Abstract
Objective
To evaluate the relationship between direct cognitive assessment introduced with the Medicare Annual Wellness Visit (AWV) and new diagnoses of dementia, and to determine if effects vary by race.
Data Sources
Medicare Limited Data Set 5% sample claims 2003‐2014 and the HRSA Area Health Resources Files.
Study Design
Instrumental Variable approach estimating the relationship between AWV utilization and new diagnoses of dementia using county‐level Welcome to Medicare Visit rates as an instrument.
Data Collection/Extraction Methods
Three hundred twenty‐four thousand three hundred and eighty‐five fee‐for‐service Medicare beneficiaries without dementia when the AWV was introduced in 2011.
Principal Findings
Annual Wellness Visit utilization was associated with an increased probability of new dementia diagnosis with effects varying by racial group (categorized as white, black, Hispanic/Latino, or Asian based on Social Security Administration data). Hazard ratios (95% confidence intervals) for new dementia diagnosis within 6 months of AWV utilization were as follows: 2.34 (2.13, 2.58) white, 2.22 (1.71, 2.89) black, 4.82 (2.94, 7.89) Asian, and 6.14 (3.70, 10.19) Hispanic (P < .001 for each). Our findings show that estimates that do not control for selection underestimate the effect of AWV on new diagnoses.
Conclusions
Dementia diagnosis rates increased with AWV implementation with heterogenous effects by race and ethnicity. Current recommendations by the United States Preventive Services Task Force state that the evidence is insufficient to recommend for or against screening for cognitive impairment in older adults.
Keywords: cognitive impairment screening, dementia screening, Medicare Annual Wellness Visit
What is Known on This Topic?
Medicare established the Annual Wellness Visit, a preventive care visit requiring direct cognitive assessment as one of its components.
Evidence is currently insufficient to recommend for or against screening for cognitive impairment in older adults.
Previous studies of the effects of the Medicare Annual Wellness Visit on dementia diagnosis rates have not accounted for unmeasured confounders.
What This Study Adds?
We found that diagnosis of dementia increased because of the Annual Wellness Visit.
Hispanic/Latino beneficiaries experienced a larger increase in diagnosis rates than Asian, black and white beneficiaries.
1. INTRODUCTION
The burden of dementia is high and growing as the US population ages. In the United States, an estimated 5.8 million people are living with Alzheimer's disease, the most common type of dementia and the fifth‐leading cause of death in Americans age 65 and older. 1 Despite evidence of decreasing incidence and prevalence of Alzheimer's disease and other dementias, the total number of people with dementia will continue to increase due to population aging, 2 , 3 particularly the growth of the population age 85 and older in whom the prevalence of dementia is approximately 34%. 4 The number of people living with Alzheimer's disease is projected to reach 14 million by 2050 in the absence of significant advances in prevention or treatment. 5
Criteria for the diagnosis of dementia were updated by the National Institute on Aging and Alzheimer's Association in 2011. 6 Despite significant progress in the development of biomarkers of disease, dementia remains a clinical diagnosis. Screening for dementia has not been widely performed, since screening is typically promoted only for conditions that can be cured or have a substantial reduction in morbidity and/or mortality with earlier treatment initiation. 7 There is no cure for dementia, and current treatment options provide only modest symptomatic benefits in some patients. In 2020, the United States Preventive Services Task Force rated dementia screening with an “I” (“insufficient evidence to recommend for or against screening”). 8 The task force stated that “although there is insufficient evidence to recommend for or against screening for cognitive impairment, there may be important reasons to identify cognitive impairment early.” Early detection of cognitive impairment could allow for the treatment of reversible causes, it could improve adherence to medical treatment plans, and it may also help patients and families in advance care planning. The task force recommended clinicians to “remain alert to early signs or symptoms of cognitive impairment,” even though potential harms exist, such as labeling a person with an illness that has no effective treatment and the possibly of increased depression. These recommendations were informed by a literature review that found no empirical evidence demonstrating potential harms or benefits from screening. 9
In 2010, the Patient Protection and Affordable Care Act introduced the Medicare Annual Wellness Visit (AWV), a preventive care‐focused visit that requires direct cognitive assessment as one of its components, despite uncertainty about the value of screening for cognitive impairment in older adults. The AWV additionally includes a health risk assessment; establishing a current list of healthcare providers and medications; reviewing medical and family history, risk factors for depression, functional ability; measuring height, weight, and blood pressure; establishing a screening schedule and list of risk factors with recommended or current interventions; providing personalized health advice, appropriate referrals and advance care planning services. 10 The AWV became available in 2011, without copayment, to all beneficiaries past their first year of Medicare enrollment. Initial uptake of the AWV has been low, especially among racial and ethnic minority beneficiaries, but utilization has been increasing over time. 11 , 12 Since over half of all dementia cases are undetected when screening is not conducted regularly, 13 the AWV has the potential to increase the diagnosis rate of dementia in the Medicare population. Previous research has found that factors contributing to missed diagnoses include provider attitudes, problems with patient‐provider communication, deficits in education, and limitations of resources in the health system. 14
Differences in incidence and prevalence of dementia by race and ethnicity, as well as stage at diagnosis, evaluation, and treatment are common in the US population. 15 , 16 Compared to nonminority elders, minority patients are diagnosed at later stages of dementia. 15 Latino patients present with a more severe stage of disease and greater degree of impairment at time of diagnosis compared to non‐Latino white patients, but survive longer with dementia than non‐Latino white patients. 17 One study found that nearly half of all people who had a positive dementia screening result did not participate in further assessment (eg, neuropsychological testing, medical record review, or caregiver interview) and also found that refusal of assessment differed by age only for black patients, with black patients 80 and older more likely to refuse further assessment for dementia. 18 A qualitative study that assessed the reasons why black Americans underutilize dementia services found that developing trust with the black American community was a critical step to improving their outreach and education efforts. 19
Given the high rates of undiagnosed dementia, utilization of Medicare AWVs that require direct cognitive assessment may lead to more diagnoses at earlier disease stages. However, empirically estimating the effect of the AWV on diagnoses is challenging because of self‐selection of patients into seeking the AWV and provider self‐selection into offering or promoting the AWV to patients. The goal of this study was to estimate the causal relationship between AWV utilization and new diagnoses of dementia using an approach that exploits exogenous variation in county‐level rates of a preventive service, the Welcome to Medicare visit. We hypothesized that AWV utilization would be associated with an increased likelihood of receiving a new diagnosis of dementia. Because rates of undiagnosed dementia are higher in racial and ethnic minority elders, 20 , 21 a secondary goal was to estimate the effect of the AWV by race and ethnicity.
2. METHODS
2.1. Conceptual framework and empirical strategy
Beneficiaries who utilize an AWV are likely to be different from those who do not, and these differences in characteristics and behaviors may be associated with both the likelihood of receiving a new dementia diagnosis and also the stage at the time of first diagnosis. Similarly, evidence shows that provider adoption of the AWV has varied by geographical region. 22 Furthermore, since rates of both AWV utilization 11 and undiagnosed dementia 20 , 21 differ by race and ethnicity, the relationship between AWV utilization and new dementia diagnosis may be confounded by factors related to race and ethnicity. The main challenge of estimating the effect of the visit is that some of these confounding factors are not observable in claims or survey data. For example, healthier beneficiaries without symptoms of dementia may be more interested in screening and preventive services (the so‐called “worried well”), and thus more likely to use the AWV. As a consequence, an observational analysis would find that those who use the AWV are less likely to be diagnosed with dementia. On the other hand, it is possible that beneficiaries who are sicker and more likely to have dementia, use an AWV because they have a regular source of care and go to the doctor often which increases the likelihood that they are approached to schedule the visit.
To overcome this problem, we applied an instrumental variables (IV) approach using county‐level Welcome to Medicare Visit (WMV) rates as an instrument for AWV utilization. 23 The WMV is a preventive care examination available once to beneficiaries who are within their first twelve months of Medicare enrollment. The WMV includes many of the same components as the AWV, but it does not include direct cognitive assessment. We hypothesized that county‐level WMV utilization rates would be predictive of AWV utilization because the WMV measures area‐level uptake of preventive care and provider willingness to conduct preventive care visits for Medicare beneficiaries, but the WMV utilization rates would not be associated with the probability of receiving a new dementia diagnosis conditional on other factors that can be observed.
We estimated the model using two‐stage residual inclusion (2SRI). 24 , 25 In the first stage, we modeled the probability of utilizing the AWV as a function of beneficiary demographics, health status, socioeconomic factors, healthcare access, and the instrument, county‐level race‐specific utilization rates of the WMV, described in detail below. The validity of this instrument hinges on two conditions: (a) how well it predicts individual AWV utilization and (b) whether it can be validly excluded from the main equation (ie, the WMV is not a confounder). Specifically, this implies that the WMV is unrelated to the likelihood of receiving a new dementia diagnosis after controlling for covariates. The first condition can be tested using standard specification tests. 26 , 27 We expected that county‐level WMV rates would predict an individual's AWV utilization because county‐level WMV utilization will, in part, measure willingness of local providers to conduct and promote such preventive visits. The second condition, known as the exclusion restriction, must also be met for county‐level WMV to be a valid instrument. The exclusion restriction requires that the association between county‐level WMV and AWV utilization is not confounded by other factors such as an individual's health status and healthcare utilization behaviors. The exclusion restriction could be violated if county‐level WMV rates were associated with the probability of a dementia diagnosis, conditional on other covariates. Instead, the only variation in county‐level WMV rates that is relevant to an individual's dementia diagnosis must operate through the relationship with an individual's AWV utilization. Our approach is similar to that of Hadley and colleagues who used area‐level medical spending to predict healthcare utilization. 28
IV estimates are interpreted as local average treatment effects because they reflect variation in the treatment that is influenced by the instrument. Thus, county‐level WMV utilization rates should not be expected to impact the probability of utilizing an AWV for all individuals. For example, there may be some beneficiaries who, due to personal characteristics, would never utilize an AWV; such people may not seek preventive care regardless of its availability. Other beneficiaries may be more engaged in their health care and would always utilize an AWV, regardless of the rate by which county residents utilize the WMV, and regardless of Medicare coverage levels for such visits. However, some beneficiaries may utilize the AWV only due to the availability and popularity of preventive visits in their county, and the willingness of providers to conduct these visits. Thus, individual AWV utilization for these people is likely to be responsive to county‐level WMV rates. These beneficiaries represent the so‐called “compliers” 23 or the “marginal patient,” and our estimates of the effect of the AWV on dementia diagnosis only applies to this group of beneficiaries. Thus, we estimate the causal effect of utilizing an AWV on the probability of receiving a new dementia diagnosis among beneficiaries who would use a Medicare‐covered wellness visit because preventive visits are commonly practiced in their county of residence.
3. DATA
The sample was drawn from the Medicare Limited Data Set (LDS) 5% random sample from 2003 through 2014; sample size and exclusions are presented in Figure S1. The data include Medicare claims linked to Centers for Medicare and Medicaid Services administrative records containing demographic and enrollment information. We limited the sample to continuously enrolled fee‐for‐service (FFS) beneficiaries age 65 and older, because claims for services paid under a health maintenance organization are incomplete in the LDS dataset. Misclassification of race and ethnicity data in the LDS (provided by the Social Security Administration) is acknowledged. However, substantial efforts to reduce racial and ethnic misclassification have been made, which precede the collection of the data used in our analysis. 29 Racial and ethnic misclassification still varies by group, with high sensitivity for correctly classifying black and white beneficiaries, but lower sensitivity for Asian and Pacific Islander and Hispanic/Latino beneficiaries. 29
We also used the HRSA Area Health Resources File 30 which contains county‐level measures of population, demographic composition, education, income and other financial indicators, and healthcare resources. Variables from the Area Health Resource File were merged on to the LDS data by county and year.
3.1. Sample selection
The index date of the analysis was January 1, 2011. We included beneficiaries who were age 65 in the years 2003 to 2008, and who had no dementia diagnosis as of January 1, 2011. In other words, we only included beneficiaries who were at risk of receiving a first dementia diagnosis when the AWV became available January 1, 2011, and we followed them forward from this time point when the beneficiaries were 68‐76 years old. Applying a look‐back period from entry to Medicare to at least 3 years forward provided us greater certainty that any diagnoses of dementia occurring in 2011 or later were in fact new diagnoses, since prevalence of dementia at age 65 is low but increases with age. The years of data used to apply exclusion criteria and for the analyses for each age cohort are displayed in Figure 1.
FIGURE 1.
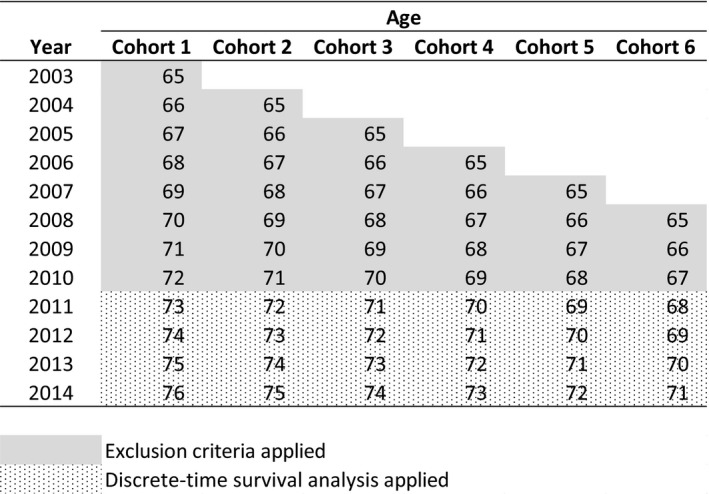
Medicare LDS 2003‐2014: Data Used. Continuously enrolled fee‐for‐service Medicare beneficiaries who were age 65 in years 2003 to 2008, and as of January 1, 2011 had no evidence of cognitive impairment were included in the analysis. Exclusion criteria (any claims with diagnostic codes reflecting a dementia diagnosis) were applied through the end of 2010. Years 2011 and later were included in the discrete‐time survival analysis. Beneficiaries were followed until 2014 if the beneficiary survived without dementia, or until the year of dementia diagnosis or death
4. VARIABLES AND EMPIRICAL SPECIFICATION
4.1. Dependent variables
Dementia was identified using the first occurrence of an ICD‐9 code for dementia in the list of active diagnoses using the same diagnostic codes used by the Medicare Chronic Conditions Data Warehouse for dementia. 31 Claims records were used to identify procedure codes for the AWV (G0438 or G0439). We created a set of dummy variables for AWV used within the past one to six months with the expectation that some beneficiaries who screened positive for dementia during the AWV would require additional assessment prior to diagnosis, thus diagnoses related to utilizing an AWV could occur after the actual visit. We created indicator variables for AWV utilization within the past six months for each racial and ethnic group to allow variation in AWV utilization effects by race/ethnicity. We used the indicators for AWV utilization within six months in the primary analysis to allow enough time to receive follow‐up care necessary for a diagnosis .
4.2. Instrumental variables
The specified instruments were county‐level measures of race‐specific WMV utilization rates for a given month. We used four instruments since we were interested in the AWV effect for each of the four racial/ethnic groups (ie, AWV by race/ethnicity interactions). These rates were calculated as the number of beneficiaries with a claim for a WMV in a given county in a given month, divided by the number of WMV‐eligible beneficiaries (ie, beneficiaries aged 65‐years) for a given racial/ethnic group. WMV utilization was identified using the procedure code G0402 for 2011‐2014.
4.3. Covariates
Demographic variables included time‐invariant (fixed) covariates: sex, race/ethnicity (categorized as white, black, Hispanic/Latino or Asian based on Social Security Administration data in the LDS denominator file), cohort (defined by age at study baseline), interactions between sex and race/ethnicity, interactions between cohort and sex; and time‐varying covariates: county‐level measures of the proportion of aged people in the county overall, the county‐level percent of people in the beneficiary's racial/ethnic group, and the percentage of people who are non‐English speaking in the county. Socioeconomic variables were all time‐varying and included overall and race/ethnicity‐specific county‐level measures of income (categorized as: less than $10 000; $10 000 to $14 999; $15 000 to $24 999; $25 000 to $49 999; $50 000 to $99 999 and $100 000 or greater), education (categorized as: less than high school, high school diploma, any education beyond high school), percent of people age ≥ 65 who are in deep poverty, and the median home value (as a continuous measure). Health status variables included time‐varying indicators for common chronic conditions (myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, mild liver disease, moderate to severe liver disease, diabetes without complications, diabetes with complications, paraplegia and hemiplegia, renal disease, cancer, metastatic cancer, HIV/AIDS) identified using the Charlson comorbidity index, 32 excluding dementia (since it was a dependent variable), identified using diagnostic codes in inpatient and outpatient Medicare claims. Healthcare access and utilization variables included the beneficiary's utilization of outpatient visits, inpatient stays, and length of inpatient stays in the year prior to the year of the observation year (eg, an observation in 2012 would have healthcare utilization measures from 2011), the county‐level proportion of Medicare beneficiaries enrolled in FFS Medicare, and a county‐level measure of the percent of the population that is urban dwelling (all time‐varying). County‐level measures were merged on to the LDS from the Area Health Resource File.
4.4. Model specifications
We used discrete‐time survival (DTS) models 33 , 34 to measure the association between new dementia diagnosis and AWV utilization by race interactions controlling for the covariates described above. DTS allows for time‐varying covariates within the structure of a survival model (note that most of our covariates were time‐varying). DTS uses logistic regression by aggregated (ie, discrete) time intervals rather than treating time as continuous. Our DTS approach used a dataset with one record per beneficiary per month from January 2011 until dementia diagnosis, death, or censoring (after December 2014). In the first stage, we fit a logit model with AWV utilization as the outcome, instruments (race/ethnicity‐specific county‐level WMV rates) as independent variables, and all covariates used in the observational and second‐stage models (indicator variables for each month are also included). We then calculated the residual (predicted minus observed AWV utilization) which was included in the second‐stage model. In the second stage, we fit a logit model with new dementia diagnosis as the outcome, adjusted for the same set of covariates used in the observational and first‐stage models, and also adjusted for the residual. After fitting the second‐stage model, we generated hazard ratios (HRs), which can be interpreted as the relative change in dementia diagnosis rates associated with a change in relation to a reference category for categorical variables, or differences in the rate of dementia diagnosis for a unit change for continuous variables. We tested the null hypothesis of no association between new diagnosis and AWV utilization within six months for each racial/ethnic group.
In sensitivity analyses, we evaluated AWV utilization within one month through twelve months to explore if our findings were sensitive to follow‐up time. We also include overall (not race‐specific) estimates for AWV utilization within 6 months, and a falsification test that analyses just the first month of outcomes (January 2011) where we would not expect to yet see an association between AWV utilization and new dementia diagnosis. In additional sensitivity analyses, we analyzed the association between AWV and subsequent neuropsychological testing using a similar approach as Ganguli et al 35 identifying visits with procedure codes 99201‐5 or 99241‐5 conducted by providers with specialty codes for psychiatry (26, 27, 62, 68, 80) or neurology (13). Although neuropsychological testing may be performed to help confirm and better characterize subjective or objective cognitive impairment, it is not required for diagnosis. Use of neuropsychological testing reflects clinician discretion, patient preference and access to testing, which is often limited. However, an association would indicate that the AWV resulted in increased consultation with specialists because of a diagnosis or to confirm a diagnosis.
5. RESULTS
5.1. Sample characteristics
The analysis sample included a total of 14 258 865 person‐months of observation for 324 485 beneficiaries. Sample characteristics are presented in Table 1. Ninety percent of the beneficiaries were white. Black beneficiaries were the largest racial/ethnic minority group, comprising 7.3% of the sample. Asian and Hispanic beneficiaries represented 1.5% and 1.1% of the sample, respectively. Due to small sample sizes and missing data, other racial/ethnic minority groups were excluded from the analyses. During the study period, 8.2% of beneficiaries died, and Black beneficiaries had the largest proportion of deaths. AWV utilization increased over time for all racial groups and was highest for white beneficiaries.
Table 1.
Sample characteristics (n = 324 485)
| Variable | Overall | White | Black | Asian | Hispanic |
|---|---|---|---|---|---|
| At baseline (Index Date = January 1, 2011) | |||||
| Age cohort, col.% | |||||
| Age 73 at baseline | 13.95% | 14.07% | 13.07% | 13.21% | 10.96% |
| Age 72 at baseline | 15.08% | 15.21% | 14.18% | 12.92% | 13.17% |
| Age 71 at baseline | 15.38% | 15.39% | 15.18% | 15.51% | 15.30% |
| Age 70 at baseline | 16.57% | 16.50% | 17.09% | 17.08% | 18.00% |
| Age 69 at baseline | 18.00% | 17.90% | 18.72% | 19.37% | 18.99% |
| Age 68 at baseline | 21.03% | 20.92% | 21.77% | 21.91% | 23.57% |
| Total, row % | 100% | 90.04% | 7.33% | 1.49% | 1.14% |
| Female, col. % | 51.97% | 51.71% | 54.33% | 56.75% | 51.16% |
| Health status (Comorbidities), col.% | |||||
| Myocardial infarction | 5.31% | 5.43% | 4.60% | 2.88% | 3.43% |
| Congestive heart failure | 11.64% | 11.22% | 17.05% | 9.71% | 11.87% |
| Peripheral vascular disease | 16.31% | 16.02% | 20.26% | 13.96% | 16.32% |
| Cerebrovascular disease | 17.59% | 17.55% | 19.11% | 15.66% | 14.08% |
| Chronic pulmonary disease | 26.65% | 26.94% | 25.14% | 21.30% | 20.39% |
| Rheumatic disease | 5.47% | 5.40% | 6.18% | 5.10% | 6.50% |
| Peptic ulcer disease | 2.87% | 2.75% | 3.46% | 6.16% | 3.83% |
| Mild liver disease | 7.78% | 7.65% | 8.02% | 13.44% | 9.36% |
| Moderate to severe liver disease | 0.47% | 0.46% | 0.46% | 0.50% | 0.78% |
| Diabetes without complications | 29.99% | 28.70% | 42.97% | 38.37% | 37.01% |
| Diabetes with complications | 9.32% | 8.57% | 17.34% | 11.08% | 14.78% |
| Paraplegia and hemiplegia | 1.37% | 1.28% | 2.46% | 1.41% | 1.51% |
| Renal disease | 8.81% | 8.24% | 15.86% | 8.48% | 9.25% |
| Cancer | 15.94% | 16.08% | 16.65% | 10.04% | 8.42% |
| Metastatic cancer | 2.28% | 2.28% | 2.55% | 1.85% | 1.38% |
| HIV/AIDS | 0.11% | 0.08% | 0.49% | 0.12% | 0.19% |
| During follow‐up (January 1, 2011 – December 31, 2014) | |||||
| Outcome, col. % | |||||
| Died | 8.18% | 8.05% | 10.14% | 6.08% | 8.70% |
| New dementia diagnosis | 4.69% | 4.55% | 6.67% | 3.60% | 4.96% |
| Censored (alive at last follow‐up) | 87.12% | 87.40% | 83.20% | 90.32% | 86.34% |
| AWV utilization, col.% | |||||
| 2011 | 8.74% | 9.11% | 5.47% | 6.68% | 3.34% |
| 2012 | 11.89% | 12.33% | 8.04% | 9.80% | 5.17% |
| 2013 | 14.79% | 15.29% | 10.33% | 12.63% | 7.35% |
| 2014 | 17.82% | 18.33% | 13.52% | 15.28% | 8.84% |
| During 2011 | |||||
| WMV utilization rate per 1000 eligible beneficiaries, mean (SD) | 26.19 (30.67) | 27.93 (32.98) | 14.58 (78.92) | 10.67 (60.70) | 6.83 (67.63) |
| Annual healthcare utilization mean (SD) | |||||
| Outpatient visits | 15.67 (16.81) | 15.86 (16.69) | 14.87 (18.29) | 12.05 (15.33) | 11.15 (16.43) |
| Inpatient stays | 0.23 (0.71) | 0.22 (0.71) | 0.28 (0.84) | 0.12 (0.51) | 0.18 (0.64) |
| Inpatient days | 1.17 (5.46) | 1.14 (5.33) | 1.65 (7.13) | 0.60 (3.78) | 1.02 (4.95) |
| County‐level demographic factors, mean (SD) | |||||
| Percent Non‐English‐speaking residents | 0.03 (0.03) | 0.03 (0.03) | 0.04 (0.03) | 0.07 (0.04) | 0.08 (0.05) |
| Proportion of FFS beneficiaries in county | 0.83 (0.23) | 0.83 (0.23) | 0.84 (0.23) | 0.68 (0.25) | 0.72 (0.22) |
| Percent Urban population in county | 74.97 (27.31) | 73.79 (27.52) | 83.56 (24.39) | 94.23 (11.87) | 90.42 (15.24) |
| County‐level socioeconomic factors | |||||
| Education—percent of area residents at level, mean (sd) | |||||
| Less than high school | 11.10 (7.89) | 9.32 (4.69) | 17.68 (6.62) | 14.68 (7.25) | 38.39 (9.42) |
| High school | 88.89 (7.93) | 90.68 (4.69) | 82.31 (6.66) | 85.28 (7.46) | 61.58 (9.45) |
| College | 29.58 (13.56) | 31.00 (12.95) | 18.07 (7.55) | 49.47 (13.19) | 12.78 (5.82) |
| Income, percent of residents at level, mean (SD) | |||||
| Less than $10 000 | 0.07 (0.04) | 5.84 (2.31) | 14.63 (5.68) | 6.56 (3.74) | 8.54 (4.31) |
| $10 000 to $14 999 | 0.05 (0.02) | 4.90 (1.87) | 8.56 (3.61) | 3.89 (2.81) | 6.66 (2.73) |
| $15 000 to $24 999 | 0.11 (0.04) | 10.13 (3.11) | 14.24 (4.42) | 7.49 (4.29) | 14.00 (3.51) |
| $25 000 to $49 999 | 0.24 (0.05) | 23.39 (5.02) | 26.16 (4.51) | 17.51 (6.42) | 28.88 (4.43) |
| $50 000 to $99 999 | 0.31 (0.04) | 31.29 (3.30) | 24.43 (6.03) | 29.03 (6.26) | 28.35 (4.61) |
| $100 000 or greater | 0.23 (0.12) | 24.46 (11.38) | 11.95 (8.27) | 35.37 (12.10) | 13.58 (5.91) |
| Median home value ($100 000), mean (SD) | 2.05 (1.27) | 1.99 (1.21) | 2.08 (1.32) | 3.55 (1.80) | 2.59 (1.60) |
| Percent of dual‐eligible (medicare & Medicaid) Enrollees, mean (SD) | 21.31 (8.89) | 20.79 (8.49) | 24.33 (9.56) | 26.29 (11.61) | 29.40 (13.27) |
324 485 fee‐for‐service (FFS) Medicare beneficiaries who were aged 68‐73 years in 2011, and had no evidence of a dementia diagnosis as of January 1, 2011 when the Annual Wellness Visit (AWV) was introduced. Utilization and health status measures are dervied from Medicare claims data. Socioeconomic measures are from the Area Health Resources File.
Abbreviation: WMV, Welcome to Medicare Visit.
5.2. First stage
As hypothesized, race‐specific AWV utilization was associated with WMV rate. The joint test of the significance of all four race‐specific WMV rates produced large F‐values, 2435.58 and 2295.16, respectively (Table 2). The estimates of the coefficients on race‐specific WMV utilization rates were statistically significant for each racial group, although the HR is notably large for white (HR: 101.53) beneficiaries compared with the groups which had more modest HRs of 1.69, 1.43, and 1.16 for black, Asian, and Hispanic beneficiaries, respectively.
Table 2.
Hazard Ratios (HR) for the primary independent variables for the outcomes of new dementia diagnosis
| 2SRI | Logit | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dementia | |||||||||
| First‐stage AWV utilization model | Second‐stage diagnosis model | Observational diagnosis model | |||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| AWV utilization within 6 mo | |||||||||
| White | 2.34 | 2.13, 2.58 | <.001 | 0.87 | 0.81, 0.94 | <.001 | |||
| Black | 2.22 | 1.71, 2.89 | <.001 | 0.81 | 0.63, 1.05 | .11 | |||
| Asian | 4.82 | 2.94, 7.89 | <.001 | 1.74 | 1.06, 2.83 | .03 | |||
| Hispanic | 6.14 | 3.70, 10.19 | <.001 | 2.19 | 1.32, 3.61 | .002 | |||
| Race‐Specific WMV utilization (exogenous identifying variables) | |||||||||
| White | 101.53 | 83.73, 123.12 | <.001 | ||||||
| Black | 1.69 | 1.56, 1.84 | <.001 | ||||||
| Asian | 1.43 | 1.31, 1.56 | <.001 | ||||||
| Hispanic | 1.16 | 1.01, 1.33 | .04 | ||||||
| Residual | 0.28 | 0.26, 0.30 | <.001 | ||||||
Joint F‐test for all four instruments: F = 2435.58, P < .001. Estimates are adjusted for demographic and socioeconomic factors, health status, and healthcare utilization in the prior year (see Table S1 for all covariates). Stage 1 estimates indicate a strong relationship between county‐level Welcome to Medicare Visit Utilization and Annual Wellness Visit (AWV) Utilization for all racial groups. Observational estimates show a modest protective effect of AWV utilization on new dementia diagnosis for white and black beneficiaries, and a positive effect of AWV utilization on new dementia diagnosis for Hispanic and Asian beneficiaries. Second stage estimates show positive and higher (relative to observational estimates) effects of AWV utilization on new dementia diagnosis for all racial groups.
5.3. New dementia diagnosis
4.7% of beneficiaries received a new dementia diagnosis during the study period. In the observational models, the relationship to AWV utilization was allowed to vary across racial and ethnic groups, with small negative associations with dementia for white (HR: 0.87, 95% CI: 0.81, 0.94) and black (HR: 0.81, 95% CI: 0.63, 1.05) beneficiaries, and moderate positive associations with dementia for Asian (HR: 1.74, 95% CI: 1.06, 2.83) and Hispanic (HR: 2.19, 95% CI: 1.32, 3.61) beneficiaries (Table 2). Adjusting for the residual generated in the first stage, the second‐stage IV estimates found a significant increase in the probability of a new dementia diagnosis within 6 months of AWV utilization for each racial/ethnic group. The estimates for AWV utilization within 6 months became positive for white (HR: 2.34) and black (HR: 2.22) beneficiaries—a notable change from the small negative estimates in the observational model—and the estimates increased for both Asian and Hispanic beneficiaries relative to the observational estimates. The HRs for AWV utilization within 6 months are largest for Hispanic and Asian beneficiaries; 4.82 and 6.14, respectively.
5.4. Sensitivity analyses
Table S2 and Figure 2 present regression results for the sensitivity analyses for the outcome of dementia diagnosis which vary the exposure time for the AWV. AWV utilization had the largest positive effect on new dementia diagnosis within the first month of utilization for white and Hispanic beneficiaries, and within three months of utilization for black and Asian beneficiaries. However, within a given racial group estimates remained relatively similar across the exposure periods. Table S3 presents regression results for all races combined, which are similar to the estimates for white beneficiaries, since they make up a large majority of the sample. Table S4 presents a falsification test that analyses only the first month of observations for January 2011. In this first month of the AWV becoming available, AWV utilization was associated with WMV utilization in the first stage (P < .001), but AWV utilization was not associated with new dementia diagnoses in the second stage (P = .20).
FIGURE 2.
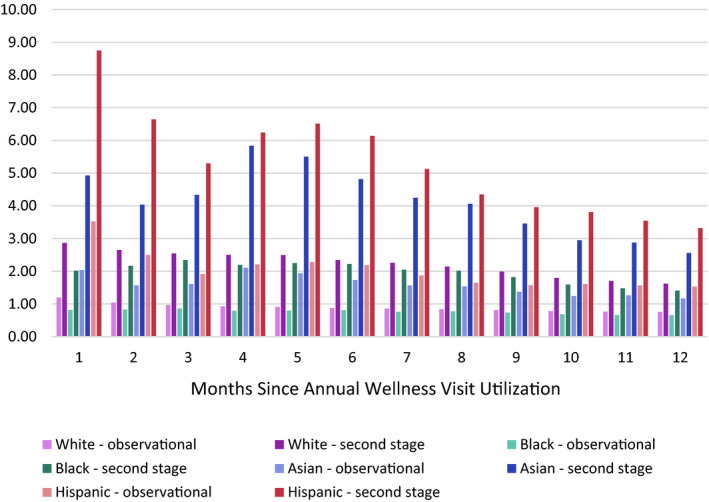
Hazard ratios for sensitivity analyses for dementia. Estimates are adjusted for demographic and socioeconomic factors, health status, and healthcare utilization in the prior year. Second‐stage estimates indicated a greater hazard of receiving a new dementia diagnosis following AWV utilization, relative to observational estimates, at all time points following AWV use from 1 to 12 months and across all racial groups [Color figure can be viewed at wileyonlinelibrary.com]
Table S5 presents regression results for the analysis of AWV utilization and subsequent neuropsychological testing. The hazard ratios indicate an increased rate of neuropsychological testing following AWV utilization, which was higher for Hispanic beneficiaries compared with Asian, Black, and white beneficiaries.
6. DISCUSSION
This study found that AWV utilization was associated with an increase in the probability of receiving a new diagnosis of dementia. Using race/ethnicity‐specific county‐level WMV utilization as an instrument for AWV utilization, we applied an IV approach to account for selection in both the patient and provider level and found that county‐level WMV utilization was a strong predictor of individual AWV utilization. If selection is not taken into account, the effect of AWV utilization on new diagnoses is underestimated in observational models and in some instances even switched direction, although the magnitude of this underestimation varied across racial and ethnic groups. Beneficiaries who use AWVs (ie, preventive care) tend to be healthier than those who do not, thus failure to account for endogeneity will bias the estimates downwards. The effect of the AWV varied across racial and ethnic groups; the AWV had the greatest impact on new dementia diagnoses among Hispanic/Latino beneficiaries whose diagnosis rates were over 6‐times higher when using the AWV. This heterogenous finding by race and ethnicity could be explained by pre‐AWV differences in dementia prevalence and incidence across racial and ethnics groups and also differences in the proportion of undiagnosed cases.
Accounting for selection may explain the significant increases in dementia diagnoses associated with AWV utilization that our study found, while another study that matched on observed beneficiary characteristics did not find the AWV significantly increased dementia diagnosis rates. 36 Together these two studies suggest that healthier beneficiaries (with less dementia) are more likely to self‐select into using an AWV. Dementia is unique from other chronic conditions addressed in the AWV in that it has no disease‐modifying treatments, it has not been regularly screened for (eg, depression is screened for and treatable). Thus, finding that the AWV has a causal effect on dementia diagnosis rates contributes to the debate about policy that promotes screening for cognitive impairment. Our results indicate that the AWV resulted in an increase in dementia diagnoses, yet the value of an increase in diagnoses has not been demonstrated because of insufficient evidence on harms and benefits. 9 One implication of our study is that if new evidence more clearly demonstrates that screening is either not beneficial, or is harmful, the requirement for direct cognitive assessment as part of the AWV should be reassessed, perhaps recommending targeted screening, or redirecting resources toward more clearly beneficial interventions. Considering the uncertainty surrounding the value of screening, our finding that the AWV increased the diagnosis of dementia to a greater extent in Hispanic/Latino beneficiaries must be interpreted with caution. Future research should examine the effects of the AWV on disparities in situations in which the value of screening is well established (eg, colorectal or breast cancer). However, our findings show that future research must take self‐selection of patients and providers into account.
Our study design has several strengths. First, the large sample size provided ample power to detect the effects of AWV utilization despite AWV utilization being low during the study period. The LDS also provided a random, representative sample of FFS Medicare beneficiaries in whom cognitive assessment may have an impact. Given the exclusion criteria used in this study our results are generalizable to Medicare FFS beneficiaries who survive until at least age 68 without receiving a diagnosis of dementia, and those who would be considered “compliers” (ie, beneficiaries who use the AWV because preventive care is available and practiced in their area). The group of “compliers” are a relevant group when it comes to evaluating this coverage expansion since they are the beneficiaries who will use the AWV because preventive care is available in their county. Second, our use of IV accounts for selection that is likely to be present in the AWV but is not observed in datasets. This instrument is analogous to other studies that used area‐level healthcare utilization measures as an instrument for individual‐level healthcare utilization. 28 Finally, the use of the DTS analysis provided allowed us to take into account time‐varying covariates.
These results should be interpreted with consideration to the study limitations. First, AWV utilization is an imperfect measure of cognitive assessment. While direct cognitive assessment is a required component of the AWV, the specific screening method or instrument is left to the discretion of the provider, although the Alzheimer's Association has published recommendations on how to implement this part of the AWV. 37 Due to this variation in practice, the effects of AWV utilization should not be interpreted as the effect of rigorous systematic screening with a validated screening tool, which might be expected to produce much larger effect sizes, although finding an effect suggests that cognitive assessment is performed. Second, effect sizes in this study may also be underestimated due to the additional evaluation that may be needed to establish a diagnosis, as noted above. For example, not all patients who screen positive for dementia will have access to or complete recommended evaluation and follow‐up, such as specialty consultation, neuroimaging, or neuropsychological testing. Our findings show a smaller effect on follow‐up neuropsychological visits. Third, our estimates may not be generalizable to other age cohorts of Medicare beneficiaries beyond those who turned 65 during 2003‐2008 due to differences in comorbid conditions, educational attainment, and other characteristics that may affect the risk of dementia. Fourth, we estimated a local average treatment effect which is generalizable only to people who would use an AWV because preventive care is practiced and readily available where they live, that is, the “compliers,” and it is possible that the IV approach did not fully correct for unmeasured confounding and that our estimates still have some degree of bias. Fifth, our results may be limited by potential productivity spillovers, in that providers in areas with high rates of preventive visits like the AWV and WMV may get better at delivering preventive care, and in turn, may then have a healthier group of Medicare beneficiaries relative to areas with lower rates of preventive visits. 38 However, the decades‐long time course for the development of dementia suggests this is unlikely to be a substantial source of bias. Sixth, we were unable to measure the impact of accountable care organizations (ACOs) because ACOs became increasingly common at the same time AWV utilization was increasing. However, both ACO penetration and AWV utilization are associated with higher education, wealth, and more urban beneficiaries. 39 Other researchers have attempted to identify relevant follow‐up care associated with screening positive during the AWV, 35 but this approach has imperfect specificity because these visit types are also used for investigating other conditions, and because this follow‐up care may not always be ordered by the primary care physician or if ordered the beneficiary may not always complete the recommended follow‐up. Follow‐up care that may be relevant to dementia workup (neuropsychological testing) was found to be modestly associated with AWV utilization in previous research. 35 Finally, since our analysis focused on one component of the AWV, we cannot comment on how the AWV has impacted other outcomes which be impacted by the many components of the AWV.
Many people with dementia are undiagnosed, yet screening has not been common practice prior to the introduction of the Medicare AWV. Our results suggest that the AWV is identifying new cases of dementia earlier. Given the low utilization rates of the AWV observed in our study and in others, the total number of new cases identified as a result of the AWV is relatively low, but are likely to increase over time as utilization of the AWV continues to increase, 22 , 40 , 41 despite the uncertain value of screening for cognitive impairment in older adults. 8
Supporting information
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENT
Joint Acknowledgement/Disclosure Statement: The University of Colorado supported this work. The authors have no conflicts of interest.
Lind KE, Hildreth K, Lindrooth R, Morrato E, Crane LA, Perraillon MC. The effect of direct cognitive assessment in the Medicare annual wellness visit on dementia diagnosis rates. Health Serv Res. 2021;56:193–203. 10.1111/1475-6773.13627
REFERENCES
- 1. Centers for Disease Control and Prevention . Mortality From Alzheimer’s Disease in the United States. Data for 2000 and 2010. Data Briefs ‐ Number 116 ‐ March 2013. Published March 2013. http://www.cdc.gov/nchs/data/databriefs/db116.htm. Accessed August 6, 2015 [Google Scholar]
- 2. Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Int Med. 2017;177(1):51. 10.1001/jamainternmed.2016.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deckers K, van Boxtel MPJ, Schiepers OJG, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30(3):234‐246. 10.1002/gps.4245 [DOI] [PubMed] [Google Scholar]
- 4. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778‐1783. 10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alzheimer’s disease facts and figures. Alzheimers Dement J Alzheimers Assoc. 2019;15(3):321‐387. 10.1016/j.jalz.2019.01.010 [DOI] [Google Scholar]
- 6. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2011;7(3):263‐269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moyses Szklo F, Nieto J. Epidemiology Beyond the Basics, 2nd edn. Burlington, MA: Jones and Bartlett Publishers; 2007. [Google Scholar]
- 8. Owens DK, Davidson KW, Krist AH, et al. Screening for cognitive impairment in older adults: US preventive services task force recommendation statement. JAMA. 2020;323(8):757‐763. 10.1001/jama.2020.0435 [DOI] [PubMed] [Google Scholar]
- 9. Patnode CD, Perdue LA, Rossom RC, et al. Screening for cognitive impairment in older adults: updated evidence report and systematic review for the US preventive services task force. JAMA. 2020;323(8):764‐785. 10.1001/jama.2019.22258 [DOI] [PubMed] [Google Scholar]
- 10. Preventive visit & yearly wellness exams | Medicare.gov. https://www.medicare.gov/coverage/preventive‐visit‐and‐yearly‐wellness‐exams.html. Accessed December 2, 2015 [Google Scholar]
- 11. Lind KE, Hildreth K, Lindrooth R, Crane L, Moratto E, Perraillon M. Ethnoracial disparities in Medicare annual wellness visit utilization. Med Care. 2018;56:761‐766. 10.1097/MLR.0000000000000962 [DOI] [PubMed] [Google Scholar]
- 12. Lind KE, Hildreth KL, Perraillon MC. Persistent disparities in Medicare’s annual wellness visit utilization. Med Care. 2019;57:1. [DOI] [PubMed] [Google Scholar]
- 13. Boustani M, Peterson B, Hanson L, Harris R, Lohr KN, U.S. Preventive Services Task Force . Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138(11):927‐937. [DOI] [PubMed] [Google Scholar]
- 14. Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009;23(4):306‐314. 10.1097/WAD.0b013e3181a6bebc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehta KM, Yaffe K, Pérez‐Stable EJ, et al. Race/ethnic differences in AD survival in US Alzheimer’s Disease Centers. Neurology. 2008;70(14):1163‐1170. 10.1212/01.wnl.0000285287.99923.3c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mehta KM, Yin M, Resendez C, Yaffe K. Ethnic differences in acetylcholinesterase inhibitor use for Alzheimer disease. Neurology. 2005;65(1):159‐162. 10.1212/01.wnl.0000167545.38161.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Livney MG, Clark CM, Karlawish JH, et al. Ethnoracial differences in the clinical characteristics of Alzheimer’s disease at initial presentation at an urban Alzheimer’s disease center. Am J Geriatr Psychiatry. 2011;19(5):430‐439. 10.1097/JGP.0b013e3181f7d881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boustani M, Perkins AJ, Fox C, et al. Who refuses the diagnostic assessment for dementia in primary care? Int J Geriatr Psychiatry. 2006;21(6):556‐563. 10.1002/gps.1524 [DOI] [PubMed] [Google Scholar]
- 19. Danner DD, Smith CD, Jessa P, Hudson J. African Americans with memory loss: findings from a community clinic in Lexington, Kentucky. Nurs Clin North Am. 2008;43(3) :437‐447. 10.1016/j.cnur.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yaffe K, Falvey C, Harris TB, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. 10.1136/bmj.f7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whitmer RA, Mayeda ER, Quesenberry CP Jr, Lu W, Glymour M. Ethnic and racial disparities in ten‐year cumulative prevalence of dementia and Alzheimer’s disease. Alzheimers Dement. 2014;10(4, Supplement):P152. 10.1016/j.jalz.2014.04.121 [DOI] [Google Scholar]
- 22. Ganguli I, Souza J, McWilliams JM, Mehrotra A. Trends in use of the US medicare annual wellness visit, 2011–2014. JAMA. 2017;317(21):2233‐2235. 10.1001/jama.2017.4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996;9(434):444‐455. [Google Scholar]
- 24. Terza JV, Basu A, Rathouz PJ. Two‐stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008;27(3):531‐543. 10.1016/j.jhealeco.2007.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Terza JV. Two‐stage residual inclusion estimation in health services research and health economics. Health Serv Res. 2018;53(3):1890‐1899. 10.1111/1475-6773.12714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Staiger D, Stock JH. Instrumental variables regression with weak instruments. Econometrica. 1997;65(3):557‐586. 10.2307/2171753 [DOI] [Google Scholar]
- 27. Stock J, Yogo M. Testing for Weak Instruments in Linear IV Regression. National Bureau of Economic Research, Inc; 2002. http://econpapers.repec.org/paper/nbrnberte/0284.htm [Google Scholar]
- 28. Hadley J, Waidmann T, Zuckerman S, Berenson RA. Medical spending and the health of the elderly. Health Serv Res. 2011;46(5):1333‐1361. 10.1111/j.1475-6773.2011.01276.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eicheldinger C, Bonito A. More accurate racial and ethnic codes for medicare administrative data. Health Care Financ Rev. 2008;29(3):27‐42. [PMC free article] [PubMed] [Google Scholar]
- 30. Health Resources & Services Administration . Area Health Resources Files. data.HRSA.gov | Health Resources & Services Administration. Published 2019. https://data.hrsa.gov/topics/health‐workforce/ahrf. Accessed September 14, 2019. [Google Scholar]
- 31. Centers for Medicare and Medicaid . Condition Categories ‐ Chronic Conditions Data Warehouse. Published 2015. https://www.ccwdata.org/web/guest/condition‐categories. Accessed December 3, 2015 [Google Scholar]
- 32. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 33. Pierce DA, Stewart WH, Kopecky KJ. Distribution‐free regression analysis of grouped survival data. Biometrics. 1979;35(4):785‐793. 10.2307/2530110 [DOI] [PubMed] [Google Scholar]
- 34. Prentice RL. Regression analysis of censored survival data with extensions to include competing risks and case‐control studies. Environ Int. 1978;1(6):321‐329. 10.1016/0160-4120(78)90007-7 [DOI] [Google Scholar]
- 35. Ganguli I, Souza J, McWilliams JM, Mehrotra A. Association of medicare’s annual wellness visit with cancer screening, referrals, utilization and spending. Health Aff Proj Hope. 2019;38(11):1927‐1935. 10.1377/hlthaff.2019.00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fowler NR, Campbell NL, Pohl GM, et al. One‐year effect of the Medicare annual wellness visit on detection of cognitive impairment: a cohort study. J Am Geriatr Soc. 2018;66(5):969‐975. 10.1111/jgs.15330 [DOI] [PubMed] [Google Scholar]
- 37. Cordell CB, Borson S, Boustani M, et al. Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement. 2013;9(2):141‐150. 10.1016/j.jalz.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 38. Chandra A, Staiger DO. Productivity spillovers in healthcare: evidence from the treatment of heart attacks. J Polit Econ. 2007;115:103‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muhlestein D, Saunders RS, Richards R, McClellan MB. Recent Progress In The Value Journey: Growth Of ACOs And Value‐Based Payment Models In 2018 | Health Affairs. Health Affairs Blog. Published August 14, 2018. https://www.healthaffairs.org/do/10.1377/hblog20180810.481968/full/. Accessed March 14, 2020 [Google Scholar]
- 40. Hu J, Jensen GA, Nerenz D, Tarraf W. Medicare’s annual wellness visit in a large health care organization: who is using it? Ann Intern Med. 2015;163(7):567‐568. 10.7326/L15-5145 [DOI] [PubMed] [Google Scholar]
- 41. Chung S, Lesser LI, Lauderdale DS, Johns NE, Palaniappan LP, Luft HS. Medicare annual preventive care visits: use increased among fee‐for‐service patients, but many do not participate. Health Aff (Millwood). 2015;34(1):11‐20. 10.1377/hlthaff.2014.0483 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material


