Abstract
Objectives
Totally implantable central venous access ports (port-a-caths) are increasingly used for the safe administration of chemotherapy; however, their use is associated with complications. This study reviews patterns of complications, reasons for premature removal and the duration of the use of port-a-caths in patients receiving cancer treatment at Sultan Qaboos University Hospital (SQUH) and compares the infection rate with the literature and the researchers’ experiences.
Methods
This retrospective follow-up study included patients who had received cancer treatment through a port-a-cath and were admitted to SQUH between January 2007 and April 2019. Demographic features, underlying diagnosis, clinical stage, treatment, duration of use and the cause of premature removal of the port-a-cath were recorded.
Results
A total of 516 port-a-caths were inserted in 482 cancer patients. The majority of devices were implanted by interventional radiologists (n = 459; 89.0%) and the right internal jugular vein was most frequently accessed (n = 396; 76.7%). The mean indwelling time of a port-a-cath was 288 days (range: 3–1,872 days) for patients with complications and 550 days (range: 7–3,123 days) for patients without complications. Port-a-cath-related infection was the main complication (n = 63; 12.2%). Patient age, gender, treatment intent, underlying diagnosis, clinical stage, chemotherapy regimen, number of treatment courses, operator implanting the port, the type of micro-organism isolated from the port-a-cath and body mass index were significant factors affecting catheter indwelling time (P <0.05). On multivariate analysis, however, none of the factors was found to be significant.
Conclusion
Infection was the most common complication necessitating port-a-cath removal. The infection rate was much lower than the researchers’ previous experience and compares favorably with several published reports.
Keywords: Port-A-Cath, Vascular Access Ports, Catheter-Related Infections, Cancer, Oman
Advances in Knowledge
- The port-a-cath-related infection rate was nearly halved when comparing across the two studied time periods; this is most likely due to better nursing standards.
- These results support the use of port-a-caths in cancer patients and provide a benchmark for regional cancer treating hospitals.
Application to Patient Care
- Venous access becomes a significant problem in most cancer patients who require long-term cancer care. Port-a-cath insertion though has associated risk of infections and other side effects as evidenced by results of our study, but those can be reduced with diligent care
- Most patients could complete their entire chemotherapy courses without side effects and avoiding the need for repeated cannulation and thrombophlebitis a common complication associated with chemotherapy administration in peripheral veins.
- With time, acceptance for port-a-cath is increasing among Omani patients as can be seen with increasing numbers.
Totally implantable central venous access ports are effective for long-term venous access and improved patient safety; they are also known as port-a-caths. Besides administering anticancer therapy, port-a-caths are used to administer blood, blood products and nutrition, and to draw blood.1 Port-a-caths impact daily activities minimally and result in better patient quality of life (QOL).2 Port-a-caths contribute to better patient QOL because they are situated subcutaneously; hence, they do not affect range of motion or impede daily activities.3 In the past, port-a-caths were implanted in operation theatres, but their more recent insertion by interventional radiologists in outpatient settings through a relatively simple procedure has reduced costs.4 Port-a-caths require minimal maintenance, but challenges include insertion-related complications (e.g. pneumothorax, haemothorax, accidental arterial puncture and cardiac arrhythmia) and late complications (e.g. bloodstream infection [BSI], thrombosis, catheter dysfunction, pocket infection and port-inversion).5–7 The incidence of infectious complications is generally <10% and most are preventable by scrupulous care.4,8
Infection is the most common complication associated with port-a-caths.5,7 Staphylococcus and Candida are the most frequently isolated microorganisms and enter the port-a-cath through the exit site and colonise the catheter. Another source of infection is the port hub; this avenue of infection commonly occurs during blood sampling or flushing. The healthcare provider and patient education plays a pivotal role in continuous and successful long-term port-a-cath care.7
A previous study reported infection as the most common cause for premature removal of port-a-caths.4 With better aseptic techniques and standardised procedures, infection rates have declined.8 This follow-up study aimed to review patterns of complications, reasons for premature removal and the duration of the use of port-a-caths in patients receiving cancer treatment. In addition, this study reports follow-up data and examines the incidence of complications over two time periods.4 To the best of the researchers’ knowledge, this is the largest dataset on port-a-cath-associated complications reported from the Gulf Cooperation Council (GCC) region.
Methods
This retrospective follow-up study included consecutive adult patients with solid tumours who had a port-a-cath inserted for the purpose of receiving cancer treatment between January 2007 and April 2019 at Sultan Qaboos University Hospital (SQUH) in Muscat, Oman. This dataset includes previously published results from the time period of January 2007 to February 2013.10 Electronic patient records (EPR) were accessed to collect the study variables including patient age, gender, body mass index (BMI), diagnosis, type of operator (i.e. interventional radiologist, general surgeon, anaesthetist), dates of insertion and removal, complications, indwelling time and current patient status (i.e. alive or dead). Indwelling time was calculated from the time of implantation until the date of removal due to complications, treatment completion, death or until April 30th, 2019, whichever came first. The Radiology Department at SQUH maintains a logbook of all the patients who undergo port-a-cath insertion or removal. A clinical nurse specialist (CNS) updates the information on the EPR.
The Vital-Port port-a-cath (Cook Medical, Bloomington, Indiana, USA) type was used until the first quarter of 2018. Subsequently, this was replaced by the Power-Port port-a-cath (Bard Access Systems Inc., Salt Lake City, Utah, USA). All implanted port-a-caths were single lumen and the internal jugular vein (IJV) was used for venous access. The right IJV was the preferred port-a-cath entry point except in patients with right-sided breast cancer. To accomplish insertion, the IJV was punctured using ultrasound guidance under local anaesthesia.
Once the port had been inserted, the track from the incision to the site of the venous puncture was anaesthetised and a track was created from the incision to the puncture site. Using fluoroscopic guidance, an estimate was made of the required length of the catheter so that the tip of the catheter would lie low in the superior vena cava (SVC). Neither prophylactic antibiotics nor routine anti-coagulation therapy was administered.
Senior specialists, CNS, specialists from the medical oncology unit and trainees rotating through Medical Oncology were allowed to access the port-a-cath. All rotating doctors had a proper introduction and demonstration of needle insertion before permission was granted for them to undertake independent work. When accessing the port-a-cath, the procedure described by Dal Molin et al. was adopted.9
The Society of Interventional Radiology Technology guidelines were followed to report port-a-cath-associated complications.8,10 Complications were divided into three groups and were considered periprocedural complications if they occurred within the first 24 hours of procedure; early, if they occurred within the first 30 days; or late, if complications were noticed 30 days or more after insertion. Catheter-related infections were also defined per the guidelines and were reported as a local infection or a BSI.10
Statistical Package for the Social Sciences (SPSS), Version 20 (IBM, Corp., Armonk, New York, USA) was used to analyse the data. Log-rank univariate analysis was performed using indwelling time as the dependent factor. Kaplan and Meier’s method was used to calculate differences in the port-a-cath’s duration of implantation. The Cox-regression method was used for multivariate analysis. In addition, data were compared across two different time periods: time period one was January 2007 to February 2013 and time period two was March 2013 to April 2019.10
The Institutional Medical Research and Ethics Committee at the Sultan Qaboos University Hospital approved the study (MREC Approval #1929).
Results
A total of 516 port-a-caths were implanted in 482 patients during the study period. Of this total, 34 patients had a second port-a-cath implanted. Of the 516 port-a-caths, 473 (91.7%) were placed at SQUH. The majority of procedures were performed by an interventional radiologist (n = 459; 89.0%). Only 11 (2.1%) port-a-caths were implanted by an anaesthetist and three (0.6%) by a general surgeon.
The median age of patients was 49.0 years (range: 13–83 years) and the majority (n = 338; 65.5%) were female. Median BMI was 26.7 kg/m2. Breast cancer (n = 205; 39.7%) was the most common diagnosis followed by colon cancer (n = 143; 27.7%) and gastric cancer (n = 50; 9.7%). The majority of patients (n = 425; 82.4%) had clinical stage III/IV disease at the time of diagnosis. More than 50% of patients received one line of chemotherapy, while the remaining patients received multiple lines of treatment through the same port-a-cath. Two patients did not receive cytotoxic chemotherapy at all. The most frequent treatment intention was palliative (n = 323; 62.6%). An interventional radiologist most commonly inserted the port-a-cath (n = 459; 89.0%) [Table 1].
Table 1.
Characteristics and diagnoses of patients with solid tumours who had a port-a-cath implanted at Sultan Qaboos University Hospital, Oman from January 2007 to April 2019 (N = 516)
| Characteristic | n (%) |
|---|---|
| Gender | |
| Male | 178 (34.5) |
| Female | 338 (65.5) |
| BMI category | |
| Below normal (<18.5) | 51 (9.9) |
| Normal (18.5–24.9) | 167 (32.4) |
| Overweight (25–30) | 142 (27.5) |
| Obese (>30) | 156 (30.2) |
| Median in kg/m2 | 26.7 |
| Diagnosis | |
| Breast cancer | 205 (39.7) |
| Colon cancer | 143 (27.7) |
| Gastric cancer | 50 (9.7) |
| Sarcoma | 22 (4.3) |
| Ovarian cancer | 17 (3.3) |
| Pancreatic cancer | 16 (3.1) |
| Lung cancer | 13 (2.5) |
| Other | 50 (9.7) |
| Cancer disease stage | |
| I | 18 (3.5) |
| II | 73 (14.1) |
| III | 138 (26.7) |
| IV | 287 (55.6) |
| Interventionist | |
| Interventional radiologist | 459 (89.0) |
| Port-a-cath implanted outside Oman | 43 (8.3) |
| Anaesthetist | 11 (2.1) |
| Surgeon | 3 (0.6) |
| Treatment intention | |
| Curative | 193 (37.4) |
| Palliative | 323 (62.6) |
BMI = body mass index.
As it was the choice of the interventional radiologist to choose the best site for implantation, the majority of the port-a-caths were implanted through the right IJV (76.7%) due to ease of access. The vast majority of patients (73.8%) had the tip of the port-a-cath inserted in the SVC.
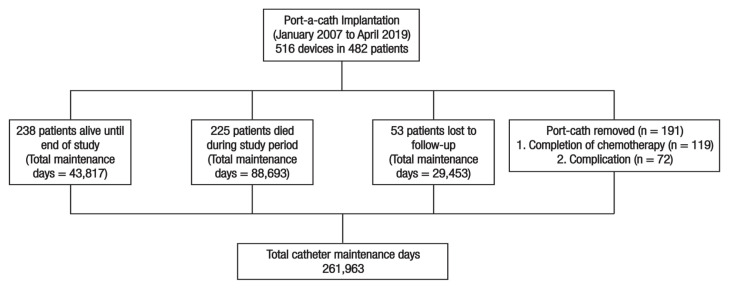
Overall, port-a-caths were used for a total of 261,963 days in all patients included in this study [Figure 1]. The mean indwelling time was 508 ± 123 days (range: 3–3,123 days); the mean duration of use of port-a-cath in patients who developed complications was 288 ± 325 days (range: 3–1,872 days) and, for those who did not develop a complication, the mean duration of use was 550 ± 194 days (range: 7–3,123 days). The most commonly isolated organism was Staphylococcus aureus (n = 14; 2.7%). A total of 119 (23.1%) devices were removed after treatment was completed [Table 2].
Figure 1.
Overview of outcomes of patients’ with solid tumours who underwent port-a-cath implantation at Sultan Qaboos University Hospital, Oman from January 2007 to April 2019.
Table 2.
Reasons for removal of port-a-cath in patients with solid tumours at Sultan Qaboos University Hospital, Oman from January 2007 to April 2019 (N = 516)
| Reason for removal* | n (%) |
|---|---|
| Complication† | 72 (14.0) |
| Infection | 59 (11.4) |
| Infection + blocked | 4 (0.8) |
| Blocked | 9 (1.7) |
| Skin rupture | 9 (1.7) |
| Vessel thrombosis | 3 (0.6) |
| Catheter migration | 1 (0.2) |
| Haematoma | 1 (0.2) |
| Catheter leak | 1 (0.2) |
| Completed chemotherapy | 119 (22.3) |
| Organism isolated | |
| Staphylococcus aureus | 14 (2.7) |
| Staphylococcus hemolyticus | 6 (1.2) |
| Klebsiella pneumonie | 6 (1.2) |
| Multi-resistant Pseudomonas aureguinosa | 4 (0.8) |
| Pseudomonas aureguinosa | 4 (0.8) |
| Multi-resistant Klebsiella | 3 (0.6) |
| Acinetobacter | 2 (0.4) |
| Candida | 2 (0.4) |
| Escherichia coli | 2 (0.4) |
| Escherichia faecalis | 1 (0.2) |
| Proteus vulgaris | 1 (0.2) |
| Streptococcus mitis | 1 (0.2) |
| Bacillus cereus | 1 (0.2) |
| Ochrobacum anthropi | 1 (0.2) |
| No organism isolated | 16 (3.1) |
| Chemotherapy regimen | |
| AC → D±T | 107 (20.7) |
| Multiple lines (no bevacizumab) | 165 (32.0) |
| Multiple lines with bevacizumab | 64 (12.4) |
| FOLFOX4 ± bevacizumab | 82 (15.9) |
| Other Regimens | 98 (20.0) |
AC = Adriamycin and Cyclophosphamide; D±T = Docetaxel ± Trastuzumab; FOLFOX4 = Folinic Acid, 5-Fluoruracil and Oxaliplatin.
The remaining port-a-caths were not removed.
Patients could have more than one complication.
Port-a-caths were removed prematurely in 72 (14.0%) patients due to complications (0.27/1,000 catheter days). Complications recorded within the 30 days of port-a-cath implantation included infection, skin dehiscence, haematoma and catheter leakage or blockage. The most common reason for removing a port-a-cath was infection (n = 63; 12.2%; 0.20/1,000 catheter days). BSI was documented in 3.7% of patients. Other reasons for removal included catheter blockage (n = 13; 2.5%), skin dehiscence (n = 5; 1.0%) and venous thrombosis (n = 3; 0.6%). The overall median time to develop an infection was 89 days from the date of port-a-cath insertion. The median time to develop a port-a-cath-related infection was 246 days (range: 3–1,872 days), 210 days (range: 24–890 days) to develop a catheter blockage, 62 days (range: 14–306 days) for skin rupture and 206 days (range: 110–278 days) for a thrombosis [Table 3]. No patient developed pneumothorax, arterial puncture or acute bleeding after the procedure. Of the remaining devices, 119 (23.1%) were removed after completion of the intended treatment while 173 (33.53%) are still implanted in patients who are under follow-up or are receiving therapy at the time of publication.
Table 3.
Time to port-a-cath complication and type of complication in patients with solid tumours
| Complication type | Early complications (within 30 days) | Late complications (after 30 days) | Total complications | Mean days to complication | |||
|---|---|---|---|---|---|---|---|
| n (%) | Per 1,000 catheter days | n (%) | Per 1,000 catheter days | n (%) | Per 1,000 catheter days | ||
| Infection + Blocked | 23 (4.4) | 0.05 | 40 (7.7) | 0.148 | 63 (12.2) | 0.20 | 246 |
| Catheter blockage | 5 (0.9) | 0.003 | 4 (0.8) | 0.041 | 9 (1.7) | 0.045 | 210 |
| Skin dehiscence | 3 (0.6) | 0.007 | 6 (1.2) | 0.011 | 9 (1.7) | 0.020 | 62 |
| Venous thrombosis | 0 (0) | 0 | 3 (0.6) | 0.131 | 3 (0.6) | 0.131 | 206 |
| Catheter migration | 0 (0) | 0 | 1 (0.2) | 0.003 | 1 (0.2) | 0.003 | 155 |
| Haematoma | 0 (0) | 0 | 1 (0.2) | 0.003 | 1 (0.2) | 0.003 | 17 |
| Catheter leak | 1 (0.2) | 0.003 | 0 (0) | 0 | 1 (0.2) | 0.003 | 17 |
Analysis of incidence of complications over two time periods (January 2007 to February 2013 versus March 2013 to April 2019) revealed a reduction in the complication rates from 25.6% in time period one to 11.2% in time period two. Similarly, the infection rate decreased from 16.2% to 8.2% over the two time periods [Table 4].10
Table 4.
Comparison of port-a-cath complications of over two time periods in patients with solid tumours at Sultan Qaboos University Hospital, Oman from January 2007 to April 2019
| Variable | n (%) | |
|---|---|---|
| Time period one* (January 2007 to February 2013) n = 117 |
Time period two (March 2013 to April 2019) n = 399 |
|
| Mean time port-a-cath in place for all patients in days (range) | 354 (3–1,876) | 495 (7–32,216) |
| Mean time port-a-cath in place for patients with complication(s) in days (range) | 252 (3–1,876) | 285 (8–1,148) |
| Reason for removal | ||
| Complication† | 30 (25.6%) | 45 (11.2%) |
| Infection | 19 (16.2%) | 33 (8.2%) |
| Infection + blocked | 2 (1.7%) | 2 (0.5) |
| Blocked | 4 (3.4%) | 4 (1.0%) |
| Skin rupture | 4 (3.4%) | 3 (0.75%) |
| Vessel thrombosis | 0 (0%) | 3 (0.8%) |
| Catheter migration | 0 (0%) | 1(0.3%) |
| Haematoma | 0 (0%) | 1(0.3%) |
| Catheter leak | 1 (0.9%) | 0 (0%) |
Some data previously reported in: D’Souza PC, Kumar S, Kakaria A, Al-Sukaiti R, Zahid KF, Furrukh M, et al. Use of port-a-cath in cancer patients: A single-center experience.10
Patients could have more than one complication.
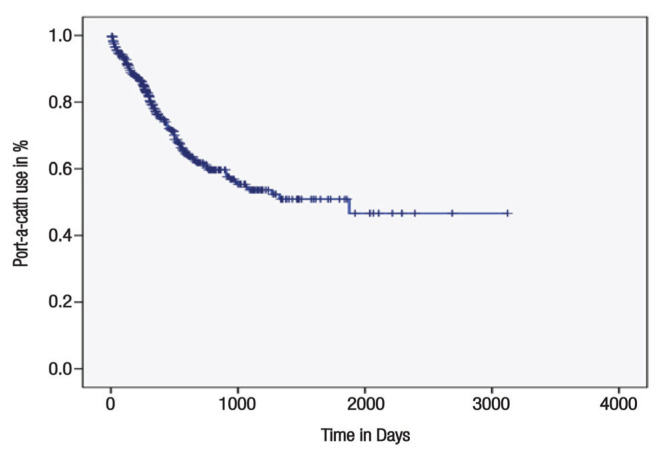
At one-year follow-up, median duration of port-a-cath use was 361 days [Figure 2]. On the log rank analysis, patient age, gender, treatment intent (curative versus palliative), underlying diagnosis, disease stage, rate of complication, chemotherapy regimen, number of treatment lines, operator implanting the port, organism isolated from the port and BMI were significant factors affecting indwelling time of a port-a-cath (P <0.05 each). On Cox regression multivariate analysis, none of the factors significantly affected the indwelling time of a port-a-cath.
Figure 2.
Duration of port-a-cath use in patients with solid tumours at Sultan Qaboos University Hospital, Oman from January 2007 to April 2019.
Discussion
To the best of the researchers’ knowledge, this study represents the largest examination of use patterns, outcomes and complication rates for port-a-caths in cancer patients in the Middle East. A total of 516 port-a-caths were inserted in 482 patients. The mean indwelling time of port-a-cath was 508 days but was shorter for those who developed complications compared to those who did not (288 versus 551 days). The overall complication rate was 14.0%, and the infection rate was 12.2%. Over the two study periods, both the complication rate and the infection rate dropped significantly, suggesting a learning curve for the institution’s healthcare workers.
Various studies have reported advantages of port-a-caths as opposed to tunnelled or peripheral catheters in relation to indwelling time, cost and complications. The reported mean indwelling time has been reported between 9–16.6 months; the current study reveals a similar indwelling time of 16.7 months (508 days).8,11,12 This time is an improvement from the mean of 354 days reported previously for SQUH and is consistent with contemporary literature.4
In the current study, approximately 12.2% of the port-a-caths were removed prematurely because of infection. This finding is consistent with the published literature, which suggests that the most common reason for premature removal of port-a-caths is infection.13 Over the two time periods in the current study, the infection rate dropped from 16.2% to 8.2%.10 The infection rate in the second study period is consistent with rates reported previously in the literature (i.e. 1.7–9.3%).14–16
Central line-associated BSIs often require long-term antibiotics, device removal and prolonged hospital stays. In the current study, 3.7% of port-a-caths had to be removed because of BSI, which is significantly lower than the previously reported (13–34%).2,12 Port-a-caths were also removed in patients with repeated infections or continuous fever despite negative blood and urine cultures and adequate antibiotic administration. Prophylactic anticoagulant agents (warfarin, unfractionated or low molecular weight heparin) are occasionally administered prior to port-a-cath placements; however, there is mounting evidence against this practice and, at SQUH, prophylactic antibiotics are not administered.10,20,21
Overall, in the current study, the complication rate was 14.0% (0.27/1,000 catheter days) compared to 11.8% (0.41/1,000 catheter days) as previously reported.3 Catheter blockage and thrombosis were also significant complication requiring port-a-cath removal. Catheter blockage and thrombosis has been reported to occur in around 3.0–8.5% of cases; whereas, only 2.3% patients in this cohort had non-thrombotic obstruction and 0.6% had thrombotic occlusion.17,18 Cancer patients are already at increased risk of thrombosis and implantation of port-a-cath further increases this risk.19 Prophylactic anti-coagulation has been extensively studied, and routine prophylaxis with anticoagulants is not recommended.20,21 SQUH doctors observe guidelines for the management of port-a-caths, so prophylactic anticoagulants are not routinely used.22–24
Comparing the outcomes of port-a-caths placed by interventional radiologists versus surgeons, two studies have reported no difference in complication rates between the two groups; however, ports placed by interventional radiologists have been found to be more cost effective.25,26 In the current study, only three port-a-caths were placed by surgeons. Because the vast majority of placements were done by interventional radiologists, a statistical comparison was not possible.
This study has several limitations. First, this study was retrospective in nature and therefore several parameters, such as patient symptoms after port-a-cath insertion, could not be captured. The primary aim of this study, however, was to assess the prevalence of complications such as BSI, catheter blockage, thrombosis and port-a-cath-insertion related complications including haematoma, pneumothorax and arterial puncture. All these complications are considered sentinel events so the data were available. Second, it is possible that complications such as reversible blockage of a port-a-cath were underreported. However, such complications are not considered of clinical significance. Finally, the study was carried out as a follow-up study over a long time period in a single centre and standard of care evolved over time. This factor provided an opportunity to study the complication rate over two time periods of more than six years each. Importantly, this study represents the largest body of data reported from the GCC and provides important baseline data which could serve as a benchmark for future studies.
Conclusion
To the best of the researchers’ knowledge, this study is the largest from the Middle East which demonstrates the utility and success of ultrasound and fluoroscopy guidance for port-a-cath placement as an outpatient procedure by interventional radiologists. The periprocedural complications were low. Although infection rates remain a concern, the rate reduced by half over the study’s second time period, reflecting better nursing care. Outcomes improve by improving care processes.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
FUNDING
No funding was received for this study.
References
- 1.Tabatabaie O, Kasumova GG, Eskander MF, Critchlow JF, Tawa NE, Tseng JF. Totally implantable venous access devices: A review of complications and management strategies. Am J Clin Oncol. 2017;40:94–105. doi: 10.1097/coc.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 2.Toure A, Vanhems P, Lombard-Bohas C, Cassier P, Pere-Verge D, Souquet JC, et al. Totally implantable central venous access port infections in patients with digestive cancer: Incidence and risk factors. Am J Infect Control. 2012;40:935–9. doi: 10.1016/j.ajic.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Teichgraber UK, Kausche S, Nagel SN, Gebauer B. Outcome analysis in 3,160 implantations of radiologically guided placements of totally implantable central venous port systems. Eur Radiol. 2011;21:1224–32. doi: 10.1007/s00330-010-2045-7. [DOI] [PubMed] [Google Scholar]
- 4.D’Souza PC, Kumar S, Kakaria A, Al-Sukaiti R, Zahid KF, Furrukh M, et al. Use of port-a-cath in cancer patients: A single-center experience. J Infect Dev Ctries. 2014;8:1476–82. doi: 10.3855/jidc.4155. [DOI] [PubMed] [Google Scholar]
- 5.Yildizeli B, Lacin T, Batirel HF, Yuksel M. Complications and management of long-term central venous access catheters and ports. J Vasc Access. 2004;5:174–8. doi: 10.1177/112972980400500407. [DOI] [PubMed] [Google Scholar]
- 6.Yu XY, Xu JL, Li D, Jiang ZF. Late complications of totally implantable venous access ports in patients with cancer: Risk factors and related nursing strategies. Medicine (Baltimore) 2018;97:e12427. doi: 10.1097/md.0000000000012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matey L, Camp-Sorrell D. Venous access devices: Clinical rounds. Asia Pac J Oncol Nurs. 2016;3:357–64. doi: 10.4103/2347-5625.196480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn SJ, Kim HC, Chung JW, An SB, Yin YH, Jae HJ, et al. Ultrasound and fluoroscopy-guided placement of central venous ports via internal jugular vein: Retrospective analysis of 1254 port implantations at a single center. Korean J Radiol. 2012;13:314–23. doi: 10.3348/kjr.2012.13.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dal Molin A, Rasero L, Guerretta L, Perfetti E, Clerico M. The late complications of totally implantable central venous access ports: The results from an Italian multicenter prospective observation study. Eur J Oncol Nurs. 2011;15:377–81. doi: 10.1016/j.ejon.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Silberzweig JE, Sacks D, Khorsandi AS, Bakal CW. Reporting standards for central venous access. J Vasc Interv Radiol. 2003;14:S443–52. doi: 10.1097/01.rvi.0000094617.61428.bc. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Ryu DY, Jung HJ, Lee SS. Evaluation of complications of totally implantable central venous port system insertion. Exp Ther Med. 2019;17:2013–8. doi: 10.3892/etm.2019.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang TY, Lee KD, Chen PT, Chen MC, Chen YY, Huang CE, et al. Incidence and risk factors for central venous access port-related infection in Chinese cancer patients. J Formos Med Assoc. 2015;114:1055–60. doi: 10.1016/j.jfma.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Fischer L, Knebel P, Schroder S, Bruckner T, Diener MK, Hennes R, et al. Reasons for explantation of totally implantable access ports: A multivariate analysis of 385 consecutive patients. Ann Surg Oncol. 2008;15:1124–9. doi: 10.1245/s10434-007-9783-z. [DOI] [PubMed] [Google Scholar]
- 14.Granziera E, Scarpa M, Ciccarese A, Filip B, Cagol M, Manfredi V, et al. Totally implantable venous access devices: Retrospective analysis of different insertion techniques and predictors of complications in 796 devices implanted in a single institution. BMC Surg. 2014;14:27. doi: 10.1186/1471-2482-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma LI, Liu Y, Wang J, Chang Y, Yu L, Geng C. Totally implantable venous access port systems and associated complications: A single-institution retrospective analysis of 2,996 breast cancer patients. Mol Clin Oncol. 2016;4:456–60. doi: 10.3892/mco.2016.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shim J, Seo TS, Song MG, Cha IH, Kim JS, Choi CW, et al. Incidence and risk factors of infectious complications related to implantable venous-access ports. Korean J Radiol. 2014;15:494–500. doi: 10.3348/kjr.2014.15.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbetakis N, Asteriou C, Kleontas A, Tsilikas C. Totally implantable central venous access ports. Analysis of 700 cases. J Surg Oncol. 2011;104:654–6. doi: 10.1002/jso.21990. [DOI] [PubMed] [Google Scholar]
- 18.Caers J, Fontaine C, Vinh-Hung V, De Mey J, Ponnet G, Oost C, et al. Catheter tip position as a risk factor for thrombosis associated with the use of subcutaneous infusion ports. Support Care Cancer. 2005;13:325–31. doi: 10.1007/s00520-004-0723-1. [DOI] [PubMed] [Google Scholar]
- 19.Busch JD, Herrmann J, Heller F, Derlin T, Koops A, Adam G, et al. Follow-up of radiologically totally implanted central venous access ports of the upper arm: Long-term complications in 127,750 catheter-days. AJR Am J Roentgenol. 2012;199:447–52. doi: 10.2214/AJR.11.7970. [DOI] [PubMed] [Google Scholar]
- 20.Chaukiyal P, Nautiyal A, Radhakrishnan S, Singh S, Navaneethan SD. Thromboprophylaxis in cancer patients with central venous catheters. A systematic review and meta-analysis. Thromb Haemost. 2008;99:38–43. doi: 10.1160/TH07-07-0446. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham MS, White B, Hollywood D, O’Donnell J. Primary thromboprophylaxis for cancer patients with central venous catheters--A reappraisal of the evidence. Br J Cancer. 2006;94:189–94. doi: 10.1038/sj.bjc.6602917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonczek R, Nurse BA. Management of Port-a-Cath devices in long-term acute care hospitals. Rehabil Nurs. 2012;37:307–11. doi: 10.1002/rnj.57. [DOI] [PubMed] [Google Scholar]
- 23.Karthaus M, Kretzschmar A, Kroning H, Biakhov M, Irwin D, Marschner N, et al. Dalteparin for prevention of catheter-related complications in cancer patients with central venous catheters: Final results of a double-blind, placebo-controlled phase III trial. Ann Oncol. 2006;17:289–96. doi: 10.1093/annonc/mdj059. [DOI] [PubMed] [Google Scholar]
- 24.Sousa B, Furlanetto J, Hutka M, Gouveia P, Wuerstlein R, Mariz JM, et al. Central venous access in oncology: ESMO Clinical Practice Guidelines. Ann Oncol. 2015;26:v152–68. doi: 10.1093/annonc/mdv296. [DOI] [PubMed] [Google Scholar]
- 25.LaRoy JR, White SB, Jayakrishnan T, Dybul S, Ungerer D, Turaga K, et al. Cost and morbidity analysis of chest port insertion: Interventional Radiology Suite Versus Operating Room. J Am Coll Radiol. 2015;12:563–71. doi: 10.1016/j.jacr.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sticca RP, Dewing BD, Harris JD. Outcomes of surgical and radiologic placed implantable central venous access ports. Am J Surg. 2009;198:829–33. doi: 10.1016/j.amjsurg.2009.04.031. [DOI] [PubMed] [Google Scholar]