparities have significantly more impact than previously recognized. That women are more likely than men to have prolonged post-transplant length of stay, in addition to significantly more health care utilization in the days prior to transplant, suggests additional implications of gender disparities in pre-transplant clinical characteristics that are not accurately captured by MELDNa or other traditional markers of disease severity. Thus, to reduce these disparities, we should develop pre-transplant interventions targeted specifically at preventing hospitalization among women on the liver transplant waitlist. In addition, efforts should be focused on developing improved tools to better characterize the severity of end-stage liver disease in women, possibly through further revisions to the MELDNa allocation system. Such changes may serve to reduce both waitlist disparities and the disproportionate burden of health care utilization by women compared with men in the peri-transplant period. In a field as advanced as liver transplantation, no form of gender-based disparity is acceptable. Future research on gender-specific prognosis and management strategies is essential in reducing disparities, reducing the cost of care, and improving outcomes and quality of life for all patients undergoing liver transplant.
Acknowledgements:
We would like to acknowledge David Goldberg, MD, MSCE for his input regarding data analysis.
Funding:
This study was funded by NIA Research Project Grant (R01AG059183, Lai), NIA Paul B. Beeson Career Development Award in Aging (K23AG048337, Lai), and NIDDK National Research Service Award Hepatology Training Grant (T32DK060414, Rubin).
Abbreviations:
- aIRR
adjusted incidence rate ratio
- aOR
adjusted odds ratio
- CI
confidence interval
- DD
deceased donor
- DRI
Donor Risk Index
- HCC
Hepatocellular Carcinoma
- ICU
intensive care unit
- IRR
incidence rate ratio
- KPS
Karnofsky Performance Status
- LOS
length of stay
- MELD
Model for End-Stage Liver Disease
- MELDNa
Model for End-Stage Liver Disease including serum sodium
- NASH
nonalcoholic steatohepatitis
- OR
odds ratio
- UNOS/OPTN
United Network for Organ Sharing/Organ Procurement and Transplantation Network
- US
United States
Footnotes
Disclosure:
The authors declare no conflicts of interest.
REFERENCES
- 1.Sarkar M, Watt KD, Terrault N, Berenguer M. Outcomes in liver transplantation: does sex matter? J Hepatol. 2015;62(4):946–955. doi: 10.1016/j.jhep.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300(20):2371–2378. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai JC, Terrault NA, Vittinghoff E, Biggins SW. Height contributes to the gender difference in wait-list mortality under the MELD-based liver allocation system. Am J Transplant. 2010;10(12):2658–2664. doi: 10.1111/j.1600-6143.2010.03326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathur AK, Schaubel DE, Gong Q, Guidinger MK, Merion RM. Sex-based disparities in liver transplant rates in the United States. Am J Transplant. 2011;11(7):1435–1443. doi: 10.1111/j.1600-6143.2011.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Schaubel DE, Messersmith EE, Guidinger MK, Merion RM. Factors that affect deceased donor liver transplantation rates in the United States in addition to the Model for End-stage Liver Disease score. Liver Transpl. 2012;18(12):1456–1463. doi: 10.1002/lt.23548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mindikoglu AL, Emre SH, Magder LS. Impact of estimated liver volume and liver weight on gender disparity in liver transplantation. Liver Transpl. 2013;19(1):89–95. doi: 10.1002/lt.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers RP, Shaheen AAM, Aspinall AI, Quinn RR, Burak KW. Gender, renal function, and outcomes on the liver transplant waiting list: assessment of revised MELD including estimated glomerular filtration rate. J Hepatol. 2011;54(3):462–470. doi: 10.1016/j.jhep.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Cullaro G, Sarkar M, Lai JC. Sex-based disparities in delisting for being “too sick” for liver transplantation. Am J Transplant. 2018;18(5):1214–1219. doi: 10.1111/ajt.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin JB, Sinclair M, Rahimi RS, Tapper EB, Lai JC. Women on the liver transplantation waitlist are at increased risk of hospitalization compared to men. World J Gastroenterol. 2018;25(8):980–988. doi: 10.3748/wjg.v25.i8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathur AK, Schaubel DE, Zhang H, Guidinger MK, Merion RM. Disparities in liver transplantation: the association between donor quality and recipient race/ethnicity and sex. Transplantation. 2014;97(8):862–869. doi: 10.1097/01.tp.0000438634.44461.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruns H, Lozanovski VJ, Schultze D, et al. Prediction of postoperative mortality in liver transplantation in the era of MELD-based liver allocation: a multivariate analysis. PLoS ONE. 2014;9(6):e98782. doi: 10.1371/journal.pone.0098782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 13.Axelrod DA, Schnitzler M, Salvalaggio PR, Swindle J, Abecassis MM. The economic impact of the utilization of liver allografts with high donor risk index. Am J Transplant. 2007;7(4):990–997. doi: 10.1111/j.1600-6143.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- 14.Rana A, Hardy MA, Halazun KJ, et al. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. 2008;8(12):2537–2546. doi: 10.1111/j.1600-6143.2008.02400.x. [DOI] [PubMed] [Google Scholar]
- 15.Weismüller TJ, Negm A, Becker T, et al. The introduction of MELD-based organ allocation impacts 3-month survival after liver transplantation by influencing pretransplant patient characteristics. Transpl Int. 2009;22(10):970–978. doi: 10.1111/j.1432-2277.2009.00915.x. [DOI] [PubMed] [Google Scholar]
- 16.Washburn WK, Meo NA, Halff GA, Roberts JP, Feng S. Factors influencing liver transplant length of stay at two large-volume transplant centers. Liver Transpl. 2009;15(11):1570–1578. doi: 10.1002/lt.21858. [DOI] [PubMed] [Google Scholar]
- 17.Foxton MR, Al-Freah MAB, Portal AJ, et al. Increased model for end-stage liver disease score at the time of liver transplant results in prolonged hospitalization and overall intensive care unit costs. Liver Transpl. 2010;16(5):668–677. doi: 10.1002/lt.22027. [DOI] [PubMed] [Google Scholar]
- 18.DiMartini A, Cruz RJ, Dew MA, et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl. 2013;19(11):1172–1180. doi: 10.1002/lt.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaltenborn A, Hartmann C, Salinas R, et al. Risk factors for short- and long-term mortality in liver transplant recipients with MELD score ≥30. Ann Transplant. 2015;20:59–69. doi: 10.12659/AOT.892322. [DOI] [PubMed] [Google Scholar]
- 20.Bittermann T, Makar G, Goldberg DS. Early post-transplant survival: Interaction of MELD score and hospitalization status. J Hepatol. 2015;63(3):601–608. doi: 10.1016/j.jhep.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rana A, Witte ED, Halazun KJ, et al. Liver transplant length of stay (LOS) index: A novel predictive score for hospital length of stay following liver transplantation. Clin Transplant. 2017;31(12). doi: 10.1111/ctr.13141. [DOI] [PubMed] [Google Scholar]
- 22.Smith JO, Shiffman ML, Behnke M, et al. Incidence of prolonged length of stay after orthotopic liver transplantation and its influence on outcomes. Liver Transpl. 2009;15(3):273–279. doi: 10.1002/lt.21731. [DOI] [PubMed] [Google Scholar]
- 23.Showstack J, Katz PP, Lake JR, et al. Resource utilization in liver transplantation: effects of patient characteristics and clinical practice. NIDDK Liver Transplantation Database Group. JAMA. 1999;281(15):1381–1386. [DOI] [PubMed] [Google Scholar]
- 24.Hicks R, Tingley D. Causal mediation analysis. Stata J. 2011;11(4):605–619. doi: 10.1177/1536867X1201100407. [DOI] [Google Scholar]
- 25.Ayloo S, Pentakota SR, Molinari M. Trends of characteristics and outcomes of donors and recipients of deceased donor liver transplantation in the United States: 1990 to 2013. World J Transplant. 2018;8(5):167–177. doi: 10.5500/wjt.v8.i5.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwong AJ, Goel A, Mannalithara A, Kim WR. Improved posttransplant mortality after share 35 for liver transplantation. Hepatology. 2018;67(1):273–281. doi: 10.1002/hep.29301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montano-Loza AJ, Meza-Junco J, Baracos VE, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20(6):640–648. doi: 10.1002/lt.23863. [DOI] [PubMed] [Google Scholar]
- 28.Rogal SS, Mankaney G, Udawatta V, et al. Pre-Transplant Depression Is Associated with Length of Hospitalization, Discharge Disposition, and Survival after Liver Transplantation. PLoS ONE. 2016;11(11):e0165517. doi: 10.1371/journal.pone.0165517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundaram V, Lim J, Tholey DM, et al. The Braden Scale, A standard tool for assessing pressure ulcer risk, predicts early outcomes after liver transplantation. Liver Transpl. 2017;23(9):1153–1160. doi: 10.1002/lt.24789. [DOI] [PubMed] [Google Scholar]
- 30.Allen AM, Heimbach JK, Larson JJ, et al. Reduced Access to Liver Transplantation in Women: Role of Height, MELD Exception Scores, and Renal Function Underestimation. Transplantation. 2018;102(10):1710–1716. doi: 10.1097/TP.0000000000002196. [DOI] [PMC free article] [PubMed] [Google Scholar]


