Abstract
Background
Diffuse large B‐cell lymphoma (DLBCL) patients refractory to rituximab‐based immunochemotherapy have a dismal prognosis. However, the definition of refractory DLBCL remains inconsistent and no large cohort study data is available from Asian countries. To validate the definition and outcomes of refractory DLBCL in China, we conducted a multicenter, retrospective cohort study.
Methods
The REtrospective AnaLysis of Treatment REspoNse of refractory DLBCL (REAL‐TREND) study was performed using real‐world data from 8 centers in China. DLBCL patients with curative intent were included in the REAL‐TREND dataset. Overall survival (OS) was estimated using the Kaplan‐Meier method and compared by the log‐rank test. Due to heterogeneity in response rates among different centers, the response rates of refractory patients were pooled using random‐effect models. Multivariate survival analysis was performed using the Cox regression model.
Results
A total of 2778 DLBCL patients diagnosed between January, 2010 and December, 2015 were enrolled to this study. After validating previous definitions, the SCHOLAR‐1 study was most suitable to define refractory DLBCL. The estimated 5‐year cumulative incidence of refractory patients was 20% (95% confidence Interval [CI] = 18%‐22%). After the determination of refractory disease, overall response rate and complete remission rate were 30% (95% CI = 22%‐38%) and 9% (95% CI = 4%‐15%), respectively. Patients with either no response to immunochemotherapy or relapse within 12 months after stem‐cell transplantation had inferior survival with a median OS of 5.9 months (95% CI = 5.5‐7.1 months) and 2‐year OS rate of 16% (95% CI = 12%‐20%). International prognostic index score 4‐5 (hazard ratio [HR] = 2.22; 95% CI = 1.47‐3.35), central nervous system relapse (HR = 1.43; 95% CI = 1.04‐1.97), and best response status (HR = 2.68; 95% CI = 1.42‐5.03 for partial remission. HR = 5.97, 95% CI = 3.21‐11.11 for stable disease/progressive disease) were independent unfavorable prognostic factors.
Conclusions
This is the first large‐scale Asian cohort study focusing on outcomes of refractory DLBCL. The definition of the SCHOLAR‐1 study identifies patients with homogenously inferior survival, thus is appropriate to select refractory DLBCL. Due to poor clinical outcomes in the rituximab era, patients with refractory DLBCL may be potential candidates for novel treatment modalities.
Keywords: diffuse large B‐cell lymphoma, multicenter cohort study, refractory, relapse, rituximab, immunochemotherapy, treatment response, prognosis
Abbreviations
- CAR‐T
chimeric antigen receptor T
- CI
confidence Interval
- CNS
central nervous system
- CR
complete remission
- CRR
complete remission rate
- CT
computed tomography
- DLBCL
diffuse large B‐cell lymphoma
- ECOG PS
Eastern Cooperative Oncology Group performance status
- HR
hazard ratio
- IPI
international prognostic index
- OS
overall survival
- ORR
overall response rate
- PD
progressive disease
- PET‐CT
positron emission tomography/computed tomography
- PR
partial remission
- R‐CHOP
rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
- REAL‐TREND
REtrospective AnaLysis of Treatment REspoNse of refractory DLBCL
- SCT
stem‐cell transplantation
- SD
stable disease
- WHO
World Health Organization
1. INTRODUCTION
Diffuse large B‐cell lymphoma (DLBCL) is the most common subtype of non‐Hodgkin lymphoma and ranks seventh as the most common cancers worldwide [1]. Rituximab, an anti‐CD20 monoclonal antibody, in combination with conventional chemotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone [R‐CHOP]) have significantly improved clinical outcomes and become the standard of care in newly diagnosed patients with DLBCL [2]. However, in the era of rituximab‐based immunochemotherapy, 4%‐12% of the patients are primarily refractory to first‐line R‐CHOP [3, 4, 5]. Among those responding to R‐CHOP, 17%‐40% of the patients will either relapse after complete remission (CR) or progress after partial remission (PR) [6, 7, 8] and 12%‐60% of them would become refractory to second‐ or third‐line therapy, including autologous stem‐cell transplantation (SCT).
The outcomes of refractory DLBCL are generally dismal, but the definition of refractory DLBCL remains inconsistent. Coiffier et al. [9] defined refractory DLBCL as non‐responders to first‐line treatment or patients relapsed within 12 months after CR. Cheson et al. [10] defined refractory DLBCL as non‐responders to second‐line/third‐line treatment or patients who had second‐line or third‐line CR/PR but progressed within 6 months. Hitz et al. [11] defined primary refractory DLBCL as non‐responders to first‐line treatment or patients relapsed within 3 months after CR/PR. As recently demonstrated by the SCHOLAR‐1 study, refractory DLBCL were defined as progressive disease or stable disease as best response to chemotherapy or relapse ≤12 months after autologous SCT[12]. Determination of disease refractoriness is essential for the risk stratification of DLBCL in the context of rituximab‐based immunochemotherapy. Upon second‐line treatment, if patients achieve CR or PR, the eligible patients will undergo high‐dose chemotherapy followed by autologous SCT. For those ineligible patients and those who remained SD or PD to second‐line treatment, they should be considered for novel treatment modalities, such as chimeric antigen receptor T (CAR‐T) therapy and clinical trials of targeted agents, instead of receiving alternative regimens of salvage chemotherapy. Of note, outcomes of refractory DLBCL are mainly from European and North American countries and no data of large‐scale cohort study is available from Asian countries.
To validate the definition and outcomes of refractory DLBCL in China, we conducted a multicenter retrospective cohort study (REtrospective AnaLysis of Treatment REspoNse of refractory DLBCL [REAL‐TREND]) to evaluate previous definitions and to investigate the real‐world incidence, response rate, and prognostic factors of refractory DLBCL.
2. MATERIALS AND METHODS
2.1. Patient selection for the REAL‐TREND dataset
A total of 2778 consecutive patients with newly diagnosed DLBCL were enrolled between January 2010 and December 2015 at 8 centers in China, including Shanghai Rui Jin Hospital (Shanghai, Shanghai, China), Fujian Medical University Union Hospital (Fuzhou, Fujian, China), the First Affiliated Hospital of Medical School of Zhejiang University (Hangzhou, Zhejiang, China), Xinqiao Hospital (Chongqing, Chongqing, China), Wuhan Union Hospital (Wuhan, Hubei, China), Shanghai Ninth People's Hospital (Shanghai, Shanghai, China), Huashan Hospital Affiliated to Fudan University (Shanghai, Shanghai, China), and the First Affiliated Hospital of Soochow University (Suzhou, Jiangsu, China). Pathological diagnosis was established according to the World Health Organization (WHO) classification [13]. The data, including patient characteristics at diagnosis and determination of refractory status, treatment modality and response to each line of therapy, date of diagnosis, date of relapse or progression, and date of death or last follow‐up, were collected from the electronic medical records by a team of research coordinators who had received the same training regarding how to perform a medical chart review following a standardized data collection protocol. A central electronic database was built for collecting the study data online. A data surveillance team performed monthly data quality control and gave feedback to all 8 centers.
When data collection was complete, a patient selection committee systematically identified patients treated with curative intent, which was defined as receiving rituximab‐based immunochemotherapy in at least one adequate line of therapy. Standard R‐CHOP‐based regimens were used by all centers as first‐line treatment, while regimens for second‐ or later‐line therapy could be varied from centers. Patients with primary central nervous system (CNS) lymphoma, patients with primary mediastinal large B‐cell lymphoma, and patients receiving CAR‐T therapy or palliative care were excluded.
The covariates for the clinical characteristics and subgroup analyses within the refractory DLBCL dataset were age, gender, Eastern Cooperative Oncology Group performance status (ECOG PS), Ann Arbor stage, international prognostic index (IPI) score, treatment modalities (immunochemotherapy plus SCT, immunochemotherapy, and palliative treatment) after the determination of refractory disease and CNS relapse defined as a secondary CNS invasion that occurred either before or after the determination of refractory disease.
All study activities were approved by the Institutional Review Boards of all participating centers, and informed consent was obtained in accordance with the Declaration of Helsinki.
2.2. Validation of previous definitions of refractory DLBCL
Within the REAL‐TREND dataset, 4 definitions of refractory DLBCL were applied: Definition 1 reported by Coiffier et al. [9], first‐line SD/PD or relapse within 12 months after CR; Definition 2 reported by Cheson et al. [10], second‐line/third‐line SD/PD or relapse within 6 months after second‐line/third‐line CR/PR; Definition 3 reported by Hitz et al. [11], first‐line SD/PD or relapse within 3 months after CR/PR; Definition 4 reported by the SCHOLAR‐1 study [12], SD/PD to any line of therapy or relapse within 12 months after SCT. The definition for identifying patients with homogenous overall survival (OS) within subgroups will be used for further analysis of incidence, response rate, and prognostic factors of refractory DLBCL.
2.3. Incidence and outcomes of refractory DLBCL
The incidence of refractory DLBCL was defined as the number of patients with refractory DLBCL that occurred during a specified period of time. The outcomes were OS, overall response rate (ORR), and complete remission rate (CRR) after the determination of refractory DLBCL. The treatment response was evaluated according to the Lugano criteria [14]. Assessment of treatment response was evaluated by follow‐up clinical, radiological, or laboratory studies, as determined by the clinicians [15]. Ambiguous cases were clarified by consulting institutional investigators and disagreements were resolved by consensus. ORR and CRR were calculated according to the best treatment response after the determination of refractory disease.
2.4. Statistical analysis
Categorical variables were summarized using frequencies and percentages. Continuous variables were summarized using median and ranges. Missing data in any variable analyzed were summarized using frequencies. OS was defined as the time interval from the date of event to the date of death or last follow‐up. Patients were followed up for survival per institution standard procedures. Patients who were alive at the time of data collection were censored at the date of the last follow‐up. In the REAL‐TREND dataset, OS was estimated by the Kaplan‐Meier method, and comparison of survival curves was performed using the log‐rank test. Five‐year cumulative incidence of refractory DLBCL was estimated from diagnosis to the determination of refractory status or last follow‐up, as reported previously [16]. In the refractory DLBCL dataset, the heterogeneity of the ORR was first tested across the participant centers by Cochran's Q and I² statistics. If the variation in response rates across centers was significant, the ORR and CRR would be pooled using random‐effect models. Otherwise, fix‐effect models would be used. Survival data would be pooled directly if the log‐rank test showed no significant difference across center, otherwise stratified by center. Univariable analysis of the covariates was performed using the Cox proportional hazards regression analysis. Statistically significant covariates in univariable analysis were included in the multivariable analysis. All analyses were performed using R version 3.4.3 (R Foundation, Vienna, Austria) and two‐sided P values and 95% confidence intervals were reported. Significance was indicated at P <0.05.
3. RESULTS
3.1. Clinical characteristics and remission status of the REAL‐TREND dataset
A total of 2778 DLBCL patients were enrolled in this study. Among them, 2342 patients were included in the REAL‐TREND dataset. The clinical characteristics of the REAL‐TREND cohort were summarized in Supplementary Table S1. The mean age at diagnosis was 54 years old (range = 14‐93 years), with a 56.4% male composition. Overall, 412 (17.6%) patients had ECOG PS 2‐4, 1276 (54.5%) had advanced disease (Ann Arbor stage III‐IV), and 689 (29.4%) had IPI scores ≥3.
Within the REAL‐TREND cohort, 2138 (91.3%) patients were evaluated by positron emission tomography/computed tomography (PET‐CT) using the Lugano PET‐CT criteria, and the remaining 204 (8.7%) patients by CT using Lugano CT criteria. No statistically significant difference in ORR and CRR was observed between patients evaluated by PET‐CT and by CT (Supplementary Table S2).
As illustrated in Figure 1, 181 (7.7%) patients failed to respond to first‐line treatment, including 33 (18.2%) with SD and 148 (81.8%) with PD. Additionally, 2161 (92.3%) patients responded to first‐line treatment, including 1714 (79.3%) patients achieved CR and 447 (20.7%) patients achieved PR (CRR = 73%; ORR = 92%). Among them, 89 (4.1%) patients underwent consolidation SCT. Afterwards, 438 (20.3%) patients relapsed or progressed, including 16 (3.7%) who relapsed within 12 months after SCT. Among them, 119 (27.2%) patients who underwent palliative care were excluded, while the other 319 (72.8%) patients received second‐line treatment. In the group that received second‐line treatment, 66 (20.7%) of them achieved CR and 138 (43.3%) achieved PR (ORR = 64%; CRR = 21%; 42 [20.6%] of them underwent second‐line salvage SCT). In comparison, 115 (36.1%) patients failed to respond, including 40 (34.8%) with SD and 75 (65.2%) with PD. Finally, excluding 17 (21.3%) patients who later underwent palliative care, the remaining 63 (78.8%) patients received third‐line or later‐line treatment. Among them, 9 (14.3%) patients achieved CR and 21 (33.3%) achieved PR (ORR = 48%; CRR = 14%), while 33 (52.4%) failed to respond, including 9 (27.3%) with SD and 24 (72.7%) with PD.
FIGURE 1.
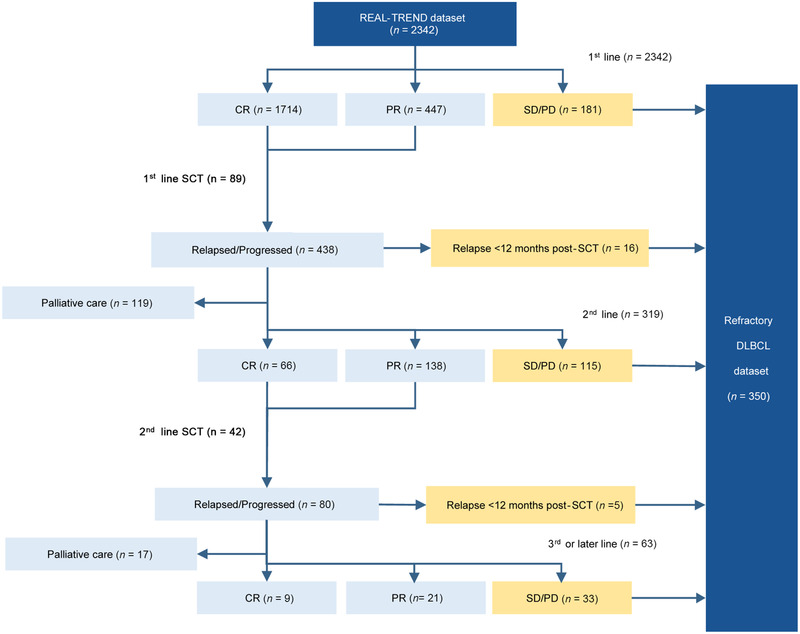
Flow diagram of patients included in the REAL‐TREND dataset and refractory DLBCL dataset.
Abbreviations: REAL‐TREND, REtrospective AnaLysis of Treatment REspoNse of refractory DLBCL; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; SCT, stem cell transplantation; DLBCL, diffuse large B‐cell lymphoma
3.2. Validation of previous definitions of refractory DLBCL in the REAL‐TREND dataset
As defined by Definition 1 (reported by Coiffier et al. [9]), internal heterogeneity was observed within subgroups, where patients with first‐line SD/PD had significantly worse OS than those who relapsed within 12 months after CR (median OS: 7.1 months [95% confidence Interval {CI} = 5.9‐9.0 months] vs. 22.8 months [95% CI = 19.6‐36.6 months]; P < 0.001; Figure 2A). Similar results were shown according to Definition 2 (reported by Cheson et al. [10]; median OS: second‐line/third‐line SD/PD, 5.8 months [95% CI = 5.1‐7.1 months]; relapsed within 6 months after second‐line/third‐line CR/PR, 14.1 months [95% CI = 10.9‐72.8 months]; P < 0.001; Figure 2B) and Definition 3 (reported by Hitz et al. [11]; median OS: first‐line SD/PD, 7.1 months [95% CI = 5.7‐9.0 months]; relapsed within 3 months after CR/PR, 14.9 months [95% CI = 9.9‐52.2 months], P < 0.001; Figure 2C). Only Definition 4 (reported in SCHOLAR‐1 [12]) identified patients with homogenously inferior survival, and there was no significant difference of OS between patients with SD/PD to any line of therapy and patients who relapsed within 12 months after SCT (median OS = 5.9 months [95% CI = 5.5‐7.1 months] vs. 5.9 months [95% CI = 3.2 months to not reached]; P = 0.564; Figure 2D). Thus, Definition 4 was applied to select refractory DLBCL patients for the following analysis.
FIGURE 2.
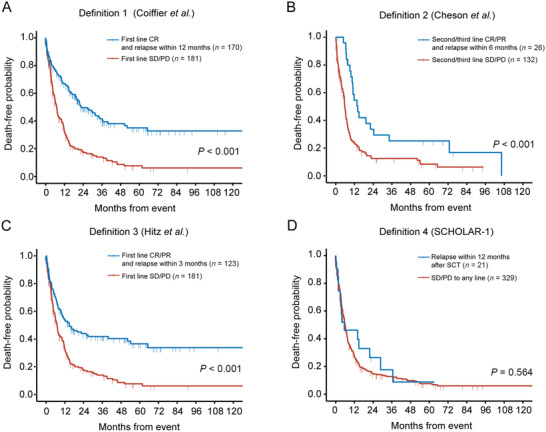
Validation of previous definitions of refractory DLBCL in the REAL‐TREND dataset. A. Overall survival of Definition 1 (Coiffier et al.). B. Overall survival of Definition 2 (Cheson et al.). C. Overall survival of Definition 3 (Hitz et al.). D. Overall survival of Definition 4 (SCHOLAR‐1).
Abbreviations: CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; SCT, stem‐cell transplantation
3.3. Incidence and clinical features of refractory DLBCL
The incidence rate of refractory DLBCL in the REAL‐TREND dataset was 14.9% (350/2342), including 181 (51.7%) primary refractory patients, 148 (42.3%) refractory patients to second or later‐line therapy, and 21 (6.0%) relapsed patients within 12 months after SCT. The estimated 5‐year cumulative incidence of refractory DLBCL was 20% (95% CI = 18%‐22%). The clinical characteristics of the patients with refractory DLBCL at the determination of refractory disease are summarized in Table 1. Overall, 104 (29.7%) patients had ECOG PS 2‐4, 197 (56.3%) had advanced disease (Ann Arbor stage III‐IV), and 138 (39.4%) had IPI scores ≥3. After the determination of refractory disease, 245 (70.0%) of patients received salvage chemotherapy and 30 (8.6%) further underwent SCT.
TABLE 1.
Clinical characteristics of 350 refractory DLBCL patients
| Characteristics | DLBCL cases (%) | |
|---|---|---|
| Age (years) | ||
| >60 | 135 (38.6) | |
| ≤60 | 215 (61.4) | |
| Gender | ||
| Male | 208 (59.4) | |
| Female | 142 (40.6) | |
| ECOG PS | ||
| 0‐1 | 208 (59.4) | |
| 2‐4 | 104 (29.7) | |
| Unavailable | 38 (10.9) | |
| Ann Arbor stage | ||
| I‐II | 128 (36.6) | |
| III‐IV | 197 (56.3) | |
| Unavailable | 25 (7.1) | |
| IPI score | ||
| 0‐1 | 73 (20.9) | |
| 2 | 76 (21.7) | |
| 3 | 87 (24.9) | |
| 4‐5 | 51 (14.6) | |
| Unavailable | 63 (18.0) | |
| Refractory category | ||
| Primary refractory | 181 (51.7) | |
| Refractory to second‐ or later‐line therapy | 148 (42.3) | |
| Relapse ≤12 months post‐SCT | 21 (6.0) | |
| Treatment modality after refractory disease (%) | ||
| Immunochemotherapy + SCT | 30 (8.6) | |
| Immunochemotherapy | 215 (61.4) | |
| Palliative treatment | 105 (30.0) | |
Abbreviations: DLBCL, diffuse large B‐cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, international prognostic index; SCT, stem‐cell transplantation.
3.4. Response rate and prognostic factors after the determination of refractory DLBCL
The variation in response rates across centers was significant, indicating that it was due to heterogeneity rather than chance (I² = 61.6%; Q = 19.1; P = 0.008). Then, the pooled ORR and CRR were estimated with random‐effects models. Survival data were pooled directly using observed values since the log‐rank test showed no significant difference across centers (P = 0.562). The pooled ORR after determination of refractory disease was 30% (95% CI = 22%‐38%) and the CRR was 9% (95% CI = 4%‐15%). Primary refractory patients had an ORR of 39% (95% CI = 26%‐52%) and CRR of 16% (95% CI = 3%‐28%). Patients’ refractory to second‐ or later‐line therapy had an ORR of 18% (95% CI = 13%‐24%) and CRR of 5% (95% CI = 2%‐8%). Patients who relapsed within 12 months after SCT had an ORR of 24% (95% CI = 9%‐39%) and CRR of 15% (95% CI = 2%‐28%).
Refractory DLBCL patients had a median OS of 5.9 months (95% CI = 5.5‐7.1 months) and 2‐year OS rate of 16% (95% CI = 12%‐20%). As shown in Table 2, univariable analysis identified the clinical characteristics of refractory diseases, including age (median OS: age >60 years old, 4.0 months [95% CI = 3.5‐5.6 months]; age ≤60 years old, 7.1 months [95% CI = 6.1‐9.0 months]), ECOG PS (median OS: ECOG PS 0‐1, 7.3 months [95% CI = 6.2‐9.3 months]; ECOG PS 2‐4, 3.5 months [95% CI = 2.5‐4.5 months]), Ann Arbor stage (median OS: stage I‐II, 7.5 months [95% CI = 6.3‐11.0 months]; stage III‐IV, 4.6 months [95% CI = 3.8‐6.1 months]), IPI score (median OS: IPI 0‐1, 8.1 months [95% CI = 6.3‐11.8 months]; IPI 2, 7.0 months [95% CI = 6.0‐11.8 months]; IPI 3, 4.2 months [95% CI = 3.6‐7.4 months]; IPI 4‐5, 2.5 months [95% CI = 1.3‐3.7 months]; Figure 3A) and CNS relapse (median OS: with CNS relapse, 4.5 months [95% CI = 3.6‐7.1 months]; without CNS relapse, 6.1 months [95% CI = 5.5‐7.4 months]; Figure 3B) had significant impact on OS. After the determination of refractory disease, 215 (61.4%) patients received immunochemotherapy, 30 (8.6%) patients received immunochemotherapy plus salvage SCT, and 105 (30.0%) patients received palliative care. According to treatment modalities, patients treated with immunochemotherapy plus salvage SCT had longer survival time (median OS: 14.1 months [95% CI = 11.1 months to not reached]) than those who received immunochemotherapy (median OS: 6.7 months [95% CI = 5.8‐7.8 months]) or palliative treatment (median OS: 2.8 months [95% CI = 1.8‐3.7 months]; P < 0.001; Figure 3C). The best response achieved after the determination of refractory disease also had significant impact on OS (P < 0.001; Figure 3D). Patients who achieved CR had a median OS of 50.4 (95% CI = 39.2 months to not reached), remarkably superior to patients who achieved PR (median OS: 12.2 months [95% CI = 11.3‐18.5 months]) or patients with SD/PD (median OS: 4.0 months [95% CI = 3.6‐5.0 months]).
TABLE 2.
Univariable and multivariable analysis of overall survival from refractory DLBCL
| Characteristics | Univariable analysis | Multivariable analysis* | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | |||||
| ≤60 | Reference | ‐ | ‐ | ‐ | |
| >60 | 1.50 (1.19‐1.90) | 0.001 | ‐ | ‐ | |
| Gender | |||||
| Male | Reference | ‐ | ‐ | ‐ | |
| Female | 0.80 (0.63‐1.01) | 0.059 | ‐ | ‐ | |
| ECOG PS | |||||
| 0‐1 | Reference | ‐ | ‐ | ‐ | |
| 2‐4 | 1.87 (1.45‐2.41) | <0.001 | ‐ | ‐ | |
| Unavailable | 1.16 (0.79‐1.70) | 0.461 | ‐ | ‐ | |
| Ann Arbor stage | |||||
| I‐II | Reference | ‐ | ‐ | ‐ | |
| III‐IV | 1.33 (1.04‐1.70) | 0.025 | ‐ | ‐ | |
| Unavailable | 1.30 (0.81‐2.09) | 0.270 | ‐ | ‐ | |
| IPI score | |||||
| 0‐1 | Reference | ‐ | Reference | ‐ | |
| 2 | 0.94 (0.65‐1.35) | 0.727 | 0.97 (0.67‐1.41) | 0.874 | |
| 3 | 1.53 (1.09‐2.15) | 0.015 | 1.23 (0.87‐1.75) | 0.236 | |
| 4‐5 | 3.43 (2.31‐5.10) | <0.001 | 2.22 (1.47‐3.35) | <0.001 | |
| Unavailable | 1.05 (0.72‐1.52) | 0.818 | 0.95 (0.65‐1.38) | 0.773 | |
| Refractory category | |||||
| Primary refractory | Reference | ‐ | ‐ | ‐ | |
| Refractory to second‐ or later‐line therapy | 1.26 (0.99‐1.60) | 0.061 | ‐ | ‐ | |
| Relapse ≤12 months post‐SCT | 0.94 (0.55‐1.60) | 0.829 | ‐ | ‐ | |
| Treatment modality after refractory disease (%) | |||||
| Immunochemotherapy + SCT | Reference | ‐ | Reference | ‐ | |
| Immunochemotherapy | 1.95 (1.21‐3.13) | 0.006 | 0.79 (0.47‐1.35) | 0.394 | |
| Palliative treatment | 3.89 (2.37‐6.38) | <0.001 | 1.07 (0.60‐1.90) | 0.811 | |
| CNS relapse | |||||
| Absent | Reference | ‐ | Reference | ‐ | |
| Present | 1.47 (1.08‐2.00) | 0.015 | 1.43 (1.04‐1.97) | 0.029 | |
| Best response after refractory disease | |||||
| CR | Reference | ‐ | Reference | ‐ | |
| PR | 2.60 (1.43‐4.72) | 0.002 | 2.68 (1.42‐5.03) | 0.002 | |
| SD/PD | 6.79 (3.92‐11.76) | <0.001 | 5.97 (3.21‐11.11) | <0.001 | |
Since age, ECOG PS and Ann Arbor stage were factors integrated into IPI scores, they were not included in multivariable analysis even though univariable analysis showed significant risks.
Abbreviations: HR, hazard ratio; CI, confidence interval; IPI, international prognostic index; CNS, central nervous system; SCT, stem‐cell transplantation; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease.
FIGURE 3.
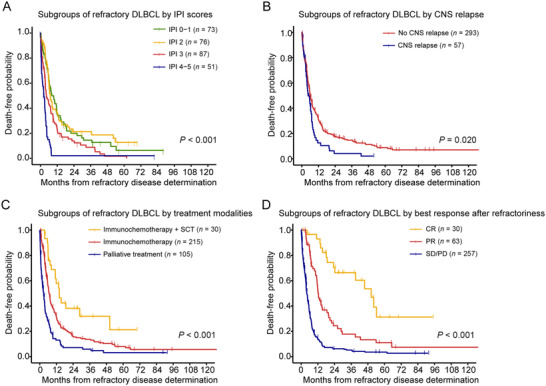
Stratified analysis of the overall survival of refractory DLBCL. Overall survival is shown for subgroups by refractory IPI scores (A), subgroups by CNS relapse (B), subgroups by treatment modalities (C), and subgroups by best response after refractory disease (D).
Abbreviations: IPI, international prognostic index; SCT, stem‐cell transplantation; CNS, central nervous system; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease.
Since age, ECOG PS and Ann Arbor stage were factors integrated into IPI scores, they were not included in multivariable analysis. Multivariate analysis showed that IPI score 4‐5 (Hazard ratio [HR] = 2.22; 95% CI = 1.47‐3.35), CNS relapse (HR = 1.43; 95% CI = 1.04‐1.97) and best response after the determination of refractory disease (PR: HR = 2.68; 95% CI = 1.42‐5.03; SD/PD: HR = 5.97, 95% CI = 3.21‐11.11) were independent unfavorable prognostic factors for OS (Table 2).
4. DISCUSSION
Refractory disease resulted in poor clinical outcomes in DLBCL. However, there have been discrepancies in the definition of refractory DLBCL among clinical studies due to different therapeutic strategies. In the group of Definition 1 [9], a large proportion of patients were treated with chemotherapy, while patients from Definition 2, 3, and SCHOLAR‐1 study received rituximab‐containing immunochemotherapy. This could have induced bias in the outcomes of relapsed and refractory patients, as reported in the CORAL study that whether patients had prior rituximab in the first‐line treatment exerts significant impacts on prognosis [17]. For SD/PD patients, Definition 1 and 3 include patients who had first‐line SD/PD [9, 11], while Definition 2 refers to patients with second‐ or third‐line SD/PD [10]. Our results showed that no significant difference was observed between first‐line and second/third‐line SD/PD, which is consistent with the SCHOLAR‐1 study that defines refractory DLBCL as SD/PD to any line of immunochemotherapy. Indeed,, it has been reported that patients with first‐line SD/PD had dismal outcomes, with a median OS of only 10 months when treated with salvage regimens or SCT[18]. For relapsed patients, Definition 1 includes patients who relapsed within 12 months after CR [9], while Definition 2 and 3 refer to patients who relapsed within 3 or 6 months after CR/PR [10, 11]. In our study, relapsed patients presented with relatively favorable survival, irrespective of the relapse time, except for those who relapsed within 12 months after salvage SCT, as described by the SCHOLAR‐1 study [12]. Together, within the REAL‐TREND dataset, we demonstrated that the definition of the SCHOLAR‐1 study was most suitable to define refractory DLBCL with homogenously poor clinical outcomes in the era of rituximab‐based immunochemotherapy.
Based on the definition of refractory DLBCL from the SCHOLAR‐1 study, the cumulative 5‐year incidence of refractory DLBCL was 20% within the REAL‐TREND dataset. Overall, clinical characteristics of refractory DLBCL patients were similar as the SCHOLAR‐1 study [12], with a comparable poor response rate (REAL‐TREND: ORR = 30%; CRR = 9%; SCHOLAR‐1: ORR = 26%; CRR = 7%) and OS (REAL‐TREND: median OS = 5.9 months; 2‐year OS rate = 16%; SCHOLAR‐1: median OS = 6.3 months; 2‐year OS rate = 20%). Only patients with refractory DLBCL who achieved CR after salvage therapy had a chance for long‐term survival (median OS = 50.4 months), while SD/PD and PR resulted in inferior prognosis (median OS = 4.0 and 12.2 months, respectively). This was also comparable with the SCHOLAR‐1 study where patients who achieved CR after salvage therapy had significantly better OS than patients with SD/PD or PR (median OS = 14.9, 4.6, and 6.9 months, respectively). Therefore, outcomes of refractory DLBCL were dismal and independent of the ethnic background of patients. Immunochemotherapy and salvage SCT had limited effects on refractory DLBCL, and novel treatment modalities should be applied. For example, CAR‐T therapy has shown a CRR of 40%‐58% and durable responses in heavily pretreated patients [19, 20, 21, 22].
The strength of this study is the real‐world design, which minimized selection bias and allowed to depict the incidence and outcomes of refractory DLBCL in China. Although R‐CHOP‐based regimens were used by all centers as first‐line treatment, regimens for second‐ or later‐line therapy varied among centers due to lack of a standard regimen after the failure of first‐line treatment [23, 24]. Thus, response rates may have varied from centers but were minimized by standard data collection procedures and adjusted using proper statistical methods.
In summary, the REAL‐TREND study is the largest real‐world study of refractory DLBCL in Asian countries. The definition made by the SCHOLAR‐1 study is the most suitable to identify refractory DLBCL.. With this definition, 20% of patients with newly diagnosed DLBCL would become refractory within 5 years, and the median OS of these patients was fewer than 6 months. Effective alternative therapies for refractory DLBCL are urgently needed.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Review Boards of all 8 participating centers. Each participant signed informed consent before participating in this study following the Declaration of Helsinki.
CONSENT FOR PUBLICATION
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
This study was supported, in part, by research funding from the National Natural Science Foundation of China (81670176 to L.W., 81520108003, and 81830007 to W.Z.), the Chang Jiang Scholars Program (T2015055 to W.Z.), the Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant Support (20152206 to L.W. and 20152208 to W.Z.), the Clinical Research Plan of SHDC (SHDC2020CR1032B to W.Z.), the Multicenter Clinical Research Project by Shanghai Jiao Tong University School of Medicine (DLY201601 to W.Z.), the Collaborative Innovation Center of Systems Biomedicine (to W.Z.), and the Samuel Waxman Cancer Research Foundation (to W.Z.). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
AUTHORS' CONTRIBUTIONS
W.Z., L.W., and P.X. designed the study. L.W., J.H., W.Q., X.Z., Y.H., Q.Z., B.C., H.Y., and D.W. provided executive support and active data surveillance and performed the patient selection process. S.W., X.Z., J.W., Y.L., G.C., Y.T., Y.M., and H.H. provided data management. S.W., P.X., and C.C.H.C designed the statistical analysis plan and performed the analyses. L.W., S.W., and W.Z. interpreted the results and prepared the manuscript. All authors reviewed the manuscript critically and approved the content.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
Not applicable.
Wang S, Wang L, Hu J, et al. Outcomes in refractory diffuse large B‐cell lymphoma: results from a multicenter real‐world study in China. Cancer Commun. 2021;41:229–239. 10.1002/cac2.12126
DATA AVAILABILITY STATEMENT
Eight clinical centers from the Multicenter Hematology‐Oncology Protocols Evaluation System (M‐HOPES) network in China participated in this study. All data have been deposited in the data repository of the Shanghai Clinical Quality Control Center for Hematology (http://www.shxyzk.com/) and are accessible to researchers meeting the criteria for data access.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69(1):7‐34. 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2. Wang L, Qin W, Huo Y‐J, Li X, Shi Q, Rasko JEJ et al. Advances in targeted therapy for malignant lymphoma. Signal Transduction and Targeted Therapy. 2020;5(1). 10.1038/s41392-020-0113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large‐B‐cell lymphoma. N Engl J Med. 2002;346(4):235‐42. 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 4. Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K et al. CHOP‐like chemotherapy plus rituximab versus CHOP‐like chemotherapy alone in young patients with good‐prognosis diffuse large‐B‐cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. The Lancet Oncology. 2006;7(5):379‐91. 10.1016/s1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 5. Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B‐cell non‐Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14‐day versus 21‐day cycles. Lancet (London, England). 2013;381(9880):1817‐26. 10.1016/s0140-6736(13)60313-x. [DOI] [PubMed] [Google Scholar]
- 6. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S et al. Long‐term outcome of patients in the LNH‐98.5 trial, the first randomized study comparing rituximab‐CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116(12):2040‐5. 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B‐cell lymphoma in British Columbia. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(22):5027‐33. 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 8. Arboe B, Olsen MH, Gorlov JS, Duun‐Henriksen AK, Dalton SO, Johansen C et al. Treatment intensity and survival in patients with relapsed or refractory diffuse large B‐cell lymphoma in Denmark: a real‐life population‐based study. Clin Epidemiol. 2019;11:207‐16. 10.2147/CLEP.S178003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coiffier B, Salles G, Bosly A, Gaulard P, Haioun C, Casasnovas O et al. Characteristics of Refractory and Relapsing Patients with Diffuse Large B‐Cell Lymphoma. Blood. 2008;112:2589‐.18779413 [Google Scholar]
- 10. Cheson BD, Bartlett NL, Vose JM, Lopez‐Hernandez A, Seiz AL, Keating AT et al. A phase II study of the survivin suppressant YM155 in patients with refractory diffuse large B‐cell lymphoma. Cancer. 2012;118(12):3128‐34. 10.1002/cncr.26510. [DOI] [PubMed] [Google Scholar]
- 11. Hitz F, Connors JM, Gascoyne RD, Hoskins P, Moccia A, Savage KJ et al. Outcome of patients with primary refractory diffuse large B cell lymphoma after R‐CHOP treatment. Annals of Hematology. 2015;94(11):1839‐43. 10.1007/s00277-015-2467-z. [DOI] [PubMed] [Google Scholar]
- 12. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J et al. Outcomes in refractory diffuse large B‐cell lymphoma: results from the international SCHOLAR‐1 study. Blood. 2017;130(16):1800‐8. 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jaffe E, Swerdlow SHCE, Campo E, Pileri S, Thiele J, Harris N et al. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues. 2008.
- 14. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(27):3059‐68. 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ et al. Revised response criteria for malignant lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(5):579‐86. 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 16. Gray RJ. A Class of $K$‐Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Statist. 1988;16(3):1141‐54. 10.1214/aos/1176350951. [DOI] [Google Scholar]
- 17. Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M et al. Salvage regimens with autologous transplantation for relapsed large B‐cell lymphoma in the rituximab era. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(27):4184‐90. 10.1200/jco.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Telio D, Fernandes K, Ma C, Tsang R, Keating A, Crump M et al. Salvage chemotherapy and autologous stem cell transplant in primary refractory diffuse large B‐cell lymphoma: outcomes and prognostic factors. Leukemia & lymphoma. 2012;53(5):836‐41. 10.3109/10428194.2011.643404. [DOI] [PubMed] [Google Scholar]
- 19. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP et al. Primary Analysis of Juliet: A Global, Pivotal, Phase 2 Trial of CTL019 in Adult Patients with Relapsed or Refractory Diffuse Large B‐Cell Lymphoma. Blood. 2017;130:577. [Google Scholar]
- 20. Abramson JS, Gordon LI, Palomba ML, Lunning MA, Arnason JE, Forero‐Torres A et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. Journal of Clinical Oncology. 2018;36(15_suppl):7505. 10.1200/JCO.2018.36.15_suppl.7505. [DOI] [Google Scholar]
- 21. Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO et al. Long‐term safety and activity of axicabtagene ciloleucel in refractory large B‐cell lymphoma (ZUMA‐1): a single‐arm, multicentre, phase 1‐2 trial. The Lancet Oncology. 2019;20(1):31‐42. 10.1016/s1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B‐Cell Lymphoma. N Engl J Med. 2019;380(1):45‐56. 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 23. Gisselbrecht C, Van Den Neste E. How I manage patients with relapsed/refractory diffuse large B cell lymphoma. British journal of haematology. 2018;182(5):633‐43. 10.1111/bjh.15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sarkozy C, Sehn LH. Management of relapsed/refractory DLBCL. Best Practice & Research Clinical Haematology. 2018;31(3):209‐16. 10.1016/j.beha.2018.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Data Availability Statement
Eight clinical centers from the Multicenter Hematology‐Oncology Protocols Evaluation System (M‐HOPES) network in China participated in this study. All data have been deposited in the data repository of the Shanghai Clinical Quality Control Center for Hematology (http://www.shxyzk.com/) and are accessible to researchers meeting the criteria for data access.


