Abstract
Homing-based gene drives, engineered using CRISPR/Cas9, have been proposed to spread desirable genes throughout populations. However, invasion of such drives can be hindered by the accumulation of resistant alleles. To limit this obstacle, we engineer a confinable population modification home-and-rescue (HomeR) drive in Drosophila targeting an essential gene. In our experiments, resistant alleles that disrupt the target gene function were recessive lethal and therefore disadvantaged. We demonstrate that HomeR can achieve an increase in frequency in population cage experiments, but that fitness costs due to the Cas9 insertion limit drive efficacy. Finally, we conduct mathematical modeling comparing HomeR to contemporary gene drive architectures for population modification over wide ranges of fitness costs, transmission rates, and release regimens. HomeR could potentially be adapted to other species, as a means for safe, confinable, modification of wild populations.
Research organism: D. melanogaster
Introduction
Effective insect control strategies are necessary for preventing human diseases, such as malaria and dengue virus, and protecting crops from pests. These challenges have fostered the development of innovative population control technologies such as Cas9/guideRNA (Cas9/gRNA) homing-based gene drives (HGDs) (Champer et al., 2016; Esvelt et al., 2014) which have been laboratory-tested for either population modification (Adolfi et al., 2020; Carballar-Lejarazú et al., 2020; Gantz et al., 2015; Li et al., 2020; Pham et al., 2019) to spread desirable traits that can impair the mosquitoes’ ability to transmit pathogens (e.g. Buchman et al., 2020; Buchman et al., 2019; Hoermann et al., 2020; Isaacs et al., 2012; Marshall et al., 2019) or population suppression (Hammond et al., 2016; Kyrou et al., 2018; Simoni et al., 2020) to reduce and eliminate wild disease-transmitting populations of mosquitoes. Despite significant progress, HGDs are still an emerging technology that can suffer from the formation of resistant alleles, hindering their efficacy (Adolfi et al., 2020; Carballar-Lejarazú et al., 2020; Gantz et al., 2015; Hammond et al., 2016; Kandul et al., 2020; Kyrou et al., 2018; Li et al., 2020; Pham et al., 2019; Simoni et al., 2020).
In CRISPR/Cas9, the Cas9 endonuclease cuts a programmed DNA sequence complementary to a user-defined short guide RNA molecule (gRNA). To engineer an HGD, leveraging creative designs originally proposed by Burt, 2003, CRISPR components are integrated at the target site in the genome. These components are configured so that when they cut the recipient wildtype (wt) allele, it is repaired via homology-directed repair (HDR) in heterozygotes, using the donor allele (i.e. allele harboring the HGD) as a template for DNA repair. This enables the HGD to home, or copy, itself into the recipient allele (Alphey et al., 2020; Champer et al., 2016; Esvelt et al., 2014) (referred to as homing from hereon). This general architecture for HGD was quickly adopted, and many HGDs were developed in several insect species (Gantz et al., 2015; Hammond et al., 2016; Kandul et al., 2020; Kyrou et al., 2018; Li et al., 2020; Simoni et al., 2020; Verkuijl et al., 2020). However, it soon became widely apparent that HGDs unintentionally promote the formation of resistant alleles through mutagenic repair. When these alleles are positively selected, they can hinder HGD spread in laboratory cage populations (Champer et al., 2017; Hammond et al., 2017; Kandul et al., 2020; KaramiNejadRanjbar et al., 2018; Oberhofer et al., 2018), with one exception that targeted a conserved sex determination gene for population suppression (Kyrou et al., 2018; Simoni et al., 2020). This resistance arises from Cas9/gRNA-directed DNA cuts being repaired by alternative DNA end-joining (EJ) repair pathways, including non-homologous (NHEJ) and microhomology-mediated end-joining (MMEJ), which can introduce insertions or deletions (indels) at the target site(s). Many of these indels produce loss-of-function (LOF) alleles, which can be selected against if deleterious to the organism. However, functional in-frame EJ-induced indel alleles can also be generated, which are unrecognized by the same Cas9/gRNA complex and become drive resistant alleles. When resistant alleles are induced in germ cells, they are heritable and can hinder spread of HGDs (Champer et al., 2017; Hammond et al., 2017; Kandul et al., 2020; KaramiNejadRanjbar et al., 2018; Oberhofer et al., 2018). Both induced and naturally existing resistant alleles can pose significant challenges to engineering a stable HGD capable of spreading and persisting long term in a population.
To overcome the accumulation of drive resistant alleles, CRISPR-based toxin-antidote (TA) drives, in which embryos are essentially ‘poisoned’ and only those embryos harboring the TA genetic cassette are rescued, were described (Figure S8 in Kandul et al., 2019) and engineered (Champer et al., 2020a; Oberhofer et al., 2020a, Oberhofer et al., 2020b, Oberhofer et al., 2019). Generally these designs utilize a toxin consisting of a non-HGD harboring multiple gRNAs targeting a vital gene, and an ‘addictive’ antidote that is a re-coded, cleavage-immune version of the targeted gene. These TA-based drives are Mendelianly transmitted and spread instead by killing progeny that fail to inherit the drive (e.g. 50% perish from heterozygous mother). Alternative HDR-based TA designs were also described (Champer et al., 2016; Esvelt et al., 2014), modeled (Noble et al., 2017), and recently tested in mosquitoes (Adolfi et al., 2020) targeting a recessive non-essential eye pigmentation gene, and in Drosophila melanogaster targeting either haplosufficient (i.e. the non-functional allele is recessive as a single functional copy of the target gene is sufficient for normal function) genes (Terradas et al., 2021) or a rare haploinsufficient (i.e. the non-functional allele is dominant as a single functional copy of the target gene is not sufficient for normal function) gene (Champer et al., 2020b), each demonstrating drive capacity.
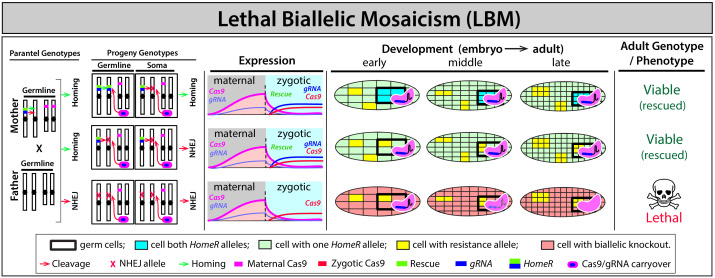
Building upon prior work, here we describe the development of a home-and-rescue (HomeR) split-drive (i.e. Cas9 separated from the drive) targeting an essential, haplosufficient gene in D. melanogaster. We demonstrate that the accumulation of EJ-induced resistant alleles can be reduced by strategically following four design criteria. First, designing the HGD to target the 3’ coding sequence of an essential gene required for insect viability. Second, encoding a dominant rescue of the endogenous target gene into HomeR. Third, using an exogenous 3’ UTR to prevent expected deleterious recombination events between the drive and the endogenous target gene. Fourth, by exploiting a process we previously first described as lethal biallelic mosaicism (LBM) in which maternal carryover of Cas9/gRNA complexes contributes to RNA-guided dominant biallelic disruption of an essential target gene throughout development thereby ensuring recessive non-functional resistant alleles result in dominant deleterious/lethal mutations that can get negatively selected out of a population (Kandul et al., 2019). Importantly, individuals that inherit the drive allele express a dominant re-coded rescue and are protected from LBM. We demonstrate that efficient cleavage of the target sequence by HomeR and rescue are requisites to achieve nearly ~100% transmission in the presence of Cas9, which is accomplished mostly by homing in trans-heterozygous females. Further, we perform multigenerational population drive experiments demonstrating long-term stability and efficient Cas9-dependent drive. Finally, we conduct comprehensive mathematical modeling to demonstrate that HomeR can outperform contemporary gene drive systems for population modification over wide ranges of fitness, transmission rates, introduction frequencies, and release regimens. Given the simplistic design, this system could be adapted to other species.
Results
Selection of PolG2 as a HomeR drive target
To develop a HomeR-based drive, we first identified an essential haplosufficient gene to target. We chose DNA Polymerase gamma subunit 2 (PolG2, DNA polymerase γ 35 kDa, CG33650), required for the replication and repair of mitochondrial DNA (mtDNA; Carrodeguas and Bogenhagen, 2000; Carrodeguas et al., 2001) whose LOF results in lethality (Iyengar et al., 2002). PolG2 encodes the small subunit of the mtDNA polymerase gamma, acting together with the large subunit 1 (PolG1, DNA polymerase γ 125 kDa, CG8987) for the replication and repair of the mitochondrial genome (Carrodeguas and Bogenhagen, 2000; Carrodeguas et al., 2001). PolG2 is a short conserved gene with an ~130 amino acids (AA) C-terminal domain (cd02426; Lu et al., 2020) sharing ~55% AA identity with the human PolG2 (Lecrenier et al., 1997; Figure 1—figure supplement 1A–B). Importantly, Drosophila PolG2 LOF mutations are known to confer lethality at the early pupal stage (Iyengar et al., 2002). The C-terminal location of the functional domain in PolG2 facilitates minimal re-coding, making PolG2 an optimal target for a HomeR gene drive (Figure 1A, Figure 1—figure supplement 1A).
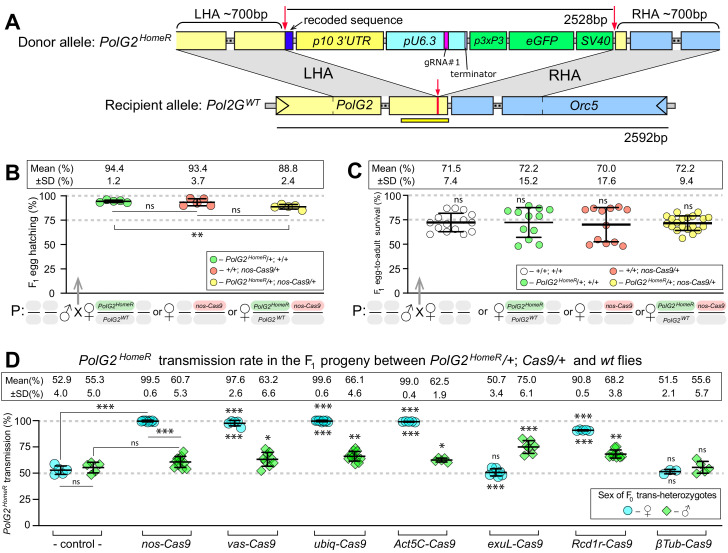
Figure 1. HomeR homes in the presence of Cas9 and biases its transmission in females to nearly 100%.
(A) Schematic maps of the PolG2 donor allele harboring HomeR integrated at the gRNA#1PolG2 cut site (PolG2HomeR, Figure 1—figure supplement 1A), and the recipient wildtype (wt) allele (PolG2WT) encompassing the area spanning PolG2 and Orc5 (CG7833) genes. To facilitate site-specific integration, the HomeR element is surrounded by the left and right homology arms (LHA and RHA) from the Cas9/guideRNA (Cas9/gRNA) cut site (red arrows and lines) in the wt allele. The re-coded 3’ end sequence of PolG2 is shown in dark blue. The yellow line under the PolG2WT recipient allele depicts the location of the C-terminal domain (Figure 1—figure supplement 1A). (B) Embryonic lethality of PolG2WT alleles cannot result in the nearly 100% transmission of PolG2HomeR. (C) The egg-to-adult survival rate indicates that the developmental lethality of PolG2WT alleles also cannot account for the preferential transmission of PolG2HomeR. Therefore, the homing of PolG2HomeR into PolG2WT alleles causes the super-Mendelian transmission of HomeR. (D) PolG2HomeR supports super-Mendelian transmission in conjunction with different Cas9 transgenes and/or maternal carryover of Cas9 protein (Figure 1—figure supplement 3). Trans-heterozygous females (♀) and males (♂) harboring paternal Cas9 expressed under different promoters were mated to wt flies of the opposite sex, and F1 progeny were scored for the GFP dominant marker of PolG2HomeR. The transmission rate was compared to that in PolG2HomeR/PolG2WT; +/+ flies without Cas9 (control) of the corresponding sex (statistical significance indicated above data points). In addition, the transmission rate by trans-heterozygous females was compared to that of trans-heterozygous males for each Cas9 promoter (statistical significance indicated below data points). Notably, while PolG2HomeR can bias its transmission in both sexes, the highest transmission rate is achieved in Drosophila females: 99.6 ± 0.6% in ♀ vs. 75.0 ± 6.1% in ♂. Plots show the mean ± SD over at least five biological replicates. Statistical significance was estimated using a two-sided Student’s t test with equal variance (p ≥ 0.05ns, p<0.05*, p<0.01**, and p<0.001***).
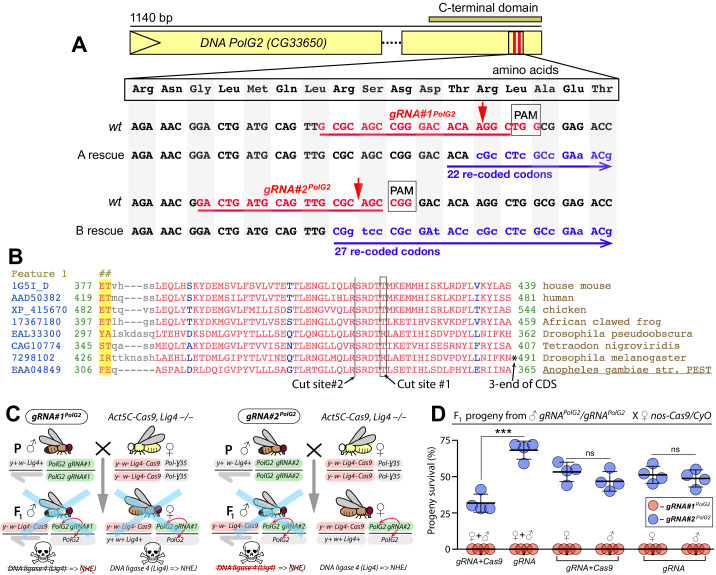
Figure 1—figure supplement 1. Assessing gRNAs for Cas9-mediated cleavage of PolG2.
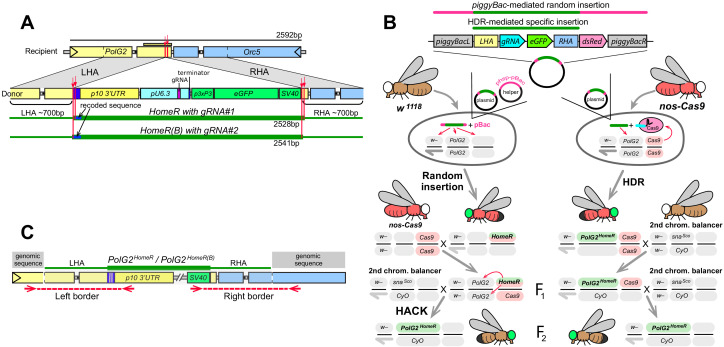
Figure 1—figure supplement 2. Site-specific integration of HomeR constructs at the PolG2 locus.
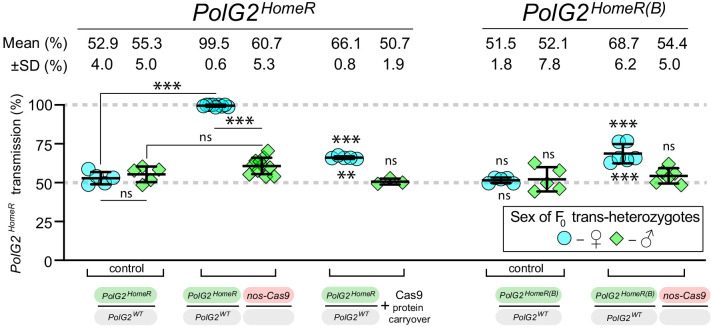
Figure 1—figure supplement 3. The gRNA efficiency to guide Cas9-mediated cleavage limits the transmission rate for PolG2HomeR.
Figure 1—figure supplement 4. Mechanism of lethal biallelic mosaicism (LBM).
Assessment of gRNAs targeting PolG2
Given that separate gRNAs can result in varying degrees of cleavage efficiencies (Kandul et al., 2019), we tested two separate gRNAs targeting the C-terminal domain of PolG2 (gRNA#1PolG2 and gRNA#2PolG2) (Figure 1—figure supplement 1A–B). According to the D. melanogaster Genetic Reference Panel 2 (DGRP2) that includes natural variation in genome architecture among 205 D. melanogaster lines (Huang et al., 2014; Mackay et al., 2012), both gRNA target sequences are completely devoid of any single nucleotide polymorphisms (SNPs) indicating a high degree of conservation. To genetically assess the efficiency of Cas9/gRNA-mediated cleavage induced by each gRNA, we crossed these established gRNA lines to two separate Cas9 expressing lines including: (i) a previously characterized ubiquitously expressing Cas9 line (Port et al., 2014) in the DNA ligase four null genetic background (Act5C-Cas9; Lig4–/–) (Zhang et al., 2014), and a (ii) germline-enriched Cas9 driven by the nanos promoter (nos-Cas9) (Kandul et al., 2020; Kandul et al., 2019; Figure 1—figure supplement 1CD). We tested the Cas9/gRNA-mediated cleavage in a Lig4–/– background, to decrease the activity of DNA repair by the NHEJ pathway (McVey et al., 2004). As the Lig4 gene is located on the X chromosome, maternal Lig4– alleles will be inherited by all male progeny, making them hemizygous Lig4– mutants, while females are heterozygous Lig4–/+.
We observed that the genetic cross between either gRNA#1PolG2 or gRNA#2PolG2 homozygous males to Act5C-Cas9, Lig4 –/– homozygous females was lethal for all male progeny (Figure 1—figure supplement 1C, Supplementary file 2). Notably, gRNA#1PolG2 also induced lethality in trans-heterozygous females harboring Act5C-Cas9 in the Lig4 +/– genetic background, suggesting that gRNA#1PolG2 is likely more potent. Furthermore, we found that the Cas9 protein deposited by nos-Cas9/+ females without inheritance of the nos-Cas9 transgene, referred to as maternal carryover (Kandul et al., 2019; Lin and Potter, 2016), was sufficient to ensure lethality of the F1 progeny harboring gRNA#1PolG2, while gRNA#2PolG2 induced lethality only in a fraction of the F1 gRNA#2PolG2/nos-Cas9 trans-heterozygous flies, independent of sex (Figure 1—figure supplement 1D, Supplementary file 3). Sanger sequencing of trans-heterozygous pupae revealed expected mutations at PolG2 gRNA target sites. As we previously first described, the mechanism ensuring lethality results from a dominant process we coined LBM (Kandul et al., 2019), in which maternal carryover/zygotic expression results in mosaic target gene cleavage throughout development leading to wide scale loss of target gene function which can be detrimental to viability of the organism if essential genes are targeted. Taken together, these results indicate that both tested gRNAs induced cleavage of the PolG2 target sequences, though gRNA#1PolG2 induced greater cleavage than gRNA#2PolG2 as evidenced by complete lethality of females and males with both sources of Cas9.
Development of split HomeR drives with encoded rescue
Using these characterized gRNAs described above (gRNA#1PolG2 or gRNA#2PolG2), we engineered two Pol2 HomeR (HomeRPolG2 and HomeR(B)PolG2) drives, respectively. Fitting with the split-drive (i.e. two-locus) design (Champer et al., 2020a; Kandul et al., 2020; Li et al., 2020), neither HomeRPolG2 nor HomeR(B)PolG2 include the Cas9 gene and thus are inherently confinable drives (Akbari et al., 2015; Champer et al., 2016; Esvelt et al., 2014; Marshall and Akbari, 2018). To mediate HDR, both HomeR constructs include left and right homology arms (LHA and RHA) matching the sequences surrounding the cut site of the corresponding gRNA. The LHA includes a carefully re-coded sequence of 22 or 27 AA downstream from the cut site #1 or #2 (Figure 1—figure supplement 1A–B), and a p10 3’ UTR to support robust expression of the re-coded PolG2 and to prevent gene conversion between the rescue HomeR allele and the endogenous allele, which proved problematic in previous drive design architectures (Champer et al., 2020a; Champer et al., 2020b). Additionally, we included a dominant 3xP3-eGFP-SV40 marker gene to visually track the presence of HomeR (Figure 1—figure supplement 2).
Two different approaches were used to generate transgenic lines harboring site-specific integrations of HomeRPolG2 and HomeR(B)PolG2 constructs at the corresponding cut sites in PolG2 (termed PolG2HomeR and PolG2HomeR(B) when integrated into genome). In the first approach, the constructs were initially randomly integrated into the genome and then relocated precisely into the PolG2 cut sites via Homology Assisted CRISPR Knock-in (HACK; Gantz and Akbari, 2018; Lin and Potter, 2016). In the second approach, the constructs were directly integrated into the PolG2 cut sites by injecting them into nos-Cas9 embryos (Kandul et al., 2019; Figure 1—figure supplement 2B). Using both approaches, multiple independent transgenic lines of each PolG2HomeR and PolG2HomeR(B) were generated. To confirm that PolG2HomeR or PolG2HomeR(B) lines were indeed inserted precisely at the corresponding target site in PolG2, we assessed their ability for super-Mendelian inheritance in the presence of Cas9 in trans. Establishment of pure breeding, viable homozygous stocks of PolG2HomeR/PolG2HomeR and PolG2HomeR(B)/PolG2HomeR(B), demonstrated a functional rescue of wt PolG2 function. Finally, we Sanger-sequenced the junction sites (Figure 1—figure supplement 2C) and molecularly confirmed the precision of HDR-mediated insertions.
Assessment of germline transmission and cleavage rates
To assess the effect of gRNA-mediated cleavage efficiency on the inheritance of HomeR, we compared the two HomeRs, as they harbored two distinct gRNA sequences that differed in cleavage efficiencies. The PolG2HomeR and PolG2HomeR(B) lines encode gRNA#1PolG2 and RNA#2PolG2, respectively, with slightly different LHA and RHA corresponding to their respective gRNA cut sites (Figure 1—figure supplement 2A). We found that PolG2HomeR/+; nos-Cas9/+ trans-heterozygous females crossed to wt males transmitted PolG2HomeR to 99.5 ± 0.6% of progeny, while PolG2HomeR(B)/+; nos-Cas9/+ females transmitted the corresponding PolG2HomeR(B) to a significantly lower fraction of F1 progeny (68.7 ± 6.2%, two-sided Student’s t test with equal variances, p<0.0001; Figure 1—figure supplement 3). Genetic crosses of either PolG2HomeR/+; nos-Cas9/+ or PolG2HomeR(B)/+; nos-Cas9/+ trans-heterozygous males to wt females did not result in significant biased transmission to F1 progeny (60.7 ± 5.3% vs. 52.9 ± 4.0% or 54.3 ± 4.0% vs. 51.5 ± 1.8%, respectively, two-sided Student’s t test with equal variances, p>0.05; Figure 1—figure supplement 3, Supplementary file 4–5). Maternal carryover of Cas9 protein by nos-Cas9/+ females significantly increased transmission of PolG2HomeR by F1 PolG2HomeR/CyO females, 66.1 ± 0.8% vs. 52.9 ± 4.0% (two-sided Student’s t test with equal variances, p<0.001; Figure 1—figure supplement 3, Supplementary file 4). These results suggest that the higher efficiently of gRNA#1PolG2 to guide the Cas9-mediated PolG2 disruption, which results in lethality (Figure 1—figure supplement 1C–D), likely contributes to the higher transmission rates of PolG2HomeR, and underscores the importance of selecting an efficient gRNA for engineering gene drives.
HomeR biases its inheritance predominantly by homing
We hypothesized that either homing (indicating allelic conversion) in germ cells (Figure 1A) or ‘destruction’ of wt alleles in the progeny of trans-heterozygous PolG2HomeR/+; Cas9/+ females via LBM (Figure 1—figure supplement 4; Kandul et al., 2019) could contribute to biased PolG2HomeR transmission rates. LBM contributes to dominant biallelic disruption of the target gene throughout development thereby ensuring recessive non-functional resistant alleles (R2 type) result in dominant deleterious/lethal mutations that can get selected out of a population (Figure 1—figure supplement 4). Previously, destruction of the wt allele in conjunction with maternal carryover of a ‘toxin’ was used to engineer gene drives based on an ‘addictive’ TA approach (Champer et al., 2020a; Oberhofer et al., 2020a, Oberhofer et al., 2019). In these TA drives, one half of the F1 progeny did not inherit the TA cassette, that is, not rescued, and were killed—ensuring survival of only progeny inheriting the drive resulting in a rapid spread of the genetic cassette throughout laboratory populations.
To explore the mechanism resulting in the super-Mendelian inheritance of PolG2HomeR, we determined the egg hatching and egg-to-adult survival rates for the progeny of trans-heterozygous females and compared it to those of females heterozygous for PolG2HomeR or just Cas9 (Figure 1B,C). The hatching rate of F1 eggs generated by PolG2HomeR/PolG2WT; nos-Cas9/+ trans-heterozygous females crossed to wt males was reduced by 5.6% as compared to that of PolG2HomeR/PolG2WT; +/+ heterozygous females (88.8 ± 2.4% vs. 94.4 ± 1.2%; two-sided Student’s t test with equal variances, p<0.004, Figure 1B) and slightly lower than +/+; nos-Cas9/+ heterozygous females (88.8 ± 2.4% vs. 93.4 ± 3.7%; two-sided Student’s t test with equal variances, p=0.052; Figure 1B, Supplementary file 6), suggesting some degree of embryo killing. Furthermore, we observed no significant difference among egg-to-adult survival rates estimated for four female types crossed to wt males: 72.2 ± 9.4% for PolG2HomeR/PolG2WT; nos-Cas9/+ ♀, 72.2 ± 15.2% for PolG2HomeR/+ ♀, 70.0 ± 17.6% for nos-Cas9/+ ♀, and 71.5 ± 7.4% for wt ♀ (Figure 1C, Supplementary file 7). Taken together, these data indicate that only a small fraction of PolG2WT alleles transmitted by trans-heterozygous females were ‘destroyed’ via LBM—meaning mutated and not complemented by the paternal PolG2WT allele, since it was also mutated by Cas9/gRNA maternal carryover (Figure 1—figure supplement 4). Therefore, the HomeR transmission of 99.5% by the PolG2HomeR/PolG2WT; nos-Cas9/+ females could not be explained simply by the ‘destruction’ of PolG2WT alleles, which would result in the lethality of 50% progeny as in cleave and rescue (ClvR; Oberhofer et al., 2020a; Oberhofer et al., 2020b; Oberhofer et al., 2019) and toxin-antidote recessive embryo (TARE; Champer et al., 2020a) drives. Instead, HomeR biases its transmission predominantly by homing (i.e. allelic conversion of PolG2WT into PolG2HomeR) from trans-heterozygous females (Figure 1A).
HomeR exhibits the strong transmission bias from females
The split-drive design facilitates testing of different Cas9 promoters. Therefore, we quantified the transmission of PolG2HomeR from either trans-heterozygous females or males harboring PolG2HomeR in combination with four Cas9 promoters active in germ cells of both sexes (Figure 1D). Nanos (nos) and vasa (vas) promoters were previously described as germline-specific promoters active in both sexes (Hay et al., 1988; Sano et al., 2002; Van Doren et al., 1998), though recent evidence indicates ectopic expression in somatic tissues from both nos-Cas9 and vas-Cas9 (Kandul et al., 2020; Kandul et al., 2019). The Ubiquitin 63E (ubiq) and Actin 5C (Act5C) promoters support strong expression in both somatic and germ cells (Kandul et al., 2020; Kandul et al., 2019; Port et al., 2014; Preston et al., 2006). Since maternal carryover of the Cas9 protein was shown to induce a ‘shadow drive’ two generations later (Guichard et al., 2019; Kandul et al., 2020), we used trans-heterozygous flies that inherited paternal Cas9 to quantify the transmission of PolG2HomeR. Trans-heterozygous females harboring PolG2HomeR together with nos-Cas9, vas-Cas9, ubiq-Cas9, or Act5C-Cas9 crossed to wt males biased transmission of PolG2HomeR to nearly ~100% of F1 progeny (99.5 ± 0.6%, 97.6 ± 2.6%, 99.6 ± 0.6%, and 99.0 ± 0.4%, respectively, vs. 52.9 ± 4.0% by PolG2HomeR/PolG2WT; +/+ females, two-sided Student’s t test with equal variances, p<0.001; Figure 1D). Note that the corresponding trans-heterozygous males only modestly biased PolG2HomeR transmission from 55.3 ± 5.0% of F1 progeny to 60.7 ± 5.3% (p>0.05), 63.2 ± 6.6% (p<0.03), 66.1 ± 4.6% (p<0.004), and 62.0 ± 1.7% (p<0.017, two-sided Student’s t test with equal variances, Figure 1D, Supplementary file 4), respectively.
To assess whether males could support robust homing, we investigated three alternative male-specific promoters. We established the Drosophila exuperantia (CG8994) large fragment (exuL) promoter for an early male-specific expression. The Rcd-1 related (Rcd1r, CG9573; Chan et al., 2013) and βTubulin 85D (βTub; Chan et al., 2011; Michiels et al., 1989) promoters support an early and late, respectively, testis-specific expression in Drosophila males. We found that only exuL-Cas9 induced the male-specific super-Mendelian inheritance of PolG2HomeR; trans-heterozygous males, but not females, transmitted PolG2HomeR to more than 50% of F1 progeny (75.0 ± 6.1% vs. 55.3 ± 5.0% in ♂, p<0.0001; and 50.7 ± 3.4% vs. 52.8 ± 4.0% in ♀, p>0.05, two-sided Student’s t test with equal variances; Figure 1D, Supplementary file 4). To our surprise, Rcd1r-Cas9 induced super-Mendelian inheritance of PolG2HomeR in both trans-heterozygous males and females (68.2 ± 3.8% vs. 55.3 ± 5.0% in ♂, p<0.002; and 90.8 ± 0.5% vs. 52.8 ± 4.0% in ♀, p>0.0001, two-sided Student’s t test with equal variances; Figure 1D). Finally, βTub-Cas9 did not induce changes in transmission of PolG2HomeR by either trans-heterozygous males or females (55.6 ± 5.7% vs. 55.3 ± 5.0% in ♂, p=0.55; and 51.5 ± 2.1% vs. 52.9 ± 4.0% in ♀, p=0.94, two-sided Student’s t test with equal variances; Figure 1D, Supplementary file 4). These results suggest that Drosophila males bias PolG2HomeR transmission; however, this bias is substantially lower than the nearly ~100% transmission of PolG2HomeR in females.
Induced resistant alleles do not impede drive invasion
We reasoned that insertion of HomeR into an essential gene could enable spread into a population by biasing transmission while also inhibiting the accumulation of LOF resistant alleles (R2 type, PolG2R2) through a combination of slow-acting Mendelian selection and by LBM (Figure 1—figure supplement 4). However, functional resistant alleles (R1) that alter the AA sequence of the target can indeed still be generated (e.g. by EJ repair resulting from in-frame indels, or nonsynonymous base substitutions) and may hinder drive spread. While these mutations may partially rescue the function of the target gene resulting in viability, it has not escaped our attention that they may still confer detrimental fitness costs to their carriers resulting from incompletely preserving the function of the essential target gene due to the altered AA sequence(s) in a critical domain and may result in negative selection within a population. We therefore refer to these here as ‘non-silent R1’ or ‘non-silent PolGR1’ mutations. On the contrary, resistant alleles that alter the nucleotide sequence, but not the AA sequence, can also be generated, and these are referred to here as ‘silent R1’ or ‘silent PolGR1 mutations’. These silent R1 mutations are expected to be especially problematic to drive spread as these would not be predicted to impose fitness costs on homozygous carriers, and therefore they would be expected to spread at the expense of the drive. Notwithstanding, the generation of both types of R1 functional resistant alleles (non-silent/silent) is a shared problem of all HGDs developed to date.
To explore this potential, in addition to spread and stability, we initiated three multigenerational cage populations of heterozygous PolG2HomeR/+ flies (50% allelic frequency) in the nos-Cas9/nos-Cas9 genetic background and assessed if induced resistant alleles could impede drive invasion and stability in 10 discrete generations (Figure 2A). Functional resistant alleles (R1) were expected to be generated, especially at the earlier generations initiated with 50% wt alleles, and would be straightforward to score in our assay by a simple loss of the dominant GFP marker. Note that any viable GFP-negative (GFP–) adult flies must have at least one functional PolG2 allele to survive (either PolG2WT or PolG2R1).
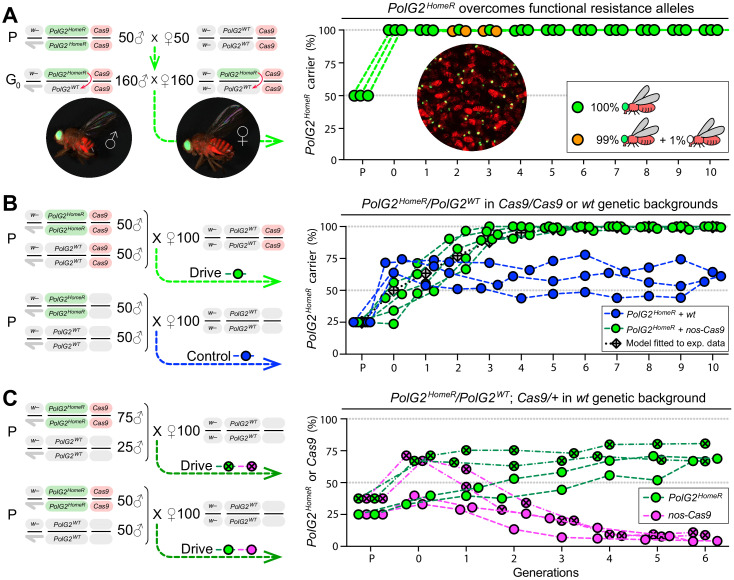
Figure 2. Induced resistant alleles do not impede the Cas9-dependent spread of PolG2HomeR.
(A) To explore the fate of induced functional resistant alleles (R1), three population cages were seeded with PolG2HomeR/PolG2WT heterozygous flies in nos-Cas9/nos-Cas9 genetic background and run for 10 discrete generations. PolG2HomeR and nos-Cas9 were tagged by dominant eye-specific GFP and body-specific dsRed, respectively. Images of an individual male (♂), female (♀), and a group of flies are shown. In total, nine PolG2HomeR-negative flies, as determined by the absence of eye-specific GFP, were identified at generations 2 and 3 in populations #1 and #3. After these flies participated in seeding the next generation, they were isolated and genotyped. Two functional PolG2R1 resistant alleles identified in these flies (Figure 2—figure supplement 1A) were not sampled in flies collected at generation 10 by Illumina amplicon sequencing (Figure 2—figure supplement 1B). (B) The PolG2HomeR allele spread efficiently in the homozygous Cas9 genetic background. Population drives were seeded with 50 PolG2HomeR/PolG2HomeR ♂, 50 wt ♂, and 100 wt virgin ♀ in the presence (green points) or absence (blue points) of nos-Cas9, and the carrier frequency of PolG2HomeR was scored at each discrete generation. The model for the HomeR population replacement drive (gray diamonds and a black dotted line) was fitted to the empirical data of the PolG2HomeR spread in the presence of nos-Cas9 (green points). After 10 generations, the PolG2HomeR allele spread from the introductory frequency of 25% to the carrier frequency of 99.9 ± 0.3% in the presence of nos-Cas (green points) or continued to drift at 60.8 ± 3.8% without the Cas9 transgene (blue points; p<0.0001, a two-sided Student’s t test with equal variance). (C) The invasion of PolG2HomeR is limited by the fitness of the Cas9 transgene. Double homozygous males at frequencies of 75% or 50% were released into the wt genetic background to establish four drive populations with mixtures of trans-heterozygous and wt flies at above or below 50%, respectively, in generation 0. Both PolG2HomeR and nos-Cas9 transgenes were scored at each generation by the GFP (green points) and dsRed (purple points) markers. The carrier frequency of nos-Cas9 decreased from 69.1% to 9.9% or from 36.4% to 4.0% in six generations confining the spread of PolG2HomeR.
Figure 2—figure supplement 1. PolG2 resistant alleles induced by PolG2HomeR in experimental populations.
In early generations 2 and 3, we sampled nine such flies lacking PolG2HomeR in two out of three populations (#1 and #3; Figure 2A, Supplementary file 8). To ensure that the PolG2R1 alleles had a chance to transmit and compete with PolG2HomeR alleles, these flies were transferred among the subsequent generation and allowed to mate with other flies and lay eggs in each population lineage before genotyping. As expected, we determined that each fly indeed harbored at least one PolG2R1 allele that rescued viability. Two distinct non-silent PolG2R1 alleles were identified, with one non-silent PolG2R1 type induced independently in two populations (Figure 2—figure supplement 1A). Since we did not find any fly without the PolG2HomeR allele after generation 3, it can be noted that the identified non-silent PolG2R1 resistant alleles did not impede drive invasion/stability. Despite this, we cannot rule out the possibility that these and other resistant alleles were still present at low frequencies in populations masked by PolG2HomeR alleles.
To further explore the diversity of resistant alleles remaining after 10 generations, we performed next-gen sequencing on 60 randomly selected GFP+ flies (note that each fly had at least one copy of PolG2HomeR) from each population to identify and quantify mutant PolG2 alleles, which did not harbor the large insert of HomeRPolG2 (~2.5 kb; Figure 1A). From nearly 150,000 sequence reads generated, we did not identify the two previously sampled functional non-silent PolG2R1 alleles (Figure 3—figure supplement 1A). Instead, we found two novel non-silent PolG2R1 in-frame indel alleles, 18 and 9 bp deletions, in populations #2 and #3 (Figure 2—figure supplement 1B). Additionally, we found 11 LOF PolG2R2 alleles harboring out-of-frame indels ranging from a 1 bp insertion to a 23 bp deletion (Figure 2—figure supplement 1B). Two PolG2R2 alleles, 2 and 4 bp deletions, were also seen in the genotyped flies at generations 2 and 3, suggesting that these may have persisted in the populations. The relative abundance of each allele can be used to extrapolate the minimum number of resistant alleles sampled in the 60 heterozygous and/or homozygous flies. We inferred that at least 9, 5, and 17 resistant alleles persisted for 10 generations and were rescued by the PolG2HomeR allele in 60 sampled flies from populations #1, #2, and #3, respectively (Figure 2—figure supplement 1B). Since a single PolG2HomeR allele rescues the wt function of PolG2 and can mask the opposite allele at the PolG2 locus from slow-acting purifying selection, it is not surprising that LOF resistant alleles can be found persisting in the population.
HomeR spreads in a Cas9-dependent manner
To evaluate drive efficacy in the presence of Cas9, we established five drive and three control (‘no-drive’) populations by seeding 50 homozygous PolG2HomeR males and 50 wt males together with 100 wt virgin females in the presence or absence of Cas9, respectively (Figure 2B). The introduction ratio of PolG2HomeR to PolG2WT was 1:2 (or 25% allele frequency) in the parental generation (P). Both types of homozygous PolG2HomeR males with and without Cas9 were able to compete with the corresponding wt males and sired 41.0 ± 12.4% (Supplementary file 9) and 70.0 ± 5.5% (Supplementary file 10) of progeny, respectively. Notably, the PolG2HomeR males were significantly less competitive with wt males for female mates in the nos-Cas9 genetic background than in the wt genetic background (p=0.01, two-sided Student’s t test with equal variances; Figure 2B). Nevertheless, the PolG2HomeR allele spread to the carrier frequency of 96.7 ± 4.4% in the presence of nos-Cas9 vs. 56.9 ± 11.6% without the Cas9 transgene in a time span of four generations (p=0.0004, a two-sided Student’s t test with equal variance; Supplementary file 9–10). At generation 10, the PolG2HomeR allele was fixed in four out of five drive populations and continued to drift at moderate frequency in three control populations in the absence of Cas9: 99.9 ± 0.3% vs. 60.8 ± 3.8%, respectively (p<0.0001, a two-sided Student’s t test with equal variance; Figure 2B, Supplementary file 9–10), underscoring Cas9 dependence for drive.
A few GFP– flies harboring PolG2R1 alleles appeared over multiple generations in drive populations #4 and #5 (Supplementary file 9). To assess the fertility of viable PolG2R1 carriers, we collected 7 GFP– females and 7 GFP– males at generation 9 and individually crossed them to wt flies of the corresponding sex. Interestingly, we found that the GFP– males were fertile, while each tested GFP– female died in 3 days without producing progeny suggesting that the sampled PolG2R1 allele(s) incurred fitness costs to female carriers. Dead females were genotyped and we identified four non-silent R1 alleles that rescued their ‘short-lived’ viability (Figure 2—figure supplement 1C). Notably one non-silent R1 allele was already sampled in the GFP– flies from heterozygous population #1 (R1#2 in Figure 2—figure supplement 1C). Each tested GFP– male was fertile, and four genotype males had the same non-silent allele identified in the females (R1#3 in Figure 2—figure supplement 1C). In summary, all in-frame resistant alleles identified in this study resulted in AA changes and are non-silent and importantly we did not sample any silent PolG2R1 mutant alleles.
Fitting a mathematical model of CRISPR/Cas9-based homing drive to the observed cage data in Figure 2B (see 'Materials and methods'), we found the data to be consistent with cleavage efficiencies in females and males of 99.2% (95% credible interval [CrI] 96.4–100%) and 99.6% (95% CrI 98.2–100%), respectively, and a frequency of accurate HDR, given cleavage, in females and males of 99.5% (95% CrI 97.8–100%) and 9.6% (95% CrI 8.0–10.0%), respectively. When accurate HDR did not occur, the data were consistent with 2.9% (95% CrI 1.9–4.0%) of resistant alleles being in-frame, and the remainder being out-of-frame or otherwise costly LOF alleles. Individuals having the HomeR system were found to have a negligible fitness cost of 0.3% (95% CrI 0.0–1.4%), while individuals homozygous for the LOF allele were modeled as completely unviable. The fitted parameter estimates are consistent with parameters estimated from individual pair crossings (Figure 1D).
The spread of HomeR is confined by the fitness of Cas9
In the split-drive (i.e. two-locus) design, the continued spread of HomeR is contingent on the availability of Cas9 which ensures confinability. Therefore, to explore the invasion potential of HomeR under a limited supply of Cas9, we seeded additional drive populations with double homozygous and wt flies, and scored both PolG2HomeR and Cas9 at each generation. Four populations with PolG2HomeR/PolG2HomeR; nos-Cas9/nos-cas9 and wt (PolG2WT/PolG2WT; +/+) males mixed at 1:1 (two replicates; 25% allele frequency) or 3:1 ratios (two replicates; 37.5% allele frequency) and 100 wt virgin females were seeded (Figure 2C). These ratios generated two population types with frequencies of trans-heterozygous flies below and above 50% in the subsequent generation: 36.4 ± 4.8% and 69.1 ± 3.0% (generation 0 in Figure 2C, Supplementary file 11). After tracking these populations for six generations, we found that frequencies of the PolG2HomeR allele gradually increased to 67.9 ± 1.3% and 73.7 ± 9.8%, respectively, and persisted near these frequencies. Notably, we also observed that the frequency of nos-Cas9 decreased each generation down to 4.1 ± 0.2% or 9.9 ± 1.6% in two population types by generation 6 (Figure 2C, Supplementary file 11). These results suggest that the nos-Cas9 incurred fitness costs on its carrier and was therefore negatively selected out from the population. Furthermore, these experiments underscore the significant fitness cost Cas9 can impose, and indicate that a single release, at the introduction thresholds used here, would be insufficient to achieve fixation of PolG2HomeR given such immense costs to Cas9. We therefore use mathematical modeling to explore multi-release scenarios (below).
Modeling indicates that HomeR is an efficacious gene drive
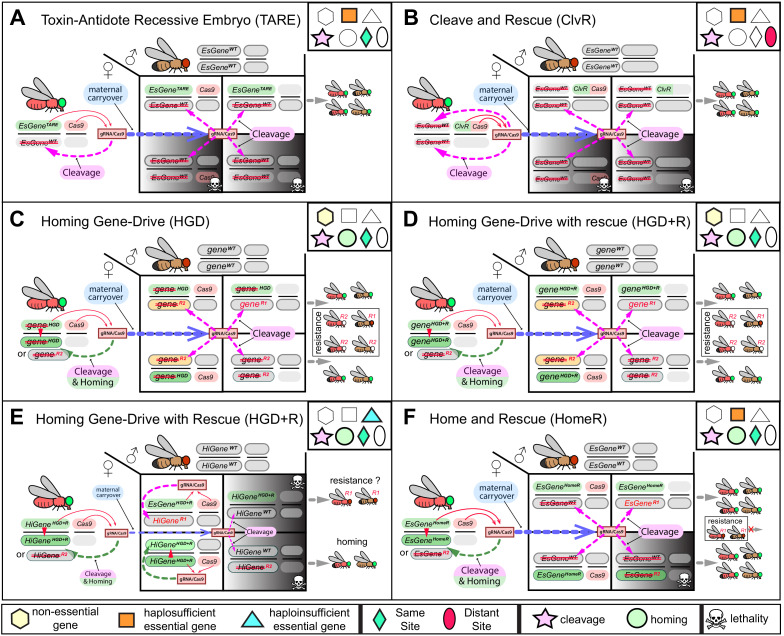
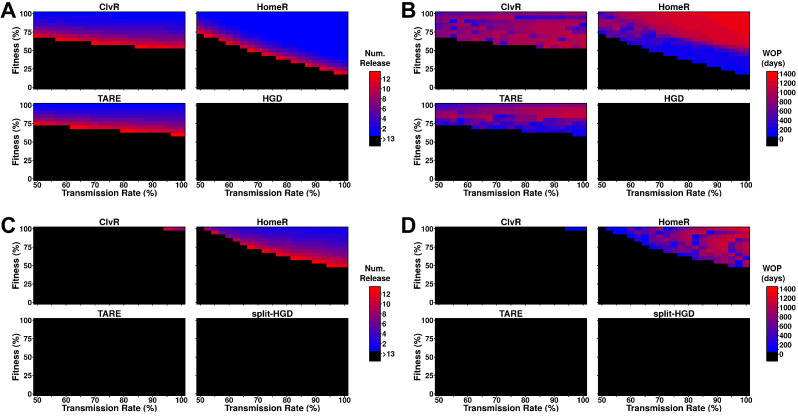
To compare the performance of HomeR against contemporary gene drive systems for population modification, we modeled one- (i.e. autonomous, linked-Cas9) and two-locus (i.e. split-drive) versions of ClvR (Faber et al., 2020; Oberhofer et al., 2020a, Oberhofer et al., 2020b, Oberhofer et al., 2019), the one-locus TARE system from Champer et al., 2020a, as well as a two-locus TARE configuration based on their design, an HGD targeting a non-essential gene (Gantz et al., 2015; Hammond et al., 2016), and HomeR (for mechanistic comparisons of these systems, see Figure 3—figure supplement 1). In each case, we first simulated population spread of each gene drive system for an ideal parameterization (see 'Materials and methods' for more details) and included additional simulations for HomeR under current experimentally derived parameters (HomeR-exp; Figure 3A and C, parameters consistent with Figure 2C). To gauge behavior across a range of scenarios, we performed simulations for a range of fitness costs (implemented as female fecundity reduction) and drive system transmission rates (implemented by varying the cleavage rate), providing heatmaps of the expected performance for each drive system at each parameter combination (Figure 3B and D). Drive efficacy, the outcome in these comparisons, is defined as the expected fraction of individuals that carry the effector allele, in either heterozygous or homozygous form, at 20 generations following a 25% release of male homozygotes for each drive system.
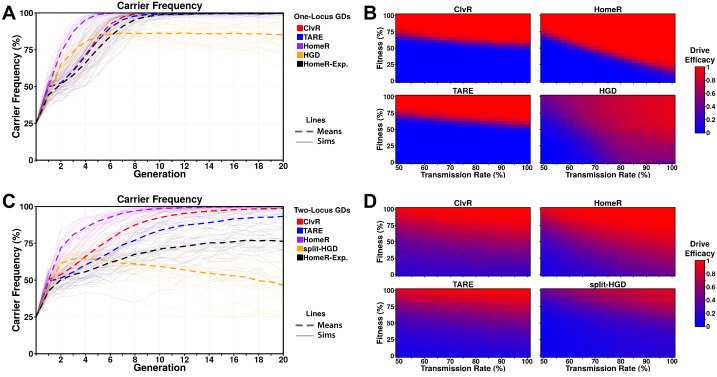
Figure 3. Performance of contemporary gene drive systems for population modification with a single release.
(A) Simulations of carrier frequency trajectories (i.e. heterozygotes and homozygotes) for one-locus versions of ClvR, TARE, HomeR, and HGD for ideal parameters (see 'Materials and methods'), and HomeR for experimental parameters (HomeR-Exp, see 'Materials and methods'). Twenty-five repetitions (lighter lines) were used to calculate the average behavior of each drive (thicker, dashed lines). Populations were initialized with 50% wildtype (+/+) adult females, 25% wildtype (+/+) adult males, and 25% drive homozygous (drive/drive) males. (B) Heatmaps depicting drive efficacy for one-locus versions of ClvR, TARE, HomeR, and HGD for a range of fitness and transmission rate parameter values. Fitness costs were incorporated as a dominant, female-specific fecundity reduction. Transmission rate was varied based on cleavage rate, using HDR rates consistent with ideal parameters, when applicable (see 'Materials and methods'). Drive efficacy is defined as the average carrier frequency at generation 20 (approximately 1 year, given a generation period of 2-3 weeks) based on 100 stochastic simulations with the same initial conditions as (A). (C) Simulations of carrier frequency trajectories for two-locus (split-drive) versions of ClvR, TARE, HomeR, and HGD for ideal parameters (see 'Materials and methods'), and HomeR for experimental parameters (HomeR-Exp, see 'Materials and methods'). Twenty-five repetitions (lighter lines) were used to calculate the average behavior of each drive (thicker, dashed lines). Populations were initialized with 50% wildtype (+/+; +/+) adult females, 25% wildtype (+/+; +/+) males, and 25% drive homozygous (Cas9/Cas9; gRNA/gRNA) males. (D) Heatmaps depicting drive efficacy for two-locus versions of ClvR, TARE, HomeR, and HGD for a range of fitness and transmission rate parameter values, implemented as in panel (B), with initial conditions given in (C).
Figure 3—figure supplement 1. Mechanistic comparison of contemporary split-drives for population modification.
When one-locus gene drive (GD) systems are compared for ideal parameter values, HomeR outperforms all other GDs in terms of speed of spread, and reaches near fixation in terms of carrier frequency, as do ClvR and TARE (Figure 3A). HGD displays a similar speed of spread to HomeR initially; however, fitness costs from the targeted gene disruption and LOF (R2) alleles slow the introgression and allow functional resistance alleles (R1) to build up over time, preventing fixation. The HomeR design overcomes this fitness reduction and R2 allele build-up by rescuing the wt function of a targeted essential gene (Figure 3—figure supplement 1). ClvR and TARE perform similarly to each other for ideal parameter values, but reach near carrier fixation ~4 generations after HomeR does for ideal parameter values (Figure 3A). When experimental parameters are used for HomeR (HomeR-Exp, in Figure 3A), it reaches near carrier fixation a generation after ClvR; almost on-par with ideal ClvR and TARE systems and significantly better than HGD. HomeR also reaches near carrier fixation for the widest range of fitness and transmission rate parameter values (Figure 3B). As an HGD, HomeR drives to high carrier frequencies provided its inheritance bias (or transmission rate) exceeds its associated fitness cost. In contrast, drive efficacy of ClvR and TARE is strongly dependent on fitness cost and weakly dependent on transmission rate. Indeed, ClvR and TARE can each only tolerate fitness costs less than ~20% (Figure 3B). This is a consequence of their design, employing a TA scheme, which induces a significant fecundity reduction (Figure 3—figure supplement 1A–B) in addition to other fitness costs. A one-locus HGD also exhibits efficacy across a wide range of parameter combinations, but its efficacy is reduced compared to HomeR due to the build-up of R2 and R1 alleles (Figure 3—figure supplement 1C–D), which can potentially block spread of the HGD in large populations (Figure 3B).
In two-locus simulations, Cas9 is separated from drive in all designs and undergoes independent assortment during gametogenesis. The effects of this design change are evident (Figure 3C). Under the same experimental conditions as one-locus simulations, there is significantly more variation in behavior of two-locus GDs, with a reduced speed of introgression into the population and slightly reduced overall efficacy. Nevertheless, general trends remain the same; HomeR with ideal parameters is more capable than comparable drives, though current experimental realizations require improvement. TARE performs significantly worse in a split configuration (Champer et al., 2020a). ClvR, when completely unlinked, also performs significantly worse, in agreement with results from Oberhofer et al., 2020a. Exploring the performance under a range of parameters, we found reduced overall efficacy for all drives (Figure 3D), but an increased range of lower efficacy for ClvR, HomeR, and TARE. This is consistent with fitness costs applied to the Cas9 locus, which is now separated from the effector gene and gRNAs. HomeR demonstrates the widest range of achieving efficacy as well as the widest range of high expected efficacy.
Exploration of multi-release scenarios
To probe the ability of these drive designs to modify field populations, we implemented an overlapping generation model (Sanchez et al., 2019), performing weekly male releases into a naive population, and tested if the frequency of females carrying the effector allele reached 95% of the female population, and how long that carrier frequency remained above 95%. One-locus constructs of ClvR, TARE, and HomeR were consistently able to reach this threshold, though HomeR achieved these thresholds over the widest range of transmission rates and fitness costs (Figure 4A). HGD never reached this threshold because of R2 allele build-up. This does not indicate that HGD cannot be effective at lower thresholds (indeed, during testing it was), but that even low rates of resistance generation are problematic. HomeR was the only one to consistently remain above a 95% carrier frequency for over 100 days (Figure 4B).
Figure 4. Performance of contemporary gene drive systems for population modification with multiple releases.
(A) Simulations of one-locus designs of ClvR, TARE, HomeR, and HGD in an ecologically consistent model (see 'Materials and methods'). Weekly releases of drive homozygous males (20% of the population size) were performed for up to 13 weeks (3 months, approximately one field season), and the female population was then tested for carrier frequency above 95% at any point within the subsequent 4 years. (B) Same setup as (A), but now the female populations were measured for how many days the carrier frequency remained above 95%, starting at the first release, lasting up to 4 years. This indicates the window where a disease-refractory allele could provide protection (window-of-protection [WOP]). (C) Simulations of two-locus (split-drive) versions of ClvR, TARE, HomeR, and HGD in an ecologically consistent model (see 'Materials and methods'). This time, only the first release was homozygous for Cas9 and the gene drive. Supplementary releases included only Cas9. Male releases were 20% of the total population size, and the female population was measured for drive carrier frequency, not Cas9 frequency, above 95%. (D) Using the data from (C), we applied the method from (B) to measure the WOP, where the drive carrier frequency remained above 95% in the female population. This only measures the drive frequency, not females who carry the Cas9 allele. All simulations contained 100 stochastic repetitions.
Two-locus designs showed significantly reduced ability to reach 95% carrier frequency in females, often requiring more and larger (20% of the total population size) releases to be effective (Figure 4C). For split-drive designs, only the first release was homozygous Cas9 and gene drive, while supplementary releases were homozygous Cas9 only (Faber et al., 2020; Oberhofer et al., 2020a, Oberhofer et al., 2020b, Oberhofer et al., 2019). A similar pattern of efficacy is seen for ClvR, TARE, and HomeR, but by splitting the HGD and maintaining the fitness effects on the Cas9 allele, it is now able to reach 95% introgression over a small parameter range. Additionally, as fitness costs are predominantly associated with the Cas9 allele and not the effector gene, all constructs were adequate at maintaining effector allele frequency in the population over a long period of time (Figure 4D). Taken together, these results suggest that multi-releases would be sufficient to ensure HomeR spreads and persists stably in a population.
Discussion
We have engineered a system we term HomeR, for population modification that mitigates some issues related to drive resistance. To limit the potential for inducing functional resistant alleles, an essential gene required for insect viability was strategically targeted. Multigenerational population drive experiments indicate that PolG2HomeR can spread and persist efficiently in the presence of Cas9, and this persistence is not impacted by induced resistant alleles, including functional resistant alleles (non-silent R1), mitigating a major challenge for population modification HGDs that are not designed to target essential genes.
The re-coded rescue strategy that we used to develop HomeR was also used in previous Drosophila TA non-HGDs (Champer et al., 2020a; Oberhofer et al., 2020a, Oberhofer et al., 2020b, Oberhofer et al., 2019; Figure 3—figure supplement 1A–B) and recent HGDs in both Drosophila (Champer et al., 2020b) and Anopheles stephensi (Adolfi et al., 2020), though each of these examples suffered from potential drawbacks. For example, both the haplolethal HGD (Champer et al., 2020b) and the TARE design (Champer et al., 2020a) share similar problematic design architectures that can be unstable as they are susceptible to functional resistant alleles induced via recombination between the promoter including sequences 5’ of the coding sequence and 3’ UTR regions, which are identical between the re-coded sequence and the wt sequence (Figure 2—figure supplement 1C in Champer et al., 2020a and Figure 2 in Champer et al., 2020b). Moreover, a haplolethal HGD (Champer et al., 2020b) that biases transmission in females requires a strict germline-specific promoter that limits maternal carryover, otherwise LBM, either mono- or biallelic, may result in dominant negative fitness costs to its carrier (Figure 3—figure supplement 1E) and impede drive spread. In fact, our efforts to find such promoters in Drosophila proved exceedingly difficult—with previously tested ‘germline-specific’ promoters such as nanos and vasa showing significant somatic activity at multiple insertion sites (Kandul et al., 2020; Kandul et al., 2019). The recent HGD in A. stephensi (Adolfi et al., 2020) was designed to target and rescue a non-essential gene for viability (i.e. the eye pigmentation kynurenine hydroxylase gene), whose disruption was pleiotropic and only partially costly to female fecundity and survival (Adolfi et al., 2020; Gantz et al., 2015; Pham et al., 2019). Notwithstanding, this drive spread efficiently in small, multigenerational laboratory population cages under several release thresholds; however, many drives did not reach, nor maintain, complete fixation presumably due to the viability and partial fertility of drive generated homozygous LOF resistant alleles (Figure 3—figure supplement 1D), underscoring the critical importance of targeting a recessive essential gene for such drives especially for larger releases. Comparatively, the ClvR system is quite stable, however, it can be cumbersome to engineer—requiring re-coding of the essential rescue gene, including all target sequences within the coding sequence (lacking introns), and uses an exogenous promoter and 3’ UTR, necessitating precise titration of expression from a distal genomic location with exogenous sequences to guarantee rescue without imposing deleterious fitness costs. These features may be difficult to accomplish for essential genes requiring complex regulatory elements and networks not directly adjacent to the target gene. In contrast to the aforementioned drives, (i) HomeR relies on the endogenous promoter sequence of the target gene to facilitate rescue expression which significantly simplifies the design and ensures endogenous expression of the rescue using native regulatory machinery, (ii) creatively designed to target the 3’ end of the essential gene to limit the degree of re-coding required for the rescue, (iii) an exogenous 3’ UTR to prevent deleterious recombination, and (iv) exploits LBM (Kandul et al., 2019) by targeting an essential gene to ensure recessive non-functional resistant alleles result in dominant deleterious/lethal mutations that are actively selected out of a population (Figure 1—figure supplement 4), four important design features that distinguish HomeR from other population modification drives.
Our findings are congruent with previous studies demonstrating reduced homing in Drosophila males (Chan et al., 2013; Chan et al., 2011; Windbichler et al., 2011). We tested multiple Cas9 lines supporting Cas9 expression in early and/or late germ cells with different levels of specificity and have not achieved high levels of homing as reported in Anopheline mosquito males (Gantz et al., 2015; Kyrou et al., 2018). Achiasmatic meiosis in Drosophila males likely correlates with the weak activity of the HDR pathway (Preston et al., 2006), which in turn results in inefficient homing in Drosophila males. Mosquito males have chiasmatic meiosis and recombination (Kitzmiller, 1976) that require active HDR machinery in primary spermatocytes, possibly contributing to efficient homing. Reduced homing efficacy in Drosophila males should be accounted for when designing HGDs in other species exhibiting achiasmatic meiosis, such as Drosophila suzukii, an invasive fruit pest.
Results from independent multigenerational population cage experiments indicate that HomeR spreads and persists efficiently in the nos-Cas9 genetic background (Figure 2B). As expected, a single copy of the HomeR inserted at an essential gene provides sufficient rescue and complements the corresponding LOF allele. The PolG2HomeR allele persisted for 10 generations in control cage populations without Cas9 and its frequency drifted >50%, underscoring the lack of major fitness costs to PolG2HomeR. The LOF alleles complemented by PolG2HomeR also persisted for many generations after a carrier frequency reached 100%. Once LOF alleles are complemented by the PolG2HomeR allele, it takes several generations for LOF alleles to combine as lethal homozygotes and be negatively selected out of the population. The slow-acting elimination of LOF alleles takes especially long time by HGDs targeting non-essential genes or genes whose disruption does not cause complete lethality or sterility of homozygous carriers (Figure 3—figure supplement 1D; Adolfi et al., 2020; Gantz et al., 2015) underscoring the importance of targeting essential genes.
Functional resistant (R1) alleles are a problematic feature shared universally by many kinds of gene drives. These alleles can still be induced even when an essential gene required for insect viability is targeted (Figure 2—figure supplement 1). However, it should be noted that here we did not identify any silent R1 mutations (i.e. mutations that change the DNA sequence but not the protein AA sequence) which would be expected to be fitness neutral. Each identified in-frame non-silent PolG2R1 allele we found changed at least one AA and thus may still affect the fitness of its carrier, especially since we are targeting an essential gene, preventing such alleles from accumulating at the expense of the drive. Indeed, we observed that functional R1 alleles imposed fitness costs on seven female carriers sampled in drive populations #4 and #5. This fitness cost likely limits their accumulation and results in negative selection out of the population, in favor of the PolG2HomeR alleles, over multiple generations (Figure 2A–B) again underscoring the importance of targeting an essential gene. Nevertheless, multiplexing by encoding additional gRNAs into HomeR may further diminish the probability of inducing functional resistant alleles and further increase drive stability, spread, and persistence (Champer et al., 2018; KaramiNejadRanjbar et al., 2018; Marshall et al., 2017; Oberhofer et al., 2018).
Splitting HomeR into two genetic loci (HomeR and Cas9) integrated on different chromosomes serves as an important molecular containment mechanism (Akbari et al., 2015; Long et al., 2020). The HomeR element is able to home into wt alleles and bias its transmission. However, the Cas9 element, which is inherited Mendelianly, is required for its homing. Therefore, the independent assortment of Cas9 and HomeR limits the spread of HomeR and acts as a genetic ‘brake’ for the invasion of HomeR. The spread dynamic of split-HGDs resembles that of high-threshold drives and thus requires a high introduction rate for HomeR to spread into a local population and prevents its spread into neighboring populations, which is an important feature for confining drive spread and may be necessary for initial field testing of gene drives (Adelman et al., 2017; Akbari et al., 2015; Friedman et al., 2020; Kandul et al., 2020; Li et al., 2020; Raban and Akbari, 2017; Raban et al., 2020). Moreover, HomeR can be further confined by fitness costs to either the HomeR drive itself or to the Cas9 element, and our experiments revealed that the Cas9 element imposed significant fitness costs that can impede drive invasion (Figure 2C). Notwithstanding, even with significant fitness costs, multiple releases of the HomeR could still enable drive spread and long-term persistence as evidenced by mathematical models (Figure 4). As an additional safety measure, if unintended consequences arise, HomeR’s spread can be reversed by reintroduction of insects harboring wt alleles of the gene targeted. Notwithstanding, if desired, HomeR could facilely be converted into a non-localized gene drive by incorporating the Cas9 into the HomeR drive cassette and our modeling illustrates that it could perform quite well under this configuration (Figure 3A,B, Figure 4A,B). Taken together, the split-drive design of HomeR is a safe localized gene drive technology that could be widely adopted and implemented for local population control, and if a non-localized drive is desired for more wide scale spread, HomeR could be adapted for that purpose too.
In sum, HomeR combines promising aspects of current population modification drives—confinablity, high transmission of HGDs, and resilience to EJ generated resistant alleles (R2 type and R1 type that induces a fitness cost) similar to TA drives (Figure 3—figure supplement 1). Modeling illustrates success of both design aspects in linked or split-drive form, demonstrating robust behavior over a range of parameter combinations (Figures 3–4). This underscores its stability and resilience to EJ alleles, overcoming a significant hurdle for current HGD designs. Given the simplicity of the HomeR design, it could be universally adapted to a wide range of species including human disease vectors in the future.
Materials and methods
Key resources table.
| Reagent type (species) or resource |
Designation | Source or reference |
Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (D. melanogaster) | gRNA#1PolG2 | 159674 | 91378 | This publication |
| Strain, strain background (D. melanogaster) | gRNA#2PolG2 | 159675 | n/a | This publication |
| Strain, strain background (D. melanogaster) | HomeRPolG2 | 159676 | Gene drives cannot be deposited at BDSC | This publication |
| Strain, strain background (D. melanogaster) | HomeR(B)PolG2 | 159677 | Gene drives cannot be deposited at BDSC | This publication |
| Strain, strain background (D. melanogaster) | nos-Cas9 | 112685 | 79004 | 30622266 |
| Strain, strain background (D. melanogaster) | vas-Cas9 | 112686 | 79005 | 30622266 |
| Strain, strain background (D. melanogaster) | Uniq-Cas9 | 112687 | 79006 | 30622266 |
| Strain, strain background (D. melanogaster) | Act5C-Cas9 | n/a | 54590 | 25002478 |
| Strain, strain background (D. melanogaster) | exuL-Cas9 | 159671 | 91375 | This publication |
| Strain, strain background (D. melanogaster) | Rcd1r-Cas9 | 159673 | 91377 | This publication |
| Strain, strain background (D. melanogaster) | bTub-Cas9 | 159672 | 91376 | This publication |
Selection of Cas9/gRNA target sites
We inserted a HomeR in DNA Polymerase gamma subunit 2 (PolG2 or Pol-γ35, CG33650). PolG2 is an essential gene required for insect viability. The C-terminal domain of PolG2 is located at the end of the coding sequence, which facilitates its re-coding (Figure 1—figure supplement 1A–B). We PCR-amplified a 413-base fragment of the domain with 1073A.S1F and 1073A.S2R from multiple Drosophila strains (w1118, Canton S, Oregon R, nos-Cas9; Kandul et al., 2019) and used the consensus sequence along with the tool CHOPCHOP v2 (Labun et al., 2016) to choose two gRNA targets sites that minimize off-target cleavage. In addition, we used the DGRP2 (http://dgrp2.gnets.ncsu.edu) that includes natural variation in genome architecture among 205 D. melanogaster genetic reference panel lines (Huang et al., 2014; Mackay et al., 2012) to explore SNPs found inside both gRNA target sequences.
Design and assembly of genetic constructs
We used Gibson enzymatic assembly to build all genetic constructs (Gibson et al., 2009). To assemble both gRNA constructs, we used the previously described sgRNASxl plasmid (Kandul et al., 2019; Addgene #112688) harboring the mini-white gene and attB docking site. We removed the fragment encompassing the U6.3 promoter and gRNA scaffold by AscI and SacII digestion, and cloned it back as two fragments overlapping at a novel gRNA sequence (Figure 1—figure supplement 1A). Both gRNA#1PolG2 and gRNA#2PolG2 plasmids targeting PolG2 are deposited at http://www.addgene.org/ (#159774 and #159675).
We assembled two HomeRPolG2 constructs using two tested gRNAs (Figure 1—figure supplement 2A). Each HomeRPolG2 was built around a specific gRNA, with matching LHA and RHA: HomeRPolG2 harbored gRNA#1PolG2, and HomeR(B) had gRNA#2PolG2. We digested the nos-Cas9 plasmid (Kandul et al., 2019; Addgene #112685) with AvrII and AscI, preserving the backbone containing the piggyBac left and right sequences that encompass the Opie-dsRed-SV40 marker gene. The HomeR construct was assembled between Opie-dsRed-SV40 and piggyBacR in three steps. First, we cloned the gRNA#1 or #2 from the corresponding plasmid together with the 3xP3-eGFP-SV40 marker gene, to tag site-specific insertion of GDe. Then, we cloned three fragments: (i) LHA, which was amplified from the Drosophila genomic DNA; (ii) the re-coded fragment downstream from the gRNA cut site, which was PCR-amplified from the dePolG2 gBlock custom synthesized by IDT (Supplementary file 1); (iii) the p10 3’ UTR to provide robust expression (Pfeiffer et al., 2012) of the re-coded PolG2 rescue. Finally, we cloned RHA, which was PCR-amplified from genomic DNA, corresponding to each specific gRNA cut site. Importantly, the re-coding was carefully designed to ensure the translation of the re-coded DNA sequence in the wt amino acid sequence of Pol2 with respect to Drosophila codon usage bias. Both HomeRPolG2 and HomeR(B)PolG2 plasmids, targeting the PolG2 locus, are deposited at http://www.addgene.org/ (#159676 and #159677).
To assemble the three constructs for testis-specific Cas9 expression, we used a plasmid harboring the hSpCas9-T2A-GFP, the Opie2-dsRed transformation marker, and both piggyBac and attB-docking sites, which were previously used to establish Cas9 transgenic lines in Aedes aegypti (Li et al., 2017) and D. melanogaster (Kandul et al., 2020; Kandul et al., 2019). We removed the Ubiquitin 63E promoter from the ubiq-Cas9 plasmid (Addgene #112686) (Kandul et al., 2019) by digesting it with SwaI at +27°C and then with NotI at +37°C, and cloned a promoter fragment amplified from the Drosophila genomic DNA. The Drosophila exuperantia (CG8994) 783 bp fragment (exuL) upstream of the exuperantia gene was amplified with ExuL.1F and ExuL.2R primers (Supplementary file 1) and cloned to assemble the exuL-Cas9 plasmid. The Rcd-1 related (Rcd1r, CG9573; Chan et al., 2013) and β-Tubulin 85D (βTub; Chan et al., 2011; Michiels et al., 1989) promoters support early and late, respectively, testis-specific expression in Drosophila males. The 937-base-long fragment upstream of Rcd1r was amplified with 1095.C1F and 1095.C2R primers and cloned to assemble the Rcd1r-Cas9 plasmid. The 481-base-long fragment upstream of βTub was amplified with βTub.1F and βTub.2R primers (Supplementary file 1) and cloned to build the βTub-Cas9 plasmid. Three plasmids for testis-specific Cas9 expression are deposited at http://www.addgene.org/ (#159671–159773).
Fly maintenance and transgenesis
Flies were maintained under standard conditions: 26°C with a 12 hr/12 hr light/dark cycle. Embryo injections were performed by Rainbow Transgenic Flies, Inc. We used φC31-mediated integration (Groth et al., 2004) to insert the gRNA#1 and gRNA#2 constructs at the P{CaryP}attP1 site on the second chromosome (BDSC #8621), and the exuL-Cas9, βTub-Cas9, and Rcd1r-Cas9 constructs at the PBac{y+-attP-3B}KV00033 on the third chromosome (BDSC #9750). Two methods were used to generate the site-specific insertion of HomeRPolG2 or HomeR(B)PolG2 constructs at the gRNA#1PolG2 or gRNA#2PolG2 cut sites, respectively, inside the PolG2 gene via HDR. First, we injected the mixture of HomeR and helper phsp-pBac, carrying the piggyBac transposase (Handler and Harrell, 1999), plasmids (500 and 250 ng/µl, respectively, in 30 µl) into w1118 embryos. Random insertions of HomeRPolG2 and HomeR(B)PolG2, assessed by double (eye-specific GFP and body-specific dsRed) fluorescence (Figure 1—figure supplement 2B), established with this injection were genetically crossed to nos-Cas9/nos-Cas9 (BDSC #79004; Kandul et al., 2019) flies to ‘relocate’ HomeRPolG2 or HomeR(B)PolG2 to the corresponding gRNA cut site via HACK (Lin and Potter, 2016). A few site-specific PolG2HomeR and PolG2HomeR(B) lines tagged with only eye-specific GFP fluorescence were recovered. Second, we injected HomeRPolG2 or HomeR(B)PolG2 plasmids directly into nos-Cas9/nos-Cas9 (BDSC #79004; Kandul et al., 2019) embryos, generating multiple independent, site-specific insertions for each PolG2HomeR (Figure 1—figure supplement 2B). Recovered transgenic lines were balanced on the second and third chromosomes using single-chromosome balancer lines (w1118; CyO/snaSco for II and w1118; TM3, Sb1/TM6B, Tb1 for III) or a double-chromosome balancer line (w1118; CyO/Sp; Dr/TM6C, Sb1). While both techniques (random insertion/HACK and HDR) worked to generate site-directed insertions, all subsequent analysis was performed exclusively on lines derived from the HDR-based transgenesis approach.
We established three homozygous lines of PolG2HomeR and PolG2HomeR(B) from independent insertion lines, and confirmed the precision of site-specific insertions by sequencing the borders between HomeR constructs and the Drosophila genome (Figure 1—figure supplement 2C). The 1118-base-long fragment overlapping the left border was PCR-amplified with 1076B.S9F and 1076B.S2R and was sequenced with 1076B.S3F and 1076B.S4R primers. The same-length fragment at the right border was amplified with 1073A.S1F and 1076B.S10R and was sequenced with 1076B.S7F and 1076B.S8R primers (Supplementary file 1).
Fly genetics and imaging
Flies were examined, scored, and imaged on a Leica M165FC fluorescent stereomicroscope equipped with a Leica DMC2900 camera. We assessed the transmission rate of HomeR by following its eye-specific GFP fluorescence, while the inheritance of Cas9 was tracked via body-specific dsRed fluorescence (Figure 2A). All genetic crosses were done in fly vials using groups of 10 males and 10 females.
RNAPolG2 cleavage assay
To assess the cleavage efficiency of each gRNA targeting the C-terminal domain of PolG2, we genetically crossed 10 w1118; gRNA#1PolG2 or w1118; gRNA#2PolG2 homozygous males to 10 y1, Act5C-Cas9, w1118, Lig4 (Zhang et al., 2014) (BDSC #58492) homozygous females, and scored the lethality of F1 males (Figure 1—figure supplement 1C). The F1 males would then inherit the X chromosome from their mothers, expressing gRNA#1PolG2 or gRNA#2PolG2 with Act5C-Cas9 in a Lig4-null genetic background, and this results in male lethality when a tested gRNA directs cleavage of the PolG2 locus. To assess the induced lethality in the Lig4+/+ genetic background, we crossed 10 y1, Act5C-Cas9, w1118 (BDSC #54590; Port et al., 2014) flies to 10 U6.3-gRNA#1PolG2 flies in both directions, and scored survival of trans-heterozygous and heterozygous F1 progeny. To measure the Cas9/gRNA-directed cleavage of PolG2 by maternally deposited Cas9 protein in the Lig4+ background, the same homozygous males were genetically crossed to w1118/w1118; nos-Cas9/CyO females (Figure 1—figure supplement 1D), and the F1 progeny, harboring gRNA#1PolG2 or gRNA#2PolG2, were scored and compared to each other.
Assessment of PolG2HomeR transmission rates
To compare transmission rates of PolG2HomeR and PolG2HomeR(B), we first established trans-heterozygous parent flies by genetically crossing PolG2HomeR/PolG2HomeR; +/+ or PolG2HomeR(B)/PolG2HomeR(B); +/+ females to +/+; nos-Cas9/nos-Cas9 males. We then assessed the transmission rates by trans-heterozygous parent females and males crossed to wt flies. For controls, we estimated the transmission rates of HomeRPolG2 and HomeR(B)PolG2 in the absence of Cas9, by heterozygous PolG2HomeR/+ or PolG2HomeR(B)/+ females and males crossed to wt flies (Figure 1—figure supplement 3). To explore the effect of maternally deposited Cas9 protein on transmission of PolG2HomeR, we generated heterozygous PolG2HomeR/CyO embryos containing Cas9 protein deposited by nos-Cas9/CyO mothers and estimated the transmission of PolG2HomeR by females and males raised from these embryos and crossed to wt flies. We tested five different Cas9 lines—supporting germline (vas-Cas9), ubiquitous (ubiq-Cas9, Act5C-Cas9), and early (exuL-cas9, Rcd1r-Cas9) or late testes-specific expression (βTub-Cas9)—together with the strongest HomeR, PolG2HomeR. To control for position effect variegation, each Cas9 transgene was inserted at the same attP docking site on the third chromosome, except for Act5C-Cas9 that was integrated on the X chromosome (Port et al., 2014). Ten trans-heterozygous females or males, generated by crossing homozygous PolG2HomeR females to homozygous Cas9 males, were genetically crossed to wt flies and the transmission of PolG2HomeR was quantified in their F1 progeny (Figure 1D).
Egg hatching and egg-to-adult survival rates
To identify the mechanism of the super-Mendelian transmission of PolG2HomeR, we assessed the percentage of F1 hatched eggs laid by trans-heterozygous PolG2HomeR/+; nos-Cas9/+ females genetically crossed to wt males and compared it to those hatched from two types of heterozygous females: PolG2HomeR/+; +/+ ♀ and +/+; nos-Cas9/+ ♀ (Figure 1B). We collected virgin females and aged them for 3 days inside food vials supplemented with a yeast paste, then five groups of 25 virgin females of each type were transferred into vials with fresh food containing 25 wt males and allowed to mate overnight (12 hr) in the dark. Then, all males were removed from the vials, while females were transferred into small embryo collection cages (Genesee Scientific 59–100) with grape juice agar plates. After 12 hr of egg laying, a batch of at least 200 laid eggs was counted for each sample group and incubated for 24 hr at 26°C before the number of unhatched eggs was counted. To assess the egg-to-adult survival rate, at least 12 groups of 75 eggs were collected to each type of tested progeny and transferred to individual vials. The emerged flies from each vial were counted (Figure 1C), and their sex and fluorescence were scored (Supplementary file 7).
‘Fishing’ for functional resistant alleles, PolG2R1
To explore the generation and accumulation of functional resistant alleles induced by EJ, we initiated three populations by crossing 50 +/+; nos-Cas9/nos-Cas9 females and 50 PolG2HomeR/PolG2HomeR; nos-Cas9/nos-Cas9 (Figure 2A) males in 0.3 l plastic bottles (VWR Drosophila Bottle 75813–110). Parent (P) flies were removed after 6 days, and their progeny were allowed to develop, eclose, and mate for 13–15 days. This established a 100% heterozygous PolG2HomeR/+; nos-Cas9/+ population in every bottle at the next generation (G0). Each generation, around 250–350 emerged flies were anesthetized using CO2, and their genotypes with respect to PolG2HomeR (presence or absence) were determined using the dominant eye-specific GFP marker. Then they were transferred to a fresh bottle and allowed to lay eggs for 6 days before removing them, and the cycle was repeated. Three populations were maintained in this way for 11 generations, which corresponds to 10 generations of gene drive. Note that any fly scored without the PolG2HomeR allele was transferred into a fresh bottle to ensure any PolG2 resistant or wt alleles could be passed to the next generation. We retrieved and froze the flies for genotyping only after 6days to ensure sufficient time for breeding. We expected that if PolG2R1 alleles were frequently generated and did not incur fitness costs, they would persist and accumulate over a few generations at the expense of PolG2HomeR. However, as we did not find any fly without the PolG2HomeR allele after G3, we stopped populations after 10 generations and froze 60 flies after G10 for further sequence analysis.
HomeR population drives in the Cas9 and wt genetic backgrounds
For HomeR drives in the nos-Cas9/nos-Cas9 genetic background, we seeded five experimental (Cas9+) drives and three control (Cas9–) drives with 50 homozygous PolG2HomeR/PolG2HomeR males and 50 wt males together with 100 wt virgin females in 0.3 l plastic bottles. Seeded flies either encoded Cas9 (experimental drive) or not (control or ‘no-drive’, Figure 2B). For HomeR drives in the wt genetic background, we seeded four drive populations with 100 wt virgin females and double homozygous (PolG2HomeR/PolG2HomeR; nos-Cas9/nos-Cas9) males mixed with wt males at the ratios of 1:1 (two populations with 25% of PolG2HomeR) or 3:1 (two populations with 37.5% of PolG2HomeR, Figure 2C). Note that the PolG2HomeR males were competing with wt males for female mates, and their mating competitiveness could be scored by the dominant 3xP3-GFP marker of PolG2HomeR in their progeny at generation 0 (G0). Both types of homozygous PolG2HomeR males with and without Cas9 were able to compete with the corresponding wt males for female mates resulting in the increase of PolG2HomeR from 25% or 37.5% in parents to nearly 50% or 70%, respectively, at generation 0 (Figure 2B–C). The discrete-generation populations were maintained and scored as described above. Each generation, around 250–350 emerged flies were anesthetized using CO2, and their genotypes were scored for the presence or absence of PolG2HomeR (eye-specific GFP) and nos-Cas9 (body-specific dsRed). Then they were transferred to a fresh bottle and allowed to lay eggs for 6 days before removing them, and the cycle was repeated.
Sequencing of induced resistant alleles
To analyze the molecular changes that caused functional in-frame (R1) and LOF (R2) resistant mutations in PolG2, we PCR-amplified the 232-base-long genomic region containing both gRNA#1PolG2 and gRNA#2PolG2 cut sites using 1073A.S3F and 1073A.S4R primers (Supplementary file 1). For PCR genotyping from a single fly, we followed the single-fly genomic DNA prep protocol (Kandul et al., 2019). PCR amplicons were purified using the QIAquick PCR purification kit (QIAGEN), subcloned into the pCR2.1-TOPO plasmid (Thermo Fisher), and at least seven clones were sequenced in both directions by Sanger sequencing at Retrogen and/or Genewiz to identify both alleles in each fly. Sequence AB1 files were aligned against the corresponding wt sequence of PolG2 in SnapGene 4.
To explore the diversity of resistant alleles persisting after 10 generations of PolG2HomeR in a 100% heterozygous population, we froze 60 flies (30 ♀ and 30 ♂), each harboring at least one copy of the dominant marker of PolG2HomeR, from each lineages after G10 (Figure 2A). Using these flies, we quantified any resistant and wt alleles remaining in the population via Illumina sequencing of heterogeneous PCR amplicons at the PolG2 locus. Note that PCR amplicons did not include the PolG2HomeR allele due to its length (Figure 1A). Additionally, this assay will not be able to accurately distinguish between germline and somatic mutations as whole flies were used. DNA was extracted using the DNeasy Blood and Tissue Kit (QIAGEN). To analyze heterogeneous PCR products, we used the Amplicon-EZ service by Genewiz and followed the Genewiz guidelines for sample preparation. In brief, Illumina adapters were added to the 1073A.S3F and 1073A.S4R primers to simplify the library preparation, PCR products were purified using QIAquick PCR purification kit (QIAGEN), around 50,000 one-direction reads covering the entire amplicon length were generated, and relative abundances of recovered SBS and indel alleles at the gRNA#2PolG2 cut site were inferred using Galaxy tools (Afgan et al., 2018). Amplicon-EZ data from Genewiz were first uploaded to Galaxy.org. A quality control was performed using FASTQC. Sequence data were then paired and aligned against the PolG2WT sequence using Map with BWA-MEM under ‘Simple Illumina mode’. The SBS and indel alleles were detected using FreeBayes, with the parameter selection level set to ‘simple diploid calling’.
Model fitting to cage experiment data
Empirical data from the HomeR population replacement experiments were used to parameterize a model of CRISPR-based homing gene drive including resistant allele formation. Model fitting was carried out for all five gene drive cage experiments using Markov chain Monte Carlo (MCMC) methods in which estimated parameters related to cleavage efficiencies in females and males, accurate HDR frequencies given cleavage in females and males, the proportion of resistant alleles that are in-frame and cost-free, and the fitness cost associated with having the HomeR system. We considered discrete generations, random mixing, and Mendelian inheritance rules at the gene drive locus, with the exception that for adults heterozygous for the homing allele (denoted by ‘H’) and wt allele (denoted by ‘W’), a proportion, c, of the W alleles are cleaved, while a proportion, 1 c, remain as W alleles. Of those that are cleaved, a proportion, pHDR, are subject to accurate HDR and become H alleles, while a proportion, (1-pHDR), become resistant alleles. Of those that become resistant alleles, a proportion, pRES, become in-frame, functional, cost-free resistant alleles (denoted by ‘R’), while the remainder, (1-pRES), become out-of-frame, non-functional, or otherwise costly resistant alleles (denoted by ‘B’). The values of c and pHDR were allowed to vary depending on whether the HW individual is female or male. The fitness cost associated with the HomeR system, sH,F, was assumed to be female-specific. These considerations allowed us to calculate expected genotype frequencies in the next generation, and to explore the parameter values that maximize the likelihood of the experimental data. The model fitting framework is described in full in S1 text of Pham et al., 2019.
Comparative modeling of gene drive systems
Comparative gene drive simulations were performed using a discrete-generation version of the Mosquito Gene Drive Explorer (MGDrivE) modeling framework (Sanchez et al., 2019). The first generation was seeded with 400 adults, 50% wt females, 25% wt males, and 25% homozygous gene drive males. At each generation, adult females mate with males, thereby obtaining a composite mated genotype (their own, and that of their mate) with mate choice following a multinomial distribution determined by adult male genotype frequencies. Egg production by mated adult females then follows a Poisson distribution, proportional to the genotype-specific lifetime fecundity of the adult female. Offspring genotype follows a multinomial distribution informed by the composite mated female genotype and the inheritance pattern of the gene drive system. Sex distribution of offspring follows a binomial distribution, assuming equal probability for each sex. Female and male adults from each generation are then sampled equally to seed the next generation, with a sample size of 400 individuals (200 female and 200 male), following a multivariate hypergeometric distribution. Twenty-five repetitions were run for each drive in the trace plots (Figure 3A and C), and 100 repetitions were run for each parameter combination in the heatmaps (Figure 3B and D).
The inheritance pattern is captured by the ‘inheritance cube’ module of MGDrivE (Sanchez et al., 2019). ClvR and TARE constructs were implemented to match their published descriptions (Champer et al., 2020a; Oberhofer et al., 2020a, Oberhofer et al., 2019). HomeR and HGD were implemented as one- or two-locus systems following equivalent inheritance rules. When Cas9 and gRNAs co-occur in the same individual, wt alleles are cleaved at a rate cF (cM) (female- (male-) specific cleavage), with 1-cF (1 cM) remaining wt. Given cleavage, successful HDR occurs at a rate chF (chM), with 1-chF (1-chM) alleles undergoing some form of EJ. Of these, a proportion, crF (crM), are in-frame EJ alleles, while the remainder, 1-crF (1-crM), are LOF alleles. Maternal carryover (maternal deposition, or maternal perdurance) was modeled to occur in zygotes of mothers having both Cas9 and gRNAs, impacting a proportion, dF, of zygotes. Of the wt alleles in impacted zygotes, a proportion, drF, become in-frame EJ alleles, while the remainder, 1-drF, become LOF alleles. These inheritance rules apply to both HomeR and HGD, with differing fitness costs.
ClvR (Oberhofer et al., 2020a; Oberhofer et al., 2019) was modeled using a 99% cleavage rate in female and male germ cells, as well as in embryos from maternal carryover. For two-locus ClvR, the two loci were assumed to undergo independent assortment (≥50 cM separation), as was assumed for all two-locus systems in this analysis. For both configurations, it was assumed that 0.1% of cleaved alleles were converted to functional resistant alleles (R1 type), and the rest became LOF alleles (R2 type). In addition to the 50% egg-hatching reduction due to the non-homing drive (Figure 3—figure supplement 1A–B), an additional 5% reduction in fecundity was applied to females that harbored Cas9. For consistency, TARE, HGD, and HomeR (for ideal parameters) also used a cleavage rate of 99% in females and males, though TARE demonstrated lower maternal carryover (Champer et al., 2020a), and was modeled with 95% cleavage. HGD and HomeR (for ideal parameters), which rely on HDR, were simulated with 90% HDR rates in females and males. Cleaved alleles that did not undergo HDR were assumed to be R1 alleles with proportion 0.5%, and R2 LOF alleles the remainder of the time. TARE and HomeR were also modeled with a small (5%) fitness reduction, applied as a reduction of female fecundity. Since an HGD does not provide a rescue for a disrupted target gene, its carriers demonstrate higher fitness costs and were assigned a 20% fitness reduction with the assumption that the HGD is inserting into a non-lethal gene that imposes a low/moderate fitness cost. Experimentally derived parameters for HomeR differed from ideal parameters in two ways: (i) there was no HDR in males (although cleavage remained the same) and (ii) 1% of EJ-repaired wt alleles were converted into R1 alleles (cf. 0.5% for the ideal case).
To determine the number of releases required to introgress effector genes into 95% of the female portion of a population, and ascertain how long that introgression could be effective (up to 4 years; Figure 4), we performed simulations using the full version of MGDrivE (Sanchez et al., 2019), implementing overlapping generations and density-dependent growth effects on aquatic stages. All gene drive characteristics were maintained as stated above. For one-locus designs, male releases, up to 13, were performed at 20% of the total population size (10,000). For two-locus designs, the first release was males homozygous for Cas9 and gene drive, but subsequent releases were only homozygous for Cas9. The two-locus releases were 20% of the total population size. Life cycle parameters are: 2 days for egg maturation, 5 days for larval maturation, 1 day for pupal maturation, and an expected adult lifespan of 11 days (Li et al., 2020). All simulations were performed, analyzed, and plotted in R (R Development Core Team, 2017). Code is available upon request.
Statistical analysis
Statistical analysis was performed in JMP 8.0.2 by SAS Institute Inc, and graphs were constructed in Prism 8.4.1 for MacOS by GraphPad Software LLC. At least three biological replicates were used to generate statistical means for comparison. p-values were calculated using a two-sample Student’s t test with equal or unequal variance.
Gene drive safety measures
All gene drive crosses were performed in accordance with protocols approved by the Institutional Biosafety Committee at UCSD, in which gene drive experiments were performed in a high-security ACL2 barrier facility in plastic vials that were autoclaved prior to being discarded, in accordance with currently suggested guidelines for the laboratory confinement of gene drive systems (Akbari et al., 2015; National Academies of Sciences, Engineering, and Medicine et al., 2016).
Ethical conduct of research
We have complied with all relevant ethical regulations for animal testing and research and conformed to the UCSD institutionally approved biological use authorization protocol (BUA #R2401).
Acknowledgements
This work was supported in part by funding from a DARPA Safe Genes Program Grant (HR0011-17-2-0047), and NIH awards (R21RAI149161A, R01AI151004, DP2AI152071) awarded to OSA. The functional characterization of the ExuL promoter was done by OSA while at Caltech working with Bruce A Hay.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Omar S Akbari, Email: oakbari@ucsd.edu.
Claude Desplan, New York University, United States.
Patricia J Wittkopp, University of Michigan, United States.
Funding Information
This paper was supported by the following grants:
Defense Advanced Research Projects Agency HR0011-17-2-0047 to Omar S Akbari.
National Institutes of Health R21RAI149161A to Omar S Akbari.
National Institutes of Health R01AI151004 to Omar S Akbari.
National Institutes of Health DP2AI152071 to Omar S Akbari.
Additional information
Competing interests
is a consultant for Agragene.
No competing interests declared.
is a founder of Agragene, Inc, has an equity interest, and serves on the company's Scientific Advisory Board.
Author contributions
Conceptualization, Formal analysis, Supervision, Investigation, Methodology, Writing - original draft, Writing - review and editing.
Data curation, Investigation, Writing - review and editing.
Formal analysis, Investigation, Visualization, Methodology, Writing - review and editing.
Formal analysis, Supervision, Funding acquisition, Investigation, Methodology, Writing - review and editing.
Conceptualization, Formal analysis, Supervision, Funding acquisition, Investigation, Methodology, Writing - original draft, Project administration, Writing - review and editing.
Additional files
Data availability
All data are represented fully within the tables and figures. The gRNA#1PolG2, gRNA#2PolG2, HomeRPolG2, HomeR(B)PolG2, exuL-Cas9, Rcd1r-Cas9, and βTub-Cas9 plasmids and corresponding fly lines are deposited at http://www.addgene.org/ (159671-159677) and the Bloomington Drosophila Stock Center (91375-91378), respectively.
References
- Adelman Z, Akbari O, Bauer J, Bier E, Bloss C, Carter SR, Callender C, Denis AC, Cowhey P, Dass B, Delborne J, Devereaux M, Ellsworth P, Friedman RM, Gantz V, Gibson C, Hay BA, Hoddle M, James AA, James S, Jorgenson L, Kalichman M, Marshall J, McGinnis W, Newman J, Pearson A, Quemada H, Rudenko L, Shelton A, Vinetz JM, Weisman J, Wong B, Wozniak C. Rules of the road for insect gene drive research and testing. Nature Biotechnology. 2017;35:716–718. doi: 10.1038/nbt.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolfi A, Gantz VM, Jasinskiene N, Lee H-F, Hwang K, Terradas G, Bulger EA, Ramaiah A, Bennett JB, Emerson JJ, Marshall JM, Bier E, James AA. Efficient population modification gene-drive rescue system in the malaria mosquito anopheles stephensi. Nature Communications. 2020;11:19426-0. doi: 10.1038/s41467-020-19426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Grüning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekrutenko A, Blankenberg D. The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Research. 2018;46:W537–W544. doi: 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari OS, Bellen HJ, Bier E, Bullock SL, Burt A, Church GM, Cook KR, Duchek P, Edwards OR, Esvelt KM, Gantz VM, Golic KG, Gratz SJ, Harrison MM, Hayes KR, James AA, Kaufman TC, Knoblich J, Malik HS, Matthews KA, O'Connor-Giles KM, Parks AL, Perrimon N, Port F, Russell S, Ueda R, Wildonger J. BIOSAFETY. Safeguarding gene drive experiments in the laboratory. Science. 2015;349:927–929. doi: 10.1126/science.aac7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey LS, Crisanti A, Randazzo FF, Akbari OS. Opinion: standardizing the definition of gene drive. PNAS. 2020;117:30864–30867. doi: 10.1073/pnas.2020417117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A, Gamez S, Li M, Antoshechkin I, Li HH, Wang HW, Chen CH, Klein MJ, Duchemin JB, Paradkar PN, Akbari OS. Engineered resistance to zika virus in transgenic Aedes aegypti expressing a polycistronic cluster of synthetic small RNAs. PNAS. 2019;116:3656–3661. doi: 10.1073/pnas.1810771116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A, Gamez S, Li M, Antoshechkin I, Lee S-H, Wang S-W, Chen C-H, Klein MJ, Duchemin J-B, Crowe JE, Paradkar PN, Akbari O. Broad dengue neutralization in mosquitoes expressing an engineered antibody. SSRN Electronic Journal. 2020;16:e1008103. doi: 10.1371/journal.ppat.1008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2003;270:921–928. doi: 10.1098/rspb.2002.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballar-Lejarazú R, Ogaugwu C, Tushar T, Kelsey A, Pham TB, Murphy J, Schmidt H, Lee Y, Lanzaro GC, James AA. Next-generation gene drive for population modification of the malaria vector mosquito, anopheles gambiae. PNAS. 2020;117:22805–22814. doi: 10.1073/pnas.2010214117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrodeguas JA, Theis K, Bogenhagen DF, Kisker C. Crystal structure and deletion analysis show that the accessory subunit of mammalian DNA polymerase gamma, pol gamma B, functions as a homodimer. Molecular Cell. 2001;7:43–54. doi: 10.1016/S1097-2765(01)00153-8. [DOI] [PubMed] [Google Scholar]
- Carrodeguas JA, Bogenhagen DF. Protein sequences conserved in prokaryotic aminoacyl-tRNA synthetases are important for the activity of the processivity factor of human mitochondrial DNA polymerase. Nucleic Acids Research. 2000;28:1237–1244. doi: 10.1093/nar/28.5.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champer J, Buchman A, Akbari OS. Cheating evolution: engineering gene drives to manipulate the fate of wild populations. Nature Reviews Genetics. 2016;17:146–159. doi: 10.1038/nrg.2015.34. [DOI] [PubMed] [Google Scholar]
- Champer J, Reeves R, Oh SY, Liu C, Liu J, Clark AG, Messer PW. Novel CRISPR/Cas9 gene drive constructs reveal insights into mechanisms of resistance allele formation and drive efficiency in genetically diverse populations. PLOS Genetics. 2017;13:e1006796. doi: 10.1371/journal.pgen.1006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champer J, Liu J, Oh SY, Reeves R, Luthra A, Oakes N, Clark AG, Messer PW. Reducing resistance allele formation in CRISPR gene drive. PNAS. 2018;115:5522–5527. doi: 10.1073/pnas.1720354115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champer J, Lee E, Yang E, Liu C, Clark AG, Messer PW. A toxin-antidote CRISPR gene drive system for regional population modification. Nature Communications. 2020a;11:1082. doi: 10.1038/s41467-020-14960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champer J, Yang E, Lee E, Liu J, Clark AG, Messer PW. A CRISPR homing gene drive targeting a haplolethal gene removes resistance alleles and successfully spreads through a cage population. PNAS. 2020b;117:24377–24383. doi: 10.1073/pnas.2004373117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YS, Naujoks DA, Huen DS, Russell S. Insect population control by homing endonuclease-based gene drive: an evaluation in Drosophila melanogaster. Genetics. 2011;188:33–44. doi: 10.1534/genetics.111.127506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YS, Huen DS, Glauert R, Whiteway E, Russell S. Optimising homing endonuclease gene drive performance in a semi-refractory species: Drosophila melanogaster experience. PLOS ONE. 2013;8:e54130. doi: 10.1371/journal.pone.0054130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esvelt KM, Smidler AL, Catteruccia F, Church GM. Concerning RNA-guided gene drives for the alteration of wild populations. eLife. 2014;3:e03401. doi: 10.7554/eLife.03401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber NR, McFarlane GR, Chris Gaynor R, Pocrnic I, Whitelaw CBA. Novel combination of CRISPR-based gene drives eliminates resistance and localises spread. bioRxiv. 2020 doi: 10.1101/2020.08.27.266155. [DOI] [PMC free article] [PubMed]
- Friedman RM, Marshall JM, Akbari OS. Gene drives new and improved. Issues in Science and Technology. 2020;36:72–78. [Google Scholar]
- Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, Macias VM, Bier E, James AA. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito anopheles stephensi. PNAS. 2015;112:E6736–E6743. doi: 10.1073/pnas.1521077112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz VM, Akbari OS. Gene editing technologies and applications for insects. Current Opinion in Insect Science. 2018;28:66–72. doi: 10.1016/j.cois.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard A, Haque T, Bobik M, Xu XS, Klanseck C, Kushwah RBS, Berni M, Kaduskar B, Gantz VM, Bier E. Efficient allelic-drive in Drosophila. Nature Communications. 2019;10:1640. doi: 10.1038/s41467-019-09694-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, Katsanos D, Gribble M, Baker D, Marois E, Russell S, Burt A, Windbichler N, Crisanti A, Nolan T. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector anopheles gambiae. Nature Biotechnology. 2016;34:78–83. doi: 10.1038/nbt.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond AM, Kyrou K, Bruttini M, North A, Galizi R, Karlsson X, Kranjc N, Carpi FM, D'Aurizio R, Crisanti A, Nolan T. The creation and selection of mutations resistant to a gene drive over multiple generations in the malaria mosquito. PLOS Genetics. 2017;13:e1007039. doi: 10.1371/journal.pgen.1007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler AM, Harrell RA. Germline transformation of Drosophila melanogaster with the piggyBac transposon vector. Insect Molecular Biology. 1999;8:449–457. doi: 10.1046/j.1365-2583.1999.00139.x. [DOI] [PubMed] [Google Scholar]
- Hay B, Jan LY, Jan YN. A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell. 1988;55:577–587. doi: 10.1016/0092-8674(88)90216-4. [DOI] [PubMed] [Google Scholar]
- Hoermann A, Tapanelli S, Capriotti P, Masters EKG, Habtewold T, Christophides GK, Windbichler N. Converting endogenous genes of the malaria mosquito into simple non-autonomous gene drives for population replacement. bioRxiv. 2020 doi: 10.1101/2020.05.09.086157. [DOI] [PMC free article] [PubMed]
- Huang W, Massouras A, Inoue Y, Peiffer J, Ràmia M, Tarone AM, Turlapati L, Zichner T, Zhu D, Lyman RF, Magwire MM, Blankenburg K, Carbone MA, Chang K, Ellis LL, Fernandez S, Han Y, Highnam G, Hjelmen CE, Jack JR, Javaid M, Jayaseelan J, Kalra D, Lee S, Lewis L, Munidasa M, Ongeri F, Patel S, Perales L, Perez A, Pu L, Rollmann SM, Ruth R, Saada N, Warner C, Williams A, Wu YQ, Yamamoto A, Zhang Y, Zhu Y, Anholt RR, Korbel JO, Mittelman D, Muzny DM, Gibbs RA, Barbadilla A, Johnston JS, Stone EA, Richards S, Deplancke B, Mackay TF. Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Research. 2014;24:1193–1208. doi: 10.1101/gr.171546.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs AT, Jasinskiene N, Tretiakov M, Thiery I, Zettor A, Bourgouin C, James AA. Transgenic anopheles stephensi coexpressing single-chain antibodies resist Plasmodium falciparum development. PNAS. 2012;109:E1922–E1930. doi: 10.1073/pnas.1207738109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar B, Luo N, Farr CL, Kaguni LS, Campos AR. The accessory subunit of DNA polymerase gamma is essential for mitochondrial DNA maintenance and development in Drosophila melanogaster. PNAS. 2002;99:4483–4488. doi: 10.1073/pnas.072664899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandul NP, Liu J, Sanchez C HM, Wu SL, Marshall JM, Akbari OS. Transforming insect population control with precision guided sterile males with demonstration in flies. Nature Communications. 2019;10:84. doi: 10.1038/s41467-018-07964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandul NP, Liu J, Buchman A, Gantz VM, Bier E, Akbari OS. Assessment of a split homing based gene drive for efficient knockout of multiple genes. G3: Genes, Genomes, Genetics. 2020;10:827–837. doi: 10.1534/g3.119.400985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KaramiNejadRanjbar M, Eckermann KN, Ahmed HMM, Sánchez C HM, Dippel S, Marshall JM, Wimmer EA. Consequences of resistance evolution in a Cas9-based sex conversion-suppression gene drive for insect pest management. PNAS. 2018;115:6189–6194. doi: 10.1073/pnas.1713825115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzmiller JB. Genetics, cytogenetics, and evolution of mosquitoes. Advances in Genetics. 1976;18:315–433. doi: 10.1016/s0065-2660(08)60441-1. [DOI] [PubMed] [Google Scholar]
- Kyrou K, Hammond AM, Galizi R, Kranjc N, Burt A, Beaghton AK, Nolan T, Crisanti A. A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged anopheles gambiae mosquitoes. Nature Biotechnology. 2018;36:1062–1066. doi: 10.1038/nbt.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labun K, Montague TG, Gagnon JA, Thyme SB, Valen E. CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Research. 2016;44:W272–W276. doi: 10.1093/nar/gkw398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrenier N, Van Der Bruggen P, Foury F. Mitochondrial DNA polymerases from yeast to man: a new family of polymerases. Gene. 1997;185:147–152. doi: 10.1016/S0378-1119(96)00663-4. [DOI] [PubMed] [Google Scholar]
- Li M, Bui M, Yang T, White B, Akbari O. Germline Cas9 expression yields highly efficient genome engineering in a major worldwide disease vector, aedes aegypti. bioRxiv. 2017 doi: 10.1101/156778. [DOI] [PMC free article] [PubMed]
- Li M, Yang T, Kandul NP, Bui M, Gamez S, Raban R, Bennett J, Sánchez C HM, Lanzaro GC, Schmidt H, Lee Y, Marshall JM, Akbari OS. Development of a confinable gene drive system in the human disease vector aedes aegypti. eLife. 2020;9:e51701. doi: 10.7554/eLife.51701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Potter CJ. Editing transgenic DNA components by inducible gene replacement in Drosophila melanogaster. Genetics. 2016;203:1613–1628. doi: 10.1534/genetics.116.191783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KC, Alphey L, Annas GJ, Bloss CS, Campbell KJ, Champer J, Chen CH, Choudhary A, Church GM, Collins JP, Cooper KL, Delborne JA, Edwards OR, Emerson CI, Esvelt K, Evans SW, Friedman RM, Gantz VM, Gould F, Hartley S, Heitman E, Hemingway J, Kanuka H, Kuzma J, Lavery JV, Lee Y, Lorenzen M, Lunshof JE, Marshall JM, Messer PW, Montell C, Oye KA, Palmer MJ, Papathanos PA, Paradkar PN, Piaggio AJ, Rasgon JL, Rašić G, Rudenko L, Saah JR, Scott MJ, Sutton JT, Vorsino AE, Akbari OS. Core commitments for field trials of gene drive organisms. Science. 2020;370:1417–1419. doi: 10.1126/science.abd1908. [DOI] [PubMed] [Google Scholar]
- Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Marchler GH, Song JS, Thanki N, Yamashita RA, Yang M, Zhang D, Zheng C, Lanczycki CJ, Marchler-Bauer A. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Research. 2020;48:D265–D268. doi: 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay TF, Richards S, Stone EA, Barbadilla A, Ayroles JF, Zhu D, Casillas S, Han Y, Magwire MM, Cridland JM, Richardson MF, Anholt RR, Barrón M, Bess C, Blankenburg KP, Carbone MA, Castellano D, Chaboub L, Duncan L, Harris Z, Javaid M, Jayaseelan JC, Jhangiani SN, Jordan KW, Lara F, Lawrence F, Lee SL, Librado P, Linheiro RS, Lyman RF, Mackey AJ, Munidasa M, Muzny DM, Nazareth L, Newsham I, Perales L, Pu LL, Qu C, Ràmia M, Reid JG, Rollmann SM, Rozas J, Saada N, Turlapati L, Worley KC, Wu YQ, Yamamoto A, Zhu Y, Bergman CM, Thornton KR, Mittelman D, Gibbs RA. The Drosophila melanogaster Genetic Reference Panel. Nature. 2012;482:173–178. doi: 10.1038/nature10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM, Buchman A, Sánchez C HM, Akbari OS. Overcoming evolved resistance to population-suppressing homing-based gene drives. Scientific Reports. 2017;7:3776. doi: 10.1038/s41598-017-02744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM, Raban RR, Kandul NP, Edula JR, León TM, Akbari OS. Winning the Tug-of-War between effector gene design and pathogen evolution in vector population replacement strategies. Frontiers in Genetics. 2019;10:1072. doi: 10.3389/fgene.2019.01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM, Akbari OS. Can CRISPR-Based gene drive be confined in the wild? A question for molecular and population biology. ACS Chemical Biology. 2018;13:424–430. doi: 10.1021/acschembio.7b00923. [DOI] [PubMed] [Google Scholar]
- McVey M, Radut D, Sekelsky JJ. End-joining repair of double-strand breaks in Drosophila melanogaster is largely DNA ligase IV independent. Genetics. 2004;168:2067–2076. doi: 10.1534/genetics.104.033902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F, Gasch A, Kaltschmidt B, Renkawitz-Pohl R. A 14 bp promoter element directs the testis specificity of the Drosophila beta 2 tubulin gene. The EMBO Journal. 1989;8:1559–1565. doi: 10.1002/j.1460-2075.1989.tb03540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Board on Life Sciences; Division on Earth and Life Studies. Committee on Gene Drive Research in Non-Human Organisms: Recommendations for Responsible Conduct Gene drives on the horizon: advancing science, navigating uncertainty, and aligning research with public values. PNAS. 2016;28:23405. doi: 10.17226/23405. [DOI] [PubMed] [Google Scholar]
- Noble C, Olejarz J, Esvelt KM, Church GM, Nowak MA. Evolutionary dynamics of CRISPR gene drives. Science Advances. 2017;3:e1601964. doi: 10.1126/sciadv.1601964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhofer G, Ivy T, Hay BA. Behavior of homing endonuclease gene drives targeting genes required for viability or female fertility with multiplexed guide RNAs. PNAS. 2018;115:E9343–E9352. doi: 10.1073/pnas.1805278115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhofer G, Ivy T, Hay BA. Cleave and rescue, a novel selfish genetic element and general strategy for gene drive. PNAS. 2019;116:6250–6259. doi: 10.1073/pnas.1816928116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhofer G, Ivy T, Hay BA. 2-Locus cleave and rescue selfish elements harness a recombination rate-dependent generational clock for self limiting gene drive. bioRxiv. 2020a doi: 10.1101/2020.07.09.196253. [DOI]
- Oberhofer G, Ivy T, Hay BA. Gene drive and resilience through renewal with next generation cleave and rescue selfish genetic elements. PNAS. 2020b;117:9013–9021. doi: 10.1073/pnas.1921698117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Truman JW, Rubin GM. Using translational enhancers to increase transgene expression in Drosophila. PNAS. 2012;109:6626–6631. doi: 10.1073/pnas.1204520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TB, Phong CH, Bennett JB, Hwang K, Jasinskiene N, Parker K, Stillinger D, Marshall JM, Carballar-Lejarazú R, James AA. Experimental population modification of the malaria vector mosquito, anopheles stephensi. PLOS Genetics. 2019;15:e1008440. doi: 10.1371/journal.pgen.1008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F, Chen HM, Lee T, Bullock SL. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. PNAS. 2014;111:E2967–E2976. doi: 10.1073/pnas.1405500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston CR, Flores CC, Engels WR. Differential usage of alternative pathways of double-strand break repair in Drosophila. Genetics. 2006;172:1055–1068. doi: 10.1534/genetics.105.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . Vienna, Austria: R Foundation for Statistical Computing; 2017. https://www.R-project.org/ [Google Scholar]
- Raban RR, Marshall JM, Akbari OS. Progress towards engineering gene drives for population control. The Journal of Experimental Biology. 2020;223:jeb208181. doi: 10.1242/jeb.208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raban R, Akbari OS. Gene drives may be the next step towards sustainable control of malaria. Pathogens and Global Health. 2017;111:399–400. doi: 10.1080/20477724.2017.1453587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez HM, Sl W, Bennett JB, Marshall JM. MGDrivE: a modular simulation framework for the spread of gene drives through spatially-explicit mosquito populations. bioRxiv. 2019 doi: 10.1101/350488. [DOI]
- Sano H, Nakamura A, Kobayashi S. Identification of a transcriptional regulatory region for germline-specific expression of vasa gene in Drosophila melanogaster. Mechanisms of Development. 2002;112:129–139. doi: 10.1016/S0925-4773(01)00654-2. [DOI] [PubMed] [Google Scholar]
- Simoni A, Hammond AM, Beaghton AK, Galizi R, Taxiarchi C, Kyrou K, Meacci D, Gribble M, Morselli G, Burt A, Nolan T, Crisanti A. A male-biased sex-distorter gene drive for the human malaria vector anopheles gambiae. Nature Biotechnology. 2020;38:0508-1. doi: 10.1038/s41587-020-0508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terradas G, Buchman AB, Bennett JB, Shriner I, Marshall JM, Akbari OS, Bier E. Inherently confinable split-drive systems in Drosophila. bioRxiv. 2021 doi: 10.1101/2020.09.03.282079. [DOI] [PMC free article] [PubMed]
- Van Doren M, Williamson AL, Lehmann R. Regulation of zygotic gene expression in Drosophila primordial germ cells. Current Biology. 1998;8:243–246. doi: 10.1016/S0960-9822(98)70091-0. [DOI] [PubMed] [Google Scholar]
- Verkuijl SAN, González E, De Ang JX, Li M, Kandul NP, Anderson M, Akbari OS, Bonsall M, Alphey L. A CRISPR endonuclease gene drive reveals two distinct mechanisms of inheritance Bias. bioRxiv. 2020 doi: 10.1101/2020.12.15.421271. [DOI] [PMC free article] [PubMed]
- Windbichler N, Menichelli M, Papathanos PA, Thyme SB, Li H, Ulge UY, Hovde BT, Baker D, Monnat RJ, Burt A, Crisanti A. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature. 2011;473:212–215. doi: 10.1038/nature09937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Koolhaas WH, Schnorrer F. A Versatile Two-Step CRISPR- and RMCE-Based Strategy for Efficient Genome Engineering in Drosophila. G3: Genes, Genomes, Genetics. 2014;4:2409–2418. doi: 10.1534/g3.114.013979. [DOI] [PMC free article] [PubMed] [Google Scholar]